Abstract
Ocular inflammation is one of the consequences of infection with the protozoan parasite Toxoplasma gondii. Even if lesions are self-healing in immunocompetent persons, they pose a lifetime risk of reactivation and are a serious threat to vision. As there are virtually no immunological data on reactivating ocular toxoplasmosis, we established a model of direct intravitreal injection of parasites in previously infected mice with a homologous type II strain. Two different mouse strains with variable ability to control retinal infection were studied in order to describe protective and deleterious reaction patterns. In Swiss-Webster mice, which are already relatively resistant to primary infection, no peak of parasite load was observed upon reinfection. In contrast, the susceptible inbred strain C57BL/6 showed high parasite loads after 7 days, as well as marked deterioration of retinal architecture. Both parameters were back to normal on day 21. C57BL/6 mice also reacted with a strong local production of inflammatory and Th1-type cytokines, like interleukin-6 (IL-6), IL-17A, and gamma interferon (IFN-γ), while Swiss-Webster mice showed only moderate expression of the Th2 cytokine IL-31. Interestingly, rapid intraocular production of anti-Toxoplasma antibodies was observed in Swiss-Webster but not in C57BL/6 mice. We then localized the cellular source of different immune mediators within the retina by immunofluorescence. Finally, neutralization experiments of IFN-γ or IL-6 demonstrated the respective protective and deleterious roles of these cytokines for parasite control and retinal integrity during reinfection. In conclusion, we developed and immunologically characterized a promising mouse model of reactivating ocular toxoplasmosis.
INTRODUCTION
Ocular toxoplasmosis (OT), a sequel of infection with the apicomplexan parasite Toxoplasma gondii, is a major cause of visual impairment worldwide, responsible for 30 to 50% of posterior uveitis in immunocompetent people. While OT was considered until recently as being exclusively due to congenital infection, screening of Toxoplasma-seropositive persons in Europe and North America revealed that 1 to 2% of this population presents retinal toxoplasmic lesions (1). Thus, possible reactivation of these ocular infections in postnatally infected individuals poses a hitherto underestimated problem for the health systems in these countries (2) and even more so in regions like South America (3, 4). At the moment, no treatment has so far achieved a consistently reduced risk of reactivation, except perhaps in high-risk patients (5, 6). To establish more specific, immune-based interventions, we need to elucidate the physiopathology of OT, which is so far largely ignored. Therefore, we wanted to gather novel data on the immune responses involved in retinal T. gondii reactivation. The long-term objective of this model is to open new therapeutic perspectives of human OT.
The main obstacle for thorough research of ocular reactivation is the absence of a suitable mouse model (4). After oral or intraperitoneal (i.p.) infection, mice develop ocular lesions and have detectable parasite persistence afterward, but no reactivation of inflammation was observed in any mouse strain. Some studies achieved reactivation in primates or rabbits following general immunodepression of the animals (7, 8), and others provoked tachyzoite release and reactivation by the administration of neutralizing antibodies (Abs) against T cells or central cytokines (9). However, these models clearly do not represent reactivation in fully immunocompetent persons and do not permit immunological studies. More promising, direct ocular infection has recently been established, with injections in the anterior eye part (10) or the vitreous (2, 11, 12). Here, we injected parasites in the vitreous of previously infected mice to model as closely as possible spontaneous liberation of tachyzoites from retinal cysts, which has been suggested as the principal mechanism of reactivation in vivo (13). We validated this reinfection model to investigate some immunological aspects of congenital Toxoplasma infection (14). However, as congenital and postnatally acquired Toxoplasma infections are clinically clearly two distinct entities (15), the immunological base of the disease is likely to differ considerably. In the present study, we set up a mouse model for reactivation of adult-acquired toxoplasmosis and study a large panel of immune factors. Because there are no data on mouse strain-dependent susceptibility for ocular reinfection, we studied outbred Swiss-Webster mice, which are relatively resistant to a primary infection, and inbred C57BL/6 mice, known to be susceptible to Toxoplasma infection by different routes (10, 16), in order to describe protective and detrimental immune responses. Finally, we proved the protective and detrimental roles of gamma interferon (IFN-γ) and interleukin-6 (IL-6) by local neutralization studies. The results presented here will constitute a valuable base for testing targeted immune intervention.
MATERIALS AND METHODS
Mice and parasites.
Female outbred Swiss-Webster mice, as well as inbred C57Bl/6J mice, were purchased from Centre d'Elevage R. Janvier (Le Genest-Saint-Isle, France) and were partially bred in our own animal housing facility (accreditation no. A67-482-37). Animals were bred under specific-pathogen-free conditions, according to national and local regulations. Animal studies in our laboratory were approved by the local ethics committee (project no. AL/77/84/02/13).
Cysts of the avirulent PRU strain of T. gondii (haplotype 2) were obtained from the brains of Swiss-Webster female mice previously infected perorally. The brain was homogenized in 1 ml of phosphate-buffered saline (PBS). The amount of cysts was determined microscopically. Tachyzoites of the PRU strain were maintained in human THP1 monocyte cultures. Tachyzoites of the virulent RH strain (haplotype I) were maintained by weekly passages in mice. Parasites were originally obtained from the French Biological Resource Center Toxoplasma (CRB Toxoplasma; Laboratoire de Parasitologie, CHU Reims, Reims, France).
Reinfection model.
Female mice at 5 weeks of age were infected intraperitoneally with a cyst suspension of the avirulent PRU strain of T. gondii (5 cysts/mouse). In preliminary experiments, we verified that retinal tissues were infected in all mice 4 weeks after infection. At this time point, mice were injected intravitreally with a solution of 2,000 tachyzoites in 5 μl sterile PBS per eye, using 30-gauge needles (BD Microlance 3; Becton Dickinson, Ireland) on a 25-μl syringe (702LT; Hamilton, Bonaduz, Switzerland), during general anesthesia with isoflurane (Forène; Laboratoire Abbott, Paris France). Mice of the control group received 5 μl PBS intravitreally.
Aqueous humor was collected at different time points after reinfection by anterior chamber paracentesis (approximately 5 μl/mouse), pooled, and stored at −80°C until analysis. Eyes were enucleated, and retinas were dissected and finally stored in pools at −20°C for transcript analyses. Pools of whole eyes were used for DNA extraction and parasite quantification. Each pool consisted of five animals (10 eyes).
Quantification of parasite load.
DNA was extracted from pools of whole eyes using the QIAamp DNA tissue minikit (Qiagen, Courtaboeuf, France), eluted with 200 μl elution buffer, and stored at −20°C. Real-time PCR specific for the B1 gene of T. gondii was performed on a LightCycler (Roche Diagnostics, Mannheim, Germany) using FastStart DNA master Sybr green (Roche) as described before (17).
Histopathology of the retina.
Eyes were enucleated and immediately preserved in 4% buffered formaldehyde. Fixed eyes were embedded in paraffin, serially cut into 5-μm sections, and stained with hematoxylin and eosin, as described before (12). Ocular pathology was scored according to reference 10: 0, normal histology; 1, mild inflammation without necrosis; 2, obvious inflammation without necrosis; 3, strong inflammation with necrosis.
Cytokine and chemokine profiles in aqueous humor.
The Bio-Plex mouse cytokine 23-plex and Th17 8-plex assays (Bio-Rad, Marne-la-Coquette, France) were used according to the manufacturer's recommendations to measure cytokine and chemokine levels in aqueous humor. The assay plate layout consisted of a standard series, one blank well, and duplicates of 20-μl AqH samples, diluted to 50 μl with Bio-Plex mouse serum diluent. Data were analyzed with Bio-Plex Manager software v1.1 (Bio-Rad). The fold induction index was calculated as the ratio of the cytokine concentration in reinfected mice to the mean cytokine concentration of PBS-injected mice.
Aqueous humor anti-Toxoplasma IgG antibody enzyme-linked immunosorbent assay (ELISA).
A 96-well plate was coated with 200 μl of crude Toxoplasma antigen (1 μg/ml) in a bicarbonate buffer (Sigma) at 4°C overnight. Then, the plate was incubated with 1% bovine serum albumin (BSA) for 1 h, followed by a 5% trehalose buffer for 30 min, separated by a wash step with PBS-0.05% Tween 20. Aqueous humor was diluted to 1/25 and serum controls at 1/5 in a dilution buffer and incubated 1 h at 37°C. After a wash step, 100 μl of goat anti-mouse IgG-horseradish peroxidase (HRP; Beckman Coulter; 1/50,000) was deposited. Finally, TMB substrate (3,3′,5,5′-tetramethylbenzidine) was given to the wells for 15 min at 37°C, and the reaction was stopped with 50 μl of H2SO4. The plate was read in a spectrophotometer at 450 nm, with 630 nm as the background. An index was calculated by dividing the optical density value for infected mice by that for noninfected mice.
Immunofluorescence localization of immune markers in retina sections.
Cryocut sections of whole eyes were prepared as previously described (18). Briefly, eyes were fixed in 3% paraformaldehyde, placed in increasing concentrations of sucrose, and embedded in Tissue-Tek OCT compound (Sakura Fintek, France). Then, 15-μm-thick sections were prepared, incubated in 4% paraformaldehyde or acetone, autofluorescence quenched with glycine, and permeabilized for 10 min in 0.1% Triton X-100 and blocked with 3% BSA. Sections were incubated with primary antibody (Table 1) overnight at 4°C, with a corresponding Alexa 488- or 546-conjugated secondary Ab (Invitrogen) for 45 min, and finally with Hoechst 33342 stain for 1 min. All Abs were diluted in 1% BSA. Between each step, sections were washed with PBS three times for 5 min. After sections were mounted, fluorescence was visualized on an Axio Observer Z1 HSDI epifluorescence microscope (Zeiss, Le Pecq, France) and evaluated with AxioVision v5 software (Zeiss).
TABLE 1.
Antibodies used for immunofluorescence
| Antibody target | Host | Sourcea |
|---|---|---|
| Vimentin (C-20) | Rabbit polyclonal | SC |
| IFN-γ (R4-6A2) | Rat monoclonal | SC |
| IL-6 | Rabbit polyclonal | Ab |
| IL-17 (H-132) | Rabbit polyclonal | SC |
| IL-23 (H-113) | Rabbit polyclonal | SC |
SC, Santa Cruz Biotechnology; Ab, Abcam.
IL-6 and IFN-γ neutralization studies.
C57BL/6 mice were infected with cysts and reinfected with tachyzoites as described before. Concomitantly, 3 μg anti-IL-6 monoclonal antibody (MAb; MP5-20F3) or 200 pg anti-IFN-γ MAb (H22.1L) was given with the same intravitreal injection. Mice of the control groups received 3 μg of rat IgG2a Ab or 200 pg of hamster IgG, as appropriate. Antibodies were purchased from eBioscience SAS (Paris, France) for IL-6 neutralization and from Santa Cruz Biotechnology (Dallas, TX, USA) for IFN-γ neutralization. Histological analysis and parasite and cytokine quantification were performed as described before.
Statistical analysis.
For the quantification of the antibody response, three independent experiments were performed and a t test was calculated. P values of <0.05 were considered statistically significant. Quantification of cytokines was done with pools of 10 eyes, due to small volumes. Means ± standard deviations (SD) from duplicate measures are shown. Due to the multiplex nature of these experiments, a threshold of 10-fold induction following reinfection which could be reproduced in at least one separate experiment was set to describe certain upregulation. A t test with false discovery exclusion (GraphPad 6) was used to calculate statistical significance between experimental groups.
RESULTS
Variable susceptibility of mouse strains.
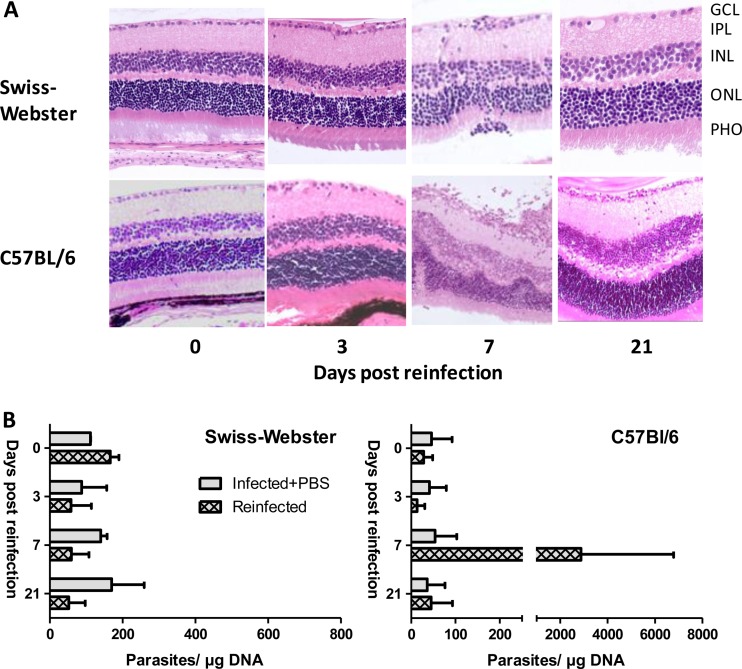
We first compared the different kinetics of reinfection in resistant Swiss-Webster and susceptible C57BL/6 mice. We chose histological evaluation and kinetics of intraocular parasite load as the primary readout, as macroscopic signs of ocular pathology (Tyndall effect in the anterior chamber, due to the presence of inflammatory cells), as observed following primary intraocular infection (19), were not observed in either strain (data not shown). We first looked at histological sections (Fig. 1A). In Swiss-Webster mice, the retinal architecture stayed intact throughout the experiment. On day 7, a slight thickening of the ganglion cell layer and some migrating cells in the inner plexiform layer were observed (inflammatory score of 1), both returning to normal (score of 0) by day 21. In sharp contrast, C57BL/6 mice showed considerable destructuration of the retina on day 7 (score of 2), with numerous migrating cells throughout the retina. However, most of these pathological changes were not visible anymore on day 21.
FIG 1.
C57BL/6 mice but not Swiss-Webster mice are susceptible to ocular T. gondii reinfection. Previously infected mice were reinfected by intraocular injection of 2,000 T. gondii PRU strain tachyzoites. (A) Hematoxylin and eosin staining of retina sections showing transient alteration of the retinal architecture of C57BL/6 but not Swiss-Webster mice. GCL, ganglion cell layer; IPL, inner plexiform layer; INL, inner nuclear layer; ONL, outer nuclear layer; PHO, photoreceptors. (B) Ocular parasite load, determined by T. gondii-specific quantitative PCR on DNA extracted from whole eyes, showing a transient peak on day 7 postinfection (p.i.) only in C57BL/6 mice. Values shown are means ± SD from two independent experiments with pools of 10 eyes. Similar results were obtained in other independent experiments.
The strain-dependent susceptibility was further assessed by parasite multiplication. As shown in Fig. 1B, no parasite multiplication was seen in Swiss-Webster mice, the initial inoculum visible on day 0 was resorbed, and no parasite peak was noted afterward. In sharp contrast, there was a strong peak in C57BL/6 mice on day 7 after reinfection. Afterward, the parasite load returned to baseline levels, comparable to the parasite load of PBS-injected mice. These results show that, in susceptible mouse strains, homologous Toxoplasma injected in the eyes of previously infected mice can indeed find their host cells and proliferate despite the present immune response. However, the immune system of the animals is rapidly capable of limiting this multiplication in any mouse strain.
Local immune response in reinfected eyes.
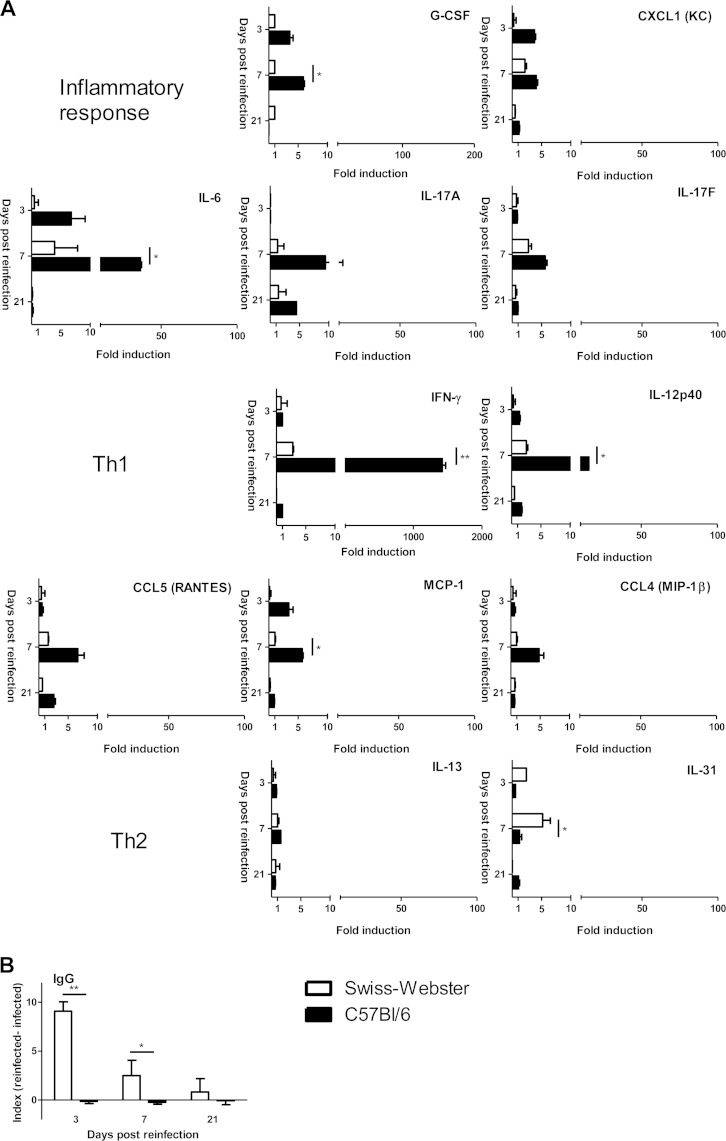
Next, we wanted to see if ocular reinfection elicits any measurable local cytokine production and whether the apparently different parasitological outcome between strains is correlated with different immune response patterns. We first studied cytokine levels in aqueous humor using the Bio-Plex technique (Fig. 2). We observed clearly different reaction patterns of the Swiss-Webster and C57BL/6 strains. In the aqueous humor of Swiss-Webster mice, even if some cytokines, like IFN-γ and IL-17A, were slightly more present in reinfected mice, IL-31 was the only cytokine which was somewhat upregulated to higher levels than in C57BL/6 mice but did also not exceed 10-fold induction. In C57BL/6 mice, a completely contrasting response pattern was observed. A clear inflammatory and Th1 pattern was observed, characterized by strongly enhanced production of IL-6 and IFN-γ and, to a lesser extent, IL-17 and IL-12. In contrast, Th2 levels stayed low, with only IL-13 showing minimal upregulation. Interestingly, enhanced IL-10 levels following infection were not visible in either mouse strain (data not shown).
FIG 2.
The ocular immune response to reinfection differs considerably between Swiss-Webster and C57BL/6 mouse strains. Previously infected mice were reinfected by intraocular injection of 2,000 T. gondii PRU strain tachyzoites, and the immune response was measured in aqueous humor pools of 10 eyes. (A) Cytokine and chemokine concentrations, determined by Bio-Plex assay. Values are means ± SD from duplicate measures in pools of 10 eyes from one representative experiment, expressed as the fold increase of T. gondii-reinfected mice over PBS sham-injected mice. (B) Anti-Toxoplasma IgG antibody titers in aqueous humor of intravitreally reinfected mice, determined in pools of 10 eyes. Values are expressed as the optical density difference between T. gondii-reinfected and PBS sham-injected mice (means ± SD from three independent experiments). Statistical analysis was performed as described in Materials and Methods. *, P < 0.05; ** P < 0.01.
We also investigated the local antibody production upon reinfection, as an additional possible means of controlling intraocular reactivation. Interestingly, Swiss-Webster mice, which had a low cytokine profile, showed a considerably higher T. gondii-specific antibody level in reinfected than in PBS-injected control mice as soon as day 3 after reinfection, indicating rapid antibody production (Fig. 2B). This antibody level returned to nearly preinjection levels by day 21 after reinfection. In contrast, antibody levels in C57BL/6 mice stayed at the background level, i.e., as in PBS-injected control mice.
Together, these results suggest that even resistant Swiss-Webster mice did show an immunological reaction, centered mainly on a Th2 response and antibody production. In contrast, the strong multiplication of parasites in susceptible C57BL/6 mice led to upregulation of inflammatory and Th1 cytokines, which enabled the local immune system to rapidly control parasite multiplication.
Cellular localization of cytokine production.
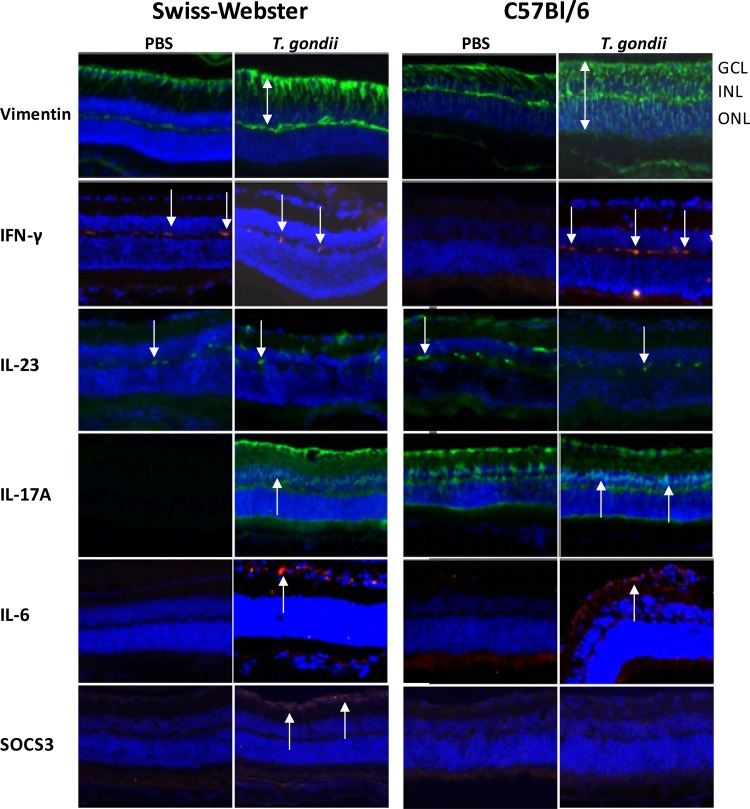
We next aimed to localize the production of central immune factors in both mouse strains by immunofluorescence on retina cryocuts (Fig. 3). We first evaluated the expression of vimentin, an activation marker of Muller cells. In PBS sham-injected animals, residual activation of Muller cells resulting from the primary infection is visible. Vimentin staining is, however, largely increased in both strains following T. gondii reinjection. Interestingly, in Swiss-Webster mice, only the interior parts of Muller cells were stained, whereas vimentin expression in reinfected C57BL/6 mice spans the entire length of Muller cells throughout the retina. IFN-γ and IL-23 seem to be expressed in microglial cells, with a visible increase of fluorescence visible only in reinfected C57BL/6 mice. We cannot exclude that some of these cells are invading monocytes instead of microglial cells, as both cell types are identified by the same marker proteins. Further experiments are needed to address this interesting question. IL-17 expression is already visible in Muller cells of control mice of both strains, in the absence of reinfection. Reinfection induced additional expression in the inner nuclear layer, which contains mainly neuronal bipolar cells. This expression is stronger in C57BL/6 mice than in Swiss-Webster mice. IL-6 is expressed following reinfection in the ganglion layer of both mouse strains. In contrast, the negative cytokine regulator SOCS3 was expressed only in the ganglion layer of reinfected Swiss-Webster mice but completely absent in C57BL/6 retinas. Neither strain showed SOCS3 expression in PBS-injected eyes.
FIG 3.
Differential expression of vimentin (Muller cells) and key immune mediators in the retinas of Swiss-Webster and C57BL/6 mice. Previously infected mice were intravitreally T. gondii reinfected or PBS sham injected. Retinas were examined on day 7 p.i. Double arrows show the different extents of retinal vimentin expression in the two mouse strains, and arrows indicate cytokine-expressing cells. Arrows in the IL-17A images specifically show cytokine expression in the inner nuclear layer, only visible in reinfected mice. Of note, SOCS3 expression is observed only in reinfected Swiss-Webster mice but not in C57BL/6 mice. GCL, ganglion cell layer; INL, inner nuclear layer; ONL, outer nuclear layer.
Neutralization studies.
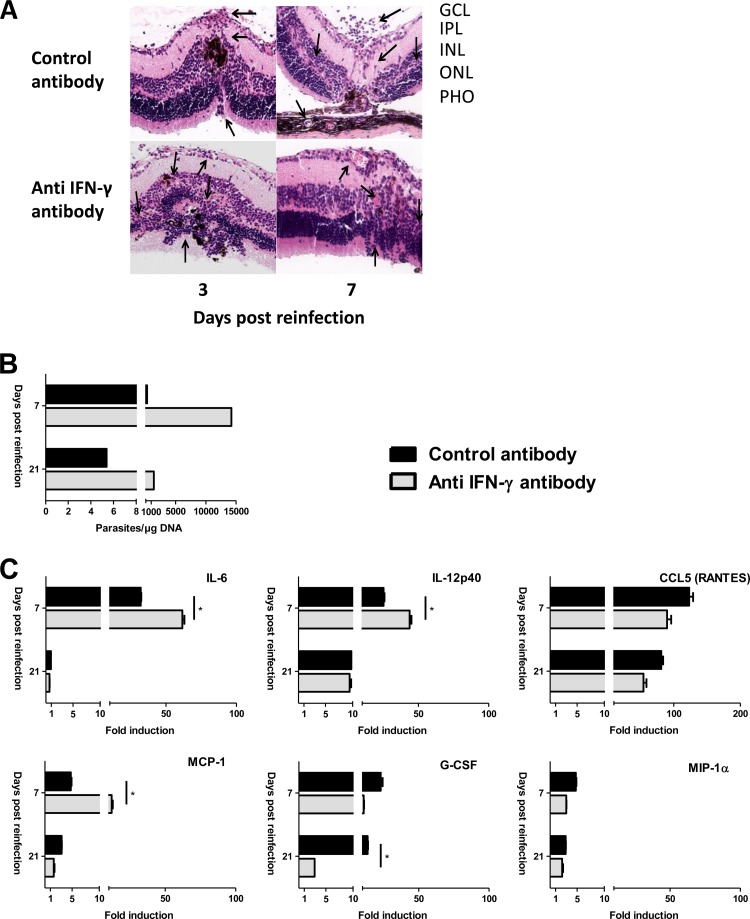
IFN-γ and IL-6 were most strongly upregulated in susceptible C57BL/6 mice. The protective role of IFN-γ for systematic as well as ocular toxoplasmosis is well established. In contrast, no data exist for reactivation or reinfection settings. As for IL-6, which is at the crossroad of protective Th1 and probably detrimental TH17 responses (19), the positive or negative role in ocular defense is entirely unclear. To clarify their roles in our reinfection model, we injected a neutralizing antibody at the same time as the reinfection dose in C57BL/6 mice. Histological sections showed similar or even aggravated retinal distortion on days 3 and 7 in the anti IFN-γ-treated mice (a score of 3 compared to a score of 2 in control mice) (Fig. 4A). IFN-γ neutralization also led to a considerably higher parasite load on day 7 (Fig. 4B). Parasite loads were diminished on day 21 but were still higher than those in control mice, where very low levels were observed at that time point. Quantification of cytokine levels in aqueous humor showed higher levels of some but not all inflammatory cytokines and chemokines (Fig. 4C). Levels of Th2 and regulatory cytokines, such as IL-4, IL-13, and IL-10, were unchanged (data not shown).
FIG 4.
Intraocular neutralization of IFN-γ impairs local parasite control in C57BL/6 mice. Anti IFN-γ or IgG isotype control antibody was injected intravitreally, concomitant with T. gondii reinfection, in previously infected C57BL/6 mice. (A) Hematoxylin and eosin staining of retina sections showing a slightly aggravated retinal distortion upon IFN-γ neutralization. GCL, ganglion cell layer; IPL, inner plexiform layer; INL, inner nuclear layer; ONL, outer nuclear layer; PHO, photoreceptors. (B) Ocular parasite load, as determined by T. gondii-specific quantitative PCR on DNA extracted from pools of 10 whole eyes per group. (C) Cytokine and chemokine concentrations in aqueous humor, determined by Bio-Plex assay. Values are means ± SD from duplicate measures in pools of 10 eyes from one representative experiment, expressed as the fold increase of reinfected over PBS sham-injected mice. Statistical analysis was performed as described in Materials and Methods. *, P < 0.05.
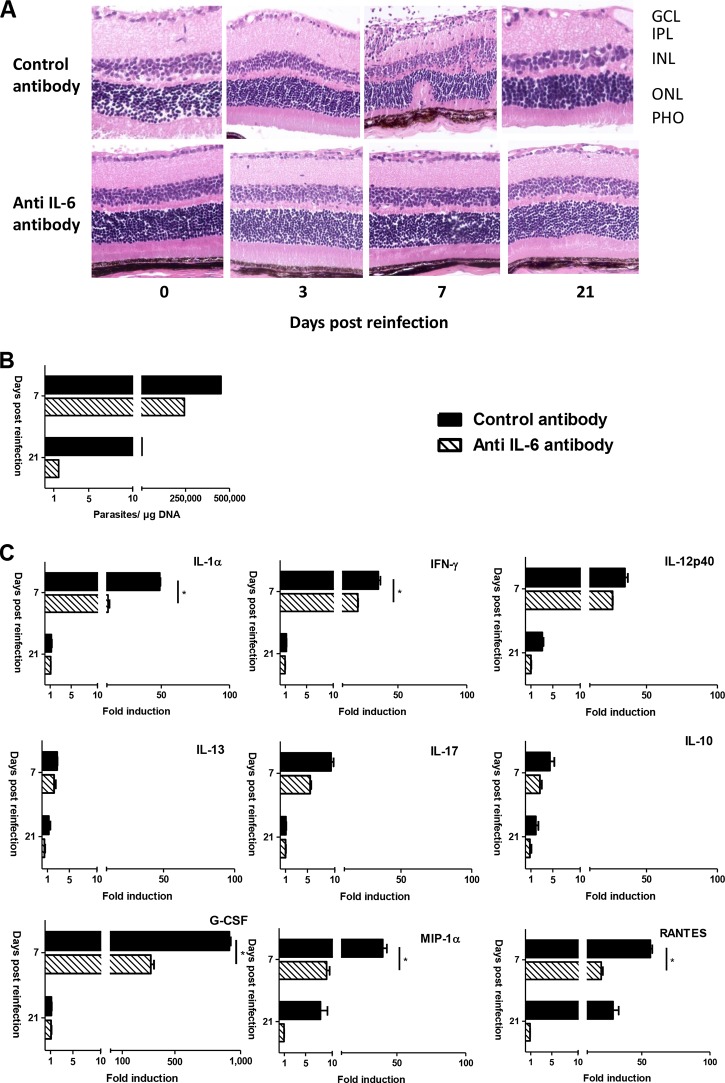
For IL-6 neutralization, we observed an opposing pattern. Histological sections show an absence of retinal distortion on day 7 in anti-IL-6-treated mice (a score of 0 versus a score of 2 in control mice) (Fig. 5A). Furthermore, neutralization of IL-6 led to a 35% lower parasite peak on day 7 and apparently also to faster recovery, as judged by parasite load on day 21 (Fig. 5B). Cytokine level analysis in aqueous humor showed downregulation of nearly all investigated factors, inflammatory and anti-inflammatory (Fig. 5C). These results indicate a protective role for IFN-γ and a detrimental role of intraocular IL-6 production on parasite control and induction of pathological responses in a reinfection setting.
FIG 5.
Intraocular neutralization of IL-6 ameliorates retinal pathology and parasite control in C57BL/6 mice. Anti-IL-6 or IgG isotype control antibody was injected intravitreally, concomitant with T. gondii reinfection, in previously infected C57BL/6 mice. (A) Hematoxylin and eosin staining of retinal sections showing nearly complete absence of retinal disorganization upon IL-6 neutralization. GCL, ganglion cell layer; IPL, inner plexiform layer; INL, inner nuclear layer; ONL, outer nuclear layer; PHO, photoreceptors. (B) Ocular parasite load, as determined by T. gondii-specific quantitative PCR on DNA extracted from pools of 10 whole eyes per group. (C) Cytokine and chemokine concentrations in aqueous humor, determined by Bio-Plex assay. Values are means ± SD from duplicate measures in pools of 10 eyes from one representative experiment, expressed as the fold increase of reinfected over PBS sham-injected mice. Statistical analysis was performed as described in Materials and Methods. *, P < 0.05.
DISCUSSION
The lifelong risk of reactivation of ocular toxoplasmic lesions represents a constant threat to visual capacity. The development of a more specific, immune-based intervention is hindered by the lack of data about immunological control mechanisms in OT reactivation, due mainly to the absence of an adequate mouse model and of conclusive human studies. We present here the setup and first immunological characterization of such a mouse model of intraocular T. gondii reactivation. The comparison of a resistant mouse strain and a susceptible mouse strain allowed us to characterize protective and deleterious response patterns. We observed both parasite load and inflammatory response, as our human studies in European and South American patients suggested that both factors can determine the gravity of retinal destruction, depending probably on the virulence of the parasite strain (20). Our results validate our local injection model, as it provoked a visible but transient disorganization in the retinal structure, as well as a transient parasite multiplication in susceptible C57BL/6 mice, recalling the transient nature of human OT reactivation (15). In contrast, resistant Swiss-Webster mice showed neither of these signs, which again reminds of their general resistance to Toxoplasma infection as well as of the majority of European OT patients in whom reactivation is rarely observed. Retinal inflammation in C57BL/6 mice was clearly correlated with transient parasite multiplication, showing that the elicited immune response in this strain is unable to control the initial parasite attack, even in this reinfection setting. Microscopic studies in mice showed infrequent spontaneous cyst rupture (21), which could then be immediately controlled or permit a transient parasite replication phase and new retinal lesions. Our models could thus represent cases of the two clinical extremes of controlled and uncontrolled parasite reactivation.
Analysis of aqueous humor allowed an immunological dissection of these protective and detrimental responses. The overall weak response observed in the resistant Swiss-Webster mice might just be a consequence of little productive infection of retinal cells. However, the marked increase of the Th2 cytokine IL-31 and pronounced rapid antibody production demonstrate an active antiparasitic process. IL-31 has been associated with inflammatory processes in Th2-dependent pathologies, such as asthma (22). This suggests that the resistance against reinfection of Swiss-Webster mice is indeed mediated by an active local immune response, possibly mediated by antibody or other specific Th2-type mechanisms. Further studies are needed to identify extra- or intracellular antiparasitic mechanisms, maybe even including a very early induction of IFN-γ or other, more classical Th1-type mechanisms not visible in our setting. In contrast, C57BL/6 mice cannot block active infection of retinal cells. The resulting parasite multiplication then elicits an inflammatory/Th1-type response which subsequently contains the infection, apparently using the same mechanisms as in primary infection. Remarkably, production of the highly inflammatory IL-17A increased sharply following reinfection, indicating that a secondary challenge of the ocular immune network in these mice does provoke a pathological process similar to what we observed during primary infection in Swiss-Webster mice (19).
Immunofluorescence detection of different immune mediators indicates cellular localization of key cytokines during reinfection but also gives further mechanistic details of this inflammation process. The more general vimentin staining of Muller cells in C57BL/6 mice, as well as the generally stronger cytokine expression in C57BL/6 mice than in Swiss-Webster mice, shows the enhanced activation of this mouse strain. In contrast, the observed SOCS3 staining in the ganglion cell layer of Swiss-Webster mice demonstrates once more that the resistance against reinfection in this strain is an active process of the retinal immune system. The SOCS3 gene was also one of the genes that were upregulated in Swiss-Webster mice but not C57BL/6 mice in an mRNA microarray experiment (our unpublished data), which also confirms our immunofluorescence detection in this mouse strain. Sustained activation of SOCS3 is associated with an active anti-inflammatory process by inhibiting mainly STAT3-dependent gene activation (23). Interestingly, SOCS3 expression was recently described in T. gondii-infected ex vivo macrophages of C57BL/6 mice, whereas we did not observe SOCS3 in this mouse strain. This contradiction can be due to specific immune reactions during reinfection, requiring a primed immune system, or to the specific immune response in the immune-privileged ocular environment. Consequently, this result opens the way for in-depth investigation of the exact cellular origin and regulation of this crucial immune regulator in the eye.
Our neutralization studies demonstrated the respective protective and detrimental roles of IFN-γ and IL-6, two cytokines strongly upregulated in C57BL/6 mice. IFN-γ is well established as a central mediator of resistance during T. gondii infection (24) and reinfection of pregnant mice (25). This cytokine is also likely to be of central importance in primary ocular infection, but the particular immune-compromised environment in the eye has to be considered (4). The more aggravated retinal pathology and higher parasite loads upon IFN-γ neutralization proved that C57BL/6 mice use this cytokine also in the case of reinfection to limit parasite multiplication in the retina. The different cytokine pattern found in aqueous humor upon IFN-γ neutralization additionally demonstrates that IFN-γ is in the center of the dynamic immune response. Especially, the higher levels of IL-6 and IL-12/23 p40 after IFN-γ neutralization indicate negative feedback mechanisms through IFN-γ to limit inflammation. In contrast, levels of the CCL5 (RANTES) and granulocyte colony-stimulating factor (G-CSF) chemokines were lower when IFN-γ was neutralized and are thus probably downstream products of IFN-γ signaling in our model.
The effects of IL-6 neutralization were very different. Most importantly, the considerably ameliorated retinal structure and the lower parasite burden following neutralization revealed a detrimental effect of IL-6. The cytokine analysis revealed that IL-6 is, in our model, at least partially responsible for IL-17 production, which we have described to be detrimental in acute OT (19). However, unlike these previous results with IL-17, neutralization of IL-6 also diminished the expression of several Th1-related mediators and did not enhance Th2 cytokines, such as IL-13. The multiple interactions of this pleiotropic cytokine merit further investigation. IL-6 has been demonstrated to be necessary for anti-T. gondii immunity (26), but an imbalance of IL-6 signaling through the gp130 receptor subunit could make IL-6 a pathological rather than protective factor (27). The key factor deciding between these extremes, also during T. gondii infection, is probably SOCS3 (28). Given the negative roles of IL-6 and IL-17, which we constantly found in the eye, this is probably best explained by the special immune-privileged situation and therefore distinct immune regulation of the eye. Interestingly, we recently observed enhanced ocular production of both IL-6 and IL-13 in Colombian OT patients compared to that in European patients (20). Even if the crucial role of the infecting parasite strain must be considered when comparing with South American infections, this observation could link the cytokine pattern observed in our ocular model to clinical features observed during OT. Furthermore, the crucial role of IL-6 for pathology could be a point of therapeutic intervention using IL-6 neutralizing antibodies, which is already broadly discussed for various inflammatory pathologies (29).
In summary, our newly established reinfection mouse models shows key characteristics of T. gondii OT reactivation or the absence of such active reactivation. The choice to specifically inject parasites and neutralizing antibody directly in the eye allowed us to look at key immune factors specifically in this particular organ. Our immunological characterization of resistance and susceptibility paves the way to evaluate the possibility of immune-based intervention in OT patients.
ACKNOWLEDGMENTS
Our work has been supported by the Fondation de Recherche Médicale (Retinal Physiopathology program), the PHRC program (grant no. 2007-3964), Université de Strasbourg, and Hôpitaux Universitaires de Strasbourg. É.R. received a Berthe Fouassier Ph.D. scholarship from the Fondation de France (grant no. 12165 and 2012-32622).
REFERENCES
- 1.Holland GN. 2003. Ocular toxoplasmosis: a global reassessment. Part I: epidemiology and course of disease. Am J Ophthalmol 136:973–988. doi: 10.1016/j.ajo.2003.09.040. [DOI] [PubMed] [Google Scholar]
- 2.Delair E, Monnet D, Grabar S, Dupouy-Camet J, Yera H, Brezin AP. 2008. Respective roles of acquired and congenital infections in presumed ocular toxoplasmosis. Am J Ophthalmol 146:851–855. doi: 10.1016/j.ajo.2008.06.027. [DOI] [PubMed] [Google Scholar]
- 3.de-la-Torre A, Gonzalez-Lopez G, Montoya-Gutierrez JM, Marin-Arango V, Gómez-Marín JE. 2011. Quality of life assessment in ocular toxoplasmosis in a Colombian population. Ocular Immunol Inflamm 19:262–266. doi: 10.3109/09273948.2011.582220. [DOI] [PubMed] [Google Scholar]
- 4.Pfaff AW, de-la-Torre A, Rochet E, Brunet J, Sabou M, Sauer A, Bourcier T, Gomez-Marin JE, Candolfi E. 2014. New clinical and experimental insights into Old World and neotropical ocular toxoplasmosis. Int J Parasitol 44:99–107. doi: 10.1016/j.ijpara.2013.09.007. [DOI] [PubMed] [Google Scholar]
- 5.Felix JP, Lira RP, Zacchia RS, Toribio JM, Nascimento MA, Arieta CE. 2014. Trimethoprim-sulfamethoxazole versus placebo to reduce the risk of recurrences of Toxoplasma gondii retinochoroiditis: randomized controlled clinical trial. Am J Ophthalmol 157:762–766 e1. doi: 10.1016/j.ajo.2013.12.022. [DOI] [PubMed] [Google Scholar]
- 6.Garweg JG, Stanford MR. 2013. Therapy for ocular toxoplasmosis—the future. Ocular Immunol Inflamm 21:300–305. doi: 10.3109/09273948.2013.779724. [DOI] [PubMed] [Google Scholar]
- 7.Holland GN, O'Connor GR, Diaz RF, Minasi P, Wara WM. 1988. Ocular toxoplasmosis in immunosuppressed nonhuman primates. Invest Ophthalmol Vis Sci 29:835–842. [PubMed] [Google Scholar]
- 8.O'Connor GR, Nozik RA. 1971. Studies on experimental ocular toxoplasmosis in the rabbit. 3. Recurrent inflammation stimulated by systemic administration of antilymphocyte serum and normal horse serum. Arch Ophthalmol 85:718–722. [DOI] [PubMed] [Google Scholar]
- 9.Gazzinelli RT, Brezin A, Li Q, Nussenblatt RB, Chan CC. 1994. Toxoplasma gondii: acquired ocular toxoplasmosis in the murine model, protective role of TNF-alpha and IFN-gamma. Exp Parasitol 78:217–229. doi: 10.1006/expr.1994.1022. [DOI] [PubMed] [Google Scholar]
- 10.Lu F, Huang S, Hu MS, Kasper LH. 2005. Experimental ocular toxoplasmosis in genetically susceptible and resistant mice. Infect Immun 73:5160–5165. doi: 10.1128/IAI.73.8.5160-5165.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Charles E, Callegan MC, Blader IJ. 2007. The SAG1 Toxoplasma gondii surface protein is not required for acute ocular toxoplasmosis in mice. Infect Immun 75:2079–2083. doi: 10.1128/IAI.01685-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lahmar I, Guinard M, Sauer A, Marcellin L, Abdelrahman T, Roux M, Mousli M, Moussa A, Babba H, Pfaff AW, Candolfi E. 2010. Murine neonatal infection provides an efficient model for congenital ocular toxoplasmosis. Exp Parasitol 124:190–196. doi: 10.1016/j.exppara.2009.09.010. [DOI] [PubMed] [Google Scholar]
- 13.Melzer TC, Cranston HJ, Weiss LM, Halonen SK. 2010. Host cell preference of Toxoplasma gondii cysts in murine brain: a confocal study. J Neuroparasitol 1:N100505. doi: 10.4303/jnp/N100505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sauer A, Rochet E, Lahmar I, Brunet J, Sabou M, Bourcier T, Candolfi E, Pfaff AW. 2013. The local immune response to intraocular Toxoplasma re-challenge: less pathology and better parasite control through Treg/Th1/Th2 induction. Int J Parasitol 43:721–728. doi: 10.1016/j.ijpara.2013.04.004. [DOI] [PubMed] [Google Scholar]
- 15.Bosch-Driessen LE, Berendschot TT, Ongkosuwito JV, Rothova A. 2002. Ocular toxoplasmosis: clinical features and prognosis of 154 patients. Ophthalmology 109:869–878. doi: 10.1016/S0161-6420(02)00990-9. [DOI] [PubMed] [Google Scholar]
- 16.Johnson AM. 1984. Strain-dependent, route of challenge-dependent, murine susceptibility to toxoplasmosis. Z Parasitenkd 70:303–309. doi: 10.1007/BF00927816. [DOI] [PubMed] [Google Scholar]
- 17.Pfaff AW, Mousli M, Senegas A, Marcellin L, Takikawa O, Klein JP, Candolfi E. 2008. Impact of foetus and mother on IFN-gamma-induced indoleamine 2,3-dioxygenase and inducible nitric oxide synthase expression in murine placenta following Toxoplasma gondii infection. Int J Parasitol 38:249–258. doi: 10.1016/j.ijpara.2007.07.007. [DOI] [PubMed] [Google Scholar]
- 18.Charles E, Joshi S, Ash JD, Fox BA, Farris AD, Bzik DJ, Lang ML, Blader IJ. 2010. CD4 T-cell suppression by cells from Toxoplasma gondii-infected retinas is mediated by surface protein PD-L1. Infect Immun 78:3484–3492. doi: 10.1128/IAI.00117-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sauer A, Pfaff AW, Villard O, Creuzot-Garcher C, Dalle F, Chiquet C, Pelloux H, Speeg-Schatz C, Gaucher D, Prevost G, Bourcier T, Candolfi E. 2012. Interleukin 17A as an effective target for anti-inflammatory and antiparasitic treatment of toxoplasmic uveitis. J Infect Dis 206:1319–1329. doi: 10.1093/infdis/jis486. [DOI] [PubMed] [Google Scholar]
- 20.de-la-Torre A, Sauer A, Pfaff AW, Bourcier T, Brunet J, Speeg-Schatz C, Ballonzoli L, Villard O, Ajzenberg D, Sundar N, Grigg ME, Gomez-Marin JE, Candolfi E. 2013. Severe South American ocular toxoplasmosis is associated with decreased IFN-gamma/IL-17a and increased IL-6/IL-13 intraocular levels. PLoS Negl Trop Dis 7:e2541. doi: 10.1371/journal.pntd.0002541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ferguson DJ, Hutchison WM, Pettersen E. 1989. Tissue cyst rupture in mice chronically infected with Toxoplasma gondii. An immunocytochemical and ultrastructural study. Parasitol Res 75:599–603. [DOI] [PubMed] [Google Scholar]
- 22.Cornelissen C, Luscher-Firzlaff J, Baron JM, Luscher B. 2012. Signaling by IL-31 and functional consequences. Eur J Cell Biol 91:552–566. doi: 10.1016/j.ejcb.2011.07.006. [DOI] [PubMed] [Google Scholar]
- 23.Carow B, Rottenberg ME. 2014. SOCS3, a major regulator of infection and inflammation. Front Immunol 5:58. doi: 10.3389/fimmu.2014.00058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Suzuki Y, Orellana MA, Schreiber RD, Remington JS. 1988. Interferon-gamma: the major mediator of resistance against Toxoplasma gondii. Science 240:516–518. doi: 10.1126/science.3128869. [DOI] [PubMed] [Google Scholar]
- 25.Abou-Bacar A, Pfaff AW, Letscher-Bru V, Filisetti D, Rajapakse R, Antoni E, Villard O, Klein JP, Candolfi E. 2004. Role of gamma interferon and T cells in congenital Toxoplasma transmission. Parasite Immunol 26:315–318. doi: 10.1111/j.0141-9838.2004.00713.x. [DOI] [PubMed] [Google Scholar]
- 26.Suzuki Y, Rani S, Liesenfeld O, Kojima T, Lim S, Nguyen TA, Dalrymple SA, Murray R, Remington JS. 1997. Impaired resistance to the development of toxoplasmic encephalitis in interleukin-6-deficient mice. Infect Immun 65:2339–2345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Handel U, Brunn A, Drogemuller K, Muller W, Deckert M, Schluter D. 2012. Neuronal gp130 expression is crucial to prevent neuronal loss, hyperinflammation, and lethal course of murine Toxoplasma encephalitis. Am J Pathol 181:163–173. doi: 10.1016/j.ajpath.2012.03.029. [DOI] [PubMed] [Google Scholar]
- 28.Whitmarsh RJ, Gray CM, Gregg B, Christian DA, May MJ, Murray PJ, Hunter CA. 2011. A critical role for SOCS3 in innate resistance to Toxoplasma gondii. Cell Host Microbe 10:224–236. doi: 10.1016/j.chom.2011.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kang S, Tanaka T, Kishimoto T. 2015. Therapeutic uses of anti-interleukin-6 receptor antibody. Int Immunol 27:21–29. doi: 10.1093/intimm/dxu081. [DOI] [PubMed] [Google Scholar]