Abstract
Human brucellosis is a protean disease with a diversity of clinical signs and symptoms resulting from infection with Brucella species. Recent reports suggest a cross-regulation between adrenal steroids (cortisol and dehydroepiandrosterone [DHEA]) and the immune system. Monocytes and macrophages are the main replication niche for Brucella. Therefore, we investigated the role of adrenal hormones on the modulation of the immune response mediated by macrophages in B. abortus infection. Cortisol treatment during B. abortus infection significantly inhibits cytokine, chemokine, and MMP-9 secretion. In contrast, DHEA treatment had no effect. However, DHEA treatment increases the expression of costimulatory molecules (CD40, CD86), the adhesion molecule CD54, and major histocompatibility complex class I (MHC-I) and MHC-II expression on the surface of B. abortus-infected monocytes. It is known that B. abortus infection inhibits MHC-I and MHC-II expression induced by gamma interferon (IFN-γ) treatment. DHEA reverses B. abortus downmodulation of the MHC-I and -II expression induced by IFN-γ. Taken together, our data indicate that DHEA immune intervention may positively affect monocyte activity during B. abortus infection.
INTRODUCTION
Several years of investigation have revealed a close relationship between the immune and endocrine systems. Substantial evidence supporting the bidirectional communication between the neuroendocrine and immune systems now exists (1). Immune cell subsets express receptors for many of the hormones secreted by the endocrine system, and likewise, receptors for cytokines and growth factors have been identified on cells of the neuroendocrine systems (2). Thus, the many lines of communication between the immune and the endocrine systems could have a profound impact on immune function, disease development, and susceptibility to infections (3).
Human brucellosis is characterized by a great diversity of clinical manifestations resulting from infection with Brucella species (4). It is chiefly an inflammatory disease. Inflammation is present both in the acute and chronic phases of the disease and in practically all of the organs affected. Clinical signs of such inflammation are undulant fever, endocarditis, arthritis, osteomyelitis, meningitis, pleocytosis, cellular infiltration of the joints, orchitis, nephritis, hepatic granuloma, etc. (5).
Brucella abortus infection elicits a vigorous Th1 immune response, which also activates cytotoxic T lymphocytes (6–9). Despite the recognition that Th1 immunity is crucial for the control of Brucella infection, several aspects of the immune response, i.e., how the innate immune response shapes the adaptive Th1 immune response and why Th1 immunity is not sufficient to completely eradicate the infection, constitute a conundrum. Macrophages/monocytes were recognized to play an important role in resistance to facultative intracellular bacteria such as Brucella, and activation of macrophages by specifically committed T lymphocytes formed the basis for cell-mediated immunity. However, B. abortus is endowed with a panoply of survival stratagems to evade immune responses and survive inside macrophages, creating a safe niche of replication inside the host (10–13).
Several cytokines that are produced during B. abortus infection, most of them secreted by macrophages (such as interleukin-1 [IL-1], IL-2, IL-6, IL-8, tumor necrosis factor alpha [TNF-α], and gamma interferon [IFN-γ]), are known to affect the release of anterior pituitary hormones by acting on the hypothalamus and/or the pituitary gland, resulting in an increase of glucocorticoid secretion (14). Glucocorticoids suppress the secretion of IL-2 (15, 16), which inhibits proliferation of activated Th1 cells. This phenomenon could also contribute to the persistence and chronicity of Brucella infection. Recent reports suggest a cross-regulation between adrenal steroids (glucocorticoids and dehydroepiandrosterone [DHEA]) and the modulation of the immune response (17). The effects of DHEA are often opposed by the other important adrenal steroid, cortisol (18). The cortisol/DHEA ratio is abnormal under various pathological conditions such as AIDS, rheumatoid arthritis, and tuberculosis (18–20). In previous studies, significantly higher levels of cortisol in serum were reported in patients with acute brucellosis than in brucellosis patients at the end of antibiotic treatment or than in healthy subjects (21). In addition, adrenal glands are enlarged in patients with acute brucellosis but return to normal size after therapy (21). Of note, Th1 responses are preponderant during acute human brucellosis, but they decline in the chronic phase (12). Thus, it is reasonable to hypothesize that the neuroendocrine system could modulate immune responses during Brucella infection, particularly influencing the macrophage response to infection and explaining, at least in part, the progression of the infection to chronicity.
In this study, we sought to investigate the relevance of the adrenal axis in the modulation of monocyte function in the context of B. abortus infection, a disease in which the induction of an effective cellular response would be an effective immune response to eradicate the infection.
MATERIALS AND METHODS
DHEA and cortisol assessment in patients.
Human blood was collected either from healthy male adult individuals (n = 23) or from patients with active acute brucellosis (n = 23) from Hospital F.J. Muñiz, Buenos Aires, Argentina. Patients and control subjects were age and sex matched. Their ages ranged between 28 and 60 years. The means ± standard errors of age were 48.30 ± 10.17 years for control individuals and 46.35 ± 8.95 years for Brucella-infected patients. Written informed consent was obtained in accordance with the local Ethics Committee.
Brucellosis was diagnosed on the basis of clinical, serologic, bacteriologic, and epidemiologic data. Disease activity was defined by the presence of typical signs and symptoms (e.g., fever, splenomegaly, lymphadenopathy, myalgia, arthralgia, and hepatic involvement). All patients showed positive results in classic serologic tests, including standard tube agglutination, 2-mercaptoethanol, Rose bengal, complement fixation, and Huddleson tests. All patients were classified as having acute disease (duration of illness, 28 to 300 days; median, 60 days). We used the criteria for discrimination between acute and chronic disease duration as defined by Young (22).
To assess cortisol and DHEA-S levels in plasma, EDTA-anticoagulated blood samples were drawn. After centrifugation, plasma aliquots were preserved at −80°C. Cortisol and DHEA-S were measured by electrochemiluminescence tests (automatic analyzer ADVIA CentaurH XP Immunoassay System; Siemens Healthcare Diagnostics, Deerfield, IL, USA). All hormone determinations were performed in the same blood samples, collected between 8:00 and 8:30 a.m. because of the circadian rhythms.
Bacterial culture.
B. abortus S2308 was grown overnight in 10 ml of tryptic soy broth (Merck, Buenos Aires, Argentina) with constant agitation at 37°C. Bacteria were harvested and the inocula were prepared as described previously (23). All live Brucella manipulations were performed in biosafety level 3 facilities located at the Instituto de Investigaciones Biomédicas en Retrovirus y SIDA (Buenos Aires, Argentina).
Cells and media.
Unless otherwise specified, all experiments were performed at 37°C in a 5% CO2 atmosphere using standard medium composed of RPMI 1640 supplemented with 2 mM l-glutamine, 10% heat-inactivated fetal bovine serum (FBS) (Gibco-BRL Life Technologies, Grand Island, NY), 100 U of penicillin per ml, and 100 μg of streptomycin per ml. THP-1 cells were obtained from the American Type Culture Collection (Manassas, VA) and were cultured as previously described (24). To induce maturation, cells were cultured in the presence of 0.05 μM 1,25-dihydroxyvitamin D3 (vitamin D3; Calbiochem-Nova Biochem International, La Jolla, CA) for 48 to 72 h.
Human neutrophils were isolated from venous blood from healthy human volunteers by a Ficoll-Paque (GE Healthcare, Uppsala, Sweden) gradient, followed by the sedimentation of erythrocytes in 6% dextran and hypotonic lysis (25). Neutrophils were harvested, washed twice with sterile phosphate-buffered saline (PBS), and resuspended at a concentration of 1 × 106 cells/ml in RPMI 1640 medium supplemented with 5% FBS, 1 mM glutamine, 100 U/ml penicillin, and 100 μg/ml streptomycin. Cell viability was 98%, as determined by trypan blue exclusion. The purity of the final neutrophil preparation was 95%, as assessed by morphological examination after Giemsa staining and flow cytometry light scatter patterns as described previously (26).
Cellular infection.
Vitamin D3-treated THP-1 cells, at a concentration of 1 × 106 cells/ml, were incubated in round-bottom polypropylene tubes (Sarstedt, Numbrecht, Germany), infected at different multiplicities of infection (MOI) in the presence or absence of DHEA (1 × 10−8 M) and cortisol (1 × 10−6 M), and incubated for 1 h at 37°C in a 5% CO2 atmosphere. Cells were extensively washed with RPMI to remove extracellular bacteria and were incubated in medium supplemented with 100 μg/ml of gentamicin and 50 μg/ml of streptomycin to kill extracellular bacteria in the presence or absence of DHEA and cortisol at the indicated concentrations. THP-1 cells and culture supernatants were harvested at 24 or 48 h to determine cytokine production, matrix metalloproteinases (MMP) secretion, and major histocompatibility complex class I (MHC-I), MHC-II, CD54, CD40, and CD86 surface expression. As was required, experiments were performed in the presence of IFN-γ (150 U/ml; Endogen). Culture of THP-1 with cortisol and DHEA alone resulted in no changes in the above-mentioned molecules.
To monitor Brucella intracellular survival, cells were lysed with a sterile solution of 0.1% (vol/vol) Triton X-100 in H2O, and serial dilutions of lysates were rapidly plated on tryptic soy agar plates to enumerate CFU.
Migration assay.
Cell migration was evaluated by using 96-well microchemotaxis plates with 5-μm-pore-diameter polycarbonate filters (Corning, Corning, NY). Neutrophils (106 cells/ml) were placed in the upper well of the chambers, and the indicated stimuli (culture supernatants from B. abortus-infected THP-1 cells) were placed in the lower wells. Migration was scored by counting the number of neutrophils that had reached the bottom well after 2 h. Migration toward N-formyl-l-methionyl-l-leucyl-l-phenylalanine (fMLP, 10−7 M; Sigma-Aldrich) served as a positive control. The number of migrating cells was expressed as follows: chemoattractant index = number of migrating cells to the tested medium/number of migrating cells to fresh culture medium.
Flow cytometry.
At the end of culture, cells were washed and incubated with fluorescein isothiocyanate-labeled (FITC) anti-human HLA-DR monoclonal antibody (MAb) (clone L243; BD Pharmingen, San Diego, CA), FITC-labeled anti-human HLA-ABC (clone G46-2.6; BD Pharmingen), phycoerythrin (PE)-labeled anti-human CD40 (BD Pharmingen clone 5C3), PE-labeled anti-human CD86 [BD Pharmingen clone 2331(FUN-1)], PE-labeled anti-human CD54 (BD Pharmingen clone HA58), or isotype-matched control antibody (Ab) for 30 min on ice. The cells were then washed and analyzed with a FACScan flow cytometer (Becton-Dickinson, Franklin Lakes, NJ), using CellQuest software (Becton-Dickinson). Results were expressed as mean fluorescence intensities (arithmetic means ± standard errors of the means).
Zymography.
Gelatinase activity was assayed by the method of Hibbs et al. (27) with modifications, as described previously (23).
Gelatinase activity under native conditions.
Gelatinase activity in unprocessed culture supernatants (native conditions) was measured using a gelatinase/collagenase fluorometric assay kit (EnzChek; Invitrogen, Carlsbad, CA) according to the manufacturer's instructions. The EnzChek kit contains DQ gelatin, a fluorescein-conjugated gelatin so heavily labeled with fluorescein that fluorescence is quenched. When this substrate is digested by gelatinases or collagenases, it yields highly fluorescent peptides, and the fluorescence increase is proportional to proteolytic activity. Collagenase purified from Clostridium histolyticum provided in the assay kit serves as a control enzyme. Plates were read in a fluorescence plate reader (Victor3; Perkin-Elmer, Waltham, MA).
Measurement of cytokine concentrations.
Secretion of transforming growth factor-β1 (TGF-β1), TNF-α, IL-1β, IL-6, IL-8, and monocyte chemotactic protein 1 (MCP-1) in the supernatants was quantified by enzyme-linked immunosorbent assay (ELISA; BD Biosciences, San Jose, CA).
Stimulation with recombinant cytokines.
As indicated, infections were performed in the presence of human recombinant IL-6, IL-1β, and TNF-α (R&D Systems) at 50 ng/ml.
Neutralization of TGF-β1.
Neutralization experiments were performed with an antibody against human TGF-β1 (IgG, rabbit polyclonal; Abcam Inc., Cambridge, MA) or its isotype control (rabbit IgG). To perform neutralizing experiments, anti-TGF-β1 antibody was included during the infection at a final concentration of 20 mg/ml.
mRNA preparation and quantitative PCR.
RNA was extracted using the Quick-RNA MiniPrep kit (Zymo Research), and 1 μg of RNA was subjected to reverse transcription using Improm-II reverse transcriptase (Promega). PCR analysis was performed with the Mx3000P real-time PCR detection system (Stratagene) using SYBR green as fluorescent DNA binding dye. The primer sets used for amplification were as follows: GAPDH (glyceraldehyde-3-phosphate dehydrogenase) sense, 5′-CGACCACTTTGTCAAGCTCA-3′; GAPDH antisense, 5′-TTACTCCTTGGAGGCCATGT-3′; CIITA (class II transactivator) sense, 5′-CCGACACAGACACCATCAAC-3′; CIITA antisense, 5′-TTTTCTGCCCAACTTCTGCT-3′. The amplification cycles for GADPH and CIITA were 95°C for 30 s, 55°C for 60 s, and 72°C for 60 s. All primer sets yielded a single product of the correct size. Relative expression levels were normalized against GADPH.
Statistical analysis.
Statistical analysis was performed with one-way analysis of variance, followed by a post hoc Tukey test, or using the nonparametric Wilcoxon matched-pair test for paired samples using GraphPad Prism 5.0 software. The data are represented as means ± standard errors of the means (SEM).
RESULTS
DHEA and cortisol in brucellosis patients.
Cortisol and DHEA have opposite effects on immune responses (28), and they are imbalanced in certain infectious diseases and pathologies (19, 29, 30). Thus, we decided to investigate cortisol and DHEA levels in patients with acute brucellosis. Cortisol and DHEA concentrations in plasma showed no significant differences between patients with acute brucellosis and control subjects (Fig. 1A and B). An increased cortisol-to-DHEA ratio is observed in infectious diseases (19, 29, 30). Further analyses of the cortisol/DHEA ratio revealed that B. abortus-infected patients had a much higher ratio than healthy controls (Fig. 1C). These results indicate that the hypothalamic-pituitary-adrenal (HPA) axis is activated during Brucella infection.
FIG 1.
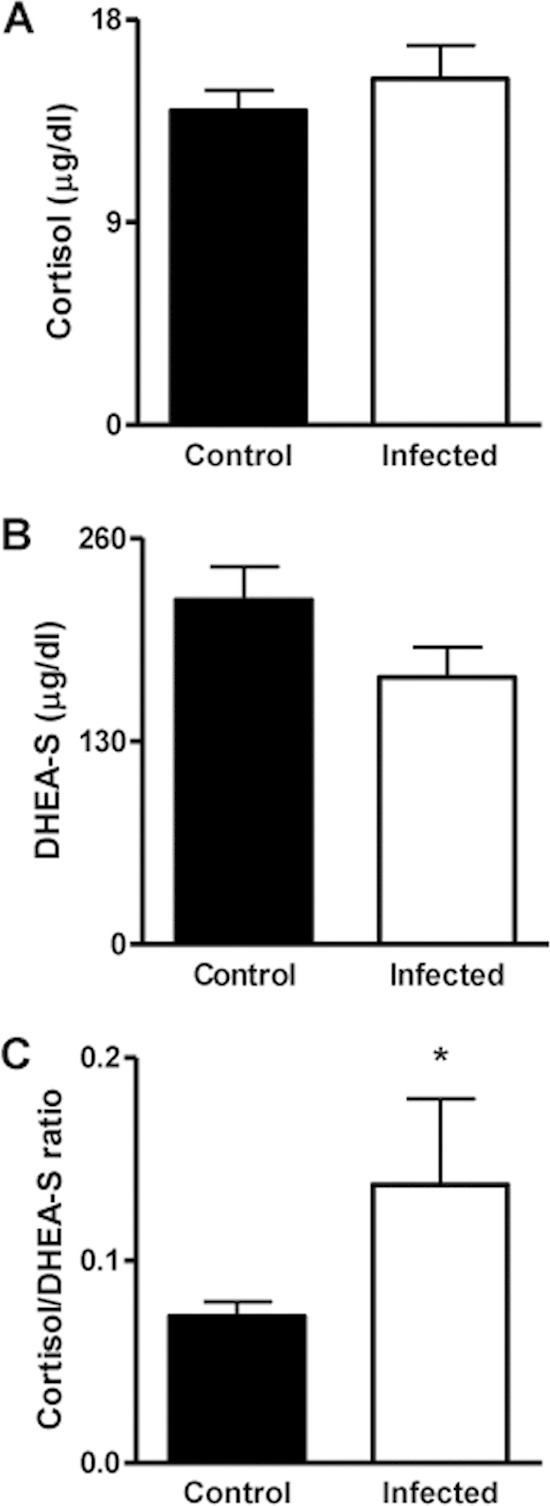
HPA axis-related hormones. Cortisol (A) and DHEA-S (B) levels in plasma from Brucella-infected patients and noninfected patients (control) and the corresponding cortisol/DHEA-S ratio (C). Bars indicate the means ± standard errors of the means (SEM) for each group. *, P < 0.1.
Cortisol inhibits proinflammatory cytokine and chemokine production induced by B. abortus infection.
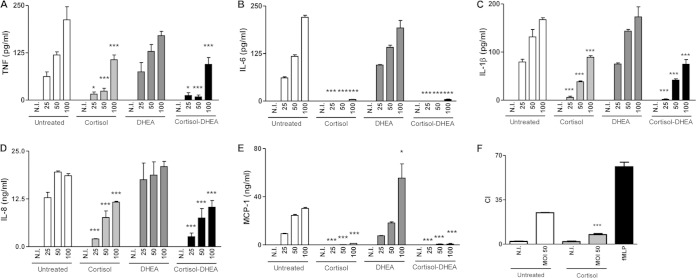
Adrenal hormones participate in the modulation of the function of several immune cells (29). Monocytes/macrophages are the main replication niche for Brucella and the powerhouse of cytokine production (31, 32). Thus, we hypothesized that their function would be influenced by cortisol and DHEA during infection with B. abortus. When THP-1 monocytes were infected with B. abortus in the presence of cortisol, these cells secreted significantly lower quantities of proinflammatory cytokines (TNF-α, IL-1β, and IL-6) and chemokines (IL-8 and MCP-1) than did untreated infected cells. In contrast, treatment with DHEA had no effect on proinflammatory cytokine and chemokine secretion in response to B. abortus infection. In addition, when the two hormones were administered together, DHEA did not reverse the inhibitory effect induced by cortisol treatment on the production of immune mediators (Fig. 2). Although cortisol treatment did not abrogate the production of IL-8, this reduction was sufficient to inhibit the migration of neutrophils (Fig. 2F). These results indicate that cortisol reduces proinflammatory cytokine and chemokine secretion induced by B. abortus infection in monocytes and DHEA cannot reverse this effect.
FIG 2.
Cortisol inhibits proinflammatory cytokine and chemokine production induced by B. abortus infection. (A to E) Cytokine and chemokine production by THP-1 monocytes infected or not with B. abortus at different MOI treated or not with cortisol (1 × 10−6 M), DHEA (1 × 10−8 M), or cortisol plus DHEA (1 × 10−6 M and 1 × 10−8 M, respectively). Levels of TNF-α (A), IL-6 (B), IL-1β (C), IL-8 (D), and MCP-1 (E) were measured at 24 h postinfection (p.i.). (F) Migration of human neutrophils induced by culture supernatants from Brucella-infected or noninfected (N.I.) THP-1 monocytes treated or not with cortisol (1 × 10−6 M), as measured in a microchemotaxis plate. Supernatants were added pure. Migrated cells were counted at 2 h, and results are expressed as chemoattractant index (CI) (the number of cells that migrated to conditioned medium divided by the number of cells that migrated to fresh culture medium). Migration toward N-formyl-l-methionyl-l-leucyl-phenylalanine (fMLP) served as a positive control. Data are given as means ± SEM from at least 4 individual experiments. Significance of results for treated versus untreated cells: *, P < 0.1; ***, P < 0.001.
Adrenal steroids modulate B. abortus intracellular replication in THP-1 cells.
Since cortisol treatment had an effect on cytokine and chemokine production, experiments were conducted to investigate whether cortisol and DHEA treatment could modify the capacity of B. abortus to replicate inside THP-1 monocytes. B. abortus replicated in THP-1 monocytes in the presence of cortisol and DHEA at 24 h postinfection. However, cortisol significantly (P < 0.01) increased the capacity of B. abortus to replicate in monocytes with respect to untreated cells. In contrast, DHEA did not significantly modify the intracellular B. abortus replication with respect to untreated cells. When infection experiments were performed in the presence of both DHEA and cortisol, there were no differences in intracellular bacterial survival with respect to untreated cells (Fig. 3A). To determine whether cortisol treatment affects intracellular replication or increases the bacterial uptake, we determined the number of intracellular bacteria between 2 h and 48 h postinfection. Our results indicated that at early time points there were no differences in the number of intracellular bacteria between cortisol-treated and untreated cells, indicating that cortisol treatment does not interfere in bacterial uptake. Yet, differences arise after 24 h, indicating that cortisol does interfere with bacterial replication (Fig. 3B).
FIG 3.
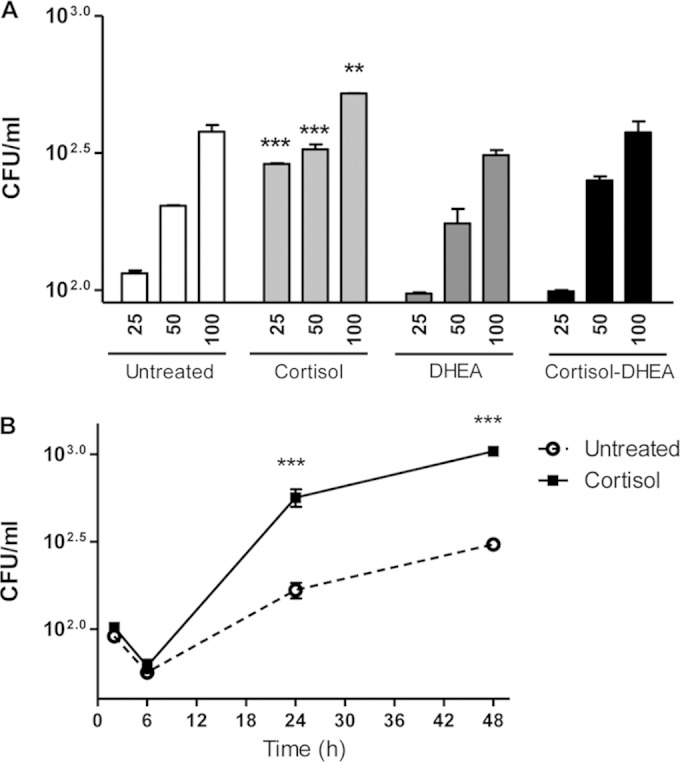
Infection and replication of B. abortus within THP-1 monocytes. After infection at different MOI in the presence or not of cortisol (1 × 10−6 M), DHEA (1 × 10−8 M), or cortisol plus DHEA (1 × 10−6 M and 1 × 10−8 M, respectively), cells were incubated with antibiotics to kill extracellular bacteria. Cell lysates obtained at 24 h p.i. were plated onto agar to determine intracellular CFU (A). At 2, 6, 24, and 48 h postinfection, THP-1 cells treated or not with cortisol were plated on agar to determine intracellular CFU (B). Data are given as means ± SEM from at least 4 individual experiments. Significance of results for treated versus untreated cells: **, P < 0.01; ***, P < 0.001.
As cortisol and DHEA can be present in any organism before Brucella infects the monocytes, experiments were conducted to determine whether cortisol and DHEA pretreatment could alter intracellular replication. To this end, THP-1 monocytes were pretreated with cortisol, DHEA, or both DHEA and cortisol during 24 h and then were infected with B. abortus. Our results indicated that hormone pretreatment does not alter intracellular replication compared with that in untreated cells (not shown).
Adrenal steroids modulate gelatinase secretion induced by B. abortus infection.
We previously demonstrated that THP-1 cells secrete MMP-9 in response to B. abortus infection (23, 33). Therefore, we investigated the effects of cortisol and DHEA on MMP-9 secretion upon infection with B. abortus. Cortisol inhibited MMP-9 secretion induced by B. abortus infection, as measured by zymography. In contrast, DHEA increased MMP-9 secretion. However, when cells were infected in the presence of both DHEA and cortisol, MMP-9 activity was similar to that produced by B. abortus-infected cells. These results indicate that cortisol inhibited B. abortus-induced MMP-9 secretion and DHEA was able to reverse the inhibitory effect induced by cortisol (Fig. 4A).
FIG 4.
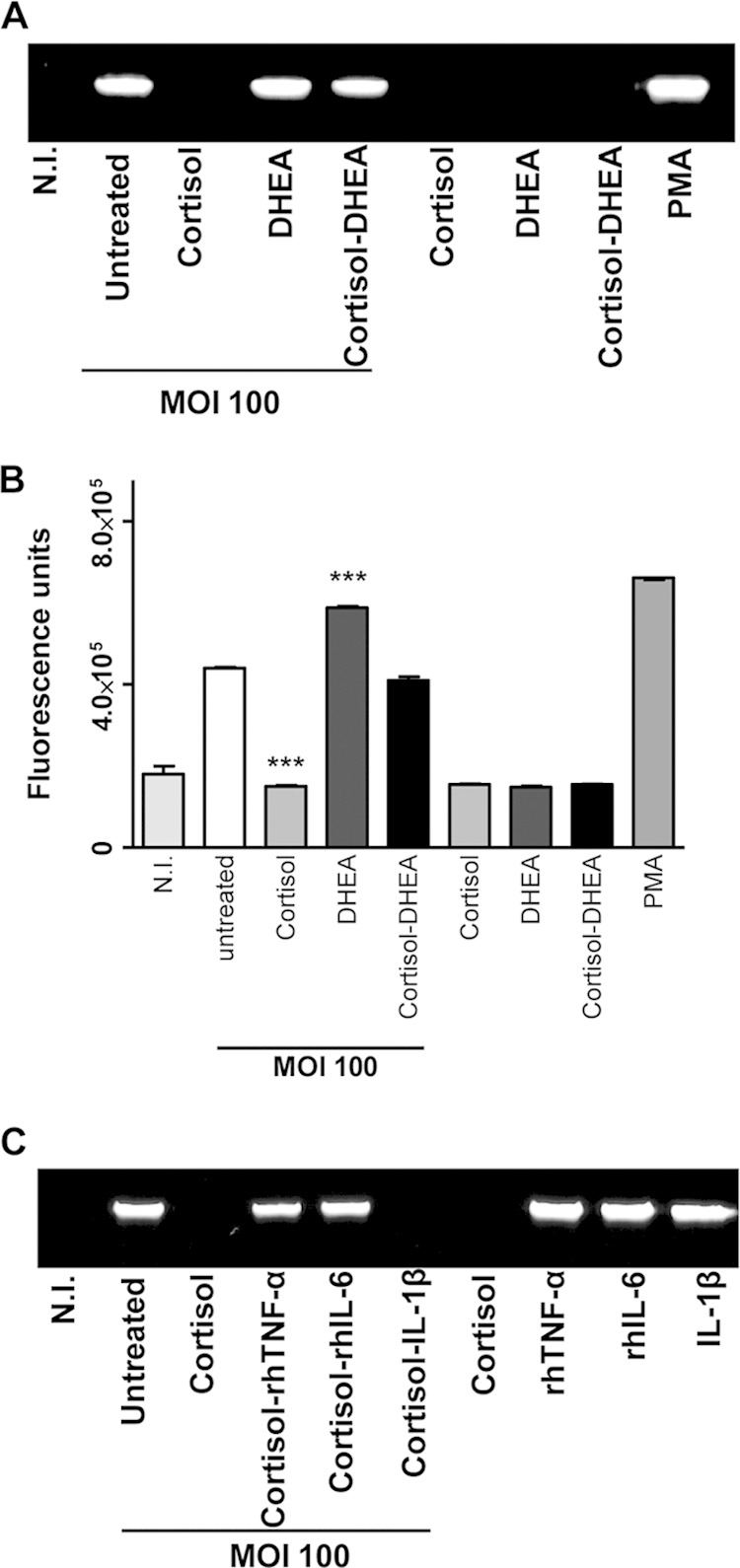
Adrenal steroids modulate MMP-9 secretion induced by B. abortus infection. MMP-9 production by THP-1 monocytes infected with B. abortus at an MOI of 100 in the presence or not of cortisol (1 × 10−6 M), DHEA (1 × 10−8 M), or cortisol plus DHEA (1 × 10−6 M and 1 × 10−8 M, respectively). (A) MMP-9 production at 24 h p.i. by B. abortus-infected THP-1 monocytes was determined in supernatants by zymography. PMA, phorbol myristate acetate. (B) Supernatants were incubated with a fluorescein-conjugated gelatin substrate (DQ; Invitrogen) that produces highly fluorescent peptides when gelatin is digested. Data are expressed in fluorescence units indicated by a fluorometer. (C) MMP-9 production in culture supernatants from B. abortus-infected THP-1 monocytes (MOI, 100) treated with cortisol (1 × 10−6 M) in the presence or not of recombinant human (rh) TNF-α, IL-6, and IL-1β. Data are given as means ± SEM from at least 4 individual experiments. Significance of results for treated versus untreated cells: ***, P < 0.001.
MMPs are counterbalanced by the activity of tissue inhibitors, including TIMPs (tissue inhibitors of metalloproteinases) (33). Therefore, the net gelatinase or collagenase activity in a complex sample, such as culture supernatants, depends on the balance between MMP and TIMP activities. This net activity is not revealed by zymography, since MMP-TIMP complexes may dissociate during gel electrophoresis. To assess whether B. abortus infection in the presence of DHEA and cortisol modified net gelatinase activity, culture supernatants from THP-1 monocytes infected with B. abortus in the presence or not of DHEA and cortisol were incubated with a nonfluorescent gelatin fluorescein conjugate, and the fluorescence unmasked as a consequence of gelatin degradation was measured in a fluorometer. Similar results to those determined by zymography were obtained when MMP-9 activity was measured by fluorometry (Fig. 4B), indicating that cortisol and DHEA can modulate gelatinase activity instead of modulating the secretion of TIMPs.
Cortisol inhibition of MMP-9 secretion induced by B. abortus infection is reversed by exogenous TNF-α and IL-6.
Proinflammatory cytokines have been previously involved in MMP production in different cell types (34–39). To determine whether TNF-α, IL-6, and IL-1β were involved in the downmodulation of MMP-9 secretion induced by cortisol in B. abortus-infected monocytes, recombinant cytokines were added exogenously at the time of treatment. Recombinant human TNF-α and IL-6 reversed the inhibitory effect on MMP-9 secretion induced by cortisol in B. abortus-infected monocytes. In contrast, IL-1β could not reverse the secretion of MMP-9 downmodulated by cortisol (Fig. 4C). This indicates that cortisol downmodulates MMP-9 secretion indirectly, by dampening TNF-α and IL-6 production.
DHEA increases MHC-I and MHC-II expression in B. abortus-infected monocytes.
As already reported (7), B. abortus infection did not induce changes in expression of MHC-I and MHC-II molecules in monocytes/macrophages (Fig. 5). However, cortisol and DHEA could modulate this expression in the context of bacterial infections (29, 40, 41). The presence of DHEA during B. abortus infection induced the expression of MHC-I and MHC-II molecules on THP-1 cells. Cortisol treatment had no effect on the expression of these molecules when it was added during B. abortus infection. When infection experiments were performed in the presence of both cortisol and DHEA simultaneously, we observed a significant increase of MHC-I and MHC-II expression over that seen in B. abortus-infected untreated cells or cells treated with cortisol alone (Fig. 5).
FIG 5.
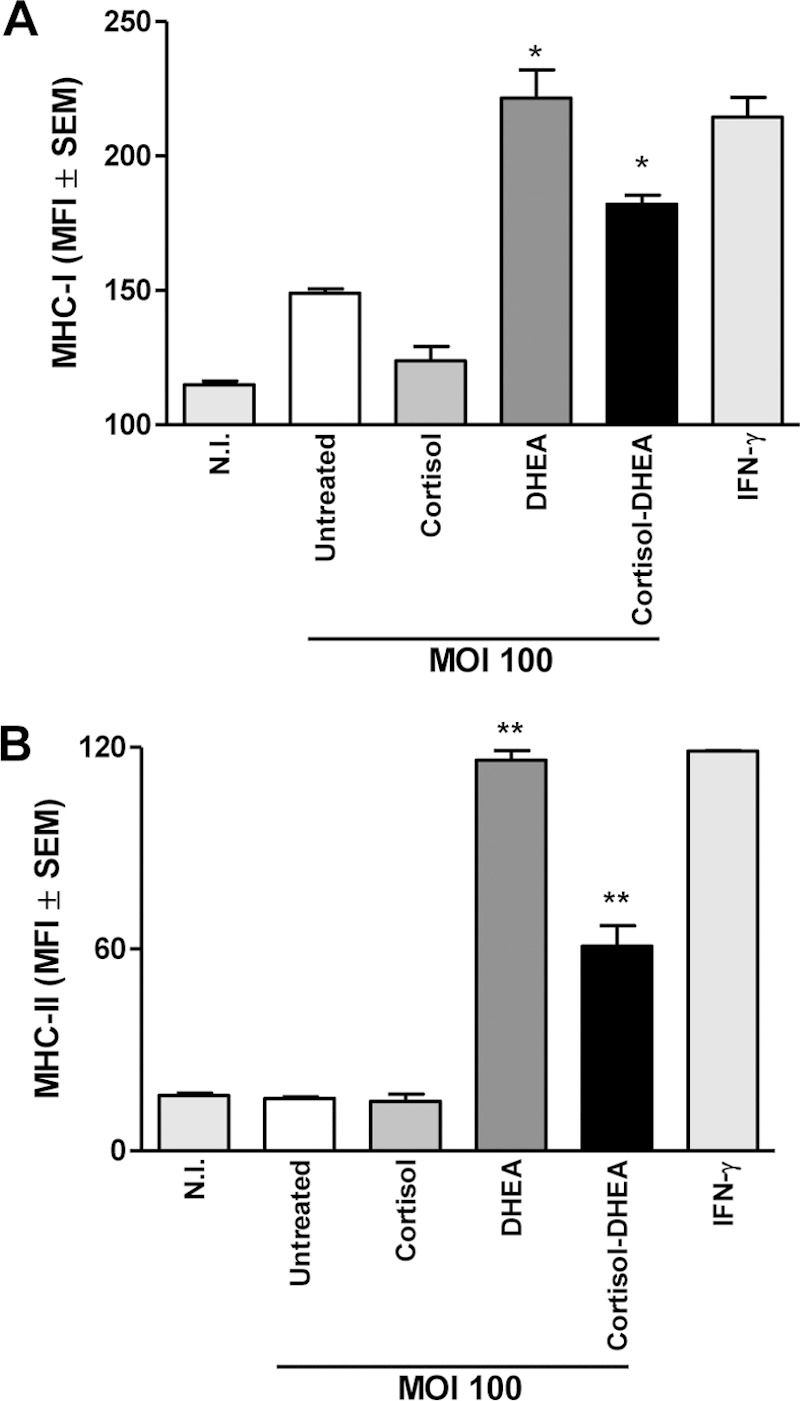
DHEA increases MHC-I and MHC-II expression in B. abortus-infected monocytes. MHC-I (A) and MHC-II (B) expression by B. abortus-infected THP-1 monocytes (MOI, 100) in the presence or not of cortisol (1 × 10−6 M), DHEA (1 × 10−8 M), or cortisol plus DHEA (1 × 10−6 M and 1 × 10−8 M, respectively). Data are given as means ± SEM from at least 4 individual experiments. Significance of results for treated versus untreated cells: *, P < 0.1; **, P < 0.01.
DHEA reverses the effect of cortisol on CD40, CD86, and CD54 expression in B. abortus-infected monocytes.
Costimulatory molecules (CD40 and CD86) and the key molecule involved in cellular recruitment during immune responses, the intercellular adhesion molecule-1 (CD54), are increased in B. abortus-infected monocytes (42). Cortisol treatment significantly inhibited the expression of B. abortus-induced CD40, CD86, and CD54. When infection experiments were performed in the presence of DHEA, the levels of CD40, CD86, and CD54 were similar to those observed in untreated B. abortus-infected cells. Interestingly, when infection experiments were performed in the presence of cortisol and DHEA simultaneously, we observed that CD40, CD86, and CD54 levels were similar to those induced by untreated B. abortus-infected cells (Fig. 6). This indicated that in the case of costimulatory and CD54 molecules DHEA could reverse the effect of cortisol.
FIG 6.
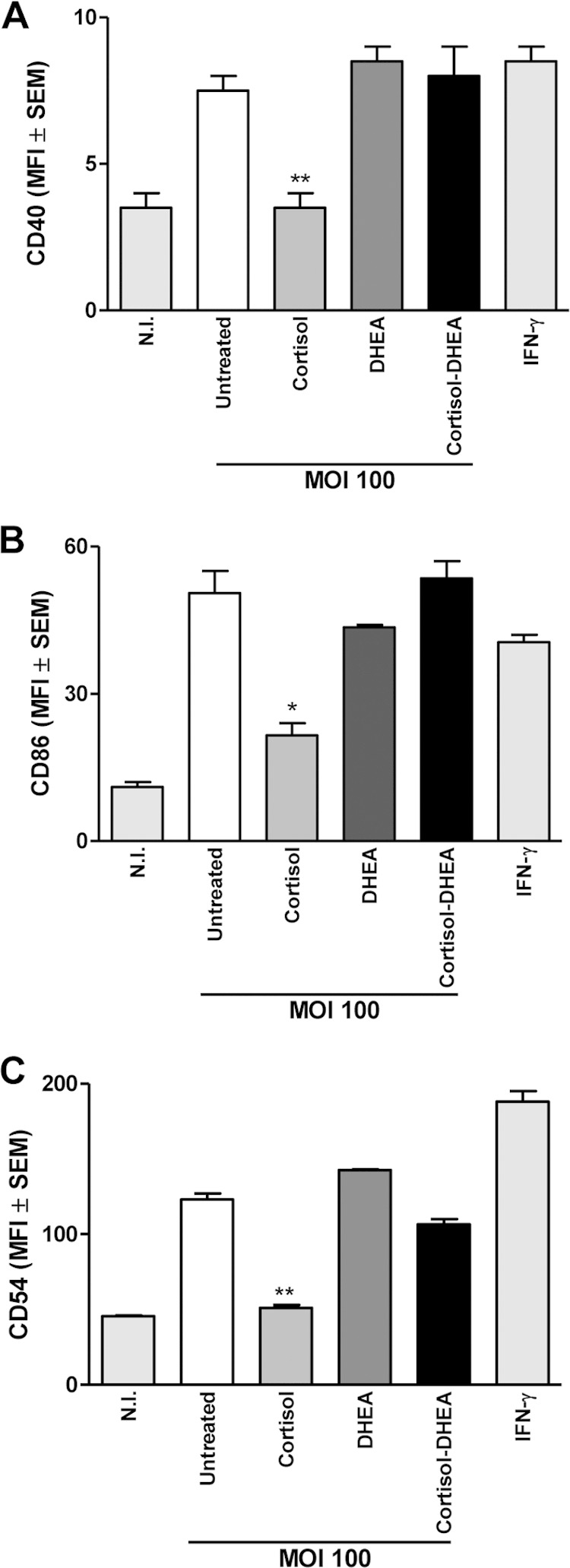
DHEA induces CD40, CD86, and CD54 expression on B. abortus-infected monocytes. CD40 (A), CD86 (B), and CD54 (C) expression by B. abortus-infected THP-1 monocytes (MOI, 100) in the presence or not of cortisol (1 × 10−6 M), DHEA (1 × 10−8 M), or cortisol plus DHEA (1 × 10−6 M and 1 × 10−8 M, respectively). Data are given as means ± SEM from at least 4 individual experiments. Significance of results for treated versus untreated cells: *, P < 0.1; **, P < 0.01.
DHEA reverses B. abortus downmodulation of MHC-I and MHC-II expression induced by IFN-γ.
IFN-γ has a critical role in protective immunity against Brucella (6, 7). The strategies that allow B. abortus to survive for prolonged periods inside macrophages could be explained at least in part by the inhibition of the IFN-γ-induced expression of MHC-I and MHC-II molecules (43, 44). We hypothesized that this phenomenon could be modulated by cortisol and DHEA. Our results indicated that treatment with DHEA resulted in significant recovery of the inhibited IFN-γ-induced MHC-I and MHC-II expression mediated by B. abortus infection. Cortisol had no effect on the downmodulation of IFN-γ-induced MHC-I and MHC-II expression induced by B. abortus. When the two hormones were administered together, the effect was similar to that of DHEA alone (Fig. 7). Therefore, DHEA could reverse the inhibition mediated by B. abortus of IFN-γ-induced MHC-I and -II expression.
FIG 7.
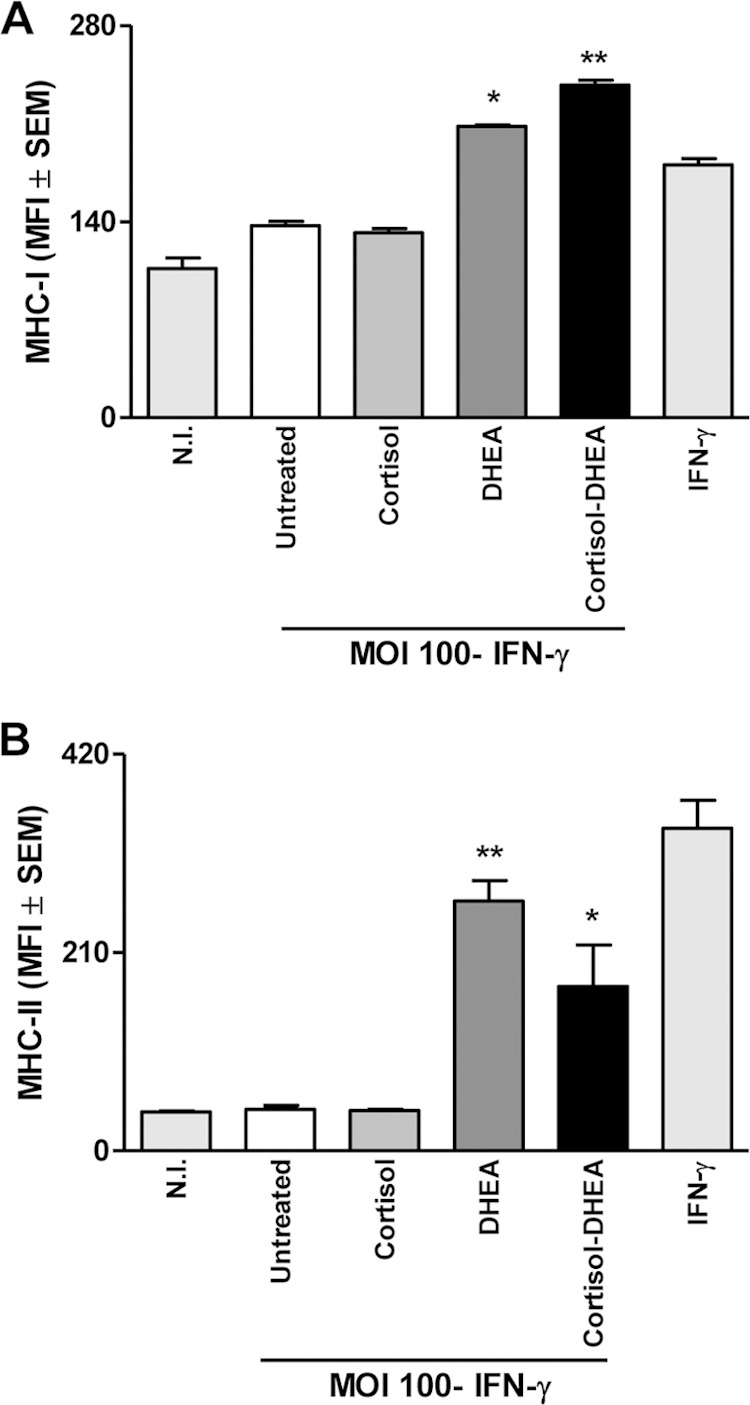
DHEA reverses B. abortus downmodulation of MHC-I and II expression induced by IFN-γ. B. abortus downmodulates the IFN-γ induced expression of MHC-I and MHC-II molecules. THP-1 cells were infected with B. abortus at an MOI of 100 in the presence of IFN-γ (150 U/ml) and in the presence or not of cortisol (1 × 10−6 M), DHEA (1 × 10−8 M), or cortisol plus DHEA (1 × 10−6 M and 1 × 10−8 M, respectively) for 48 h. MHC-I (A) and MHC-II (B) expression was assessed by flow cytometry. MFI, mean fluorescence intensity. Nonspecific binding was determined using a control isotype antibody. Data are given as means ± SEM from at least 4 individual experiments. Significance of results for treated versus untreated cells: *, P < 0.1; **, P < 0.01.
DHEA induces CIITA expression.
Transcription of MHC-II genes depends on a specific non-DNA-binding protein, the class II transactivator (CIITA) (45). Therefore, experiments were conducted to determine whether the CIITA expression is modulated by DHEA and cortisol during B. abortus infection in the presence or not of IFN-γ. Our results indicated that CIITA expression increased when B. abortus infection was performed in the presence of DHEA and independent of the presence of IFN-γ (Fig. 8A and B, respectively). When the two hormones were administered together, the effect was similar to that of DHEA alone. Therefore, CIITA expression can be modulated by DHEA during B. abortus infection. These results indicate that MHC-II expression can be modulated by DHEA via regulation of CIITA expression.
FIG 8.
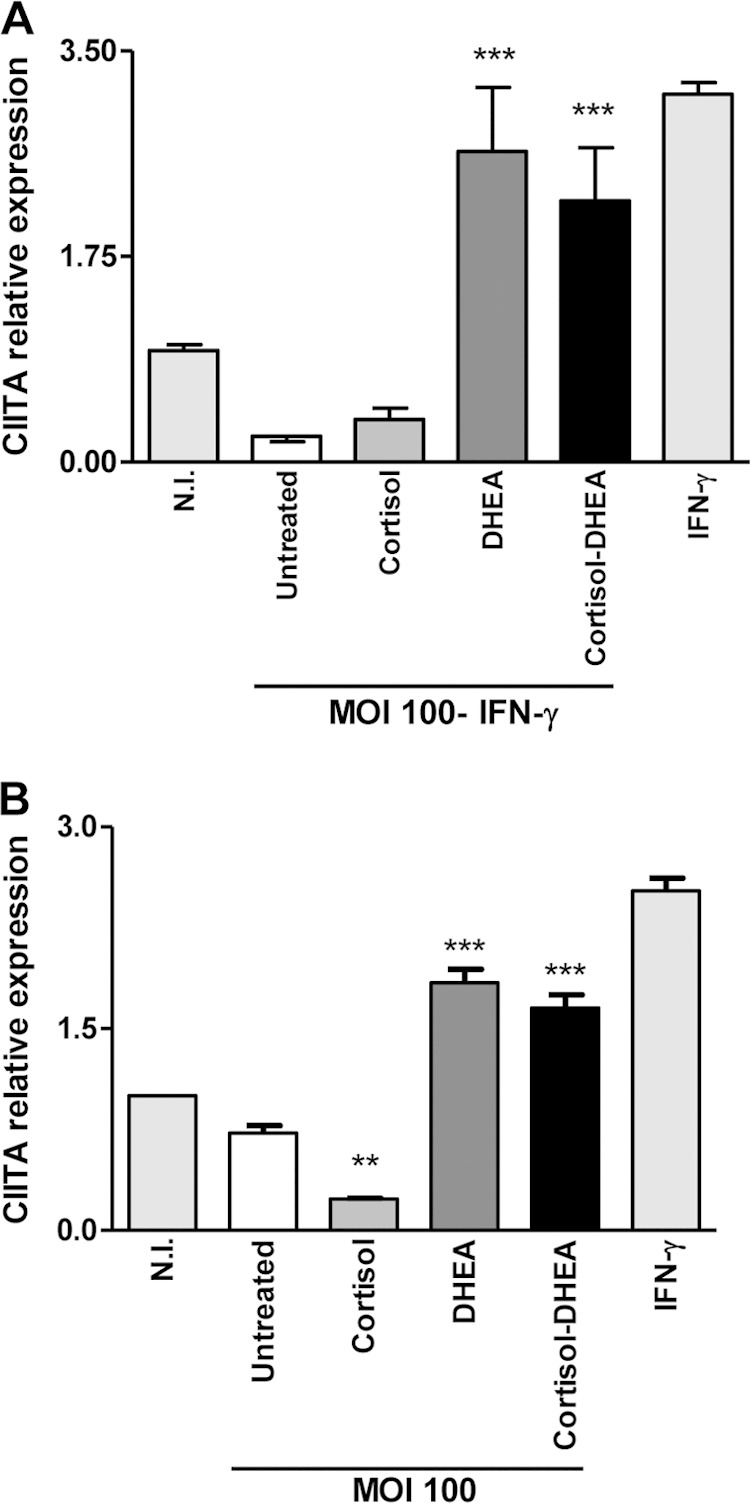
DHEA induces CIITA expression. CIITA expression was determined by quantitative real-time PCR (qRT-PCR) in THP-1 cells infected with B. abortus at an MOI of 100 in the presence or not of cortisol (1 × 10−6 M), DHEA (1 × 10−8 M), or cortisol plus DHEA (1 × 10−6 M and 1 × 10−8 M, respectively) in the presence of IFN-γ (150 U/ml) for 48 h (A) and without IFN-γ for 24 h (B). Data are given as means ± SEM from at least 4 individual experiments. Significance of results for treated versus untreated cells: **, P < 0.01; ***, P < 0.001.
TGF-β1 contributes to the inhibition of MHC-II expression induced by IFN-γ during B. abortus infection in the presence of cortisol.
Previously, we demonstrated that IL-6 is involved in the downmodulation of IFN-γ-induced MHC-II in B. abortus infection (43). The presence of cortisol during B. abortus infection significantly inhibits IL-6 secretion (Fig. 2B). However, B. abortus infection was able to induce MHC-II downmodulation even in the presence of cortisol (Fig. 7B). This result indicates that another mediator is involved in such a phenomenon.
It has been demonstrated in a variety of cell types that TGF-β1 downmodulated MHC-II expression (46, 47). Also, in chronic Brucella infection patients, it has been demonstrated that circulating TGF-β1 levels were markedly elevated compared with those from Brucella-negative healthy subjects; and this upregulation correlated with diminished lymphoproliferative responses (48).
In infection experiments performed in the presence of cortisol, we found a significant increase in TGF-β1 secretion (Fig. 9A). In these assays, THP-1 monocytes were infected with B. abortus in the presence or absence of neutralizing antibodies to TGF-β1 or the isotype control. Neutralization of TGF-β1 resulted in significant (P < 0.001) recovery of the inhibition of IFN-γ-induced MHC-II expression mediated by B. abortus in the presence of cortisol (Fig. 9B). This result indicates that TGF-β1 is involved in the inhibition of MHC-II expression induced by IFN-γ during B. abortus infection in the presence of cortisol.
FIG 9.
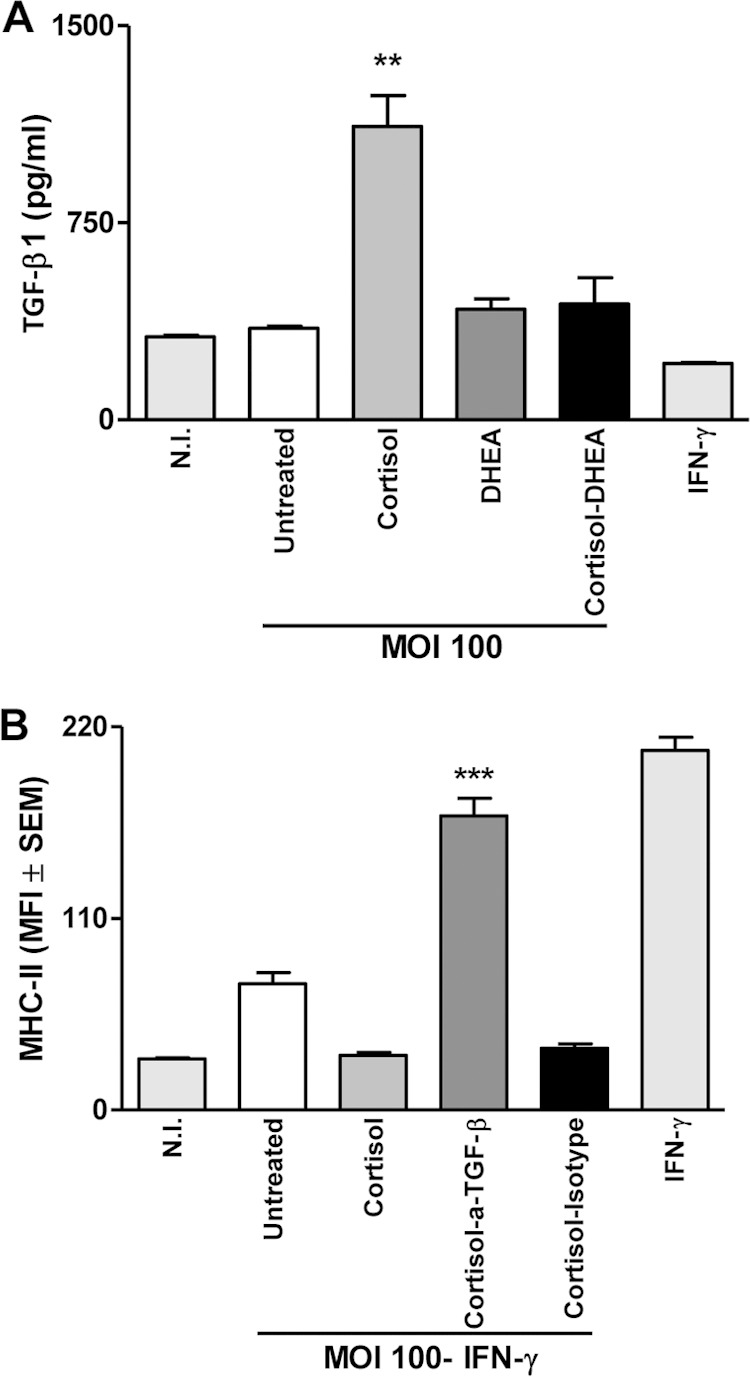
TGF-β1 and MHC-II expression by B. abortus-infected THP-1 cells. THP-1 cells were infected with B. abortus (MOI, 100) in the presence of IFN-γ (150 U/ml) and cortisol (1 × 10−6 M). TGF-β1 expression by B. abortus-infected THP-1 monocytes (MOI, 100) (A) and MHC-II (B) expression was assessed by flow cytometry. MFI, mean fluorescence intensity. Nonspecific binding was determined using a control isotype antibody. Data are given as means ± SEM from at least 4 individual experiments. **, P < 0.01 versus untreated cells; ***, P < 0.001 versus cortisol-treated cells.
DISCUSSION
Host control of brucellosis requires a set of cells and components of the immune system that together promote a complex response against Brucella spp. (49, 50). However, the role of the adrenal axis in the immune response against Brucella infection has not yet been reported.
Under healthy conditions, DHEA secretion is synchronized with cortisol in response to adrenocorticotropic hormone (ACTH) and corticotrophin-releasing hormone (CRH), while in critical illness, a remarkable decrease of DHEA secretion has been observed (28). The physiological role of DHEA is poorly understood, but there are clear indications that it modulates the immune response and influences both pro- and anti-inflammatory cytokine release (51–54).
The hypothalamus adrenal axis is activated during many bacterial and viral infections, resulting in an increase in circulating glucocorticoid levels. This activation is dependent on the inflammatory cytokines (55, 56). In previous studies, it has been demonstrated that cortisol levels were more elevated in patients with acute brucellosis than in healthy individuals and patients with remission (21). In concordance, our results indicated that the cortisol/DHEA ratio in infected patients is much higher than in healthy controls. This indicated that the hypothalamus adrenal axis is activated in Brucella-infected patients.
So far, there are no reports describing the effect of adrenal hormones on monocyte/macrophage function in the context of Brucella infection. Adrenal hormones participate in the modulation of the function of several immune cells. We hypothesize that cell function would be influenced by cortisol and DHEA, modulating both innate and adaptive immune responses to B. abortus. Moreover, the effects of cortisol and DHEA on monocyte/macrophage function have been demonstrated in other infection models, showing changes in cytokine production, antigen presentation, and costimulatory molecule expression (57–59).
Cortisol treatment significantly inhibited B. abortus-induced CD40, CD86, and CD54 expression. Interestingly, when infection experiments were performed in the presence of cortisol and DHEA simultaneously, we observed that CD40, CD86, and CD54 levels were similar to those induced by untreated B. abortus-infected cells. Interestingly, the presence of DHEA during B. abortus infection induces the expression of MHC-I and MHC-II molecules. When infection experiments were performed in the presence of cortisol and DHEA simultaneously, we also observed a significant increase of MHC-I and MHC-II. This indicated that DHEA could positively modulate monocyte function during B. abortus infection.
IFN-γ has a critical role in protective immunity against Brucella, and this cytokine enhances both the microbicidal and antigen-presenting functions of macrophages (60–63). In our laboratory, we demonstrated that B. abortus infection inhibits MHC-I and MHC-II expression induced by IFN-γ, contributing in this way to the chronicity of Brucella infection (43, 44). Our results indicated that DHEA immune intervention could positively affect monocyte function, resulting in a significant recovery of the inhibited IFN-γ-induced MHC-I and -II expression mediated by B. abortus infection. In addition, DHEA could also reverse the inhibition of IFN-γ-induced MHC-I and -II expression mediated by B. abortus infection in the presence of cortisol.
TGF-β1 is an important mediator of immune regulation (64), and its expression is always associated with immune suppression. In patients with chronic Brucella infection, it has been demonstrated that circulating TGF-β1 is abundantly secreted in sera and peripheral blood mononuclear cells (PBMCs) of patients with chronic brucellosis. The increased activity of TGF-β1 at both mRNA and protein levels may underlie the depressed function of T cell responses with consequent prolongation of the disease course (48). Accordingly, our results indicate that cortisol induces TGF-β1 secretion by B. abortus-infected monocytes and that this cytokine is involved in the inhibition of MHC-II expression as was demonstrated using specific neutralizing antibodies. In addition, transcription of MHC-II genes depends on a specific non-DNA-binding protein, the class II transactivator (CIITA) (45). CIITA expression can be inhibited by TGF-β1, as was demonstrated in different cell types (46, 47). In concordance, our results indicate that cortisol treatment induces TGF-β1 secretion and does not induce CIITA expression in B. abortus-infected monocytes. In contrast, DHEA treatment inhibits TGF-β1 secretion with concomitant induction of CIITA expression.
Our data demonstrated that DHEA could reverse the effect of cortisol in the context of Brucella infection, since we observed an increment of expression of adhesion molecules and molecules involved in antigen presentation and T cell costimulation. However, DHEA treatment had no effect on proinflammatory cytokine and chemokine production.
Taken together, our results indicate that DHEA immune intervention may positively affect monocyte function since we observed the induction of a proinflammatory milieu, incremented expression of the MHC-I and MHC-II molecules and costimulatory molecules, and adhesion enhancement.
Finally, the present study constitutes the first analysis on the immune-endocrine alterations in brucellosis and provides an initial background for studying in more detail the role of DHEA during the infection. Thus, the supplementation or cotreatment with DHEA or derivatives in addition to antibiotics therapy can be considered as a new possible treatment against Brucella.
ACKNOWLEDGMENTS
We thank Horacio Salomón and the staff of the Instituto de Investigaciones Biomédicas en Retrovirus y Sida (INBIRS) for their assistance with biosafety level 3 laboratory use. We also thank Oscar Bottasso for the seminal ideas.
This work was supported by grants PICT2010-1501, PICT2011-1200, and PICT2012-2252 from Agencia Nacional de Promoción Científica y Tecnológica (ANPCYT, Argentina) and by grants UBACYT 20020090200012 and 20020120100128 from Universidad de Buenos Aires. M.V.G., P.C.A.B., and L.N.V. are recipients of a fellowship from CONICET. P.B., G.H.G. and M.V.D. are members of the Research Career of CONICET. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
REFERENCES
- 1.Pittman QJ. 2011. A neuro-endocrine-immune symphony. J Neuroendocrinol 23:1296–1297. doi: 10.1111/j.1365-2826.2011.02176.x. [DOI] [PubMed] [Google Scholar]
- 2.Petrovsky N. 2001. Towards a unified model of neuroendocrine-immune interaction. Immunol Cell Biol 79:350–357. doi: 10.1046/j.1440-1711.2001.01029.x. [DOI] [PubMed] [Google Scholar]
- 3.Procaccini C, Pucino V, De Rosa V, Marone G, Matarese G. 2014. Neuro-endocrine networks controlling immune system in health and disease. Front Immunol 5:143. doi: 10.3389/fimmu.2014.00143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Young EJ. 1995. An overview of human brucellosis. Clin Infect Dis 21:283–289, 290. doi: 10.1093/clinids/21.2.283. [DOI] [PubMed] [Google Scholar]
- 5.Pappas G, Akritidis N, Bosilkovski M, Tsianos E. 2005. Brucellosis. N Engl J Med 352:2325–2336. doi: 10.1056/NEJMra050570. [DOI] [PubMed] [Google Scholar]
- 6.Zhan Y, Kelso A, Cheers C. 1993. Cytokine production in the murine response to brucella infection or immunization with antigenic extracts. Immunology 80:458–464. [PMC free article] [PubMed] [Google Scholar]
- 7.Murphy EA, Sathiyaseelan J, Parent MA, Zou B, Baldwin CL. 2001. Interferon-gamma is crucial for surviving a Brucella abortus infection in both resistant C57BL/6 and susceptible BALB/c mice. Immunology 103:511–518. doi: 10.1046/j.1365-2567.2001.01258.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Oliveira SC, Splitter GA. 1995. CD8+ type 1 CD44hi CD45 RBlo T lymphocytes control intracellular Brucella abortus infection as demonstrated in major histocompatibility complex class I- and class II-deficient mice. Eur J Immunol 25:2551–2557. doi: 10.1002/eji.1830250922. [DOI] [PubMed] [Google Scholar]
- 9.He Y, Vemulapalli R, Zeytun A, Schurig GG. 2001. Induction of specific cytotoxic lymphocytes in mice vaccinated with Brucella abortus RB51. Infect Immun 69:5502–5508. doi: 10.1128/IAI.69.9.5502-5508.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Eisenschenk FC, Houle JJ, Hoffmann EM. 1999. Mechanism of serum resistance among Brucella abortus isolates. Vet Microbiol 68:235–244. doi: 10.1016/S0378-1135(99)00075-9. [DOI] [PubMed] [Google Scholar]
- 11.Velasco J, Bengoechea JA, Brandenburg K, Lindner B, Seydel U, Gonzalez D, Zahringer U, Moreno E, Moriyon I. 2000. Brucella abortus and its closest phylogenetic relative, Ochrobactrum spp, differ in outer membrane permeability and cationic peptide resistance. Infect Immun 68:3210–3218. doi: 10.1128/IAI.68.6.3210-3218.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Giambartolomei GH, Delpino MV, Cahanovich ME, Wallach JC, Baldi PC, Velikovsky CA, Fossati CA. 2002. Diminished production of T helper 1 cytokines correlates with T cell unresponsiveness to Brucella cytoplasmic proteins in chronic human brucellosis. J Infect Dis 186:252–259. doi: 10.1086/341449. [DOI] [PubMed] [Google Scholar]
- 13.Celli J. 2006. Surviving inside a macrophage: the many ways of Brucella. Res Microbiol 157:93–98. doi: 10.1016/j.resmic.2005.10.002. [DOI] [PubMed] [Google Scholar]
- 14.Haddad JJ, Saade NE, Safieh-Garabedian B. 2002. Cytokines and neuro-immune-endocrine interactions: a role for the hypothalamic-pituitary-adrenal revolving axis. J Neuroimmunol 133:1–19. doi: 10.1016/S0165-5728(02)00357-0. [DOI] [PubMed] [Google Scholar]
- 15.Boumpas DT, Anastassiou ED, Older SA, Tsokos GC, Nelson DL, Balow JE. 1991. Dexamethasone inhibits human interleukin 2 but not interleukin 2 receptor gene expression in vitro at the level of nuclear transcription. J Clin Invest 87:1739–1747. doi: 10.1172/JCI115192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sierra-Honigmann MR, Murphy PA. 1992. T cell receptor-independent immunosuppression induced by dexamethasone in murine T helper cells. J Clin Invest 89:556–560. doi: 10.1172/JCI115620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McEwen BS, Biron CA, Brunson KW, Bulloch K, Chambers WH, Dhabhar FS, Goldfarb RH, Kitson RP, Miller AH, Spencer RL, Weiss JM. 1997. The role of adrenocorticoids as modulators of immune function in health and disease: neural, endocrine and immune interactions. Brain Res Brain Res Rev 23:79–133. doi: 10.1016/S0165-0173(96)00012-4. [DOI] [PubMed] [Google Scholar]
- 18.Loria RM. 1997. Antiglucocorticoid function of androstenetriol. Psychoneuroendocrinology 22(Suppl 1):S103–S108. doi: 10.1016/S0306-4530(97)00005-X. [DOI] [PubMed] [Google Scholar]
- 19.Christeff N, Melchior JC, Mammes O, Gherbi N, Dalle MT, Nunez EA. 1999. Correlation between increased cortisol:DHEA ratio and malnutrition in HIV-positive men. Nutrition 15:534–539. doi: 10.1016/S0899-9007(99)00111-2. [DOI] [PubMed] [Google Scholar]
- 20.Bozza VV, D'Attilio L, Mahuad CV, Giri AA, del Rey A, Besedovsky H, Bottasso O, Bay ML. 2007. Altered cortisol/DHEA ratio in tuberculosis patients and its relationship with abnormalities in the mycobacterial-driven cytokine production by peripheral blood mononuclear cells. Scand J Immunol 66:97–103. doi: 10.1111/j.1365-3083.2007.01952.x. [DOI] [PubMed] [Google Scholar]
- 21.Yildiz O, Gokce C, Alp E, Durak AC, Aygen B, Kelestimur F, Doganay M. 2005. Investigation of the hypothalamo-pituitary-adrenal axis and changes in the size of adrenal glands in acute brucellosis. Endocr J 52:183–188. doi: 10.1507/endocrj.52.183. [DOI] [PubMed] [Google Scholar]
- 22.Young EJ. 1989. Clinical manifestations of human brucellosis, p 97 In Young EJ, Corbel MJ (ed), Brucellosis: clinical and laboratory aspects. CRC Press, Boca Raton, FL. [Google Scholar]
- 23.Delpino MV, Barrionuevo P, Scian R, Fossati CA, Baldi PC. 2010. Brucella-infected hepatocytes mediate potentially tissue-damaging immune responses. J Hepatol 53:145–154. doi: 10.1016/j.jhep.2010.02.028. [DOI] [PubMed] [Google Scholar]
- 24.Giambartolomei GH, Zwerdling A, Cassataro J, Bruno L, Fossati CA, Philipp MT. 2004. Lipoproteins, not lipopolysaccharide, are the key mediators of the proinflammatory response elicited by heat-killed Brucella abortus. J Immunol 173:4635–4642. doi: 10.4049/jimmunol.173.7.4635. [DOI] [PubMed] [Google Scholar]
- 25.Boyum A. 1968. Separation of leukocytes from blood and bone marrow. Scand J Clin Lab Invest Suppl 97:7. [PubMed] [Google Scholar]
- 26.O'Neill S, O'Neill AJ, Conroy E, Brady HR, Fitzpatrick JM, Watson RW. 2000. Altered caspase expression results in delayed neutrophil apoptosis in acute pancreatitis. J Leukoc Biol 68:15–20. [PubMed] [Google Scholar]
- 27.Hibbs MS, Hasty KA, Seyer JM, Kang AH, Mainardi CL. 1985. Biochemical and immunological characterization of the secreted forms of human neutrophil gelatinase. J Biol Chem 260:2493–2500. [PubMed] [Google Scholar]
- 28.Vermes I, Beishuizen A. 2001. The hypothalamic-pituitary-adrenal response to critical illness. Best Pract Res Clin Endocrinol Metab 15:495–511. doi: 10.1053/beem.2001.0166. [DOI] [PubMed] [Google Scholar]
- 29.Perez AR, Bottasso O, Savino W. 2009. The impact of infectious diseases upon neuroendocrine circuits. Neuroimmunomodulation 16:96–105. doi: 10.1159/000180264. [DOI] [PubMed] [Google Scholar]
- 30.Angerami M, Suarez G, Pascutti MF, Salomon H, Bottasso O, Quiroga MF. 2013. Modulation of the phenotype and function of Mycobacterium tuberculosis-stimulated dendritic cells by adrenal steroids. Int Immunol 25:405–411. doi: 10.1093/intimm/dxt004. [DOI] [PubMed] [Google Scholar]
- 31.Pei J, Kahl-McDonagh M, Ficht TA. 2014. Brucella dissociation is essential for macrophage egress and bacterial dissemination. Front Cell Infect Microbiol 4:23. doi: 10.3389/fcimb.2014.00023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kohler S, Michaux-Charachon S, Porte F, Ramuz M, Liautard JP. 2003. What is the nature of the replicative niche of a stealthy bug named Brucella? Trends Microbiol 11:215–219. doi: 10.1016/S0966-842X(03)00078-7. [DOI] [PubMed] [Google Scholar]
- 33.Brinckerhoff CE, Matrisian LM. 2002. Matrix metalloproteinases: a tail of a frog that became a prince. Nat Rev Mol Cell Biol 3:207–214. doi: 10.1038/nrm763. [DOI] [PubMed] [Google Scholar]
- 34.Alsalameh S, Amin RJ, Kunisch E, Jasin HE, Kinne RW. 2003. Preferential induction of prodestructive matrix metalloproteinase-1 and proinflammatory interleukin 6 and prostaglandin E2 in rheumatoid arthritis synovial fibroblasts via tumor necrosis factor receptor-55. J Rheumatol 30:1680–1690. [PubMed] [Google Scholar]
- 35.Harris SG, Padilla J, Koumas L, Ray D, Phipps RP. 2002. Prostaglandins as modulators of immunity. Trends Immunol 23:144–150. doi: 10.1016/S1471-4906(01)02154-8. [DOI] [PubMed] [Google Scholar]
- 36.Nagase H, Brew K. 2003. Designing TIMP (tissue inhibitor of metalloproteinases) variants that are selective metalloproteinase inhibitors. Biochem Soc Symp 2003(70):201–212. [DOI] [PubMed] [Google Scholar]
- 37.Panagakos FS, Kumar S. 1994. Modulation of proteases and their inhibitors in immortal human osteoblast-like cells by tumor necrosis factor-alpha in vitro. Inflammation 18:243–265. doi: 10.1007/BF01534267. [DOI] [PubMed] [Google Scholar]
- 38.Uchida M, Shima M, Shimoaka T, Fujieda A, Obara K, Suzuki H, Nagai Y, Ikeda T, Yamato H, Kawaguchi H. 2000. Regulation of matrix metalloproteinases (MMPs) and tissue inhibitors of metalloproteinases (TIMPs) by bone resorptive factors in osteoblastic cells. J Cell Physiol 185:207–214. doi:. [DOI] [PubMed] [Google Scholar]
- 39.Wright KM, Friedland JS. 2004. Regulation of monocyte chemokine and MMP-9 secretion by proinflammatory cytokines in tuberculous osteomyelitis. J Leukoc Biol 75:1086–1092. doi: 10.1189/jlb.0903433. [DOI] [PubMed] [Google Scholar]
- 40.Straub RH, Cutolo M, Buttgereit F, Pongratz G. 2010. Energy regulation and neuroendocrine-immune control in chronic inflammatory diseases. J Intern Med 267:543–560. doi: 10.1111/j.1365-2796.2010.02218.x. [DOI] [PubMed] [Google Scholar]
- 41.Hazeldine J, Arlt W, Lord JM. 2010. Dehydroepiandrosterone as a regulator of immune cell function. J Steroid Biochem Mol Biol 120:127–136. doi: 10.1016/j.jsbmb.2009.12.016. [DOI] [PubMed] [Google Scholar]
- 42.Zaitseva M, Golding H, Manischewitz J, Webb D, Golding B. 1996. Brucella abortus as a potential vaccine candidate: induction of interleukin-12 secretion and enhanced B7.1 and B7.2 and intercellular adhesion molecule 1 surface expression in elutriated human monocytes stimulated by heat-inactivated B. abortus. Infect Immun 64:3109–3117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Barrionuevo P, Cassataro J, Delpino MV, Zwerdling A, Pasquevich KA, Garcia Samartino C, Wallach JC, Fossati CA, Giambartolomei GH. 2008. Brucella abortus inhibits major histocompatibility complex class II expression and antigen processing through interleukin-6 secretion via Toll-like receptor 2. Infect Immun 76:250–262. doi: 10.1128/IAI.00949-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Barrionuevo P, Delpino MV, Pozner RG, Velasquez LN, Cassataro J, Giambartolomei GH. 2013. Brucella abortus induces intracellular retention of MHC-I molecules in human macrophages down-modulating cytotoxic CD8(+) T cell responses. Cell Microbiol 15:487–502. doi: 10.1111/cmi.12058. [DOI] [PubMed] [Google Scholar]
- 45.Ting JP, Zhu XS. 1999. Class II MHC genes: a model gene regulatory system with great biologic consequences. Microbes Infect 1:855–861. [PubMed] [Google Scholar]
- 46.Czarniecki CW, Chiu HH, Wong GH, McCabe SM, Palladino MA. 1988. Transforming growth factor-beta 1 modulates the expression of class II histocompatibility antigens on human cells. J Immunol 140:4217–4223. [PubMed] [Google Scholar]
- 47.Reimold AM, Kara CJ, Rooney JW, Glimcher LH. 1993. Transforming growth factor beta 1 repression of the HLA-DR alpha gene is mediated by conserved proximal promoter elements. J Immunol 151:4173–4182. [PubMed] [Google Scholar]
- 48.Elfaki MG, Al-Hokail AA. 2009. Transforming growth factor beta production correlates with depressed lymphocytes function in humans with chronic brucellosis. Microbes Infect 11:1089–1096. doi: 10.1016/j.micinf.2009.08.001. [DOI] [PubMed] [Google Scholar]
- 49.Baldwin CL, Goenka R. 2006. Host immune responses to the intracellular bacteria Brucella: does the bacteria instruct the host to facilitate chronic infection? Crit Rev Immunol 26:407–442. doi: 10.1615/CritRevImmunol.v26.i5.30. [DOI] [PubMed] [Google Scholar]
- 50.Golding B, Scott DE, Scharf O, Huang LY, Zaitseva M, Lapham C, Eller N, Golding H. 2001. Immunity and protection against Brucella abortus. Microbes Infect 3:43–48. doi: 10.1016/S1286-4579(00)01350-2. [DOI] [PubMed] [Google Scholar]
- 51.Blum CA, Mueller C, Schuetz P, Fluri F, Trummler M, Mueller B, Katan M, Christ-Crain M. 2013. Prognostic value of dehydroepiandrosterone-sulfate and other parameters of adrenal function in acute ischemic stroke. PLoS One 8:e63224. doi: 10.1371/journal.pone.0063224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Oberbeck R, Dahlweid M, Koch R, van Griensven M, Emmendorfer A, Tscherne H, Pape HC. 2001. Dehydroepiandrosterone decreases mortality rate and improves cellular immune function during polymicrobial sepsis. Crit Care Med 29:380–384. doi: 10.1097/00003246-200102000-00029. [DOI] [PubMed] [Google Scholar]
- 53.Oberbeck R, Kobbe P. 2010. Dehydroepiandrosterone (DHEA): a steroid with multiple effects. Is there any possible option in the treatment of critical illness? Curr Med Chem 17:1039–1047. doi: 10.2174/092986710790820570. [DOI] [PubMed] [Google Scholar]
- 54.Schmitz D, Kobbe P, Wegner A, Hammes F, Oberbeck R. 2010. Dehydroepiandrosterone during sepsis: does the timing of administration influence the effectiveness. J Surg Res 163:e73–77. doi: 10.1016/j.jss.2010.05.017. [DOI] [PubMed] [Google Scholar]
- 55.Bornstein SR, Chrousos GP. 1999. Clinical review 104: adrenocorticotropin (ACTH)- and non-ACTH-mediated regulation of the adrenal cortex: neural and immune inputs. J Clin Endocrinol Metab 84:1729–1736. doi: 10.1210/jcem.84.5.5631. [DOI] [PubMed] [Google Scholar]
- 56.Demirdag K, Ozden M, Kalkan A, Godekmerdan A, Sirri Kilic S. 2003. Serum cytokine levels in patients with acute brucellosis and their relation to the traditional inflammatory markers. FEMS Immunol Med Microbiol 39:149–153. doi: 10.1016/S0928-8244(03)00207-4. [DOI] [PubMed] [Google Scholar]
- 57.Mouithys-Mickalad A, Deby-Dupont G, Mathy-Hartert M, Habraken Y, Nys M, Henrotin Y, Lamy M, Deby C. 2004. Effects of glucocorticoids on the respiratory burst of Chlamydia-primed THP-1 cells. Biochem Biophys Res Commun 318:941–948. doi: 10.1016/j.bbrc.2004.04.120. [DOI] [PubMed] [Google Scholar]
- 58.Le Tulzo Y, Pangault C, Amiot L, Guilloux V, Tribut O, Arvieux C, Camus C, Fauchet R, Thomas R, Drenou B. 2004. Monocyte human leukocyte antigen-DR transcriptional downregulation by cortisol during septic shock. Am J Respir Crit Care Med 169:1144–1151. doi: 10.1164/rccm.200309-1329OC. [DOI] [PubMed] [Google Scholar]
- 59.Billing AM, Fack F, Turner JD, Muller CP. 2011. Cortisol is a potent modulator of lipopolysaccharide-induced interferon signaling in macrophages. Innate Immun 17:302–320. doi: 10.1177/1753425910369269. [DOI] [PubMed] [Google Scholar]
- 60.Cooper AM, Dalton DK, Stewart TA, Griffin JP, Russell DG, Orme IM. 1993. Disseminated tuberculosis in interferon gamma gene-disrupted mice. J Exp Med 178:2243–2247. doi: 10.1084/jem.178.6.2243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Flynn JL, Chan J. 2001. Immunology of tuberculosis. Annu Rev Immunol 19:93–129. doi: 10.1146/annurev.immunol.19.1.93. [DOI] [PubMed] [Google Scholar]
- 62.Flynn JL, Chan J, Triebold KJ, Dalton DK, Stewart TA, Bloom BR. 1993. An essential role for interferon gamma in resistance to Mycobacterium tuberculosis infection. J Exp Med 178:2249–2254. doi: 10.1084/jem.178.6.2249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Schneeberger EE, DeFerrari M, Skoskiewicz MJ, Russell PS, Colvin RB. 1986. Induction of MHC-determined antigens in the lung by interferon-gamma. Lab Invest 55:138–144. [PubMed] [Google Scholar]
- 64.Wahl SM, McCartney-Francis N, Mergenhagen SE. 1989. Inflammatory and immunomodulatory roles of TGF-beta. Immunol Today 10:258–261. doi: 10.1016/0167-5699(89)90136-9. [DOI] [PubMed] [Google Scholar]