Abstract
To investigate whether activated autologous platelet-rich plasma (PRP) can promote proliferation and osteogenic differentiation of human adipose-derived stem cells (hASCs) in vitro. hASCs were isolated from lipo-aspirates, and characterized by specific cell markers and multilineage differentiation capacity after culturing to the 3rd passage. PRP was collected and activated from human peripheral blood of the same patient. Cultured hASCs were treated with normal osteogenic inductive media alone (group A, control) or osteogenic inductive media plus 5%, 10%, 20%, 40%PRP (group B, C, D, E, respectively). Cell proliferation was assessed by CCK-8 assay. mRNA expression of osteogenic marker genes including alkaline phosphatase (ALP), osteopontin (OPN), osteocalcin (OCN) and core binding factor alpha 1 (Cbfa1) were determined by Real-Time Quantitative PCR Analysis (qPCR). Data revealed that different concentrations of activated autologous PRP significantly promoted hASCs growth in the proliferation phase compared to the without PRP group and resulted in a dose-response relationship. At 7-d and 14-d time point of the osteogenic induced stage, ALP activity in PRP groups gradually increased with the increasing of concentrations of PRP and showed that dose-response relationship. At 21-d time point of the osteogenic induced stage, PRP groups make much more mineralization and mRNA relative expression of ALP, OPN, OCN and Cbfa1 than that without PRP groups and show that dose-response relationship. This study indicated that different concentrations of activated autologous PRP can promote cell proliferation at earlier stage and promote osteogenic differentiation at later stage of hASCs in vitro. Moreover, it displayed a dose-dependent effect of activated autologous PRP on cell proliferation and osteogenic differentiation of hASCs in vitro.
Keywords: Adipose-derived stem cells, cell therapy, induced differentiation, platelet-rich plasma, cell proliferation, osteogenic differentiation
Introduction
Nowadays, autologous bone transfers have been considered the standard method for approaching congenital and acquired bone defects in humans [1]. However, the morbidity of the procedure remains a drawback that has driven development of new strategies based on bone engineering, which may induce bone formation with less morbidity and similar efficiency [2,3]. The concept of tissue engineering was described by Lalan S et al as an “interdisciplinary field that applies the principles of engineering and life sciences toward the development of biologic tissues that restore, maintain, or improve tissue function” [4]. Conceptually, it can be divided into three interconnecting elements: (1) the biomaterial matrix carrier/scaffold, (2) the cells in the matrix/scaffold, and (3) an environment or recipient site where this association resides [5].
There are constant efforts to maximize the role of each of these elements to enhance the capacity of a chosen strategy to regenerate needed tissue. Concerning bone tissue engineering (BTE), advances in technology of biomaterials and characterization of mesenchymal stem cell (MSC) immunophenotype have contributed tremendously to this area [6]. As strategies of BTE continue to gain more solid scientific footing, they may fulfill an important gap in plastic and reconstructive surgery and may create opportunities to heal osseous defects with high efficiency and low morbidity.
In the last decade, more and more researches have focused on mesenchymal stem cells isolated from bone marrow in vitro and in vivo. However, bone marrow procurement causes considerable discomfort to the patient and yields a relatively small number of harvested cells. Animal and clinical studies have shown that adipose-derived stem cells (ASCs) are capable of repairing damaged skeletal tissue or large-bone segmental defects [7,8]. ASCs represent a readily available abundant supply of mesenchymal stem cells from lipo-aspirates [9]. ASCs are simply expanded to large numbers in vitro, when compared with bone marrow-derived mesenchymal stromal cells (BMSCs) [9] and there is less cell heterogeneity in ASCs than there is in BMSCs due to the mixture of hematopoietic and mesenchymal stem cells [10,11]. Similar to BMSCs, Different studies described ASCs’ plasticity towards chondrocytes, osteoblasts, adipocytes, myocytes and neural phenotype by different inductive culture systems [12-17]. Although BMSCs are considered as a valuable source for bone tissue regeneration in human diseases [18,19], the capacity of autologous BMSCs to differentiate along functional bone-forming osteoblasts remains relatively limited for bone regeneration in vivo [20]. An important issue for efficient bone regeneration is therefore to make ASCs a promising source of skeletal progenitor cells, to promote their osteogenic potential for in vivo bone regeneration [21]. In this regard, the regulatory mechanism of osteogenesis, and ways to improve osteogenic differentiation of ASCs need to be determined in detail. Osteogenesis is defined by a series of events, which starts with a commitment to an osteogenic lineage by mesenchymal cells. However, the differentiation potency of ASCs toward osteogenic cell lines is not as strong enough as BMSCs. In order to promote their differentiation ability into osteogenic cell lines as well as elevating their proliferative capacity, in this study, we introduced activated autologous platelet-rich plasma (PRP) was used as a supplement in cell cultures to observe its ability to promote proliferation and osteogenic differentiation of human adipose-derived stem cells (hASCs) in vitro.
Materials and methods
Patient consent and ethical approval
The present study was approved by the institutional ethical review board of Southern Medical University (Guangzhou, Guangdong, China, Ref #: 5105150378). Written informed consent was provided by the donor patient.
Isolation and characterization of hASCs
hASCs were isolated via abdominal lipoaspirate which obtained from six patients undergoing routine liposuction procedures for cosmetic reasons (Table 1). For each sample, 150 g of the fat portion of the lipo-aspirates was digested with 0.1% collagenase (Invitrogen, USA) at 37°C for 60 min by vigorous shaking. The digested samples were passed through an 200 μm mesh and centrifuged at 300× g for 5 min, the cell pellet (SVF) and floating adipocytes were resuspended with Dulbecco’s modified Eagle media (DMEM, Invitrogen, USA), then plus 10% fetal bovine serum and 1% Penicillin-Streptomycin solution (FBS, Gibco, USA) to removal the dopant and supernatant fluid. The isolated cells were resuspended with DMEM and then seeded onto culture dishes and incubated at 37°C in 5% carbon dioxide. After 24 hours, media changes were performed every 3 days. The initial media change resulted in removal of nonadherent cells. The morphology and expansion of cultured cells were observed using a contrast phase microscope. Cells were harvested at 80-90% confluence and passaged at a ratio of 1:3. The third passage hASCs were cultured using adipogenic, chondrogenic and neurogenic inductive media (Table 2) in order to confirm the multilineage differentiation capacity of them. The induced hASCs was detected using relevant histological assays.
Table 1.
Information of patients
| SN of patients | gender | Age (year) | body weight (Kg) |
|---|---|---|---|
| NO.1 | female | 31 | 68 |
| NO.2 | female | 28 | 70 |
| NO.3 | female | 35 | 75 |
| NO.4 | female | 40 | 77 |
| NO.5 | female | 26 | 72 |
| NO.6 | female | 32 | 75 |
Table 2.
Multilineage induction of ASCs
| Induction component and time | Characterization | |
|---|---|---|
| Adipogenic | DMEM-HG, 10%FBS, 200 μM indomethacin, 0.5 mM IBMX, 1 μM dexamethasone, 10 μM insulin, 1% antibiotic/antimycotic, 2 weeks | Oil red O staining |
| Neurogenic | pre-induction medium (DMEM-HG, 10%FBS, 1 mM BME, 1% antibiotic/antimycotic), for 24 hours, then replaced by the neurogenic inductive medium (DMEM-HG, 5 mM BME, 1% antibiotic/antimycotic), for 6 days | Immunohistochemical staining for NSE |
| Chondrogenic | DMEM-HG, 10%FBS, 10 ng/ml TGF-β1, 50 ng/ml IGF-1, 6.25 μM transferrin, 0.1 μM dexamethasone, 1% antibiotic/antimycotic, 2 weeks | Alcian blue staining and Immunohistochemical staining for collagen type II |
DMEM-HG: Dulbecco’s modified eagle medium-high glucose; FBS: Fetal Bovine Serum; IBMX: isobutyl-methylxanthine; BME: mercaptoethanol; TGF-β1: transforming growth factor-β1; IGF-1: insulin-like growth factor 1.
Immunofluorescence and immunohistochemistry
To identify specific cellular surface markers, CD29, CD31, CD34, CD44, CD49d, CD73, CD90 and CD105 immunofluorescence staining was performed on hASCs at the third passage. The primary antibodies used were monoclonal mouse anti-human (1:200, Sigma, Santa Clara, CA, USA), and the secondary antibodies were goat anti-mouse IgG-Cy3 (CD29, CD31, CD34 and CD90) or IgG-FITC (CD44, CD49d, CD73 and CD105) (all 1:100, Sigma). The nuclei of CD29, CD31, CD34, CD44 and CD73 stained hASCs were counterstained with 4’, 6-diamidino-2-phenylindole, dihydrochloride (DAPI), whereas the nuclei of CD49d and CD105 were counterstained with propidium iodide (PI), the nuclei of CD90 were counterstained with methyl green.
Preparation of PRP and measurement of growth factor concentrations
Platelet-rich plasma (PRP) were obtained from venous blood of the same patient underwent liposuction. Patient’s 10 ml venous blood was drawn from the antecubital vein in sterile Vacutainer containing 1 ml Citrate Phosphate Dextrose- Adenine (CPDA) as anticoagulant and centrifuged at 250× g for 15 minutes. After the first centrifugation, two layers were seen clearly in the Vacutainer. The upper yellow layer was consisting of platelet rich and poor plasma and lower red layer was consisting of erythrocytes and leukocytes. Then the complete upper yellow layer and lower red layer’s top 1-2 mm part was transferred into plain Vacutainer. After the second centrifugation at 200× g for 15 minutes, approximately 2 ml was plasma rich from platelets at bottom of Vacutainer and the upper rest was plasma poor from platelets. The part of platelet poor plasma was discarded and remaining plasma at bottom was stored in platelet agitator till its use. The PRP was activated with 10% CaCl2 solution (Sigma, St. Louis, MO, USA), 1 part CaCl2 stock solution was added to 9 parts PRP to mixing together. Each PRP sample was incubated for 1 hr at 37°C [22]. The resulting supernatants from the clot preparation were referred to as activated PRP. The PRP were stored at -80°C until use. The concentrations of platelet-derived growth factor (PDGF), transforming growth factor β (TGF-β), vascular endothelial growth factor (VEGF), fibroblast growth factor (FGF) and insulin-like growth factor (IGF-1) were measured with commercially available Quantikine colorimetric sandwich ELISA kits (R&D Systems, Minneapolis, MN, USA) according to the manufacturer’s instructions.
Effect of PRP on hASC proliferation
For the cell proliferation assays, hASCs were cultured in 96-well plates at 1×104 cells per well with growth culture medium. Twenty-four hours later, cells were switched to different concentration PRP containing media for up to 7 days. The proliferation of hASCs was determined by the CCK-8 (Kumamoto, Japan) and measured by microplate reader scanning (ELx800, BioTek) at 450 nm as previously described elsewhere of our study [23].
Alkaline phosphatase enzyme activity (ALP)
The ALP is a generally used marker for early osteogenic differentiation, so each group construct was analyzed at 7 and 14 days after cell seeding. To assess ALP, cells were plated in 24-well plates at density of 5×104 cells per well in 200 μL of growth medium for each group. The cell lysate (0.1 mL) was mixed with 0.5 mL p-nitrophenol phosphate substrate solution (Sigma, USA) and 0.5 mL alkaline phosphatase buffer solution (Sigma, USA). After incubation at 37°C for 15 min, 10 mL of 0.05 N NaOH were added to stop the reaction. The production of p-nitrophenol in the presence of ALP was measured by monitoring light absorbance of the solution at 405 nm at 1 min increments. The slope of the absorbance versus time plot was used to calculate the ALP activity.
Osteogenic differentiation
Harvested hASCs from passage 3, were resuspended in osteogenic-based culture medium (high glucose Dulbecco’s modified Eagle medium (Gibco), 1% antibiotic/antimycotic (Sigma), 0.1 μM dexamethasone (Sigma), 50 μM ascorbate-2-phosphate (Sigma), 10 mM glycerophosphate (Sigma), supplemented with 0, 5, 10, 20 and 40%PRP (group A, B, C, D, E, respectively). Each group’s cells of hASCs at P3 were cultured in T25 flasks at a cell density of 5×104 cells/cm2 in the osteogenic induction medium. The medium was replaced every 2 days. In the control group (group A), normal osteogenic medium was used containing no PRP. To evaluate the effect of PRP in osteogenesis, we applied PRP in four different concentrations (5%, 10%, 20% and 40%) to plus in the normal osteogenic medium. All other conditions and components of the osteogenic medium remained the same. The cells were kept in osteogenic medium up to 3 weeks.
Staining for mineralization
The mineralization of the extracellular matrix (ECM) is a characteristic of late osteogenic differentiation. So alizarin red S staining was used to evaluate hASCs mineralization in all groups after 3 weeks of incubation, the mineral deposition in the extracellular matrix was visualized by staining with alizarin red S. Briefly, the cells were washed with PBS and fixed with 70% ethanol for 1 hour at -20°C. The cells were then rinsed in distilled water, stained with 40 mM alizarin red S (Sigma-Aldrich) at pH 4.2 with rotation for 10 min at room temperature, and subsequently washed with distilled water. Cetylpyridinium chloride (Sigma-Aldrich) was used to extract the dye, followed by absorbance measurement at 540 nm with a microplate reader (Victor 1420).
Real-time quantitative PCR analysis (qPCR)
At 21 days, total RNA was extracted from each group by the TRIzol method strictly following the manufacturer’s protocol (Invitrogen, USA). The first-strand cDNA was synthesized from 1 μg RNA with virus reverse transcriptase (TaKaRa, Japan), and used for quantitative real-time PCR. Expression levels of representative genes, including ALP, OPN, OCN and Cbfa1 were quantified with an ABI 7300 real-time PCR system (Applied Biosystems, USA) and SYBR green PCR reaction mix (TaKaRa, Japan). Primers and annealing temperature for each gene are listed in Table 3. The program used was 95°C for 5 minutes, 40 cycles of 95°C for 15 seconds, annealing temperature for 1 minute, and 72°C for 30 s. Melting analysis and agarose gel electrophoresis were performed to confirm the specificity of the PCR products. The relative expression levels of genes were analyzed using the 2-ΔΔCt method by normalizing with GAPDH housekeeping gene expression, and presented as fold increase relative to the control group.
Table 3.
Primer sequences
| Gene Name (human) | Forward primer sequence (5’ to 3’) | Reverse primer sequence (5’ to 3’) |
|---|---|---|
| ALP | TGGAGCTTCAGAAGCTCAACACCA | ATCTCGTTGTCTGAGTACCAGTCC |
| OPN | CACCTGTGCCATACCAGTTAA | GGTGATGTCCTCGTCTGTAGCATC |
| OCN | AGGTGCGAAGCCCAGCGGTGCA | CCTGGAGAGGAGCAGAACTGGG |
| Cbfa1 | GAGTAGGTGTCCCGCCTCAGAACCC | TCTGAAGCACCTGCCTGGCTCTTCT |
| GAPDH | GAAGGTGAAGGTCGGAGTC | GAAGATGGTGATGGGATTTC |
Statistical analysis
All statistical analyses were performed using SPSS® version 16.0 software (SPSS, Inc., Chicago, IL, USA). The results of Experiments in Figures 4, 5, 7 are expressed as means ± SD, and assessed using One-Way ANOVA, respectively. Differences at P<0.05 were considered statistically significant.
Figure 4.
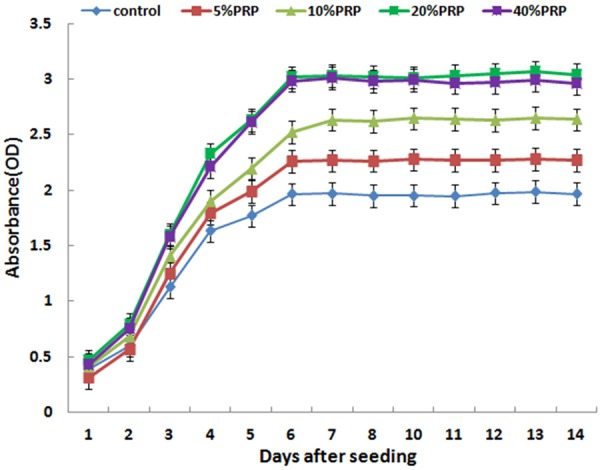
Results of the cell proliferation assay using the CCK-8 test. The test groups (B-5%PRP, C-10%PRP, D-20%PRP and E-40%PRP) displayed a significantly higher value for absorbance compared with the control group (A) at every time point starting from day 3 of the study. Results are the mean ± SD, n=6; *P<0.05, as assessed using ANOVA.
Figure 5.
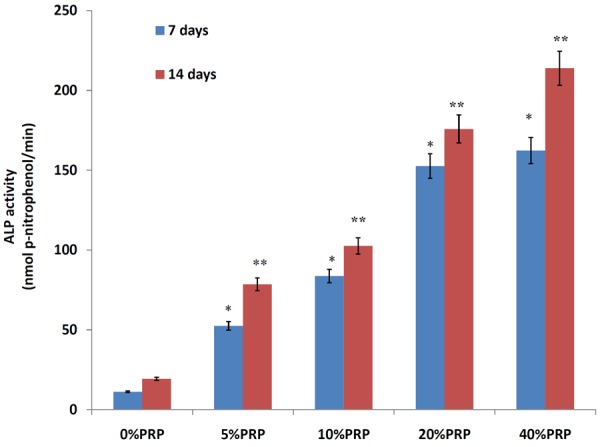
Detection of ALP activities for control, 5%PRP, 10%PRP, 20%PRP, 40%PRP groups at 7, 14 day after cell seeding. Results are shown as mean ± SD, n = 6 for each group; *P < 0.05, **P < 0.05, as assessed using ANOVA.
Figure 7.
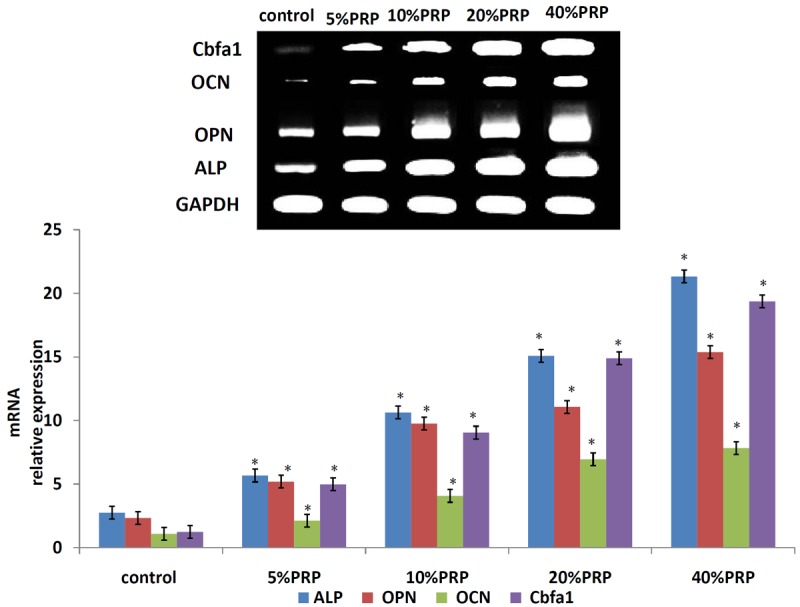
The mRNA-relative expression analysis of osteogenic marker genes ALP, OPN, OCN and Cbfa1 in 0%PRP osteogenic inductive group, 5%PRP osteogenic inductive group, 10%PRP osteogenic inductive group, 20%PRP osteogenic inductive group, 40%PRP osteogenic inductive group. Results are shown as mean ± SD, n=6 for each group; *P<0.05, as assessed using ANOVA.
Results
Characterization and multipotency of hASCs
Following initial isolation and expansion, homogeneous hASCs that grew in a monolayer with spindle-shaped morphology were observed following culturing for one to two weeks (Figure 1A). These hASCs presented a strong proliferation capacity. HASCs reached 80-90% confluency 7 days after initial seeding for the first passage. Confluency within 3-4 days with a 1:3 split ratio. These observations demonstrated that hASCs resemble bone mesenchymal stem cells (BMSCs) in terms of morphology and proliferation capacity. The isolated hASCs, subconfluent hASCs at passage 3 were cultured for 1-2 weeks with adipogenic, chondrogenic and neurogenic induction media as listed in Table 2. The lineage-specific cell morphology was observed following 2, 2 and 1 weeks of inductive culturing for adipocytes, chondrocytes and neuron-like cells, respectively. Positive staining of oil Red-O, Alcian blue staining, collagen type II and NSE immunohistochemical staining typically indicate adipocytes, chondrocytes or neuron-like cells, respectively. Thus, lineage-specific histological staining was performed with these dyes and the results confirmed that hASCs were differentiated into adipocytes, chondrocytes and neuron-like cells following relevant inductive culturing (Figure 1B-E). Positive staining was not observed in any of the control groups (Figure 1F-H). These results validated the multipotency of hASCs.
Figure 1.
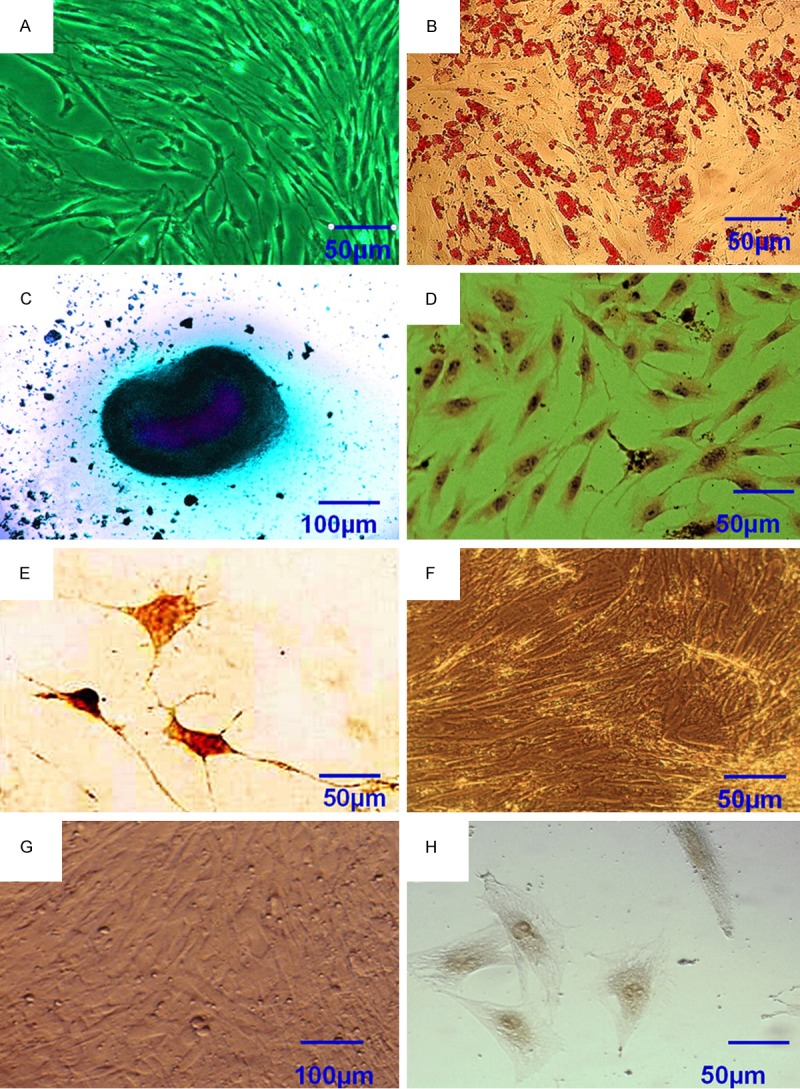
Characterization of hASCs prior to and following induction. (A) hASCs at passage 3 were marked with the following: (B) Positive for oil red O staining following adipogenic induction for 2 weeks; (C) Positive for Alcian blue staining following chondrogenic induction for 2 weeks; (D) Positive for immunohistochemical staining of collagen type II; (E) Positive for immunohistochemical staining of neuron-specific enolase (NSE); (F) Negative for oil red O staining following adipogenic induction for 2 weeks; (G) Negative for Alcian blue staining following chondrogenic induction for 2 weeks; (H) Negative for immunohistochemical staining of NSE. Scale bars=50 µm (A, B, D-F and H); 100 µm (C and G).
It has been reported that MSCs derived from different tissue sources express similar but nonidentical patterns of cell surface markers, possibly due to differences in tissue source and donor age [24-26]. CD105, CD90 and CD44 are the main three positive markers for MSCs. While CD34 is the most frequently reported negative marker, CD49d have also been reported as a positive marker and a negative marker, respectively [24,25]. In order to define the cellular features of hASCs, all abovementioned cell surface markers were analyzed in hASCs isolated in the present study. HASCs at the third passage were subjected to immunofluorescence staining with antibodies against these marker proteins. The immunofluorescence staining results demonstrated that these hASCs express CD29, CD44, CD49d, CD73, CD90 and CD105 (>90% cells are positive for staining, Figure 2A, 2D-H), but not express CD31 or CD34 (<5% cells are positive for staining; Figure 2B and 2C).
Figure 2.
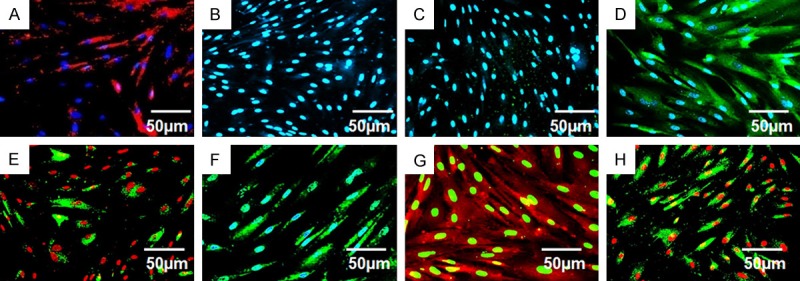
Cellular surface markers of hASCs by Immunofluorescence or immunohistochemistry detection. A. Positive for CD29 staining; B. Negative for CD31 staining; C. Negative for CD34 staining; D. Positive for CD44 staining; E. Positive for CD49d staining; F. Positive for CD73 staining; G. Positive for CD90 staining; H. Positive for CD105 staining. The nuclei in panel E, H were counterstained with propidium iodide (red), nuclei in panel G were counterstained with methyl green and nuclei in other panels were counterstained with 4’,6-diamidino-2-phenylindole dihydrochloride (blue). Scale bars: 50 μm.
Effect of PRP on hASC proliferation
The PRP was prepared from the same patient’s venous whole blood (VWB) by the double centrifugation approach as described in the Materials and methods section (Figure 3). The platelet density was markedly increased in PRP compared with that in the original VWB. The mean values of the platelet density were (129.65±19.24) ×109/L and (852.63±37.95) 109/L in VWB and PRP, respectively, revealing a 657.64% increase in PRP. Consistently, the concentrations of essential growth factors, including PDGF, TGF-β1, VEGF, FGF and IGF-1 in PRP were increased >100-fold in PRP compared with VWB.
Figure 3.
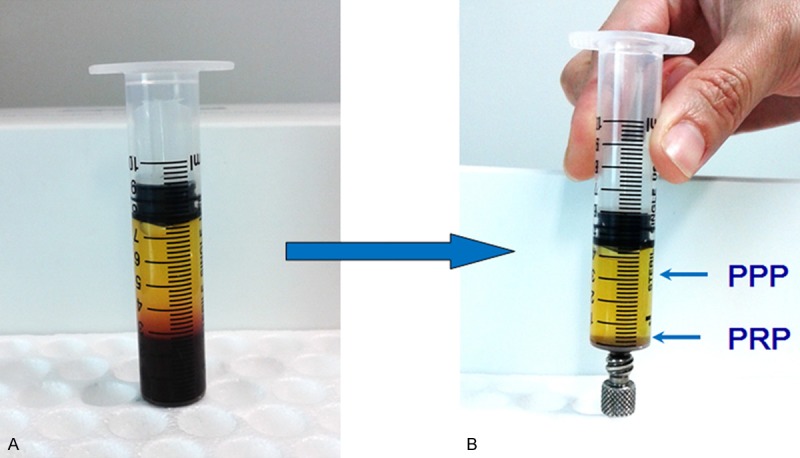
Isolation of PRP and PPP from venous whole blood. A. Preparation tube following the first centrifugation. The upper phase containing platelets were transferred into a new tube for a second centrifugation. Blood cell elements were in the lower phase. B. Preparation tube following the second centrifugation. The lower phase was collected as PRP. The small red cell pellet at the bottom was not collected into the final PRP preparation. PRP, platelet-rich plasma; PPP, platelet-poor plasm.
In order to examine the effect of PRP on hASC proliferation during osteogenic differentiation, growth curves were established using the CCK-8 for hASCs cultured in basic osteogenic inductive medium without (control group) or with 5%, 10%, 20%, and 40%PRP (test groups), which were designated as the test group and the control group, respectively. A proliferation phase in the first 6 days and a subsequent morphological conversion phase were observed during the differentiation process for the test and the control groups (Figure 4). These data further revealed that different concentrations of activated autologous PRP significantly promoted hASCs growth in the proliferation phase. Following initial equivalent seeding, the absorbance values of the test group, which were positively associated with the cell densities, were significantly greater than those of the control group (*P<0.05) between day 3 and day 6 of induction (Figure 4). The proliferation difference between the test group and control group continued to increase until the cells reached the growth platform on day 6, when morphology conversion started.
Alkaline phosphatase enzyme (ALP) activity
The ALP activity of each group was assessed at 7 days and 14 days after cell seeding. As shown in Figure 5, at the 7-day time point, the ALP activity was enhanced respectively 4.69-fold, 7.47-fold, 13.63-fold and 14.49 in 5%, 10%, 20%, and 40%PRP group (test groups) compared to that in control group (*P<0.05). at the14-day time point, the ALP activity was enhanced respectively 4.07-fold, 5.32-fold, 9.11-fold and 11.08 in 5%, 10%, 20%, and 40%PRP group (test groups) compared to that in control group (**P<0.05). ALP activity in test groups gradually increased with the increasing of concentration of PRP and showed a dose-response relationship (Figure 5).
Alizarin red S staining
After cell seeding at 21 days, more obvious positive staining of Alizarin red S and the number of mineralized nodules in test groups was observed than that in control group. Similar to the ALP activity, PRP increased mineralization the most in group B, C, D and E at day 21, but the difference between group D (20%PRP) and E (40%PRP) was not significant (Figure 6). Overall, mineralization was very weak under without PRP conditions (group A, Figure 6) and there are no mineralizations in negative control group (group F, Figure 6). In PRP cultures, group D (20%PRP) and E (40%PRP) resulted in a significantly higher mineralization than group B (5%PRP) and C (10%PRP) at the 21-day time point. Although the difference between group D (20%PRP) and E (40%PRP) in test groups was not significant in the quantitative analysis of mineralization, but the qualitative Alizarin Red staining showed more intense mineralization by group E than group D (Figure 6). The result indicated that hASCs supplement with PRP make much more mineralization than that without PRP and show that dose-response relationship.
Figure 6.
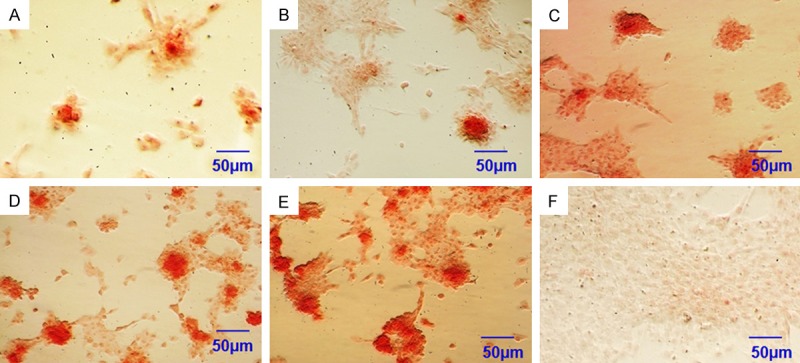
Alizarin red S staining for (A) 0%PRP osteogenic inductive group, (B) 5%PRP osteogenic inductive group, (C) 10%PRP osteogenic inductive group, (D) 20%PRP osteogenic inductive group, (E) 40%PRP osteogenic inductive group at 21 days after cell seeding. (F) negative control group (without osteogenic inductive group), Bar=50 μm.
Real-time quantitative PCR analysis
At day 21 after induction for osteogenic differentiation, the mRNA relative expression of osteogenic marker genes ALP, OPN, OCN and Cbfa1 in test groups were higher compared to control group respectively, from 1.94 times to 15.61 times more than those of control group (*P < 0.05, Figure 7).
Discussion
Recent progress in the field of bone tissue engineering has led to new and exciting research concerning regenerative medicine. This interdisciplinary field is focused on the development of biological substitutes that restore, maintain or improve tissue function by applying the principles of engineering and the life sciences [27,28]. The primary target of clinical therapeutic strategies is the regeneration of bone for skeletal reconstruction of large bone defects created by trauma, infection, tumor resection and skeletal abnormalities, or cases in which the regenerative process is compromised, including avascular necrosis, atrophic non-union and osteoporosis. Strategies that stimulate bone healing to reduce or treat complications are becoming more important, due to the increase in life expectancy and ageing of the world population. Cell-based approaches for bone formation and regeneration are widely considered the most effective, as they are able to efficiently sustain the physiologic osteogenic process in vivo. Indeed, the most promising field for ASCs application is represented by bone reconstruction/regeneration [27-29]. At the same time, activated autologous PRP as a promising therapy in regenerative medicine has been proved to have great potential in stimulating cell growth or differentiation. The applications of PRP have spread into many fields of clinical medicine for over 30 years. Soon later its applications extended across many fields including periodontal [30,31] and oral and maxillofacial surgery [32,33], aesthetic plastic surgery [34], spinal fusion [35], heart bypass surgery [36], and treatment of soft-tissue wounds or ulcers [37,38]. The effects of PRP therapy depend on various growth factors released from the activated platelets, such as transforming growth factor (TGF)-β, PDGF, VEGF, and SDF-1. PRP can be combined with cell-based therapies such as adipose-derived stem cells, regenerative cell therapy, and transfer factors therapy. While this is a relatively new concept, the strategy is appealing as the regenerative matrix graft delivers a potent trilogy of regenerative cells, fibrin matrix, growth factor or Chinese herbal drugs [17,39]. The applications are similar to those for PRP alone with the added benefit of regenerative cell enrichment.
Mesenchymal stem cells (MSCs) derived from adipose tissue have been shown to be functionally similar to bone marrow derived MSC (BMSC). However, the relative abundance of MSCs in adipose tissue compared to bone marrow, the relative ease of obtaining large volumes of tissue and the ability to rapidly isolate the stromal vascular fraction (SVF), makes adipose tissue an attractive source of MSCs, particularly when liposuction operation becomes more and more popular in the past decades. Work from our studies demonstrated the complexity of hASCs populations by showing that they are composed of several subpopulations that express different levels of hASCs markers and exhibit distinctive differentiation potentials. In our study, hASCs subpopulations were isolated using immunofluorescence positive staining specific for CD29, CD44, CD49d, CD73, CD90 and CD105, negative staining specific for CD31 and CD34. Moreover, cultured with specific adipogenic, chondrogenic and neurogenic media in order to evaluate their differentiation potential into these lineages and consistent with the studies have extensively described the differentiation potential and function of hASCs in vitro [40,41].
PRP has been discovered capable of promoting ASCs differentiation toward chondrogenic, osteogenic lineage as well as promoting ASCs proliferation in vitro. Here, we chose hASCs which may be the best cell source for regenerative medicine, to investigate the influences of PRP on osteogenic differentiation of them. While the effects of the PRP supplements have been largely studied with BMSCs and ASCs [42,43], one of the major shortcomings in all of these in vitro studies has been the lack of comparison between different concentration conditions of PRP. Accordingly, we aimed to optimize the osteogenic culturing conditions for the in vitro induction of hASCs by testing different concentrations of PRP in osteogenic-based culture medium as well as in a without PRP medium functioning as a control. As hypothesized, the differential effect of PRP media was evident in hASCs proliferation and osteogenic differentiation. The mechanism of PRP therapy is attributed to various growth factors released from the activated platelets. Many of these cytokines can stimulate cell growth, migration, mobilization, and differentiation [44-46]. Through these factors, PRP can exert its ability to promote tissue regeneration. The functions of several growth factors have been figured out for a long time, some of them have been proved for clinical use. However, tissue regeneration is a complex, multigenic event regulated by a cascade of factors. Application of a single factor usually cannot yield satisfactory therapeutic effects. Therefore, the employment of PRP have great advantages over single use of a growth factor: it is a natural reservoir of growth factors prepared to perform multiple functions during different process of tissue regeneration, according to diverse requirements. Furthermore, it can be extracted from autologous venous blood, which is much safer than the application of exogenous cytokines. Besides from its clinical applications or in vivo therapeutical effects, the influences of PRP on in vitro cultured cells have drawn a lot attention in recent years. It has been demonstrated that PRP is capable of promoting proliferation and differentiation of several kind of cells in vitro, including bone marrow-derived mesenchymal cells and ASCs. This phenomenon greatly inspired scientists to introduce PRP into researches of regenerative medicine, because the administration of stem cells in regenerative medicine commonly requires cell expansion or differentiation in vitro, but supplement of exogenous cytokines or serums into cell culture system always has security and ethical concerns. Therefore, considering its safety and effectiveness, autologous PRP can be a powerful tool to solve problems like this and thus facilitate the application of stem cells in regenerative medicine. In our studies, according to the cell growth curves, different concentration supplement of PRP could promote cell proliferation when hASCs were undergoing osteogenic differentiation in the osteogenic-based culture medium. Comparison of the cell growth in 5%PRP, 10%PRP, 20%PRP and 40%PRP maintenance media revealed a slightly higher growth rate in hASCs cultured in osteogenic-based culture medium than in without PRP medium, whereas the highest growth rate was achieved in 20%PRP. It is indicated that the lower concentration of PRP could promote the proliferation of hASCs (groups B and C) and the high concentration of PRP (groups D and E) could promote osteogenic differentiation of hASCs. On the other hand, in the current study we administered 5%, 10%, 20% and 40%PRP into the osteogenic-based culture medium to cultured hASCs and found that this supplement significantly enhanced the mRNA expression level of osteogenic specific factors such as ALP (relative density from 2.06 to 7.75 times), OPN (relative density from 2.23 to 6.60 times), OCN (relative density from 1.94 to 7.18 times) and Cbfa1 (relative density from 4.02 to 15.61 times) when hASCs were maintained in osteogenic inductive conditioned medium for 21 days compared to the control group. Thus, different concentrations of PRP can promote osteogenic differentiation in hASCs. In addition, the cell growth curves showed that supplementation with PRP promoted cell proliferation within 7 d when hASCs were undergoing osteogenic differentiation. These findings not only demonstrate the potential use of PRP for bone regeneration but also support the theory that PRP has a broad range of effects on cell differentiation and proliferation. In a certain concentration range of PRP, the results displayed a dose-dependent effect of PRP on proliferation and osteogenic differentiation of hASCs in vitro.
Conclusions
In summary, our data show that the different concentration of PRP have a significant effect on hASCs behavior; and demonstrated that activated autologous PRP has an obvious effect of stimulating cell proliferation of hASCs in vitro. It also has a significant potential of promoting osteogenic differentiation of hASCs. Moreover, the results displayed a dose-dependent effect of PRP on proliferation and osteogenic differentiation of hASCs in vitro. These effects of activated autologous PRP may facilitate the potential use of hASCs for bone regeneration.
Acknowledgements
This work was financially supported by the China Postdoctoral Science Foundation (NO. 20090450910), the Medical Scientific Research Foundation of Guangdong Province, China (No. A2011739 and A2012814) and the scientific research & technology development program of Guangxi (2015BC12041). The authors thank the Research Center of Tissue Engineering, Southern Medical University for their support, and a special thanks is due to Professor Shan Jiang and Lab Technician Pei-Ran Zhao.
Disclosure of conflict of interest
None.
References
- 1.Raposo-Amaral CE, Bueno DF, Almeida AB, Jorgetti V, Costa CC, Gouveia CH, Vulcano LC, Fanganiello RD, Passos-Bueno MR, Alonso N. Is bone transplantation the gold standard for repair of alveolar bone defects? J Tissue Eng. 2014;5:2041731413519352. doi: 10.1177/2041731413519352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yamada Y, Nakamura S, Ito K, Umemura E, Hara K, Nagasaka T, Abe A, Baba S, Furuichi Y, Izumi Y, Klein OD, Wakabayashi T. Injectable bone tissue engineering using expanded mesenchymal stem cells. Stem Cells. 2013;31:572–80. doi: 10.1002/stem.1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kim J, Bae WG, Choung HW, Lim KT, Seonwoo H, Jeong HE, Suh KY, Jeon NL, Choung PH, Chung JH. Multiscale patterned transplantable stem cell patches for bone tissue regeneration. Biomaterials. 2014;35:9058–67. doi: 10.1016/j.biomaterials.2014.07.036. [DOI] [PubMed] [Google Scholar]
- 4.Lalan S, Pomerantseva I, Vacanti JP. Tissue engineering and its potential impact on surgery. World J Surg. 2001;25:1458–66. doi: 10.1007/s00268-001-0131-3. [DOI] [PubMed] [Google Scholar]
- 5.Holzwarth JM, Ma PX. Biomimetic nanofibrous scaffolds for bone tissue engineering. Biomaterials. 2011;32:9622–9. doi: 10.1016/j.biomaterials.2011.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nagata M, Hoshina H, Li M, Arasawa M, Uematsu K, Ogawa S, Yamada K, Kawase T, Suzuki K, Ogose A, Fuse I, Okuda K, Uoshima K, Nakata K, Yoshie H, Takagi R. A clinical study of alveolar bone tissue engineering with cultured autogenous periosteal cells: coordinated activation of bone formation and resorption. Bone. 2012;50:1123–9. doi: 10.1016/j.bone.2012.02.631. [DOI] [PubMed] [Google Scholar]
- 7.Li X, Yao J, Wu L, Jing W, Tang W, Lin Y, Tian W, Liu L. Osteogenic induction of adipose-derived stromal cells: not a requirement for bone formation in vivo. Artif Organs. 2010;34:46–54. doi: 10.1111/j.1525-1594.2009.00795.x. [DOI] [PubMed] [Google Scholar]
- 8.Fan J, Park H, Lee MK, Bezouglaia O, Fartash A, Kim J, Aghaloo T, Lee M. Adipose-Derived Stem Cells and BMP-2 Delivery in Chitosan-Based 3D Constructs to Enhance Bone Regeneration in a Rat Mandibular Defect Model. Tissue Eng Part A. 2014;20:2169–79. doi: 10.1089/ten.tea.2013.0523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zuk PA, Zhu M, Ashjian P, De Ugarte DA, Huang JI, Mizuno H, Alfonso ZC, Fraser JK, Benhaim P, Hedrick MH. Human Adipose Tissue Is a Source of Multipotent Stem Cells. Mol Biol Cell. 2002;13:4279–95. doi: 10.1091/mbc.E02-02-0105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Reyes M, Lund T, Lenvik T, Aguiar D, Koodie L, Verfaillie CM. Purification and ex vivo expansion of postnatal human marrow mesodermal progenitor cells. Blood. 2001;98:2615–25. doi: 10.1182/blood.v98.9.2615. [DOI] [PubMed] [Google Scholar]
- 11.Majumdar MK, Thiede MA, Haynesworth SE, Bruder SP, Gerson SL. Human marrow-derived mesenchymal stem cells (MSCs) express hematopoietic cytokines and support long-term hematopoiesis when differentiated toward stromal and osteogenic lineages. J Hematother Stem Cell Res. 2000;9:841–8. doi: 10.1089/152581600750062264. [DOI] [PubMed] [Google Scholar]
- 12.Zhang K, Zhang Y, Yan S, Gong L, Wang J, Chen X, Cui L, Yin J. Repair of an articular cartilage defect using adipose-derived stem cells loaded on a polyelectrolyte complex scaffold based on poly(l-glutamic acid) and chitosan. Acta Biomater. 2013;9:7276–88. doi: 10.1016/j.actbio.2013.03.025. [DOI] [PubMed] [Google Scholar]
- 13.Liu TM, Martina M, Hutmacher DW, Hui JH, Lee EH, Lim B. Identification of common pathways mediating differentiation of bone marrow- and adipose tissue-derived human mesenchymal stem cells into three mesenchymal lineages. Stem Cells. 2007;25:750–60. doi: 10.1634/stemcells.2006-0394. [DOI] [PubMed] [Google Scholar]
- 14.Wei X, Li G, Yang X, Ba K, Fu Y, Fu N, Cai X, Li G, Chen Q, Wang M, Lin Y. Effects of bone morphogenetic protein-4 (BMP-4) on adipocyte differentiation from mouse adipose-derived stem cells. Cell Prolif. 2013;46:416–24. doi: 10.1111/cpr.12044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Geng J, Liu G, Peng F, Yang L, Cao J, Li Q, Chen F, Kong J, Pang R, Zhang C. Decorin promotes myogenic differentiation and mdx mice therapeutic effects after transplantation of rat adipose-derived stem cells. Cytotherapy. 2012;14:877–86. doi: 10.3109/14653249.2012.688944. [DOI] [PubMed] [Google Scholar]
- 16.James AW, Leucht P, Levi B, Carre AL, Xu Y, Helms JA, Longaker MT. Sonic Hedgehog influences the balance of osteogenesis and adipogenesis in mouse adipose-derived stromal cells. Tissue Eng Part A. 2010;16:2605–16. doi: 10.1089/ten.tea.2010.0048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xu FT, Li HM, Yin QS, Cui SE, Liu DL, Nan H, Han ZA, Xu KM. Effect of ginsenoside Rg1 on proliferation and neural phenotype differentiation of human adipose-derived stem cells in vitro. Can J Physiol Pharmacol. 2014;92:467–75. doi: 10.1139/cjpp-2013-0377. [DOI] [PubMed] [Google Scholar]
- 18.Prins HJ, Braat AK, Gawlitta D, Dhert WJ, Egan DA, Tijssen-Slump E, Yuan H, Coffer PJ, Rozemuller H, Martens AC. In vitro induction of alkaline phosphatase levels predicts in vivo bone forming capacity of human bone marrow stromal cells. Stem Cell Res. 2014;12:428–40. doi: 10.1016/j.scr.2013.12.001. [DOI] [PubMed] [Google Scholar]
- 19.Honda H, Tamai N, Naka N, Yoshikawa H, Myoui A. Bone tissue engineering with bone marrow-derived stromal cells integrated with concentrated growth factor in Rattus norvegicus calvaria defect model. J Artif Organs. 2013;16:305–15. doi: 10.1007/s10047-013-0711-7. [DOI] [PubMed] [Google Scholar]
- 20.Petite H, Viateau V, Bensaïd W, Meunier A, de Pollak C, Bourguignon M, Oudina K, Sedel L, Guillemin G. Tissue-engineered bone regeneration. Nat Biotechnol. 2000;18:959–63. doi: 10.1038/79449. [DOI] [PubMed] [Google Scholar]
- 21.Hamidouche Z, Fromigué O, Ringe J, Häupl T, Vaudin P, Pagès JC, Srouji S, Livne E, Marie PJ. Priming integrin alpha5 promotes human mesenchymal stromal cell osteoblast differentiation and osteogenesis. Proc Natl Acad Sci U S A. 2009;106:18587–91. doi: 10.1073/pnas.0812334106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Roussy Y, Bertrand Duchesne MP, Gagnon G. Activation of human platelet-rich plasmas: effect on growth factors release, cell division and in vivo bone formation. Clin Oral Implants Res. 2007;18:639–48. doi: 10.1111/j.1600-0501.2007.01385.x. [DOI] [PubMed] [Google Scholar]
- 23.Li HM, Han ZA, Liu DL, Zhao PR, Liang SW, Xu KM. Autologous platelet-rich plasma promotes neurogenic differentiation of human adipose-derived stem cells in vitro. Int J Neurosci. 2013;123:184–90. doi: 10.3109/00207454.2012.742077. [DOI] [PubMed] [Google Scholar]
- 24.Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–7. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 25.Gronthos S, Franklin DM, Leddy HA, Robey PG, Storms RW, Gimble JM. Surface protein characterization of human adipose tissue-derived stromal cells. J Cell Physiol. 2001;189:54–63. doi: 10.1002/jcp.1138. [DOI] [PubMed] [Google Scholar]
- 26.Hass R, Kasper C, Böhm S, Jacobs R. Different populations and sources of human mesenchymal stem cells (MSC): A comparison of adult and neonatal tissue-derived MSC. Cell Commun Signal. 2011;9:12. doi: 10.1186/1478-811X-9-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rosa AL, de Oliveira PT, Beloti MM. Macroporous scaffolds associated with cells to construct a hybrid biomaterial for bone tissue engineering. Expert Rev Med Devices. 2008;5:719–28. doi: 10.1586/17434440.5.6.719. [DOI] [PubMed] [Google Scholar]
- 28.Romagnoli C, Brandi ML. Adipose mesenchymal stem cells in the field of bone tissue engineering. World J Stem Cells. 2014;6:144–52. doi: 10.4252/wjsc.v6.i2.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yang P, Huang X, Wang C, Dang X, Wang K. Repair of bone defects using a new biomimetic construction fabricated by adipose-derived stem cells, collagen I, and porous beta-tricalcium phosphate scaffolds. Exp Biol Med (Maywood) 2013;238:1331–43. doi: 10.1177/1535370213505827. [DOI] [PubMed] [Google Scholar]
- 30.Moghe S, Saini N, Moghe A. Platelet-rich plasma in periodontal defect treatment after extraction of impacted mandibular third molars. Natl J Maxillofac Surg. 2012;3:139–43. doi: 10.4103/0975-5950.111344. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 31.Tobita M, Uysal CA, Guo X, Hyakusoku H, Mizuno H. Periodontal tissue regeneration by combined implantation of adipose tissue-derived stem cells and platelet-rich plasma in a canine model. Cytotherapy. 2013;15:1517–26. doi: 10.1016/j.jcyt.2013.05.007. [DOI] [PubMed] [Google Scholar]
- 32.Albanese A, Licata ME, Polizzi B, Campisi G. Platelet-rich plasma (PRP) in dental and oral surgery: from the wound healing to bone regeneration. Immun Ageing. 2013;10:23. doi: 10.1186/1742-4933-10-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Daif ET. Effect of autologous platelet-rich plasma on bone regeneration in mandibular fractures. Dent Traumatol. 2013;29:399–403. doi: 10.1111/edt.12021. [DOI] [PubMed] [Google Scholar]
- 34.Cervelli V, Palla L, Pascali M, De Angelis B, Curcio BC, Gentile P. Autologous platelet-rich plasma mixed with purified fat graft in aesthetic plastic surgery. Aesthetic Plast Surg. 2009;33:716–21. doi: 10.1007/s00266-009-9386-0. [DOI] [PubMed] [Google Scholar]
- 35.Okamoto S, Ikeda T, Sawamura K, Nagae M, Hase H, Mikami Y, Tabata Y, Matsuda K, Kawata M, Kubo T. Positive effect on bone fusion by the combination of platelet-rich plasma and a gelatin β-tricalcium phosphate sponge: a study using a posterolateral fusion model of lumbar vertebrae in rats. Tissue Eng Part A. 2012;18:157–66. doi: 10.1089/ten.TEA.2011.0283. [DOI] [PubMed] [Google Scholar]
- 36.Okamura T, Koh E, Yokoyama S. Effect of autologous platelet-rich plasma (PRP) in cardiac surgery. Kyobu Geka. 2001;54:397–400. [PubMed] [Google Scholar]
- 37.Jiritano F, Serraino GF, Rossi M, Dominijanni A, Brescia A, Renzulli A. Ventricular assist device driveline infection: treatment with platelet-rich plasma. Ann Thorac Surg. 2013;96:e37–8. doi: 10.1016/j.athoracsur.2013.01.093. [DOI] [PubMed] [Google Scholar]
- 38.Akhundov K, Pietramaggiori G, Waselle L, Darwiche S, Guerid S, Scaletta C, Hirt-Burri N, Applegate LA, Raffoul WV. Development of a cost-effective method for platelet-rich plasma (PRP) preparation for topical wound healing. Ann Burns Fire Disasters. 2012;25:207–13. [PMC free article] [PubMed] [Google Scholar]
- 39.Woo SH, Jeong HS, Kim JP, Koh EH, Lee SU, Jin SM, Kim DH, Sohn JH, Lee SH. Favorable vocal fold wound healing induced by platelet-rich plasma injection. Clin Exp Otorhinolaryngol. 2014;7:47–52. doi: 10.3342/ceo.2014.7.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Park Y, Jung MK, Yoon SY, Lee HR, Hur DY, Kim D, Yang Y, Kim TS, Kim S, Yoon SR, Park HJ, Bang SI, Cho DH. The combination of DHEA, histamine, and insulin increases adipogenic differentiation and enhances tissue transplantation outcome in mice. Biotechnol Appl Biochem. 2013;60:356–64. doi: 10.1002/bab.1100. [DOI] [PubMed] [Google Scholar]
- 41.Gong L, Zhou X, Wu Y, Zhang Y, Wang C, Zhou H, Guo F, Cui L. Proteomic analysis profile of engineered articular cartilage with chondrogenic differentiated adipose tissue-derived stem cells loaded polyglycolic acid mesh for weight-bearing area defect repair. Tissue Eng Part A. 2014;20:575–87. doi: 10.1089/ten.tea.2013.0205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cui SE, Li HM, Liu DL, Nan H, Xu KM, Zhao PR, Liang SW. Human breast adipose‑derived stem cells: Characterization and differentiation into mammary gland-like epithelial cells promoted by autologous activated platelet-rich plasma. Mol Med Rep. 2014;10:605–14. doi: 10.3892/mmr.2014.2280. [DOI] [PubMed] [Google Scholar]
- 43.Kishimoto S, Ishihara M, Mori Y, Takikawa M, Hattori H, Nakamura S, Sato T. Effective expansion of human adipose-derived stromal cells and bone marrow-derived mesenchymal stem cells cultured on a fragmin/protamine nanoparticles-coated substratum with human platelet-rich plasma. J Tissue Eng Regen Med. 2013;7:955–64. doi: 10.1002/term.1488. [DOI] [PubMed] [Google Scholar]
- 44.Ricco S, Renzi S, Del Bue M, Conti V, Merli E, Ramoni R, Lucarelli E, Gnudi G, Ferrari M, Grolli S. Allogeneic adipose tissue-derived mesenchymal stem cells in combination with platelet rich plasma are safe and effective in the therapy of superficial digital flexor tendonitis in the horse. Int J Immunopathol Pharmacol. 2013;26:61–8. doi: 10.1177/03946320130260s108. [DOI] [PubMed] [Google Scholar]
- 45.Sánchez-González DJ, Méndez-Bolaina E, Trejo-Bahena NI. Platelet-rich plasma peptides: key for regeneration. Int J Pept. 2012;2012:532519. doi: 10.1155/2012/532519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sánchez-Ilárduya MB, Trouche E, Tejero R, Orive G, Reviakine I, Anitua E. Time-dependent release of growth factors from implant surfaces treated with plasma rich in growth factors. J Biomed Mater Res A. 2013;101:1478–88. doi: 10.1002/jbm.a.34428. [DOI] [PubMed] [Google Scholar]


