Abstract
To understand the effect of previous malaria exposure on antiparasite immune responses is important for developing successful immunization strategies. Controlled human malaria infections (CHMIs) using cryopreserved Plasmodium falciparum sporozoites provide a unique opportunity to study differences in acquisition or recall of antimalaria immune responses in individuals from different transmission settings and genetic backgrounds. In this study, we compared antiparasite humoral and cellular immune responses in two cohorts of malaria-naive Dutch volunteers and Tanzanians from an area of low malarial endemicity, who were subjected to the identical CHMI protocol by intradermal injection of P. falciparum sporozoites. Samples from both trials were analyzed in parallel in a single center to ensure direct comparability of immunological outcomes. Within the Tanzanian cohort, we distinguished one group with moderate levels of preexisting antibodies to asexual P. falciparum lysate and another that, based on P. falciparum serology, resembled the malaria-naive Dutch cohort. Positive P. falciparum serology at baseline was associated with a lower parasite density at first detection by quantitative PCR (qPCR) after CHMI than that for Tanzanian volunteers with negative serology. Post-CHMI, both Tanzanian groups showed a stronger increase in anti-P. falciparum antibody titers than Dutch volunteers, indicating similar levels of B-cell memory independent of serology. In contrast to the Dutch, Tanzanians failed to increase P. falciparum-specific in vitro recall gamma interferon (IFN-γ) production after CHMI, and innate IFN-γ responses were lower in P. falciparum lysate-seropositive individuals than in seronegative individuals. In conclusion, positive P. falciparum lysate serology can be used to identify individuals with better parasite control but weaker IFN-γ responses in circulating lymphocytes, which may help to stratify volunteers in future CHMI trials in areas where malaria is endemic.
INTRODUCTION
In 2012, Plasmodium falciparum malaria caused an estimated 207 million cases and 627,000 deaths, of which 90% occurred in children under 5 years of age and in pregnant women in sub-Saharan Africa (1). Major control efforts have been implemented with some success (2, 3), but malaria eradication will likely require a safe and highly protective vaccine. Subunit vaccines have thus far shown moderate efficacy at best. RTS,S is the only vaccine candidate in phase 3 trials but, despite averting substantial numbers of malaria cases (4), shows only 30 to 50% reduction in clinical disease after 12 months depending on both age and malaria endemicity and even less after 18 months (5–7). These results stress the need for more effective second-generation vaccines. Key requirements are not only the identification of novel immunogens but also a better understanding of protection-related immune responses. This includes the effect of previous malaria exposure on immune responses upon reexposure or vaccination (8, 9).
During the past 3 decades, controlled human malaria infection (CHMI) trials have become an indispensable tool not only in assessing the efficacy of candidate vaccines (10, 11) but also in evaluating immune responses induced by exposure to the malaria parasite (12–15). CHMI trials have so far been performed in countries were malaria is not endemic in previously unexposed individuals (11, 16–19). A logical next step is to study the potential differences in the acquisition, maintenance, or recall of immune responses in individuals from different transmission settings and genetic backgrounds (20, 21). The availability of aseptic, purified, cryopreserved, live P. falciparum sporozoites (PfSPZs; PfSPZ Challenge) (22) opens up opportunities to carry out CHMI trials in countries where malaria is endemic, since it bypasses the need for infecting local Anopheles mosquitoes with P. falciparum or importing P. falciparum-infected mosquitoes to the trial site. The first PfSPZ Challenge trial in malaria-naive Dutch volunteers demonstrated an infectivity rate of 83% after intradermal injections, independent of the dose given (23). Recently, PfSPZ Challenge was used for the first time during a CHMI trial in healthy adult male Tanzanian volunteers, resulting in similar infection rates (24). As a follow-up, we here present results of the malaria-specific humoral and cellular immune responses in Tanzanians and Dutch volunteers who were inoculated intradermally with the same number of live PfSPZs during these CHMI studies.
MATERIALS AND METHODS
Human ethics statement.
The Dutch trial (23) was approved by the Central Committee for Research Involving Human Subjects of The Netherlands (NL31858.091.10) and registered at Clinicaltrials.gov, identifier NCT 01086917. The Tanzanian trial (24) was approved by institutional review boards of the Ifakara Health Institute (IHI/IRB/No25), the National Institute for Medical Research Tanzania (NIMR/HQ/R.8a/Vol.IX/1217), the Ethikkommission beider Basel (EKBB), Basel, Switzerland (EKBB 319/11), and the Tanzanian Food and Drug Administration (reference no. CE.57/180/04A/50) and registered at Clinicaltrials.gov, identifier NCT 01540903. All study teams complied with the Declaration of Helsinki and good clinical practice, including monitoring of data, and all volunteers gave written informed consent.
Clinical trial design.
Samples for immunological analysis were obtained from two CHMI trials (23, 24).
The first trial, performed at Radboud University Medical Center, Nijmegen, The Netherlands, was composed of 18 healthy Dutch subjects between the ages of 19 and 30 years with no history of malaria. Any volunteer who was positive for P. falciparum serology or had resided in an area where malaria is endemic within the previous 6 months was excluded from the trial. Three groups (n = 6 per group) were infected by intradermal injections of 2,500, 10,000, or 25,000 cryopreserved PfSPZs (NF54 strain). By day 21, 15/18 volunteers had developed parasites detectable by positive blood thick smear (TS), 5/6 in each group (23). There were no differences in parasite densities at diagnosis between the three dose groups (23). For immunological analysis, nine P. falciparum-positive volunteers of the 10,000 (n = 4) and 25,000 (n = 5) PfSPZ dose groups were selected based on availability of plasma and peripheral blood mononuclear cells (PBMCs).
The second trial was carried out in Bagamoyo, Tanzania, with volunteers residing in Dar es Salaam (an area where malaria is hypoendemic). Twenty-four males between 20 to 35 years of age were enrolled and confirmed to be free of parasites by real-time quantitative PCR (qPCR). Subjects with a self-reported history of clinical malaria in the previous 5 years were excluded. The volunteers were divided into two groups with 12 volunteers per group and infected by intradermal injections of either 10,000 or 25,000 PfSPZs (NF54 strain). A total of 21/24 became both qPCR and blood smear positive by day 21 after infection (24). The three P. falciparum-negative volunteers were excluded from analysis in the present study.
PBMCs, citrate anticoagulated plasma samples from Dutch volunteers, and serum samples from Tanzanian volunteers were collected and cryopreserved 1 day before challenge (pre-CHMI) and after treatment (post-CHMI; day 35 and day 28 after infection).
DNA extraction and qPCR analysis.
A total of 5 μl Zap-Oglobin II lytic reagent (Beckman Coulter) was added to 500 μl of EDTA blood, after which the samples were mixed and stored at −80°C.
DNA extraction and quantification of parasitemia by qPCR in the Dutch CHMI trial were performed in Nijmegen as described previously (25), with slight modifications. Briefly, after thawing, samples were spiked with murine white blood cells as an extraction control, and DNA was extracted with a MagnaPure LC isolation station. For detection of the extraction control and P. falciparum, primers for the murine albumin gene and P. falciparum 18R rRNA were used as described previously (25). Additionally, the P. falciparum 18R rRNA TaqMan MGB probe AAC AAT TGG AGG GCA AG–6-carboxyfluorescein (FAM) was used.
DNA extraction and qPCR in the Tanzanian trial were carried out at the Leiden University Medical Center, Leiden, The Netherlands, as described previously (26). Phocine herpesvirus 1 (PhHV-1) was added to the isolation lysis buffer to serve as an internal control. For quantification of PhHV, the primers GGGCGAATCACAGATTGAATC and GCGGTTCCAAACGTACCAA and the probe Cy5-TTTTTATGTGTCCGCCACCATCTGGATC were used.
P. falciparum (NF54 strain) standard curves for both qPCR assays were prepared in Nijmegen by titration of ring-stage-infected red blood cells (RBC) in uninfected human blood. The two qPCR assays in both sites were confirmed to yield the same results when quantifying the P. falciparum content in sequential samples from four CHMI volunteers.
Parasite material for immunological analysis.
The P. falciparum NF54 strain used in both CHMI trials is the parental strain of the 3D7 clone (27). P. falciparum (NF54 strain) blood-stage parasites were cultured in RPMI 1640 containing 10% human A+ serum and a 5% hematocrit erythrocyte suspension in a semiautomated culture system and regularly screened for mycoplasma contamination. For in vitro stimulation assays, asynchronous parasites harvested at a parasitemia of approximately 10 to 20% were purified by centrifugation on a 63% Percoll density gradient to obtain mature asexual stages. This resulted in concentrations of parasitemia levels of about 80 to 90%, consisting of more than 95% schizonts/mature trophozoites. P. falciparum-infected RBC (PfRBC) were washed twice in RPMI, cryopreserved in glycerol-containing freeze medium, and used upon thawing in stimulation assays. Mock-cultured uninfected erythrocytes (uRBC) were obtained similarly and served as the control.
P. falciparum lysate for enzyme-linked immunosorbent assay (ELISA) was prepared by extracting purified schizonts/mature trophozoites with 1% sodium desoxycholate and 2.5 μl phenylmethanesulfonyl fluoride protease inhibitor for 15 min at room temperature (RT).
Recombinant and synthetic proteins.
Recombinant proteins of circumsporozoite protein (CSP) and liver-stage antigen 1 (LSA-1) were used to probe humoral responses toward preerythrocytic stages, while crude P. falciparum lysates were used to assess antibody reactivity toward blood stages. Apical membrane protein 1 (AMA-1) and exported protein 1 (EXP-1) are expressed in both preerythrocytic and asexual stages.
Full-length P. falciparum NF54 CSP with repeats was produced in Escherichia coli by Gennova Biopharmaceuticals Ltd., Pune, India. A recombinant LSA-1 construct, LSA-NRC, was expressed in E. coli, incorporating the N- and C-terminal regions of the protein and two of the centrally placed 17-amino-acid repeats for the 3D7 LSA-1 sequence (PlasmoDB-PF3D7_1036400) (28). Both the N- and C-terminal regions as well as the repeats are highly conserved between NF54 and 3D7. The major difference is the greater number of repeats, which are the primary target of anti-LSA-1 antibodies (29), in the NF54 sequence than in the 3D7 sequence (30). Amino acids 25 to 545 of codon-optimized AMA-1 of the P. falciparum FVO strain were expressed in the methylotrophic yeast Pichia pastoris (31, 32). A peptide covering the C-terminal amino acids 73 to 162 of the integral parasitophorous vacuolar membrane protein EXP-1 (Swiss-Prot Database primary accession number P04926) was chemically synthesized using solid-phase 9-fluorenylmethoxy carbonyl (Fmoc) chemistry and differs from the 3D7 sequence only by a single amino acid in position 160 (33).
ELISA to assess antibody reactivity.
Ninety-six-well Polystyrene flat-bottom plates (Nunc Maxisorp; Thermo Scientific) were coated with 2 μg/ml of CSP, EXP-1, and AMA-1, 0.25 μg/ml of LSA-1, or P. falciparum lysate at the equivalent of 20,000 PfRBC/well in phosphate-buffered saline (PBS) and incubated overnight at 4°C. Plates were blocked with 5% milk in PBS. All of the following washing steps were carried out with PBS-0.05% Tween (PBST). Using 1% milk in PBST, plasma or serum samples were serially diluted in duplicate starting at 1:50 to 1:800 for protein antigen and 1:250 to 1:4,000 for P. falciparum lysate and incubated for 3 h at room temperature. Bound IgG was detected using horseradish peroxidase (HRP)-conjugated anti-human IgG (Thermo Scientific; diluted 1:60,000 in sample buffer). Plates were developed using tetramethylbenzidine (TMB) peroxidase substrate (tebu-bio). The reaction was stopped using an equal volume of 0.2 M H2SO4, and absorbance was measured with a spectrophotometer plate reader at 450 nm (Anthos 2001 ELISA plate reader).
A serial dilution of a pool of sera from 100 hyperimmune Tanzanian (HIT) (20) individuals living in an area where malaria is highly endemic was used as a reference standard and was included on each plate. The reactivity for each antigen in undiluted HIT serum was defined as 100 arbitrary units (AUs). Optical density (OD) values were converted into AUs by using the four-parameter logistic curve fit using the Auditable Data Analysis and Management System for ELISA (ADAMSEL-v1.1; http://www.malariaresearch.eu/content/software).
For each antigen, all time points of an individual volunteer were assayed on the same plate. To determine whether Tanzanians had a positive P. falciparum serology (by recognition of P. falciparum lysate), the mean (+2 standard deviations [SD]) baseline antibody titer against P. falciparum lysate of the Dutch volunteers was used as the cutoff for positivity.
In vitro PBMC stimulation assay to assess cellular responses.
Venous whole blood was collected into citrated Vacutainer CPT cell preparation tubes (Becton Dickinson). PBMCs were obtained by density gradient centrifugation, washed three times in cold PBS, counted, frozen at 107 cells/ml in fetal calf serum (FCS) with 10% dimethyl sulfoxide, and stored in vapor-phase nitrogen. After being thawed, PBMCs were counted and cultured at a concentration of 500,000 cells/well in a 96-well round-bottom plate and stimulated in duplicate at a ratio of 1:2 with 106 P. falciparum NF54-infected RBC or uRBC for either 24 h or 6 days in a total volume of 200 μl.
Flow cytometry.
Cells were stained and analyzed by flow cytometry either directly ex vivo or after 24 h or 6 days of in vitro stimulation. Cells were stained first for viability with LIVE/DEAD fixable Aqua dead cell stain (Invitrogen) or fixable viability dye eFluor 780 (eBioscience) and later with three different staining panels for surface markers: for the 24-h stain, CD3 PerCP (UCH1; BioLegend), CD56-phycoerythrin (PE) (HCD56; Biolegend), anti-T-cell receptor (TCR) Pan γ/δ-PE (IMMU510; Beckman Coulter), CD4 Pacific Blue (OKT4; Beckman Coulter), CD8 allophycocyanin (APC)-H7 (SK1; BD Pharmingen), CD45RO energy coupled dye (ECD) (UCH1; Beckman Coulter), and CD62L PeCy7 (DREG56; eBioscience); for the ex vivo stain, the only changes from the previous stain were CD3 V500 (clone SP342; BD Horizon) and CD8 PerCP (RPA-T8; BioLegend); and for the third-panel 6-day stain, CD3 PeCy7 (OKT3; BioLegend) and CD8 PerCP (RPA-T8; BioLegend). For intracellular staining, cells were incubated with different monoclonal antibodies (MAbs) depending on the staining panels. After 30 min of incubation at RT, cells were washed and permeabilized with Foxp3 fix/perm buffer (eBioscience) for 30 min on ice and stained in permeabilization buffer (eBioscience) with IFN-γ fluorescein isothiocyanate (FITC) (4S.B3; eBioscience; 24-h stain) and Foxp3 eF660 (PCH101; eBioscience; ex vivo stain) or Ki67 FITC (B56; BD Pharmingen) and Foxp3 eF660 (PCH101; eBioscience; 6-day stain). Cells were collected on a CyAn ADP 9-color flow cytometer (Dako/Beckman Coulter) and analyzed using FlowJo software (Tree Star, Inc.) version 9.6. All assays were conducted with the same batches of PfRBC and uRBC, with all time points of one volunteer assayed in one experiment to prevent interassay variations. Natural killer T cells (NKT) and gamma delta T cells (γδT) were analyzed in the same gate and henceforth are referred to as NKT-γδT cells.
Statistical analysis.
Statistical analysis was performed in GraphPad Prism 5. Differences within the cohorts and between time points were analyzed per volunteer by a Wilcoxon matched-pairs signed-rank test and those between groups were analyzed by a Mann-Whitney U test. The relationship between baseline antibody titers and the increase in antibody titers was analyzed by Spearman correlation, and P values of <0.05 were considered statistically significant. Cellular responses were corrected for the background by subtracting responses to uRBC from responses to PfRBC for each sample; resulting negative values were set to zero.
RESULTS
Tanzanian volunteers have higher baseline antibody titers than Dutch subjects.
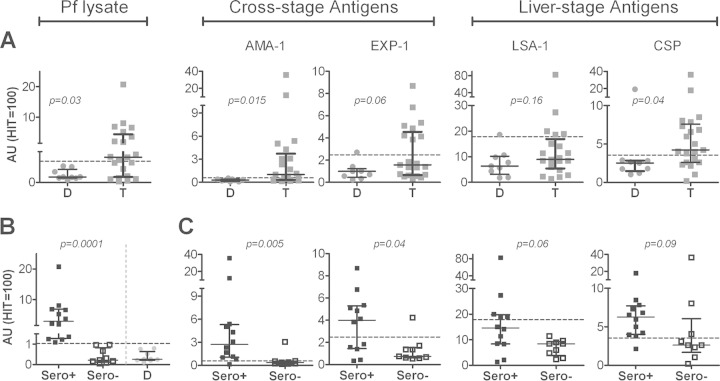
Pre-CHMI antibody titers were significantly higher in Tanzanian than in the malaria-naive Dutch volunteers for crude P. falciparum lysate (P < 0.03), with 12/21 Tanzanians having titers higher than the mean (+2 SD) titers of Dutch volunteers. This was also true for pre-CHMI antibodies to the individual parasite antigens AMA-1 (P < 0.015; 13/21) and CSP (P < 0.04; 15/21), and the same trend was found for EXP-1 (P < 0.06; 8/21) (Fig. 1A). Elevated LSA-1 antibody titers were found in 5/21 Tanzanians, but there was no significant difference between Dutch and Tanzanians at the group level (P < 0.16). Within the Tanzania cohort, there was a wide range of pre-CHMI antibody responses, with some volunteers showing only low responses, comparable to those of the Dutch cohort. To address whether there was a general division into high and low responders to malaria antigens, we stratified Tanzanian individuals based on their reactivity to P. falciparum lysate (containing a large number of late-liver- and blood-stage antigens) (Fig. 1B). Compared to their P. falciparum lysate-seronegative counterparts (n = 9), seropositive Tanzanians (n = 12) had significantly higher antibody titers against the cross-stage antigens AMA-1 (P = 0.005; 7.6-fold higher median titer) and EXP-1 (P = 0.04; 5.4-fold higher). The same trend was found for the sporozoite antigen CSP (P = 0.09; 2.4-fold higher) and the liver-stage antigen LSA-1 (P = 0.06; 1.7-fold) (Fig. 1C).
FIG 1.
Baseline malaria-specific antibody titers indicate previous exposure in Tanzanian volunteers. Antibody reactivity against crude P. falciparum lysate and the P. falciparum antigens AMA-1, EXP-1, LSA-1, and CSP was tested prior to malaria infection. A pool of sera from 100 hyperimmune Tanzanians (HIT) was used as a reference. Reactivity for each antigen in undiluted HIT serum was set at 100 arbitrary units (AUs). (A) Responses between Dutch (D; circles; n = 9) and Tanzanian (T; squares; n = 21) cohorts were compared using a Mann-Whitney U test. (B) Tanzanian volunteers were stratified based on P. falciparum lysate recognition at baseline as Sero+ (n = 12; black box plots) or Sero− (n = 9; white box plots), using the mean + 2 SD of Dutch volunteers (gray circles) as a cutoff for positivity. (C) Pre-CHMI responses of seropositive and seronegative Tanzanians were analyzed by ELISA for individual P. falciparum antigens and compared by Mann-Whitney U test. Scatter plots show individual data points, horizontal lines indicate the median of the group, and error bars indicate the interquartile range (IQR). Dashed lines indicate the mean + 2 SD of antibody titers in Dutch volunteers for each respective antigen.
P. falciparum lysate seropositivity prior to CHMI is associated with reduced initial blood-stage parasitemia.
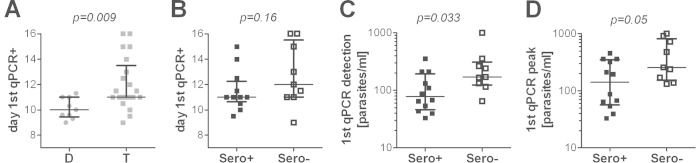
We next assessed whether preexisting humoral responses might be associated with the control of parasites in Tanzanian volunteers. In line with stronger humoral responses in the Tanzanian cohort than in the Dutch cohort at baseline, Tanzanian volunteers became qPCR positive significantly later than the Dutch volunteers (Fig. 2A), with a median prepatent period of 11.0 days (interquartile range [IQR], 11.0 to 13.5) in Tanzanians and 10.0 days (9.5 to 11.0) in Dutch volunteers (P = 0.009). Similarly, prepatency by TS was also longer in Tanzanians (median [IQR], 13.7 days [12.75 to 16.7]) than in the Dutch (12.6 days [12.3 to 14]) (P = 0.035). The time between detection by qPCR and by TS was comparable for both cohorts (median [IQR] for Tanzanians, 2.6 days [2.2 to 3.15]; Dutch, 3.0 days [2.0 to 3.15]; P = 0.88), but Dutch volunteers had a significantly higher peak parasite density (median number of parasites/milliliter [IQR] for Tanzanians, 12,000 [6,800 to 15,000]; Dutch, 74,000 [26,000 to 190,000]; P = 0.02), possibly due to slight differences in the thick smear protocol between the two sites. Within the Tanzanian cohort, there was no significant difference in time to qPCR-detectable parasitemia between volunteers who were either P. falciparum lysate seropositive or seronegative at baseline (P = 0.16) (Fig. 2B), nor was there a difference in prepatency by TS (P = 0.41) or time between detection by qPCR and TS (P = 0.15). However, seropositive Tanzanian volunteers had a significantly lower parasite load at the time of first qPCR-detectable parasitemia than their seronegative counterparts (P = 0.033) (Fig. 2C). This difference remained evident, but became smaller, by the time the first peak in parasite load was reached (P = 0.05; Fig. 2D). Across all Tanzanian volunteers, pre-CHMI antibody titers for CSP (Pearson r = 0.45, P = 0.04), but no other antigens, correlated significantly with prepatancy by qPCR (see Fig. S1 in the supplemental material).
FIG 2.
Preexposure to malaria is associated with difference in prepatency and parasitemia after CHMI. Parasitemia after CHMI was determined by qPCR. The day of first parasite detection by qPCR after PfSPZ injection is shown for Dutch (D; gray circles; n = 9) compared to Tanzanians (T; gray squares; n = 21) (A) and for P. falciparum-seropositive (n = 12; black squares) and -seronegative (n = 9; white squares) Tanzanian volunteers (B). The parasite load (number of P. falciparum parasites per milliliter of blood) at the first day of qPCR-detectable parasitemia (C) and the time of first peak parasite density (D) are shown for P. falciparum-seropositive (n = 12; black squares) and -seronegative (n = 9; white squares) Tanzanian volunteers. Data are shown as median ± IQR. Groups were compared by a Mann-Whitney U test.
P. falciparum-specific antibody responses are more efficiently increased in Tanzanians than in Dutch volunteers after CHMI.
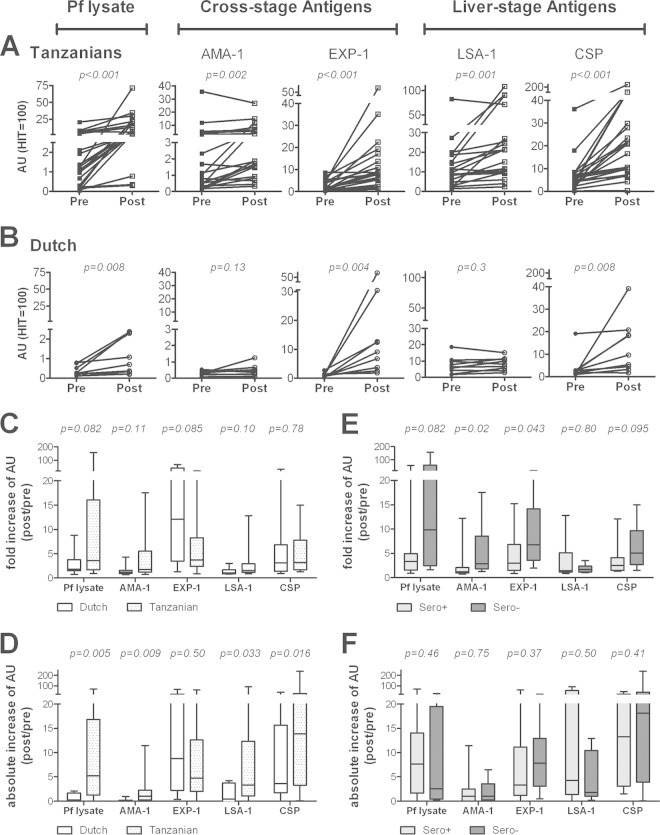
Post-CHMI, antibody responses increased significantly in the Tanzanian volunteers for P. falciparum lysate (P < 0.001; 15/21 with a >3-fold increase in titers), AMA-1 (P = 0.002; 6/21), EXP-1 (P < 0.0001; 14/21), LSA-1 (P = 0.001; 5/21), and CSP (P < 0.001; 11/21) (Fig. 3A). In contrast, the Dutch volunteers showed significant increased titers only against EXP-1 (P = 0.004; 7/9), CSP (P = 0.008; 6/9), and P. falciparum lysate (P = 0.008; 3/9), while responses to LSA-1 and AMA-1 remained low and unaltered (Fig. 3B). Compared to the Dutch, Tanzanians showed a trend for stronger induction or boosting of responses based on the overall fold increase in titers to P. falciparum lysate, AMA-1, and LSA-1 (Fig. 3C) and significantly higher absolute increases in titers for these antigens (Fig. 3D). Volunteers in both cohorts were subjected to CHMI with either 10,000 or 25,000 PfSPZs. However, the only significant difference in antibody responses between the two dose groups was a slightly higher response in the 25,000 than in the 10,000 dose group for P. falciparum lysate in Dutch volunteers (P = 0.04) and a similar trend for the Tanzanians for CSP (P = 0.07) (see Fig. S2 in the supplemental material). Post-CHMI, P. falciparum lysate-seronegative Tanzanians had a significantly stronger fold increase in antibody titers against AMA-1 (P = 0.02) and EXP-1 (P = 0.04) than seropositive individuals, with a similar trend for P. falciparum lysate (P = 0.082) and CSP (P = 0.095), but no difference in LSA-1 responses (P = 0.80) (Fig. 3E). For the entire cohort, the fold increase of P. falciparum lysate (P = 0.01) and AMA-1 (P = 0.001) responses upon CHMI correlated negatively with the baseline response. The absolute increase in titers, however, was not different for any of the antigens between the two groups (Fig. 3F). Notably, P. falciparum lysate-seronegative Tanzanians thereby also showed a greater absolute increase in antibody titers than malaria-naive Dutch volunteers.
FIG 3.
Increased humoral responses in Dutch and Tanzanians after CHMI. Antibody titers were measured 4 weeks (Tanzanians; squares; n = 21) (A) and 5 weeks (Dutch; circles; n = 9) (B) after intradermal infection with PfSPZs. Graphs show the antibody reactivity (AUs) pre-CHMI (black) and post-CHMI (white) against crude P. falciparum lysate, AMA-1, EXP-1, LSA-1, and CSP. Plots show individual data points, with lines connecting the two time points for each volunteer. Responses in the two cohorts pre- and post-CHMI were compared by using a Wilcoxon matched-pairs signed-rank test. The fold increase (ratio of AU post-CHMI/AU pre-CHMI) (C, E) and absolute increase in antibody reactivity (AU post-CHMI minus AU pre-CHMI) (D, F) were calculated for Dutch versus Tanzanian volunteers (C, D) and in Tanzanian volunteers classified as P. falciparum lysate seropositive or seronegative at baseline (E, F). Data are shown as whisker plots, with boxes indicating the median and IQR, and whiskers indicating the minimum/maximum values. Responses were compared using a Mann-Whitney U test.
Dutch but not Tanzanian volunteers show cellular recall responses induced by CHMI.
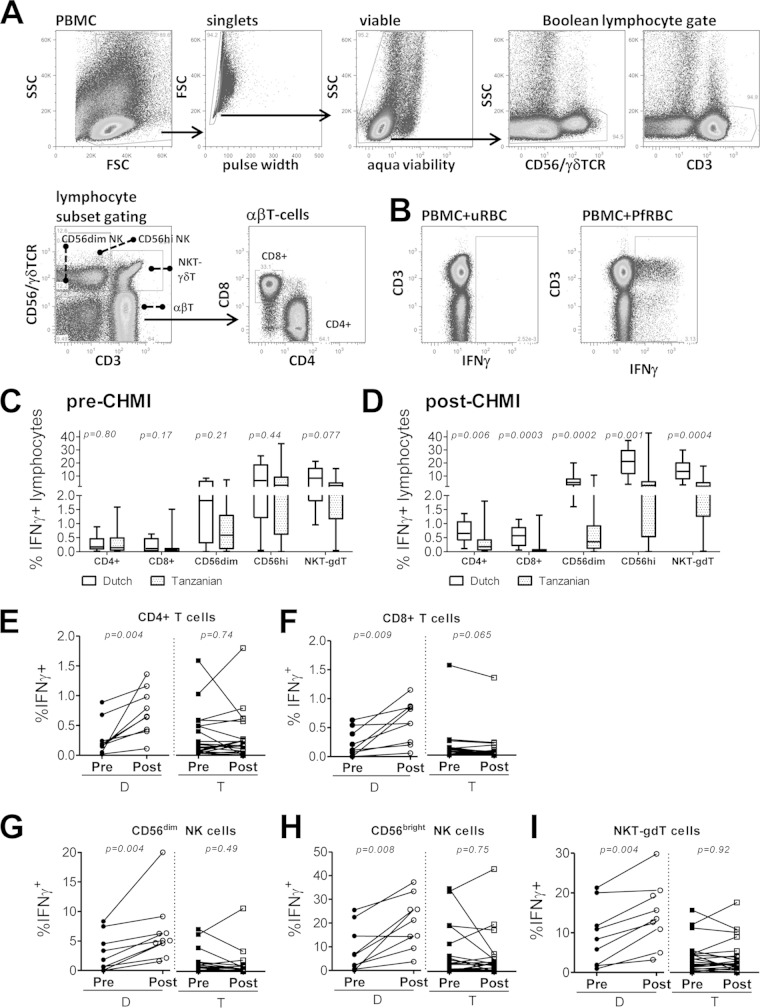
To analyze the acquisition of cellular responses after CHMI, we investigated in vitro IFN-γ responses upon incubation with PfRBC for 24 h (Fig. 4A and B). At baseline, P. falciparum-specific IFN-γ responses were comparable between Dutch and Tanzanians for all cell subsets except for NKT-γδT cells, which showed slightly higher responses in the Dutch (P = 0.077) (Fig. 4C). Post-CHMI, previously malaria-naive Dutch volunteers showed significant increases in P. falciparum-specific IFN-γ production by all lymphocyte subsets analyzed (CD4+, P = 0.004; CD8+, P = 0.009; CD56dim, P = 0.004; CD56bright NK, P = 0.008; NKT-γδT, P = 0.004) (Fig. 4E to I). In contrast, Tanzanian volunteers showed little or no increase in IFN-γ-producing cells (CD4+, P = 0.74; CD8+, P = 0.065; CD56dim, P = 0.49; CD56bright NK, P = 0.75; NKT-γδT, P = 0.92). In both cohorts and at both time points, CD56bright NK cells showed significantly higher IFN-γ responses than CD56dim NK cells (Dutch pre/post-CHMI, P = 0.004; Tanzanian pre/post-CHMI, P ≤ 0.0001). Significantly increased IFN-γ responses were found in both the effector (CD4+, P = 0.004; CD8+, P = 0.008) and central (CD4+, P = 0.004; CD8+, P = 0.012) memory T-cell compartments in the Dutch cohort, while the Tanzanians showed no such increase (see Fig. S3 in the supplemental material). As a result, post-CHMI IFN-γ responses in the Dutch were significantly higher for all cell subsets than in the Tanzanian cohort (Fig. 4D). Within the Tanzanian cohort, there was an overall trend for higher P. falciparum-specific pre-CHMI IFN-γ responses in those volunteers that had particularly short prepatency by qPCR (see Fig. S1 in the supplemental material). CD8+ T cells from Dutch (P = 0.04) but not Tanzanian (P = 0.47) volunteers showed increased proliferative responses post-CHMI (see Fig. S4A in the supplemental material). The proliferation of CD4+ T cells in response to PfRBC was not significantly altered post-CHMI in either cohort (P = 0.25 in Dutch and P = 0.11 in Tanzanians) (see Fig. S4B).
FIG 4.
Dutch but not Tanzanian volunteers show increased P. falciparum-specific in vitro IFN-γ production after CHMI. PBMCs collected pre- and post-CHMI from Dutch (D; circles; n = 9; post = 35 days after CHMI) and Tanzanian (T; squares; n = 21; post = 28 days after CHMI) volunteers were stimulated with PfRBC for 24 h. (A) After stimulation, cells were stained for surface expression of CD3, CD4, γδTCR, CD8, and CD56 to gate lymphocyte subsets. NKT and γδT were gated as a combined population. (B) Intracellular IFN-γ is shown for total lymphocytes after 24 h of uRBC or PfRBC stimulation. Graphs show the proportions of cells with P. falciparum-specific IFN-γ production, comparing Dutch and Tanzanian volunteers pre-CHMI (C) and post-CHMI (D), and comparing pre- and post-CHMI responses within each cohort for CD4+ T cells (E), CD8+ T cells (F), CD56dim NK cells (G), CD56bright NK cells (H), and NKT-γδT cells (I). For each volunteer, all time points were measured in a single experiment. Data are shown as whisker plots with boxes indicating the median with IQR and whiskers the minimum/maximum values (C and D) or individual data points with lines connecting the two time points for each volunteer (E to I). All responses were corrected for the background by subtracting responses to uRBC. Responses pre-CHMI and post-CHMI were compared using a Wilcoxon matched-pairs signed-rank test.
Analyzing the lymphocyte subset compositions in both cohorts, we found that they were largely stable between pre- and post-CHMI time points, with the exception of a prominent and significant post-CHMI increase of the NKT-γδT proportion in the Dutch volunteers and a clear decrease of CD56dim NK proportions in both cohorts. There was further no significant difference in the proportions of CD4+ CD45RO+ Foxp3+ T cells between the two cohorts, which could have explained differences in responsiveness (see Table S1 in the supplemental material). For the Tanzanian cohort, we observed a significant reduction of Foxp3+ T cells post-CHMI (P = 0.008), while for the Dutch volunteers there was no change (P = 0.44). Stimulation with the phorbol myristate acetate (PMA) and ionomycin mitogens showed that lymphocytes of both Dutch and Tanzanian volunteers were fully functional and able to produce IFN-γ at comparable levels both pre- and post-CHMI (see Fig. S4C).
Reduced innate IFN-γ responses in Tanzanian volunteers are associated with preexisting humoral immunity and thus potential preexposure.
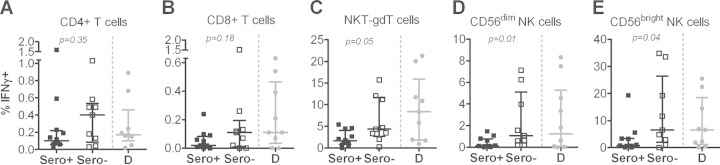
Finally, we analyzed whether the degree of preexposure reflected by differences in malaria-specific humoral immunity might affect cellular recall responses to PfRBC. Cells from P. falciparum lysate-seronegative Tanzanian volunteers had significantly higher responses to PfRBC (pre-CHMI) than those from seropositive Tanzanian volunteers (NKT-γδT, P = 0.05; CD56dim, P = 0.01; CD56bright NK, P = 0.04) and were of a magnitude comparable to those observed in the Dutch volunteers (Fig. 5). CD4+ and CD8+ T cells showed a similar pattern, although the difference between seropositive and seronegative Tanzanians did not reach statistical significance (Fig. 5A and B). Post-CHMI, these differences remained, and there was no increase in these responses in either of the two groups.
FIG 5.
P. falciparum-specific in vitro IFN-γ production by innate lymphocytes from Tanzanian volunteers is dependent on baseline serological status. PBMCs collected from Tanzanian volunteers who either had a positive P. falciparum (Sero+; n = 12; black squares) or negative P. falciparum (Sero−; n = 9; white squares) serology prior to challenge (baseline) were stimulated with PfRBC for 24 h and stained for intracellular IFN-γ. Panels show IFN-γ production by lymphocyte subsets: (A) CD4+ T cells; (B) CD8+ T cells; (C) NKT-γδT cells; (D) CD56dim; (E) CD56bright NK cells. Data are shown as median ± IQR of responses corrected for the background by subtracting responses to uRBC. Differences in responses between the seropositive and seronegative groups were analyzed by a Mann-Whitney U test. P. falciparum-specific IFN-γ responses of pre-CHMI PBMCs from malaria-naive Dutch volunteers (D; n = 9; gray circles), who had negative P. falciparum serology, are shown as a comparator.
DISCUSSION
In the present study, we performed a side-by-side comparison of antimalarial immune responses initiated or recalled by CHMI in two cohorts of young adults, from The Netherlands and Tanzania, with different malaria exposure histories and genetic backgrounds. Recruitment of Tanzanian volunteers took place in Dar es Salaam, an urban area where malaria is hypoendemic. Inclusion criteria into this CHMI trial were tailored to ensure minimal recent exposure to P. falciparum: Tanzanian volunteers had not experienced a clinical episode of malaria for 5 years, as self-reported, and were confirmed free of parasites by qPCR before CHMI took place. Nevertheless, more than 50% of the 21 Tanzanian volunteers had a positive P. falciparum lysate serology before CHMI based on P. falciparum lysate ELISA, which is a standard exclusion criterion in Dutch CHMI trials. In line with this, increased baseline antibody titers for the P. falciparum antigens CSP, LSA-1, EXP-1, and AMA-1 were found in the Tanzania cohort and particularly in those with positive serology for P. falciparum lysate. This clearly indicates previous exposure to the malaria parasite in this cohort. Asymptomatic infections, which can often occur in people living in areas with low malarial endemicity (34), in the 5 years of no self-reported clinical malaria preceding CHMI might have led to a maintenance of higher antibody responses.
As might be expected, preexisting antibodies to P. falciparum antigens appeared to have an effect on the outcome of CHMI. Tanzanians had a significantly longer prepatency based on qPCR detection than the previously malaria-naive Dutch. Furthermore, P. falciparum lysate-seropositive Tanzanians had a lower parasite load at the time of first detection by qPCR and at the time of the first peak of parasite load than seronegative Tanzanians. The first peak of blood-stage parasitemia can be used as a proxy for parasite liver load (16, 35). Our results might therefore indicate the emergence of fewer parasites from the liver and hence better control of either initiation or progression of liver-stage infection in preexposed individuals within the Tanzanian cohort and in Tanzanians than in the Dutch cohort. In line with such an effect of preexisting antisporozoite immunity would be the observation that those Tanzanian volunteers with a longer prepatancy by qPCR also had higher baseline antibody titers against the sporozoite antigen CSP. However, the first detection by qPCR in both the Dutch and Tanzanians after intradermal PfSPZ injection was uncharacteristically late compared to that for infection by mosquito bite routinely conducted in The Netherlands and elsewhere (11, 16, 18, 19, 23, 24, 35). This is likely due to less efficient liver-stage infection by this route and hence a low initial blood-stage load that reaches the qPCR detection limit only later. A lower first detectable parasitemia in seropositive Tanzanians than in seronegative Tanzanians might therefore additionally be attributed to the control of blood-stage replication prior to qPCR detection. Antibodies directed against AMA-1, for instance, are known to interfere with blood-stage multiplication (36) but can also confer protection against liver-stage infection (37). Since recognition of both cross-stage and liver-stage antigens was stronger in the Tanzanian cohort than in the Dutch, and particularly in the seropositive individuals, both possibilities remain open. Our data, however, support antibody control of blood-stage replication only during the initial phase of sub-qPCR-detectable parasitemia: while parasite multiplication based on PCR data could not be directly compared due to the high variability of the amplification dynamics (24), there was no difference between prepatency by TS and qPCR in the two Tanzanian groups.
Upon CHMI, Dutch volunteers showed a slight but significant induction of humoral responses against CSP, P. falciparum lysate, and EXP-1, while Tanzanians boosted responses to all antigens examined. Reactivity to the CSP sporozoite antigen is expected after PfSPZ inoculation and consistent with previous findings (38, 39). Increased antibody responses against P. falciparum lysate and EXP-1 likely reflect cross-stage reactivity between late-liver-stage and blood-stage merozoites, while exposure to developing liver stages (LSA-1) in naive volunteers appears insufficient to induce a detectable antibody response. Similarly, limited exposure to blood-stage-expressed AMA-1, due to early curative treatment, appears to prevent induction of detectable titers in naive volunteers, which is consistent with results even after multiple CHMIs (39). Consistent with preexposure, Tanzanians showed on the group level a greater increase in titers for P. falciparum lysate, AMA-1, and LSA-1 than the previously malaria-naive Dutch. Within the Tanzanian cohort, P. falciparum lysate-seronegative Tanzanians showed a similar absolute and accordingly much greater fold increase in antibody titers than their seropositive counterparts. At baseline, these volunteers had significantly lower responses to most parasite antigens than their seropositive counterparts and largely resembled the malaria-naive Dutch cohort. The fact that this group showed a greater increase in antibody titers than the Dutch strongly suggests that despite largely negative P. falciparum serology, these individuals have a stable P. falciparum-specific memory B-cell repertoire (40, 41). Given the same increase in absolute antibody titers, their memory B-cell repertoire appears to be of a magnitude similar to that of the P. falciparum lysate-seropositive Tanzanians, despite lower circulating plasma antibody levels.
Of note, humoral responses were assessed using a number of recombinant or synthetic proteins, which are not fully identical to the sequences of these proteins expressed by the NF54 strain used in both CHMI trials. While most antigens used in this study were of the 3D7 sequence and thus closely resemble the NF54 sequence, AMA-1 responses were assessed using the FVO strain sequence. AMA-1 is known for its extensive antigenic polymorphism and to cause strain-specific immunity (42, 43). Nevertheless, there is a significant antigenic overlap between AMA-1 alleles allowing for cross-reactivity across different strains, both in terms of functional activity and recognition, which affects mainly the magnitude of the response (42, 44–46). Based on these data, we consider it unlikely that assessment of AMA-1 responses using the NF54 or 3D7 sequence would have yielded different qualitative results. However, absolute titers would likely have been higher when using the AMA-1 sequence homologous to the CHMI strain.
Another potential confounder is the fact that the P. falciparum strains that Tanzanian volunteers were naturally exposed to prior to CHMI with P. falciparum NF54 are unknown. As any study examining preexposed individuals, analysis of antigen-specific responses against polymorphic antigens has therefore the additional limitation that it is difficult to match this unknown exposure history. This has to especially be taken into account when investigating CHMI-induced boosting of potential preexisting responses in this cohort, which might be masked by using antigens of different strain origins. However, despite the fact that Tanzanians likely experienced exposure to a variety of P. falciparum strains prior to CHMI, there was (i) a clear division into seropositive and seronegative individuals not just by total P. falciparum NF54 lysate but also by all individual antigens analyzed and (ii) a clearly stronger increase in antibody titers in Tanzanians than in malaria-naive Dutch volunteers to several antigens, including AMA-1. We cannot exclude, however, that this might have even been more pronounced when using antigens of different strain origins for analysis.
Although the Tanzanian volunteers in the present study showed evidence of humoral immune memory, parasite-specific IFN-γ production by adaptive T-cell subsets was not higher than in malaria-naive Dutch volunteers at baseline and remained unchanged after CHMI. In contrast, previously naive Dutch volunteers showed a significant increase in IFN-γ production by CD4 and CD8 T-cell subsets after a primary infection, consistent with what has been shown previously (47, 48). The lack of increased proliferative and Th1 responses 1 month after CHMI in Tanzanian volunteers could be partially due to immunosuppression following exposure to blood-stage parasites during CHMI. Such T-cell immunosuppression is well described for malaria (49–58) and, although usually resolved within 2 weeks (52, 53), can persist for more than 4 weeks (50). That Dutch volunteers are not equally affected by this might be due to the fact that such immunosuppressive effects appear to be more pronounced in immune than in nonimmune donors, as shown elsewhere (59). T regulatory cells (Tregs) are one potential mediator of suppressed IFN-γ production by adaptive cells. Increased Treg numbers during or after malaria are a well-reported phenomenon (60), and malaria parasites can enhance the suppressive activity of Tregs (61). While Dutch and Tanzanian volunteers had similar Treg proportions, we cannot exclude that Tregs in Tanzanian volunteers might be functionally more active, potentially due to past priming in malaria infections. That this apparent lack of a Th1 immune response in the Tanzanian cohort is malaria specific is supported by the fact Dutch and Tanzanian volunteers showed similar Th1 responses to mitogen stimulation both pre- and post-CHMI. Nevertheless, an additional influence of genetic background, which may explain differential P. falciparum-specific responses described in settings where malaria is endemic, cannot be excluded (62, 63).
NK cells are rapidly activated by malaria parasites, contribute to the early IFN-γ response during blood-stage infection (64), and can eliminate infected erythrocytes in vivo in a contact-dependent manner (65). Importantly, there is a functional dichotomy: the rarer CD56bright cells are more prominent in lymphatic tissues and are superior cytokine producers, while the CD56dim subset harbors a stronger cytotoxic potential (66–68). However, NK cells have usually been examined as one population, and the exact contributions of CD56dim and CD56bright NK cell subsets to malaria immunity thus remain to be established. An in vitro study on PBMCs from malaria-naive donors found greater IFN-γ production by CD56bright than by CD56dim NK cells only in response to cytokines but not upon PfRBC stimulation (69). However, consistent with their generally reported greater cytotoxic potential, only CD56dim cells showed degranulation upon PfRBC stimulation (69). Our findings that CD56bright NK cells show a greater IFN-γ response than CD56dim NK cells to PfRBC both before and after a primary malaria infection, as well as a memory-like effect of this response, are in line with findings from a previous CHMI trial (70). Tanzanian volunteers showed the same functional difference between the two NK cell subsets but no memory effect in either subset after CHMI. It was previously shown that depletion of αβ T cells abrogates memory-like IFN-γ responses of innate cells otherwise observed after CHMI (47, 70). Therefore, the absence of increased PfRBC-specific αβ T-cell responses post-CHMI in Tanzania volunteers might be one reason why P. falciparum-specific IFN-γ production by NK cells and other innate lymphocytes (NKT and γδ T cells) was also not increased post-CHMI. The fact that P. falciparum lysate-seronegative Tanzanians had higher innate IFN-γ responses than did seropositive Tanzanians already at baseline is a further indication that reduced Th1 responses are likely linked to the degree of previous malaria exposure. Of note, the proportion of CD56bright cells remained unaltered after CHMI, while the CD56dim subset had a smaller contribution to the PBMC compartment post-CHMI in both cohorts. It remains to be established whether this means that, in contrast to memory-like IFN-γ production, other NK cell functions, such as migration out of the circulation and engagement in antimalaria immune responses at other sites, such as the spleen, are unaffected and fully functional in preexposed individuals.
Within the Tanzanian cohort, there was no association of preexisting P. falciparum-specific IFN-γ responses by T-cell subsets or innate lymphocytes with the parasitological outcome of CHMI, i.e., prepatency or parasite load at first detection by qPCR. If at all, those with higher IFN-γ responses to blood-stage PfRBC had a shorter prepatency. This does not, however, exclude that cellular responses play a role in the prolonged prepatency of Tanzanian volunteers and specifically those with positive P. falciparum serology. One possible reason for the lack of such an association is that PfRBC were chosen as a stimulus for P. falciparum-specific responses. This was done due to the relatively large antigenic overlap between blood-stage and (late) liver-stage parasites (71, 72). However, responses to sporozoite and early-liver-stage antigens, which may be more relevant when assessing responses responsible for reducing the parasite load by targeting the earlier stages of infection, are likely to be missed using PfRBC as a stimulus. Moreover, our analysis was restricted to P. falciparum-specific IFN-γ production, and it is likely that other responses, for instance, degranulation as a proxy for cytotoxicity, may be more relevant readouts (71). Finally, the only accessible compartment for analysis of cellular responses in these human trials was peripheral blood. P. falciparum-specific responses, and particularly those responsible for reducing liver infection, might, however, be enriched or primarily located in other sites, for instance, in tissue-resident memory cells in the liver (73, 74). This might be even more pronounced in preexposed individuals, where such a tissue-resident memory population might have already been established.
Noteworthy, the differential immune responses described herein may explain why Tanzanian volunteers reported fewer clinical symptoms, such as fever, than their Dutch counterparts during CHMI after PfSPZ injection (24). On the one hand, preexisting antibody responses may reduce the initial parasite load and mediate antidisease immunity. Additionally, the relatively lower P. falciparum-specific cellular Th1 responses observed in the Tanzanian cohort than in the Dutch cohort might also be beneficial. Dutch volunteers preexposed to infected mosquito bites under chloroquine prophylaxis exhibited stronger IFN-γ production and earlier clinical symptoms when reexposed to blood-stage parasites than malaria-naive volunteers during their first infection (15). Thus, a shift away from Th1 responses in preexposed volunteers may make them less vulnerable to fever and other inflammation-induced symptoms.
In conclusion, our data show that previous malaria exposure is associated with some degree of parasite control during liver-stage or early-blood-stage infection after CHMI, humoral immune memory, and reduced antiparasite Th1 responses in the circulating lymphocyte compartment. Positive P. falciparum lysate serology can be used to identify individuals with better parasite control but weaker peripheral blood Th1 responses, which may help to stratify volunteers in future CHMI trials in areas where malaria is endemic. However, assessment of memory B-cell responses might be explored as a potentially better tool than serology to define preexposure per se. Important questions to be addressed in future studies include (i) which readouts other than Th1 responses should be used to determine immunization-induced cellular immunity and (ii) how differences in preexisting malaria-specific immune responses affect the outcome of whole parasite immunization and vaccination approaches in areas where malaria is endemic.
Supplementary Material
ACKNOWLEDGMENTS
We thank all the trial volunteers and the staff from the Radboud University Medical Center, the Ifakara Health Institute/Bagamoyo Research and Training Centre, Sanaria Inc., and the Swiss Tropical and Public Health Institute, all of whom made this study possible. We thank S. Singh (Gennova Biopharmaceuticals), D. Lanar, and G. Corradin for providing recombinant and synthetic CSP, LSA-1, and EXP-1 proteins, respectively. We thank M. van de Vegte-Bolmer, R. Siebelink-Stoter, and W. Graumans for culture and isolation of PfRBC.
S.L.H. is chief executive and scientific officer at Sanaria Inc. B.K.L.S., P.F.B., and E.R.J. are employees of Sanaria Inc., which manufactured and provided PfSPZ Challenge, and thus do have a potential conflict of interest. There are no other conflicts of interest for all other authors.
This work was supported by Top Institute (TI) Pharma (grant number T4-102), the FP7-founded European Virtual Institute of Malaria Research (EVIMalaR; grant agreement number 242095), the Tanzanian Commission on Science and Technology (COSTECH), the Ifakara Health Institute, and the Swiss Tropical Public Health Institute. A.S. received a long-term postdoctoral fellowship from the European Molecular Biology Organization (EMBO). The development and manufacturing of cryopreserved P. falciparum sporozoites (PfSPZ Challenge) were further supported by Small Business Innovation Research (SBIR) (grants R44AI058375-03, -04, -05, -05S1) from the National Institute of Allergy and Infectious Diseases at the National Institutes of Health (NIAID/NIH), USA, and through agreement number 07984 from the Program for Appropriate Technology in Health (PATH) Malaria Vaccine Initiative (with funds from the Bill and Melinda Gates Foundation). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
J.M.O., S.S., C.C.H., E.M.B., M.R, C.A.D., S.L.H., S.A., R.W.S., and A.S. designed the studies and experiments. S.S., E.M.B., and M.R. performed the clinical studies and collected clinical data. J.M.O., C.C.H., K.T., and A.S. conducted experiments. J.M.O., C.C.H., and A.S. analyzed the data. M.M., P.F.B., B.K.L.S., E.R.J., S.L.H., and A.S. collected/prepared/contributed vital reagents. J.M.O., C.C.H., R.W.S., and A.S. interpreted the data. J.M.O., C.C.H., R.W.S., and A.S. wrote the manuscript. All authors read and approved the final manuscript.
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/IAI.03069-14.
REFERENCES
- 1.WHO. 2013. World malaria report. WHO, Geneva, Switzerland. [Google Scholar]
- 2.Feachem RG, Phillips AA, Hwang J, Cotter C, Wielgosz B, Greenwood BM, Sabot O, Rodriguez MH, Abeyasinghe RR, Ghebreyesus TA, Snow RW. 2010. Shrinking the malaria map: progress and prospects. Lancet 376:1566–1578. doi: 10.1016/S0140-6736(10)61270-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mendis K, Rietveld A, Warsame M, Bosman A, Greenwood B, Wernsdorfer WH. 2009. From malaria control to eradication: the WHO perspective. Trop Med Int Health 14:802–809. doi: 10.1111/j.1365-3156.2009.02287.x. [DOI] [PubMed] [Google Scholar]
- 4.RTS,S Clinical Trials Partnership. 2014. Efficacy and safety of the RTS,S/AS01 malaria vaccine during 18 months after vaccination: a phase 3 randomized, controlled trial in children and young infants at 11 African sites. PLoS Med 11:e1001685. doi: 10.1371/journal.pmed.1001685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.RTS,S Clinical Trials Partnership, Agnandji ST, Lell B, Fernandes JF, Abossolo BP, Methogo BG, Kabwende AL, Adegnika AA, Mordmuller B, Issifou S, Kremsner PG, Sacarlal J, Aide P, Lanaspa M, Aponte JJ, Machevo S, Acacio S, Bulo H, Sigauque B, Macete E, Alonso P, Abdulla S, Salim N, Minja R, Mpina M, Ahmed S, Ali AM, Mtoro AT, Hamad AS, Mutani P, Tanner M, Tinto H, D'Alessandro U, Sorgho H, Valea I, Bihoun B, Guiraud I, Kabore B, Sombie O, Guiguemde RT, Ouedraogo JB, Hamel MJ, Kariuki S, Oneko M, Odero C, Otieno K, Awino N, McMorrow M, Muturi-Kioi V, Laserson KF, Slutsker L, et al. 2012. A phase 3 trial of RTS,S/AS01 malaria vaccine in African infants. N Engl J Med 367:2284–2295. doi: 10.1056/NEJMoa1208394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bejon P, White MT, Olotu A, Bojang K, Lusingu JPA, Salim N, Otsyula NN, Agnandji ST, Asante KP, Owusu-Agyei S, Abdulla S, Ghani AC. 2013. Efficacy of RTS,S malaria vaccines: individual-participant pooled analysis of phase 2 data. Lancet Infect Dis 13:319–327. doi: 10.1016/S1473-3099(13)70005-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bojang KA, Milligan PJM, Pinder M, Vigneron L, Alloueche A, Kester KE, Ballou WR, Conway DJ, Reece WHH, Gothard P, Yamuah L, Delchambre M, Voss G, Greenwood BM, Hill A, McAdam KPWJ, Tornieporth N, Cohen JD, Doherty T. 2001. Efficacy of RTS,S/AS02 malaria vaccine against Plasmodium falciparum infection in semiimmune adult men in The Gambia: a randomised trial. Lancet 358:1927–1934. doi: 10.1016/S0140-6736(01)06957-4. [DOI] [PubMed] [Google Scholar]
- 8.Struik SS, Riley EM. 2004. Does malaria suffer from lack of memory? Immunol Rev 201:268–290. doi: 10.1111/j.0105-2896.2004.00181.x. [DOI] [PubMed] [Google Scholar]
- 9.Doolan D, Dobaño C, Baird J. 2009. Acquired immunity to malaria. Clin Microbiol Rev 22:13. doi: 10.1128/CMR.00025-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sauerwein R, Roestenberg M, Moorthy V. 2011. Experimental human challenge infections can accelerate clinical malaria vaccine development. Nat Rev Immunol 11:57–64. doi: 10.1038/nri2902. [DOI] [PubMed] [Google Scholar]
- 11.Stoute JA, Slaoui M, Heppner DG, Momin P, Kester KE, Desmons P, Wellde BT, Garcon N, Krzych U, Marchand M. 1997. A preliminary evaluation of a recombinant circumsporozoite protein vaccine against Plasmodium falciparum malaria. RTS,S Malaria Vaccine Evaluation Group. N Engl J Med 336:86–91. [DOI] [PubMed] [Google Scholar]
- 12.Clyde D. 1975. Immunization of man against falciparum and vivax malaria by use of attenuated sporozoites. Am J Trop Med Hyg 24:397–401. [DOI] [PubMed] [Google Scholar]
- 13.Hoffman S, Goh L, Luke T, Schneider I, Le T, Doolan D, Sacci J, de la Vega P, Dowler M, Paul C, Gordon D, Stoute J, Church L, Sedegah M, Heppner D, Ballou W, Richie T. 2002. Protection of humans against malaria by immunization with radiation-attenuated Plasmodium falciparum sporozoites. J Infect Dis 185:1155–1164. doi: 10.1086/339409. [DOI] [PubMed] [Google Scholar]
- 14.Kester K, McKinney D, Tornieporth N, Ockenhouse C, Heppner D, Hall T, Krzych U, Delchambre M, Voss G, Dowler M, Palensky J, Wittes J, Cohen J, Ballou W, RTS,S Malaria Vaccine Evaluation Group. 2001. Efficacy of recombinant circumsporozoite protein vaccine regimens against experimental Plasmodium falciparum malaria. J Infect Dis 183:640–647. doi: 10.1086/318534. [DOI] [PubMed] [Google Scholar]
- 15.Bijker E, Bastiaens G, Teirlinck A, van Gemert GJ, Graumans W, van de Vegte-Bolmer M, Siebelink-Stoter R, Arens T, Teelen K, Nahrendorf W, Remarque E, Roeffen W, Jansens A, Zimmerman D, Vos M, van Schaijk B, Wiersma J, van der Ven AJ, de Mast Q, van Lieshout L, Verweij J, Hermsen C, Scholzen A, Sauerwein RW. 2013. Protection against malaria after immunization by chloroquine prophylaxis and sporozoites is mediated by preerythrocytic immunity. Proc Natl Acad Sci U S A 110:7862–7867. doi: 10.1073/pnas.1220360110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Roestenberg M, O'Hara GA, Duncan CJA, Epstein JE, Edwards NJ, Scholzen A, van der Ven AJAM, Hermsen CC, Hill AVS, Sauerwein RW. 2012. Comparison of clinical and parasitological data from controlled human malaria infection trials. PLoS One 7:e38434. doi: 10.1371/journal.pone.0038434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Engwerda CR, Minigo G, Amante FH, McCarthy JS. 2012. Experimentally induced blood stage malaria infection as a tool for clinical research. Trends Parasitol 28:515–521. doi: 10.1016/j.pt.2012.09.001. [DOI] [PubMed] [Google Scholar]
- 18.Lyke KE, Laurens M, Adams M, Billingsley PF, Richman A, Loyevsky M, Chakravarty S, Plowe CV, Sim BK, Edelman R, Hoffman SL. 2010. Plasmodium falciparum malaria challenge by the bite of aseptic Anopheles stephensi mosquitoes: results of a randomized infectivity trial. PLoS One 5:e13490. doi: 10.1371/journal.pone.0013490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hodgson SH, Ewer KJ, Bliss CM, Edwards NJ, Rampling T, Anagnostou NA, de Barra E, Havelock T, Bowyer G, Poulton ID, de Cassan S, Illingworth JJ, Douglas AD, Mange PB, Collins KA, Roberts R, Gerry S, Berrie E, Moyle S, Colloca S, Cortese R, Sinden RE, Gilbert SC, Bejon P, Lawrie AM, Nicosia A, Faust SN, Hill AV. 2015. Evaluation of the efficacy of ChAd63-MVA vectored vaccines expressing CS & ME-TRAP against controlled human malaria infection in malaria naive individuals. J Infect Dis 211:1076–1086. doi: 10.1093/infdis/jiu579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Roestenberg M, McCall M, Hopman J, Wiersma J, Luty A, van Gemert G, van de Vegte-Bolmer M, van Schaijk B, Teelen K, Arens T, Spaarman L, de Mast Q, Roeffen W, Snounou G, Rénia L, van der Ven A, Hermsen C, Sauerwein R. 2009. Protection against a malaria challenge by sporozoite inoculation. N Engl J Med 361:468–477. doi: 10.1056/NEJMoa0805832. [DOI] [PubMed] [Google Scholar]
- 21.Seder RA, Chang LJ, Enama ME, Zephir KL, Sarwar UN, Gordon IJ, Holman LA, James ER, Billingsley PF, Gunasekera A, Richman A, Chakravarty S, Manoj A, Velmurugan S, Li M, Ruben AJ, Li T, Eappen AG, Stafford RE, Plummer SH, Hendel CS, Novik L, Costner PJ, Mendoza FH, Saunders JG, Nason MC, Richardson JH, Murphy J, Davidson SA, Richie TL, Sedegah M, Sutamihardja A, Fahle GA, Lyke KE, Laurens MB, Roederer M, Tewari K, Epstein JE, Sim BK, Ledgerwood JE, Graham BS, Hoffman SL, VRC 312 Study Team. 2013. Protection against malaria by intravenous immunization with a nonreplicating sporozoite vaccine. Science 341:1359–1365. doi: 10.1126/science.1241800. [DOI] [PubMed] [Google Scholar]
- 22.Hoffman SL, Billingsley PF, James E, Richman A, Loyevsky M, Li T, Chakravarty S, Gunasekera A, Chattopadhyay R, Li M, Stafford R, Ahumada A, Epstein JE, Sedegah M, Reyes S, Richie TL, Lyke KE, Edelman R, Laurens MB, Plowe CV, Sim BK. 2010. Development of a metabolically active, non-replicating sporozoite vaccine to prevent Plasmodium falciparum malaria. Hum Vaccin 6:97–106. doi: 10.4161/hv.6.1.10396. [DOI] [PubMed] [Google Scholar]
- 23.Roestenberg M, Bijker E, Sim B, Billingsley P, James E, Bastiaens G, Teirlinck A, Scholzen A, Teelen K, Arens T, van der Ven A, Gunasekera A, Chakravarty S, Velmurugan S, Hermsen C, Sauerwein R, Hoffman S. 2013. Controlled human malaria infections by intradermal injection of cryopreserved Plasmodium falciparum sporozoites. Am J Trop Med Hyg 88:5–13. doi: 10.4269/ajtmh.2012.12-0613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shekalaghe S, Rutaihwa M, Billingsley PF, Chemba M, Daubenberger CA, James E, Mpina M, Ali Juma O, Schindler T, Huber E, Gunasekera A, Manoj A, Simon B, Savarino E, Church LW, Hermsen CC, Sauerwein RW, Plowe CV, Venkatesan M, Sasi P, Lweno O, Mutani P, Hamad A, Mohammed A, Urassa A, Mzee T, Padilla D, Ruben A, Lee Sim BK, Tanner M, Abdullah S, Hoffman SL. 2014. Controlled human malaria infection of Tanzanians by intradermal injection of aseptic, purified, cryopreserved Plasmodium falciparum sporozoites. Am J Trop Med Hyg 91:471–480. doi: 10.4269/ajtmh.14-0119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hermsen CC, Telgt DS, Linders EH, van de Locht LA, Eling WM, Mensink EJ, Sauerwein RW. 2001. Detection of Plasmodium falciparum malaria parasites in vivo by real-time quantitative PCR. Mol Biochem Parasitol 118:247–251. doi: 10.1016/S0166-6851(01)00379-6. [DOI] [PubMed] [Google Scholar]
- 26.Adegnika AA, Verweij JJ, Agnandji ST, Chai SK, Breitling LP, Ramharter M, Frolich M, Issifou S, Kremsner PG, Yazdanbakhsh M. 2006. Microscopic and sub-microscopic Plasmodium falciparum infection, but not inflammation caused by infection, is associated with low birth weight. Am J Trop Med Hyg 75:798–803. [PubMed] [Google Scholar]
- 27.Walliker D, Quakyi IA, Wellems TE, McCutchan TF, Szarfman A, London WT, Corcoran LM, Burkot TR, Carter R. 1987. Genetic analysis of the human malaria parasite Plasmodium falciparum. Science 236:1661–1666. doi: 10.1126/science.3299700. [DOI] [PubMed] [Google Scholar]
- 28.Hillier CJ, Ware LA, Barbosa A, Angov E, Lyon JA, Heppner DG, Lanar DE. 2005. Process development and analysis of liver-stage antigen 1, a preerythrocyte-stage protein-based vaccine for Plasmodium falciparum. Infect Immun 73:2109–2115. doi: 10.1128/IAI.73.4.2109-2115.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fidock DA, Gras-Masse H, Lepers JP, Brahimi K, Benmohamed L, Mellouk S, Guerin-Marchand C, Londono A, Raharimalala L, Meis JF, Langsley G, Roussilhon C, Tartar A, Druilhe P. 1994. Plasmodium falciparum liver stage antigen-1 is well conserved and contains potent B and T cell determinants. J Immunol 153:190–204. [PubMed] [Google Scholar]
- 30.Zhu J, Hollingdale MR. 1991. Structure of Plasmodium falciparum liver stage antigen-1. Mol Biochem Parasitol 48:223–226. doi: 10.1016/0166-6851(91)90117-O. [DOI] [PubMed] [Google Scholar]
- 31.Kocken C, Withers-Martinez C, Dubbeld M, van der Wel A, Hackett F, Valderrama A, Blackman M, Thomas A. 2002. High-level expression of the malaria blood-stage vaccine candidate Plasmodium falciparum apical membrane antigen 1 and induction of antibodies that inhibit erythrocyte invasion. Infect Immun 70:4471–4476. doi: 10.1128/IAI.70.8.4471-4476.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Faber B, Remarque E, Kocken C, Cheront P, Cingolani D, Xhonneux F, Jurado M, Haumont M, Jepsen S, Leroy O, Thomas A. 2008. Production, quality control, stability and pharmacotoxicity of cGMP-produced Plasmodium falciparum AMA1 FVO strain ectodomain expressed in Pichia pastoris. Vaccine 26:6143–6150. doi: 10.1016/j.vaccine.2008.08.055. [DOI] [PubMed] [Google Scholar]
- 33.Meraldi V, Nebié I, Moret R, Cuzin-Ouattara N, Thiocone A, Doumbo O, Esposito F, Traoré A, Corradin G, Terenzi S. 2002. Recognition of synthetic polypeptides corresponding to the N- and C-terminal fragments of Plasmodium falciparum Exp-1 by T-cells and plasma from human donors from African endemic areas. Parasite Immunol 24:141–150. doi: 10.1046/j.1365-3024.2002.00447.x. [DOI] [PubMed] [Google Scholar]
- 34.Bottius E, Guanzirolli A, Trape J-F, Rogier C, Konate L, Druilhe P. 1996. Malaria: even more chronic in nature than previously thought; evidence for subpatent parasitaemia detectable by the polymerase chain reaction. Trans R Soc Trop Med Hyg 90:15–19. doi: 10.1016/S0035-9203(96)90463-0. [DOI] [PubMed] [Google Scholar]
- 35.Roestenberg M, de Vlas SJ, Nieman AE, Sauerwein RW, Hermsen CC. 2012. Efficacy of preerythrocytic and blood-stage malaria vaccines can be assessed in small sporozoite challenge trials in human volunteers. J Infect Dis 206:319–323. doi: 10.1093/infdis/jis355. [DOI] [PubMed] [Google Scholar]
- 36.Arnot DE, Cavanagh DR, Remarque EJ, Creasey AM, Sowa MP, Morgan WD, Holder AA, Longacre S, Thomas AW. 2008. Comparative testing of six antigen-based malaria vaccine candidates directed toward merozoite-stage Plasmodium falciparum. Clin Vaccine Immunol 15:1345–1355. doi: 10.1128/CVI.00172-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schussek S, Trieu A, Apte SH, Sidney J, Sette A, Doolan DL. 2013. Immunization with apical membrane antigen 1 confers sterile infection-blocking immunity against Plasmodium sporozoite challenge in a rodent model. Infect Immun 81:3586–3599. doi: 10.1128/IAI.00544-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Felgner PL, Roestenberg M, Liang L, Hung C, Jain A, Pablo J, Nakajima-Sasaki R, Molina D, Teelen K, Hermsen CC, Sauerwein R. 2013. Pre-erythrocytic antibody profiles induced by controlled human malaria infections in healthy volunteers under chloroquine prophylaxis. Sci Rep 3:3549. doi: 10.1038/srep03549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nahrendorf W, Scholzen A, Bijker EM, Teirlinck AC, Bastiaens GJ, Schats R, Hermsen CC, Visser LG, Langhorne J, Sauerwein RW. 2014. Memory B-cell and antibody responses induced by Plasmodium falciparum sporozoite immunization. J Infect Dis 210:1981–1990. doi: 10.1093/infdis/jiu354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ndungu FM, Lundblom K, Rono J, Illingworth J, Eriksson S, Farnert A. 2013. Long-lived Plasmodium falciparum specific memory B cells in naturally exposed Swedish travelers. Eur J Immunol 43:2919–2929. doi: 10.1002/eji.201343630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ndungu FM, Olotu A, Mwacharo J, Nyonda M, Apfeld J, Mramba LK, Fegan GW, Bejon P, Marsh K. 2012. Memory B cells are a more reliable archive for historical antimalarial responses than plasma antibodies in no-longer exposed children. Proc Natl Acad Sci U S A 109:8247–8252. doi: 10.1073/pnas.1200472109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kennedy MC, Wang J, Zhang Y, Miles AP, Chitsaz F, Saul A, Long CA, Miller LH, Stowers AW. 2002. In vitro studies with recombinant Plasmodium falciparum apical membrane antigen 1 (AMA1): production and activity of an AMA1 vaccine and generation of a multiallelic response. Infect Immun 70:6948–6960. doi: 10.1128/IAI.70.12.6948-6960.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Remarque EJ, Faber BW, Kocken CH, Thomas AW. 2008. Apical membrane antigen 1: a malaria vaccine candidate in review. Trends Parasitol 24:74–84. doi: 10.1016/j.pt.2007.12.002. [DOI] [PubMed] [Google Scholar]
- 44.Drew DR, Hodder AN, Wilson DW, Foley M, Mueller I, Siba PM, Dent AE, Cowman AF, Beeson JG. 2012. Defining the antigenic diversity of Plasmodium falciparum apical membrane antigen 1 and the requirements for a multi-allele vaccine against malaria. PLoS One 7:e51023. doi: 10.1371/journal.pone.0051023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Terheggen U, Drew DR, Hodder AN, Cross NJ, Mugyenyi CK, Barry AE, Anders RF, Dutta S, Osier F, Elliott SR, Senn N, Stanisic DI, Marsh K, Siba PM, Mueller I, Richards JS, Beeson JG. 2014. Limited antigenic diversity of Plasmodium falciparum apical membrane antigen 1 supports the development of effective multi-allele vaccines. BMC Med 12:183. doi: 10.1186/s12916-014-0183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Remarque EJ, Faber BW, Kocken CH, Thomas AW. 2008. A diversity-covering approach to immunization with Plasmodium falciparum apical membrane antigen 1 induces broader allelic recognition and growth inhibition responses in rabbits. Infect Immun 76:2660–2670. doi: 10.1128/IAI.00170-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Teirlinck A, McCall MB, Roestenberg M, Scholzen A, Woestenenk R, de Mast Q, van der Ven AJ, Hermsen C, Luty A, Sauerwein R. 2011. Longevity and composition of cellular immune responses following experimental Plasmodium falciparum malaria infection in humans. PLoS Pathog 7:e1002389. doi: 10.1371/journal.ppat.1002389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Teirlinck A, Roestenberg M, van de Vegte-Bolmer M, Scholzen A, Heinrichs M, Siebelink-Stoter R, Graumans W, van Gemert GJ, Teelen K, Vos M, Nganou-Makamdop K, Borrmann S, Rozier Y, Erkens MA, Luty A, Hermsen C, Sim B, van Lieshout L, Hoffman S, Visser L, Sauerwein R. 2013. NF135.C10: a new Plasmodium falciparum clone for controlled human malaria infections. J Infect Dis 207:656–660. doi: 10.1093/infdis/jis725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Riley EM, Andersson G, Otoo LN, Jepsen S, Greenwood BM. 1988. Cellular immune responses to Plasmodium falciparum antigens in Gambian children during and after an acute attack of falciparum malaria. Clin Exp Immunol 73:17–22. [PMC free article] [PubMed] [Google Scholar]
- 50.Ho M, Webster HK, Looareesuwan S, Supanaranond W, Phillips RE, Chanthavanich P, Warrell DA. 1986. Antigen-specific immunosuppression in human malaria due to Plasmodium falciparum. J Infect Dis 153:763–771. doi: 10.1093/infdis/153.4.763. [DOI] [PubMed] [Google Scholar]
- 51.Chemtai AK, Okelo GB. 1989. Suppression of T-cell proliferative response in Plasmodium falciparum malaria patients—preliminary results. East Afr Med J 66:787–791. [PubMed] [Google Scholar]
- 52.Bygbjerg IC, Jepsen S, Theander TG. 1986. Lymphocyte response to purified Plasmodium falciparum antigens during and after malaria. Acta Trop 43:55–62. [PubMed] [Google Scholar]
- 53.Theander TG, Bygbjerg IC, Andersen BJ, Jepsen S, Kharazmi A, Odum N. 1986. Suppression of parasite-specific response in Plasmodium falciparum malaria. A longitudinal study of blood mononuclear cell proliferation and subset composition. Scand J Immunol 24:73–81. [DOI] [PubMed] [Google Scholar]
- 54.Williamson WA, Greenwood BM. 1978. Impairment of the immune response to vaccination after acute malaria. Lancet i:1328–1329. [DOI] [PubMed] [Google Scholar]
- 55.Mabey DC, Brown A, Greenwood BM. 1987. Plasmodium falciparum malaria and Salmonella infections in Gambian children. J Infect Dis 155:1319–1321. doi: 10.1093/infdis/155.6.1319. [DOI] [PubMed] [Google Scholar]
- 56.Gunapala DE, Facer CA, Davidson R, Weir WR. 1990. In vitro analysis of Epstein-Barr virus: host balance in patients with acute Plasmodium falciparum malaria. I. Defective T-cell control. Parasitol Res 76:531–535. [DOI] [PubMed] [Google Scholar]
- 57.Greenwood BM, Bradley-Moore AM, Bryceson AD, Palit A. 1972. Immunosuppression in children with malaria. Lancet i:169–172. [DOI] [PubMed] [Google Scholar]
- 58.Cook IF. 1985. Herpes zoster in children following malaria. J Trop Med Hyg 88:261–264. [PubMed] [Google Scholar]
- 59.Riley EM, Jobe O, Blackman M, Whittle HC, Greenwood BM. 1989. Plasmodium falciparum schizont sonic extracts suppress lymphoproliferative responses to mitogens and antigens in malaria-immune adults. Infect Immun 57:3181–3188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Scholzen A, Minigo G, Plebanski M. 2010. Heroes or villains? T regulatory cells in malaria infection. Trends Parasitol 26:16–25. doi: 10.1016/j.pt.2009.10.004. [DOI] [PubMed] [Google Scholar]
- 61.Minigo G, Woodberry T, Piera KA, Salwati E, Tjitra E, Kenangalem E, Price RN, Engwerda CR, Anstey NM, Plebanski M. 2009. Parasite-dependent expansion of TNF receptor II-positive regulatory T cells with enhanced suppressive activity in adults with severe malaria. PLoS Pathog 5:e1000402. doi: 10.1371/journal.ppat.1000402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.McCall MB, Hopman J, Daou M, Maiga B, Dara V, Ploemen I, Nganou-Makamdop K, Niangaly A, Tolo Y, Arama C, Bousema JT, van der Meer JW, van der Ven AJ, Troye-Blomberg M, Dolo A, Doumbo OK, Sauerwein RW. 2010. Early interferon-gamma response against Plasmodium falciparum correlates with interethnic differences in susceptibility to parasitemia between sympatric Fulani and Dogon in Mali. J Infect Dis 201:142–152. doi: 10.1086/648596. [DOI] [PubMed] [Google Scholar]
- 63.Torcia MG, Santarlasci V, Cosmi L, Clemente A, Maggi L, Mangano VD, Verra F, Bancone G, Nebie I, Sirima BS, Liotta F, Frosali F, Angeli R, Severini C, Sannella AR, Bonini P, Lucibello M, Maggi E, Garaci E, Coluzzi M, Cozzolino F, Annunziato F, Romagnani S, Modiano D. 2008. Functional deficit of T regulatory cells in Fulani, an ethnic group with low susceptibility to Plasmodium falciparum malaria. Proc Natl Acad Sci U S A 105:646–651. doi: 10.1073/pnas.0709969105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Inoue S, Niikura M, Mineo S, Kobayashi F. 2013. Roles of IFN-gamma and gammadelta T cells in protective immunity against blood-stage malaria. Front Immunol 4:258. doi: 10.3389/fimmu.2013.00258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Chen Q, Amaladoss A, Ye W, Liu M, Dummler S, Kong F, Wong LH, Loo HL, Loh E, Tan SQ, Tan TC, Chang KT, Dao M, Suresh S, Preiser PR, Chen J. 2014. Human natural killer cells control Plasmodium falciparum infection by eliminating infected red blood cells. Proc Natl Acad Sci U S A 111:1479–1484. doi: 10.1073/pnas.1323318111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Jacobs R, Hintzen G, Kemper A, Beul K, Kempf S, Behrens G, Sykora KW, Schmidt RE. 2001. CD56bright cells differ in their KIR repertoire and cytotoxic features from CD56dim NK cells. Eur J Immunol 31:3121–3127. doi:. [DOI] [PubMed] [Google Scholar]
- 67.Cooper MA, Fehniger TA, Caligiuri MA. 2001. The biology of human natural killer-cell subsets. Trends Immunol 22:633–640. doi: 10.1016/S1471-4906(01)02060-9. [DOI] [PubMed] [Google Scholar]
- 68.Poli A, Michel T, Theresine M, Andres E, Hentges F, Zimmer J. 2009. CD56bright natural killer (NK) cells: an important NK cell subset. Immunology 126:458–465. doi: 10.1111/j.1365-2567.2008.03027.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Korbel DS, Newman KC, Almeida CR, Davis DM, Riley EM. 2005. Heterogeneous human NK cell responses to Plasmodium falciparum-infected erythrocytes. J Immunol 175:7466–7473. doi: 10.4049/jimmunol.175.11.7466. [DOI] [PubMed] [Google Scholar]
- 70.McCall MB, Roestenberg M, Ploemen I, Teirlinck A, Hopman J, de Mast Q, Dolo A, Doumbo O, Luty A, van der Ven A, Hermsen C, Sauerwein R. 2010. Memory-like IFN-γ response by NK cells following malaria infection reveals the crucial role of T cells in NK cell activation by P. falciparum. Eur J Immunol 40:3472–3477. doi: 10.1002/eji.201040587. [DOI] [PubMed] [Google Scholar]
- 71.Bijker EM, Teirlinck AC, Schats R, van Gemert GJ, van de Vegte-Bolmer M, van Lieshout L, IntHout J, Hermsen CC, Scholzen A, Visser LG, Sauerwein RW. 2014. Cytotoxic markers associate with protection against malaria in human volunteers immunized with Plasmodium falciparum sporozoites. J Infect Dis 210:1605–1615. doi: 10.1093/infdis/jiu293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Tarun AS, Peng X, Dumpit RF, Ogata Y, Silva-Rivera H, Camargo N, Daly TM, Bergman LW, Kappe SH. 2008. A combined transcriptome and proteome survey of malaria parasite liver stages. Proc Natl Acad Sci U S A 105:305–310. doi: 10.1073/pnas.0710780104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Nganou-Makamdop K, van Gemert GJ, Arens T, Hermsen CC, Sauerwein RW. 2012. Long term protection after immunization with P. berghei sporozoites correlates with sustained IFNgamma responses of hepatic CD8+ memory T cells. PLoS One 7:e36508. doi: 10.1371/journal.pone.0036508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Tse SW, Cockburn IA, Zhang H, Scott AL, Zavala F. 2013. Unique transcriptional profile of liver-resident memory CD8+ T cells induced by immunization with malaria sporozoites. Genes Immun 14:302–309. doi: 10.1038/gene.2013.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.