Abstract
SasX is a recently described surface protein of Staphylococcus aureus that is linked to the epidemic success of hospital-associated methicillin-resistant clones, in particular in Asia. It enhances nasal colonization and virulence in skin and lung infection models. Here, we evaluated the potential of SasX as a vaccine component in passive and active immunization efforts using mouse infection models. We found that SasX induced a specific immune response predominantly based on IgG1 antibodies. Active immunization with recombinant SasX or passive immunization with rabbit polyclonal anti-SasX IgG significantly decreased the size of lesions caused by S. aureus in a skin infection model. Furthermore, active immunization reduced acute lung injury in a lung infection model. Moreover, active or passive immunization significantly reduced S. aureus colonization in a nasal colonization model. Finally, anti-SasX IgG enhanced the susceptibility of S. aureus to killing by human neutrophils. We conclude that SasX is a potential target for therapeutics or vaccines designed to moderate colonization and infection by sasX-positive epidemic strains of S. aureus.
INTRODUCTION
Staphylococcus aureus is a major global source of morbidity and mortality (1), causing more than 11,000 deaths per year in the United States alone (2). It is particularly notorious as a dangerous hospital-associated pathogen. Treatment of S. aureus infections is severely complicated by antibiotic resistance (3). In particular, resistance to methicillin in methicillin-resistant S. aureus (MRSA), which in many countries occurs in more than half of infectious S. aureus isolates, is a major health care concern. In addition, many S. aureus strains are resistant to a wide variety of other antibiotics, leaving only very limited options for treatment. In an era that has seen a broad withdrawal of pharmaceutical companies from the much-needed development of novel antibiotics, researchers in companies and academia are again beginning to attempt vaccine development against S. aureus. A working vaccine against S. aureus infections is not available, and multiple reasons have been discussed for why an anti-S. aureus vaccine is difficult to find (4, 5). These include first and foremost the large arsenal of immune evasion factors of S. aureus, which target both acquired and innate immune defenses (6). However, there is promising evidence indicating that passive and active vaccination against S. aureus should in principle be possible, notwithstanding the fact that we still do not completely understand the mechanisms the immune system uses for protective immunity against S. aureus (4).
Infective S. aureus and MRSA isolates are very diverse regarding geographical origin and time of isolation. Over the years, specific predominant MRSA clones arose and were thereafter replaced by others, in a scenario of epidemic waves (7). Furthermore, infections in different geographic areas are characterized by a divergent and often endemic composition of prevalent S. aureus and MRSA lineages. This situation requires an adaptation of vaccine targets to specific, predominant infectious clones.
The sequence type 239 (ST239) lineage of MRSA isolates is the predominant lineage causing hospital-associated infections in Asia (8). Furthermore, it has caused outbreaks in other geographical locations (9). We previously showed that many ST239 isolates harbor a lysogenic prophage expressing a surface protein, SasX, which is associated with disease severity in skin and lung infections (10). It also facilitates nasal colonization, from which infection can originate. Notably, the sasX gene has been spreading at a considerable rate among ST239 and other MRSA lineages in Chinese hospitals and is considered an important factor contributing to the pathogenic success of the ST239 lineage (10). In the present study, we evaluated active and passive immunization strategies using the SasX protein to reduce infectivity and colonization by ST239 and other sasX-containing MRSA.
MATERIALS AND METHODS
Ethics statement.
All animal work was approved by the ethics committee of Renji Hospital, Shanghai Jiaotong University School of Medicine, Shanghai, China. Human neutrophils were isolated from the heparinized venous blood of healthy individuals with a standard method (11) in accordance with a protocol approved by the ethics committee of Renji Hospital, Shanghai Jiaotong University School of Medicine, Shanghai, China. All individuals gave informed consent prior to donating blood.
Bacteria and growth conditions.
Bacteria were identified as staphylococci by classic microbiological methods: Gram's staining and catalase and coagulase activity on rabbit plasma. S. aureus strains were further categorized by biochemical characterization using the Api-Staph test (bioMérieux, Lyon, France). The MRSA clinical isolate HS770 (ST239, containing the sasX gene) was recovered from the sputum of an inpatient with pneumonia at Shanghai Hospital, China, and determined to belong to ST239 by multilocus sequence typing (MLST) (10). Isolate HS770 was grown in tryptic soy broth (TSB; Oxoid) and used in all animal work. Escherichia coli BL21 was grown in Luria-Bertani broth (LB; Oxoid). When necessary, media were supplemented with ampicillin (100 μg/ml for E. coli) or chloramphenicol (10 μg/ml) for S. aureus.
Construction and purification of recombinant SasX protein.
The sasX gene was cloned, overexpressed, and purified as a glutathione S-transferase (GST) fusion protein as described elsewhere (10). To obtain pure, recombinant SasX (rSasX) for use in immunization experiments, the GST part was removed by thrombin digestion (Fig. 1A and B).
FIG 1.
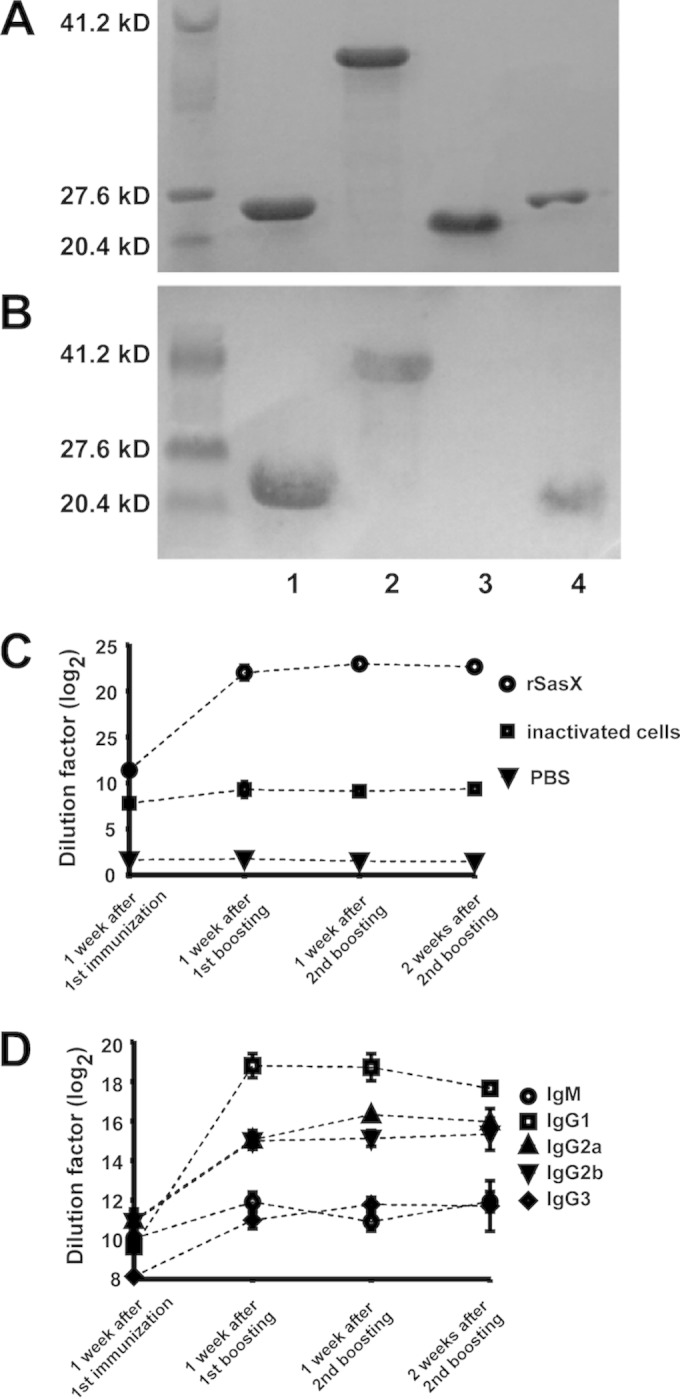
Recombinant SasX can induce specific immune responses. (A and B) Production and purification of rSasX. Lane 1, purified GST protein; 2, purified GST-SasX fusion protein; 3, purified rSasX protein after thrombin digestion, which was used for immunization and vaccination; 4, purified GST-tagged protein after thrombin digestion. The molecular mass marker is at the left. SDS-PAGE with Coomassie blue staining is shown in panel A, and a Western blot with anti-GST antibodies is shown in panel B. (C) IgG titers against rSasX in serum determined by ELISA. (D) Serum concentrations of antibody subtypes determined by ELISA. The mice were immunized three times at 12-day intervals (days 0, 12, and 24), and sera were obtained from the mice 1 week after the first immunization, 1 week after the first boosting, 1 week after the second boosting, and 2 weeks after the second boosting. Inactivated S. aureus cells and rSasX were emulsified in Freund's adjuvant. The control group was injected with only PBS.
Production of rabbit antisera and purification of anti-SasX IgG.
Purified rSasX protein was used as an immunogen for the production of rabbit polyclonal antisera (provided by Youke Biotech Company, Shanghai, China). Anti-SasX IgG was purified using the protein A affinity column Hitrap rProteinA FF (GE Healthcare) using an AKTA purifier (GE Healthcare) according to the manufacturer's specifications.
Active immunization.
Female BALB/c mice were used for active immunization. All mice were 5 to 6 weeks of age. The mice were randomly allocated to 3 treatment groups as follows: (i) rSasX plus Freund's adjuvant (Sigma), (ii) inactivated S. aureus HS770 plus Freund's adjuvant, and (iii) phosphate-buffered saline (PBS) (control group). Purified rSasX protein was dissolved in PBS and emulsified in Freund's adjuvant; the emulsions (100 μl each) contained 50 μg of protein. The inactivated S. aureus vaccine was prepared as follows. MRSA HS770 was cultured overnight at 37°C in TSB, harvested by centrifugation, resuspended in sterilized PBS, and adjusted to 1 × 106 CFU/ml. The bacteria were then inactivated at 37°C for 24 h in 0.2% (wt/vol) formaldehyde and emulsified at a 1:1 ratio in complete Freund's adjuvant (CFA). The mice were immunized by intramuscular injection of 50 μg of purified rSasX or inactivated S. aureus vaccine in CFA on day 0, followed by a boost with the same antigens in incomplete Freund's adjuvant (IFA) at 12-day intervals (days 0, 12, and 24). Blood samples were collected on days 7, 19, 31, and 38, and the sera were harvested and stored at −80°C. The control group was injected with PBS by following the same protocol.
Detection of specific IgG by ELISA.
Enzyme-linked immunosorbent assays (ELISAs) were used to determine the titers of IgG against rSasX. Ninety-six-well ELISA plates (JET BIOFIL; JET Biotech Company, Guangzhou, China) were coated with 100 μl of rSasX at a concentration of 1 μg/ml overnight at 4°C in carbonate/bicarbonate buffer (pH 9.6). Then the plates were blocked using 100 μl carbonate/bicarbonate buffer (pH 9.6) containing fetal calf serum (FCS) (10% [wt/vol]) overnight at 4°C. The serum samples were added at serial dilutions and incubated for 1 h at 37°C. Horseradish peroxidase (HRP)-conjugated goat anti-mouse IgG antibodies (1:2,000) were then added, and the mixture was incubated for 1 h at 37°C. A total of 100 μl chromogenic substrate solution (42 mM tetramethylbenzidine and 0.01% hydrogen peroxide) was added to the plates. Plates were incubated at 37°C for 10 min. Then the enzymatic reaction was stopped by the addition of 50 μl of 2 N hydrofluoric acid. Three washes using PBS with 0.05% Tween 20 were performed between each step. The optical density (OD) at 450 nm/630 nm was read on a microplate reader (BioTek Instruments, Winooski, VT). The method used to determine the titers of antibodies against the SasX in inactivated S. aureus was the same as that described above for rSasX, except that the coated antigen was inactivated S. aureus.
Detection of the subtypes of antibodies.
ELISAs were performed as in the method described for the detection of specific IgG, except the secondary antibody was mouse anti-IgG1–HRP (1:500), mouse anti-IgG2a–HRP (1:500), mouse anti-IgG2b–HRP (1:500), mouse anti-IgG3–HRP (1:500), or mouse anti-IgM–HRP (1:500) (Southern Biotech).
Mouse cytokine detection.
Cytokine concentrations in the blood or supernatant of the homogenized tissues of mice were detected using a Bio-Plex Pro assay kit (Bio-Rad Laboratories). A total of 50 μl of antibody-conjugated beads was added to the assay plate. The samples were diluted 1:4, 50 μl of diluted samples, standards, the blank, and controls was added to the plate, and the plate was incubated in the dark at room temperature (RT) with shaking at 300 rpm for 30 min and then washed 3 times with 100 μl wash buffer. A total of 25 μl biotinylated antibody was added to the plate, which was incubated in the dark at RT with shaking at 300 rpm for 30 min and then washed 3 times with 100 μl wash buffer. A total of 50 μl streptavidin-phycoerythrin (PE) was added to the plate. The plate was again incubated in the dark at RT with shaking at 300 rpm for 10 min and then washed 3 times with 100 μl wash buffer. Finally, the plate was read using a Bio-Plex protein array reader. Bio-Plex Manager 6.0 software was used for Bio-Plex Pro assay data acquisition and analysis.
Skin abscess model in mice after active immunization.
The skin abscess model was performed 1 week after the first boosting during the active immunization procedure. Before the experiments, the mice were shaved around the site of injection. The HS770 isolate was grown to mid-exponential phase, washed once with sterile PBS, and then resuspended in PBS at 1 × 109 CFU/100 μl. Mice (n = 15 per group) were anesthetized with isoflurane and inoculated with 100 μl PBS containing 109 live S. aureus cells in the right flank by subcutaneous injection. Ten mice were chosen randomly out of the 15 mice per group once subcutaneous injection of S. aureus was finished to measure abscess formation and were examined with a caliper at 24-h intervals for a total of 10 days. We applied length (L) and width (W) values to calculate the area of abscesses with the formula L × W. On the fourth day after infection, the remaining five mice per group were killed, the abscess regions were excised, and part of the excised tissue material was fixed in 10% formalin (Sigma). Paraffin embedding, hematoxylin and eosin (H&E) staining, and toluidine blue (TB) staining were performed as described previously (10, 12–14). Abscess material samples of 0.05 g were homogenized in 0.5 ml PBS for the measurement of cytokines. All animals were euthanized after completion of the entire procedure.
Skin abscess model after passive immunization.
Female BALB/c mice were used for passive immunization. All mice were 5 to 6 weeks of age. The mice were randomly allocated to 2 treatment groups (n = 15 per group), as follows: (i) rabbit polyclonal anti-SasX IgG and (ii) rabbit preimmune serum. The mice received 5 mg total IgG purified from either the rabbit preimmune serum or the polyclonal anti-SasX IgG in 500 μl PBS via intraperitoneal injection. Twenty-four hours later, the mice received another 5 mg total IgG via intraperitoneal injection. Twenty-four hours later, the mice in each group were anesthetized with isoflurane and inoculated with 100 μl PBS containing 109 live HS770 bacterial cells in the left flank by subcutaneous injection. Subsequent steps were the same as for the active immunization procedure.
Lung infection model in mice after active immunization.
The lung infection model was performed 1 week after the first boosting during the active immunization procedure. Isolate HS770 was grown to mid-exponential growth phase, washed, and resuspended in sterile PBS at 5 × 107 CFU/μl. Mice (n = 15 per group) were anesthetized with isoflurane. The inoculum, which contained 109 CFU/20 μl, was pipetted into the nares of the anesthetized mice without touching the nose. Mice were euthanized at 24 h (n = 4), 48 h (n = 8), or 72 h (n = 3) after infection. The lungs were excised and washed with saline. The right lungs were homogenized in 0.5 ml of TSB, the homogenized lung tissue was plated in triplicate on tryptic soy agar (TSA) containing 5% sheep blood, and the supernatant of the homogenized lung tissue was used for cytokine detection. The left lung was fixed in 4% formalin (Sigma). Paraffin embedding and H&E staining were performed as previously described (10, 12).
Nasal colonization model in mice after active immunization.
The nasal colonization model was performed 1 week after the first boosting during the active immunization procedure. Isolate HS770 was grown to mid-exponential growth phase, washed, and resuspended in sterile PBS at 106 CFU/μl. Mice (n = 24 per group) were anesthetized with isoflurane. The inoculum, which contained 107 CFU in 10 μl of PBS, was pipetted slowly into the nares of the anesthetized mice without touching the pipette tip to the nose. Three days and 7 days after inoculation, 12 mice per group were chosen randomly, euthanized, and evaluated for nasal carriage of S. aureus. The nasal region was wiped externally with 70% ethanol, and the nasal tissue was homogenized in 0.5 ml of TSB. The total number of S. aureus CFU per nose was assessed by plating 100 μl of diluted nasal suspensions on TSB agar containing penicillin (100 μg/ml).
Nasal colonization model in mice after passive immunization.
The passive immunization procedure was performed as described above for the skin abscess model. For the preventive approach, 24 h after immunization, the mice in each group were anesthetized and received S. aureus bacteria as described above for the active immunization procedure in the nasal colonization model. On day 7 after inoculation, all mice were euthanized and evaluated for nasal carriage of S. aureus as described above. For the treatment experiment, procedures were the same except that mice first were given S. aureus in the nose and received 10 mg of preimmune serum or the polyclonal anti-SasX IgG serum via intraperitoneal injection 24 h afterwards.
Opsonophagocytic killing.
Isolated human neutrophils were adjusted to 5 × 106/ml in RPMI 1640. The complement source (rabbit serum; Sigma) was adsorbed three times with HS770 bacteria at 4°C for 30 min in order to remove S. aureus-specific antibodies. After adsorption, the complement solution was centrifuged and filter sterilized. HS770 bacteria were grown to mid-exponential phase, washed once with sterile PBS, and resuspended in RPMI 1640 at 5 × 107 CFU/ml. The opsonophagocytic killing assay was performed by mixing 100 μl (each) of the neutrophil suspension, HS770 cells, dilutions of rabbit polyclonal anti-SasX IgG or rabbit preimmune serum, and the complement source. The reaction mixture was incubated at 30°C for 90 min. The samples were taken at 0 min, 30 min, 60 min, and 90 min and plated on TSA containing 5% sheep blood for detection of surviving S. aureus. Percent survival was determined relative to control reactions, to which no neutrophils were added. The lysis of neutrophils after phagocytosis was measured using a lactate dehydrogenase (LDH) cytotoxicity detection kit according to the manufacturer's protocol (Roche) as described elsewhere (15). Samples were diluted 1:20 for the assays.
Statistics.
Statistical analysis was performed using Graph-Pad Prism, version 6.02, with unpaired t tests. Error bars show the standard error of the mean (SEM).
RESULTS
Vaccination with rSasX induces specific immune responses.
To test whether rSasX induces specific immune responses in mice, IgG titers were measured after three rounds of vaccination (Fig. 1C). Compared to inactivated S. aureus immunization with the same adjuvant, rSasX induced a much stronger antibody response. In addition, rSasX stimulated a more rapid increase in antibody levels than inactivated S. aureus. Furthermore, we measured the serum levels of antibody subtypes directed against rSasX following the administration of rSasX emulsified in Freund's adjuvant (Fig. 1D). The subtype of antibodies induced by rSasX was predominantly IgG1. The titers of IgG1 induced by rSasX peaked at 1 week after the first boosting. These results show that rSasX is strongly immunogenic in mice, causing a rapid and substantial production of specific antibodies.
Opsonic activity of rabbit polyclonal anti-SasX IgG.
For passive immunization efforts, we developed a polyclonal anti-SasX antiserum in rabbits and tested it first for its potential to stimulate killing after opsonophagocytosis. Opsonophagocytic killing by neutrophils is the primary mechanism by which S. aureus is cleared by the host's immune system (16). Opsonophagocytosis assays with human neutrophils can be used to assess the function of antistaphylococcal antibodies, because an in vitro opsonic reaction is initiated when bacterial cells, functional antibodies, and an exogenous complement source are mixed together. Measurement of S. aureus survival after opsonophagocytosis in vitro showed that a significantly lower number of bacteria survived upon the addition of purified rabbit polyclonal anti-SasX IgG than in rabbit preimmune serum-treated cultures (Fig. 2A). We also found a significant reduction in neutrophil lysis as measured by release of lactate dehydrogenase (LDH) upon the addition of rabbit polyclonal anti-SasX IgG, which can be interpreted as a result of a lower number of bacteria being present to lyse neutrophils by mechanisms such as toxin-mediated lysis (17) (Fig. 2B). These findings demonstrate that anti-SasX antibodies promote elimination of sasX-positive MRSA ST239 by human neutrophils.
FIG 2.
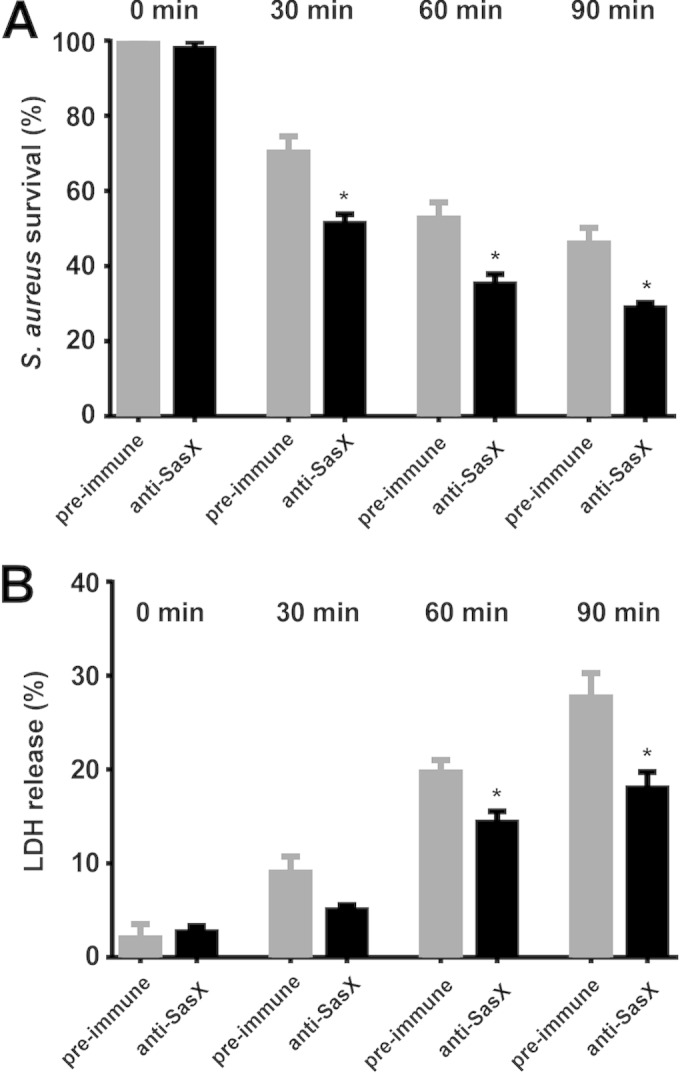
Rabbit polyclonal anti-SasX IgG enhances the susceptibility of MRSA ST239 toward elimination by human neutrophils. (A) Survival of S. aureus (isolate HS770) after in vitro opsonophagocytosis by human neutrophils. Bacteria were incubated for 90 min with neutrophils, the complement source, and dilutions of rabbit polyclonal anti-SasX IgG or rabbit preimmune serum. The number of surviving bacteria was determined by dilution plating. *, P < 0.05. (B) In vitro neutrophil lysis after opsonophagocytosis of S. aureus cells. Neutrophil lysis was determined by release of lactate dehydrogenase (LDH). *, P < 0.05.
Active or passive immunization with rSasX reduces skin infection in mice.
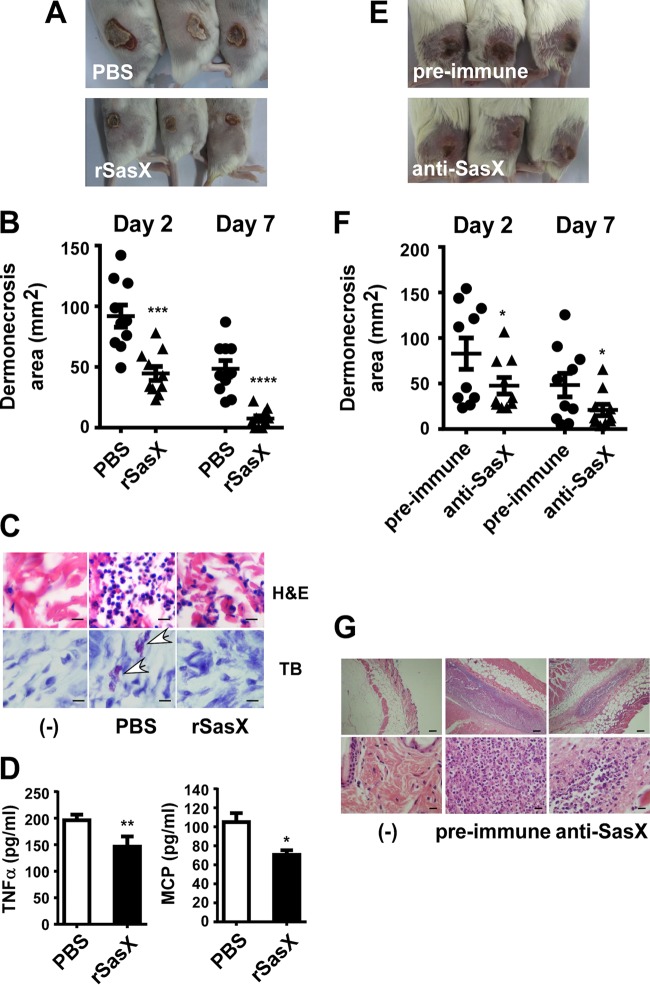
We first analyzed whether active immunization with rSasX reduces skin infection due to MRSA ST239 using a mouse abscess model. Skin infections (wounds and abscesses) were the second most frequent type of infection caused by sasX-positive S. aureus in our previous study (10). Skin lesions of mice infected with MRSA isolate HS770 (ST239) were significantly smaller after active immunization with rSasX plus Freund's adjuvant than lesions of mice in PBS control-immunized groups (Fig. 3A and B). Furthermore, we measured the infiltration of neutrophils and mast cells, as they have been implicated in a variety of inflammatory conditions and play an important role in host defense against staphylococcal infection (14, 18). In the group of mice immunized with rSasX, the degree of skin tissue destruction and the degree of neutrophil and mast cell infiltration were strongly reduced compared to those in PBS control mice (Fig. 3C). It has been reported that mast cells modulate the influx of neutrophils and monocytes, and thus clearance of bacterial infection, through release of chemoattractants, such as tumor necrosis factor alpha (TNF-α) (13, 18). Concentrations of TNF-α and monocyte chemoattractant protein 1 (MCP-1) in the supernatant of homogenized abscess tissue were significantly lower in the rSasX-immunized group than in the PBS control group (Fig. 3D). These results show that active immunization with rSasX against sasX-positive MRSA ST239 results in reduced symptoms of skin disease, including dermonecrosis and inflammatory reactions.
FIG 3.
Active and passive immunization with SasX reduces dermonecrosis caused by MRSA ST239. To measure the outcome of active immunization, mice were challenged with 109 live S. aureus CFU 1 week after the first boosting with rSasX (or the PBS control), and dermonecrotic lesions were measured with a caliper at 24-h intervals for a total of 10 days. (A) Representative skin lesions on day 4; (B) dermonecrosis areas on days 2 and 7 after infection. ***, P < 0.001; ****, P < 0.0001. (C) H&E and TB staining of abscess skin tissues harvested on day 4. (−), control without bacterial challenge. Top row (H&E staining), neutrophils appear purple; bottom row (TB staining), mast cells appear purple (white arrows). Scale bars, 20 μm. (D) Cytokine concentrations in abscess tissue. The concentrations of the TNF-α and MCP-1 cytokines were measured by ELISA in abscess samples on day 4 after infection. *, P < 0.05; **, P < 0.01. To measure the outcome of passive immunization, mice received a total of 10 mg IgG of either rabbit preimmune serum or polyclonal anti-SasX IgG via intraperitoneal injection in 24-h intervals. Twenty-four hours later, the mice were inoculated with 100 μl PBS containing 109 live MRSA HS770 bacteria in the left flank by subcutaneous injection, and dermonecrotic lesions were measured with a caliper at 24-h intervals for a total of 10 days. (E) Representative skin lesions on day 4; (F) dermonecrosis areas on days 2 and 7 after infection. *, P < 0.05. (G) H&E staining of abscess skin tissues harvested on day 4. Scale bars, 20 μm (bottom row) and 200 μm (top row); (−), control without bacterial challenge.
We then also evaluated whether passive immunization with polyclonal anti-SasX IgG antibodies obtained from rabbits could reduce skin infection due to MRSA ST239. After 2 and 7 days of skin infection, mice that had received anti-SasX antibodies had significantly smaller lesions than mice that had received preimmune serum as a control (Fig. 3E and F). Furthermore, the treated mice showed reduced neutrophil infiltration and skin tissue destruction compared to those of the mice treated with preimmune serum (Fig. 3G). These results showed that passive immunization with anti-SasX antibodies can reduce skin infection due to sasX-positive MRSA ST239 and indicate that the protection achieved with active immunization was antibody mediated.
Active immunization with rSasX reduces lung infection in mice.
In addition to infections of the skin, S. aureus is most commonly involved in respiratory infections (1). In the specific case of infections with sasX-positive S. aureus, respiratory infections were the predominant type of infections caused (10). Therefore, we determined whether active immunization against rSasX would moderate the severity of acute lung injury. Lung wet weight/body weight ratios (Fig. 4A), macroscopic and histological examination (Fig. 4B), bacterial counts (Fig. 4C), and the degree of inflammation in the lung as assessed by TNF-α and MCP-1 concentrations (Fig. 4D) showed that active immunization with rSasX reduces acute lung injury and inflammation during lung infection with sasX-positive MRSA ST239.
FIG 4.
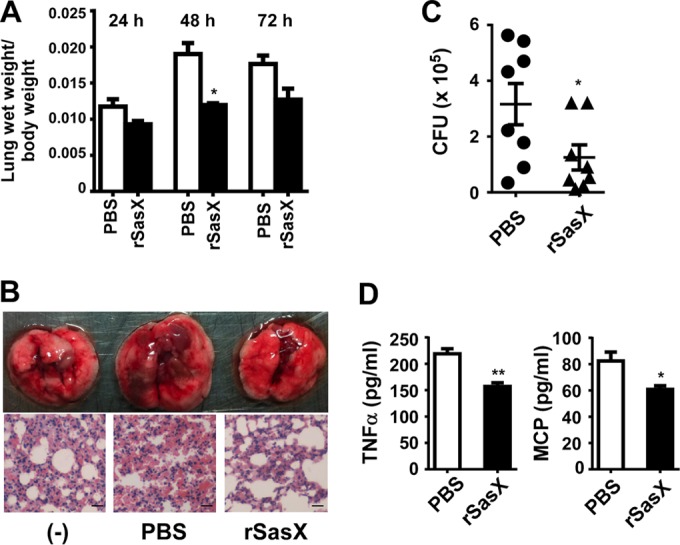
Active immunization with SasX reduces acute lung injury caused by MRSA ST239 in a lung infection model. Mice were challenged by introducing 109 live MRSA HS770 CFU into the nares 1 week after the first boosting with rSasX (or the PBS control). At 24, 48, and 72 h after inoculation, groups of mice were euthanized. (A) Lung wet weight/body weight ratios. *, P < 0.05. (B) The left lungs of mice euthanized at 48 h after infection were fixed in 4% formalin for H&E staining. Scale bars, 50 μm. (C and D) The right lungs of mice euthanized at 48 h after infection were homogenized, and the homogenized lung tissue was used for CFU (C) and cytokine detection (D). *, P < 0.05; **, P < 0.01.
Active and passive immunization with rabbit polyclonal anti-SasX IgG reduces nasal colonization in mice.
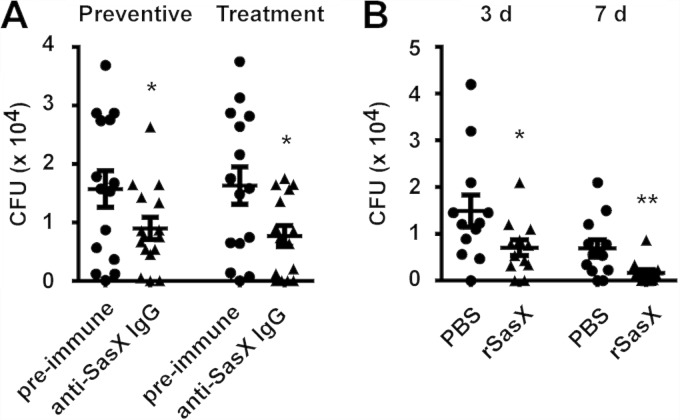
Nares are the predominant location of S. aureus colonization in the human body (19). Our previous study demonstrated that the SasX surface protein promotes nasal colonization (10). Therefore, we tested whether active immunization with rSasX or passive immunization with rabbit polyclonal anti-SasX IgG can reduce nasal colonization in a preventive or treatment fashion. The determination of bacterial counts after 7 days of inoculation of the noses of mice showed that rabbit polyclonal anti-SasX IgG had a significant preventive effect regarding subsequent colonization, compared with PBS and rabbit preimmune serum (Fig. 5A). Notably, anti-SasX IgG could also reduce colonization in a treatment fashion, i.e., once colonization was established. These results show that nasal colonization of sasX-positive MRSA ST239 can be suppressed using anti-SasX antibodies. Similarly, using an active immunization approach, vaccination with rSasX reduced subsequent colonization by sasX-positive MRSA ST239 (Fig. 5B).
FIG 5.
Active or passive immunization with SasX reduces nasal colonization caused by MRSA ST239 in a nasal colonization model. (A) Passive immunization. Mice received a total of 10 mg IgG of either rabbit preimmune serum or polyclonal anti-SasX IgG in 24-h intervals via intraperitoneal injection 24 h before (preventive effect) or after (treatment effect) nasal colonization with 107 CFU of MRSA strain HS770. At 7 days after inoculation, all mice were euthanized and evaluated for nasal carriage of S. aureus. Numbers are total CFU per nose. *, P < 0.05. (B) Active immunization. Mice were challenged by introducing 107 live bacterial cells of MRSA strain HS770 into the nares 1 week after the first boosting with rSasX (or the PBS control). Three days and 7 days after inoculation, 12 mice per group were euthanized and evaluated for nasal carriage of S. aureus. Numbers are total CFU per nose. *, P < 0.05; **, P < 0.01.
DISCUSSION
S. aureus vaccine development is aimed at reducing the severity of S. aureus infections via two possible routes. Vaccines may reduce pathogen virulence during infection and allow the immune system to clear off the infection. Alternatively, S. aureus vaccines may reduce colonization by S. aureus, which is linked to subsequent infection (4, 20). In addition to fulfilling common prerequisites for optimal vaccine targets, such as accessibility on the surface of S. aureus, the SasX protein has been demonstrated to significantly impact both S. aureus nasal colonization and in vivo virulence in major S. aureus infection types (10). It is thus a potential target for both anti-S. aureus vaccination strategies aimed at colonization and/or infection.
Only a limited number of virulence determinants in S. aureus are widely distributed among virtually all S. aureus strains, such as alpha-toxin or phenol-soluble modulins (17). In ongoing efforts to use these as vaccination targets, alpha-toxin toxoid, for example, has proven to represent a very promising vaccination agent in efforts to reduce S. aureus skin and lung infection (21). In addition, we believe that virulence determinants that are found to strongly impact colonization and virulence phenotypes in MRSA strains with considerable epidemiological importance, wide geographical distribution, and likely further spread, such as SasX, should also be analyzed as potential vaccine targets. Other surface proteins, such as ClfA or IsdA, which impact nasal colonization in animal models, have previously shown success as immunogens in experimental animal colonization studies (22–24). For these reasons, we here analyzed whether active and passive immunization with SasX as the target reduces S. aureus skin and lung infection and has the potential to reduce S. aureus nasal colonization.
We found that SasX was strongly immunogenic in mice with a dominant production of IgG1 subtype antibodies. This suggests a Th2-dominant response. The induction of a high proportion of IgG1 is a typical feature of protein immunization (25). In addition, IgG1 was previously shown to be the most efficient opsonization isotype for neutrophils (26); therefore, the higher titers of total IgG and the dominant IgG1 subtype might be responsible for the superior protection conferred by SasX immunization than by immunization with inactivated S. aureus.
In our study, active immunization with SasX significantly reduced acute skin and lung infection in mice, and passive immunization with IgG purified from a rabbit polyclonal antiserum developed against SasX also reduced acute skin infection. Furthermore, active or passive immunization reduced nasal colonization in mice, with passive immunization being successful in both treatment and prevention fashions. Moreover, anti-SasX antibodies promoted elimination of sasX-positive MRSA by human neutrophils. These results suggest that SasX could be a valuable component in vaccine strategies targeting epidemic S. aureus hospital MRSA infections in Asia, among which the SasX protein is frequently expressed. Nowadays, such strategies are commonly set up to develop a multivalent vaccine aimed at a selection of targets with predominant effects on virulence and/or colonization (27). Furthermore, our present results underscore our previous findings indicating a significant impact of SasX on acute types of S. aureus infection as well as nasal colonization (10).
ACKNOWLEDGMENTS
This study was supported by the National Natural Science Foundation of China (grants 81322025, 81171623, and 81371875), Shanghai Shuguang Talent Project (12SG03), the Shanghai Committee of Science and Technology, China (14140901000), and the Foundation for Innovative Research Groups of the National Natural Science Foundation of China (grant no. 81421001) (to M.L.) and the Intramural Research Program of the National Institute of Allergy and Infectious Diseases, the National Institutes of Health (to M.O.).
REFERENCES
- 1.Lowy FD. 1998. Staphylococcus aureus infections. N Engl J Med 339:520–532. doi: 10.1056/NEJM199808203390806. [DOI] [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention. 2013. Antibiotic resistance threats in the United States, 2013. CDC, Atlanta, GA: http://www.cdc.gov/drugresistance/threat-report-2013. [Google Scholar]
- 3.Lowy FD. 2003. Antimicrobial resistance: the example of Staphylococcus aureus. J Clin Invest 111:1265–1273. doi: 10.1172/JCI200318535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schaffer AC, Lee JC. 2009. Staphylococcal vaccines and immunotherapies. Infect Dis Clin North Am 23:153–171. doi: 10.1016/j.idc.2008.10.005. [DOI] [PubMed] [Google Scholar]
- 5.DeLeo FR, Otto M. 2008. An antidote for Staphylococcus aureus pneumonia? J Exp Med 205:271–274. doi: 10.1084/jem.20080167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rooijakkers SH, van Kessel KP, van Strijp JA. 2005. Staphylococcal innate immune evasion. Trends Microbiol 13:596–601. doi: 10.1016/j.tim.2005.10.002. [DOI] [PubMed] [Google Scholar]
- 7.DeLeo FR, Chambers HF. 2009. Reemergence of antibiotic-resistant Staphylococcus aureus in the genomics era. J Clin Invest 119:2464–2474. doi: 10.1172/JCI38226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu Y, Wang H, Du N, Shen E, Chen H, Niu J, Ye H, Chen M. 2009. Molecular evidence for spread of two major methicillin-resistant Staphylococcus aureus clones with a unique geographic distribution in Chinese hospitals. Antimicrob Agents Chemother 53:512–518. doi: 10.1128/AAC.00804-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Holden MT, Lindsay JA, Corton C, Quail MA, Cockfield JD, Pathak S, Batra R, Parkhill J, Bentley SD, Edgeworth JD. 2010. Genome sequence of a recently emerged, highly transmissible, multi-antibiotic- and antiseptic-resistant variant of methicillin-resistant Staphylococcus aureus, sequence type 239 (TW). J Bacteriol 192:888–892. doi: 10.1128/JB.01255-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li M, Du X, Villaruz AE, Diep BA, Wang D, Song Y, Tian Y, Hu J, Yu F, Lu Y, Otto M. 2012. MRSA epidemic linked to a quickly spreading colonization and virulence determinant. Nat Med 18:816–819. doi: 10.1038/nm.2692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kobayashi SD, Voyich JM, Buhl CL, Stahl RM, DeLeo FR. 2002. Global changes in gene expression by human polymorphonuclear leukocytes during receptor-mediated phagocytosis: cell fate is regulated at the level of gene expression. Proc Natl Acad Sci U S A 99:6901–6906. doi: 10.1073/pnas.092148299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li M, Cheung GY, Hu J, Wang D, Joo HS, DeLeo FR, Otto M. 2010. Comparative analysis of virulence and toxin expression of global community-associated methicillin-resistant Staphylococcus aureus strains. J Infect Dis 202:1866–1876. doi: 10.1086/657419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Malaviya R, Ikeda T, Ross E, Abraham SN. 1996. Mast cell modulation of neutrophil influx and bacterial clearance at sites of infection through TNF-alpha. Nature 381:77–80. doi: 10.1038/381077a0. [DOI] [PubMed] [Google Scholar]
- 14.Nakamura Y, Oscherwitz J, Cease KB, Chan SM, Munoz-Planillo R, Hasegawa M, Villaruz AE, Cheung GY, McGavin MJ, Travers JB, Otto M, Inohara N, Nunez G. 2013. Staphylococcus delta-toxin induces allergic skin disease by activating mast cells. Nature 503:397–401. doi: 10.1038/nature12655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Voyich JM, Otto M, Mathema B, Braughton KR, Whitney AR, Welty D, Long RD, Dorward DW, Gardner DJ, Lina G, Kreiswirth BN, DeLeo FR. 2006. Is Panton-Valentine leukocidin the major virulence determinant in community-associated methicillin-resistant Staphylococcus aureus disease? J Infect Dis 194:1761–1770. doi: 10.1086/509506. [DOI] [PubMed] [Google Scholar]
- 16.Rigby KM, DeLeo FR. 2012. Neutrophils in innate host defense against Staphylococcus aureus infections. Semin Immunopathol 34:237–259. doi: 10.1007/s00281-011-0295-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Otto M. 2014. Staphylococcus aureus toxins. Curr Opin Microbiol 17:32–37. doi: 10.1016/j.mib.2013.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Malaviya R, Ikeda T, Ross EA, Jakschik BA, Abraham SN. 1995. Bacteria—mast cell interactions in inflammatory disease. Am J Therap 2:787–792. [DOI] [PubMed] [Google Scholar]
- 19.Wertheim HF, Melles DC, Vos MC, van Leeuwen W, van Belkum A, Verbrugh HA, Nouwen JL. 2005. The role of nasal carriage in Staphylococcus aureus infections. Lancet Infect Dis 5:751–762. doi: 10.1016/S1473-3099(05)70295-4. [DOI] [PubMed] [Google Scholar]
- 20.von Eiff C, Becker K, Machka K, Stammer H, Peters G. 2001. Nasal carriage as a source of Staphylococcus aureus bacteremia. Study Group N Engl J Med 344:11–16. doi: 10.1056/NEJM200101043440102. [DOI] [PubMed] [Google Scholar]
- 21.Hilliard JJ, Datta V, Tkaczyk C, Hamilton M, Sadowska A, Jones-Nelson O, O'Day T, Weiss WJ, Szarka S, Nguyen V, Prokai L, Suzich J, Stover CK, Sellman BR. 2015. Anti-alpha toxin monoclonal antibody and antibiotic combination therapy improves disease outcome and accelerates healing in a Staphylococcus aureus dermonecrosis model. Antimicrob Agents Chemother 59:299–309. doi: 10.1128/AAC.03918-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pozzi C, Wilk K, Lee JC, Gening M, Nifantiev N, Pier GB. 2012. Opsonic and protective properties of antibodies raised to conjugate vaccines targeting six Staphylococcus aureus antigens. PLoS One 7:e46648. doi: 10.1371/journal.pone.0046648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wertheim HF, Walsh E, Choudhurry R, Melles DC, Boelens HA, Miajlovic H, Verbrugh HA, Foster T, van Belkum A. 2008. Key role for clumping factor B in Staphylococcus aureus nasal colonization of humans. PLoS Med 5:e17. doi: 10.1371/journal.pmed.0050017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Clarke SR, Brummell KJ, Horsburgh MJ, McDowell PW, Mohamad SA, Stapleton MR, Acevedo J, Read RC, Day NP, Peacock SJ, Mond JJ, Kokai-Kun JF, Foster SJ. 2006. Identification of in vivo-expressed antigens of Staphylococcus aureus and their use in vaccinations for protection against nasal carriage. J Infect Dis 193:1098–1108. doi: 10.1086/501471. [DOI] [PubMed] [Google Scholar]
- 25.Maurer M, von Stebut E. 2004. Macrophage inflammatory protein-1. Int J Biochem Cell Biol 36:1882–1886. doi: 10.1016/j.biocel.2003.10.019. [DOI] [PubMed] [Google Scholar]
- 26.Schlageter AM, Kozel TR. 1990. Opsonization of Cryptococcus neoformans by a family of isotype-switch variant antibodies specific for the capsular polysaccharide. Infect Immun 58:1914–1918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Otto M. 2010. Novel targeted immunotherapy approaches for staphylococcal infection. Expert Opin Biol Ther 10:1049–1059. doi: 10.1517/14712598.2010.495115. [DOI] [PMC free article] [PubMed] [Google Scholar]