Abstract
Th17 cells constitute a subset of CD4+ T lymphocytes that play a crucial role in protection against extracellular bacteria and fungi. They are also associated with tissue injury in autoimmune and inflammatory diseases. Here, we report that serpin from the tick Ixodes ricinus, IRS-2, inhibits Th17 differentiation by impairment of the interleukin-6 (IL-6)/STAT-3 signaling pathway. Following activation, mature dendritic cells produce an array of cytokines, including the pleiotropic cytokine IL-6, which triggers the IL-6 signaling pathway. The major transcription factor activated by IL-6 is STAT-3. We show that IRS-2 selectively inhibits production of IL-6 in dendritic cells stimulated with Borrelia spirochetes, which leads to attenuated STAT-3 phosphorylation and finally to impaired Th17 differentiation. The results presented extend the knowledge about the effect of tick salivary serpins on innate immunity cells and their function in driving adaptive immune responses.
INTRODUCTION
Ticks are bloodsucking arthropods, major vectors of human pathogens like Borrelia burgdorferi and tick-borne encephalitis virus. Ticks from the family Ixodidae (hard ticks) require several days to fully engorge. During feeding, ixodid ticks remain tightly attached to their host (1, 2). To avoid attack from the host immune system during the feeding period, tick saliva contains two groups of molecules, the first with antihemostatic and the second with immunomodulatory properties. These groups include both proteinaceous and nonprotein molecules (3). One group of immunomodulatory proteins is represented by serine proteinase inhibitors (serpins), a large superfamily of structurally related, but functionally diverse, proteins that control essential proteolytic pathways (4, 5). Recently, three serine protease inhibitors, namely, purified human urinary trypsin inhibitor (UTI) and two synthetic serpins, gabextate mesilate (FOY) and nafamostat mesilate (FUT), which are widely used in treatment of acute inflammatory disorders, such as disseminated intravascular coagulation (DIC), have been shown to attenuate allergic airway inflammation and remodeling in a murine model of chronic asthma. These effects were associated with inhibition of Th2 cytokines (interleukin-4 [IL-4], IL-5, IL-6, and IL-13) and Th17 cell functions. These serpins also inhibited NF-κB activation in lung tissues (6).
Until now, more than 60 serpins have been identified at the sequence level in ixodid ticks, but only two serpins from Ixodes ricinus have been further functionally characterized (7–9). The first known I. ricinus serpin, Iris (I. ricinus immunosuppressor), is known to preferentially target leukocyte elastase. It also interferes with the contact phase coagulation pathway, fibrinolysis, and disrupts platelet adhesion. Moreover, Iris has the ability to modulate both innate and adaptive immunity. It affects T lymphocyte and macrophage responsiveness, and it induces a Th2-type response and inhibits the production of proinflammatory cytokines. Interestingly, it was shown that the anti-inflammatory properties of the protein are independent of its proteolytic activity and are mediated through its exosite domain (10–13).
IRS-2, the second described serpin from I. ricinus, targets cathepsin G and chymase. Both enzymes are part of the acute inflammatory response and are produced by activated neutrophils (cathepsin G) and mast cells (chymase). Moreover, IRS-2 is able to inhibit swelling and the migration of neutrophils into the inflamed tissue (14). The effects of IRS-2 on other cells of innate and acquired immunity have not been described so far.
Dendritic cells (DCs) are known as antigen-presenting cells and play a critical role in initiating and modulating the immune response. With their ability to recognize, process, and present antigens on their surfaces and thus activate T lymphocytes, DCs form a unique link between innate and acquired immunity (15, 16). Depending upon the recognized pathogens and other stimuli produced by activated DCs, such as cytokines and chemokines, T lymphocytes differentiate into cytotoxic CD8+ or helper CD4+ cells, which can further differentiate into various subsets (17). The IL-6/STAT-3 signaling pathway leads to differentiation of CD4+ T lymphocytes into the Th17 subset. IL-6, a pleiotropic cytokine produced by dendritic cells in response to invading pathogens, binds to IL-6 receptors on T cells and activates the signaling pathway, leading to phosphorylation of the transcription factor STAT-3, an essential molecule for Th17 differentiation (18, 19). Th17 cells participate in host defense against extracellular bacteria and fungi by mediating the recruitment of neutrophils and macrophages into infected tissues. It is also known that regulation of Th17 cells plays a significant role in the pathogenesis of various inflammatory and autoimmune disorders (20–22). Moreover, it was shown that Th17 cells are involved in the development of severe destructive arthritis caused by the Lyme disease spirochete B. burgdorferi (23).
The objective of this study was to analyze the effect of a tick salivary serpin on dendritic cells and its consequences for the development of proinflammatory cells, like Th17 lymphocytes.
MATERIALS AND METHODS
Experimental animals.
Specific-pathogen-free C57BL/6 mice (6- to 10-week-old females) were purchased from Charles River Laboratories. The animals were maintained under standard conditions in the animal house facility of the Institute of Parasitology, Biology Centre AS CR, České Budějovice. All experiments were performed with permission of the Czech animal ethics committee.
Recombinant IRS-2.
Recombinant serpin from I. ricinus, IRS-2, was overexpressed in Escherichia coli BL21(DE3) pLysS cells. The expressed protein accumulated in inclusion bodies, which were separated. Refolded and concentrated IRS-2 was purified using a standard chromatographic method (fast protein liquid chromatography [FPLC]) (14, 24). Lipopolysaccharide (LPS) contamination was removed by Arvys Proteins Company using the detergent-based method.
Bacteria.
B. burgdorferi sensu stricto ATCC 35211 isolated from I. ricinus was grown in Barbour-Stoenner-Kelly-H (BSK-H) medium (Sigma-Aldrich) supplemented with 6% rabbit serum at 34°C. The number of spirochetes was calculated by dark-field microscopy according to the method of Magnuson et al. (25). The fourth to sixth passages were used in the experiments.
Splenic DC isolation.
Isolated mouse spleens were minced with scissors, digested in RPMI containing 0.25 mg/ml Liberase DL (Roche) and 0.2 mg/ml DNase I (Roche) at 37°C for 30 min, and passed through a 70-μm nylon cell strainer (BD Falcon). The dendritic cells were isolated using magnetic beads conjugated with anti-CD11c antibody (Ab) and magnetically activated cell sorting (MACS) column separation following the manufacturer's instructions (Miltenyi Biotec). The purified dendritic cells were cultured in RPMI 1640 medium supplemented with 10% heat-inactivated fetal calf serum (FCS), 50 μM 2-mercaptoethanol, 100 μg/ml penicillin, and 100 U/ml streptomycin (all from Sigma-Aldrich). The purity of the isolated dendritic cells (∼90% CD11c+ cells) was determined by subsequent fluorescence-activated cell sorter (FACS) analysis.
CD4+ T cell isolation.
The fourth day after subcutaneous infection of mice with 1 × 105 Borrelia spirochetes, isolated mouse spleens were passed through a 70-μm nylon cell strainer (BD Falcon), and CD4+ T cells were isolated using magnetic beads conjugated with anti-CD4 Ab and MACS column separation following the manufacturer's instructions (Miltenyi Biotec). Purified CD4+ T cells were cultured in RPMI 1640 supplemented with 10% heat-inactivated fetal calf serum (FCS), 50 μM 2-mercaptoethanol, 100 μg/ml penicillin, and 100 U/ml streptomycin (all from Sigma-Aldrich). The purity of the isolated CD4+ T cells (≥90% CD4+ CD62Lhigh) was determined by FACS analysis.
Specific activation of CD4+ T lymphocytes.
Purified splenic DCs were seeded at 5 × 104 cells per well in 96-well plates and stimulated with Borrelia spirochetes (5 × 105 per well) and IRS-2 (6 μM). After 24 h incubation, the medium was removed, and 3 × 105 freshly isolated Borrelia-primed CD4+ T lymphocytes in 200 μl of culture medium were added to each well. The T cells were incubated with DCs for 3 days before restimulation with phorbol myristate acetate (PMA) (20 ng/ml) and ionomycin (1 μM) (both Sigma-Aldrich). Cell-free culture supernatants for IL-17 and IL-9 assessment were harvested at 2, 6, 12, 24, and 48 h after restimulation.
To determine the number of IL-17-producing Th cells, Borrelia-exposed DCs and Borrelia-primed CD4+ T cells were cocultured as described above. On day 5 of coculture, the cells were restimulated with PMA and ionomycin and, after an additional 2 h, treated with monensin (2 μM; eBiosciences). The cells were then incubated for 4 h before staining was performed with anti-IL-17 antibody conjugated with phycoerythrin (PE) (eBioscience).
Cytokine measurement.
Freshly isolated dendritic cells were seeded at 2 × 105 cells per well on 96-well plates. The next day, the DCs were stimulated with B. burgdorferi spirochetes at a multiplicity of infection (MOI) of 10 (2 × 106 per well) in the presence or absence of IRS-2 (6 μM). Cell-free culture supernatants were harvested 2, 9, 12, 24, or 48 h after stimulation and used for detection of IL-1β, IL-6, IL-10, and tumor necrosis factor alpha (TNF-α) with Ready-Set-Go! enzyme-linked immunosorbent assay (ELISA) kits (eBioscience) following the manufacturer's instructions. Cell-free culture supernatants for IL-17 and IL-9 assessment were prepared as described in “Specific activation of CD4+ T lymphocytes” above, and the amount of cytokines was measured with a Ready-Set-Go! ELISA kit (eBioscience) following the manufacturer's instructions. All reactions were performed in triplicate.
RNA extraction, quantitative real-time PCR, and mRNA half-life determination.
To assess relative mRNA expression, DCs were seeded at 2 × 106 cells per well in 24-well plates. The next day, the DCs were stimulated with Borrelia spirochetes at an MOI of 10 (2 × 107 per well) in the presence or absence of IRS-2 (6 μM) and incubated for 6 or 12 h. RNA was then isolated with the Nucleospin RNA II kit (Macherey-Nagel) following the manufacturer's instructions. The quality and concentration of the isolated RNA were assessed by measurement on a Nanophotometer P-330 (Implen). cDNA was synthesized with the High-Capacity RNA-to-cDNA kit (Applied Biosystems). Real-time PCR analysis was performed with a TaqMan gene expression set (Applied Biosystems) containing primers and probe specific for IL-6 and β-actin using a Rotor Gene 3000 and Rotor-Gene 6.0.19 software (Corbett Research). The relative expression of IL-6 mRNA was determined by the comparative threshold cycle (CT) method (26), where the mouse β-actin gene was used as a housekeeping gene (Applied Biosystems). All reactions were performed in triplicate.
Immunoblotting.
Freshly isolated dendritic cells were seeded at 1 × 106 cells per well in 24-well plates. The next day, the DCs were stimulated with Borrelia spirochetes at an MOI of 10 (1 × 107 per well) in the presence or absence of IRS-2 (6 μM). Following stimulation (15, 30, and 60 min for C/EBP, phosphorylated NF-κB [p-NF-κB], p-CREB, p-p-38, and p-ERK1/2 and 6 and 16 h for p-STAT-3), the cells were lysed in a modified RIPA buffer (1% Nonidet P-40, 0.25% sodium deoxycholate, 1 mM EGTA, 150 mM NaCl, and 50 mM Tris-HCl, pH 7.5) in the presence of protease and phosphatase inhibitors (10 μg/ml aprotinin, 1 μg/ml leupeptin, 1 mM phenylmethylsulfonyl fluoride [PMSF], 1 μg/ml pepstatin, 25 mM NaF, and 2 mM NaVO3). The protein extracts, mixed with Laemmli sample buffer, were separated by SDS-PAGE and transferred to Immobilon-P membranes. Following blocking in Tris-buffered saline (TBS)-containing 5% fat-free milk, the blots were incubated overnight with the antibodies against C/EBP, phospho-STAT-3 (Tyr705), phospho-NF-κB (Ser536), phospho-CREB (Ser133), phospho-p38 (Thr180), and phospho-ERK1/2 (Thr202) (all from Cell Signaling) and GAPDH (glyceraldehyde-3-phosphate dehydrogenase) or β-actin (Santa Cruz Biotechnology). The proteins were visualized using enhanced chemiluminescence (Pierce), and their abundances were analyzed using a charge-coupled-device (CCD) imaging system (ChemiDoc MP Imaging System) and Image Lab software v. 4.1 (Bio-Rad).
To assess the level of phosphorylated STAT-3 in T lymphocytes, freshly isolated dendritic cells were seeded at 1. 5 × 105 per well in a 96-well plate. After 6 h, the DCs were stimulated with Borrelia spirochetes (1 × 107 per well) in the presence or absence of IRS-2 (6 μM). The next day, cell-free culture supernatants were harvested and added to freshly isolated Borrelia-primed CD4+ T lymphocytes (9 × 105 per well). Following stimulation (15 and 30 min), the cells were lysed, and Western blotting was performed as described above with phospho-STAT-3 (Tyr705) and β-actin antibodies.
Flow cytometry.
CD4+ cells were prepared and stimulated as described in “Specific activation of CD4+ T lymphocytes” above. After 4 h of restimulation with PMA, ionomycin, and monensin, the cells were harvested (using cold 5 mM EDTA in PBS) and stained with anti-CD4 antibody (conjugated with allophycocyanin [APC]; eBioscience). After washing, the cells were fixed and permeabilized with a Foxp3/transcription factor staining buffer set (eBioscience) and labeled with anti-IL-17A antibody (conjugated with PE; eBioscience). The prepared cells were resuspended in cold PBS with 1% FCS. Flow cytometry was performed on a FACSCanto II cytometer using FACS Diva software v. 5.0 (BD Biosciences).
Statistical analysis.
One-way analysis of variance (ANOVA) followed by a Bonferroni test in GraphPad Prism, version 5.0, was used to compare the differences between control and treated groups. A P value of ≤0.05 was considered statistically significant.
RESULTS
IRS-2 selectively inhibits IL-6 production by DCs upon stimulation with Borrelia spirochetes.
Cytokines produced by activated DCs play a key role in shifting the immune response toward particular Th subsets. To investigate the effects of IRS-2 on the production of different pro- and anti-inflammatory cytokines by DCs, immature DCs were stimulated with Borrelia spirochetes in the presence or absence of IRS-2 for 9, 12, 24, or 48 h, and the production of IL-1β, IL-6, IL-10, and TNF-α was measured.
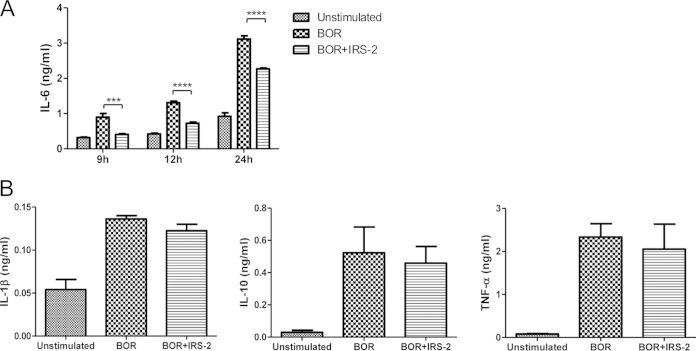
Serpin significantly inhibited the production of IL-6 in DCs (Fig. 1A), whereas the production of other cytokines remained unaltered (Fig. 1B). The same inhibitory effect of IRS-2 on the production of IL-6 was also observed in the PMJ2-R cell line (macrophages) and primary neutrophils (data not shown).
FIG 1.
IRS-2 selectively inhibits IL-6 production by DCs. Splenic dendritic cells were stimulated with Borrelia spirochetes (BOR) (10 spirochetes per cell) in the presence or absence of IRS-2 (6 μM). (A) Culture supernatants were harvested 9, 12, and 24 h after stimulation, and the amount of IL-6 was determined by ELISA. Two independent experiments were performed, and data from a representative experiment are shown. The data are expressed as the mean cytokine concentrations from three wells plus standard errors of the mean (SEM). *** and ****, effects of IRS-2 on IL-6 production were significant at P values of <0.001 and <0.0001, respectively. (B) Culture supernatants were harvested 24 h (TNF-α) or 48 h (IL-1β and IL-10) after stimulation, and the presence of cytokines was detected by ELISA. Three independent experiments were performed, and the data were pooled. The data are expressed as the mean cytokine concentrations from nine wells plus SEM.
IRS-2 inhibits IL-6 production at the level of mRNA.
Gene expression can be regulated by many mechanisms at many stages, including chromatin accessibility, transcription activation, mRNA nuclear export, mRNA decay, and translation. To understand the mechanism of IL-6 decline caused by IRS-2, the expression of the IL-6 gene was measured in DCs activated with Borrelia spirochetes in the presence or absence of IRS-2. mRNA specific for IL-6 was determined by quantitative RT-PCR. As shown in Fig. 2, the IL-6 transcript level was slightly increased after as little as 6 h (no significant effect of IRS-2 was observed). However, the mRNA of IL-6 was severely suppressed by IRS-2 at a later time point (12 h). We concluded that a decline in IL-6 production is the result of impaired gene expression in IRS-2-exposed cells after activation with Borrelia spirochetes (Fig. 2).
FIG 2.
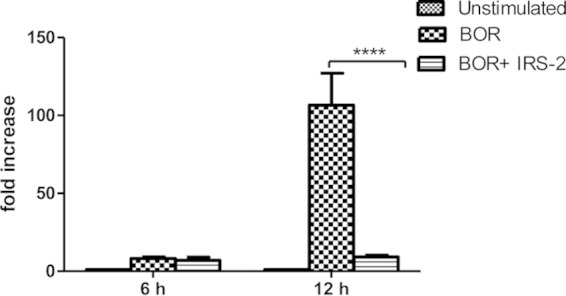
IRS-2 inhibits IL-6 production at the level of mRNA expression. Splenic dendritic cells were stimulated with Borrelia spirochetes (10 spirochetes per cell) in the presence or absence of IRS-2 (6 μM). The transcript level of IL-6 was determined by quantitative PCR (qPCR) using specific primers for IL-6. The gene expression of IL-6 was normalized to the β-actin transcript. Two independent experiments were performed, and the data were pooled. The data are expressed as the average fold IL-6 mRNA increase (plus SEM) from six wells compared with the control. ****, the effect of IRS-2 on the relative expression of IL-6 mRNA was significant at a P value of <0.0001.
Decreased stability of IL-6 mRNA is often responsible for a decline in IL-6 production. Moreover, inhibition of IL-6 production due to increased IL-6 mRNA decay was observed with another tick salivary protein (27). Therefore, we investigated whether the same mechanism could be responsible for the IRS-2-induced effect. Splenic DCs were stimulated with Borrelia spirochetes in the presence or absence of IRS-2. After 9 h, actinomycin D was added to block mRNA synthesis, cells were harvested (after 1 and 2 h), and mRNA decay was determined. The IL-6 mRNA half-life observed in the presence of IRS-2 was comparable to that of control cells stimulated only with Borrelia spirochetes (data not shown). This result suggests that the impaired gene expression of IL-6 is not due to impaired stability of IL-6 mRNA.
In our effort to reveal the mechanism of the IRS-2 effect on IL-6, we further tested whether signaling pathways leading to induction of IL-6 are affected by IRS-2.
Gene expression of IL-6 is controlled by several transcription factors and signaling molecules, including NF-κB, C/EBP, CREB, and kinases p38 and ERK1/2 (28–32); therefore, the phosphorylation of these molecules was tested. DCs were stimulated with Borrelia in the presence or absence of IRS-2 for 15, 30, and 60 min. After stimulation, cell lysates were prepared and analyzed by immunoblotting. The phosphorylation of none of these signaling molecules was inhibited by IRS-2, so we concluded that induction of the IL-6 gene is intact and does not seem to be responsible for decreased IL-6 transcript expression (data not shown).
IRS-2 impairs Th17 differentiation via inhibition of the IL-6/STAT-3 signaling pathway.
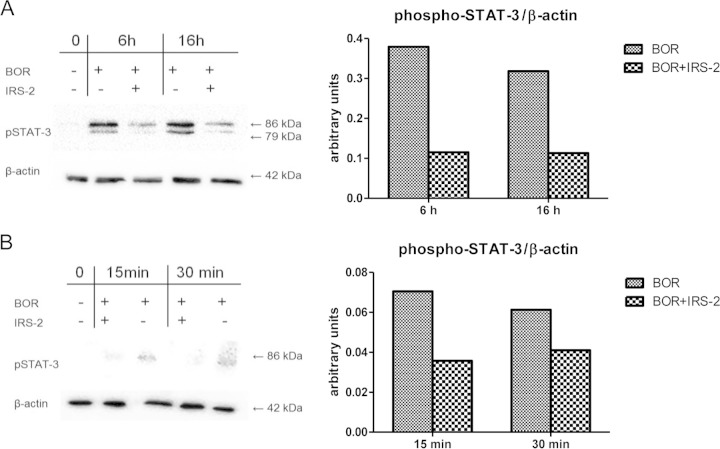
It is well known that the major transcription factor activated by IL-6 is STAT-3. STAT-3 phosphorylation is mediated through the association of IL-6 with the IL-6 receptor (IL-6R) and the signal transducer glycoprotein 130 (gp130), followed by subsequent activation of Janus kinases (19). Since the production of IL-6 in DCs was strongly inhibited by IRS-2, we expected that the phosphorylation of the STAT-3 signaling molecule would be decreased. DCs were activated with Borrelia spirochetes in the presence or absence of IRS-2, and the level of phosphorylated STAT-3 molecules was determined 6 and 16 h after activation. Indeed, a marked decrease of phospho-STAT-3 was observed (Fig. 3A). Borrelia-primed T lymphocytes were activated with supernatants from DCs (stimulated for 24 h with Borrelia spirochetes in the presence or absence of IRS-2), and the phosphorylation of STAT-3 in T lymphocytes was also decreased, likely due to diminished production of IL-6 by DCs (Fig. 3B).
FIG 3.
IRS-2 inhibits STAT-3 molecule phosphorylation. (A) Splenic dendritic cells (sDCs) were stimulated with Borrelia spirochetes (10 spirochetes per cell) in the presence or absence of IRS-2 (6 μM). Six and 16 h after stimulation, cell lysates were prepared and analyzed by immunoblotting with anti-phospho-STAT-3. The membranes were reprobed with antibody against β-actin. The phosphorylation of STAT-3 was quantified using chemiluminescence and normalized to the β-actin protein level. The two bands represent different isoforms (α and β) of pSTAT-3 that are present in DCs. (B) Freshly isolated Borrelia-primed CD4+ T cells were stimulated with 24-h supernatants from sDCs stimulated with Borrelia spirochetes (10 spirochetes per cell) in the presence or absence of IRS-2 (6 μM). Fifteen and 30 min after stimulation, cell lysates were prepared and analyzed by immunoblotting with anti-phospho-STAT-3. The membranes were reprobed with antibody against β-actin. The phosphorylation of STAT-3 was quantified using chemiluminescence and normalized to the β-actin protein level.
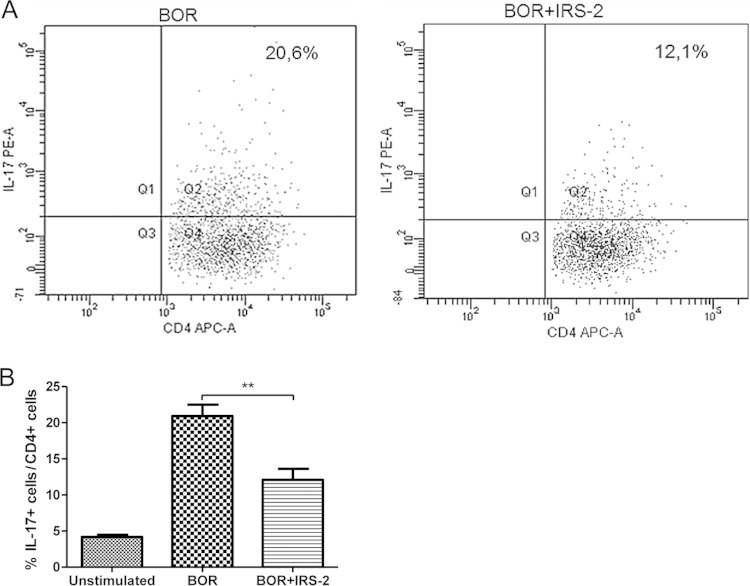
The IL-6/STAT-3 signaling pathway is known to be crucial for development of the Th17 subset (18, 20). The main effector cytokines produced by Th17 cells are IL-17 (IL-17A), which is a hallmark of the subpopulation; IL-21; IL-22; and IL-9 (22, 33, 34). We predicted that the inhibition of IL-6/STAT-3 signaling by IRS-2 could lead to impaired Th17 differentiation, and therefore, the number of Th17-producing cells and the amounts of IL-17 and IL-9 were determined. Splenic DCs were stimulated with Borrelia spirochetes in the presence or absence of IRS-2. After 24 h, freshly isolated Borrelia-primed CD4+ T cells were added to the cultured DCs, and the cells were cocultured for the next 5 days. Afterward, the cells were restimulated with PMA and ionomycin. To detect the Th17 subset, intracellular staining for IL-17A was performed, and the cells were analyzed by flow cytometry. To block cellular transport, monensin was added to the restimulated cells. As seen in Fig. 4A and B, the number of IL-17-producing CD4+ T cells was significantly decreased by IRS-2.
FIG 4.
IRS-2 reduces the number of IL-17-producing CD4+ T cells. Splenic dendritic cells were stimulated with Borrelia spirochetes (10 spirochetes per cell) in the presence or absence of IRS-2 (6 μM). After 24 h, freshly isolated Borrelia-primed CD4+ T cells were added to the cultured DCs, and the cells were cocultured for 5 days. Then, T lymphocytes were restimulated with PMA and ionomycin, treated with monensin, and stained for IL-17. (A) Flow cytometry dot plots of T lymphocytes treated with DCs stimulated with Borrelia in the presence or absence of IRS-2. Quadrant 2 (Q2) shows CD4+ IL-17+ cells. (B) The percentage of IL-17-producing cells was determined in live CD4+ cells. The data are expressed as the mean percentages of CD4+ IL-17+ cells from triplicate wells plus SEM. **, the effect of IRS-2 on the presence of IL-17-producing cells was significant at a P value of <0.01.
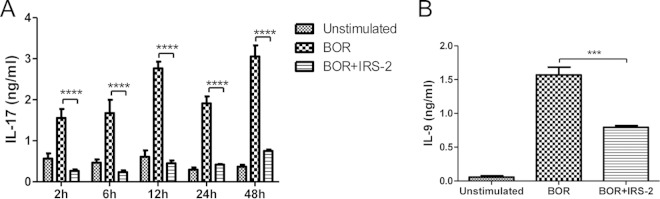
To measure the production of IL-17 and IL-9 cytokines, the coculture of DCs and Borrelia-primed CD4+ T cells lasted 3 days before restimulation with PMA and ionomycin. The supernatants were then collected at various time points and analyzed. The levels of both IL-17 and IL-9 were significantly decreased in the presence of IRS-2 (Fig. 5A and B). The reduced levels of the measured cytokines, together with the decreased number of IL-17-producing CD4+ T cells, in the presence of IRS-2 clearly indicate that IRS-2 inhibits Th17 differentiation.
FIG 5.
IRS-2 reduces levels of Th17 cytokines. Splenic dendritic cells were stimulated with Borrelia spirochetes (10 spirochetes per cell) in the presence or absence of IRS-2 (6 μM). After 24 h, freshly isolated Borrelia-primed CD4+ T cells were added to the cultured DCs, and the cells were cocultured for the next 3 days. Afterward, the cells were restimulated with PMA and ionomycin, and the production of cytokines was analyzed at various time points. (A) Cell supernatants for IL-17 assessment were harvested 2, 6, 12, 24, and 48 h after restimulation and analyzed by ELISA. The data are expressed as the mean cytokine concentrations from triplicate wells plus SEM. Two independent experiments were performed, and data from a representative experiment are shown. ****, the effect of IRS-2 on the IL-17 level was significant at a P value of <0.0001. (B) IL-9 production was assessed by ELISA 24 h after restimulation. The data are expressed as the mean cytokine concentrations from triplicate wells plus SEM. ***, the effect of IRS-2 on IL-9 production was significant at a P value of <0.001.
DISCUSSION
During coevolution with their hosts, ticks evolved various mechanisms enabling them to avoid the hosts' hemostatic and immune systems and successfully finish their blood meals.
In recent years, attention has been focused on identification and functional characterization of particular tick salivary proteins that are responsible for antihemostatic and immunomodulatory effects (3).
Thanks to this intensive research, many tick salivary substances that have immunomodulatory effects on various immune cell populations have been identified. Among these substances, molecules that can affect DC functions seem to play important roles, since DCs are among the first cells present at the site of inflammation and can further modulate or shift the immune response by driving T cell differentiation.
Here, we describe specific and extensive inhibitory effects of the tick salivary serpin IRS-2 from the hard tick I. ricinus on Th17 differentiation mediated by impairment of the IL-6/STAT-3 signaling pathway.
Proteins from the serpin superfamily are involved in fundamental biological processes, such as blood coagulation, complement activation, fibrinolysis, angiogenesis, inflammation, and tumor suppression (35, 36). From this enumeration, it is apparent that tick serpins can be expected to play a role in tick feeding, suppressing both the antihemostatic and immune responses of the host. To date, only two I. ricinus serpins have been functionally characterized (12, 14).
We showed that IRS-2 decreased IL-6 at the protein and mRNA levels in spleen dendritic cells activated by B. burgdorferi. A decrease by Sapl15, the best-studied tick salivary protein, in the IL-6 level in response to B. burgdorferi was also observed. Salp15 binds to DCs via the DC-SIGN receptor, which results in activation of the serine/threonine kinase Raf-1/mitogen-activated protein kinase (MEK)-dependent signaling pathway and subsequently in a decrease of IL-6 and TNF-α mRNA stability and impaired nucleosome remodeling at the IL-12p35 promoter in human DCs activated with B. burgdorferi (27). However, the authors point to the fact that the addition of rabbit polyclonal anti-Salp15 antibodies abrogates the capacity of I. ricinus saliva to inhibit IL-12, but not IL-6 and TNF-α, which might be due to the presence of other molecules in tick saliva that are able to block IL-6 and TNF-α. In our study, we proved that one of these molecules, which can be responsible for IL-6 inhibition, is the salivary serpin IRS-2. To reveal the possible mechanism of IRS-2 effects, mRNA for IL-6 was assessed. However, it turned out that, in contrast to Salp15, IRS-2 does not act through impaired stability of IL-6 mRNA. In addition, monitoring of signaling pathways important for induction of IL-6 did not show any defect that led us to the conclusion that gene induction is not impaired. There is a positive-feedback loop in IL-6/STAT-3 signaling (IL-6 binds to IL-6R on a cell and activates phosphorylation of the STAT-3 molecule, which in turn boosts the production of autocrine IL-6), so direct inhibition of STAT-3 phosphorylation by IRS-2 could explain the observed decrease in IL-6 mRNA expression and, subsequently, IL-6 production (37). However, this option was also excluded (data not shown). Thus, we did not reveal the precise mechanism of the IRS-2 effect.
It has been shown that tick saliva and tick salivary proteins, like Salp15, Japanin, and sialostatin L, can modulate the T cell response by modulating DC accessory functions or directly by interaction with CD4+ T cells. It was well demonstrated that tick saliva or salivary gland extract (SGE) diminishes the production of Th1-related cytokines and increases the production of Th2-related cytokines. Salp15 specifically binds to CD4 molecules on the surfaces of CD4+ T (helper) cells, which results in inhibition of T cell receptor-mediated signaling, leading to reduced IL-2 production and impaired T cell proliferation (38). Japanin, a lipocalin from Rhipicephalus appendiculatus, specifically reprograms the response of DCs to a wide variety of stimuli in vitro, altering their expression of costimulatory and coinhibitory transmembrane molecules and secretion of proinflammatory, anti-inflammatory, and T cell-polarizing cytokines (it blocks LPS-induced secretion of Th17- and Th1-promoting cytokines); it also inhibits the differentiation of DCs from monocytes (39). Recently, Horka et al. showed that cystatin from I. scapularis, sialostatin L, which also inhibits several dendritic cell functions, can inhibit IL-9 production by Th9 cells, thus preventing the development of experimental asthma (40). Another cystatin, OmC2 from the soft tick Ornithodoros moubata, can also suppress the host adaptive immune response by reducing TNF-α and IL-12 production and the proliferation of antigen-specific CD4+ T cells (41).
In line with these reports, our data show that the serpin IRS-2 is another tick salivary protein that is able to modulate T cell differentiation. We demonstrated that inhibition of Borrelia-induced IL-6 production in the presence of IRS-2 in DCs was accompanied by decreased phosphorylation of the STAT-3 signaling molecule, which is essential for the development of Th17 cells. Indeed, the impairment by IRS-2 of Th17 development was observed and was demonstrated by a decreased amount of IL-17 produced and by flow cytometry assessment of intracellular IL-17 in CD4+ T lymphocytes cocultured with activated DCs. Similar results, showing that tick saliva inhibits the Th17 subset, were reported by Skallova and colleagues, who showed that saliva-exposed DCs failed to induce efficient Th1 and Th17 polarization and promoted development of Th2 responses (42). Interestingly, treatment with Salp15, which also inhibits IL-6 production in dendritic cells, was shown to increase the differentiation of Th17 cells in vivo, as evidenced by higher IL-17 production from PLP139-151-specific CD4+ T cells isolated from the central nervous system and the periphery (43).
Th17 cells, a quite recently described subpopulation of CD4+ T lymphocytes, can be characterized by production of the hallmark cytokine IL-17. Overproliferation of Th17 cells is connected with many severe autoimmune diseases, like human psoriasis, rheumatoid arthritis, multiple sclerosis, inflammatory bowel disease, asthma, and some bacterial and fungal infections. However, it is well established that Th17 cells not only play an important role in autoimmunity, but also function in the clearance of specific types of pathogens that require a massive inflammatory response and are not adequately dealt with by Th1 or Th2 immunity. Thus, the Th17 response can be triggered by many bacteria, including Borrelia spirochetes (22). Infante-Duarte showed that B. burgdorferi lysate is able to induce massive amounts of IL-17 in T cell cultures and that microbe-induced IL-17 production can mediate infection-induced immunopathology in Lyme disease (44). Involvement of the Th17 subset in the development of severe destructive arthritis in patients with Lyme disease was also demonstrated by Burchill et al. (23). A causative protein, neutrophil-activating protein A (NapA) from B. burgdorferi, which is able to stimulate IL-17 production in synovial-fluid-derived T cells and could thus be crucial for the induction and maintenance of Lyme arthritis, was identified (45). Moreover, it is well described that synthetic or human-derived serpins, which are commonly used in the treatment of many autoimmune diseases, are able to decrease Th17 differentiation (6).
All these findings highlight the importance and potential of the I. ricinus serpin IRS-2 described here as a prospective molecule in many pharmaceutical applications.
In conclusion, here, we present a newly described ability of the I. ricinus salivary serpin IRS-2 to inhibit Th17 differentiation upon B. burgdorferi exposure via inhibition of the IL-6/STAT-3 signaling pathway, thus extending the knowledge about the effect of tick salivary serpins on innate immunity cells and their function in driving the adaptive immune response. This paper contributes to the understanding of tick saliva-mediated modulation of the host immune system.
ACKNOWLEDGMENTS
This work was supported by the Grant Agency of the Czech Republic (grant P302/12/2208 and grant 14-25799S) and by the Grant Agency of the University of South Bohemia project 155/2013/P.
REFERENCES
- 1.Anderson JF, Magnarelli LA. 2008. Biology of ticks. Infect Dis Clin North Am 22:195–215, v. doi: 10.1016/j.idc.2007.12.006. [DOI] [PubMed] [Google Scholar]
- 2.Parola P, Raoult D. 2001. Ticks and tickborne bacterial diseases in humans: an emerging infectious threat. Clin Infect Dis 32:897–928. doi: 10.1086/319347. [DOI] [PubMed] [Google Scholar]
- 3.Francischetti IM, Sa-Nunes A, Mans BJ, Santos IM, Ribeiro JM. 2009. The role of saliva in tick feeding. Front Biosci (Landmark ed) 14:2051–2088. doi: 10.2741/3363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gettins PG. 2002. Serpin structure, mechanism, and function. Chem Rev 102:4751–4804. doi: 10.1021/cr010170+. [DOI] [PubMed] [Google Scholar]
- 5.Silverman GA, Bird PI, Carrell RW, Church FC, Coughlin PB, Gettins PG, Irving JA, Lomas DA, Luke CJ, Moyer RW, Pemberton PA, Remold-O'Donnell E, Salvesen GS, Travis J, Whisstock JC. 2001. The serpins are an expanding superfamily of structurally similar but functionally diverse proteins. Evolution, mechanism of inhibition, novel functions, and a revised nomenclature. J Biol Chem 276:33293–33296. doi: 10.1074/jbc.R100016200. [DOI] [PubMed] [Google Scholar]
- 6.Lin CC, Lin LJ, Wang SD, Chiang CJ, Chao YP, Lin J, Kao ST. 2014. The effect of serine protease inhibitors on airway inflammation in a chronic allergen-induced asthma mouse model. Mediators Inflamm 2014:879326. doi: 10.1155/2014/879326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mulenga A, Khumthong R, Blandon MA. 2007. Molecular and expression analysis of a family of the Amblyomma americanum tick Lospins. J Exp Biol 210:3188–3198. doi: 10.1242/jeb.006494. [DOI] [PubMed] [Google Scholar]
- 8.Mulenga A, Khumthong R, Chalaire KC. 2009. Ixodes scapularis tick serine proteinase inhibitor (serpin) gene family; annotation and transcriptional analysis. BMC Genomics 10:217. doi: 10.1186/1471-2164-10-217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mulenga A, Tsuda A, Onuma M, Sugimoto C. 2003. Four serine proteinase inhibitors (serpin) from the brown ear tick, Rhiphicephalus appendiculatus; cDNA cloning and preliminary characterization. Insect Biochem Mol Biol 33:267–276. doi: 10.1016/S0965-1748(02)00240-0. [DOI] [PubMed] [Google Scholar]
- 10.Leboulle G, Crippa M, Decrem Y, Mejri N, Brossard M, Bollen A, Godfroid E. 2002. Characterization of a novel salivary immunosuppressive protein from Ixodes ricinus ticks. J Biol Chem 277:10083–10089. doi: 10.1074/jbc.M111391200. [DOI] [PubMed] [Google Scholar]
- 11.Prevot PP, Adam B, Boudjeltia KZ, Brossard M, Lins L, Cauchie P, Brasseur R, Vanhaeverbeek M, Vanhamme L, Godfroid E. 2006. Anti-hemostatic effects of a serpin from the saliva of the tick Ixodes ricinus. J Biol Chem 281:26361–26369. doi: 10.1074/jbc.M604197200. [DOI] [PubMed] [Google Scholar]
- 12.Prevot PP, Beschin A, Lins L, Beaufays J, Grosjean A, Bruys L, Adam B, Brossard M, Brasseur R, Zouaoui Boudjeltia K, Vanhamme L, Godfroid E. 2009. Exosites mediate the anti-inflammatory effects of a multifunctional serpin from the saliva of the tick Ixodes ricinus. FEBS J 276:3235–3246. doi: 10.1111/j.1742-4658.2009.07038.x. [DOI] [PubMed] [Google Scholar]
- 13.Prevot PP, Couvreur B, Denis V, Brossard M, Vanhamme L, Godfroid E. 2007. Protective immunity against Ixodes ricinus induced by a salivary serpin. Vaccine 25:3284–3292. doi: 10.1016/j.vaccine.2007.01.008. [DOI] [PubMed] [Google Scholar]
- 14.Chmelar J, Oliveira CJ, Rezacova P, Francischetti IM, Kovarova Z, Pejler G, Kopacek P, Ribeiro JM, Mares M, Kopecky J, Kotsyfakis M. 2011. A tick salivary protein targets cathepsin G and chymase and inhibits host inflammation and platelet aggregation. Blood 117:736–744. doi: 10.1182/blood-2010-06-293241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Banchereau J, Steinman RM. 1998. Dendritic cells and the control of immunity. Nature 392:245–252. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 16.Granucci F, Foti M, Ricciardi-Castagnoli P. 2005. Dendritic cell biology. Adv Immunol 88:193–233. doi: 10.1016/S0065-2776(05)88006-X. [DOI] [PubMed] [Google Scholar]
- 17.Lipscomb MF, Masten BJ. 2002. Dendritic cells: immune regulators in health and disease. Physiol Rev 82:97–130. doi: 10.1152/physrev.00023.2001. [DOI] [PubMed] [Google Scholar]
- 18.Kimura A, Kishimoto T. 2010. IL-6: regulator of Treg/Th17 balance. Eur J Immunol 40:1830–1835. doi: 10.1002/eji.201040391. [DOI] [PubMed] [Google Scholar]
- 19.Heinrich PC, Behrmann I, Haan S, Hermanns HM, Muller-Newen G, Schaper F. 2003. Principles of interleukin (IL)-6-type cytokine signalling and its regulation. Biochem J 374:1–20. doi: 10.1042/BJ20030407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stockinger B, Veldhoen M. 2007. Differentiation and function of Th17 T cells. Curr Opin Immunol 19:281–286. doi: 10.1016/j.coi.2007.04.005. [DOI] [PubMed] [Google Scholar]
- 21.Stockinger B, Veldhoen M, Martin B. 2007. Th17 T cells: linking innate and adaptive immunity. Semin Immunol 19:353–361. doi: 10.1016/j.smim.2007.10.008. [DOI] [PubMed] [Google Scholar]
- 22.Korn T, Bettelli E, Oukka M, Kuchroo VK. 2009. IL-17 and Th17 Cells. Annu Rev Immunol 27:485–517. doi: 10.1146/annurev.immunol.021908.132710. [DOI] [PubMed] [Google Scholar]
- 23.Burchill MA, Nardelli DT, England DM, DeCoster DJ, Christopherson JA, Callister SM, Schell RF. 2003. Inhibition of interleukin-17 prevents the development of arthritis in vaccinated mice challenged with Borrelia burgdorferi. Infect Immun 71:3437–3442. doi: 10.1128/IAI.71.6.3437-3442.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kovarova Z, Chmelar J, Sanda M, Brynda J, Mares M, Rezacova P. 2010. Crystallization and diffraction analysis of the serpin IRS-2 from the hard tick Ixodes ricinus. Acta Crystallogr Sect F Struct Biol Cryst Commun 66:1453–1457. doi: 10.1107/S1744309110032343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Magnuson HJ, Eagle H, Fleischman R. 1948. The minimal infectious inoculum of Spirochaeta pallida (Nichols strain) and a consideration of its rate of multiplication in vivo. Am J Syph Gonorrhea Vener Dis 32:1–18. [PubMed] [Google Scholar]
- 26.Livak KJ, Schmittgen TD. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods 25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 27.Hovius JW, de Jong MA, den Dunnen J, Litjens M, Fikrig E, van der Poll T, Gringhuis SI, Geijtenbeek TB. 2008. Salp15 binding to DC-SIGN inhibits cytokine expression by impairing both nucleosome remodeling and mRNA stabilization. PLoS Pathog 4:e31. doi: 10.1371/journal.ppat.0040031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Libermann TA, Baltimore D. 1990. Activation of interleukin-6 gene expression through the NF-kappa B transcription factor. Mol Cell Biol 10:2327–2334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Akira S, Isshiki H, Sugita T, Tanabe O, Kinoshita S, Nishio Y, Nakajima T, Hirano T, Kishimoto T. 1990. A nuclear factor for IL-6 expression (NF-IL6) is a member of a C/EBP family. EMBO J 9:1897–1906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wen AY, Sakamoto KM, Miller LS. 2010. The role of the transcription factor CREB in immune function. J Immunol 185:6413–6419. doi: 10.4049/jimmunol.1001829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chae HJ, Chae SW, Chin HY, Bang BG, Cho SB, Han KS, Kim SC, Tae KC, Lee KH, Kim DE, Im MK, Lee SJ, Chang JY, Lee YM, Kim HM, Kim HH, Lee ZH, Kim HR. 2001. The p38 mitogen-activated protein kinase pathway regulates interleukin-6 synthesis in response to tumor necrosis factor in osteoblasts. Bone 28:45–53. doi: 10.1016/S8756-3282(00)00413-0. [DOI] [PubMed] [Google Scholar]
- 32.Tuyt LM, Dokter WH, Birkenkamp K, Koopmans SB, Lummen C, Kruijer W, Vellenga E. 1999. Extracellular-regulated kinase 1/2, Jun N-terminal kinase, and c-Jun are involved in NF-kappa B-dependent IL-6 expression in human monocytes. J Immunol 162:4893–4902. [PubMed] [Google Scholar]
- 33.Li H, Rostami A. 2010. IL-9: basic biology, signaling pathways in CD4+ T cells and implications for autoimmunity. J Neuroimmune Pharmacol 5:198–209. doi: 10.1007/s11481-009-9186-y. [DOI] [PubMed] [Google Scholar]
- 34.Elyaman W, Bradshaw EM, Uyttenhove C, Dardalhon V, Awasthi A, Imitola J, Bettelli E, Oukka M, van Snick J, Renauld JC, Kuchroo VK, Khoury SJ. 2009. IL-9 induces differentiation of TH17 cells and enhances function of FoxP3+ natural regulatory T cells. Proc Natl Acad Sci U S A 106:12885–12890. doi: 10.1073/pnas.0812530106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.van Gent D, Sharp P, Morgan K, Kalsheker N. 2003. Serpins: structure, function and molecular evolution. Int J Biochem Cell Biol 35:1536–1547. doi: 10.1016/S1357-2725(03)00134-1. [DOI] [PubMed] [Google Scholar]
- 36.Huntington JA. 2011. Serpin structure, function and dysfunction. J Thromb Haemost 9(Suppl 1):S26–S34. doi: 10.1111/j.1538-7836.2011.04360.x. [DOI] [PubMed] [Google Scholar]
- 37.Chang Q, Daly L, Bromberg J. 2014. The IL-6 feed-forward loop: a driver of tumorigenesis. Semin Immunol 26:48–53. doi: 10.1016/j.smim.2014.01.007. [DOI] [PubMed] [Google Scholar]
- 38.Anguita J, Ramamoorthi N, Hovius JW, Das S, Thomas V, Persinski R, Conze D, Askenase PW, Rincon M, Kantor FS, Fikrig E. 2002. Salp15, an Ixodes scapularis salivary protein, inhibits CD4(+) T cell activation. Immunity 16:849–859. doi: 10.1016/S1074-7613(02)00325-4. [DOI] [PubMed] [Google Scholar]
- 39.Preston SG, Majtan J, Kouremenou C, Rysnik O, Burger LF, Cabezas Cruz A, Chiong Guzman M, Nunn MA, Paesen GC, Nuttall PA, Austyn JM. 2013. Novel immunomodulators from hard ticks selectively reprogramme human dendritic cell responses. PLoS Pathog 9:e1003450. doi: 10.1371/journal.ppat.1003450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Horka H, Staudt V, Klein M, Taube C, Reuter S, Dehzad N, Andersen JF, Kopecky J, Schild H, Kotsyfakis M, Hoffmann M, Gerlitzki B, Stassen M, Bopp T, Schmitt E. 2012. The tick salivary protein sialostatin L inhibits the Th9-derived production of the asthma-promoting cytokine IL-9 and is effective in the prevention of experimental asthma. J Immunol 188:2669–2676. doi: 10.4049/jimmunol.1100529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Salat J, Paesen GC, Rezacova P, Kotsyfakis M, Kovarova Z, Sanda M, Majtan J, Grunclova L, Horka H, Andersen JF, Brynda J, Horn M, Nunn MA, Kopacek P, Kopecky J, Mares M. 2010. Crystal structure and functional characterization of an immunomodulatory salivary cystatin from the soft tick Ornithodoros moubata. Biochem J 429:103–112. doi: 10.1042/BJ20100280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Skallova A, Iezzi G, Ampenberger F, Kopf M, Kopecky J. 2008. Tick saliva inhibits dendritic cell migration, maturation, and function while promoting development of Th2 responses. J Immunol 180:6186–6192. doi: 10.4049/jimmunol.180.9.6186. [DOI] [PubMed] [Google Scholar]
- 43.Juncadella IJ, Bates TC, Suleiman R, Monteagudo-Mera A, Olson CM Jr, Navasa N, Olivera ER, Osborne BA, Anguita J. 2010. The tick saliva immunosuppressor, Salp15, contributes to Th17-induced pathology during experimental autoimmune encephalomyelitis. Biochem Biophys Res Commun 402:105–109. doi: 10.1016/j.bbrc.2010.09.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Infante-Duarte C, Horton HF, Byrne MC, Kamradt T. 2000. Microbial lipopeptides induce the production of IL-17 in Th cells. J Immunol 165:6107–6115. doi: 10.4049/jimmunol.165.11.6107. [DOI] [PubMed] [Google Scholar]
- 45.Codolo G, Amedei A, Steere AC, Papinutto E, Cappon A, Polenghi A, Benagiano M, Paccani SR, Sambri V, Del Prete G, Baldari CT, Zanotti G, Montecucco C, D'Elios MM, de Bernard M. 2008. Borrelia burgdorferi NapA-driven Th17 cell inflammation in Lyme arthritis. Arthritis Rheum 58:3609–3617. doi: 10.1002/art.23972. [DOI] [PubMed] [Google Scholar]