Abstract
Protein energy malnutrition (PEM) increases susceptibility to infectious diseases, including tuberculosis (TB), but it is not clear how PEM influences vaccine-promoted immunity to TB. We demonstrate that PEM during low-level steady-state TB infection in a mouse model results in rapid relapse of Mycobacterium tuberculosis, as well as increased pathology, in both Mycobacterium bovis BCG-vaccinated and unvaccinated animals. PEM did not change the overall numbers of CD4 T cells in BCG-vaccinated animals but resulted in an almost complete loss of antigen-specific cytokine production. Furthermore, there was a change in cytokine expression characterized by a gradual loss of multifunctional antigen-specific CD4 T cells and an increased proportion of effector cells expressing gamma interferon and tumor necrosis factor alpha (IFN-γ+ TNF-α+ and IFN-γ+ cells). PEM during M. tuberculosis infection completely blocked the protection afforded by the H56-CAF01 subunit vaccine, and this was associated with a very substantial loss of the interleukin-2-positive memory CD4 T cells promoted by this vaccine. Similarly, PEM during the vaccination phase markedly reduced the H56-CAF01 vaccine response, influencing all cytokine-producing CD4 T cell subsets, with the exception of CD4 T cells positive for TNF-α only. Importantly, this impairment was reversible and resupplementation of protein during infection rescued both the vaccine-promoted T cell response and the protective effect of the vaccine against M. tuberculosis infection.
INTRODUCTION
In 2013, an estimated 9 million people developed clinical tuberculosis (TB), 1.5 million of whom died (1). Healthy, immunocompetent individuals are usually successful at containing the infection following exposure to Mycobacterium tuberculosis, the causative agent of TB, resulting in latent, asymptomatic infection. Over a lifetime, the risk of reactivation of latent TB is estimated to be 10% but can be drastically increased in individuals who are immunocompromised because of HIV infection, immunosuppressive treatments, or malnutrition (2). Hence, with approximately two billion people already infected with M. tuberculosis, there is a vast potential for future transmission, disease, and deaths.
Historically, malnutrition, and in particular, protein energy malnutrition (PEM), has been associated with increased susceptibility to TB, although very few studies have addressed this in clinical settings. For ethical reasons, randomized controlled trials connecting PEM to TB disease and/or reactivation of latent TB in humans have not been possible and environmental and socioeconomic risk factors such as crowding, poor housing, and limited access to health care in many developing countries have been confounding factors in observational studies (reviewed in reference 3). However, even given these limitations, there are several classical observational clinical studies that provide compelling evidence of the contribution of PEM to TB risk in regions of both high and low endemicity (reviewed in reference 4). Studies conducted with experimental animals under controlled conditions have confirmed the epidemiological data and provided a link between PEM and the development of more progressive TB infection (4–6). Studies that used animal models have also demonstrated a negative influence of PEM on Mycobacterium bovis BCG-induced immunity in the very susceptible guinea pig model (7, 8).
There are currently extensive international efforts to develop novel and more efficient TB vaccines to either replace or boost BCG (9–13). As immunity to TB is mediated by protective T cells, these vaccines employ a range of adjuvants or live-delivery systems to promote protective T cells (reviewed in references 13 and 14). However, the cellular part of the immune system has, in early studies, been reported to be sensitive to the effects of PEM (15, 16), resulting in reduced production of the T helper type 1 (Th1) cytokines gamma interferon (IFN-γ) and interleukin-2 (IL-2) (17–20). The cellular and molecular background of this reduced response is not fully understood and has been the subject of some debate (21–25).
In this study, we examined the effect of PEM on disease progression in a low-dose TB murine infection model and on vaccine-promoted immunity. We demonstrate that PEM perturbs the balance between host and pathogen but that the T cell impairment following PEM is not intrinsic to the vaccine-promoted T cell response and can be rescued by nutrient supplementation during infection.
MATERIALS AND METHODS
Ethics statement.
The experiments described here were conducted in agreement with the regulations set forward by the Danish Ministry of Justice under the animal protection committees by Danish Animal Experiments Inspectorate Permits 2004-561-868 and 2009/561-1655, with a specific extension for the protein malnutrition experiments. This is compliant with European Community directive 2010/63/EU for the care and use of laboratory animals. The Statens Serum Institut Animal Ethics Board approved these experiments.
Animals.
Six- to eight-week-old inbred female BALB/c × C57BL/6 (CB6F1) mice were obtained from Harlan Scandinavia (Allerød, Denmark). Mice were kept at the experimental animal facilities at the Statens Serum Institut. In addition, infected mice were housed in cages contained within laminar-flow safety enclosures (Scantainer; Scanbur) in a biosafety level 3 facility and provided with irradiated food (Harlan Teklad) and filtered drinking water. All animals were allowed a minimum of 1 week of acclimatization prior to commencement of experimental procedures.
Bacteria.
M. tuberculosis Erdman was grown at 37°C suspended in Sauton medium (BD Pharmingen) enriched with 0.5% sodium pyruvate, 0.5% glucose, and 0.2% Tween 80. BCG Danish strain 1331 was grown at 37°C in Middlebrook 7H9 medium (BD Pharmingen). All bacteria were grown to log phase and then stored at −80°C in growth medium at ∼5 × 108 CFU/ml. Bacteria were thawed, placed in an ultrasound bath for 5 min, and forced through a syringe to disperse bacterial clumps, washed, and diluted in phosphate-buffered saline (PBS) prior to infection.
Synthetic peptides.
Synthetic overlapping peptides (15- or 18-mers) covering the complete primary structure of ESAT-6 and TB10.4 were synthesized by standard solid-phase methods on a SyRo peptide synthesizer (MultiSynTech; New England) at JPT Peptide Technologies (Berlin, Germany) or GenScript. Peptides were lyophilized and stored dry at −20°C until reconstitution in PBS and dimethyl sulfoxide (1:1 ratio).
Experimental vaccine.
The vaccine antigen construct H56 (Ag85B-ESAT-6-Rv2660c) (11, 26) was formulated with the adjuvant CAF01 (27). The doses used were 5 μg of H56 and 100 μl of CAF01, corresponding to 250 μg of dimethyldioctadecylammonium and 50 μg of α,α′-trehalose 6,6′-dibehenate.
Histopathological, histomorphometric, and immunohistochemical analyses.
The right lung lobe of each mouse was fixed by immersion in 10% neutral buffered formalin and processed for histological examination. Cut sections were stained with hematoxylin and eosin and by Ziehl-Neelsen techniques and evaluated without prior knowledge of the stage of infection or the treatment group. Lesions were quantitated by computer-aided histomorphometry (Palmrobo software, version 1.2.3; Palm Microlaser Technologies AG Ltd., Bernried, Germany). Granuloma morphometry was carried out with the public-domain, Java-based image processing program ImageJ (imagej.nih.gov/ij/). The cross-sectional areas of 10 randomly selected granulomas from 2% protein (2%P)- and 16% protein (16%P)-fed mice were measured, as were areas within granulomas occupied by lymphocytes (at week 3) and affected by necrosis (at week 9). Percentages of granuloma area infiltrated by lymphocytes and those affected by necrosis were then calculated for each treatment group at weeks 3 and 9, respectively. Immunolabeling of inducible nitric oxide synthase (iNOS), an indicator of macrophage activation status within lesions, was carried out following dewaxing and rehydration of sections and an epitope retrieval step where tissue slides were microwaved for 20 min in a trisodium citrate solution (pH 6.0). Subsequently, treatment of sections with normal goat serum blocking solution (Vectastain ABC kit; Vector Laboratories, CA) was performed to block endogenous peroxidase. The primary antibody (rabbit polyclonal anti-mouse iNOS/NOSII; Upstate, NY) at a 1/1,000 dilution was then applied for 1 h; this was followed by the sequential application of biotinylated goat anti-rabbit IgG (Vector Laboratories) and ABC solution for 30 min, respectively, at room temperature (Vectastain ABC kit; Vector Laboratories). Visualization of target cells followed the application of a diaminobenzidine-tetrahydrochloride solution (Sigma-Aldrich, Germany) and a hematoxylin counterstain.
Experimental infections, PEM, and vaccination. (i) Relapse model.
Mice were vaccinated subcutaneously (s.c.) with BCG at 5 × 107 CFU/ml in 100 μl or the equivalent volume of saline as a control. After 6 weeks, virulent M. tuberculosis Erdman was delivered via the respiratory route at approximately 50 CFU per mouse (see Fig. 1A for the experimental outline) with an inhalation exposure system (GlasCol) or Biaera exposure system controlled via AeroMP software. Fifteen weeks after the aerosol challenge, groups of mice were fed either the standard 16%P diet (Harlan Teklad) or an isocaloric 2%P diet (Harlan Teklad) and the animals remained on their respective diet for the duration of the experiment. Lungs were harvested from mice sacrificed at various time points following PEM. Immune responses were determined at weeks 0, 3, and 9 after PEM as described below. The numbers of bacteria in the lung from weeks 6, 15, 18, 20, and 24 were determined by plating serial 3-fold dilutions of individual organ homogenates in duplicate on 7H11 medium supplemented with PANTA (Becton Dickinson, San Diego, CA) and 2 μg/ml of 2-thiophene-carboxylic acid hydrazide (TCH). The latter supplement selectively inhibits residual BCG bacterial growth. Colonies were counted after 2 to 3 weeks of incubation at 37°C. The detection limit for bacterial enumeration was 0.3 log10 CFU, and in animals where no bacteria were detected, the CFU counts were arbitrarily set at the detection limit. Bacterial numbers are expressed as log10 CFU counts. Mice were routinely weighed to track changes in weight following initiation of PEM.
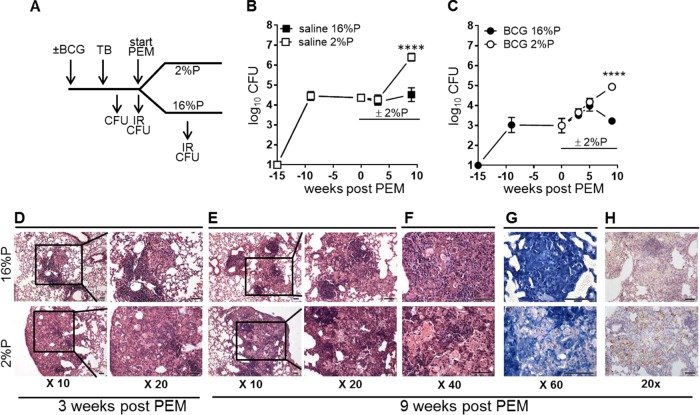
FIG 1.
PEM induces disease relapse in steady-state TB infections in both saline-treated and BCG-vaccinated mice. Mice were vaccinated with BCG or given saline 6 weeks prior to M. tuberculosis infection by the aerosol route. PEM was initiated at week 15 p.i. Lungs were harvested at various time points p.i. and after PEM for assessment of immune responses (IR) and bacterial numbers (CFU). (A) Schematic drawing of the experimental setup (see Materials and Methods for more details). For determination of bacterial loads, lungs were harvested, homogenized, and cultured for 2 to 3 weeks at 37°C before enumeration of bacteria. (B) CFU counts in TB-infected control animals with a normal diet (16%) and PEM (2%). Data are displayed as the mean ± the standard error of the mean (n = 5 or 6 mice per time point). (C) CFU counts of BCG-vaccinated animals were determined as described above. Data are displayed as the mean ± the standard error of the mean (n = 9 to 19 individual mice per time point) and are from two independent experiments. Statistical analysis was done by two-way ANOVA and a Bonferroni posttest. ****, P < 0.0001. (D to H) Photomicrographs of lung tissue from TB-infected, BCG-vaccinated mice on a normal diet (16%P, top) and PEM (2%P, bottom) groups. These were stained with hematoxylin and eosin (D to F), by the Ziehl-Neelsen method (G), and by immunohistochemistry for iNOS (H). The images shown are representative of lesions observed in four mice in each group. Scale bars, 50 μm.
(ii) Vaccination and PEM during infection.
Mice were vaccinated with 5 μg of H56 formulated in CAF01 (27) in a total volume of 200 μl per mouse. Immunizations were given s.c. at the base of the tail three times with 3-week intervals between immunizations. Negative-control mice were given three equivalent doses of saline. Six weeks after the final vaccination, mice were infected by the aerosol route with approximately 100 CFU/mouse as described above. Simultaneously, half the mice were fed the low-protein 2%P diet whereas the other half continued on the control 16%P diet. At weeks 6 and 18 postinfection (p.i.), lungs were harvested from sacrificed mice and used to assess the immune response, as described below, and the protective efficacy of the vaccine. Enumeration of bacteria in the lungs was done as described above, but the lung homogenates were cultured on 7H11 medium supplemented with PANTA (Becton Dickinson, San Diego, CA) (without TCH supplementation).
(iii) Vaccination and PEM during vaccination.
Following 1 week of acclimatization, mice were fed the 2%P diet or 16%P diet. After 2 weeks, mice were vaccinated with 5 μg of H56 formulated in CAF01 in a total volume of 200 μl per mouse, as described above, and control animals received an equivalent volume of saline. Two weeks after the final vaccination, all mice were fed the 16%P diet. Mice were bled 6 weeks after the final vaccination to assess vaccine-induced immune responses as described below. At that time, mice were infected by the aerosol route with approximately 100 CFU as described above. Protection conferred by H56 vaccination under normal (16%P) or protein-deficient (2%P) conditions was determined at weeks 6 and 18 p.i. by culturing lung homogenate as described above.
For the initial studies of PEM's influence on primary TB infection (see Fig. S1 in the supplemental material), groups of mice were fed a 2%P or 16%P diet 2 weeks prior to infection and throughout the experiment.
Cell preparation, ELISA, and flow cytometric analyses.
Lymphocytes from perfused lungs and peripheral blood mononuclear cells (PBMCs) were isolated, cultured, and used to assess immune responses (28–30). PBMCs were purified on Lympholyte cell separation medium (Cedarlane Laboratories Inc.) according to the manufacturer's protocol, followed by two RPMI washing procedures. Lung lymphocytes were obtained by passage of lungs through a 100-μm nylon cell strainer (BD Pharmingen), followed by two washing procedures with RPMI. Cells were cultured in sterile microtiter wells (96-well plates; Nunc) containing 2 × 105 cells/well for enzyme-linked immunosorbent assay (ELISA) or 1 × 106 to 2 × 106 cells/well for flow analysis. ELISA and flow cell cultures were in 200 μl of RPMI 1640 supplemented with 5 × 10−5 M 2-mercaptoethnaol, 1% pyruvate, 1% HEPES, 1% (vol/vol) premixed penicillin-streptomycin solution (Invitrogen Life Technologies), 1 mM glutamine, and 10% (vol/vol) fetal calf serum (FCS) and were restimulated with antigen at 2 μg/ml (peptide pools for ESAT-6 and TB10.4 and recombinant protein for H56). IFN-γ, tumor necrosis factor alpha (TNF-α), or IL-2 secretion was measured in supernatants after 72 h of incubation at 37°C in 5% CO2. Determination of IFN-γ secretion alone was done by ELISA as previously described (30). For determination of multiple cytokines, a multiplex cytokine release assay (Meso Scale Discovery [MSD]) was applied in accordance with the manufacturer's protocol. For intracellular staining of cytokines (ICS), cells from the blood or lungs of mice were stimulated for 1 h with 2 μg/ml antigen and subsequently incubated for 5 h at 37°C with 10 μg/ml brefeldin A (Sigma-Aldrich). Cells were washed in fluorescence-activated cell sorter (FACS) buffer (PBS containing 0.1% sodium azide and 1% FCS) before staining with a combination of the following rat anti-mouse antibodies: peridinin chlorophyll protein-Cy5.5–anti-CD8α (53-6.7, RM4-5) and allophycocyanin (APC)-Cy7–anti-CD4 (GKI.5). Cells were washed with FACS buffer before fixation and permeabilization with the BD Cytofix/Cytoperm kit (BD, San Diego, CA) according to the manufacturer's protocol before staining for intracellular cytokines with phycoerythrin (PE)-Cy7–anti-IFN-γ (XMG1.2), PE–anti-TNF-α, and APC–anti-IL-2 (JES6-5H4). After washing, cells were suspended in a 4% (wt/vol) formaldehyde solution (Bie & Berntsen), pH 7.0, for 30 min before resuspension in FACS buffer. Samples were analyzed on a six-color BD FACSCanto flow cytometer (BD Biosciences). Responses were analyzed with FlowJo Software (TreeStar, Ashland, OR) by Boolean gating of IFN-γ-, TNF-α-, and IL-2-positive CD4 T cells (gating sequence: singlets, cells, lymphocytes, CD4 versus forward scatter (FSC), cytokine versus CD4). The cytokine-responsive cell populations divided according to their cytokine profile were displayed with the representation of each individual subpopulation (bar graphs). The background levels of cytokine production measured in medium-stimulated cells were deducted (background, <0.5%).
Statistics.
Results based on individual mice were subjected to the statistical analyses indicated with Prism 6 (GraphPad Software). Briefly, differences between 2%P- and 16%P-fed mice measured over time were analyzed by two-way analysis of variance (ANOVA), followed by Bonferroni's posttest. Differences between the cytokine profiles of 2%P- and 16%P-fed mice were analyzed with a t test comparing the mean values of each T cell subpopulation. For all tests, P values of <0.05 were considered statistically significant.
RESULTS
Protein deficiency leads to relapse of steady-state TB infection.
The purpose of our studies was to establish a model for addressing the effect of PEM on protective vaccination. We investigated the influence of a 2%P diet instead of the standard 16%P diet. This diet had a significant influence on the bacterial load during a primary aerosol TB infection in the mouse model (see Fig. S1 in the supplemental material), in agreement with earlier studies of PEM (5, 17). We started by examining if a 2%P diet could induce relapse of disease when introduced to mice with an established M. tuberculosis infection. Furthermore, we wanted to address if prior BCG vaccination influenced the progression of the infection following PEM. Groups of mice (BCG-vaccinated mice or saline-treated controls) were infected with M. tuberculosis and allowed to develop a steady-state infection (week 15) before PEM was initiated (Fig. 1A, experimental outline). In both control and BCG-vaccinated mice, malnutrition resulted in very significant weight loss (see Fig. S2 in the supplemental material), in accordance with previous publications (31). The growth of M. tuberculosis in nonimmunized control mice plateaued at around 4.5 log10 CFU (Fig. 1B), whereas the bacterial levels in the BCG-vaccinated mice stabilized at approximately 3 log10 CFU (Fig. 1C). For both saline-treated and BCG-vaccinated mice, there was no effect of PEM on the bacterial load immediately following initiation of the 2%P diet (Fig. 1B and C). In fact, the CFU counts of the two groups were similar until week 5 postinitiation of PEM. However, after 9 weeks of PEM, we detected a highly significant 100-fold increase in bacterial numbers in the malnourished animals in both groups (saline, P = 0.0008; BCG, P < 0.0001).
The influence of PEM on the histopathological appearance of the lungs of BCG-vaccinated animals was investigated. Although there were no statistically significant differences between the groups in the number (week 18: 2%P, 9.3 ± 3.9; 16%P, 5.3 ± 1.7 [P = 0.1070]) or size of lesions (week 18: 2%P, 0.89 ± 0.71 µm2; 16%P, 0.51 ± 0.37 µm2 [P = 0.3914]), PEM had a qualitative influence on the composition of the pulmonary granulomas (Fig. 1D to G). Pulmonary granulomas consisted of closely apposed macrophages with interposed dense clusters/wedges of lymphocytes, but at 3 weeks after PEM, the cellular infiltrates were less compact, with significantly lower percentages of infiltrating lymphocytes in the 2%P-fed mice (18.4% ± 5.8%) than in the 16%P-fed mice (34.8% ± 3.6%; P = 0.0265) (Fig. 1D, bottom). Six weeks later (9 weeks after PEM), the difference between the two groups had developed further, with more abundant foci of necrosis (2%P, 7.2% ± 1.1%; 16%P, 0.8% ± 0.5% [P < 0.0001]), more extensive neutrophil accumulation (Fig. 1E and F), and numerous acid-fast mycobacteria in the PEM group (Fig. 1G). More intense labeling of macrophages for cytoplasmic iNOS (a measure of their activation status) was noted in 2%P-fed mouse lesions at both time points (Fig. 1H, shown only for 9 weeks after PEM).
PEM severely impairs the T cell immune response.
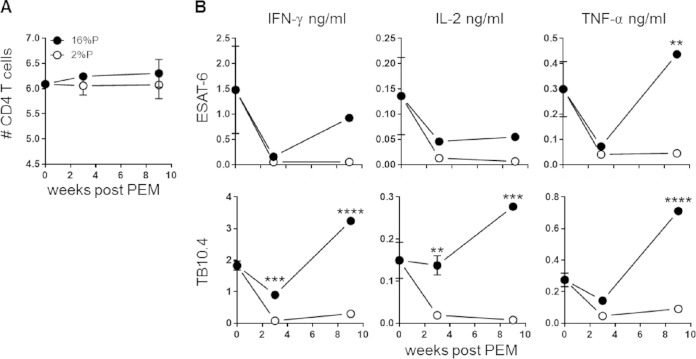
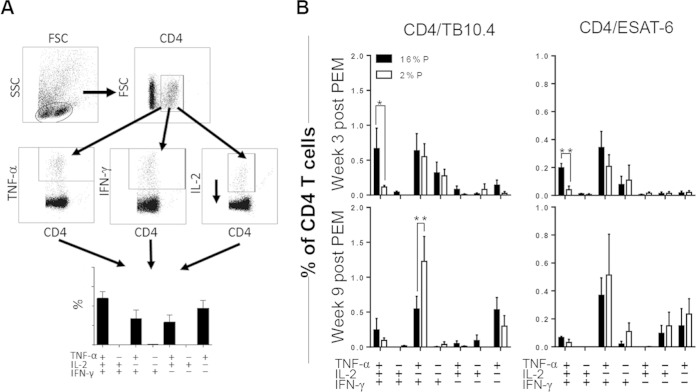
We continued by investigating if the antigen-specific CD4 T cell response is influenced by PEM. Lymphocytes isolated from perfused lungs at different time points after PEM were used for assessment of CD4 T cell responses known to be promoted by BCG. There was no significant impact of PEM on the overall CD4 T cell numbers (Fig. 2A). In contrast, there was a pronounced effect on the antigen-specific cytokine response, where the levels were significantly reduced in 2%P-fed mice. Cytokine secretion was assessed in lung lymphocytes by stimulation with peptide pools covering the entire sequence of the antigens ESAT-6 and TB10.4, both of which are immunodominant during M. tuberculosis infection (Fig. 2B). Secretion of the Th1 cytokines IFN-γ, IL-2, and TNF-α was determined in culture supernatants with a multiplex cytokine assay kit. Overall, we observed clearly diminished levels of cytokine secretion in the 2%P-fed group for both the antigens and the mitogen control (Fig. 2B, data shown only for the antigens). The difference between the 2%P- and 16%P-fed groups was most pronounced in the TB10.4-specific response, with a 10-fold reduction in the overall cytokine response in the 2%P-fed mice. Furthermore, there were marked differences in the cytokine profiles of the M. tuberculosis-specific CD4 T cells following PEM measured by ICS (Fig. 3). The various CD4 T cell subsets were enumerated on the basis of IFN-γ-, TNF-α-, and IL-2-expressing CD4 T cells and with FlowJo to perform a Boolean gating analysis (Fig. 3A, gating sequence). For both antigens, there was a significantly lower proportion of polyfunctional T cells coproducing IFN-γ, TNF-α, and IL-2 in malnourished mice, which was most pronounced at week 3 after PEM (TB10.4, P = 0.0132; ESAT6, P = 0.0082) (Fig. 3B). This was followed by increased numbers of the effector subsets producing IFN-γ or IFN-γ and TNF-α (TB10.4, P = 0.0205; ESAT6, P = 0.06649), evident after relapse at week 9 after PEM.
FIG 2.
PEM reduces antigen-specific cytokine responses. Groups of mice were vaccinated with BCG, infected with M. tuberculosis, and subsequently fed a 16%P or 2%P diet. Mice were terminated at weeks 0, 3, and 9 after PEM. (A) Lymphocytes from perfused lungs were stained for CD4 and analyzed by flow cytometry. The numbers of CD4 T cells were calculated by multiplying the percentage of CD4 cells (of the total cells in a forward scatter-side scatter plot) by the cell count from the single-cell suspensions for 2%P-fed (open circles) and 16%P-fed (closed circles) mice. Data are expressed as the mean ± the standard error of the mean (n = 4 to 6 mice per group per time point). (B) Lung lymphocytes were harvested from perfused lungs and stimulated with TB10.4 or ESAT-6 in cell culture plates for 72 h at 37°C. The production of IFN-γ, TNF-α, and IL-2 was assessed with an MSD multiplex cytokine kit. Each data point represents a pool of four mice, assessed in technical duplicates or triplicates and represents the mean ± the standard error of the mean. The results shown are representative of two independent experiments. Statistical analysis was done by two-way ANOVA and Bonferroni's multiple-comparison posttest. *, P < 0.05; **, P < 0.01; ***, P < 0.001; ****, P < 0.0001.
FIG 3.
PEM is associated with a reduced proportion of multifunctional CD4 T cells. Groups of mice were vaccinated with BCG, infected with M. tuberculosis, and subsequently fed a 16%P (n = 4 to 7 per time point) or a 2%P (n = 3 to 6 per time point) diet. Mice were sacrificed at weeks 3 (prelapse) and 9 (postlapse) after PEM. Single-cell suspensions obtained from perfused lungs were stimulated in vitro with no antigen, TB10.4, or ESAT-6 for 5 h prior to staining for CD4, TNF-α, IFN-γ, and IL-2 for multicolor ICS flow cytometric analysis. (A) Gating sequence for flow analysis. Lymphocytes were gated from the side scatter (SSC)-forward scatter (FSC) plot, followed by gating of IFN-γ-, TNF-α-, and IL-2-positive cells from the CD4-positive population. With FlowJo for Boolean gating analysis, the antigen-specific CD4 T cells were subdivided into seven distinct subsets based on their cytokine profiles. (B) The ICS data were analyzed as described in Materials and Methods, and the frequency of each CD4 subset is depicted as the mean ± the standard error of the mean for 16%P (black bars) and 2%P (white bars) mice. The results shown are from one experiment and are representative of two individual experiments. All of the background from medium-stimulated cells (<0.5%) has been subtracted. Statistical analysis was done by t test. *, P < 0.05; **, P < 0.01.
Nutritional status during the effector phase and not the priming phase is critical for vaccine performance.
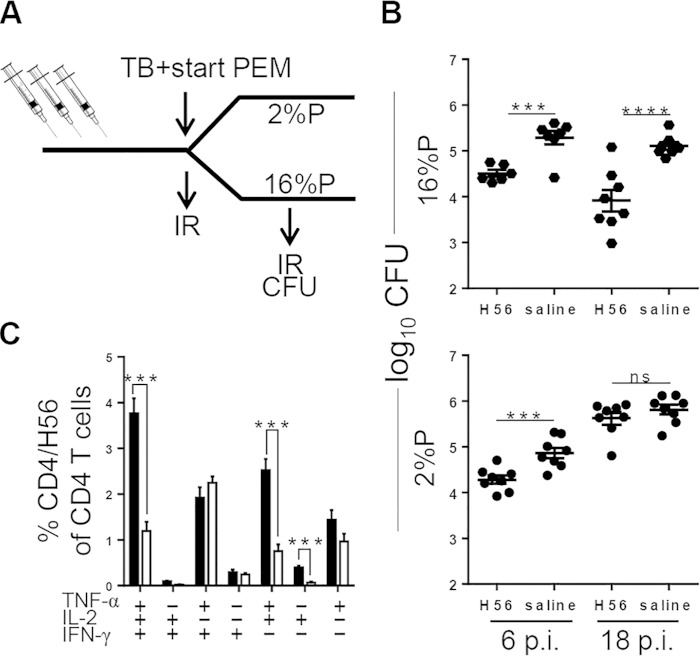
Thus far, we observed that PEM is associated with an impairment of the T cell response and disease relapse, i.e., loss of infection control and/or impairment of protection conferred by BCG. We continued by investigating the influence of PEM on the efficacy of an adjuvanted subunit vaccine, H56, with a demonstrated protective effect in a range of experimental animal models (11, 26, 32). Groups of mice were vaccinated with H56 formulated in CAF01 or given saline as a control (Fig. 4A, experimental outline). PEM was initiated at the time point of M. tuberculosis challenge, and the subsequent effect of PEM during infection was assessed. In normal animals (16%P), H56-CAF01 was protective early during infection (0.8 log10 at week 6 p.i.) and, as previously reported (11), the vaccine was more efficacious at later time points of infection (1.2 log10 at week 18 p.i.). H56 vaccination also gave significant protection against M. tuberculosis to the malnourished animals, but the effect was less than that in mice receiving normal levels of protein (0.6 log at week 6 p.i.). The vaccine protection was, furthermore, only transient, with no CFU count difference noted between vaccinated and control animals at week 18 (Fig. 4B, bottom). At that time point, the CD4 T cell response in 2%P-fed mice was dominated by IFN-γ- and TNF-α-cosecreting cells, whereas the IL-2-producing populations (IFN-γ+ TNF-α+ IL-2+, TNF-α+ IL-2+, and IL-2+) that dominated the response in normal mice were significantly reduced to very low levels in malnourished mice (IFN-γ+ TNF-α+ IL-2+, P = 0.0006; TNF-α+ IL-2+, P = 0.0008; IL-2+, P = 0.0003) (Fig. 4C).
FIG 4.
PEM during infection results in loss of vaccine-mediated protection. (A) Experimental outline. Groups of mice were vaccinated with H56 formulated in CAF01 or saline as a control. At the onset of an M. tuberculosis challenge, mice were divided into two groups fed a 2%P or 16%P diet until the end of the experiment. (B) Mice from the 2%P- and 16%P-fed groups were sacrificed at weeks 6 and 18 p.i. Assessment of H56-induced protection was done by culturing lung homogenates at 37°C and enumerating the bacteria; results are expressed as log10 CFU counts. Data are depicted as the mean ± the standard error of the mean of 8 to 16 individual mice. Statistical analysis was done by two-way ANOVA with a Bonferroni posttest for multiple comparisons. ns, not significant; ***, P < 0.001; ****, P < 0.0001. (C) At week 18 p.i., lymphocytes from perfused lungs were stimulated with H56 for 5 h before staining with fluorescently labeled anti-CD4, -TNF-α, -IFN-γ, and -IL-2 antibodies for ICS analysis and Boolean gating as described above. The cytokine-producing CD4 T cells were subdivided into seven distinct subsets dependent on the cytokine profile for both 2%P (white bars) and 16%P (black bars). Data are depicted as the mean ± the standard error of the mean of four individual mice. All background values from medium-stimulated cells (<0.5%) have been subtracted. Statistical analysis was done by t test. ns, not significant; ***, P < 0.001; ****, P < 0.0001.
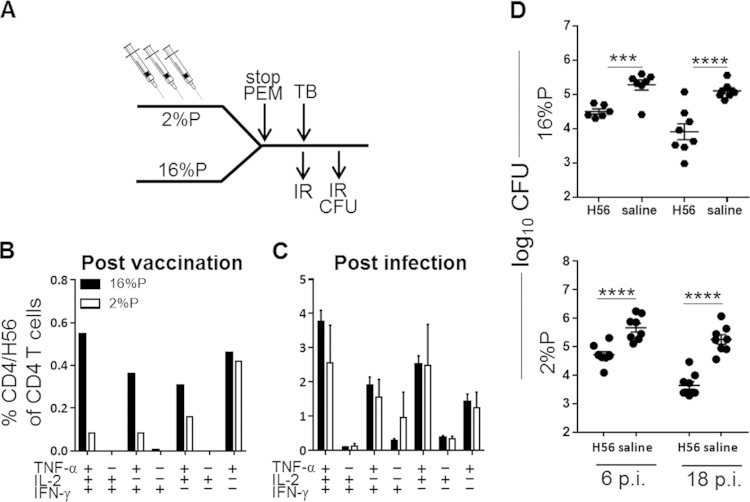
We then went on to examine if PEM during the vaccination phase has a similar detrimental effect on vaccine-conferred immunity. Groups of mice fed either the 2%P or the 16%P diet during H56 vaccination were returned to a normal diet prior to an M. tuberculosis challenge (Fig. 5A, experimental outline). The T cell response to H56 in nutritionally normal mice was dominated by multifunctional IFN-γ+ TNF-α+ IL-2+ CD4 T cells, with a proportion of TNF-α+ cells either singly or coexpressing IL-2 or IFN-γ. PEM during vaccination resulted in severe impairment of the vaccine-specific immune response (Fig. 5B) and an overall reduction in all T cell subsets (with the exception of the TNF-α+ cells). However, nutritional replenishment during infection resulted in an almost complete rescue of the antigen-specific response and a distribution very similar to that in 16%P-fed mice, as seen by intracellular multicolor flow cytometry (Fig. 5C). In agreement with the reconstitution of normal T cell responses, the mice that were malnourished during vaccination were efficiently protected against an M. tuberculosis challenge (Fig. 5D).
FIG 5.
Impairment of vaccine-induced immune response by PEM is reversed by reconstitution of the diet during infection. (A) Experimental outline. Groups of mice were fed a 2%P or 16%P diet during the vaccination period (three vaccinations given at 3-week intervals). Two weeks after the final vaccination, all mice were fed the standard 16%P diet for the rest of the experiment. (B) Groups of mice were bled postvaccination, and PBMCs were stimulated with H56 for 6 h before staining for CD4, TNF-α, IFN-γ, and IL-2 for ICS analysis. Bar graphs denote frequencies of the CD4/H56 subsets for 16%P (black bars) and 2%P (white bars). Data are displayed as the mean ± the standard error of the mean of a pool of four mice per group. (C) Groups of mice were sacrificed at week 18 p.i., and lymphocytes were obtained from perfused lungs, stimulated with H56, and stained for ICS as described above for 2%P (white bars) and 16%P (black bars, also shown in Fig. 4C). Data are depicted as the mean ± the standard error of the mean of three or four individual animals. All background values from medium-stimulated cells (<0.5%) have been subtracted. (D) Lungs were harvested at weeks 6 and 18 p.i. from mice fed 2%P and 16%P (the 16%P data are also shown in Fig. 4B) during vaccination. Bacterial numbers were assessed by culturing lung homogenates at 37°C, and results are expressed as log10 CFU counts. Data are expressed as the mean ± the standard error of the mean (n = 8 to 16 mice). Statistical analysis was done by two-way ANOVA with a Bonferroni posttest for multiple comparisons. ***, P < 0.001; ****, P < 0.0001.
Hence, despite impairment of the vaccine-induced CD4/H56 response following vaccination, reconstitution of a higher protein content prior to infection was sufficient to rescue the response and preserve H56-mediated protection. Overall, nutritional status during vaccination therefore appears to be less critical for vaccine efficacy than does nutritional status during M. tuberculosis infection.
DISCUSSION
Historically, PEM has been associated with increased susceptibility to infectious diseases, including TB (reviewed in references 33–35). In the context of TB, this connection has been based on human observational studies (4, 36, 37) supported by animal model studies (4, 5, 17, 38). Our study demonstrates that vaccine-induced T cell response impairment provoked by PEM during vaccination is reversible following replenishment of dietary protein content, which resulted in the reestablishment of vaccine-conferred immunity (Fig. 5). In contrast, PEM during M. tuberculosis infection led to diminished vaccine-mediated protection (Fig. 1C and 4B) associated with an impaired T cell response (Fig. 2, 3, and 4C). PEM led to exacerbation of disease, as reflected in increased pulmonary bacterial loads and increasing pathological changes in the 2%P-fed mice, relative to those in the 16%P-fed animals (Fig. 1D to F). This exacerbation occurred regardless of when the nutritional deficiency was imposed, i.e., during the steady-state phase of an established infection or at the challenge time point. Despite the reduced levels of IFN-γ and TNF-α (Fig. 2B), there was no obvious impairment of iNOS expression (Fig. 1H), as has been previously reported following PEM (5). In fact, immunohistochemical labeling indicated that intralesional iNOS expression within macrophages was more pronounced in granulomas of PEM mice. One interpretation of this finding is that macrophages in these lesions remained at higher levels of activation in order to control putative larger numbers of organisms. In the absence (low availability) of IFN-γ and TNF-α, iNOS was most likely maintained or induced by other proinflammatory cytokines, such as IL-1 (39), triggered by the heightened bacterial load (Fig. 1C) but obviously insufficient for the control of bacterial growth.
Malnutrition has previously been reported to have an impact on BCG-promoted immunity in the highly susceptible guinea pig model (6–8, 38). However, to date, there have been very limited studies of the influence of malnutrition on immunity to novel TB subunit vaccines (40). In one study, impaired IFN-γ secretion and complete abolition of antibody production were induced in undernourished mice vaccinated with the pVAXhsp65 DNA vaccine, but there was no report if this affected vaccine efficacy (40). Most studies with experimental animals, with the exception of one (41), have reported PEM-associated impairment of the cell-mediated immune (CMI) response to BCG (7, 8, 38). Interestingly, as in the present study, McMurray and Bartow (38) found that nutritional repletion following BCG vaccination was sufficient for animals to regain some level of protection against an M. tuberculosis challenge, although protection was only measured by the bacterial load in the spleen following aerosol infection. In addition, those authors did not explore if this finding was due to reconstitution of the CMI response. In another study, nutritional replenishment did not reverse the effect of malnutrition during vaccination against Leishmania chagasi (42). This discrepancy may relate to the fact that a more severe malnutrition protocol was employed in the study of Malafaia et al., which combined protein, zinc, and iron deprivation, which together may have a more profound and irreversible impact (reviewed in reference 34). The implications of our findings are that PEM during vaccination, despite resulting in an immediate T cell impairment (Fig. 5C), does not affect subsequent immunity to a challenge when protein is reconstituted during infection (Fig. 5B). Similarly, a previous study found no indication for postponing BCG immunization in infants despite malnutrition in utero and low birth weight and found no significant difference in the BCG-primed immune response from that of normal birth weight controls (43, 44). Conversely, H56 vaccination-promoted immunity was almost completely blocked if animals were malnourished during M. tuberculosis infection and this was associated with a massive loss of the IL-2+ subsets such as IFN-γ+ TNF-α+ IL-2+, TNF-α+ IL-2+, and IL-2+ cells (Fig. 4C). This selective loss of IL-2-producing T cell subsets expands on an earlier observation in the guinea pig model where IL-2 activity was severely impaired in malnourished animals (45), as measured by the proliferative response of an IL-2-dependent cell line to culture supernatants harvested from blood and spleen. The IL-2-producing subsets have been reported to represent central memory T cells capable of replenishing the T cells at the site of infection and therefore to be of crucial importance for the long-term containment of TB infection (46, 47). Lack of homeostatic proliferation of these memory T cells (48) could result in a gradual depletion of functional T cells and ultimately contribute to the lack of protection at the late time point, in malnourished H56 vaccinated mice (Fig. 4B). T cell activation and proliferation are both processes with high energy demands and are dependent on both glucose and amino acid availability. Leptin is a hormone whose primary role is to regulate glucose uptake and fat deposition, and reduced levels thereof have been reported in malnourished individuals (22). Recent data suggest that leptin, in addition to its metabolic functions, is essential for activation of effector T cells (21), and leptin has even been suggested as a potential vaccine adjuvant (49). It would be highly relevant in future TB PEM studies to monitor leptin levels and the potential role of leptin supplementation. The shortage of some amino acids may be of particular importance during PEM. Glutamine is a critical amino acid for T cell activation, and supplementation has been reported to increase Th1 cytokine responses (50, 51). Arginine is a precursor of nitric oxide, an important effector molecule in M. tuberculosis infection (52–54), and limitation of this amino acid may result in impaired macrophage control with M. tuberculosis growth and subsequent functional exhaustion, i.e., loss of IL-2-producing T cells (55). Both glutamine and arginine are therefore amino acids that may be of particular relevance during PEM and where the potential impact of selective supplementation would be highly relevant.
It has been difficult to delineate the effect of PEM on TB through analytical epidemiology and dissect the temporal relationships, as malnutrition is a cause of TB disease but wasting is also one of the consequences of TB (35). Very few studies provide solid evidence of the effect of PEM, besides those conducted in controlled experiments with animal models (5, 56), although some of the studies described earlier do support a link between PEM and TB disease (4, 37). Briefly, these studies associated PEM with an increased TB incidence without the influence of other socioeconomic factors, such as crowding, poor housing, and bad hygiene. In our study, we did find a direct effect of PEM on disease relapse of already infected mice (Fig. 1B and C), an experiment that, for ethical reasons, would never be approved with humans.
In summary, the data show that protein is critical for the maintenance of a functional T cell response during ongoing TB infection. Our findings indicate that PEM does not cause intrinsic defects in the T cell response promoted during vaccination, because protein supplementation restored this response. On the other hand, prolonged PEM abolished or severely impaired the vaccine promoted CD4 response, with a negative impact on host protection. Given that one-third of the global population is already infected with TB, our data provide a compelling argument for combining nutritional supplementation with vaccination in regions of high TB endemicity and poor nutritional status.
Supplementary Material
ACKNOWLEDGMENTS
We thank Camilla Haumann Rasmussen, Linda Christensen, Sandra Isling, Katja Bøgebjerg Carlsen, Merete Henriksen, and the Department for Biological Services for excellent technical assistance. Claus Aagaard and Vivi Andersen provided the H56 protein, and the Vaccine Adjuvant Research group provided CAF01. We thank Brian Cloak for his assistance in the preparation of the photomicrographs.
This work was supported by the Danish Council for Strategic Research, Centre for Nano-Vaccine (contract 0603-00322B), ADITEC (FP7/2007-2013 under grant agreement FP7-HEALTH-F2010-280873), and TBVAC2020 under grant agreement H2020-PHC-643381. The funders had no role in study design, data collection and analysis, the decision to publish, or preparation of the manuscript.
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/IAI.03030-14.
REFERENCES
- 1.World Health Organization. 2014. Global tuberculosis report 2014. World Health Organization, Geneva, Switzerland: http://apps.who.int/iris/bitstream/10665/137094/1/9789241564809_eng.pdf. [Google Scholar]
- 2.Selwyn PA, Sckell BM, Alcabes P, Friedland GH, Klein RS, Schoenbaum EE. 1992. High risk of active tuberculosis in HIV-infected drug users with cutaneous anergy. JAMA 268:504–509. doi: 10.1001/jama.268.4.504. [DOI] [PubMed] [Google Scholar]
- 3.Lienhardt C. 2001. From exposure to disease: the role of environmental factors in susceptibility to and development of tuberculosis. Epidemiol Rev 23:288–301. doi: 10.1093/oxfordjournals.epirev.a000807. [DOI] [PubMed] [Google Scholar]
- 4.Cegielski JP, McMurray DN. 2004. The relationship between malnutrition and tuberculosis: evidence from studies in humans and experimental animals. Int J Tuberc Lung Dis 8:286–298. [PubMed] [Google Scholar]
- 5.Chan J, Tian Y, Tanaka KE, Tsang MS, Yu K, Salgame P, Carroll D, Kress Y, Teitelbaum R, Bloom BR. 1996. Effects of protein calorie malnutrition on tuberculosis in mice. Proc Natl Acad Sci U S A 93:14857–14861. doi: 10.1073/pnas.93.25.14857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dai G, Phalen S, McMurray DN. 1998. Nutritional modulation of host responses to mycobacteria. Front Biosci 3:e110–122. [DOI] [PubMed] [Google Scholar]
- 7.McMurray DN, Yetley EA. 1982. Cell-mediated immunity in malnourished guinea pigs after Mycobacterium bovis BCG vaccination. Infect Immun 35:909–914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McMurray DN, Carlomagno MA, Mintzer CL, Tetzlaff CL. 1985. Mycobacterium bovis BCG vaccine fails to protect protein-deficient guinea pigs against respiratory challenge with virulent Mycobacterium tuberculosis. Infect Immun 50:555–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Knudsen NP, Norskov-Lauritsen S, Dolganov GM, Schoolnik GK, Lindenstrøm T, Andersen P, Agger EM, Aagaard C. 2014. Tuberculosis vaccine with high predicted population coverage and compatibility with modern diagnostics. Proc Natl Acad Sci U S A doi: 10.1073/pnas.1314973111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ottenhoff TH, Kaufmann SH. 2012. Vaccines against tuberculosis: where are we and where do we need to go? PLoS Pathog 8:e1002607. doi: 10.1371/journal.ppat.1002607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Aagaard C, Hoang T, Dietrich J, Cardona PJ, Izzo A, Dolganov G, Schoolnik GK, Cassidy JP, Billeskov R, Andersen P. 2011. A multistage tuberculosis vaccine that confers efficient protection before and after exposure. Nat Med 17:189–194. doi: 10.1038/nm.2285. [DOI] [PubMed] [Google Scholar]
- 12.Tameris MD, Hatherill M, Landry BS, Scriba TJ, Snowden MA, Lockhart S, Shea JE, McClain JB, Hussey GD, Hanekom WA, Mahomed H, McShane H, MVA85A 020 Trial Study Team . 2013. Safety and efficacy of MVA85A, a new tuberculosis vaccine, in infants previously vaccinated with BCG: a randomised, placebo-controlled phase 2b trial. Lancet 381:1021–1028. doi: 10.1016/S0140-6736(13)60177-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Orme IM. 2013. Vaccine development for tuberculosis: current progress. Drugs 73:1015–1024. doi: 10.1007/s40265-013-0081-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Andersen P, Woodworth JS. 2014. Tuberculosis vaccines—rethinking the current paradigm. Trends Immunol 35:387–395. doi: 10.1016/j.it.2014.04.006. [DOI] [PubMed] [Google Scholar]
- 15.Drake TE. 1971. Thymolymphatic deficiency and depression of cell-mediated immunity in protein-calorie malnutrition. Lancet ii:1322–1323. [DOI] [PubMed] [Google Scholar]
- 16.Chandra RK. 1997. Nutrition and the immune system: an introduction. Am J Clin Nutr 66:460S–463S. [DOI] [PubMed] [Google Scholar]
- 17.Dai G, McMurray DN. 1998. Altered cytokine production and impaired antimycobacterial immunity in protein-malnourished guinea pigs. Infect Immun 66:3562–3568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mengheri E, Nobili F, Crocchioni G, Lewis JA. 1992. Protein starvation impairs the ability of activated lymphocytes to produce interferon-gamma. J Interferon Res 12:17–21. doi: 10.1089/jir.1992.12.17. [DOI] [PubMed] [Google Scholar]
- 19.González-Martínez H, Rodriguez L, Najera O, Cruz D, Miliar A, Dominguez A, Sanchez F, Graniel J, González-Torres MC. 2008. Expression of cytokine mRNA in lymphocytes of malnourished children. J Clin Immunol 28:593–599. doi: 10.1007/s10875-008-9204-5. [DOI] [PubMed] [Google Scholar]
- 20.González-Torres C, González-Martínez H, Miliar A, Najera O, Graniel J, Firo V, Alvarez C, Bonilla E, Rodriguez L. 2013. Effect of malnutrition on the expression of cytokines involved in Th1 cell differentiation. Nutrients 5:579–593. doi: 10.3390/nu5020579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Saucillo DC, Gerriets VA, Sheng J, Rathmell JC, Maciver NJ. 2014. Leptin metabolically licenses T cells for activation to link nutrition and immunity. J Immunol 192:136–144. doi: 10.4049/jimmunol.1301158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Palacio A, Lopez M, Perez-Bravo F, Monkeberg F, Schlesinger L. 2002. Leptin levels are associated with immune response in malnourished infants. J Clin Endocrinol Metab 87:3040–3046. doi: 10.1210/jcem.87.7.8636. [DOI] [PubMed] [Google Scholar]
- 23.Soliman AT, El Zalabany MM, Salama M, Ansari BM. 2000. Serum leptin concentrations during severe protein-energy malnutrition: correlation with growth parameters and endocrine function. Metabolism 49:819–825. doi: 10.1053/meta.2000.6745. [DOI] [PubMed] [Google Scholar]
- 24.Howard JK, Lord GM, Matarese G, Vendetti S, Ghatei MA, Ritter MA, Lechler RI, Bloom SR. 1999. Leptin protects mice from starvation-induced lymphoid atrophy and increases thymic cellularity in ob/ob mice. J Clin Invest 104:1051–1059. doi: 10.1172/JCI6762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lord GM, Matarese G, Howard JK, Baker RJ, Bloom SR, Lechler RI. 1998. Leptin modulates the T-cell immune response and reverses starvation-induced immunosuppression. Nature 394:897–901. doi: 10.1038/29795. [DOI] [PubMed] [Google Scholar]
- 26.Hoang T, Aagaard C, Dietrich J, Cassidy JP, Dolganov G, Schoolnik GK, Lundberg CV, Agger EM, Andersen P. 2013. ESAT-6 (EsxA) and TB10.4 (EsxH) based vaccines for pre- and post-exposure tuberculosis vaccination. PLoS One 8:e80579. doi: 10.1371/journal.pone.0080579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Agger EM, Rosenkrands I, Hansen J, Brahimi K, Vandahl BS, Aagaard C, Werninghaus K, Kirschning C, Lang R, Christensen D, Theisen M, Follmann F, Andersen P. 2008. Cationic liposomes formulated with synthetic mycobacterial cordfactor (CAF01): a versatile adjuvant for vaccines with different immunological requirements. PLoS One 3:e3116. doi: 10.1371/journal.pone.0003116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Weinrich Olsen A, van Pinxteren LA, Meng Okkels L, Birk Rasmussen P, Andersen P. 2001. Protection of mice with a tuberculosis subunit vaccine based on a fusion protein of antigen 85B and ESAT-6. Infect Immun 69:2773–2778. doi: 10.1128/IAI.69.5.2773-2778.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hoang TT, Nansen A, Roy S, Billeskov R, Aagaard C, Elvang T, Dietrich J, Andersen P. 2009. Distinct differences in the expansion and phenotype of TB10.4 specific CD8 and CD4 T cells after infection with Mycobacterium tuberculosis. PLoS One 4:e5928. doi: 10.1371/journal.pone.0005928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lindenstrøm T, Agger EM, Korsholm KS, Darrah PA, Aagaard C, Seder RA, Rosenkrands I, Andersen P. 2009. Tuberculosis subunit vaccination provides long-term protective immunity characterized by multifunctional CD4 memory T cells. J Immunol 182:8047–8055. doi: 10.4049/jimmunol.0801592. [DOI] [PubMed] [Google Scholar]
- 31.Mainali ES, McMurray DN. 1998. Protein deficiency induces alterations in the distribution of T-cell subsets in experimental pulmonary tuberculosis. Infect Immun 66:927–931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lin PL, Dietrich J, Tan E, Abalos RM, Burgos J, Bigbee C, Bigbee M, Milk L, Gideon HP, Rodgers M, Cochran C, Guinn KM, Sherman DR, Klein E, Janssen C, Flynn JL, Andersen P. 2012. The multistage vaccine H56 boosts the effects of BCG to protect cynomolgus macaques against active tuberculosis and reactivation of latent Mycobacterium tuberculosis infection. J Clin Invest 122:303–314. doi: 10.1172/JCI46252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Boelaert JR, Gordeuk VR. 2002. Protein energy malnutrition and risk of tuberculosis infection. Lancet 360:1102. doi: 10.1016/S0140-6736(02)11169-X. [DOI] [PubMed] [Google Scholar]
- 34.Keusch GT. 2003. The history of nutrition: malnutrition, infection and immunity. J Nutr 133:336S–340S. [DOI] [PubMed] [Google Scholar]
- 35.Schaible UE, Kaufmann SH. 2007. Malnutrition and infection: complex mechanisms and global impacts. PLoS Med 4:e115. doi: 10.1371/journal.pmed.0040115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cegielski JP, Arab L, Cornoni-Huntley J. 2012. Nutritional risk factors for tuberculosis among adults in the United States, 1971–1992. Am J Epidemiol 176:409–422. doi: 10.1093/aje/kws007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Faber K. 1938. Tuberculosis and nutrition. Acta Tuberc Scand 12:306–309. [Google Scholar]
- 38.McMurray DN, Bartow RA. 1992. Immunosuppression and alteration of resistance to pulmonary tuberculosis in guinea pigs by protein undernutrition. J Nutr 122:738–743. [DOI] [PubMed] [Google Scholar]
- 39.Nussler AK, Billiar TR. 1993. Inflammation, immunoregulation, and inducible nitric oxide synthase. J Leukoc Biol 54:171–178. [PubMed] [Google Scholar]
- 40.Ishikawa LL, Franca TG, Chiuso-Minicucci F, Zorzella-Pezavento SF, Marra NM, Pereira PC, Silva CL, Sartori A. 2009. Dietary restriction abrogates antibody production induced by a DNA vaccine encoding the mycobacterial 65 kDa heat shock protein. Genet Vaccines Ther 7:11. doi: 10.1186/1479-0556-7-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sugawara I, Yamada H, Mizuno S. 2007. BCG vaccination enhances resistance to M. tuberculosis infection in guinea pigs fed a low casein diet. Tohoku J Exp Med 211:259–268. doi: 10.1620/tjem.211.259. [DOI] [PubMed] [Google Scholar]
- 42.Malafaia G, Serafim TD, Silva ME, Pedrosa ML, Rezende SA. 2009. Protein-energy malnutrition decreases immune response to Leishmania chagasi vaccine in BALB/c mice. Parasite Immunol 31:41–49. doi: 10.1111/j.1365-3024.2008.01069.x. [DOI] [PubMed] [Google Scholar]
- 43.Mussi-Pinhata MM, Goncalves AL, Foss NT. 1993. BCG vaccination of full-term infants with chronic intrauterine malnutrition: influence of immunization age on development of post-vaccination, delayed tuberculin hypersensitivity. Bull World Health Organ 71:41–48. [PMC free article] [PubMed] [Google Scholar]
- 44.Roth A, Jensen H, Garly ML, Djana Q, Martins CL, Sodemann M, Rodrigues A, Aaby P. 2004. Low birth weight infants and Calmette-Guerin bacillus vaccination at birth: community study from Guinea-Bissau. Pediatr Infect Dis J 23:544–550. doi: 10.1097/01.inf.0000129693.81082.a0. [DOI] [PubMed] [Google Scholar]
- 45.McMurray DN, Mintzer CL, Bartow RA, Parr RL. 1989. Dietary protein deficiency and Mycobacterium bovis BCG affect interleukin-2 activity in experimental pulmonary tuberculosis. Infect Immun 57:2606–2611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lindenstrøm T, Knudsen NP, Agger EM, Andersen P. 2013. Control of chronic Mycobacterium tuberculosis infection by CD4 KLRG1-IL-2-secreting central memory cells. J Immunol 190:6311–6319. doi: 10.4049/jimmunol.1300248. [DOI] [PubMed] [Google Scholar]
- 47.Seder RA, Darrah PA, Roederer M. 2008. T-cell quality in memory and protection: implications for vaccine design. Nat Rev Immunol 8:247–258. doi: 10.1038/nri2274. [DOI] [PubMed] [Google Scholar]
- 48.Iyer SS, Chatraw JH, Tan WG, Wherry EJ, Becker TC, Ahmed R, Kapasi ZF. 2012. Protein energy malnutrition impairs homeostatic proliferation of memory CD8 T cells. J Immunol 188:77–84. doi: 10.4049/jimmunol.1004027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.White SJ, Taylor MJ, Hurt RT, Jensen MD, Poland GA. 2013. Leptin-based adjuvants: an innovative approach to improve vaccine response. Vaccine 31:1666–1672. doi: 10.1016/j.vaccine.2013.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Carr EL, Kelman A, Wu GS, Gopaul R, Senkevitch E, Aghvanyan A, Turay AM, Frauwirth KA. 2010. Glutamine uptake and metabolism are coordinately regulated by ERK/MAPK during T lymphocyte activation. J Immunol 185:1037–1044. doi: 10.4049/jimmunol.0903586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Horio Y, Osawa S, Takagaki K, Hishida A, Furuta T, Ikuma M. 2008. Glutamine supplementation increases Th1-cytokine responses in murine intestinal intraepithelial lymphocytes. Cytokine 44:92–95. doi: 10.1016/j.cyto.2008.06.011. [DOI] [PubMed] [Google Scholar]
- 52.Scanga CA, Mohan VP, Tanaka K, Alland D, Flynn JL, Chan J. 2001. The inducible nitric oxide synthase locus confers protection against aerogenic challenge of both clinical and laboratory strains of Mycobacterium tuberculosis in mice. Infect Immun 69:7711–7717. doi: 10.1128/IAI.69.12.7711-7717.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chan J, Xing Y, Magliozzo RS, Bloom BR. 1992. Killing of virulent Mycobacterium tuberculosis by reactive nitrogen intermediates produced by activated murine macrophages. J Exp Med 175:1111–1122. doi: 10.1084/jem.175.4.1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wu G, Flynn NE, Flynn SP, Jolly CA, Davis PK. 1999. Dietary protein or arginine deficiency impairs constitutive and inducible nitric oxide synthesis by young rats. J Nutr 129:1347–1354. [DOI] [PubMed] [Google Scholar]
- 55.Wherry EJ, Blattman JN, Murali-Krishna K, van der Most R, Ahmed R. 2003. Viral persistence alters CD8 T-cell immunodominance and tissue distribution and results in distinct stages of functional impairment. J Virol 77:4911–4927. doi: 10.1128/JVI.77.8.4911-4927.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.McMurray DN, Bartow RA, Mintzer C. 1990. Malnutrition-induced impairment of resistance against experimental pulmonary tuberculosis. Prog Clin Biol Res 325:403–412. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.