Abstract
Cronobacter spp. are opportunistic pathogens that cause neonatal meningitis and sepsis with high mortality in neonates. Despite the peril associated with Cronobacter infection, the mechanisms of pathogenesis are still being unraveled. Hfq, which is known as an RNA chaperone, participates in the interaction with bacterial small RNAs (sRNAs) to regulate posttranscriptionally the expression of various genes. Recent studies have demonstrated that Hfq contributes to the pathogenesis of numerous species of bacteria, and its roles are varied between bacterial species. Here, we tried to elucidate the role of Hfq in C. sakazakii virulence. In the absence of hfq, C. sakazakii was highly attenuated in dissemination in vivo, showed defects in invasion (3-fold) into animal cells and survival (103-fold) within host cells, and exhibited low resistance to hydrogen peroxide (102-fold). Remarkably, the loss of hfq led to hypermotility on soft agar, which is contrary to what has been observed in other pathogenic bacteria. The hyperflagellated bacteria were likely to be attributable to the increased transcription of genes associated with flagellar biosynthesis in a strain lacking hfq. Together, these data strongly suggest that hfq plays important roles in the virulence of C. sakazakii by participating in the regulation of multiple genes.
INTRODUCTION
The genus Cronobacter (formerly Enterobacter sakazakii) comprises Gram-negative, rod-shaped, peritrichous, and yellow-pigmented facultative anaerobes belonging to the family Enterobacteriaceae. This family encompasses the well-known enteric pathogens Salmonella and pathogenic Escherichia coli, but Cronobacter spp. are most closely related to the genera Enterobacter and Citrobacter. Based on the original 16 biogroups of Farmer et al., E. sakazakii has been reclassified into several Cronobacter spp. according to genotypic and phenotypic evaluations (1–4). Cronobacter spp. are present in a wide range of environments, including water, soil, processed foods, and facilities (5), and are composed of seven species: Cronobacter sakazakii, Cronobacter malonaticus, Cronobacter muytjensii, Cronobacter turicensis, Cronobacter dublinensis, Cronobacter universalis, and Cronobacter condimenti (3, 4, 6).
C. sakazakii is a foodborne opportunistic pathogen that causes bacteremia, meningitis, and necrotizing enterocolitis, particularly in premature infants (7–9). Although the incidence rate is quite low, mortality is high, ranging from 40 to 80% (10–12). Despite the serious perils associated with C. sakazakii, little is known at the molecular level about the mechanism of its pathogenicity and about its virulence factors. A cell-bound zinc-containing metalloprotease, encoded by zpx, caused rounding of Chinese hamster ovary (CHO) cells, which might be important in dissemination of the pathogen into the systemic circulation (13). The outer membrane proteins A (OmpA) and OmpX are reported to be essential for adhesion to/invasion of Caco-2 and INT-407 cells (14, 15). Studies have shown that C. sakazakii can invade and translocate efficiently across cultured human intestinal epithelial cells, as well as endothelial cells, experimentally mimicking the potential path of meningitis (14, 16). A plasmid-borne outer membrane protease (Cpa) is reported to be involved in C. sakazakii protection against complement-dependent serum killing and efficient invasion by activating plasminogen into plasmin (17). Furthermore, plasmid-borne iron acquisition systems, such as an aerobacterin-like siderophore (cronobactin) and an ABC ferric-iron transporter (eitABCE), might enable Cronobacter spp. to obtain iron in a highly iron-restricted environment (18). Flagella from Cronobacter spp. can induce an inflammatory response dependent on Toll-like receptor 5 (TLR5) recognition, so they could contribute to the pathogenesis of the bacteria (19). As a global regulator, LysR-type transcriptional regulator (LTTR) in C. sakazakii impacts pathogenesis, invasion, biofilm formation, and in vivo challenge (20).
The Hfq protein, which was first discovered in E. coli nearly half a century ago as a host bacterial factor required for the RNA synthesis of bacteriophage Qβ (21), is widely conserved as an RNA chaperone in many bacterial species (22). It oligomerizes into a hexameric ring structure (23), enhances the formation of small-RNA (sRNA)–mRNA duplexes, and contributes to RNA regulation by interacting with RNA turnover enzymes, RNase E, polynucleotide phosphorylase, and poly(A) polymerase (24). Thus, Hfq is regarded as a posttranscriptional global regulator involved in the biogenesis of outer membrane proteins (OMPs) (25), quorum sensing (26), and various stress responses (27). In addition, recent studies have demonstrated the importance of Hfq in the pathogenesis of various bacteria, including Listeria monocytogenes, Yersinia pseudotuberculosis, Francisella tularensis, Salmonella enterica serovar Typhimurium, and E. coli (28–32). For example, a lack of Hfq in S. Typhimurium caused attenuated virulence in vivo, reduced host cell adhesion and invasion in vitro, defectiveness in secretion of effector proteins, chronic envelope stress (accumulation of periplasmic and outer membrane proteins), loss of motility, and reduced survival within cultured macrophages (31). Such pleiotropic functions of the hfq gene product were also observed in other bacterial species (29, 33), but the effects of hfq deletion could be different from one species to another; the deletion of hfq in S. Typhimurium and Brucella abortus reduced bacterial growth and survival within the host cells, but not in L. monocytogenes and F. tularensis (28, 30, 34).
The aim of this study was to elucidate the function of the hfq homolog of C. sakazakii in virulence or stress responses, such as motility, invasion of host epithelial cells, intracellular survival in macrophages, and resistance to hydrogen peroxide. Here, we demonstrate that Hfq is essential for virulence and stress adaptation in C. sakazakii ATCC 29544.
MATERIALS AND METHODS
Bacterial strains, plasmids, and growth.
The bacterial strains used in this study are listed in Table 1. C. sakazakii and E. coli were routinely grown in Luria-Bertani (LB) medium at 37°C with constant shaking unless otherwise indicated.
TABLE 1.
Bacterial strains and plasmids used in this study
| Strain or plasmid | Genotype and/or characteristicsa | Reference or source |
|---|---|---|
| C. sakazakii | ||
| ATCC 29544 | Wild-type strain | 15 |
| ES1001 | 29544 harboring pKD46 | 15 |
| SK001 | 29544 harboring pACYC184 | This study |
| SK002 | 29544 harboring pBAD18 | This study |
| SK003 | Δhfq | This study |
| SK004 | hfq::kan harboring pACYC184 | This study |
| SK005 | Δhfq harboring pACYC184 | This study |
| SK006 | Δhfq harboring pBAD18 | This study |
| SK007 | Δhfq harboring pHFQ | This study |
| SK008 | Δhfq harboring pHFQ-A | This study |
| E. coli | ||
| DH5α | λ− ϕ80dlacZΔM15 Δ(lacZYA-argF)U169 recA1 endA1 hsdR17(rK− mK−) supE44 thi-1 gyrA relA1 | 36 |
| Plasmids | ||
| pKD13 | oriR6K Ampr FRT Kanr FRT | 35 |
| pKD46 | oriR101 repA101(Ts) Ampr ara BADpgam-bet-exo | 35 |
| pCP20 | oripSC101(TS) Ampr Cmr cI857λ PRflp | 35 |
| pACYC184 | Tetr Cmr p15A ori | 35 |
| pBAD18 | Ampr araC PBAD pBR322 ori; expression vector | 37 |
| pHFQ | pACYC184-hfq | This study |
| pHFQ-A | pBAD18-hfq | This study |
FRT, FLP recombination target.
Construction of a Δhfq deletion mutant using the Lambda-Red recombination method.
The whole genome sequence of C. sakazakii BAA-894 was obtained from GenBank (18) and adopted to manipulate the C. sakazakii ATCC 29544 genome. Site-specific mutation of C. sakazakii ATCC 29544 was generated by the Lambda-Red recombination method, as described by Kim et al. and Datsenko and Wanner (15, 35). Briefly, the kanamycin resistance cassette from plasmid pKD13 was amplified using the following primers (Table 2): for hfq deletion mutant construction, hfq-lamb-F (5′-ATG GCT AAG GGG CAA TCT TTG CAA GAT CCG TTC CTC AAC GCG CTG CGT CGT GTA GGC TGG AGC TGC TTC G-3′) and hfq-lamb-R (5′-TTA CTC GGC GTC TTC GCT ATC CTG AGA GGC AGC GGA AGA TGG CTG TGC GTA TTC CGG GGA TCC GTC GAC C-3′). (The nucleotide sequences originating from pKD13 are underlined, and those from the C. sakazakii gene of interest are shown in italics.)
TABLE 2.
Oligonucleotides used in this study
| Oligonucleotide name | Oligonucleotide sequence (5′ to 3′) | Reference or source |
|---|---|---|
| Mutant construction | ||
| hfq-lamb-F | ATG GCT AAG GGG CAA TCT TTG CAA GAT CCG TTC CTC AAC GCG CTG CGT CGT GTA GGC TGG AGC TGC TTC G | This study |
| hfq-lamb-R | TTA CTC GGC GTC TTC GCT ATC CTG AGA GGC AGC GGA AGA TGG CTG TGC GTA TTC CGG GGA TCC GTC GAC C | This study |
| hfq-lamb-conF | GTA CAG CGA TGT GTT ACA GGT | This study |
| hfq-lamb-conR | CCA ACA AAA TAC TTT GGG TGC G | This study |
| Cloning | ||
| hfq- pACYC184-R-SalI | AAA GTC GAC GTA AAA TAG ATG TGT ACC AG | This study |
| hfq-pACYC184-F-SphI | AAA GCA TGC AGC GAT GGC GGA GAT TGT CG | This study |
| pACYC184-seq-F | GGC TCA TGA GCG CTT GTT TC | This study |
| pACYC184-seq-R | TGT CCT ACG AGT TGC ATG ATA | This study |
| hfq-pBAD18-F-EcoRI | AAA GAA TTC GGT TCA AGA GTA TAA ACA AC | This study |
| pBAD18-seq-F | GTC CAC ATT GAT TAT TTG CAC G | This study |
| pBAD18-seq-R | CAG GCT GAA AAT CTT CTC TCA T | This study |
| qRT-PCR | ||
| Control-RT-F | GGG CCT CAT GCC ATC AGA T | 70 |
| Control-RT-R | TCT CAG ACC AGC TAG GGA TCG T | 70 |
| flhC-RT-F | GCA ACT TAG CCG CGG TAG AC | This study |
| flhC-RT-R | TGA ACC AGT CCG TGG AAA AGG | This study |
| fliA-RT-F | GCA GGA ACT GGG ACG TAA CG | This study |
| fliA-RT-R | GTG TCG AGC AAC ATC TGA CGA T | This study |
| flgK-RT-F | CGC TAT GAG CAG ATG TCG AAA AT | This study |
| flgK-RT-R | GTC TGC AGG CTT TTG AAG AAA TC | This study |
| fliC-RT-F | CGT ATC GCT GGT GGT GCT AA | This study |
| fliC-RT-R | CAG CGC CAA CCT GAA TTT TC | This study |
| miaA-RT-F | GAG CAG CGT TTT CAC CAG AT | This study |
| miaA-RT-R | AGG CAT GTC CGT ATG CAA AT | This study |
| hflX-RT-F | TGT CGA AGC ATT ACA GGT GAT T | This study |
| hflX-RT-R | AAT CTC AAC GGC TTT ACC TTC A | This study |
The PCR products were transformed into the wild-type (WT) strain, C. sakazakii ATCC 29544, harboring the pKD46 plasmid, by electroporation, and cells were selected for kanamycin-resistant transformants, hfq::kan. Finally, the kanamycin resistance cassette was removed using the pCP20 plasmid, as described previously (20).
Growth curves.
C. sakazakii strains ATCC 29544 (SK001), Δhfq (SK005), and Δhfq harboring pHFQ (SK007) were cultured overnight in LB medium at 37°C and subcultured with a 1% overnight culture in 50 ml LB medium. The cultures were incubated with shaking at 220 rpm in 250-ml Scott flasks at 37°C for 14 h. Every 1 h, the optical density was measured at 600 nm (OD600).
Complementation study.
Two hfq complementation plasmids (a pACYC184 derivative, pHFQ, with its own hfq promoter and a pBAD18 derivative, pHFQ-A, under an arabinose-inducible promoter) were constructed. To make pHFQ, the entire 309-bp hfq coding sequence (accession number KC866358) and 782 bp of upstream sequence comprising miaA (accession number KC866359), which includes promoter regions of hfq, were amplified from C. sakazakii ATCC 29544 chromosomal DNA (33) with primer sets hfq-pACYC184-SphI-F (5′-AAA GCA TGC AGC GAT GGC GGA GAT TGT CG-3′) and hfq-pACYC184-SalI-R (5′-AAA GTC GAC GTA AAA TAG ATG TGT ACC AG-3′) (the artificial restriction enzyme digestion site is underlined) and cloned into pACYC184 (36). For pHFQ-A, hfq-pBAD18-EcoRI-F (5′-AAA GAA TTC GGT TCA AGA GTA TAA ACA AC-3′) and hfq-pACYC184-SalI-R (5′-AAA GTC GAC GTA AAA TAG ATG TGT ACC AG-3′) were used to amplify the hfq coding region into the pBAD18 vector (the underlined nucleotide sequences are artificially added restriction recognition sites) (37). The plasmids were used to transform the mutant to generate Δhfq harboring pHFQ and Δhfq harboring pHFQ-A.
Gentamicin protection (invasion) assay.
To determine bacterial invasion of mammalian Caco-2 cells, a gentamicin protection assay was performed as described previously (15). Briefly, bacteria were prepared by transferring a 1% inoculum from overnight cultures into fresh LB medium, followed by incubation for 2.5 h at 37°C with constant shaking. C. sakazakii cells were collected by centrifugation at 20,000 × g, washed with phosphate-buffered saline (PBS) (pH 7.4), and resuspended in 1 ml of prewarmed fresh Eagle's minimum essential medium (EMEM) (ATCC). Mammalian cells were seeded in EMEM supplemented with 20% fetal bovine serum (FBS) (Gibco, Invitrogen) in 24-well tissue culture plates with a cell density of 2 × 105 per well. The cell monolayers were incubated for 1 day, infected with bacteria at a multiplicity of infection (MOI) of 100, and incubated for 1.5 h in the presence of 5% CO2. After the cells were washed once with PBS, fresh medium containing gentamicin (100 μg/ml; Sigma) was added, and the plates were further incubated for 1.5 h, followed by three washes with PBS. Then, 500 μl of 1% Triton X-100 was added, and the plates were incubated for a further 15 min before the bacteria were collected and plated on tryptic soy agar (TSA).
Motility assay.
The C. sakazakii ATCC 29544 (SK002) and Δhfq (SK006) strains and the complemented strain (SK008) were cultured overnight in LB medium at 37°C, which was used to subculture (1% inoculum) fresh LB medium. Bacteria were grown to midexponential phase and then diluted to adjust the OD600 to 1.5. Aliquots (2 μl) of each strain were injected onto soft-agar motility plates (LB medium containing 0.3% agar) and incubated at 37°C for 7 h. A GelDoc EZ imager (Bio-Rad) was used to take a photograph.
Bacterial survival assay in animal cells.
A long-term survival assay was performed as described previously (38). Briefly, RAW264.7 murine macrophage-like cells were seeded in Dulbecco's modified Eagle medium (DMEM) (Gibco, Invitrogen) supplemented with 10% FBS in 24-well plates at a density of 5 × 105 cells per well and incubated for 1 day at 37°C with 5% CO2. C. sakazakii strains were prepared as described for the invasion assay, and cell monolayers were infected with bacteria at an MOI of 100 for 45 min at 37°C. The medium was replaced with DMEM (plus 10% FBS) supplemented with 100 μg/ml of gentamicin, and the cells were incubated for an additional 45 min. The plates were washed twice with PBS, and the cells were lysed with 1% Triton X-100, followed by serial dilution and plating onto TSA to determine the numbers of intracellular bacteria at various time points. For further study of bacterial persistence (long-term survival), the cells were replenished daily with fresh medium containing 10 μg/ml of gentamicin, and the numbers of intracellular bacteria were determined at 3, 8, 24, 48, 72, and 96 h.
Bacterial resistance against hydrogen peroxide.
A hydrogen peroxide assay was performed as described previously with minor modifications (29). A C. sakazakii culture was prepared as for the motility assay. Hydrogen peroxide (Sigma) was added to the culture to a final concentration of 100 mM, and the culture was incubated at 37°C for 10 min, followed by counting of viable cells on TSA. For quantitative real-time (qRT)-PCR analysis (see below), each sample was exposed to 100 mM H2O2 for 5 min at 37°C.
qRT-PCR.
A C. sakazakii culture was prepared as for the motility assay. Five hundred microliters of the culture was mixed with 1 ml of RNA Protect Bacteria Reagent (Qiagen), and total RNA was isolated using an RNeasy minikit (Qiagen). The RNA sample was then treated with RNase-free DNase (Ambion) to remove residual DNA. cDNA was synthesized using Omnitranscript Reverse Transcription reagents (Qiagen) and random hexamers (Invitrogen), and quantification of the cDNA was performed using 2× iQ SYBR green Supermix (Bio-Rad). The real-time amplification of the PCR products was performed using the CFX Connect Real-Time PCR Detection system (Bio-Rad). The mRNA level of each gene was divided by the mRNA level of the 16S rRNA. The mRNA expression values in strains SK005 and SK007 were further normalized to the transcription levels in the wild-type strain.
Animal study in vivo.
Three- to 4-day-old Sprague-Dawley (SD) rat pups were used to assess the virulence of the WT (SK001) and the hfq mutant (SK004) strains. Midexponential-phase bacteria were collected, washed, and resuspended in PBS. For competitive assays, a mixed inoculum of 1 × 109 CFU/ml of the fully virulent WT (SK001) and the hfq mutant (SK004) harboring a kan cassette was orally administered to rat pups. To analyze bacterial colonization in organs, all the rat pups were killed with CO2 at 20 h postinfection. The spleens and livers were aseptically removed, homogenized, and serially diluted. Bacterial loads were determined for the WT and mutant by plating onto TSA in the presence or absence of kanamycin. The results are presented as competitive-index (CI) values, which were calculated as follows: (hfqoutput/hfqinput)/(WToutput/WTinput).
TEM analysis.
Flagellar morphology was visualized by negative staining, followed by transmission electron microscopy (TEM). After being negatively stained with 2% uranyl acetate for 1 min on carbon-Formvar copper grids, flagella were examined with an energy-filtering transmission microscope (E-TEM; Libra 120, Germany) at a voltage of 120 kV.
Western blot analysis.
C. sakazakii ATCC 29544 was cultured overnight in LB medium at 37°C, which was used to subculture (1% inoculum) fresh LB medium. The bacteria were grown to midexponential phase, collected, and disrupted in B-PER (Thermo Scientific) according to the manufacturer's instructions. The protein samples were loaded on a 12% SDS-polyacrylamide gel. The separated proteins on the gel were transferred to a polyvinylidene difluoride (PVDF) membrane and blocked with 5% nonfat dry milk–1× Tris-buffered saline–Tween 20 (TBST) buffer. The membrane was probed with anti-OmpA/OmpX polyclonal antibody and anti-DnaK antibody (Enzo Life Science) as primary antibodies and then treated with anti-mouse IgG conjugated with peroxidase (Santa Cruz Biotechnology) as the secondary antibody in all of the immunoblot experiments. The chemiluminescent signals were developed with a West-Zol plus Western blot detection system (Intron Biotechnology, South Korea).
Polyclonal antibody production against OmpA or OmpX.
BALB/c mice were immunized with recombinant OmpA or OmpX that was prepared as described previously (15). Anti-OmpA or anti-OmpX sera were obtained by bleeding, stored, in a refrigerator overnight, and centrifuged at 5,000 × g for 10 min.
Statistical analysis.
Statistical analysis was conducted using the GraphPad Prism program (version 5.01). All results were analyzed by Student's unpaired t test. The data are presented as means and standard deviations. A P value of <0.05 was considered statistically significant.
Ethics statement.
This study was carried out according to the recommended protocol for the care and use of laboratory animals from the Institute of Laboratory Animal Resources at Seoul National University, based on the Korean Animal Protection Law and Korea Food and Drug Administration regulations on laboratory animals. The protocol was approved by the Committee on the Ethics of Animal Experiments of Seoul National University (Institutional Animal Care and Use Committee permit number SNU-130214-1-3).
RESULTS AND DISCUSSION
Construction and growth characteristics of the hfq mutant in C. sakazakii.
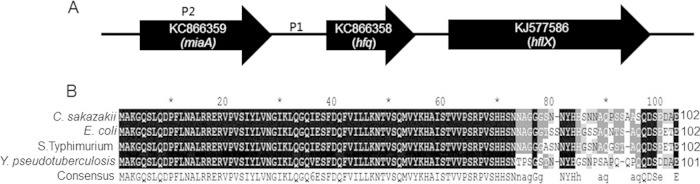
As numerous studies have demonstrated that Hfq contributes to the pathogenesis of many bacteria differently (28, 30, 34), we studied the effects of an Hfq-like protein on the pathogenesis of C. sakazakii ATCC 29544. The gene (KC866358) homologous to the hfq gene is located in a clockwise orientation in the genome of C. sakazakii ATCC 29544, as shown in Fig. 1A, and is also found in C. sakazakii ATCC BAA 894 (YP_001436319.1) and C. turicensis z3032 (YP_003212051.1) (39, 40). The gene (KC866358) showed high protein sequence similarity to the hfq genes of E. coli K-12 (89%), S. Typhimurium (91%), and Y. pseudotuberculosis (84%) (Fig. 1B). In most bacteria, the N-terminal domain of Hfq is highly conserved and is responsible for RNA binding and protein-protein interactions among Sm (the core of small nuclear ribonucleoprotein particles [snRNPs]) and Sm-like proteins (41, 42), whereas the C-terminal fragments are variable in length and amino acid composition (43). The C-terminal domain of Hfq is reported to play roles in gene regulation and protein stability (43, 44), suggesting that the variable regions at the C termini may be responsible for the differential gene regulation by Hfq observed in diverse bacterial species. To understand the roles of hfq in C. sakazakii ATCC 29544 pathogenesis, we generated an unmarked mutant lacking the entire hfq gene using the Lambda-Red recombination technique.
FIG 1.
hfq homolog in C. sakazakii ATCC 29544. (A) Genomic organizations of hfq (KC866358), miaA (KC866359), and hflX (KJ577586) in C. sakazakii ATCC 29544. The promoter regions of hfq are indicated as P1 and P2. (B) Multiple-sequence alignment of hfq sequences in Gram-negative pathogens with a functionally identified hfq gene by Clustal Omega (version 1.2.1) and Genedoc. The uppercase letters in the consensus sequence indicate conserved amino acids appearing in all the aligned sequences, and the lowercase letters indicate conserved amino acids appearing in at least two sequences. Identical and conserved residues are in black and shaded gray (dark gray, >70% conserved; light gray, >40% conserved), respectively. Every 10 amino acids are indicated by asterisks.
The loss of hfq was verified by sequencing, and we confirmed no polar effect on hflX, located downstream of hfq, by qRT-PCR (data not shown). When we compared the growth of the strains in LB medium with constant aeration at 37°C, the deletion mutant showed a slightly lower growth rate than the wild type (Fig. 2). The plasmid carrying the hfq gene complemented the reduced growth of the Δhfq strain (Fig. 2).
FIG 2.
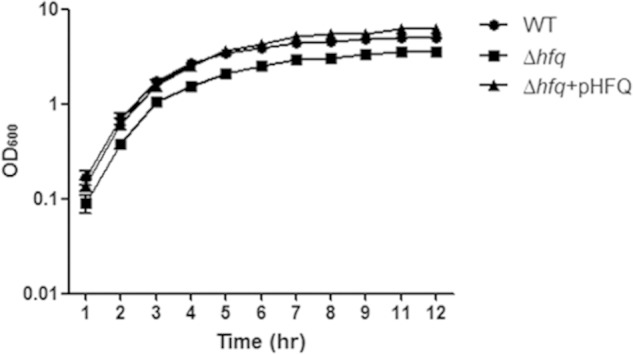
Growth of C. sakazakii Δhfq in LB medium. C. sakazakii wild-type and Δhfq strains and Δhfq harboring pHFQ were cultured in LB broth at 37°C. The OD600 value of each strain harboring the pACYC184 control plasmid or the complementation plasmid pHFQ was measured hourly. The error bars represent means and standard errors of the mean (SEM) from three independent biological replicates.
The loss of hfq attenuates colonization by C. sakazakii in rat pups.
Hfq is a global regulator known to modulate physiological fitness and pathogenesis at multiple levels in various bacteria (29, 31, 33, 45). A Klebsiella pneumoniae mutant lacking hfq was unable to disseminate into extraintestinal organs (spleen and liver) and was attenuated in induction of a systemic infection in a mouse model (33). Similarly, Vibrio cholerae hfq mutants were highly attenuated in virulence in the suckling mouse model of cholera, showing 1,000-fold less efficacy than wild-type bacteria at colonizing the murine small intestine (45).
To examine whether the hfq gene in C. sakazakii contributes to pathogenesis, an in vivo challenge assay using newborn rats was conducted as performed in other studies (38, 46). To minimize individual variations between infant rats, competitive assays were carried out by orally inoculating the bacterial mixture containing equal numbers of Δhfq and wild-type strains. At 20 h postinfection, the hfq deletion mutant was outcompeted by the wild type, with CIs of 0.05 ± 0.06 and 0.08 ± 0.09 in the spleen and liver, respectively (Fig. 3). These results suggest that hfq is critical for the ability of C. sakazakii to disseminate into deeper organs. The attenuated virulence of the Δhfq mutant might be attributable to multiple defects during the infection processes, including decreased invasion into host cells and impaired resistance against environmental stresses. To define the roles of Hfq in virulence regulation in C. sakazakii, a variety of approaches were applied, as described below.
FIG 3.
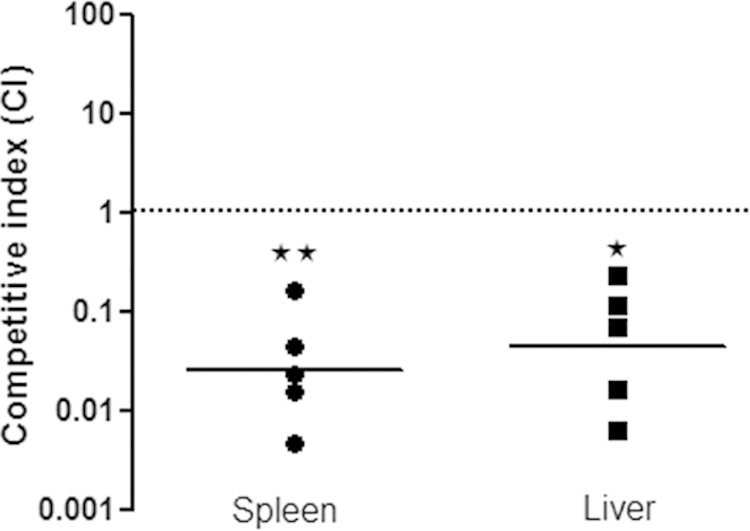
Diminished virulence of C. sakazakii due to the absence of hfq in vivo. Two- to 3-day-old rat pups were orally administered a mixture of wild-type C. sakazakii harboring pACYC184 (SK001) and an hfq::kan strain (SK004), with approximately 5 × 108 CFU/ml of each. To determine the bacterial loads in the spleen and liver, the organs were removed aseptically, homogenized at 20 h postinfection, and plated onto selective agar. The CI was calculated as follows: (Δhfqoutput/Δhfqinput)/(WToutput/WTinput). A CI of <1 indicates attenuation of virulence. Each circle or square represents the CI for a single animal in the spleen and liver of each group, respectively. The geometric means of the CIs for all the rat pups are shown as solid lines, and the asterisks indicate significant differences (**, P < 0.05; *, P < 0.1). CI values of less than 1 indicate that hfq::kan (SK004) strains were outcompeted by the wild type (SK001).
Hfq is involved in the invasion of C. sakazakii into human epithelial cells.
The loss of hfq caused a reduction in invasion ability in S. Typhimurium and E. coli (31, 47). To explore whether hfq in C. sakazakii affects its invasion ability, a gentamicin protection assay was performed using Caco-2 cells. As expected, the Δhfq strain showed approximately 3-fold-reduced invasion into these cells compared with the WT. Complementation with the plasmid harboring the hfq fully restored the invasion ability of the Δhfq strain to wild-type levels (Fig. 4A). Additionally, we checked the invasiveness of the strains in HepG2 cells, which are derived from liver epithelia, and the result was comparable to that with Caco-2 cells (data not shown), suggesting that impaired invasiveness may be common for the epithelium-derived cell lines.
FIG 4.
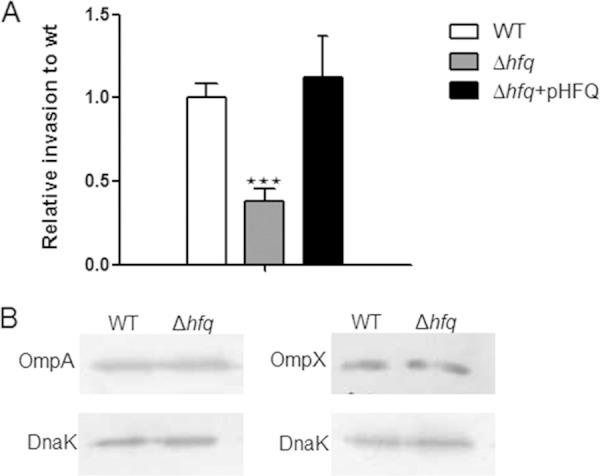
Loss of Hfq impairs the invasion of epithelial cells. (A) Confluent monolayers of eukaryotic cells were infected with C. sakazakii grown to the exponential phase and incubated for 1.5 h, followed by gentamicin treatment (100 μg/ml) for a further 1.5 h. The Caco-2 cells were lysed with 1% Triton X-100 to recover the intracellular bacteria. The data are representative of 3 independent experiments. The bars represent the means and SEM from independent experiments performed in triplicate. The asterisks indicate significant differences (***, P < 0.001). (B) Levels of OmpA and OmpX were assessed by immunoblotting in exponentially grown cells. The blots are representative of 3 independent experiments. DnaK was used as a loading control.
In Salmonella, Hfq controls Salmonella pathogenicity island I (SPI1), which encodes a type III secretion system (T3SS) and several effector proteins that are involved in the invasion of Salmonella into host cells (31). A secretion system equivalent to the T3SS has not been identified in Cronobacter; rather, it invades host cells via a receptor-mediated mechanism (14). Previously, outer membrane proteins (OmpA and OmpX) were reported to be involved in C. sakazakii invasion of human intestinal Caco-2 cells as invasins (15). In this study, the effect of Hfq on the expression of OmpA and OmpX was studied with Western blot analysis using anti-OmpA and anti-OmpX polyclonal antibodies. However, we did not observe substantial differences in OmpA and OmpX expression (Fig. 4B), suggesting that Hfq might not be associated with outer membrane protein expression. However, the possibility that Hfq may regulate other, yet-unidentified invasins in C. sakazakii ATCC 29544 cannot be ruled out.
Hfq is required for oxidative-stress resistance in C. sakazakii.
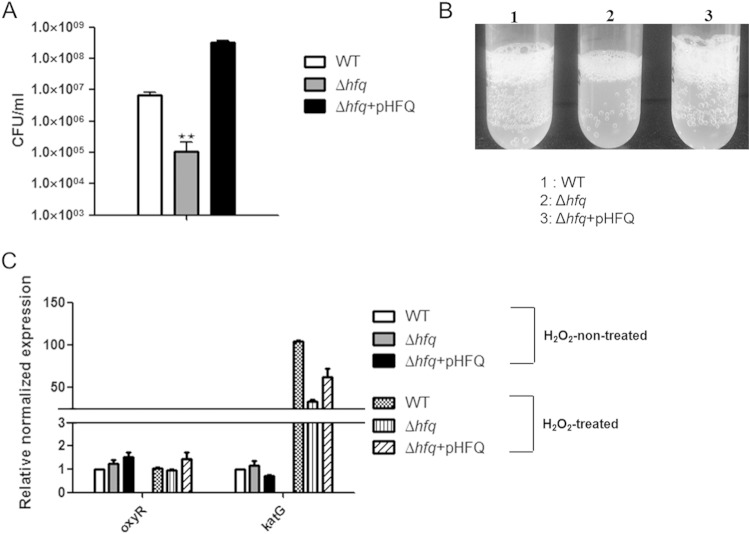
In aerobic environments, many bacteria generate or encounter reactive oxygen species (ROS) (superoxide anion, hydrogen peroxide, and hydroxyl radicals), which damage lipids, proteins, and nucleic acids (48). It is well known that pathogenic bacteria have the ability to cope with oxidative stresses, which is essential for their pathogenesis (49). Here, we tested the role of Hfq in bacterial resistance to oxidative stress by exposing wild-type and Δhfq strains of C. sakazakii to H2O2. The hfq mutant showed significantly (100-fold) lower survival than the WT in the presence of 100 mM H2O2 after 10 min of exposure (Fig. 5A). In agreement with this result, the Δhfq strain exhibited a reduction in bubble formation, which is indicative of decomposition of H2O2 into water and oxygen (Fig. 5B). katG is one of the H2O2-inducible genes and encodes hydroperoxidase I or catalase, a primary detoxifier of H2O2 in many bacteria, including C. sakazakii (50, 51). As katG is positively regulated by OxyR, which is a H2O2-inducible regulator in E. coli and S. Typhimurium (50, 52), both oxyR and katG expression levels were monitored by qRT-PCR. The addition of H2O2 significantly increased katG expression in wild-type bacteria, but the absence of Hfq decreased its expression approximately 3-fold (Fig. 5C), implying that Hfq plays an important role in inducing katG expression for protection of C. sakazakii against oxidative stress. Complementation with a plasmid containing hfq in trans restored the impaired resistance of the Δhfq strain to H2O2 (Fig. 5C). Interestingly, oxyR expression was not induced by H2O2 in both strains, suggesting that OxyR-independent katG induction may be possible, as has been reported in Neisseria gonorrhea and Bradyrhizobium japonicum (53, 54).
FIG 5.
Stress tolerance is attenuated in C. sakazakii in the absence of Hfq. (A) Survival of C. sakazakii strains harboring the pACYC184 control plasmid or a complementation plasmid, pHFQ, upon treatment with 100 mM H2O2 for 10 min. (B) Indirect catalase activities of strains treated with H2O2. 1, wild type (SK001); 2, Δhfq (SK005); 3, Δhfq harboring pHFQ (SK007). The bubbles indicate oxygen gas, produced by decomposition of H2O2. (C) qRT-PCR analysis of oxyR and katG expression levels. To obtain the expression level relative to the WT on the y axis, the mRNA level of each gene was divided by the mRNA level of the 16S rRNA. The mRNA expression values in strains SK005 and SK007 were further normalized to the transcription levels of the wild type. The bars represent means and SEM from independent experiments performed in triplicate. The data are representative of 3 independent experiments. The asterisks indicate significant differences (**, P < 0.05).
Loss of the hfq gene reduces intracellular survival of C. sakazakii in macrophage-like cells.
A previous study showed that Cronobacter spp. were able to persist or replicate within macrophages (38), and Salmonella hfq was shown to be critical for bacterial survival in cultured host macrophages (31). Thus, we examined the impact of hfq deletion on the survival of C. sakazakii within cultured macrophages.
Although the initial uptake of Δhfq bacteria by macrophages was 5-fold lower than that of the WT, clearance of the mutant (2-log-unit and 5-log-unit decreases in survival after 24 and 72 h, respectively) was significantly faster than for the WT (0.5 and 1.5 log units at the respective time points) on average (Fig. 6A). Furthermore, the hfq deletion mutants were cleared completely after 72 h postinfection, whereas WT strains (approximately 4 log CFU/ml) were still able to persist within macrophages at 96 h (Fig. 6A). A plasmid containing hfq complemented the ability to survive within macrophages (Fig. 6B). These data suggest that hfq is necessary for intracellular survival of C. sakazakii in host macrophages.
FIG 6.
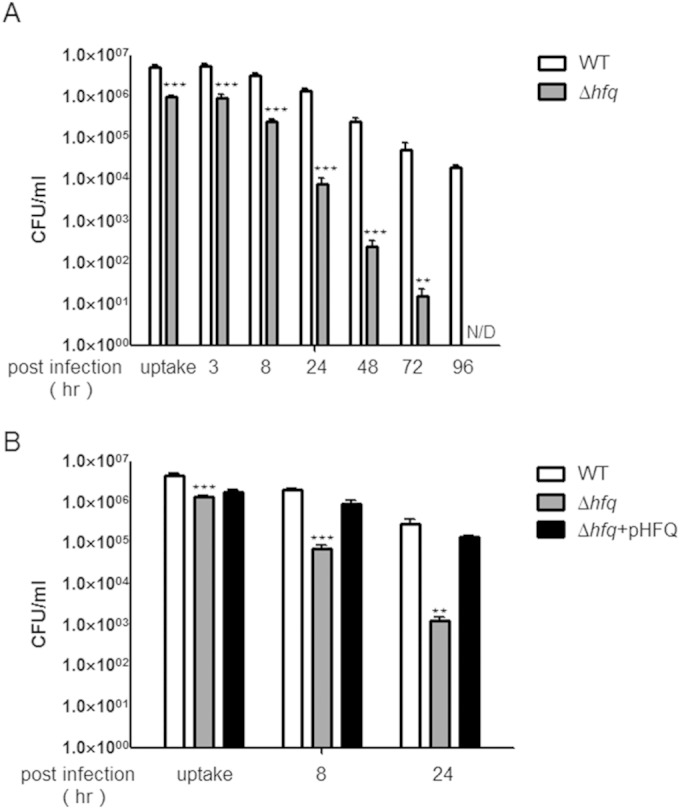
Lack of Hfq results in reduced intracellular survival of C. sakazakii in macrophage-like cells. The C. sakazakii strains harboring the pACYC184 control plasmid or a complementation plasmid, pHFQ, and the wild type or hfq mutant (SK003) were incubated with RAW264.7 macrophage-like cells. (A) Intracellular bacteria of the wild type and hfq mutant (SK003) were evaluated for the number of CFU at uptake (1.5 h) and at 8, 24, 48, 72, and 96 h postinfection. (B) Intracellular bacteria of C. sakazakii strains harboring the pACYC184 control plasmid or a complementation plasmid, pHFQ, were evaluated for the number of CFU at uptake (1.5 h) and at 8 and 24 h postinfection. The bars represent means and SEM from independent experiments performed in triplicate. The data are representative of 3 independent experiments. The asterisks indicate significant differences (***, P < 0.001; **, P < 0.05).
F. tularensis lacking katG caused increases in hydrogen peroxide production and tumor necrosis factor alpha (TNF-α) and interleukin 6 (IL-6) expression within macrophages, leading to attenuation of bacterial survival inside host cells (55). In this regard, the accelerated clearance of the Δhfq strain within host cells might be attributable, at least in part, to decreased katG expression (Fig. 5C), which causes H2O2 accumulation and stimulates inflammatory cytokine production. Another probable explanation for the attenuated Δhfq persistence inside the macrophage-like cells is that flagellar synthesis is altered by the removal of hfq, as described below.
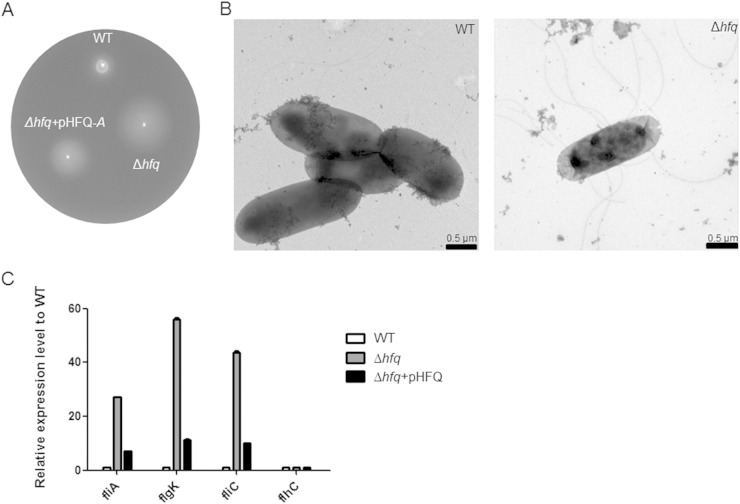
A C. sakazakii Δhfq strain is hypermotile due to higher expression of flagella.
As motility is an important virulence determinant (56), we examined the effect of Hfq on the swimming motility of C. sakazakii using 0.3% soft-agar plates. The absence of the hfq gene resulted in increased motility (Fig. 7A), and the transmission electron microscope image showed that the hfq mutant was more flagellated than the WT (Fig. 7B). These results suggested the possibility of negative regulation of flagellar synthesis by Hfq. Therefore, the expression of flagellum-associated genes was studied in the hfq mutant by qRT-PCR (Fig. 7C). The transcription of fliA, which encodes an alternative sigma factor specific for the late operons of flagellar regulons, including flgK and fliC (57), was increased 30-fold in the hfq mutant compared with the wild type. In accordance with the increased fliA transcription, genes under the control of FliA, including flgK and fliC, encoding a flagellar-hook-associated protein and a flagellin protein, respectively, showed increases in transcription in the absence of Hfq. On the other hand, the transcriptional level of flhDC, encoding a master regulator of flagellar regulons, was not affected by the lack of Hfq. Interestingly, introducing a plasmid harboring hfq into the hfq mutant could partially complement the transcription levels of fliA, flgK, and fliC (Fig. 7C) but could not recover the hypermotility (data not shown). An Acinetobacter baylyi Δhfq strain showing a retarded growth and abnormal morphology was fully complemented with the introduction of hfq and its cognate promoter region into the chromosome, while a low-copy-number plasmid harboring hfq under a leaky inducible promoter could not complement it, indicating that fine-tuning of hfq expression is important for its tight regulation (58). The failure of the pHFQ plasmid to complement the hypermotility of the hfq strain might be caused by the imbalance of Hfq production from the low-copy-number plasmid pACYC184 (59). To rule out that possibility, the pHFQ-A plasmid, in which hfq expression is under an arabinose-inducible promoter (37), was used to optimize Hfq expression and accomplished partial restoration of motility with 1.33 mM arabinose (Fig. 7A). Taken together, these data suggest that hfq in C. sakazakii is involved in the negative regulation of flagellar biosynthesis.
FIG 7.
Loss of Hfq causes enhanced motility. (A) Motility of the C. sakazakii strains harboring the pBAD18 control plasmid or the complementation plasmid pHFQ-A on semisolid agar plates. The strains were cultured on semisolid agar plates containing 1.33 mM arabinose at 37°C, and motility was monitored. The image is representative of several experiments. (B) Morphology of wild-type and Δhfq (SK003) strains imaged by transmission electron microscopy. (C) qRT-PCR of fliA, flgK, fliC, and flhC transcripts from WT (SK001) and Δhfq (SK005) strains and Δhfq harboring pHFQ (SK007). To obtain the expression level relative to that of the WT on the y axis, the mRNA level of each gene was divided by the mRNA level of the 16S rRNA. The mRNA expression values in strains SK005 and SK007 were further normalized to the transcription levels of the wild type. The bars represent means and SEM from independent experiments performed in triplicate. The data are representative of 3 independent experiments.
It is interesting that the C. sakazakii hfq mutant showed an increased motility phenotype, unlike other bacteria, i.e., Salmonella and uropathogenic E. coli (31, 32). One explanation for this phenomenon might be the variable region at the C terminus of Hfq, as shown in Fig. 1B. According to a previous report, an Hfq variant lacking its C terminus was defective in autoregulation and riboregulation (44). Therefore, C-terminal variation might enable differential gene regulation from species to species. Alternatively, the hypermotility of the hfq strain may be associated with Hfq-mediated RpoS regulation in flagellar biosynthesis. It has been reported that Hfq is required for efficient translation of the rpoS sigma factor (60, 61), and RpoS negatively regulates operons involved in flagellar biosynthesis and functions in E. coli by decreasing the amount of RpoF, which competes with RpoS in binding to the core RNA polymerase (62, 63). In the absence of RpoS, the expression of RpoF (FliA) and FliC was increased, and the surface of the bacteria was found to be more flagellated than that of the WT strain. In this regard, it is plausible that the hfq null mutation caused poor translation of rpoS in C. sakazakii and that the lowered level of RpoS resulted in higher flagellar biosynthesis and formation. Further study would be needed to shed light on the mechanisms of flagellar regulation mediated by hfq.
The ability of pathogenic microbes to survive within cultured phagocytic cells is associated with the ability to escape or remodel an innate immune response that employs antimicrobial defense mechanisms, such as oxidative burst and acidic compartmentalization (38). To establish a good niche within the host, bacteria should have the ability to modulate immune system activation by harnessing cytokine production (64, 65). It has been reported that C. sakazakii flagella and flagellin proteins stimulate the production of inflammatory cytokines (IL-8, TNF-α, and IL-10) in a dose-dependent manner (19). To dampen cytokine stimulation, pathogens, such as Pseudomonas and Salmonella, reduce flagellar synthesis after entering host cells (65–67). Bacterial flagella activate the signaling pathway by binding to TLR5 during infection (68). Indeed, secreted flagellin from Pseudomonas triggered the expression of NF-κB and secretion of IL-8. To avoid the activation of TLR5, the enzyme AprA, which cleaves flagellin, is produced (67). Likewise, flagellin from Salmonella also stimulates the activation of immune cells, leading to proinflammatory cytokine expression. However, intracellular Salmonella represses flagellar expression to modulate the immune response, whereas extracellular bacteria express flagella. Overall, it is important for pathogenic bacteria to harness flagellar expression for successful infection (65, 67). In this context, hyperflagellation of the hfq mutant (Fig. 7) might result in higher production of inflammatory cytokines. Accordingly, the increase in cytokine production might elicit an immune response, leading to accelerated clearance of intracellular bacteria or causing local inflammation and necrosis (69).
Conclusions.
In the present study, we demonstrated that the loss of Hfq remarkably attenuated C. sakazakii virulence in a rat pup model of infection due to defects in growth, invasion ability, resistance to oxidative stress, and intracellular survival. In addition, to our surprise, the Hfq-deficient strain showed hypermotility, which is a distinctive phenotype among bacteria.
ACKNOWLEDGMENT
This research was supported by a grant (14162MFDS972) from the Ministry of Food and Drug Safety, South Korea, 2015.
REFERENCES
- 1.Farmer JJ, Asbury MA, Hickman FW, Brenner DJ. 1980. Enterobacter sakazakii—a new species of Enterobacteriaceae isolated from clinical specimens. Int J Syst Bacteriol 30:569–584. doi: 10.1099/00207713-30-3-569. [DOI] [Google Scholar]
- 2.Iversen C, Lehner A, Mullane N, Marugg J, Fanning S, Stephan R, Joosten H. 2007. Identification of “Cronobacter” spp. (Enterobacter sakazakii). J Clin Microbiol 45:3814–3816. doi: 10.1128/JCM.01026-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Iversen C, Lehner A, Mullane N, Bidlas E, Cleenwerck I, Marugg J, Fanning S, Stephan R, Joosten H. 2007. The taxonomy of Enterobacter sakazakii: proposal of a new genus Cronobacter gen. nov. and descriptions of Cronobacter sakazakii comb. nov., Cronobacter sakazakii subsp. sakazakii, comb. nov., Cronobacter sakazakii subsp. malonaticus subsp. nov., Cronobacter turicensis sp. nov., Cronobacter muytjensii sp. nov., Cronobacter dublinensis sp. nov. and Cronobacter genomospecies 1. BMC Evol Biol 7:64. doi: 10.1186/1471-2148-7-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Iversen C, Mullane N, McCardell B, Tall BD, Lehner A, Fanning S, Stephan R, Joosten H. 2008. Cronobacter gen. nov., a new genus to accommodate the biogroups of Enterobacter sakazakii, and proposal of Cronobacter sakazakii gen. nov., comb. nov., Cronobacter malonaticus sp. nov., Cronobacter turicensis sp. nov., Cronobacter muytjensii sp. nov., Cronobacter dublinensis sp. nov., Cronobacter genomospecies 1, and of three subspecies, Cronobacter dublinensis subsp. dublinensis subsp. nov., Cronobacter dublinensis subsp. lausannensis subsp. nov. and Cronobacter dublinensis subsp. lactaridi subsp. nov. Int J Syst Evol Microbiol 58:1442–1447. doi: 10.1099/ijs.0.65577-0. [DOI] [PubMed] [Google Scholar]
- 5.Kandhai MC, Reij MW, Gorris LG, Guillaume-Gentil O, van Schothorst M. 2004. Occurrence of Enterobacter sakazakii in food production environments and households. Lancet 363:39–40. doi: 10.1016/S0140-6736(03)15169-0. [DOI] [PubMed] [Google Scholar]
- 6.Joseph S, Cetinkaya E, Drahovska H, Levican A, Figueras MJ, Forsythe SJ. 2012. Cronobacter condimenti sp. nov., isolated from spiced meat, and Cronobacter universalis sp. nov., a species designation for Cronobacter sp. genomospecies 1, recovered from a leg infection, water and food ingredients. Int J Syst Evol Microbiol 62:1277–1283. doi: 10.1099/ijs.0.032292-0. [DOI] [PubMed] [Google Scholar]
- 7.Drudy D, Mullane NR, Quinn T, Wall PG, Fanning S. 2006. Enterobacter sakazakii: an emerging pathogen in powdered infant formula. Clin Infect Dis 42:996–1002. doi: 10.1086/501019. [DOI] [PubMed] [Google Scholar]
- 8.Bar-Oz B, Preminger A, Peleg O, Block C, Arad I. 2001. Enterobacter sakazakii infection in the newborn. Acta Paediatr 90:356–358. doi: 10.1111/j.1651-2227.2001.tb00319.x. [DOI] [PubMed] [Google Scholar]
- 9.Joker RN, Norholm T, Siboni KE. 1965. A case of neonatal meningitis caused by a yellow Enterobacter. Dan Med Bull 12:128–130. [PubMed] [Google Scholar]
- 10.Hunter CJ, Petrosyan M, Ford HR, Prasadarao NV. 2008. Enterobacter sakazakii: an emerging pathogen in infants and neonates. Surg Infect (Larchmt) 9:533–539. doi: 10.1089/sur.2008.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mullane NR, Iversen C, Healy B, Walsh C, Whyte P, Wall PG, Quinn T, Fanning S. 2007. Enterobacter sakazakii: an emerging bacterial pathogen with implications for infant health. Minerva Pediatr 59:137–148. [PubMed] [Google Scholar]
- 12.Willis J, Robinson JE. 1988. Enterobacter sakazakii meningitis in neonates. Pediatr Infect Dis J 7:196–199. doi: 10.1097/00006454-198803000-00012. [DOI] [PubMed] [Google Scholar]
- 13.Kothary MH, McCardell BA, Frazar CD, Deer D, Tall BD. 2007. Characterization of the zinc-containing metalloprotease encoded by zpx and development of a species-specific detection method for Enterobacter sakazakii. Appl Environ Microbiol 73:4142–4151. doi: 10.1128/AEM.02729-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim KP, Loessner MJ. 2008. Enterobacter sakazakii invasion in human intestinal Caco-2 cells requires the host cell cytoskeleton and is enhanced by disruption of tight junction. Infect Immun 76:562–570. doi: 10.1128/IAI.00937-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim K, Kim KP, Choi J, Lim JA, Lee J, Hwang S, Ryu S. 2010. Outer membrane proteins A (OmpA) and X (OmpX) are essential for basolateral invasion of Cronobacter sakazakii. Appl Environ Microbiol 76:5188–5198. doi: 10.1128/AEM.02498-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Giri CP, Shima K, Tall BD, Curtis S, Sathyamoorthy V, Hanisch B, Kim KS, Kopecko DJ. 2012. Cronobacter spp. (previously Enterobacter sakazakii) invade and translocate across both cultured human intestinal epithelial cells and human brain microvascular endothelial cells. Microb Pathog 52:140–147. doi: 10.1016/j.micpath.2011.10.003. [DOI] [PubMed] [Google Scholar]
- 17.Franco AA, Kothary MH, Gopinath G, Jarvis KG, Grim CJ, Hu L, Datta AR, McCardell BA, Tall BD. 2011. Cpa, the outer membrane protease of Cronobacter sakazakii, activates plasminogen and mediates resistance to serum bactericidal activity. Infect Immun 79:1578–1587. doi: 10.1128/IAI.01165-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Grim CJ, Kothary MH, Gopinath G, Jarvis KG, Beaubrun JJ, McClelland M, Tall BD, Franco AA. 2012. Identification and characterization of Cronobacter iron acquisition systems. Appl Environ Microbiol 78:6035–6050. doi: 10.1128/AEM.01457-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cruz-Cordova A, Rocha-Ramirez LM, Ochoa SA, Gonzalez-Pedrajo B, Espinosa N, Eslava C, Hernandez-Chinas U, Mendoza-Hernandez G, Rodriguez-Leviz A, Valencia-Mayoral P, Sadowinski-Pine S, Hernandez-Castro R, Estrada-Garcia I, Munoz-Hernandez O, Rosas I, Xicohtencatl-Cortes J. 2012. Flagella from five Cronobacter species induce pro-inflammatory cytokines in macrophage derivatives from human monocytes. PLoS One 7:e52091. doi: 10.1371/journal.pone.0052091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Choi Y, Kim KP, Kim K, Choi J, Shin H, Kang DH, Ryu S. 2012. Possible roles of LysR-type transcriptional regulator (LTTR) homolog as a global regulator in Cronobacter sakazakii ATCC 29544. Int J Med Microbiol 302:270–275. doi: 10.1016/j.ijmm.2012.06.001. [DOI] [PubMed] [Google Scholar]
- 21.Franze de Fernandez MT, Eoyang L, August JT. 1968. Factor fraction required for the synthesis of bacteriophage Qbeta-RNA. Nature 219:588–590. doi: 10.1038/219588a0. [DOI] [PubMed] [Google Scholar]
- 22.Kajitani M, Ishihama A. 1991. Identification and sequence determination of the host factor gene for bacteriophage Q beta. Nucleic Acids Res 19:1063–1066. doi: 10.1093/nar/19.5.1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sauter C, Basquin J, Suck D. 2003. Sm-like proteins in Eubacteria: the crystal structure of the Hfq protein from Escherichia coli. Nucleic Acids Res 31:4091–4098. doi: 10.1093/nar/gkg480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vogel J, Luisi BF. 2011. Hfq and its constellation of RNA. Nat Rev Microbiol 9:578–589. doi: 10.1038/nrmicro2615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Guillier M, Gottesman S, Storz G. 2006. Modulating the outer membrane with small RNAs. Genes Dev 20:2338–2348. doi: 10.1101/gad.1457506. [DOI] [PubMed] [Google Scholar]
- 26.Lenz DH, Mok KC, Lilley BN, Kulkarni RV, Wingreen NS, Bassler BL. 2004. The small RNA chaperone Hfq and multiple small RNAs control quorum sensing in Vibrio harveyi and Vibrio cholerae. Cell 118:69–82. doi: 10.1016/j.cell.2004.06.009. [DOI] [PubMed] [Google Scholar]
- 27.Repoila F, Majdalani N, Gottesman S. 2003. Small non-coding RNAs, co-ordinators of adaptation processes in Escherichia coli: the RpoS paradigm. Mol Microbiol 48:855–861. doi: 10.1046/j.1365-2958.2003.03454.x. [DOI] [PubMed] [Google Scholar]
- 28.Christiansen JK, Larsen MH, Ingmer H, Sogaard-Andersen L, Kallipolitis BH. 2004. The RNA-binding protein Hfq of Listeria monocytogenes: role in stress tolerance and virulence. J Bacteriol 186:3355–3362. doi: 10.1128/JB.186.11.3355-3362.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schiano CA, Bellows LE, Lathem WW. 2010. The small RNA chaperone Hfq is required for the virulence of Yersinia pseudotuberculosis. Infect Immun 78:2034–2044. doi: 10.1128/IAI.01046-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Meibom KL, Forslund AL, Kuoppa K, Alkhuder K, Dubail I, Dupuis M, Forsberg A, Charbit A. 2009. Hfq, a novel pleiotropic regulator of virulence-associated genes in Francisella tularensis. Infect Immun 77:1866–1880. doi: 10.1128/IAI.01496-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sittka A, Pfeiffer V, Tedin K, Vogel J. 2007. The RNA chaperone Hfq is essential for the virulence of Salmonella typhimurium. Mol Microbiol 63:193–217. doi: 10.1111/j.1365-2958.2006.05489.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kulesus RR, Diaz-Perez K, Slechta ES, Eto DS, Mulvey MA. 2008. Impact of the RNA chaperone Hfq on the fitness and virulence potential of uropathogenic Escherichia coli. Infect Immun 76:3019–3026. doi: 10.1128/IAI.00022-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chiang MK, Lu MC, Liu LC, Lin CT, Lai YC. 2011. Impact of Hfq on global gene expression and virulence in Klebsiella pneumoniae. PLoS One 6:e22248. doi: 10.1371/journal.pone.0022248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Robertson GT, Roop RM Jr. 1999. The Brucella abortus host factor I (HF-I) protein contributes to stress resistance during stationary phase and is a major determinant of virulence in mice. Mol Microbiol 34:690–700. doi: 10.1046/j.1365-2958.1999.01629.x. [DOI] [PubMed] [Google Scholar]
- 35.Datsenko KA, Wanner BL. 2000. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc Natl Acad Sci U S A 97:6640–6645. doi: 10.1073/pnas.120163297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hanahan D. 1983. Studies on transformation of Escherichia coli with plasmids. J Mol Biol 166:557–580. doi: 10.1016/S0022-2836(83)80284-8. [DOI] [PubMed] [Google Scholar]
- 37.Guzman LM, Belin D, Carson MJ, Beckwith J. 1995. Tight regulation, modulation, and high-level expression by vectors containing the arabinose pBAD promoter. J Bacteriol 177:4121–4130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Townsend SM, Hurrell E, Gonzalez-Gomez I, Lowe J, Frye JG, Forsythe S, Badger JL. 2007. Enterobacter sakazakii invades brain capillary endothelial cells, persists in human macrophages influencing cytokine secretion and induces severe brain pathology in the neonatal rat. Microbiology 153:3538–3547. doi: 10.1099/mic.0.2007/009316-0. [DOI] [PubMed] [Google Scholar]
- 39.Kucerova E, Clifton SW, Xia XQ, Long F, Porwollik S, Fulton L, Fronick C, Minx P, Kyung K, Warren W, Fulton R, Feng D, Wollam A, Shah N, Bhonagiri V, Nash WE, Hallsworth-Pepin K, Wilson RK, McClelland M, Forsythe SJ. 2010. Genome sequence of Cronobacter sakazakii BAA-894 and comparative genomic hybridization analysis with other Cronobacter species. PLoS One 5:e9556. doi: 10.1371/journal.pone.0009556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stephan R, Lehner A, Tischler P, Rattei T. 2011. Complete genome sequence of Cronobacter turicensis LMG 23827, a food-borne pathogen causing deaths in neonates. J Bacteriol 193:309–310. doi: 10.1128/JB.01162-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Moller T, Franch T, Hojrup P, Keene DR, Bachinger HP, Brennan RG, Valentin-Hansen P. 2002. Hfq: a bacterial Sm-like protein that mediates RNA-RNA interaction. Mol Cell 9:23–30. doi: 10.1016/S1097-2765(01)00436-1. [DOI] [PubMed] [Google Scholar]
- 42.Kambach C, Walke S, Young R, Avis JM, de la Fortelle E, Raker VA, Luhrmann R, Li J, Nagai K. 1999. Crystal structures of two Sm protein complexes and their implications for the assembly of the spliceosomal snRNPs. Cell 96:375–387. doi: 10.1016/S0092-8674(00)80550-4. [DOI] [PubMed] [Google Scholar]
- 43.Arluison V, Folichon M, Marco S, Derreumaux P, Pellegrini O, Seguin J, Hajnsdorf E, Regnier P. 2004. The C-terminal domain of Escherichia coli Hfq increases the stability of the hexamer. Eur J Biochem 271:1258–1265. doi: 10.1111/j.1432-1033.2004.04026.x. [DOI] [PubMed] [Google Scholar]
- 44.Vecerek B, Rajkowitsch L, Sonnleitner E, Schroeder R, Blasi U. 2008. The C-terminal domain of Escherichia coli Hfq is required for regulation. Nucleic Acids Res 36:133–143. doi: 10.1093/nar/gkm985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ding YP, Davis BM, Waldor MK. 2004. Hfq is essential for Vibrio cholerae virulence and downregulates sigma(E) expression. Mol Microbiol 53:345–354. doi: 10.1111/j.1365-2958.2004.04142.x. [DOI] [PubMed] [Google Scholar]
- 46.Hunter CJ, Singamsetty VK, Chokshi NK, Boyle P, Camerini V, Grishin AV, Upperman JS, Ford HR, Prasadarao NV. 2008. Enterobacter sakazakii enhances epithelial cell injury by inducing apoptosis in a rat model of necrotizing enterocolitis. J Infect Dis 198:586–593. doi: 10.1086/590186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Simonsen KT, Nielsen G, Bjerrum JV, Kruse T, Kallipolitis BH, Moller-Jensen J. 2011. A role for the RNA chaperone Hfq in controlling adherent-invasive Escherichia coli colonization and virulence. PLoS One 6:e16387. doi: 10.1371/journal.pone.0016387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Imlay JA. 2003. Pathways of oxidative damage. Annu Rev Microbiol 57:395–418. doi: 10.1146/annurev.micro.57.030502.090938. [DOI] [PubMed] [Google Scholar]
- 49.Hassett DJ, Cohen MS. 1989. Bacterial adaptation to oxidative stress: implications for pathogenesis and interaction with phagocytic cells. FASEB J 3:2574–2582. [DOI] [PubMed] [Google Scholar]
- 50.Storz G, Tartaglia LA, Farr SB, Ames BN. 1990. Bacterial defenses against oxidative stress. Trends Genet 6:363–368. doi: 10.1016/0168-9525(90)90278-E. [DOI] [PubMed] [Google Scholar]
- 51.Alvarez-Ordonez A, Begley M, Hill C. 2012. Polymorphisms in rpoS and stress tolerance heterogeneity in natural isolates of Cronobacter sakazakii. Appl Environ Microbiol 78:3975–3984. doi: 10.1128/AEM.07835-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Storz G, Tartaglia LA, Ames BN. 1990. The OxyR regulon. Antonie Van Leeuwenhoek 58:157–161. doi: 10.1007/BF00548927. [DOI] [PubMed] [Google Scholar]
- 53.Tseng HJ, McEwan AG, Apicella MA, Jennings MP. 2003. OxyR acts as a repressor of catalase expression in Neisseria gonorrhoeae. Infect Immun 71:550–556. doi: 10.1128/IAI.71.1.550-556.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Panek HR, O'Brian MR. 2004. KatG is the primary detoxifier of hydrogen peroxide produced by aerobic metabolism in Bradyrhizobium japonicum. J Bacteriol 186:7874–7880. doi: 10.1128/JB.186.23.7874-7880.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Melillo AA, Bakshi CS, Melendez JA. 2010. Francisella tularensis antioxidants harness reactive oxygen species to restrict macrophage signaling and cytokine production. J Biol Chem 285:27553–27560. doi: 10.1074/jbc.M110.144394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Duan Q, Zhou M, Zhu L, Zhu G. 2013. Flagella and bacterial pathogenicity. J Basic Microbiol 53:1–8. doi: 10.1002/jobm.201100335. [DOI] [PubMed] [Google Scholar]
- 57.Ohnishi K, Kutsukake K, Suzuki H, Iino T. 1990. Gene fliA encodes an alternative sigma factor specific for flagellar operons in Salmonella typhimurium. Mol Gen Genet 221:139–147. [DOI] [PubMed] [Google Scholar]
- 58.Schilling D, Gerischer U. 2009. The Acinetobacter baylyi hfq gene encodes a large protein with an unusual C terminus. J Bacteriol 191:5553–5562. doi: 10.1128/JB.00490-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chang AC, Cohen SN. 1978. Construction and characterization of amplifiable multicopy DNA cloning vehicles derived from the P15A cryptic miniplasmid. J Bacteriol 134:1141–1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Brown L, Elliott T. 1996. Efficient translation of the RpoS sigma factor in Salmonella typhimurium requires host factor I, an RNA-binding protein encoded by the hfq gene. J Bacteriol 178:3763–3770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Muffler A, Fischer D, Hengge-Aronis R. 1996. The RNA-binding protein HF-I, known as a host factor for phage Qbeta RNA replication, is essential for rpoS translation in Escherichia coli. Genes Dev 10:1143–1151. doi: 10.1101/gad.10.9.1143. [DOI] [PubMed] [Google Scholar]
- 62.Makinoshima H, Aizawa S, Hayashi H, Miki T, Nishimura A, Ishihama A. 2003. Growth phase-coupled alterations in cell structure and function of Escherichia coli. J Bacteriol 185:1338–1345. doi: 10.1128/JB.185.4.1338-1345.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Patten CL, Kirchhof MG, Schertzberg MR, Morton RA, Schellhorn HE. 2004. Microarray analysis of RpoS-mediated gene expression in Escherichia coli K-12. Mol Genet Genomics 272:580–591. doi: 10.1007/s00438-004-1089-2. [DOI] [PubMed] [Google Scholar]
- 64.Finlay BB, McFadden G. 2006. Anti-immunology: evasion of the host immune system by bacterial and viral pathogens. Cell 124:767–782. doi: 10.1016/j.cell.2006.01.034. [DOI] [PubMed] [Google Scholar]
- 65.Cummings LA, Barrett SL, Wilkerson WD, Fellnerova I, Cookson BT. 2005. FliC-specific CD4+ T cell responses are restricted by bacterial regulation of antigen expression. J Immunol 174:7929–7938. doi: 10.4049/jimmunol.174.12.7929. [DOI] [PubMed] [Google Scholar]
- 66.Eriksson S, Lucchini S, Thompson A, Rhen M, Hinton JC. 2003. Unravelling the biology of macrophage infection by gene expression profiling of intracellular Salmonella enterica. Mol Microbiol 47:103–118. doi: 10.1046/j.1365-2958.2003.03313.x. [DOI] [PubMed] [Google Scholar]
- 67.Bardoel BW, van der Ent S, Pel MJ, Tommassen J, Pieterse CM, van Kessel KP, van Strijp JA. 2011. Pseudomonas evades immune recognition of flagellin in both mammals and plants. PLoS Pathog 7:e1002206. doi: 10.1371/journal.ppat.1002206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hayashi F, Smith KD, Ozinsky A, Hawn TR, Yi EC, Goodlett DR, Eng Akira JKS, Underhill DM, Aderem A. 2001. The innate immune response to bacterial flagellin is mediated by Toll-like receptor 5. Nature 410:1099–1103. doi: 10.1038/35074106. [DOI] [PubMed] [Google Scholar]
- 69.Slifka MK, Whitton JL. 2000. Clinical implications of dysregulated cytokine production. J Mol Med 78:74–80. doi: 10.1007/s001090000086. [DOI] [PubMed] [Google Scholar]
- 70.Asakura H, Morita-Ishihara T, Yamamoto S, Igimi S. 2007. Genetic characterization of thermal tolerance in Enterobacter sakazakii. Microbiol Immunol 51:671–677. doi: 10.1111/j.1348-0421.2007.tb03955.x. [DOI] [PubMed] [Google Scholar]