Abstract
A vaccine to prevent the transmission of malaria parasites from infected humans to mosquitoes is an important component for the elimination of malaria in the 21st century, yet it remains neglected as a priority of malaria vaccine development. The lead candidate for Plasmodium falciparum transmission-blocking vaccine development, Pfs25, is a sexual stage surface protein that has been produced for vaccine testing in a variety of heterologous expression systems. Any realistic malaria vaccine will need to optimize proper folding balanced against cost of production, yield, and potentially reactogenic contaminants. Here Chlamydomonas reinhardtii microalga-produced recombinant Pfs25 protein was formulated with four different human-compatible adjuvants (alum, Toll-like receptor 4 [TLR-4] agonist glucopyranosal lipid A [GLA] plus alum, squalene–oil-in-water emulsion, and GLA plus squalene–oil-in-water emulsion) and compared for their ability to induce malaria transmission-blocking antibodies. Alga-produced recombinant Pfs25 plus GLA plus squalene–oil-in-water adjuvant induced the highest titer and avidity in IgG antibodies, measured using alga-produced recombinant Pfs25 as the enzyme-linked immunosorbent assay (ELISA) antigen. These antibodies specifically reacted with the surface of P. falciparum macrogametes and zygotes and effectively prevented parasites from developing within the mosquito vector in standard membrane feeding assays. Alga-produced Pfs25 in combination with a human-compatible adjuvant composed of a TLR-4 agonist in a squalene–oil-in-water emulsion is an attractive new vaccine candidate that merits head-to-head comparison with other modalities of vaccine production and administration.
INTRODUCTION
A vaccine to prevent gametocytemic humans from infecting mosquitoes by inducing antibodies against sexual stage Plasmodium antigens, termed a transmission-blocking vaccine (TBV), was first demonstrated experimentally as proof of principle using a chicken model of Plasmodium gallinaceum more than 30 years ago (1, 2). TBV development has been stated to be a key priority toward achieving the global goals of malaria control and elimination (3, 4), yet it lags behind other malaria vaccine development despite candidate proteins such as Pfs25 being deeply studied as a TBV. Pfs25, the first molecularly cloned Plasmodium sexual stage protein, was identified more than 25 years ago and remains a lead TBV candidate (5, 6). Parasite-produced Pfs25 contains 4 epidermal growth factor (EGF)-like domains and is not glycosylated, which makes it biotechnologically challenging to produce the properly folded, conformationally correct recombinant protein(s) required for the induction of effective transmission-blocking antibodies (7–9). Heterologous expression systems used to produce Pfs25 as a recombinant subunit immunogen include Escherichia coli (10), Saccharomyces cerevisiae (11), Pichia pastoris (7, 12), baculovirus (13), Nicotiana benthamiana (14), Triticum vulgare (wheat germ) extract (6, 15, 16), and most recently, the chloroplast of the microalga Chlamydomonas reinhardtii (17, 18).
The microalga C. reinhardtii has previously been reported to produce nonglycosylated, immunogenic recombinant Pfs25 that induces transmission-blocking antibodies in combination with complete Freund's adjuvant (17) and mucosal IgA antibodies when fused with cholera toxin beta subunit (CtxB) and administered orally (18). Production in algal chloroplasts is advantageous from an economic and biological perspective. The biotechnological production and scale-up of recombinant proteins in C. reinhardtii is remarkably inexpensive, requiring just light and simple chemical nutrients. Recombinant algae can be grown in transparent plastic bags as containment, and standardized scalable methods for processing the biological materials are available (19). The chloroplast lacks the machinery to glycosylate proteins, a distinct advantage for production of Pfs25, and contains nucleus-encoded chaperones that likely assist in folding complex EGF-like domains (20). A major issue that has arisen previously in designing a Pfs25-based vaccine is the need for a strong yet human-use-compatible adjuvant to overcome the inherent lack of antigenicity of Pfs25; in a phase I clinical trial, erythema nodosum associated with the Montanide ISA 51 oil-in-water adjuvant was observed in a small number of subjects, which led to cessation of that vaccine formulation (21). New adjuvants based on squalene–oil-in-water emulsion in the context of a nontoxic lipid A-like moiety, glucopyranosyl lipid A (GLA), have been developed in extensive animal/in vitro experimentation (22–25) and used in human experimentation (26), and they have been demonstrated to have the desired effects of low reactogenicity, induction of high levels of effector antibodies, and optimized affinity maturation.
The purpose of the present study was to assess the ability of Chlamydomonas reinhardtii-produced Pfs25 to induce transmission-blocking systemic IgG antibodies when injected along with a human-compatible adjuvant, a Toll-like receptor 4 (TLR-4) agonist, i.e., GLA, plus squalene–oil-in-water emulsion. New adjuvants need to be tested in combination with recombinant Pfs25, because previous studies with Pfs25 have shown that presumed human-compatible adjuvants (Montanide ISA51) induced serious adverse events, including erythema nodosum in early-stage clinical trials (21). We hypothesized that combining a TLR-4 receptor agonist, GLA, with squalene–oil-in-water would enhance the titer, avidity, and functional transmission-blocking activity of alga-produced Pfs25 compared to non-GLA-containing adjuvants. Previously published data indicate that GLA and squalene–oil-in-water emulsion synergistically enhance acquired immune responses (27). In the experiments reported here, antibody responses against P. falciparum macrogamete and zygote Pfs25 were assessed in response to vaccination of mice with Chlamydomonas reinhardtii chloroplast-produced Pfs25 combined with alum, alum with GLA, squalene–oil-in-water emulsion alone, and GLA plus squalene–oil-in-water emulsion. Conventional and avidity enzyme-linked immunosorbent assay (ELISA), immunofluorescence assays, and standard membrane feeding assays (SMFAs) were used to measure the function of the vaccine-induced antibodies. This work is an important new step along a promising pathway for transmission-blocking vaccine development for deployment in humans.
MATERIALS AND METHODS
Ethics statement.
All procedures were approved by the University of California—San Diego (UCSD) Institutional Animal Care and Use Committee under protocol number S09277, in compliance with the USDA Animal Welfare Act (Public Law 89-544), the Health Research Extension Act of 1985 (Public Law 99-158), the Public Health Service Policy on Humane Care and Use of Laboratory Animals, and the NAS Guide for the Care and Use of Laboratory Animals (ISBN-13). The University of California—San Diego is a registered Research Facility under the Animal Welfare Act and has a current assurance on file with the Office of Laboratory Animal Welfare, in compliance with NIH policy.
Expression and purification of Pfs25.
The production of alga-Pfs25 recombinant protein was carried out as previously described (17). Briefly, the C. reinhardtii codon-optimized Pfs25 gene was synthesized, subcloned into pD1-KanR, and transformed into C. reinhardtii strain W1.1 by particle bombardment. Transformants were propagated on TAP (Tris-acetate-phosphate) agar in the presence of kanamycin and screened for the presence of alga codon-pfs25 (using gene-specific primers) and for homoplasmicity. A positive batch was used as inoculum (250 ml) to initiate 20 liters of culture in a photobioreactor. The cells were harvested and lysed, and rPfs25 protein was purified using M2 anti-FLAG resin affinity chromatography. The partially purified recombinant protein was resolved in SDS-PAGE, verified by immunoblotting with anti-mouse Pfs25 monoclonal antibody (MAb) 4B7, aliquoted, and stored at −80°C to prevent repeated freeze-thawing.
Adjuvants.
Aluminum hydroxide (alum)- and oil-water emulsion-based adjuvants are widely used in human vaccination against various infectious diseases (28, 29). Four different adjuvants were used in this study: AL007 (alum, 2 mg/ml), AL019 (TLR-4 agonist GLA plus alum, 0.1 and 2 mg/ml, respectively), EM081 (squalene–oil-in-water emulsion), and EM082 (0.25 mg/ml GLA–squalene–oil-in-water emulsion). In each immunization, 4 GLA doses (5 μg) were equal in both the AL019 and the EM082 groups of mice. Adjuvants were provided by the Infectious Disease Research Institute (IDRI), Seattle, WA, USA (http://www.idri.org).
Vaccination and serum collection.
Four- to 6-week-old female BALB/c mice were purchased from Jackson Laboratory, ME, USA, and maintained in a specific-pathogen-free environment at the UCSD. Four groups (10 females/group) of mice were injected via the intraperitoneal (i.p.) route with the initial dose of 20 μg alga-produced rPfs25 antigen plus adjuvant followed by three injections of 10 μg of antigen at 3-week intervals. A negative-control group (10 mice) was injected with 1× phosphate buffer saline (PBS) mixed with adjuvant as was done with the antigen-containing regimens. Immediately before the immunization, adjuvant was added to the recombinant protein or PBS to a final volume of 100 μl and mixed by brief vortexing. The vaccinated mice were bled 2 weeks after the final immunization, and serum samples were pooled and measured for anti-Pfs25 IgG (and subtype) in sera.
Evaluation of comparative vaccine-induced immunity by conventional ELISA.
A standard ELISA method was used to compare the anti-Pfs25 IgG titers in the serum samples from the different adjuvant groups. Protein A-purified, conformationally dependent, transmission-blocking anti-Pfs25 monoclonal antibody 4B7 was used as a positive control, and sera from each PBS plus adjuvant group were assayed. Alga-produced rPfs25 was dissolved (2 μg/ml) in 0.06 M sodium carbonate buffer (pH 9.6); 100 ng was used to coat wells by incubation overnight at 4°C in a humidified chamber. For the assay, 96-well plates were used (Immunolon 4; Dynex Technology Inc., Chantilly, VA). Superblock blocking buffer (Thermo Fisher Scientific, IL) was used for blocking, PBST (PBS containing 0.05% Tween 20) was used for washing the plate, and 1% Superblock blocking buffer in PBST was used to dilute antibodies and conjugates. Plates were sealed with a plate sealer before every incubation step. Plates were blocked with 300 μl blocking solution per well and incubated for 1 h at room temperature followed by three PBST washes. Serum samples were serially diluted (1/500 to 1/64,000) in dilution buffer; 200 μl per well was added in duplicate, and the wells were incubated overnight at 4°C. The wells were washed three times with PBST, 200 μl of diluted (1:5,000) goat anti-mouse immunoglobulin G (IgG) antibody conjugated to horseradish peroxidase was added to each well, and the mixtures were incubated for 1 h at room temperature. The plate was washed four times, 100 μl of chromogenic horseradish peroxidase (HRPO) substrate (TMB Microwell Peroxidase Substrate system; KPL, Gaithersburg, MD) was added to each well, and the reaction was stopped by the addition of 100 μl of 2 N H2SO4. The plate was read using a microplate reader (Spectramax Plus; Molecular Devices, Sunnyvale, CA) at a wavelength of 450 nm. The titers for each group/dilution were normalized against the negative-control serum (vaccination with adjuvant plus PBS alone, without recombinant Pfs25) within the same group of mice.
To determine reactivity against native parasite protein, ELISA was performed according to the method described above but using lysate of P. falciparum in vitro-cultured transmission stages (mixed gametes and zygotes) as an antigen. In this assay, 100 μl of P. falciparum transmission stage lysate (2 μg/ml in antigen dilution in coating buffer) was used as a coating agent per well in carbonate buffer, and the 1/500 serum dilution was used in the adjuvant groups. Other steps were followed as described above.
Evaluation of Pfs25-specific IgG subtypes of antibodies among the four different adjuvant groups.
ELISA plates coated with alga-produced affinity-purified rPfs25 protein as capture antigen were incubated with serum samples (1:500 dilutions) from different adjuvant (with alga-rPfs25 antigen) and control (adjuvant alone) groups in duplicate wells. Pooled serum samples from pre- and postimmunization sera (2 weeks after the final immunization) were used for the subclass typing ELISA. The Mouse Typer Subtyping kit (catalog number 172-2051; Bio-Rad Laboratories, Hercules, CA) was used to determine anti-Pfs25 subtypes (IgG1, IgG2a, IgG2b, and IgG3) containing rabbit-mouse subtype antibodies (IgG1, IgG2a, IgG2b, and IgG3), which were used to determine the comparative specific anti-Pfs25 IgG subtype level, and values were normalized against the PBS-adjuvant controls for each group. For statistical analysis, P values were calculated using Student's t test to compare the various adjuvant groups (GraphPad Prism, San Diego, CA).
Avidity ELISA.
The avidity indices of anti-Pfs25 IgG from the four different antigen-adjuvant groups were measured by inhibition of antibody binding to alga-produced rPfs25 antigen in the presence of 8 M urea, and these assays were carried out as previously published (30). Briefly, the ELISA steps were carried out as described in the previous section with protocol changes described as follows. Primary antibodies (postvaccination pool, 1/500 dilutions, 200 μl per well) were added to two sets of triplicate wells and incubated for 2 h at room temperature. The wells were then washed three times with either PBST or urea wash buffer (8 M urea in PBST, and each wash was incubated for 5 min). Then anti-mouse HRPO-conjugated secondary antibody (1/3,000 dilution and 200 μl per well) was added, incubated for 1 h at room temperature followed by washing four times with PBST, and 100 μl of chromogenic substrate (TMB) was added to each well. The plate was read using a Molecular Devices Gemini microplate reader at a wavelength of 450 nm after the reaction was stopped by 100 μl of 2 N H2SO4 in each well. The avidity index was calculated as the ratio of average urea-treated optical density to the average PBST-washed optical density.
SDS-PAGE and Western immunoblotting to determine the binding of antiserum to native parasite protein.
To determine the reactivity of antibodies to rPfs25 and native parasite protein, immunoblotting was performed as described previously (31). Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and protein transfer were carried out using Xcell Sure Lock Minicell electrophoresis and the XCell Blot II module (Life Technologies). P. falciparum gamete/zygote mixed-stage lysate was mixed with 4× sample buffer with or without 10 mM dithiothreitol (DTT; Thermo Scientific, Rockford, IL, USA) and denatured at 70°C for 10 min. The samples were resolved using 4 to 12% gradient bis-Tris gel/MOPS (morpholinepropanesulfonic acid) buffer (Life Technologies) and electroblotted to nitrocellulose. The blotted membrane was stained with Ponceau S solution (Sigma-Aldrich, St. Louis, MO); strips were cut and then blocked (Superblock, Thermo Fisher Scientific, Lake Barrington, IL) for 1 h at room temperature. The strips were washed with 0.05% TBS–Tween 20 (TBST), incubated individually in pooled serum samples (1/1,000 dilution in 1% Superblock in TBST) and anti-Pfs25 MAb (4B7, 1 mg/ml, positive control) overnight at 4°C, and washed three times. Then, the blots were incubated in 1/3,000 dilution of goat anti-mouse IgG phosphatase-labeled antibodies (Kierkegard and Perry Laboratories, Gaithersburg, MD) for 1 h and washed 4 times in TBST. Blots were developed with 5-bromo-4-chloro-3-indolyl phosphate-nitroblue tetrazolium alkaline phosphatase substrate in substrate solution (Kierkegard and Perry Laboratories).
Immunofluorescence assay.
An indirect immunofluorescence assay (IFA) was carried out using a mixture of in vitro-cultured gamete and zygote stages of P. falciparum strain NF 54 obtained from gametocytes as published previously (32, 33). Female gametes and zygotes can be distinguished by cell and nuclear size. A thin smear of parasitized red cells was made on a glass slide and air dried, and a small circle around the material was made with a hydrophobic pen (ImmunoPen; EMD Millipore, Billerica, MT). The slides were fixed in 4% paraformaldehyde for 30 min, followed by permeabilization in 1% Triton X-100 in PBS for 10 min, followed by incubation with a 1/500 dilution of test sera in 1% bovine serum albumin (BSA) in PBS, rinsed with PBS, and finally incubated with Alexa Fluor 488-labeled donkey anti-mouse IgG (Heavy + Light chains; Life Technologies, Carlsbad, CA). After 5 PBS washes, mounting medium with DAPI (4′,6-diamidino-2-phenylindole) was put onto slides (ProLong Gold Antifade Mountant with DAPI; Life Technologies), and slides were examined using an UltraView Vox spinning disk confocal microscope (PerkinElmer, MA, USA), available at the Microscopy Core, Department of Medicine, University of San Diego.
Standard membrane feeding assay.
P. falciparum transmission blocking activity was assessed by standard membrane feeding assay (SMFA). Briefly, an Anopheles stephensi colony is maintained at the University of California San Diego following the protocols described in Methods in Anopheles Research (MR4; http://www.mr4.org). For the SMFA, female mosquitoes (4 days old, 40 per cup) were placed in small containers 1 day before being administered an infectious blood meal and starved overnight. Mosquitoes were fed with mature stage V P. falciparum NF54 gametocytes (with >10 exflagellating centers per 40× field) in the presence of heat-inactivated control or immune serum samples through a custom-made glass membrane feeder. The temperatures of the feeders were maintained at 37°C using a circulating water bath. After 20 min of blood feeding, unfed/partially blood-fed mosquitoes were removed from each cup, and fully engorged mosquitoes were maintained in an environmental chamber set at 26°C and 75% relative humidity. Mosquitoes were provided daily with fresh damp cotton balls soaked in 8% sugar plus 0.05% para-amino benzoic acid (PABA) solution, and on day 8 the midguts were dissected from surviving mosquitoes. Immediately thereafter, midguts were stained with 0.1% mercurochrome in PBS for 10 min, and the number of oocysts per midgut was determined under a light microscope. The Mann-Whitney and Fisher's exact tests (Prism 5; GraphPad Software Inc., La Jolla, CA) were used to calculate statistical differences in infection rates and the geometric mean numbers of oocysts.
RESULTS
Chlamydomonas reinhardtii-produced recombinant Pfs25 plus human-use-compatible adjuvants generated high-titer IgG antibodies.
Previous experiments using complete Freund's adjuvant (not compatible with human use) in combination with Chlamydomonas reinhardtii-produced recombinant Pfs25 demonstrated as proof in principle that the microalga's chloroplast heterologous expression system is capable of producing properly folded Plasmodium falciparum transmission-blocking vaccine candidate Pfs25 (17). SDS-PAGE analysis of the purified rPfs25 shows an appropriately sized major protein band; by Western immunoblotting, the protein strongly reacted with MAb 4B7 (anti-Pfs25) (Fig. 1A). In addition to the dominant 25-kDa protein band, the anti-Pfs25 MAb 4B7 bound to predicted dimers and trimers of the rPfs25protein (Fig. 1B).
FIG 1.
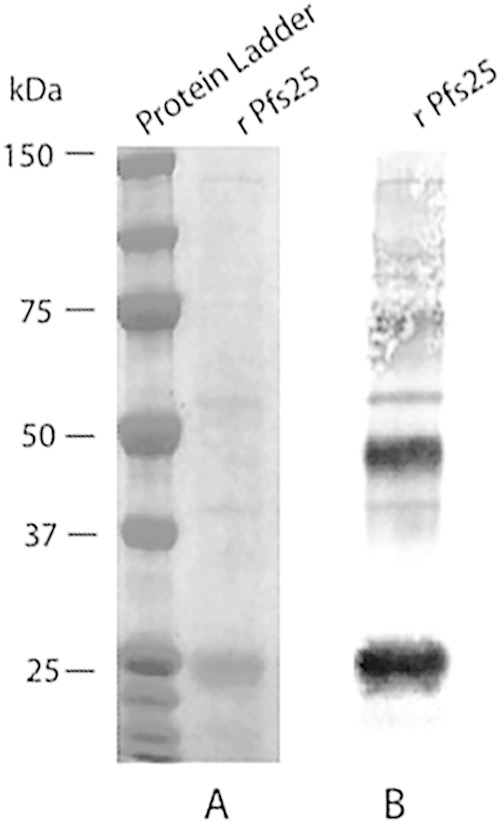
Analysis of purified alga-produced Pfs25. (A) FLAG tag affinity-purified alga-produced rPfs25 was resolved by SDS-PAGE and stained with InstantBlue. (B) Western immunoblot of FLAG tag affinity-purified alga-produced rPfs25 probed with the anti-Pfs25 MAb 4B7.
Adjuvant selection for inducing high titers is an important factor in malaria transmission-blocking vaccine development. Squalene–oil-in-water-based adjuvants (EM081 and EM082) were more efficient than were alum-based adjuvants (AL007 and AL019) in inducing high-titer anti-Pfs25 IgG antibodies against Pfs25 (Fig. 2). The results show that using the GLA-containing adjuvant EM082 was better than using the non-GLA-containing EM081 in eliciting anti-Pfs25 IgG.
FIG 2.
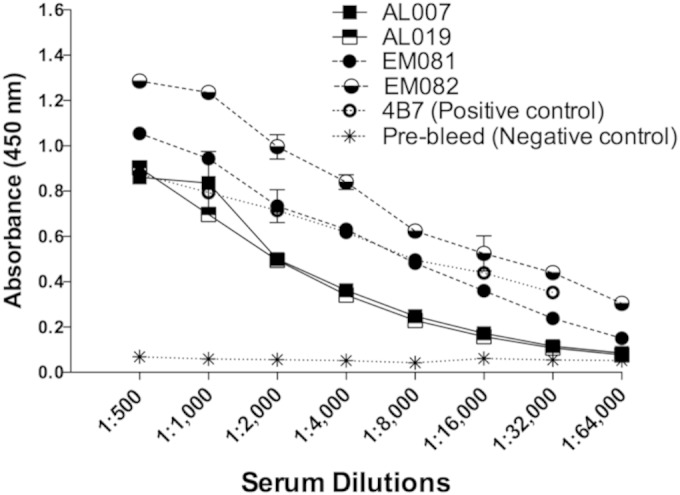
Anti-Pfs25 IgG titers in mice vaccinated with alga-produced Pfs25 antigen in combination with human-compatible adjuvants. Ten mice per group were vaccinated with or without affinity-purified alga-produced Pfs25 antigens mixed with AL007 (2 mg/ml alum), AL019 (0.5 mg/ml alum plus GLA), EM081 (squalene–oil-in-water emulsion), and EM082 (squalene–oil-in-water emulsion plus GLA). Each group of mice was vaccinated with the initial dose of 20 μg alga-rPfs25 antigen plus adjuvant, followed by 3 injections of 10 μg of antigen at 3-week intervals. The vaccinated mice were bled 2 weeks after the final immunization, sera were pooled, and anti-Pfs25 IgG titers were determined by ELISA. Titers for rPfs25 plus adjuvants are reported after subtracting signals obtained using serum from each respective adjuvant-alone group. Pfs25 monoclonal antibody secreting hybridoma (4B7) was obtained from MR4 and cultured in vitro, and the MAb was purified and used as a positive control in this assay at a stock solution concentration of 1 mg/ml. Pooled prebleed mouse sera were used as negative control. ELISA plates were coated with alga-Pfs25 antigens (100 ng/well) in sodium bicarbonate buffer, pH 9.6, and serial serum dilutions (1/500 to 1/64,000) were used to determine titers.
The anti-Pfs25 IgG titers against native parasite gamete/zygote lysates were different from those against the recombinant protein, which is important for understanding the functional effect of potential transmission-blocking antibodies. EM082 (squalene–oil-in-water plus GLA) adjuvant induced a 3-fold-higher titer than EM081 (oil-water emulsion) (Fig. 3). AL007 (2 mg/ml alum) produced significantly higher titers than did AL019 (0.5 mg/ml alum plus GLA) (Fig. 3).
FIG 3.
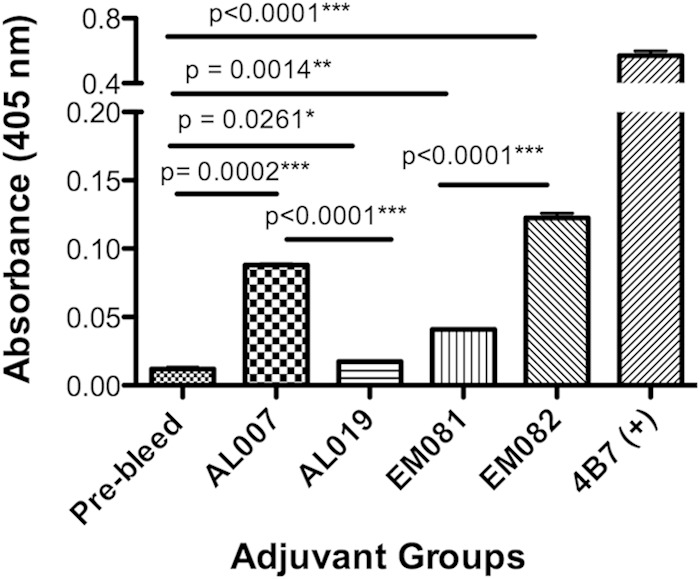
Comparison of anti-Pfs25 IgG levels among four different adjuvant groups against P. falciparum parasite lysate. Plasmodium falciparum mixed gamete/zygote stage lysate-coated ELISA plate (100 ng/well) and the pooled serum (10 mice) samples (1/500 dilutions) were used to measure anti-Pfs25 IgG levels among the four adjuvant plus alga-Pfs25-vaccinated animals AL007 (2 mg/ml alum), AL019 (0.5 mg/ml alum plus GLA), EM081 (squalene–oil-in-water emulsion), and EM082 (squalene–oil-in-water emulsion plus GLA). Titers for rPfs25 plus adjuvants are reported after subtracting signals obtained using serum from the adjuvant-alone group. Pfs25 MAb obtained from MR4 (concentration, 1 mg/ml) was used as a positive control; a sample from pooled sera of prevaccinated mice (prebleed) was used as a negative control. Statistical analysis was carried out using Student's t test between various adjuvant groups, and statistical significance is indicated by horizontal lines above the values.
Western immunoblotting using native P. falciparum gamete/zygote lysate as an antigen showed that all four adjuvant groups (AL007, AL019, EM081, and EM082) developed antibodies against both native and linear, reduction-sensitive epitopes of native Pfs25 (Fig. 4). The sera from control groups (adjuvant alone) did not react with native parasite Pfs25 under both reducing and nonreducing conditions (data not shown).
FIG 4.
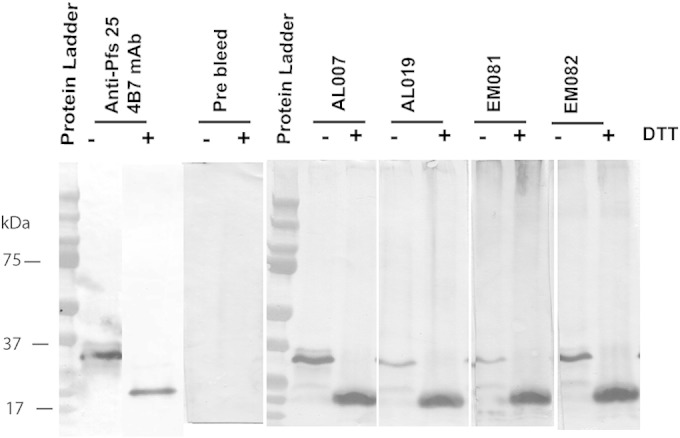
Western immunoblot analysis of P. falciparum mixed gamete/zygote lysate probed with anti-alga Pfs25 serum raised with four different adjuvants. Reduced (+DTT) and nonreduced (−DTT) mixed gamete/zygote stage lysates were resolved by SDS-PAGE, transferred to nitrocellulose membrane, and cut into individual strips. The blots were probed individually with postvaccinated sera from the four vaccine groups (AL007, AL019, EM081, and EM082), prebleed mouse sera (negative control), and anti-Pfs25 monoclonal antibodies (4B7) as a positive control under the same conditions to determine the comparative binding efficiency levels of anti-Pfs25 antibodies induced by different adjuvants.
Effect of adjuvant on anti-Pfs25 specific IgG subtypes.
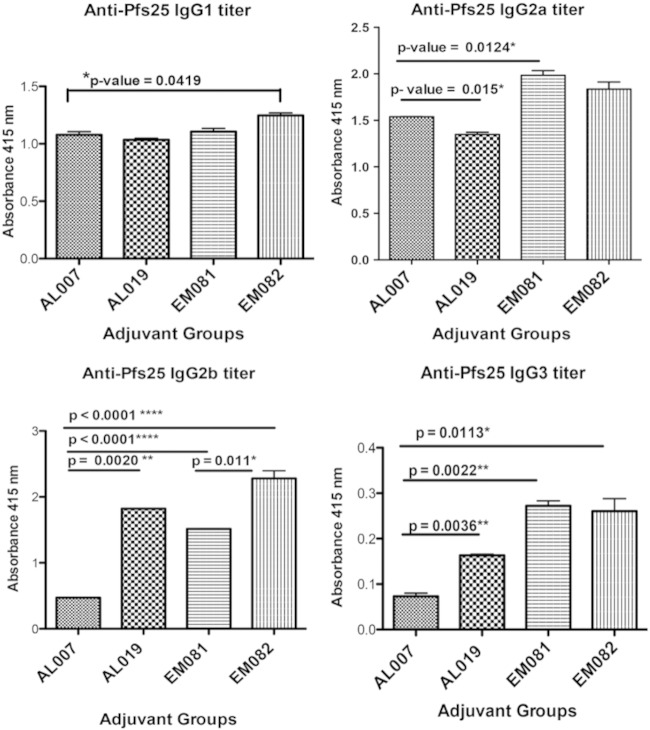
EM081- and EM082-adjuvanted antigen produced elevated levels of antigen-specific subtypes IgG1, IgG2b, and IgG3 compared to AL007 and AL019, alum-based adjuvants (Fig. 5). Addition of GLA to alum (AL019) significantly improved the IgG2b and IgG3 titers compared to those obtained with alum alone (AL007) (Fig. 5, bottom panels). Similarly, addition of GLA (EM082) to the oil and water emulsion significantly increased the IgG2b titer.
FIG 5.
Pfs25-specific IgG subtypes of antibodies along with four different adjuvant groups vaccinated with alga-produced Pfs25. ELISA plates were coated with alga-produced, affinity-purified rPfs25 recombinant protein as capture antigen (100 ng/well) in bicarbonate buffer and incubated with serum samples (1/500 dilution) from different adjuvant (with rPfs25 antigen) and control (adjuvant alone) groups in duplicate wells. Groups were AL007 (2 mg/ml alum), AL019 (0.5 mg/ml alum plus GLA), EM081 (squalene–oil-in-water emulsion), and EM082 (squalene–oil-in-water emulsion plus GLA). Pooled serum samples from pre- and postimmunization sera were used for the IgG subtyping ELISA. Statistical analysis was carried out using Student's t test between various adjuvant groups, and the values mentioned for significant differences (P value < 0.05) are marked with horizontal lines above them.
Adjuvant EM082 (squalene–oil-in-water emulsion plus GLA) increased antibody avidity.
To compare the avidity of anti-Pfs25 antisera in response to alga-produced rPfs25 combined with the four different adjuvants, IgG was quantified in the presence of 8 M urea (Fig. 6). Alga-produced rPfs25 used as an antigen for coating the wells and the avidity ELISA showed >50% relative avidity indices (RAI) for antibodies from four different adjuvant groups. However, the oil-in-water emulsion adjuvant with TLR-4 agonist (EM082) elicited antibodies with significantly higher relative binding (P < 0.001) than that seen with alum. There was no significant difference in RAI of anti-Pfs25 antibodies in the context of vaccination with AL007, AL019, or EM081 adjuvants, even though antibody titers were significantly higher against rPfs25 antigen in EM081 adjuvant.
FIG 6.
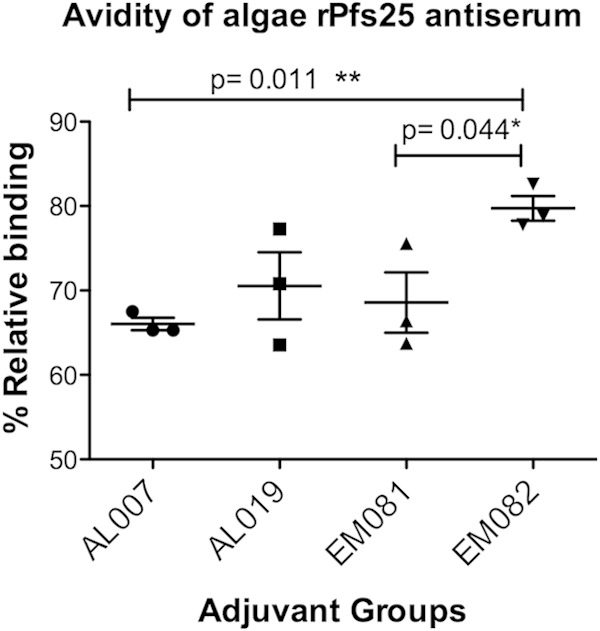
Comparison of relative avidity indexes of anti-Pfs25 antibodies against recombinant alga-Pfs25 antigen from four vaccination groups. Avidity of the anti-Pfs25 IgG was measured by continued binding to alga-Pfs25 antigen in the presence of 8 M urea compared to no urea as a control. Serum samples from alga-Pfs25 antigens vaccinated in combination with AL007 (2 mg/ml alum), AL019 (0.5 mg/ml alum plus GLA), EM081 (squalene–oil-in-water emulsion), and EM082 (squalene–oil-in-water emulsion plus GLA). The avidity index was calculated as the ratio of average optical density at 450 nm (OD450) for urea-treated samples to the average OD450 for PBS-Tween 20-treated samples. Statistical analysis was carried out using Student's t test to compare various adjuvant groups, and the values mentioned for significant differences between groups (P value < 0.05) are marked with a horizontal line above them. Vaccination with EM082 (oil-in-water plus GLA) showed a significantly higher avidity index than vaccination with alum (AL007) and than vaccination with oil-in-water emulsion without GLA (EM081).
Immunofluorescence assay to evaluate detection of native Pfs25 by the antiserum raised by four adjuvants.
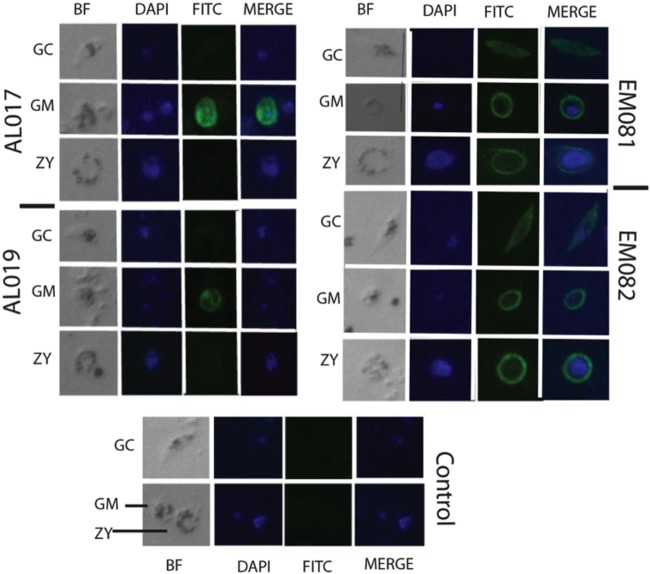
Immunofluorescence microscopy was performed to determine pattern, localization, and binding efficiency of antiserum at a fixed dilution (1/500) to native Pfs25 proteins expressed by in vitro-cultured parasites (gametocyte stage, gamete, and zygote) (Fig. 7). Interestingly, the anti-Pfs25 antiserum in response to EM081 and EM082 showed the typical rim fluorescence in female gamete and zygote stages and showed a previously observed cytoplasmic distribution of the protein in stage V gametocytes (34). The antiPfs25 antiserum raised by alum-based adjuvants (AL007 and AL019) showed a positive signal in the gamete stages but failed to show fluorescence in the zygote stages, possibly due to low-titer/low-affinity antibodies or the differential recognition of epitopes of Pfs25.
FIG 7.
Immunofluorescence microscopy images show the binding of anti-alga Pfs25 antiserum to native parasite protein expressed in the in vitro-cultured gametocyte (GC), female gamete (GM), and zygote (ZY) stages of P. falciparum. BF, Nomarski (differential interference contrast) bright-field images; DAPI, nucleus stained blue; FITC (fluorescein isothiocyanate), Pfs25 protein stained green; Merge, merged images to show both the nucleus and the Pfs25 protein localization in P. falciparum parasite stages. Serum samples from rPfs25 antigens in the four vaccine groups were used as primary antibodies for probing the specimens.
GLA plus squalene–oil-in-water emulsion most effectively induces transmission-blocking antibodies.
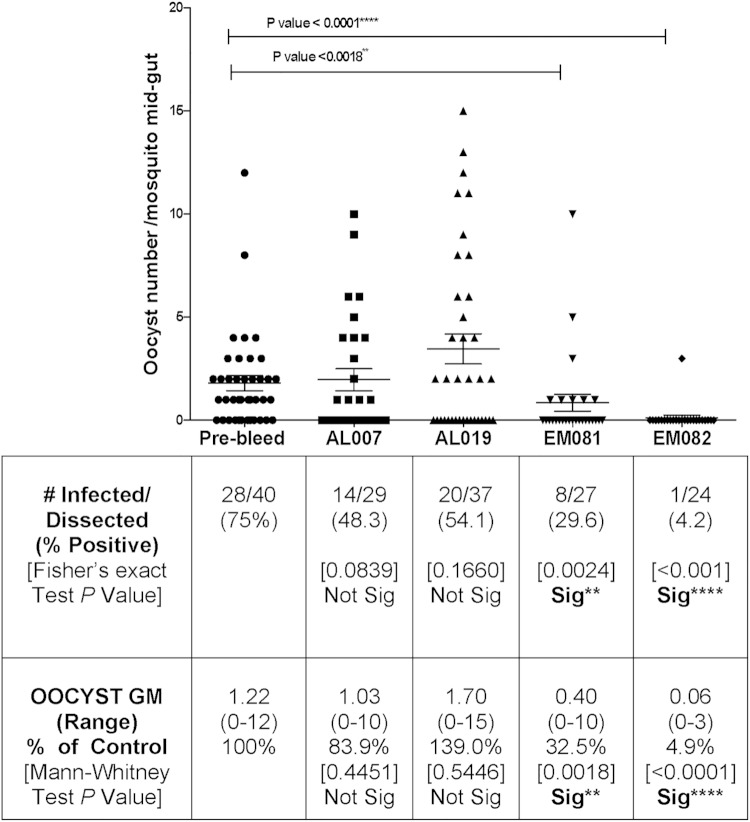
To determine whether different adjuvant formulations differentially induced transmission-blocking antibodies, mouse antisera were combined with infectious P. falciparum NF54 gametocytes in SMFA with Anopheles stephensi. We observed significant differences among the transmission-blocking effects of anti-Pfs 25 antisera induced by different adjuvants (Fig. 8). Squalene–oil-in-water emulsion led to the induction of antibodies with significantly lower oocyst counts than those observed with alum-based adjuvants. The most dramatic results were those produced by the anti-Pfs25 serum in association with GLA-containing oil-in-water (EM082) adjuvant, in which only 1 vector mosquito (of 24 mosquitoes examined) was found to be positive (4.2% infection rate compared to 75% in control; 28 positive of 40 mosquitoes examined; Fisher's exact P value, <0.0001) with only 3 oocysts (the geometric mean number of oocysts was 0.06 compared to 1.22 for the control oocysts; Mann-Whitney test P value, <0.0001).
FIG 8.
Adjuvant effect on transmission-blocking activity of anti-Pfs25 antibodies. Standard membrane feeding assays were carried out with pooled sera from four groups of mice vaccinated with alga-produced Pfs25 in combination with the four adjuvants (AL007, AL019, EM081, and EM082). Preimmune sera were used as negative controls. Oocyst counts reflect the successful development of P. falciparum inside the Anopheles stephensi mosquitoes. Two separate membrane feeds were done using serum from each group, and oocyst counts were pooled for statistical analysis. The table shows P. falciparum infectivity and oocyst counts in the different groups (AL007, AL019, EM081, and EM082). Statistical analysis (Mann-Whitney test and Fisher's exact test) was carried out using GraphPad Prism 5. Sig, significant.
DISCUSSION
Here we have shown that Chlamydomonas reinhardtii-produced recombinant Pfs25, in combination with a human-use-compatible adjuvant composed of oil-in-water–glucopyranosyl lipid A, induced high-affinity antibodies that effectively block Plasmodium falciparum infection of mosquitoes. Previous work has convincingly demonstrated that the absolute titer of anti-Pfs25 antibody raised to properly folded Pfs25 predicts transmission-blocking efficacy in standard membrane feeding assays (6, 35). The data reported here also demonstrate that avidity, as estimated by urea-ELISA, provides additional surrogate efficacy information beyond simply the titer. Finally, we found that the pattern of immunofluorescence on in vitro-cultivated P. falciparum macrogametes and zygotes using sera from mice injected with alga-produced Pfs25 in combination with different adjuvants showed specificity for the surface of parasite sexual stages as revealed by rim fluorescence. Alga-produced Pfs25 in combination with squalene–oil-in-water emulsion plus GLA adjuvant also produced the most convincingly specific surface recognition of macrogametes and zygotes, identical to what has been observed with the well-characterized transmission-blocking monoclonal antibodies 1B7 and 4B7 (36, 37). The surface IFA signal presumably reflects the efficacy of transmission-blocking anti-Pfs25 antibodies directed against the sexual stages present in the mosquito midgut soon after the exflagellation/emergence of gametes and the zygote product of fertilization.
A key finding here is that the same quantity of alga-produced Pfs25 compared head-to-head with four different, human-use-compatible adjuvants had different immunological and transmission-blocking activities. The many different systems, i.e., E. coli (10), tobacco (38, 39), Saccharomyces cerevisiae (11, 37, 40, 41), Pichia pastoris (12, 42), DNA vaccines (43, 44), wheat germ (15), insect cells via baculovirus (13, 16), protein-peptide conjugates (45), Pseudomonas aeruginosa ExoProtein A (rEPA) (46), cholera toxin adjuvant (12), and virally vectored vaccines (47, 48), by which recombinant Pfs25 has been produced have uniformly found that recombinant Pfs25 administered in animal models in the absence of adjuvant is poorly immunogenic, with relatively low IgG titers. The importance of a safe and effective adjuvant for Pfs25—regardless of the heterologous expression system—was highlighted in a phase I clinical trial in which Montanide IS151 combined with Pichia-produced Pfs25 was associated with leukemoid reactions and erythema nodosum in humans (21).
C. reinhardtii-produced recombinant Pfs25 has certain biotechnological advantages as the basis for vaccine development. Production is as simple as growing the alga in minimal growth medium plus light (17, 18). Photosynthetic growth, combined with high recombinant protein yields and the ease of scaling up production, results in a relatively inexpensive production platform (49). Standard, scalable industrial-scale cell extraction and protein purification methods are then readily applied, as has been observed with another plant-based method with hydroponic Nicotiana (14, 39). Microalgae and single-celled waterborne plants grow much more rapidly than more complex plants and can be cultured under cyclic GMP (cGMP) conditions at low cost (50). The growth of the alga-based biofuel industry has spurred interest in the present area of vaccine interest, which leverages a profitable industry to reduce production costs of alga-produced recombinant protein vaccines.
Recombinant protein malaria vaccine candidates have often been found to be poorly immunogenic (PfCSP [51], PfMSP-1 [52, 53], Pfs25 [reviewed in reference 21]), requiring an adjuvant to achieve potentially protective immune responses in the case of transmission-blocking vaccines as assessed by antibody titers and other additional assays. In these studies and others, aluminum hydroxide consistently has been found to be insufficiently potent as an adjuvant to induce high-level immune responses. Indeed, a recently reported head-to-head comparison of aluminum hydroxide adjuvant and squalene–oil-in-water emulsion–GLA in combination with E. coli-produced nearly full-length recombinant PfCSP in a mouse vaccine model—as was done in the present work—showed marked enhancement of antibody responses to nearly full-length P. falciparum circumsporozoite protein (51). The data presented here demonstrate that anti-Pfs25 antibody avidity is remarkably enhanced in the presence of squalene–oil-in-water–GLA adjuvant, as reflected by total titer, IgG subclass analysis, IFA patterns, and transmission-blocking antibody effects. These findings are particularly important because the antibody titer against properly folded Pfs25 predicts transmission-blocking activity in the standard membrane feeding assay, hence providing the most robust in vitro predictor of vaccine efficacy among malaria vaccine strategies aimed at different stages of the malaria parasite. The mechanisms by which these adjuvants—squalene–oil-in-water with or without GLA—enhance immunogenicity (antibody titer, affinity, avidity) nonetheless remain a black box, although clear correlates in terms of dendritic cell antigen presentation and enhancement of cytokine stimulation (particularly gamma interferon) have been suggested (54–56). Future work will focus on an improved mechanistic understanding of how squalene–oil-in-water–GLA and other adjuvant and delivery systems lead to protective immune responses against malaria and how such protective responses might be measured with laboratory-based assays to predict vaccine efficacy. Improving our understanding of adjuvant mechanisms remains key to improving vaccine efficacy and potency while minimizing or eliminating adverse effects.
This study has limitations that should be considered when interpreting the data presented here. First, the immunogen—alga-produced recombinant Pfs25—has not yet been produced using cGMP protocols and has not yet been scaled up or purified to a homogeneous monomeric protein. Nonetheless, any such increase in purity would likely further enhance the potency of this recombinant protein as a vaccine candidate. Second, the mouse model used to generate antisera remains a proof of principle, and vaccine-induced antibody responses in mice are different from those obtained in vaccinations of nonhuman primates and humans. Finally, the present work did not provide a head-to-head comparison of alga-produced recombinant Pfs25 with other heterologous expression systems and vaccine presentation. The work presented here does support moving ahead with scaling up the alga-produced recombinant Pfs25 platform, purifying the protein to homogeneity, carrying out vaccination in nonhuman primates, and assessing immunogenicity in terms of transmission-blocking antibodies, potency (affinity, avidity, IgG subclass, and VH and VL chain variable region usage), and finally potential adverse events in preclinical testing.
In the larger context of malaria vaccine development, a strategy to develop, validate, and deploy a vaccine aimed at preventing endemic transmission of malaria continues to be important yet neglected (4). Alga-produced Pfs25 in combination with a human-compatible adjuvant composed of a TLR-4 agonist in a squalene–oil-in-water emulsion is an attractive new vaccine candidate that merits head-to-head comparison with other modalities of vaccine production and administration.
ACKNOWLEDGMENTS
We thank Paula Maguina and Jennifer Santini, Director of the UCSD Microscopy Core Facility, for expert and essential scientific, ethics, and logistical contributions to this work.
This work was supported by U.S. Public Health Service grants and cooperative agreements through the National Institutes of Health (U19AI089681 [J.M.V.], 1R01AI067727 [J.M.V.], K24AI068903 [J.M.V.], D43TW007120 [J.M.V.], and P30NS047101), the U.S. Department of Energy through grant DE-EE-0003373, the San Diego Foundation (J.A.G.), the California Energy Commission (500-10-039), and the Bill and Melinda Gates Foundation to D.C. and S.G.R. under grant number 42387.
REFERENCES
- 1.Carter R, Chen DH. 1976. Malaria transmission blocked by immunisation with gametes of the malaria parasite. Nature 263:57–60. doi: 10.1038/263057a0. [DOI] [PubMed] [Google Scholar]
- 2.Gwadz RW. 1976. Successful immunization against the sexual stages of Plasmodium gallinaceum. Science 193:1150–1151. doi: 10.1126/science.959832. [DOI] [PubMed] [Google Scholar]
- 3.Alonso PL, Brown G, Arevalo-Herrera M, Binka F, Chitnis C, Collins F, Doumbo OK, Greenwood B, Hall BF, Levine MM, Mendis K, Newman RD, Plowe CV, Rodriguez MH, Sinden R, Slutsker L, Tanner M. 2011. A research agenda to underpin malaria eradication. PLoS Med 8:e1000406. doi: 10.1371/journal.pmed.1000406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Abdulla S, Agre P, Alonso PL, Arevalo-Herrera M, Bassat Q, Binka F, Chitnis C, Corradin G, Cowman A, Culpepper J, del Portillo H, Dinglasan R, Duffy P, Gargallo D, Greenwood B, Guinovart C, Hall BF, Herrera S, Hoffman S, Lanzavecchia A, Leroy O, Levine MM, Loucq C, Mendis K, Milman J, Moorthy V, Pleuschke G, Plowe CV, Reed S, Sauerwein R, Saul A, Schofield L, Sinden R, Stubbs J, Villafana T, Wirth D, Yadav P, Ballou R, Brown G, Birkett A, Brandt W, Brooks A, Carter T, Golden A, Lee C, Nunes J, Puijalon O, Raphael T, Richards H, Warren C, Woods C, malERA Consultative Group on Vaccines. 2011. A research agenda for malaria eradication: vaccines. PLoS Med 8:e1000398. doi: 10.1371/journal.pmed.1000398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kaslow DC, Quakyi IA, Syin C, Raum MG, Keister DB, Coligan JE, McCutchan TF, Miller LH. 1988. A vaccine candidate from the sexual stage of human malaria that contains EGF-like domains. Nature 333:74–76. doi: 10.1038/333074a0. [DOI] [PubMed] [Google Scholar]
- 6.Miura K, Takashima E, Deng B, Tullo G, Diouf A, Moretz SE, Nikolaeva D, Diakite M, Fairhurst RM, Fay MP, Long CA, Tsuboi T. 2013. Functional comparison of Plasmodium falciparum transmission-blocking vaccine candidates by the standard membrane-feeding assay. Infect Immun 81:4377–4382. doi: 10.1128/IAI.01056-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zou L, Miles AP, Wang J, Stowers AW. 2003. Expression of malaria transmission-blocking vaccine antigen Pfs25 in Pichia pastoris for use in human clinical trials. Vaccine 21:1650–1657. doi: 10.1016/S0264-410X(02)00701-6. [DOI] [PubMed] [Google Scholar]
- 8.Stowers AW ZY, Shimp RL, Kaslow DC. 2001. Structural conformers produced during malaria vaccine production in yeast. Yeast 18:137–150. doi:. [DOI] [PubMed] [Google Scholar]
- 9.Stowers AW, Keister DB, Muratova O, Kaslow DC. 2000. A region of Plasmodium falciparum antigen Pfs25 that is the target of highly potent transmission-blocking antibodies. Infect Immun 68:5530–5538. doi: 10.1128/IAI.68.10.5530-5538.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kumar R, Angov E, Kumar N. 2014. Potent malaria transmission-blocking antibody responses elicited by Plasmodium falciparum Pfs25 expressed in Escherichia coli after successful protein refolding. Infect Immun 82:1453–1459. doi: 10.1128/IAI.01438-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kaslow DC, Bathurst IC, Lensen T, Ponnudurai T, Barr PJ, Keister DB. 1994. Saccharomyces cerevisiae recombinant Pfs25 adsorbed to alum elicits antibodies that block transmission of Plasmodium falciparum. Infect Immun 62:5576–5580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Arakawa T, Komesu A, Otsuki H, Sattabongkot J, Udomsangpetch R, Matsumoto Y, Tsuji N, Wu Y, Torii M, Tsuboi T. 2005. Nasal immunization with a malaria transmission-blocking vaccine candidate, Pfs25, induces complete protective immunity in mice against field isolates of Plasmodium falciparum. Infect Immun 73:7375–7380. doi: 10.1128/IAI.73.11.7375-7380.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mlambo G, Kumar N, Yoshida S. 2010. Functional immunogenicity of baculovirus expressing Pfs25, a human malaria transmission-blocking vaccine candidate antigen. Vaccine 28:7025–7029. doi: 10.1016/j.vaccine.2010.08.022. [DOI] [PubMed] [Google Scholar]
- 14.Jones RM, Chichester JA, Mett V, Jaje J, Tottey S, Manceva S, Casta LJ, Gibbs SK, Musiychuk K, Shamloul M, Norikane J, Mett V, Streatfield SJ, van de Vegte-Bolmer M, Roeffen W, Sauerwein RW, Yusibov V. 2013. A plant-produced Pfs25 VLP malaria vaccine candidate induces persistent transmission blocking antibodies against Plasmodium falciparum in immunized mice. PLoS One 8:e79538. doi: 10.1371/journal.pone.0079538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tsuboi T, Takeo S, Iriko H, Jin L, Tsuchimochi M, Matsuda S, Han ET, Otsuki H, Kaneko O, Sattabongkot J, Udomsangpetch R, Sawasaki T, Torii M, Endo Y. 2008. Wheat germ cell-free system-based production of malaria proteins for discovery of novel vaccine candidates. Infect Immun 76:1702–1708. doi: 10.1128/IAI.01539-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tsuboi T, Takeo S, Sawasaki T, Torii M, Endo Y. 2010. An efficient approach to the production of vaccines against the malaria parasite. Methods Mol Biol 607:73–83. doi: 10.1007/978-1-60327-331-2_8. [DOI] [PubMed] [Google Scholar]
- 17.Gregory JA, Li F, Tomosada LM, Cox CJ, Topol AB, Vinetz JM, Mayfield S. 2012. Algae-produced pfs25 elicits antibodies that inhibit malaria transmission. PLoS One 7:e37179. doi: 10.1371/journal.pone.0037179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gregory JA, Topol AB, Doerner DZ, Mayfield S. 2013. Alga-produced cholera toxin-Pfs25 fusion proteins as oral vaccines. Appl Environ Microbiol 79:3917–3925. doi: 10.1128/AEM.00714-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ugwu CU, Aoyagi H, Uchiyama H. 2008. Photobioreactors for mass cultivation of algae. Bioresour Technol 99:4021–4028. doi: 10.1016/j.biortech.2007.01.046. [DOI] [PubMed] [Google Scholar]
- 20.Mayfield SP, Manuell AL, Chen S, Wu J, Tran M, Siefker D, Muto M, Marin-Navarro J. 2007. Chlamydomonas reinhardtii chloroplasts as protein factories. Curr Opin Biotechnol 18:126–133. doi: 10.1016/j.copbio.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 21.Wu Y, Ellis RD, Shaffer D, Fontes E, Malkin EM, Mahanty S, Fay MP, Narum D, Rausch K, Miles AP, Aebig J, Orcutt A, Muratova O, Song G, Lambert L, Zhu D, Miura K, Long C, Saul A, Miller LH, Durbin AP. 2008. Phase 1 trial of malaria transmission blocking vaccine candidates Pfs25 and Pvs25 formulated with Montanide ISA 51. PLoS One 3:e2636. doi: 10.1371/journal.pone.0002636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Arias MA, Van Roey GA, Tregoning JS, Moutaftsi M, Coler RN, Windish HP, Reed SG, Carter D, Shattock RJ. 2012. Glucopyranosyl lipid adjuvant (GLA), a synthetic TLR4 agonist, promotes potent systemic and mucosal responses to intranasal immunization with HIVgp140. PLoS One 7:e41144. doi: 10.1371/journal.pone.0041144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Carter D, Reed SG. 2010. Role of adjuvants in modeling the immune response. Curr Opin HIV AIDS 5:409–413. doi: 10.1097/COH.0b013e32833d2cdb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Misquith A, Fung HWM, Dowling QM, Guderian JA, Vedvick TS, Fox CB. 2014. In vitro evaluation of TLR4 agonist activity: formulation effects. Colloids Surf B Biointerfaces 113:312–319. doi: 10.1016/j.colsurfb.2013.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Coler RN, Bertholet S, Moutaftsi M, Guderian JA, Windish HP, Baldwin SL, Laughlin EM, Duthie MS, Fox CB, Carter D, Friede M, Vedvick TS, Reed SG. 2011. Development and characterization of synthetic glucopyranosyl lipid adjuvant system as a vaccine adjuvant. PLoS One 6:e16333. doi: 10.1371/journal.pone.0016333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Treanor JJ, Essink B, Hull S, Reed S, Izikson R, Patriarca P, Goldenthal KL, Kohberger R, Dunkle LM. 2013. Evaluation of safety and immunogenicity of recombinant influenza hemagglutinin (H5/Indonesia/05/2005) formulated with and without a stable oil-in-water emulsion containing glucopyranosyl-lipid A (SE+GLA) adjuvant. Vaccine 31:5760–5765. doi: 10.1016/j.vaccine.2013.08.064. [DOI] [PubMed] [Google Scholar]
- 27.Fox CB, Moutaftsi M, Vergara J, Desbien AL, Nana GI, Vedvick TS, Coler RN, Reed SG. 2013. TLR4 ligand formulation causes distinct effects on antigen-specific cell-mediated and humoral immune responses. Vaccine 31:5848–5855. doi: 10.1016/j.vaccine.2013.09.069. [DOI] [PubMed] [Google Scholar]
- 28.Bruder JT, Angov E, Limbach KJ, Richie TL. 2010. Molecular vaccines for malaria. Hum Vaccin 6:54–77. doi: 10.4161/hv.6.1.10463. [DOI] [PubMed] [Google Scholar]
- 29.Rappuoli R, Aderem A. 2011. A 2020 vision for vaccines against HIV, tuberculosis and malaria. Nature 473:463–469. doi: 10.1038/nature10124. [DOI] [PubMed] [Google Scholar]
- 30.Lai RP, Seaman MS, Tonks P, Wegmann F, Seilly DJ, Frost SD, LaBranche CC, Montefiori DC, Dey AK, Srivastava IK, Sattentau Q, Barnett SW, Heeney JL. 2012. Mixed adjuvant formulations reveal a new combination that elicit antibody response comparable to Freund's adjuvants. PLoS One 7:e35083. doi: 10.1371/journal.pone.0035083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Towbin H, Staehelin T, Gordon J. 1992. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. 1979. Biotechnology 24:145–149. [PubMed] [Google Scholar]
- 32.Bounkeua V, Li F, Chuquiyauri R, Abeles SR, McClean CM, Neyra V, Llanos-Cuentas A, Yori PP, Vinetz JM. 2011. Lack of molecular correlates of Plasmodium vivax ookinete development. Am J Trop Med Hyg 85:207–213. doi: 10.4269/ajtmh.2011.10-0729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bounkeua V, Li F, Vinetz JM. 2010. In vitro generation of Plasmodium falciparum ookinetes. Am J Trop Med Hyg 83:1187–1194. doi: 10.4269/ajtmh.2010.10-0433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Scholz SM, Simon N, Lavazec C, Dude MA, Templeton TJ, Pradel G. 2008. PfCCp proteins of Plasmodium falciparum: gametocyte-specific expression and role in complement-mediated inhibition of exflagellation. Int J Parasitol 38:327–340. doi: 10.1016/j.ijpara.2007.08.009. [DOI] [PubMed] [Google Scholar]
- 35.Miura K, Keister DB, Muratova OV, Sattabongkot J, Long CA, Saul A. 2007. Transmission-blocking activity induced by malaria vaccine candidates Pfs25/Pvs25 is a direct and predictable function of antibody titer. Malar J 6:107. doi: 10.1186/1475-2875-6-107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rener J, Graves PM, Carter R, Williams JL, Burkot TR. 1983. Target antigens of transmission-blocking immunity on gametes of Plasmodium falciparum. J Exp Med 158:976–981. doi: 10.1084/jem.158.3.976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Barr PJ, Green KM, Gibson HL, Bathurst IC, Quakyi IA, Kaslow DC. 1991. Recombinant Pfs25 protein of Plasmodium falciparum elicits malaria transmission-blocking immunity in experimental animals. J Exp Med 174:1203–1208. doi: 10.1084/jem.174.5.1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Farrance CE, Chichester JA, Musiychuk K, Shamloul M, Rhee A, Manceva SD, Jones RM, Mamedov T, Sharma S, Mett V, Streatfield SJ, Roeffen W, van de Vegte-Bolmer M, Sauerwein RW, Wu Y, Muratova O, Miller L, Duffy P, Sinden R, Yusibov V. 2011. Antibodies to plant-produced Plasmodium falciparum sexual stage protein Pfs25 exhibit transmission blocking activity. Hum Vaccin 7(Suppl):191–198. doi: 10.4161/hv.7.0.14588. [DOI] [PubMed] [Google Scholar]
- 39.Jones RM, Chichester JA, Manceva S, Gibbs SK, Musiychuk K, Shamloul M, Norikane J, Streatfield SJ, van de Vegte-Bolmer M, Roeffen W, Sauerwein RW, Yusibov V. 2015. A novel plant-produced Pfs25 fusion subunit vaccine induces long-lasting transmission blocking antibody responses. Hum Vaccin Immunother 11:124–132. doi: 10.4161/hv.34366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kaslow DC, Shiloach J. 1994. Production, purification and immunogenicity of a malaria transmission-blocking vaccine candidate: TBV25H expressed in yeast and purified using nickel-NTA agarose. Biotechnology 12:494–499. doi: 10.1038/nbt0594-494. [DOI] [PubMed] [Google Scholar]
- 41.Gozar MM, Price VL, Kaslow DC. 1998. Saccharomyces cerevisiae-secreted fusion proteins Pfs25 and Pfs28 elicit potent Plasmodium falciparum transmission-blocking antibodies in mice. Infect Immun 66:59–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shimp RL Jr, Rowe C, Reiter K, Chen B, Nguyen V, Aebig J, Rausch KM, Kumar K, Wu Y, Jin AJ, Jones DS, Narum DL. 2013. Development of a Pfs25-EPA malaria transmission blocking vaccine as a chemically conjugated nanoparticle. Vaccine 31:2954–2962. doi: 10.1016/j.vaccine.2013.04.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Grifantini R, Finco O, Bartolini E, Draghi M, Del Giudice G, Kocken C, Thomas A, Abrignani S, Grandi G. 1998. Multiplasmid DNA vaccination avoids antigenic competition and enhances immunogenicity of a poorly immunogenic plasmid. Eur J Immunol 28:1225–1232. doi:. [DOI] [PubMed] [Google Scholar]
- 44.Lobo CA, Dhar R, Kumar N. 1999. Immunization of mice with DNA-based Pfs25 elicits potent malaria transmission-blocking antibodies. Infect Immun 67:1688–1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kubler-Kielb J, Majadly F, Biesova Z, Mocca CP, Guo C, Nussenzweig R, Nussenzweig V, Mishra S, Wu Y, Miller LH, Keith JM, Liu TY, Robbins JB, Schneerson R. 2010. A bicomponent Plasmodium falciparum investigational vaccine composed of protein-peptide conjugates. Proc Natl Acad Sci U S A 107:1172–1177. doi: 10.1073/pnas.0913374107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Qian F, Wu Y, Muratova O, Zhou H, Dobrescu G, Duggan P, Lynn L, Song G, Zhang Y, Reiter K, MacDonald N, Narum DL, Long CA, Miller LH, Saul A, Mullen GE. 2007. Conjugating recombinant proteins to Pseudomonas aeruginosa ExoProtein A: a strategy for enhancing immunogenicity of malaria vaccine candidates. Vaccine 25:3923–3933. doi: 10.1016/j.vaccine.2007.02.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Da DF, Dixit S, Sattabonkot J, Mu J, Abate L, Ramineni B, Ouedraogo JB, MacDonald NJ, Fay MP, Su XZ, Cohuet A, Wu Y. 2013. Anti-Pfs25 human plasma reduces transmission of Plasmodium falciparum isolates that have diverse genetic backgrounds. Infect Immun 81:1984–1989. doi: 10.1128/IAI.00016-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Miyata T, Harakuni T, Sugawa H, Sattabongkot J, Kato A, Tachibana M, Torii M, Tsuboi T, Arakawa T. 2011. Adenovirus-vectored Plasmodium vivax ookinete surface protein, Pvs25, as a potential transmission-blocking vaccine. Vaccine 29:2720–2726. doi: 10.1016/j.vaccine.2011.01.083. [DOI] [PubMed] [Google Scholar]
- 49.Dove A. 2002. Uncorking the biomanufacturing bottleneck. Nat Biotechnol 20:777–779. doi: 10.1038/nbt0802-777. [DOI] [PubMed] [Google Scholar]
- 50.Fletcher SP, Muto M, Mayfield SP. 2007. Optimization of recombinant protein expression in the chloroplasts of green algae. Adv Exp Med Biol 616:90–98. doi: 10.1007/978-0-387-75532-8_8. [DOI] [PubMed] [Google Scholar]
- 51.Schwenk R, DeBot M, Porter M, Nikki J, Rein L, Spaccapelo R, Crisanti A, Wightman PD, Ockenhouse CF, Dutta S. 2014. IgG2 antibodies against a clinical grade Plasmodium falciparum CSP vaccine antigen associate with protection against transgenic sporozoite challenge in mice. PLoS One 9:e111020. doi: 10.1371/journal.pone.0111020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Burghaus PA, Wellde BT, Hall T, Richards RL, Egan AF, Riley EM, Ballou WR, Holder AA. 1996. Immunization of Aotus nancymai with recombinant C terminus of Plasmodium falciparum merozoite surface protein 1 in liposomes and alum adjuvant does not induce protection against a challenge infection. Infect Immun 64:3614–3619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Keitel WA, Kester KE, Atmar RL, White AC, Bond NH, Holland CA, Krzych U, Palmer DR, Egan A, Diggs C, Ballou WR, Hall BF, Kaslow D. 1999. Phase I trial of two recombinant vaccines containing the 19kd carboxy terminal fragment of Plasmodium falciparum merozoite surface protein 1 (msp-1(19)) and T helper epitopes of tetanus toxoid. Vaccine 18:531–539. doi: 10.1016/S0264-410X(99)00221-2. [DOI] [PubMed] [Google Scholar]
- 54.Leroux-Roels G. 2010. Unmet needs in modern vaccinology: adjuvants to improve the immune response. Vaccine 28(Suppl 3):C25–C36. doi: 10.1016/j.vaccine.2010.07.021. [DOI] [PubMed] [Google Scholar]
- 55.Casares S, Brumeanu TD, Richie TL. 2010. The RTS,S malaria vaccine. Vaccine 28:4880–4894. doi: 10.1016/j.vaccine.2010.05.033. [DOI] [PubMed] [Google Scholar]
- 56.Lousada-Dietrich S, Jogdand PS, Jepsen S, Pinto VV, Ditlev SB, Christiansen M, Larsen SO, Fox CB, Raman VS, Howard RF, Vedvick TS, Ireton G, Carter D, Reed SG, Theisen M. 2011. A synthetic TLR4 agonist formulated in an emulsion enhances humoral and Type 1 cellular immune responses against GMZ2–a GLURP-MSP3 fusion protein malaria vaccine candidate. Vaccine 29:3284–3292. doi: 10.1016/j.vaccine.2011.02.022. [DOI] [PubMed] [Google Scholar]