Abstract
We recently demonstrated that the secreted aspartyl proteinases (Saps), Sap2 and Sap6, of Candida albicans have the potential to induce the canonical activation of the NLRP3 inflammasome, leading to the secretion of interleukin-1β (IL-1β) and IL-18 via caspase-1 activation. We also observed that the activation of caspase-1 is partially independent from the NLRP3 activation pathway. In this study, we examined whether Sap2 and Sap6 are also able to activate the noncanonical inflammasome pathway in murine macrophages. Our data show that both Sap2 and Sap6 can activate caspase-11 through type I interferon (IFN) production. Caspase-11 cooperates to activate caspase-1, with a subsequent increase of IL-1β secretion. Endocytosis and internalization of Saps are required for the induction of type I IFN production, which is essential for induction of noncanonical inflammasome activation. Our study indicates a sophisticated interplay between caspase-1 and caspase-11 that connects the canonical and noncanonical pathways of inflammasome activation in response to C. albicans Saps.
INTRODUCTION
Only a few Candida species are known as normally harmless commensals in the gastrointestinal, genitourinary, or oropharyngeal tract of most healthy individuals, but these can also cause superficial or systemic infections (1, 2). In fact, Candida species are the fourth most common cause of hospital-acquired systemic infections associated with a mortality rate of up to 50% (3). The leading Candida species, which causes more than 50% of all cases of candidiasis, is Candida albicans.
C. albicans is equipped with an arsenal of fitness and virulence attributes which are crucial for causing infections (4). Morphological plasticity is key to the shift of C. albicans from commensalism to pathogenicity and includes the yeast-to-hypha transition and the production of pseudohyphae (filamentous forms). While pseudohyphae consist of elongated ellipsoidal cells with constrictions at the septa, true hyphae lack constrictions at the septa and express a distinct set of hypha-associated genes (5, 6). Other morphologies include white/opaque switching and chlamydospores (4).
This morphological flexibility greatly contributes to the pathogenesis of candidiasis, and the different morphological forms are believed to play different roles at the various stages of infection, including recognition by immune cells, adherence to mucosal surfaces, invasion and tissue damage, and escape from immune system surveillance (7, 8). Additional important virulence factors produced by C. albicans are the secreted aspartyl proteinases (Saps). These enzymes are encoded by a family of 10 SAP genes that have been extensively studied as key virulence determinants of C. albicans (1). Of note, Sap2 is the most abundant protease expressed by yeast cells in medium with protein as the sole source of nitrogen; expression of Sap1 and Sap3 has been associated with white/opaque switching, while Sap4 to Sap6 are hypha specific (1). Similar proteases are produced by other pathogenic Candida species, including C. parapsilosis and C. tropicalis (but not C. glabrata) (9, 10) and contribute to the virulence potential of these fungi.
We previously reported that Sap1, Sap2, Sap3, and Sap6 show different abilities to induce the secretion of proinflammatory cytokines by human monocytes. In particular Sap1, Sap2, and Sap6 were able to induce a great amount of cytokines, such as interleukin-1β (IL-1β), tumor necrosis factor alpha (TNF-α), and IL-6, by human monocytes, suggesting that Saps contribute to the pathogenesis of candidiasis by activating the innate immune system (11). We also demonstrated that the secretion of IL-1β and IL-18 is greatly mediated by the canonical activation of the NLRP3 inflammasome (12). NLRP3 influences an innate immune response by forming an intracellular multimolecular complex, called the inflammasome, which leads to upregulation of IL-1β and IL-18 (13). A variety of pathogen- and host-derived “danger” signals activate the NLRP3 inflammasome, including whole pathogens, pathogen-associated molecular patterns (PAMPs), other pathogen-associated molecules (e.g., bacterial pore-forming toxins), host-derived indicators of cellular damage (danger-associated molecular patterns, or DAMPs), and environmental irritants. Upon activation, NLRP3, via the adaptor protein ASC, induces caspase-1 activation, which cleaves pro-IL-1β and pro-IL-18 into biologically active cytokines (13, 14).
Our own data suggest that Sap2 and Sap6 proteins trigger IL-1β production through inflammasome activation (11, 12). This occurs via NLRP3 and caspase-1 activation, which in turn cleaves pro-IL-1β into secreted bioactive IL-1β. Interleukin-1β production was monitored in monocytes, in monocyte-derived macrophages, and in dendritic cells after Sap stimulation (12). Inflammasome activation required Sap internalization via a clathrin-dependent mechanism, intracellular induction of K+ efflux, and reactive oxygen species (ROS) production (12). Of note, we observed that silencing of NLRP3 did not lead to complete inhibition of IL-1β secretion. Residual NLRP3 or additional activities could contribute to production of the remaining IL-1β, which is considerable (about 20%), and we speculate that these mechanisms contribute to the full activation and IL-1β production observed.
In this study, we examined whether Sap2 and Sap6 have the capacity to activate an additional noncanonical inflammasome pathway (15, 16) that finally leads to innate immune cell activation.
MATERIALS AND METHODS
Aspartyl proteinase production.
Recombinant C. albicans aspartyl proteinases 2 and 6 (Sap2 and Sap6) were expressed as recombinant proteins using Pichia pastoris clones (17); the proteins were purified and tested negative for endotoxin contamination in a Limulus assay (E-Toxate; Sigma) with a sensitivity of 10 pg/ml of Escherichia coli lipopolysaccharide (LPS) (11).
Mice.
Female, 6- to 8-week-old, inbred C57BL/6 mice were obtained by Harlan Nossan Laboratories, Milan, Italy, and housed in the animal facility of the University of Perugia (authorization number 34/2003A).
Ethics statement.
The procedures involving the animals and their care were conducted in conformity with national and international laws and policies. All animal experiments were performed in agreement with EU Directive 2010/63, the European Convention for the Protection of Vertebrate Animals used for Experimental and other Scientific Purposes, and National Law 116/92. The protocol was approved by Perugia University Ethics Committee for animal care and use, permit number 149/2009-B.
Cells.
The murine macrophage cell line RAW 264.7 was obtained from the ATCC. Cells were maintained in Dulbecco's modified Eagle's medium (DMEM) with l-glutamine supplemented with 10% fetal calf serum (FCS) and antibiotics (complete medium) (all from EuroClone) at 37°C in 5% CO2. Peritoneal murine macrophages (PM) were collected 4 days after the intraperitoneal injection of 0.5 ml of endotoxin-free 10% thioglycolate solution (Difco) (18).
Western blot analysis.
RAW 264.7 macrophages (5 × 106/ml) were pretreated in complete medium in the presence or absence of a selective inhibitor of caspase-11 (IC-11), wedelolactone (40 μM; Santa Cruz Biotechnology) (19), or a neutralizing monoclonal antibody (MAb) to type I interferon (IFN) receptor (IFNAR1) (0.5 μg/ml; Millipore) (20) for 1 h, or chlorpromazine (10 μg/ml; Sigma) for 30 min at 37°C in 5% CO2. Cells were then stimulated in the presence or absence of 10 μg/ml LPS plus 5 mM ATP (LPS-ATP) (both from Sigma) or with Sap2 or Sap6 (each, 20 μg/ml) (11) for 30 min, 2, or 4 h at 37°C in 5% CO2. To simultaneously stop the cultures, the stimuli were added starting from 4 h to 30 min.
After incubation, cells were washed and lysed with mammalian protein extraction reagent in the presence of protease and phosphatase inhibitors (all from Pierce). Protein concentrations were determined with a bicinchoninic acid (BCA) protein assay reagent kit (Pierce). The lysates (60 μg) were separated by sodium dodecyl sulfate–10% PAGE and transferred to a nitrocellulose membrane (Pierce) for 1 h at 100 V in a blotting system (Bio-Rad). The membranes were incubated overnight with polyclonal antibodies (Abs) to caspase-11 (p20) (rabbit), caspase-1 (p10) (goat), and IL-1β (p17) (goat) (all at a dilution 1/200; Santa Cruz), NLRP3 (p117) (rabbit) (5 μg/ml; Imgenex), and caspase-8 (p18) (mouse) (dilution of 1/1,000; Cell signaling) in blocking buffer. Detection was achieved with the appropriate secondary Ab coupled to horseradish peroxidase (HRP), followed by a chemiluminescence trial kit (Chemicon International). Immunoblotting with rabbit polyclonal Ab to actin (p43) (dilution, 1/200; Santa Cruz) was used as an internal loading control. Immunoreactive bands were visualized by a ChemiDoc instrument (Bio-Rad). A quantitative analysis of the region of interest was performed using the program Fiji Is Just ImageJ.
Flow cytometry analysis.
RAW 264.7 macrophages (1 × 106/ml) were pretreated in complete medium in the presence or absence of IC-11 (40 μM) (19) for 1 h at 37°C in 5% CO2 and then stimulated in the presence or absence of LPS (10 μg/ml) plus ATP (5 mM) or with Sap2 or Sap6 (each, 20 μg/ml) for 2 h at 37°C in 5% CO2. After incubation, cells were fixed with 1.5% formalin for 10 min at room temperature (RT), washed, and permeabilized with absolute methanol (500 μl/106 cells) for 10 min on ice and incubated with Ab to caspase-11 (p20) (rabbit) (dilution, 1/50; Santa Cruz) for 20 min on ice, followed by Cy3-labeled conjugated affinity-purified secondary Ab (dilution, 1/250; Chemicon International) (21), and analyzed with a FACSCalibur flow cytometer (BD Biosciences). Autofluorescence was assessed using untreated cells. Control staining of cells with an irrelevant Ab was used to obtain background fluorescence values.
Cytokine production.
Peritoneal murine macrophages (PM) or RAW 264.7 macrophages (both, 5 × 106 cells/ml) were pretreated in complete medium in the presence or absence of IC-11 (40 μM) (19) or a neutralizing MAb to IFNAR1 (0.5 μg/ml) (Millipore) (20) for 1 h or with chlorpromazine (10 μg/ml) for 30 min at 37°C in 5% CO2. Cells were then stimulated in the presence or absence of LPS (10 μg/ml) plus ATP (5 mM) or with Sap2 or Sap6 (each, 20 μg/ml) for 2, 18, or 48 h at 37°C in 5% CO2. After incubation, the supernatants were collected and tested for IFN-α, IL-1β, and IL-18 levels by specific enzyme-linked immunosorbent assays (ELISAs; eBioscience). Cytokine titers were calculated relative to standard curves.
Statistical analysis.
The results reported in the bar graphs are the means ± standard errors of the means (SEM) from triplicate samples of three to five different experiments. Quantitative variables were tested for normal distribution and compared by means of a Student's t test. A P value of <0.05 was considered significant.
RESULTS
Saps induce caspase-11 activation.
Recently, we demonstrated that Sap2 and Sap6 are able to induce NLRP3 inflammasome activation that leads to subsequent cleavage of procaspase-1 into caspase-1 and cleavage of pro-IL-1β into IL-1β (12). Silencing NLRP3 leads to decreased production of IL-1β but not to a complete blockade. Hence, we investigated whether possible additional mechanisms that support canonical inflammasome activation are involved.
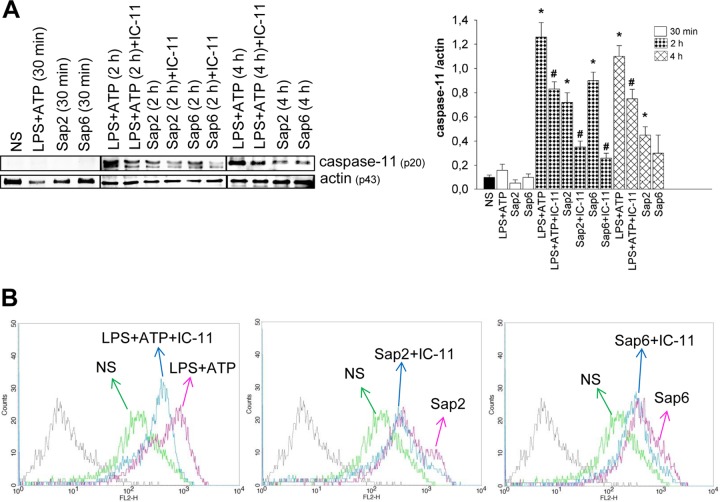
Recent studies reported that caspase-11 is a regulator of caspase-1 activation in response to enteric bacteria (22). Thus, we first analyzed whether selected Saps induce caspase-11 activation. To this end, cells of the murine macrophage cell line RAW 264.7 were treated for 30 min, 2, and 4 h with LPS-ATP as a positive control or with Sap2 or Sap6 (each, 20 μg/ml) in the presence or absence of a selective inhibitor of caspase-11 (IC-11) at a concentration of 40 μM (19). This dose of Saps was previously found to be the optimal dose to induce IL-1β production (11). The results showed that Sap2 induces caspase-11 activation at 2 and 4 h posttreatment, while Sap6 induces significant activation of caspase-11 after 2 h and that this level decreases in 4 h. LPS-ATP induced a greater amount of caspase-11 activation after 2 and 4 h. Of note, no activation of caspase-11 was observed within 30 min of incubation under all conditions. Treatment of cells with IC-11 markedly reduced Sap-induced activation of caspase-11 (Fig. 1A).
FIG 1.
Caspase-11 activation. (A) RAW 264.7 macrophages (5 × 106/ml) were pretreated for 1 h in the presence or absence of IC-11 (40 μM) and then cultured for 30 min, 2, or 4 h in the presence or absence (not stimulated, NS) of LPS (10 μg/ml) plus ATP (5 mM) or with Sap2 or Sap6 (each, 20 μg/ml) in complete medium at 37°C in 5% CO2. After incubation cell lysates were subjected to Western blotting. Membranes were incubated with Abs to caspase-11 (p20) and actin (p43). Bands of one experiment of five with similar results are shown for caspase-11 (left panel); caspase-11 (right panel) was normalized against actin. Error bars denote SEM. *, P < 0.05, for results from LPS-ATP- or Sap-treated cells versus those from untreated (NS) cells; #, P < 0.05, for results from IC-11-treated cells (with LPS-ATP or Saps) versus those from IC-11-untreated cells (LPS-ATP or Saps alone). (B) RAW 264.7 macrophages (1 × 106/ml) were cultured as described for panel A. After 2 h of stimulation, intracellular staining of cells was performed using caspase-11 (p20) Ab, followed by Cy3-labeled secondary Ab, and cells were analyzed by a FACSCalibur flow cytometer. Representative FACSCalibur histograms show the mean of fluorescence intensity of one experiment of three with similar results.
The activation of caspase-11 was also assessed by cytofluorometric analysis, and the results clearly confirmed that Sap2 and Sap6 stimulation resulted in activation of caspase-11 within 2 h of incubation and that IC-11 was able to downgrade this activation (Fig. 1B).
Involvement of caspase-11 in Sap-induced IL-1β production.
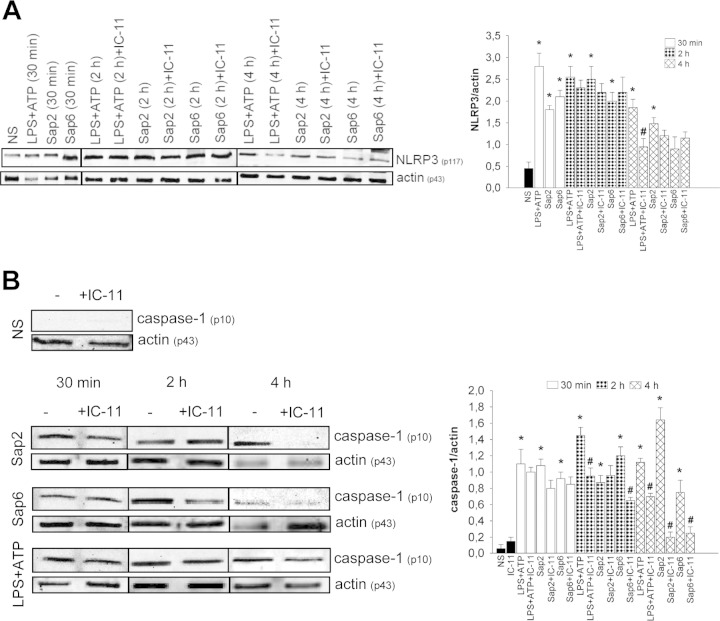
To examine whether caspase-11 influences the NLRP3 inflammasome, cells were treated with Sap2 and Sap6 in the presence or absence of IC-11, and the expression of NLRP3 was evaluated. The results shown in Fig. 2A indicate that Sap2 and Sap6 activate NLRP3 already after 30 min of stimulation and that this effect persists until 4 h. The inhibitor of caspase-11 does not modify the activation of Sap-induced NLRP3 under any condition investigated (Fig. 2A).
FIG 2.
NLRP3 and caspase-1 activation. RAW 264.7 macrophages (5 × 106/ml) were pretreated for 1 h in the presence or absence of IC-11 (40 μM) and then cultured for 30 min, 2, or 4 h in the presence or absence (NS) of LPS (10 μg/ml) plus ATP (5 mM) or with Sap2 or Sap6 (each, 20 μg/ml) in complete medium at 37°C in 5% CO2. After incubation cell lysates were subjected to Western blotting. Membranes were incubated with Abs to NLRP3 (p117), caspase-1 (p10), and actin (p43). Bands of one experiment of five with similar results are shown for NLRP3 (A, left panel) and caspase-1 (B, left panel). NLRP3 (A, right panel) and caspase-1 (B, right panel) amounts were normalized against those of actin. Error bars denote SEM. *, P < 0.05, for results from LPS-ATP- or Sap-treated cells versus those from untreated (NS) cells; #, P < 0.05, for results from IC-11-treated cells (with LPS-ATP or Saps) versus those from IC-11-untreated cells (LPS-ATP or Saps alone).
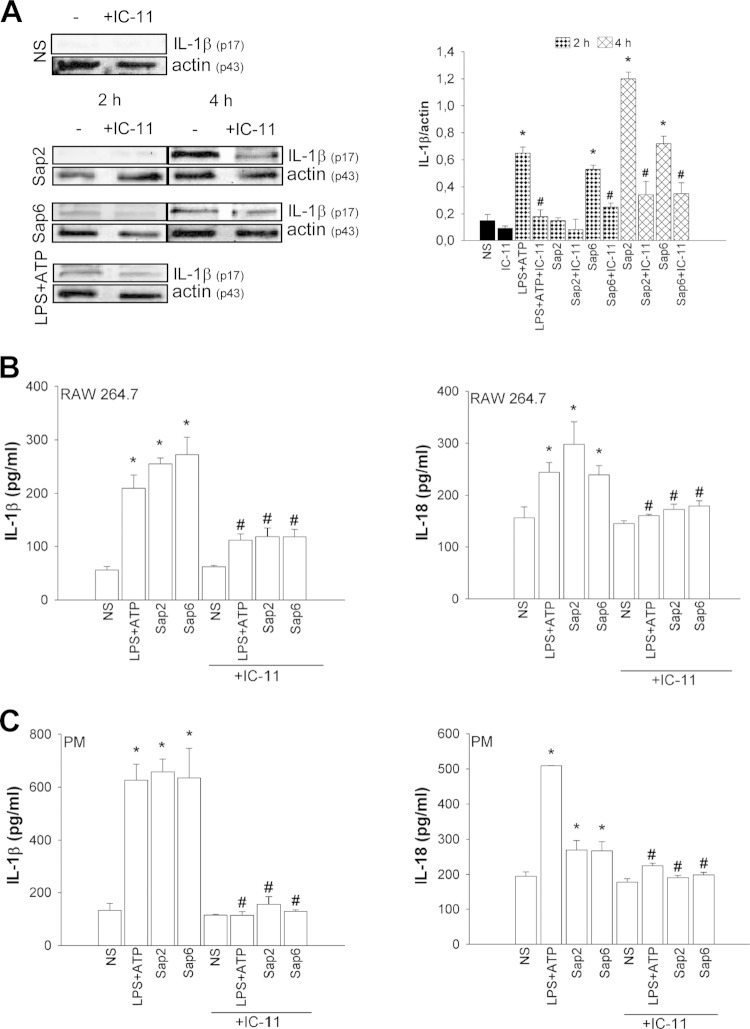
Additional experiments were performed to evaluate whether the activation of caspase-11 influences caspase-1 activation. Sap2 and Sap6 increased cleavage of caspase-1 with different kinetics. In particular, maximum activation of caspase-1 was observed after 4 h of treatment with Sap2, while Sap6 induced the optimal stimulation of caspase-1 after 2 h. Regarding the stimulation with Sap2, treatment with IC-11 produced a drastic decrease of caspase-1 activation at 4 h posttreatment while the addition of IC-11 clearly inhibited caspase-1 activation at 2 and 4 h of incubation in response to Sap6. This inhibition was substantial even after 4 h, when the Sap6-induced activation of caspase-1 declined over time, suggesting that the modest activation of caspase-11 is indeed still able to regulate caspase-1 (Fig. 2B). Notably, pro-IL-1β was not cleaved after 2 h of stimulation with Sap2 but was clearly cleaved after 4 h. At this time, the addition of IC-11 drastically reduced the amount of cleaved IL-1β. When the cells were stimulated with Sap6, treatment with IC-11 produced an inhibition of Sap6-induced IL-1β cleavage after 2 and 4 h (Fig. 3A). As previously demonstrated, IL-1β and IL-18 were secreted via NLRP3 activation (12); as a consequence, the production of these cytokines was tested in the presence or absence of IC-11. RAW 264.7 macrophages were stimulated with LPS-ATP or with Sap2 or Sap6 in the presence or absence of IC-11. The results reported in Fig. 3B show that both Saps induced the production of large amounts of IL-1β and IL-18 in culture supernatants after 48 h of treatment and that IC-11 drastically reduced the production of the two cytokines (Fig. 3B). Similar results were obtained when peritoneal murine macrophages (PM) were used (Fig. 3C). Again, addition of IC-11 caused a strong inhibition of Sap-induced IL-1β and IL-18 production (Fig. 3C). The abrogation of IL-1β production mediated by IC-11 evidenced in PM suggests that these cells are more susceptible to IC-11 than RAW 264.7 macrophages. Alternatively, secreted IL-1β is more promptly reutilized by PM than by RAW 264.7 macrophages, particularly considering that the incubation time was 48 h.
FIG 3.
IL-1β activation and cytokine production. (A) RAW 264.7 macrophages (5 × 106/ml) were pretreated for 1 h in the presence or absence of IC-11 (40 μM) and then cultured for 2 or 4 h in the presence or absence (NS) of LPS (10 μg/ml) plus ATP (5 mM) or with Sap2 or Sap6 (each, 20 μg/ml) in complete medium at 37°C in 5% CO2. After incubation, cell lysates were subjected to Western blotting. Membranes were incubated with Abs to IL-1β (p17) and actin (p43). Bands of one experiment of five with similar results are shown (left panel); IL-1β was normalized against actin (right panel). Error bars denote SEM. *, P < 0.05, for results from LPS-ATP- or Sap-treated cells versus those from untreated (NS) cells; #, P < 0.05, for results from IC-11-treated cells (with LPS-ATP or Saps) versus those from IC-11-untreated cells (LPS-ATP or Saps alone). (B and C) RAW 264.7 macrophages or PM (both, 5 × 106/ml) were pretreated for 1 h in the presence or absence of IC-11 (40 μM) and then cultured for 48 h in the presence or absence (NS) of LPS (10 μg/ml) plus ATP (5 mM) or with Sap2 or Sap6 (each, 20 μg/ml) in complete medium at 37°C in 5% CO2. After incubation, IL-1β and IL-18 levels were evaluated in culture supernatants using specific ELISAs. Error bars denote SEM of triplicate samples of three experiments. *, P < 0.05, for results from LPS-ATP-treated or Sap-treated cells versus those from untreated (NS) cells; #, P < 0.05, for results from IC-11-treated cells (with LPS-ATP or Saps) versus those from IC-11-untreated cells (LPS-ATP or Saps alone).
Regulation of caspase-11 activation by Sap-induced type I interferon.
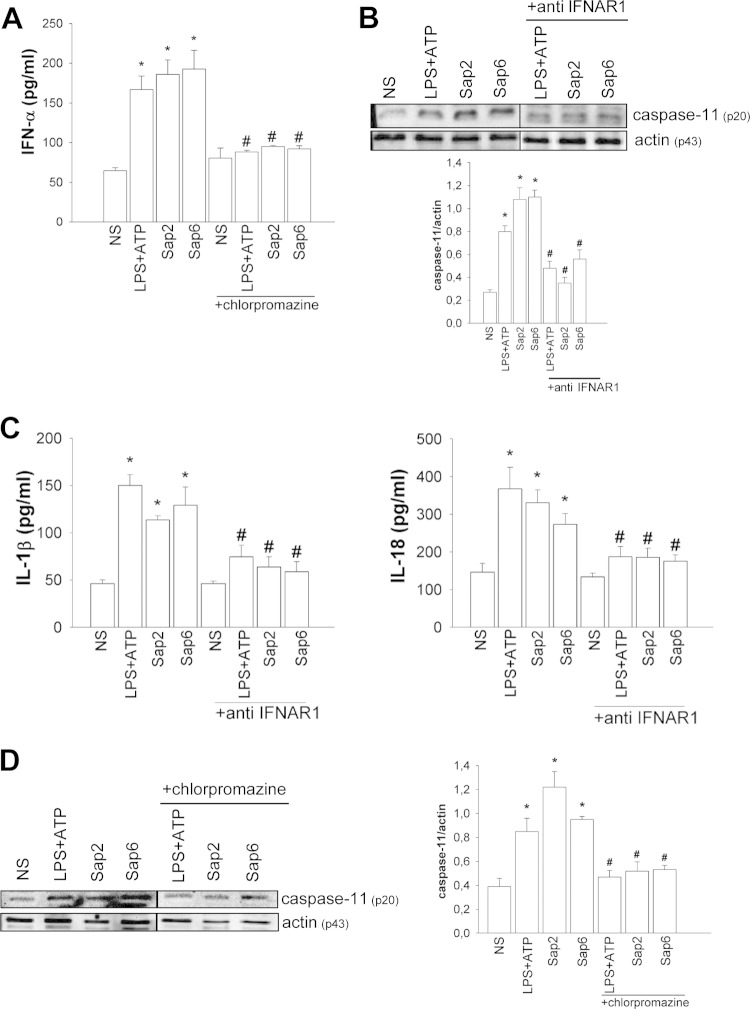
It has been reported that caspase-11 may be activated by type I interferon (IFN) (also known as IFN-α). Therefore, we analyzed whether Sap2 and Sap6 are able to induce IFN-α release. The results documented in Fig. 4A show that both Saps are able to induce significant levels of IFN-α after 2 h of incubation (Fig. 4A). This effect was also evident after 18 h of incubation (data not shown). Since we have been able to demonstrate that uptake of Sap2 and Sap6 is inhibited by the clathrin-dependent endocytosis inhibitor chlorpromazine (12), we evaluated whether chlorpromazine treatment would affect Sap-induced IFN-α production. Our results show that pretreatment with chlorpromazine significantly inhibited Sap-induced IFN-α production (Fig. 4A).
FIG 4.
Neutralizing antibody to IFNAR1 and chlorpromazine block Sap-mediated caspase-11 activation. (A) RAW 264.7 macrophages (5 × 106/ml) were pretreated for 30 min in the presence or absence (NS) of chlorpromazine (10 μg/ml) and then incubated for 2 h with LPS (10 μg/ml) plus ATP (5 mM) or with Sap2 or Sap6 (each, 20 μg/ml) in complete medium at 37°C in 5% CO2. After incubation culture supernatants were collected and tested for IFN-α production. Error bars denote SEM of triplicate samples of three experiments. *, P < 0.05, for results from LPS-ATP-treated or Sap-treated cells versus those from untreated (NS) cells; #, P < 0.05, for results from chlorpromazine-treated cells (with LPS-ATP or Saps) versus those from chlorpromazine-untreated cells (LPS-ATP or Saps alone). (B) RAW 264.7 macrophages (5 × 106/ml) were pretreated for 1 h in the presence or absence of MAb to IFNAR1 (0.5 μg/ml) and then cultured for 2 h in the presence or absence (NS) of LPS (10 μg/ml) plus ATP (5 mM) or with Sap2 or Sap6 (each, 20 μg/ml) in complete medium at 37°C in 5% CO2. After incubation cell lysates were subjected to Western blotting. Membranes were incubated with Abs to caspase-11 (p20) and actin (p43). Bands of one experiment of five with similar results are shown (upper panel). The amount of Caspase-11 was normalized against that of actin (lower panel). Error bars denote SEM. *, P < 0.05, for results from LPS-ATP-treated or Sap-treated cells versus those from untreated (NS) cells; #, P < 0.05, for results from anti-IFNAR1 treated cells (with LPS-ATP or Saps) versus those from anti-IFNAR1-untreated cells (LPS-ATP or Saps alone). (C) RAW 264.7 macrophages (5 × 106/ml) were treated as described above in complete medium at 37°C in 5% CO2. After 48 h of incubation, IL-1β and IL-18 levels were evaluated in culture supernatants using specific ELISAs. Error bars denote SEM of triplicate samples of three experiments. *, P < 0.05, for results from LPS-ATP-treated or Sap-treated cells versus those from untreated (NS) cells; #, P < 0.05, for results from anti IFNAR1-treated cells (with LPS-ATP or Saps) versus those from anti-IFNAR1-untreated cells (LPS-ATP or Saps alone). (C) Cell lysates from the experiment described for panel A were subjected to Western blotting. Membranes were incubated with Abs to caspase-11 (p20) and actin (p43). Bands of one experiment of five with similar results are shown (left panel). The amount of caspase-11 was normalized against that of actin (right panel). Error bars denote SEM. *, P < 0.05, for results from LPS-ATP-treated or Sap-treated cells versus those from untreated (NS) cells; #, P < 0.05, for results from chlorpromazine-treated cells (with LPS-ATP or Saps) versus those from chlorpromazine-untreated cells (LPS-ATP or Saps alone).
Next, the involvement of IFN-α in the activation of caspase-11 was determined. The cells were treated in the presence or absence of MAb to IFN-α receptor 1 (IFNAR1), and the activation of caspase-11 was determined. The results shown in Fig. 4B indicate that caspase-11 activation is drastically reduced by blocking IFNAR1 on RAW 264.7 macrophages.
Moreover, to verify whether the treatment with MAb to IFNAR1 also influenced the production of IL-1β and IL-18, cells were pretreated with MAb to IFNAR1 and stimulated with Saps for 48 h. The results show that, by blocking IFNAR1, Sap-induced production of both cytokines was significantly decreased (Fig. 4C). Moreover, we evaluated whether chlorpromazine treatment could affect Sap-induced caspase-11 activation. Our results clearly demonstrate that blocking endocytosis drastically reduced Sap-induced caspase-11 activation (Fig. 4D).
DISCUSSION
The noncanonical inflammasome pathway is usually activated by infection with Gram-negative bacteria, and the activation of this pathway seems to be related to the presence of LPS and, in particular, to the toxic part of LPS, the lipid A (14, 22, 23). More recently, it has been observed that selected Gram-positive bacterial pathogens, such as Streptococcus pneumoniae, also induce the production of type I IFN by host cells and that type I IFN regulates resistance and proinflammatory responses (24). Type I IFN signaling was also required for activation of caspase-1, which is important for maturation and secretion of IL-18 (24).
Compelling evidence shows that caspase-11 activation occurs in response to intracellular Gram-negative bacteria also in cells lacking LPS receptors, such as Toll-like receptor 4 (TLR4), suggesting that other LPS receptors are involved (25, 26). Therefore, even though the signal pathway of activation that is triggered via engagement of TLR4 is well described (27), alternative mechanisms that involve noncanonical activation of the inflammasome must be considered.
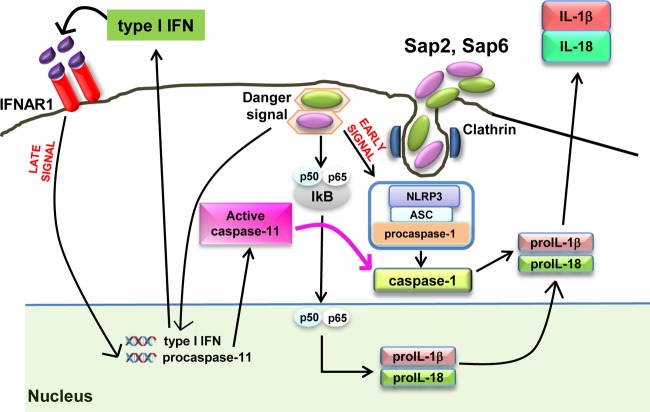
In this study, we demonstrate for the first time that secreted virulence factors of C. albicans, members of the secreted aspartyl proteinase (Sap) family, induce noncanonical activation of the inflammasome. Indeed, both purified Sap2 and Sap6 induce caspase-11 activation though with different kinetics. Cells express caspase-11 in response to both Sap2 and Sap6, and this expression is more prolonged in response to Sap2 than to Sap6. The activation of caspase-11 does not affect Sap-induced NLRP3 expression, but the selective inhibition of caspase-11 (19) results in a consistent downregulation of caspase-1 activation. These results clearly indicate that caspase-11 is involved in the activation of caspase-1, consistent with the observation that maximum caspase-1 activation is related to the concurrent presence of activated caspase-11. As a matter of fact, a trend of kinetic activation was an interplay between caspase-1 and caspase-11. For instance, in response to Sap2, the best stimulation of caspase-1 occurs after 4 h of stimulation, and at the same time a substantial activation of caspase-11 is observed. The highest stimulation of caspase-1 in response to Sap6 is observed after 2 h of stimulation, and at this time the strongest stimulation of caspase-11 is evidenced. In agreement with this hypothesis, a significant reduction of caspase-1 activation was manifested in the presence of caspase-11 inhibitor. Indeed the activation of caspase-1 in response to Sap2 after 2 h of treatment does not reflect the activation of IL-1β. This could be ascribed, at least in part, to the relative temporal difference in activation of caspase-1 and production of pro-IL-1β that are induced via different signal transduction pathways, as shown in Fig. 5.
FIG 5.
Model of canonical and noncanonical Sap-induced inflammasome activation. Sap2 and Sap6 activate the NLRP3 inflammasome pathway through early and late signals. Sap2 and Sap6 induce an early cascade of events (within 30 min), causing upstream NLRP3 inflammasome activation and downstream caspase-1-mediated cytokine production (early signal). Sap endocytosis induces the translocation of NF-κB (p50/p65) into the nucleus, leading to pro-IL-1β and pro-IL-18 synthesis. Sap2 and Sap6 subsequently induce, through type I IFN production, caspase-11 activation (within 2 h) that cooperates with the NLRP3 inflammasome, triggering downstream caspase-1-mediated cytokine production (late signal).
Moreover, a complete reduction of IC-11-induced caspase-1 activation after 4 h of Sap treatment correlated with a drastic reduction of IL-1β production, suggesting that in the late phase, in contrast to the early phase, the activation of caspase-11 is necessary for caspase-1 activation and for subsequent IL-1β secretion.
Nonetheless, caspase-11 is not necessarily required for Sap-induced caspase-1 activation mediated by NLRP3; however, caspase-11 plays a key role in potentiating and prolonging caspase-1 activation via induction of type I IFN.
Therefore, caspase-11 markedly influences caspase-1 activation and the subsequent production of IL-1β and IL-18. This was particularly evident for the IL-1β production by PM. Furthermore, the induction of caspase-11 is at least in part mediated by type I IFN secreted in response to Sap2 and Sap6. This observation is supported by the fact that the blockade of IFNAR1, before stimulation with Saps, results in a drastic reduction of caspase-11 activation.
In our previous study we observed that caspase-1 activation in response to Sap2 was maximal after 1 h and then decreased, whereas IL-1β activation in response to Sap6 was increased after 4 h. In this study, we observed that the Sap2-induced activation of caspase-1 was evident at 30 min posttreatment and reached maximum levels after 4 h. Moreover, IL-1β activation in response to Sap6 was evident just after 2 h and continued until 4 h. This apparent discrepancy could be due to different cells and the experimental setting used, such as different culture conditions including cell concentrations and greater amounts of protein analyzed by Western blotting.
Indeed, in contrast to our previous study, where freshly isolated human monocytes were used, we analyzed a murine macrophage cell line in this study. These different experimental settings could also account for the lower cytokine production observed in response to Saps. Given that caspase-8 could be involved in the activation of the inflammasome, we also tested the activation of caspase-8 in response to Saps. In our experimental system, caspase-8 is not involved in Sap-induced inflammasome activation (data not shown).
We recently demonstrated that both Sap2 and Sap6 cause increased production of IL-β and IL-18 via canonical inflammasome activation (12) and that this occurs via activation of caspase-1. We also observed that the activation of caspase-1 was partially independent of the NLRP3 activation pathway. Indeed, in this study we add new, important information by demonstrating that the increase in caspase-1 activation is supported and potentiated by caspase-11. Of note, caspase-11 does not affect NLRP3 expression, but it supports directly the activation of caspase-1. A possible scenario could involve caspase-1 that is recruited and activated early by NLRP3 (early signal, 30 min); subsequently, the activation of caspase-1 is enhanced and maintained by the increased expression of caspase-11 (late signal, 2 h) (Fig. 5). This scenario is supported by analyzing the kinetics of caspase-1 and caspase-11 expression. Caspase-1 activation is evident already after 30 min of Sap stimulation, whereas the activation of caspase-11 requires at least 2 h of stimulation. Notably, NLRP3 is already highly activated after 30 min. Thus, NLRP3 and caspase-1 activation occurs concomitantly. The activation of caspase-11 is observed later and is related to stimulation of type I IFN. This process is summarized in Fig. 5. It has been reported that TLR4/Trif-mediated type I IFN plays a pivotal role in caspase-11 activation (15). del Fresno et al. have recently reported that C. albicans induces type I IFN in dendritic cells through a Dectin-1-, Syk-, and Card9-dependent pathway (28). In addition, other research groups have shown that Ifnar−/− mice are extremely resistant to otherwise lethal Candida and Histoplasma infections (29, 30). These studies demonstrated that type I IFN induces severe kidney damage by promoting excessive recruitment and activation of inflammatory monocytes and neutrophils. However, other reports suggest that type I IFN can be beneficial as part of the host immune response to C. albicans (28, 31). Other studies related to sepsis show that type I IFN plays a critical role in promoting lethal endotoxemia (32, 33). Finally, many aspects of this pathway remain unclear, and two models in which activation is triggered by Gram-negative bacteria have been suggested: one is receptor scaffold-mediated activation and the other is related to auto-activation of procaspase-11 (15).
In the first model, caspase-11 activation is induced by type I IFN signaling, which in turn increases procaspase-11 expression via IFNAR1 and induces the expression of an inducible receptor/activator of caspase-11 (15). The second possible mechanism is auto-activation of procaspase-11. Signaling through Trif and IFN regulatory factor 3 (IRF3) induces expression of type I IFN, which induces procaspase-11 that, via auto-activation, induces caspase-11 expression (16).
In our experimental system, the activation of caspase-11 is related to release of type I IFN in response to Saps; it is noteworthy that this is the first demonstration that distinct proteins, the Saps of C. albicans, are able to induce type I IFN release. The mechanism that leads to type I IFN production and caspase-11 activation is unclear. We previously demonstrated that clathrin-dependent endocytosis, likely associated with an intracellular danger signal, is necessary for activating the canonical NLRP3 inflammasome by Saps (12). Indeed Sap endocytosis and internalization are also required for the induction of type I IFN that, in turn, interacts with IFNAR1. This pathway leads to activation of procaspase-11. Pyroptosis is a nonhomeostatic lytic model that shares some features with caspase-independent necrotic cell death (22). Pyroptosis requires enzymatic activity of caspase-1 or caspase-11 (16, 34). Caspase-1 has been involved in pyroptosis induction as well as in processing IL-1β and IL-18, whereas caspase-11 is involved only in pyroptosis induction (34). Therefore, the activity of caspase-11 in regulating IL-1β and IL-18 secretion is exclusively related to its capacity to affect activation of caspase-1. Indeed, it has recently been reported that caspase-11 mediates extracellular release of IL-1β and IL-18 indirectly through regulation of the inflammasome and/or caspase-1 (34). In our experimental setting, we demonstrate that there is no cross talk between NLRP3 and caspase-11, but caspase-11 potentiates caspase-1 activation and, as a consequence, the secretion of proinflammatory cytokines.
The activation of a noncanonical inflammasome exerted by Saps opens a novel scenario in which not only microorganisms, and particularly Gram-negative bacteria, participate in the activation of a noncanonical inflammasome but also distinct virulence factors of the pathogenic fungus C. albicans can trigger this activation. The identification of this mechanism could also open novel therapeutic strategies to dampen hyperinflammatory responses, which are characteristic attributes of a disease such as vaginal candidiasis. Since it is known that Saps are secreted in large amounts during vaginal candidiasis (35), our future studies will be devoted to validating the role of Sap secretion in inducing the noncanonical inflammasome during mucosal, in particular, vaginal, candidiasis in vivo.
ACKNOWLEDGMENT
This work was supported by Fondazione Cassa di Risparmio 2012.0128.021.
REFERENCES
- 1.Naglik JR, Challacombe SJ, Hube B. 2003. Candida albicans secreted aspartyl proteinases in virulence and pathogenesis. Microbiol Mol Biol Rev 67:400–428. doi: 10.1128/MMBR.67.3.400-428.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vecchiarelli A, Pericolini E, Gabrielli E, Pietrella D. 2012. New approaches in the development of a vaccine for mucosal candidiasis: progress and challenges. Front Microbiol 3:294. doi: 10.3389/fmicb.2012.00294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pfaller MA, Diekema DJ. 2010. Epidemiology of invasive mycoses in North America. Crit Rev Microbiol 36:1–53. doi: 10.3109/10408410903241444. [DOI] [PubMed] [Google Scholar]
- 4.Mayer FL, Wilson D, Hube B. 2013. Candida albicans pathogenicity mechanisms. Virulence 4:119–128. doi: 10.4161/viru.22913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sudbery P, Gow N, Berman J. 2004. The distinct morphogenic states of Candida albicans. Trends Microbiol 12:317–324. doi: 10.1016/j.tim.2004.05.008. [DOI] [PubMed] [Google Scholar]
- 6.Sudbery PE. 2011. Growth of Candida albicans hyphae. Nat Rev Microbiol 9:737–748. doi: 10.1038/nrmicro2636. [DOI] [PubMed] [Google Scholar]
- 7.Gow NA, Brown AJ, Odds FC. 2002. Fungal morphogenesis and host invasion. Curr Opin Microbiol 5:366–371. doi: 10.1016/S1369-5274(02)00338-7. [DOI] [PubMed] [Google Scholar]
- 8.Jacobsen ID, Wilson D, Wachtler B, Brunke S, Naglik JR, Hube B. 2012. Candida albicans dimorphism as a therapeutic target. Expert Rev Anti Infect Ther 10:85–93. doi: 10.1586/eri.11.152. [DOI] [PubMed] [Google Scholar]
- 9.de Viragh PA, Sanglard D, Togni G, Falchetto R, Monod M. 1993. Cloning and sequencing of two Candida parapsilosis genes encoding acid proteases. J Gen Microbiol 139:335–342. doi: 10.1099/00221287-139-2-335. [DOI] [PubMed] [Google Scholar]
- 10.Monod M, Togni G, Hube B, Sanglard D. 1994. Multiplicity of genes encoding secreted aspartic proteinases in Candida species. Mol Microbiol 13:357–368. doi: 10.1111/j.1365-2958.1994.tb00429.x. [DOI] [PubMed] [Google Scholar]
- 11.Pietrella D, Rachini A, Pandey N, Schild L, Netea M, Bistoni F, Hube B, Vecchiarelli A. 2010. The inflammatory response induced by aspartic proteases of Candida albicans is independent of proteolytic activity. Infect Immun 78:4754–4762. doi: 10.1128/IAI.00789-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pietrella D, Pandey N, Gabrielli E, Pericolini E, Perito S, Kasper L, Bistoni F, Cassone A, Hube B, Vecchiarelli A. 2013. Secreted aspartic proteases of Candida albicans activate the NLRP3 inflammasome. Eur J Immunol 43:679–692. doi: 10.1002/eji.201242691. [DOI] [PubMed] [Google Scholar]
- 13.Schroder K, Zhou R, Tschopp J. 2010. The NLRP3 inflammasome: a sensor for metabolic danger? Science 327:296–300. doi: 10.1126/science.1184003. [DOI] [PubMed] [Google Scholar]
- 14.Rathinam VA, Vanaja SK, Waggoner L, Sokolovska A, Becker C, Stuart LM, Leong JM, Fitzgerald KA. 2012. TRIF licenses caspase-11-dependent NLRP3 inflammasome activation by Gram-negative bacteria. Cell 150:606–619. doi: 10.1016/j.cell.2012.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vigano E, Mortellaro A. 2013. Caspase-11: the driving factor for noncanonical inflammasomes. Eur J Immunol 43:2240–2245. doi: 10.1002/eji.201343800. [DOI] [PubMed] [Google Scholar]
- 16.Broz P, Monack DM. 2013. Noncanonical inflammasomes: caspase-11 activation and effector mechanisms. PLoS Pathog 9:e1003144. doi: 10.1371/journal.ppat.1003144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Borg-von Zepelin M, Beggah S, Boggian K, Sanglard D, Monod M. 1998. The expression of the secreted aspartyl proteinases Sap4 to Sap6 from Candida albicans in murine macrophages. Mol Microbiol 28:543–554. doi: 10.1046/j.1365-2958.1998.00815.x. [DOI] [PubMed] [Google Scholar]
- 18.Gabrielli E, Pericolini E, Cenci E, Ortelli F, Magliani W, Ciociola T, Bistoni F, Conti S, Vecchiarelli A, Polonelli L. 2009. Antibody complementarity-determining regions (CDRs): a bridge between adaptive and innate immunity. PLoS One 4:e8187. doi: 10.1371/journal.pone.0008187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kobori M, Yang Z, Gong D, Heissmeyer V, Zhu H, Jung YK, Gakidis MA, Rao A, Sekine T, Ikegami F, Yuan C, Yuan J. 2004. Wedelolactone suppresses LPS-induced caspase-11 expression by directly inhibiting the IKK complex. Cell Death Differ 11:123–130. doi: 10.1038/sj.cdd.4401325. [DOI] [PubMed] [Google Scholar]
- 20.Sheehan KC, Lai KS, Dunn GP, Bruce AT, Diamond MS, Heutel JD, Dungo-Arthur C, Carrero JA, White JM, Hertzog PJ, Schreiber RD. 2006. Blocking monoclonal antibodies specific for mouse IFN-alpha/beta receptor subunit 1 (IFNAR-1) from mice immunized by in vivo hydrodynamic transfection. J Interferon Cytokine Res 26:804–819. doi: 10.1089/jir.2006.26.804. [DOI] [PubMed] [Google Scholar]
- 21.Pericolini E, Gabrielli E, Bistoni G, Cenci E, Perito S, Chow SK, Riuzzi F, Donato R, Casadevall A, Vecchiarelli A. 2010. Role of CD45 signaling pathway in galactoxylomannan-induced T cell damage. PLoS One 5:e12720. doi: 10.1371/journal.pone.0012720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kayagaki N, Warming S, Lamkanfi M, Vande Walle L, Louie S, Dong J, Newton K, Qu Y, Liu J, Heldens S, Zhang J, Lee WP, Roose-Girma M, Dixit VM. 2011. Non-canonical inflammasome activation targets caspase-11. Nature 479:117–121. doi: 10.1038/nature10558. [DOI] [PubMed] [Google Scholar]
- 23.Broz P, Ruby T, Belhocine K, Bouley DM, Kayagaki N, Dixit VM, Monack DM. 2012. Caspase-11 increases susceptibility to Salmonella infection in the absence of caspase-1. Nature 490:288–291. doi: 10.1038/nature11419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fang R, Hara H, Sakai S, Hernandez-Cuellar E, Mitsuyama M, Kawamura I, Tsuchiya K. 2014. Type I interferon signaling regulates activation of the absent in melanoma 2 inflammasome during Streptococcus pneumoniae infection. Infect Immun 82:2310–2317. doi: 10.1128/IAI.01572-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hagar JA, Powell DA, Aachoui Y, Ernst RK, Miao EA. 2013. Cytoplasmic LPS activates caspase-11: implications in TLR4-independent endotoxic shock. Science 341:1250–1253. doi: 10.1126/science.1240988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kayagaki N, Wong MT, Stowe IB, Ramani SR, Gonzalez LC, Akashi-Takamura S, Miyake K, Zhang J, Lee WP, Muszynski A, Forsberg LS, Carlson RW, Dixit VM. 2013. Noncanonical inflammasome activation by intracellular LPS independent of TLR4. Science 341:1246–1249. doi: 10.1126/science.1240248. [DOI] [PubMed] [Google Scholar]
- 27.Mogensen TH. 2009. Pathogen recognition and inflammatory signaling in innate immune defenses. Clin Microbiol Rev 22:240–273. doi: 10.1128/CMR.00046-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.del Fresno C, Soulat D, Roth S, Blazek K, Udalova I, Sancho D, Ruland J, Ardavin C. 2013. Interferon-beta production via Dectin-1-Syk-IRF5 signaling in dendritic cells is crucial for immunity to C. albicans. Immunity 38:1176–1186. doi: 10.1016/j.immuni.2013.05.010. [DOI] [PubMed] [Google Scholar]
- 29.Inglis DO, Berkes CA, Hocking Murray DR, Sil A. 2010. Conidia but not yeast cells of the fungal pathogen Histoplasma capsulatum trigger a type I interferon innate immune response in murine macrophages. Infect Immun 78:3871–3882. doi: 10.1128/IAI.00204-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Majer O, Bourgeois C, Zwolanek F, Lassnig C, Kerjaschki D, Mack M, Muller M, Kuchler K. 2012. Type I interferons promote fatal immunopathology by regulating inflammatory monocytes and neutrophils during Candida infections. PLoS Pathog 8:e1002811. doi: 10.1371/journal.ppat.1002811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Biondo C, Signorino G, Costa A, Midiri A, Gerace E, Galbo R, Bellantoni A, Malara A, Beninati C, Teti G, Mancuso G. 2011. Recognition of yeast nucleic acids triggers a host-protective type I interferon response. Eur J Immunol 41:1969–1979. doi: 10.1002/eji.201141490. [DOI] [PubMed] [Google Scholar]
- 32.Mahieu T, Park JM, Revets H, Pasche B, Lengeling A, Staelens J, Wullaert A, Vanlaere I, Hochepied T, van Roy F, Karin M, Libert C. 2006. The wild-derived inbred mouse strain SPRET/Ei is resistant to LPS and defective in IFN-beta production. Proc Natl Acad Sci U S A 103:2292–2297. doi: 10.1073/pnas.0510874103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Huys L, Van Hauwermeiren F, Dejager L, Dejonckheere E, Lienenklaus S, Weiss S, Leclercq G, Libert C. 2009. Type I interferon drives tumor necrosis factor-induced lethal shock. J Exp Med 206:1873–1882. doi: 10.1084/jem.20090213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Latz E, Xiao TS, Stutz A. 2013. Activation and regulation of the inflammasomes. Nat Rev Immunol 13:397–411. doi: 10.1038/nri3452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cassone A. 23 July 2014. Vulvovaginal Candida albicans infections: pathogenesis, immunity and vaccine prospects. BJOG doi: 10.1111/1471-0528.12994. [DOI] [PubMed] [Google Scholar]