Abstract
This study aims to investigate the role of activator protein 1 (AP-1) in the effects of 27nt-miRNA on expression of endothelial nitric oxide synthase (eNOS) gene and proliferation of endothelial cells. Cell proliferation was analyzed by cell number counting, colony formation assay and MTT assay. Cell migration and invasion was detected by transwell assay and invasion assay. Expression of eNOS and AP-1 was measured by real-time RT-PCR (mRNA level) and Western blotting (protein level). Luciferase reporter assay was performed to detect the binding of 27nt-miRNA to AP-1. Overexpression of 27nt-miRNA significantly inhibited endothelial cells proliferation, invasion and migration in vitro. And, eNOS and AP-1 expression at mRNA and protein levels were down-regulated by overexpression of 27nt-miRNA. Interestingly, overexpression of AP-1 protein partially restored eNOS expression and endothelial cell proliferation. Furthermore, the luciferase reporter assay demonstrated that AP-1 was a direct target of 27nt-miRNA. These data demonstrate that overexpression of 27nt-miRNA inhibits endothelial cell proliferation, invasion, migration, eNOS expression and AP-1 expression. Moreover, AP-1, a direct target of 27nt-miRNA, reverses the inhibitory effects of 27nt-miRNA. Thus, the effects of 27nt-miRNA might be acted through targeting AP-1.
Keywords: 27nt-miRNA, cell proliferation, endothelial nitric oxide synthase, AP-1
Introduction
MicroRNAs (miRNAs) are a kind of naturally occurring, small non-coding RNA molecules, about 21-25 nucleotides (nt) in length. MiRNAs can regulate mRNA translation and degradation through targeting protein-coding mRNAs [1,2]. Recently, it is shown that angiogenesis is also controlled by miRNAs [3,4]. In addition, miRNAs are implicated in the development of a variety of important human diseases including cancer [5,6], cardiovascular diseases [7,8] and diabetes [9]. Moreover, miRNAs posttranscriptionally regulate the expression of genes involved in important cellular processes, including differentiation [10], growth [11], and apoptosis [12]. Intronic miRNAs, another group of non-coding RNAs, were first reported in 2003 and are from the intron regions of gene transcripts. Currently, more than 90 intronic miRNAs have been identified [13,14]. Intronic miRNAs, which are structurally and functionally similar to intergenic miRNAs, also play important roles in the regulation of host gene expression [15,16].
It has been shown that nuclear transcription factors (TFs) and miRNAs are two large families of trans-acting gene regulators in multicellular genomes with extensive interactions on gene regulation [17,18]. This dynamic interactive mechanism of gene regulation by TFs and miRNAs has been supported by a few recent studies [19-22]. For example, Yeh et al [19] reported that miRNA-138 suppressed ovarian cancer cell invasion and metastasis by targeting SRY-related high mobility group box 4 and hypoxia-inducible factor-1α. Yang et al [22] found that miR-16 inhibited glioma cell growth and invasion through suppressing the nuclear factor-kappaB1/matrix metalloproteinase-9 signaling pathway. The endothelial nitric oxide synthase (eNOS) gene plays an important role in the regulation of endothelial proliferation [23]. The intronic 27-ntmiRNA is derived from the 27-base pair repeats in intron 4 of eNOS gene. Previously we reported that 27nt-miRNA overexpression significantly down-regulated eNOS mRNA level and protein expression, and decreased the eNOS transcriptional efficiency [24,25]. And, we also showed that 27nt-miRNA overexpression significantly inhibited eNOS expression and endothelial cell proliferation via inhibition of STAT3 signaling [16] or by a mechanism related to the transcription factor SP-1 [26]. These results suggest that 27nt-miRNA may regulate eNOS expression and endothelial cell proliferation through targeting TFs. The activator protein-1 (AP-1), one of the TFs, is reported to be involved in the regulation of eNOS expression [27,28]. However, whether AP-1 mediates the effect of 27nt-miRNA on eNOS expression and endothelial cell proliferation is poorly understood.
In this study, we over expressed 27nt-miRNA in human endothelial cells and investigated its effects on cell growth, invasion, migration and colony formation. And, we detected the effects of 27nt-miRNA on eNOS and AP-1 expression. In addition, the relationship between AP-1 and 27nt-miRNA was analyzed. Furthermore, the role of AP-1 in the effects mediated by 27nt-miRNA was also assessed.
Materials and methods
Reagents
Cell culture media EBM-2, fetal bovine serum (FBS), OptiMem I, G418 and RNase-free DNase were all purchased from Gibco (Invitrogen Co, Carlsbad, CA, USA). Plasmid kit, PCR kit, total RNA purification Kit, LipofectamineTM 2000 Kit, vascular endothelial growth factor (VEGF) and secondary antibodies were all obtained from Invitrogen Co. (Carlsbad, CA, USA). The pEGP-miR vector was from Cell Biolabs (San Diego, CA, USA). Dual-Luciferase® Reporter Assay System and pGL3 vector were from Promega (Madison, WI, USA). Reverse transcription (RT) kit was from MBI Fermentas (Hanover, MD, USA). Primers, siRNA synthesis kits, anti-eNOS antibody and anti-β-actin antibody were from Cell Signaling Technology (Boston, MA, USA). Anti-AP-1 antibody was from Santa Cruz (Santa Cruz, CA, USA). Methylthiazolyl blue tetrazolium (MTT) and dimethyl sulphoxide (DMSO) were from Sigma (St. Louis, MO, USA). The pCMV6-XL4-AP-1 plasmid was from OriGene Technologies, Inc. (Rockville, MD, USA). The non-coated membrane (24-well insert; pore size, 8 μm) was from BD Biosciences (San Jose, CA, USA). QCM™ 24-Well Colorimetric Cell Migration Assay Kit and polyvinylidene difluoride (PVDF) membranes were from Millipore (Billerica, MA, USA).
Construction of plasmids
For the miRNA plasmid construction, hairpin miRNA was designed based on the sequence of 27nt repeat in intron 4 of eNOS gene as previously described [29]. A mutant oligonucleotide with 3 mutations at sites 13, 14, and 15 of the 27nt sequence was also designed. A loop of 9 nucleotides (5’-UUCAAGACA-3’) was used for the production of miRNAs. A 27nt sequence from the Luciferase gene was used as control. The sequences of oligonucleotides were listed in Table 1. DNA strands (top and bottom strands) encoding pre-miRNAs were synthesized, annealed, and ligated into pEGP-miR vector. The plasmids constructed were named as the wild type 27nt-miRNA (wt-27nt-miR), the mutant 27nt-miRNA (mut-27nt-miR) and the control plasmid. The correctly constructed plasmids were verified by sequencing. The sequence in the control plasmid had no similarity to the human genome.
Table 1.
Sequences of oligonucleotides and primers used in this study
| Name | Sequence |
|---|---|
| control | 5’-GTCTGACAGTTACCAATGCTTAATCAG-3’ |
| wt-27nt-miR | 5’-GAAGTCTAGACCTGCTGCAGGGGTGAG-3’ |
| mut-27nt-miR | 5’-GAAGTCTAGACCTGCTGCAGGCCAGAG-3’ |
| eNOS Forward | 5’-AGGAACCTGTGTGACCCTCA-3’ |
| eNOS Reverse | 5’-CGAGGTGGTCCGGGTATCC-3’ |
| AP-1 Forward | 5’-AATGGGCACATCACCACTACAC-3’ |
| AP-1 Reverse | 5’-TGCTCGTCGGTCACGTTCT-3’ |
| β-actin Forward | 5’-CTGGAACGGTGAAGGTGACA-3’ |
| β-actin Reverse | 5’-AAGGGACTTCCTGTAACAATGCA-3’ |
For construction of AP-1 luciferase reporter plasmids, the wild-type AP-1 (5’-GGTCTAG-3’, wt-AP-1) and mutant AP-1 (5’-GCAGTAG-3’, mut-AP-1) were constructed into pGL3 vector. The AP-1 luciferase reporter plasmids were named as PGL3-wt-AP-1 and PGL3-mut-AP-1, respectively. The correctly constructed plasmids were confirmed by sequencing.
Cell line and cell culture
Human umbilical vein endothelial cells (HUVECs) were obtained from Cell Application (Santiago, CA, USA) and cultured in EBM-2 supplemented with 10% FBS and 1% penicillin/streptomycin. Cells were maintained at 37°C with an atmosphere of 5% CO2.
Cell transfection and establishment of stable cell lines with miRNA overexpression
For transfection, when cell confluence reached 90%, cells were transfected with 50 nM of wt-27nt-miR, mut-27nt-miR or control plasmid, or with pCMV6-XL4-AP-1 by using LipofectamineTM 2000 according to the manufacturer’s instructions. After incubating in OptiMem-I media for 4 h, the cells were then culture in fresh EBM-2 with 10% FBS.
For establishment of stable cell lines over-expressing miRNA, G418 (1 mg/ml) was added into the culture media at 2 days after 27nt-miRNA transfection. The culture medium supplemented with G418 was changed once a week. The survived cell colonies were individually picked 2 weeks later for expansion. Stable cell lines with 2-10 passages were identified by verifying the miRNA expression with northern blotting.
Cell proliferation assay
Endothelial cells stably expressing 27nt-miRNA were seeded at a concentration of 2 × 103 cells/well in 96-well plates and were cultured for 7 days. At day 1, day 2, day 3, day 4, day 5, day 6 and day 7 of culture, cells were collected by trypsinization and the cell numbers were determined using a hemocytometer.
Colony formation assay
For the colony formation assay, endothelial cells stably expressing 27nt-miRNA were seeded at a density of 1 × 103 cells/well in 6 cm petri dish. After incubation for 15 days, cells were fixed with methanol and stained with 0.1% crystal violet. Visible colonies were counted. Triplicate wells were measured for each group. The experiment was repeated three times.
MTT assay
Cell proliferation was determined using MTT assay as previously described [30]. Briefly, cells stably expressing 27nt-miRNA were seeded at a density of 2 × 104 cells/well in 96-well plates. After 24 h incubation, the culture medium was replaced with serum-free medium. After culturing with serum-free medium for 24 h, the cells were treated with VEGF (10 ng/ml) for 24 h. Then cells were transfected with pCMV6-XL4-AP-1. At 24 h after transfection, 100 μl MTT (0.5 mg/ml) was added to each well and incubated at 37°C for 4 h. The culture supernatant was carefully removed to avoid disruption of the formazan crystals and 150 μl DMSO was added to solubilize the crystals. The absorbance of viable cells was measured at 570 nm using a microplate reader (Cell Application, San Diego, CA, USA). The relative cell number was calculated based on A570 value.
In vitro migration and invasion assays
For transwell migration assays, cells were transfected with 50 nM plasmids of wt-27nt-miR, mut-27nt-miR or control plasmid. At 24 h after transfection, 2 × 105 cells were plated in the top chamber with the non-coated membrane. For invasion assays, migration of cells was assessed using the QCM™ 24-Well Colorimetric Cell Migration Assay Kit according to the manufacturer’s instructions [31]. Briefly, at 24 h post-transfection with wt-27nt-miR, mut-27nt-miR or control plasmid (50 nM each), 5 × 104 cells were plated in the top chamber with a non-coated membrane. In both assays, cells in the top chamber were cultured in medium without serum, and medium supplemented with 10% FBS was used as a chemoattractant in the lower chamber. The cells were incubated for 24 to 36 h. Cells that did not migrate or invade through the pores were removed by a cotton swab. To minimize the bias, at least three randomly selected fields were quantified using × 100 magnification. The number of migrated or invaded cells was counted and the fold change was calculated. The experiment was repeated three times.
Real-time RT-PCR analysis
Total RNA was extracted from the stable cells expressing 27nt-miRNA or those transfected with pCMV6-XL4-AP-1 plasmid using the TRIzol method. The quality and quantity of the RNA samples were assessed by standard electrophoresis and spectrophotometric methods. RNase-free DNase I was used to remove any genomic DNA contamination. The RNAs were then reverse-transcribed into cDNA and real-time RT-PCR analysis was performed as previously described [32]. The following procedure was used: initial denaturation at 95°C for 2 min, followed by 30 cycles of denaturation at 95°C for 30 s, annealing at 55°C for 30 s and extension at 72°C for 45 s. The amplified products were identified by 1.5% agarose gel electrophoresis and β-actin was used as an internal control. The 2-ΔΔCT method was used to calculate the mRNA expression levels. Relative levels of eNOS mRNA and AP-1 mRNA were calculated based on the levels of the internal control. The sequences of the primers used were listed in Table 1.
Western blotting
The cells stably expressing 27nt-miRNA or cells transfected with pCMV6-XL4-AP-1 plasmid were collected and lysed on ice for 30 min with lysis buffer. Proteins were separated by 8% SDS-PAGE and transferred to PVDF membrane. The membrane was then incubated with the primary antibodies against eNOS (1:1000), AP-1 (1:1000), and β-actin (1:5000) in 5% non-fat milk in TBST at room temperature for 1 h. After washing for three times, the membrane was incubated with secondary antibodies at room temperature for 1 h. The membrane was developed using the enhanced chemiluminescence plus reagent.
Luciferase-reporter assay
Luciferase activity was measured using the dual luciferase assay system (Promega) according to the manufacturer’s instructions. Briefly, cells were seeded in 6-well plates at a concentration of 2 × 106 cells/well. Then cells were co-transfected with pGL3-wt-AP-1 and wt-27nt-miR, or with pGL3-mut-AP-1 and wt-27nt-miR. Cells co-transfected with control plasmid and pGL3-wt-AP-1 or with control plasmid and pGL3-mut-AP-1 were used as control. After incubation for 36 h, the luciferase activity was detected.
Statistical analyses
SPSS 16.0 (SPSS, Chicago, IL, USA) was used for statistical analysis. All data were expressed as mean ± standard deviations (SD) of three independent experiments unless otherwise specified. A two-tailed Student’s t-test was used for the comparison of differences between two independent groups. One-way ANOVA was to compare the differences among multiple groups and post hoc Bonferroni correction was conducted for multiple comparisons. P < 0.05 was considered to indicate a statistically significant difference.
Results
Overexpression of 27nt-miRNA inhibits the growth of endothelial cells
To determine the effect of 27nt-miRNA overexpression on endothelial cell growth, cell proliferation curve was generated. Endothelial cells stably expressing 27nt-miRNA were seeded and cell number was counted on day 1 to day 7. As shown in Figure 1A, on day 1 and day 2, no difference was found in cell number among the three groups of wt-27nt miR, mut-27nt miR and control. From day 3 to day 7, the cell number in wt-27nt miR group was significantly lower than that in control group (0.10 ± 0.01) x 106 vs. (1.0 ± 0.10) x 106 on day 3, (0.40 ± 0.04) x 106 vs. (1.60 ± 0.12) x 106 on day 4, (0.60 ± 0.05) x 106 vs. (2.20 ± 0.20) x 106 on day 5, (0.90 ± 0.07) x 106 vs. (3.40 ± 0.35) x 106 on day 6, (1.80 ± 0.16) x 106 vs. (4.60 ± 0.42) x 106 on day 7) (P < 0.01). The cell number in mut-27nt miR group was (0.40 ± 0.04) x 106 on day 3, (0.80 ± 0.05) x 106 on day 4, (1.00 ± 0.10) x 106 on day 5, (1.50 ± 0.12) x 106 on day 6 and (2.30 ± 0.20) x 106 on day 7. Compared with control group, mut-27nt miR group had significantly lower levels of cell number from day 3 to day 7 (P < 0.01). However, the cell number in mut-27nt miR group was significantly higher than that in wt-27nt miR group from day 3 to day 7 (P < 0.01).
Figure 1.
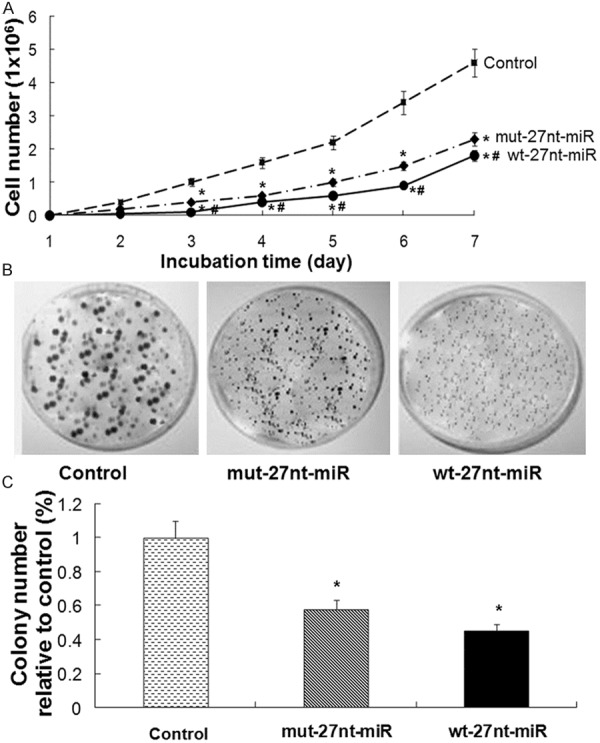
Cell proliferation and colony formation analysis. A. Endothelial cells stably transfected with the control plasmid and the plasmids expressing the wt-27nt miR and mut-27nt miR were seeded at a concentration of 2 × 103 cells/well in 96-well plates and then were counted at day 1 to day 7 of culture. Proliferation curve of cells was generated based on cell numbers. B and C. Endothelial cells stably transfected with the control plasmid and the plasmids expressing the wt-27nt miR and mut-27nt miR were seeded at a concentration of 1 × 103 cells/well in 6 cm petri dish and incubated for 15 days for colony formation. Representative images and quantitative results were shown. Data were shown as means ± SD of three independent experiments. Compared with control group, *P < 0.01; Compared with mut-27nt miR group, #P < 0.01.
To further verify the inhibitory effect of 27nt-miRNA overexpression on endothelial cell growth, colony formation assay was performed. After incubation for 15 days, cells were stained with 0.1% crystal violet. Representative morphology of colonies was shown in Figure 1B. Then, the relative colony numbers were calculated based on the colony numbers of the control group. As shown in Figure 1C, compared with control, the relative colony numbers in wt-27nt miR group was significantly decreased by 55.0% (0.45 ± 0.04 vs. 1.00 ± 0.10, P < 0.01) and by 42.0% (0.58 ± 0.05 vs. 1.00 ± 0.10, P < 0.01) in mut-27nt miR group, respectively. These results suggest that overexpression of 27nt-miRNA inhibits the growth of endothelial cells in vitro.
Overexpression of 27nt-miRNA significantly inhibits endothelial cell invasion and migration in vitro
To investigate the effects of 27nt-miRNA overexpression on cell invasion and migration, cell migration and invasion assays in vitro were conducted. Representative and quantitative results were shown in Figure 2A and 2B, respectively. The fold change was calculated by normalizing the number of migrated or invaded cells to those in the corresponding control group. For the cell migration assay, the fold change of migrated cells was significantly decreased by 55.0% in wt-27nt miR group (0.45 ± 0.04 vs. 1.00 ± 0.10, P < 0.01) and by 43.0% (0.57 ± 0.05 vs. 1.00 ± 0.10, P < 0.01) in mut-27nt miR group, respectively, compared with control group. Meanwhile, the fold change of migrated cells was significantly decreased by 26.7% in wt-27nt miR group (0.45 ± 0.04 vs. 0.57 ± 0.05, P < 0.01), compared with the mut-27nt miR group. These results suggest that overexpression of 27nt miRNA significantly suppresses the cell migration, while mutation in the 27nt miRNA could partly reverse this effect.
Figure 2.
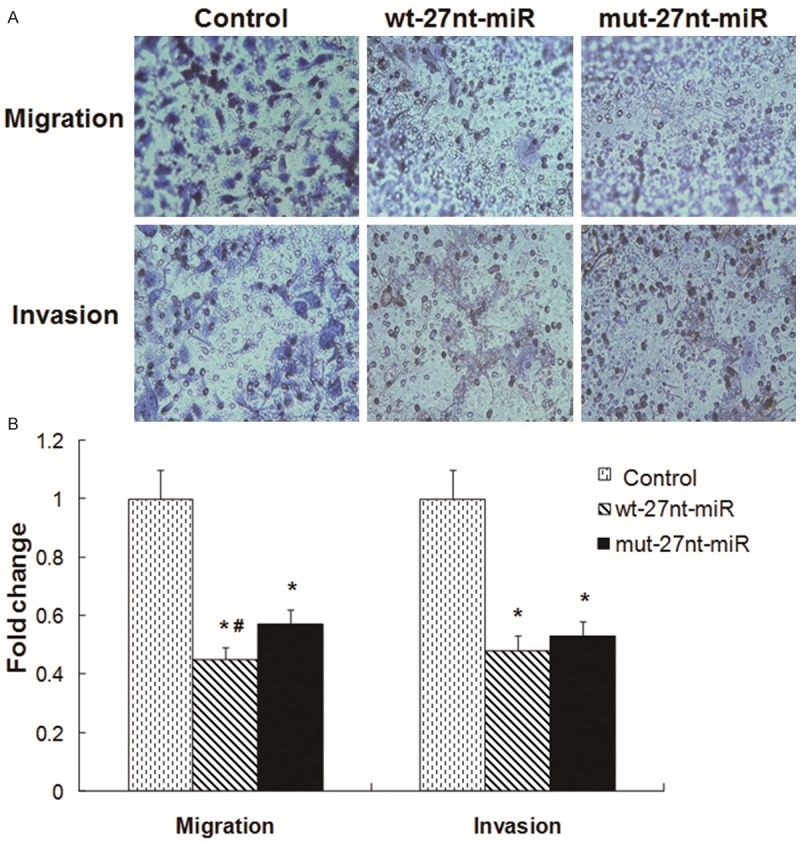
Cell migration and invasion analysis. For transwell migration assay, cells were seeded at a concentration of 2 × 105. For invasion assay, cells were seeded at a concentration of 5 × 104. The cells were incubated for 24 to 36 h. A. Representative images were shown. Magnification: × 100. B. Quantitative results were shown. Data represented the fold change in migration/invasion as compared with the migration/invasion of control group. Compared with control, *P < 0.01. Compared with mut-27nt-miR, #P < 0.01.
For the cell invasion assay, compared with control group, the fold change of invaded cells was significantly decreased by 52.0% in wt-27nt miR group (0.48 ± 0.05 vs. 1.00 ± 0.10, P < 0.01) and by 47.0% (0.53 ± 0.06 vs. 1.00 ± 0.10, P < 0.01) mut-27nt miR group, respectively. No difference was found between wt-27nt miR and mut-27nt miR groups. These results demonstrate that overexpression of 27nt-miRNA inhibits the invasion ability of endothelial cells in vitro.
Overexpression of 27nt-miRNA significantly inhibits eNOS mRNA and protein levels in the endothelial cells
To determine whether the 27nt-miRNA influences eNOS expression, real-time RT-PCR and Western blotting was performed to detect eNOS expression at mRNA level and protein level, respectively. The quantitative real-time RT-PCR results were shown in Figure 3A. Compared with control group, eNOS mRNA expression level was significantly decreased by 76.0% in wt-27nt miR group (0.24 ± 0.05 vs. 1.00 ± 0.10, P < 0.01) and by 44.0% (0.56 ± 0.07 vs. 1.00 ± 0.10, P < 0.01) in mut-27nt miR group, respectively. Meanwhile, the eNOS mRNA level in wt-27nt miR group was significantly lower than that in mut-27-ntmiRNA group (0.24 ± 0.05 vs. 0.56 ± 0.07, P < 0.01), suggesting that wt-27nt miR is more effective in inhibiting eNOS transcription.
Figure 3.
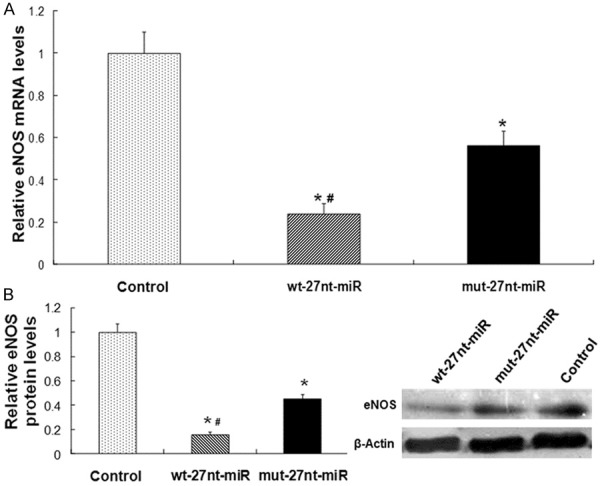
Analysis of eNOS expression. The eNOS expression at mRNA and protein level was detected by real-time RT-PCR and Western blotting analysis, respectively. A. Quantitative real-time RT-PCR results. B. Quantitative (left panel) and representative (right panel) Western blotting results. Three independent experiments were performed. Data were expressed as means ± SD. Compared with control, *P < 0.01. Compared with mut-27nt-miR, #P < 0.01.
Representative and quantitative Western blotting results were shown in Figure 3B. Consistent with the changes in the mRNA levels, eNOS protein level was decreased by 84.0% (0.16 ± 0.02 vs. 1.00 ± 0.07, P < 0.01) in wt-27nt miR group and by 55.0% (0.45 ± 0.04 vs. 1.00 ± 0.07, P < 0.01) in mut-27ntmiR group, compared with control, respectively (Figure 3B). Meanwhile, the eNOS protein level in wt-27nt miR group was significantly lower than that in mut-27-nt miRNA group (0.16 ± 0.02 vs. 0.45 ± 0.04, P < 0.01), suggesting that the inhibitory effect of wt-27nt miR on eNOS protein expression is significantly higher than that of mut-27nt miR. These data indicate that 27nt-miRNA overexpression suppresses eNOS expression at mRNA and protein levels in endothelial cells and the effect of wt-27nt miR is more effective than its mutant allele.
Overexpression of 27nt-miRNA significantly inhibits AP-1 mRNA and protein levels in the endothelial cells
To detect AP-1 expression at mRNA level and protein level after 27nt-miRNA overexpression, real-time RT-PCR and Western blotting were conducted. The results were shown in Figure 4. In Figure 4A, AP-1 mRNA level was significantly decreased by 56% (0.44 ± 0.02 vs. 1.00 ± 0.09, P < 0.01) in wt-27nt miR group, while decreased by 37% (0.63 ± 0.05 vs. 1.00 ± 0.09, P < 0.01) in mut-27nt miR group, compared with control, respectively. Further, compared with cells transfected with mut-27nt miR, AP-1 mRNA level in wt-27nt miR group was significantly lower than that in mut-27nt miR group (0.44 ± 0.02 vs. 0.63 ± 0.05, P < 0.01), suggesting that miRNA mutation only partially inhibits the transcriptional expression of AP-1. Consistently, AP-1 protein level was significantly decreased by 66% (0.34 ± 0.03 vs. 1.00 ± 0.10, P < 0.01) in wt-27nt miRNA group and by 43% (0.57 ± 0 .05 vs. 1.00 ± 0.10, P < 0.01) in mut-27nt miRNA group, respectively, compared with the control (Figure 4B). Compared with wt-27nt miRNA group, AP-1 protein level in mut-27nt miRNA group was significantly increased by 67.6% (0.34 ± 0.03 vs. 0.57 ± 0 .05, P < 0.01). These results show that overexpression of 27nt-miRNA significantly inhibits AP-1 mRNA and protein levels in the endothelial cells and that the effect of wt-27nt miR is more effective than mut-27nt miRNA.
Figure 4.
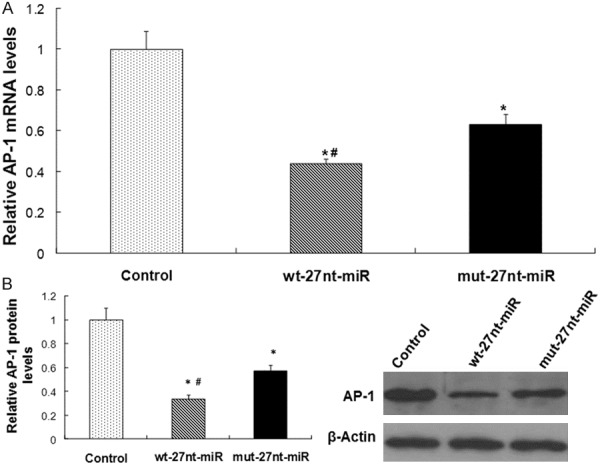
Analysis of AP-1 expression. The AP-1 expression at mRNA and protein level was detected by real-time RT-PCR and Western blotting analysis, respectively. A. Quantitative real-time RT-PCR results. B. Quantitative (left panel) and representative (right panel) Western blotting results. Three independent experiments were performed. Data were expressed as means ± SD. Compared with control, *P < 0.01. Compared with mut-27nt-miR, #P < 0.01.
The 27nt-miRNA directly binds to AP-1 mRNA
To determine whether AP-1 is a target gene of 27nt miRNA in endothelial cells, the wild type (pGL3-wt-AP-1) or mutant (pGL3-mut-AP-1) reporter plasmid was co-transfected into normal endothelial cells along with the 27nt miRNA or the control. Luciferase activity was measured after treatment. As shown in Figure 5, in cells transfected with wide type AP-1, the luciferase activity was significantly decreased by 44.9% in wt-27nt miR group (0.84 ± 0.05 vs. 1.52 ± 0.06, P < 0.01), compared with control. Meanwhile, in cells transfected with mutant AP-1, the luciferase activity did not significantly change in wt-27nt miR group (1.57 ± 0.07) compared with control (1.62 ± 0.08). These results suggest that 27nt miRNA suppresses AP-1 by directly binding to AP-1 mRNA. Therefore, AP-1 is a direct target of 27nt miRNA.
Figure 5.
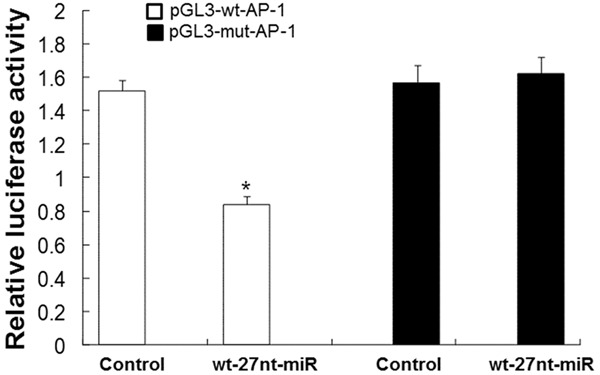
Analysis of 27nt miRNA binding to 3’UTR of AP-1 mRNA by luciferase reportor assay. The reporter plasmid (pGL3-wt-AP-1 and pGL3-mut-AP-1) was co-transfected into endothelial cells with wt-27nt-miR or control plasmid. The relative luciferase activity was calculated based on the luciferase activity in the control group. Compared with control, *P < 0.01.
The inhibitory effect of 27nt-miRNA on endothelial cell proliferation is reversed by AP-1
We previously reported that VEGF played an important role in regulating eNOS expression and endothelial cell proliferation [33]. To investigate whether AP-1 is involved in the effect of 27nt-miRNA on endothelial cell proliferation, cell proliferation was measured by MTT after VEGF treatment and transfection of wt-27nt miR and AP-1. As shown in Figure 6, cell proliferation was up-regulated by VEGF incubation. In the control cells, proliferation of cells after treatment with VEGF (235.0 ± 15.0) was increased by 113.6% than those without VEGF treatment (110 ± 8.0) and the difference was significant (P < 0.01). However, in cells transfected with wt-27nt-miR, there was no significant difference in cell proliferation between cells with VEGF treatment and those without VEGF treatment. In cells transfected with wt-27nt-miR and AP-1, the proliferation of cells with VEGF treatment was significantly increased by 102.6% (158.0 ± 9.0 vs. 78.0 ± 6.0, P < 0.01), compared with those without VEGF treatment. This data indicate that cell proliferation stimulated by VEGF is also inhibited by 27nt miRNA. After VEGF treatment, compared with control (235.0 ± 15.0), proliferation of cells transfected with wt-27nt-miR (68.0 ± 7.0) was significantly decreased by 71.0% (P < 0.01) and proliferation of cells transfected with wt-27nt-miR and AP-1 (158.0 ± 7.0) was significantly decreased by 32.8% (P < 0.01). Compared with cells transfected with wt-27nt-miR (68.0 ± 7.0), proliferation of cells transfected with wt-27nt-miR and AP-1 (158.0 ± 7.0) was significantly increased by 113.4% (P < 0.01). These results showed that the AP-1 rescued the effect of 27nt-miRNA on endothelial cell proliferation, suggesting that 27nt-miRNA might regulate endothelial cell proliferation through targeting AP-1.
Figure 6.
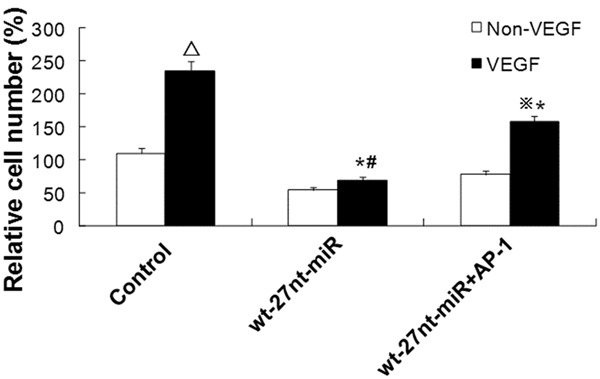
Analysis of endothelial cell proliferation after VEGF stimulation and AP-1 transfection. Endothelial cells were stimulated with or without VEGF for 24 h. Then cell proliferation of cells transfected with wt-27nt-miR and wt-27nt-miR+AP-1 was analyzed by MTT assay. In control group, compared to control cells without VEGF treatment, ΔP < 0.01. In wt-27nt-miR+AP-1 group, compared to cells without VEGF treatment, ※P < 0.01. Compared to control cells with VEGF treatment, *P < 0.01. Compared to cells in wt-27nt-miR+AP-1 group with VEGF treatment, #P < 0.01.
The eNOS expression inhibited by 27nt-miRNA are rescued by AP-1
To assess whether AP-1 is involved in the effect of 27nt-miRNA on eNOS expression, eNOS expression at protein and mRNA level was detected by real-time RT-PCR and Western blotting, respectively, after VEGF treatment and transfection of wt-27nt miR and AP-1. The quantitative results of real-time RT-PCR were shown in Figure 7A. In the control cells, expression level of eNOS mRNA in cells with VEGF treatment was significantly increased by 38.9% (1.32 ± 0.10) compared with that in cells without VEGF treatment (0.95 ± 0.08) (P < 0.01). In cells transfected with wt-27nt-miR, eNOS mRNA level with VEGF treatment was significantly increased by 173.9% (0.63 ± 0.06 vs. 0.23 ± 0.02, P < 0.01), compared with that without VEGF treatment. In cells transfected with wt-27nt-miR and AP-1, eNOS mRNA level with VEGF treatment was significantly increased by 108.9% (0.94 ± 0.07 vs. 0.45 ± 0.05, P < 0.01), compared with that without VEGF treatment. However, after VEGF stimulation, eNOS mRNA level was decreased by 47.9% (0.63 ± 0.06 vs. 1.32 ± 0.10, P < 0.01) in 27nt-miR group and by 28.9% in 27 nt-miR plus AP-1 group (0.94 ± 0.07 vs. 1.32 ± 0.10, P < 0.01), compared with control. Interestingly, eNOS mRNA level was increased by 49.2% (0.94 ± 0.07 vs. 0.63 ± 0.06, P < 0.01) in 27 nt-miR plus AP-1 group, compared with 27 nt-miR group, suggesting that Ap-1 could partly reverse the effect of 27nt-miR.
Figure 7.
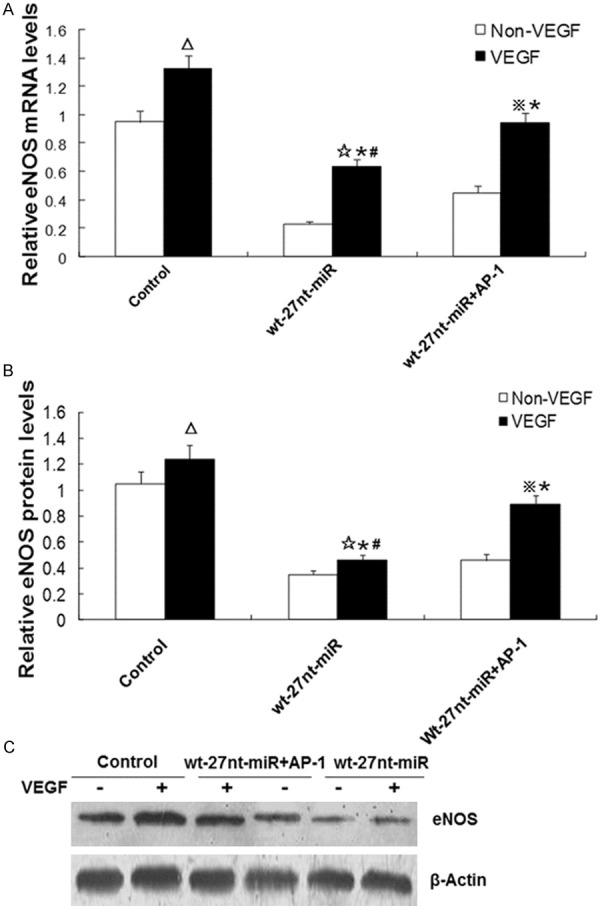
Analysis of eNOS expression after VEGF stimulation and AP-1 transfection. Endothelial cells were stimulated with or without VEGF for 24 h. Then eNOS expression at mRNA level and protein level in cells transfected with wt-27nt-miR and wt-27nt-miR+AP-1 was measured by real-time RT-PCR and Western blotting analysis, respectively. A. Quantitative real-time RT-PCR results. B. Quantitative Western blotting results. C. Representative Western blotting results. Three independent experiments were conducted and data were expressed as means ± SD. In control cells, compared to cells without VEGF treatment, ΔP < 0.01. In wt-27nt-miR group, compared to cells without VEGF treatment, ☆P < 0.01. In wt-27nt-miR+AP-1 group, compared to cells without VEGF treatment, ※P < 0.01. Compared to control cells with VEGF treatment, *P < 0.01. Compared to cells in wt-27nt-miR+AP-1 group with VEGF treatment, #P < 0.01.
To further analyze whether the AP-1 mediates the effect of 27nt-miR on eNOS protein expression, Western blotting was performed. The quantitative and representative Western blotting results were shown in Figure 7B and 7C, respectively. In control group, compared with cells without VEGF treatment (1.05 ± 0.09), the eNOS protein level in cells with VEGF treatment was significantly increased by 18.1% (1.24 ± 0.11) (P < 0.01). In cells transfected with wt-27nt-miR, the eNOS protein level with VEGF was significantly increased by 31.4% (0.46 ± 0.04 vs. 0.35 ± 0.03, P < 0.01) than that without VEGF treatment. In cells transfected with wt-27nt-miR and AP-1, the eNOS protein level after VEGF treatment was significantly increased by 31.4% (0.89 ± 0.07 vs. 0.46 ± 0.05, P < 0.01), compared with that without VEGF treatment. Under the condition of VEGF-treatment, the eNOS protein level was deceased by 62.9% (0.46 ± 0.04 vs. 1.24 ± 0.11, P < 0.01) in 27nt miR group and by 27.4% (0.89 ± 0.07 vs. 1.24 ± 0.11, P < 0.01) in 27nt miR plus AP-1 group, respectively, compared with control. Additionally, the eNOS protein level was significantly increased by 93.5% (0.89 ± 0.07 vs. 0.46 ± 0.04, P < 0.01) in 27nt miR plus AP-1 group, compared with 27nt miR group. These data indicate that AP-1 could, at least in part, reverse the inhibitory effect of 27nt-miRNA on eNOS expression at both mRNA and protein expression levels.
Discussion
Approximately 700 kinds of miRNAs have been found in human tissues since the discovery of the first miRNA in 1993 [34,35]. It has been reported that miRNAs play an important role in regulating diverse cellular processes including proliferation, apoptosis, migration and invasion [30,36]. Dysregulation of miRNAs affects normal cell growth and development, leading to a variety of disorders such as human cancer, diabetes and cardiovascular diseases [37,38]. In order to investigate the function of miRNA, it is very important to elucidate its functional targets. This usually involves analysis of changes in target proteins following either a gain or loss of function of the specific miRNA. In this study, we over-expressed 27nt-miRNA in endothelial cells and investigated its effects. We found that overexpression of 27nt-miRNA significantly inhibited growth, invasion and migration of endothelial cells in vitro. And, overexpression of 27nt-miRNA inhibited eNOS and AP-1 expression at both mRNA and protein levels in endothelial cells. Additionally, AP-1 was a direct target of 27nt-miRNA. Furthermore, the effects of 27nt-miRNA on endothelial cell proliferation and eNOS expression were reversed by AP-1.
Recently, the inhibitory role of 27nt-miRNA in endothelial cell proliferation and eNOS expression has been demonstrated [16,24-26]. In this study, we found that after transfection with wild type 27nt-miRNA, endothelial cell proliferation was significantly inhibited and eNOS expression was significantly decreased. And, these effects were partially rescued in cells transfected with mutant 27nt-miRNA. Therefore, our results further confirmed the inhibitory effects of 27nt-miRNA on endothelial cell proliferation and eNOS expression. Furthermore, overexpression of wild type 27nt-miRNA inhibited invasion and migration of endothelial cells. Although mutant 27nt-miRNA also inhibited invasion and migration of endothelial cells, its inhibitory effect was significantly lower than that of wide type 27nt-miRNA. These results suggest that 27nt-miRNA might be involved in carcinogenesis of endothelial cells. The mechanism underlying the effects of 27nt-miRNA on cell invasion and migration needs to be further elucidated.
It is reported that miRNAs can regulate the expression of TFs [39,40]. Consistently, in the present study, we found that the mRNA and protein expression of AP-1 was inhibited by 27nt-miRNA overexpression. And, this inhibition was alleviated when cells were transfected with mutant 27nt-miRNA. Furthermore, the luciferase reporter analysis showed that AP-1 mRNA was the direct target of the 27nt-miRNA. More recently, studies have shown that in cancer or diabetes cells, miRNAs can regulate cell proliferation by targeting TFs [41,42]. Meanwhile, results from our previous studies also provided further evidence that 27nt-miRNA could regulate endothelial cell proliferation via inhibiting STAT3 signaling pathway or the transcription factor SP-1 [16,26]. Additionally, AP-1 is involved in the regulation of eNOS expression [27,28]. Thus, we hypothesize that in addition to STAT3 signaling pathway and SP-1, AP-1 may also be one of the mechanisms underlying the effects of 27nt-miRNA.
To further verify this hypothesis, we introduced AP-1 into endothelial cells with 27nt-miRNA overexpression. And, to stimulate endothelial cell proliferation and eNOS expression, cells were treated with VEGF. Then, endothelial cell proliferation and eNOS expression was analyzed. The results showed that VEGF stimulated proliferation of endothelial cells and expression of eNOS. However, these effects of VEGF were inhibited by 27nt-miRNA overexpression, which further confirmed the inhibitory effect of 27nt-miRNA. Interestingly, after co-expressing AP-1 with 27nt-miRNA in endothelial cells, cell number and eNOS expression level was significantly increased. This data indicate that AP-1 reverses the inhibitory effects of 27nt-miRNA on endothelial cell proliferation and eNOS expression. Therefore, we suppose that AP-1 mediates the suppressive effects of 27nt-miRNA on endothelial cell proliferation and eNOS expression.
In conclusion, we showed that upregulation of 27nt-miRNA played an important role in the regulation of AP-1 and eNOS at both the mRNA and protein levels and reduced endothelial cell proliferation, invasion and migration. And, the 27nt-miRNA directly bound to AP-1. Furthermore, transfection of AP-1 reversed the inhibitory effects of 27nt-miRNA. These results suggest that 27nt-miRNA may exert its effects through targeting AP-1.
Acknowledgements
This study is supported by the National Natural Science Foundation of China (No. 81373403), Research Foundation of the Education Department of Guangxi Province, China (No. 2013YB049), and Guangxi Natural Science and Technology Project for Drug Discovery (No. YCSZ2013031 and No. YCSZ2014025).
Disclosure of conflict of interest
All authors declare no financial competing interests. All authors declare no non-financial competing interests.
References
- 1.Ambros V. The functions of animal microRNAs. Nature. 2004;431:350–5. doi: 10.1038/nature02871. [DOI] [PubMed] [Google Scholar]
- 2.Würdinger T, Tannous BA, Saydam O, Skog J, Grau S, Soutschek J, Weissleder R, Breakefield XO, Krichevsky AM. miR-296 regulates growth factor receptor overexpression in angiogenic endothelial cells. Cancer Cell. 2008;14:382–93. doi: 10.1016/j.ccr.2008.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Poliseno L, Tuccoli A, Mariani L, Evangelista M, Citti L, Woods K, Mercatanti A, Hammond S, Rainaldi G. MicroRNAs modulate the angiogenic properties of HUVECs. Blood. 2006;108:3068–71. doi: 10.1182/blood-2006-01-012369. [DOI] [PubMed] [Google Scholar]
- 4.Suárez Y, Fernández-Hernando C, Pober JS, Sessa WC. Dicer dependent microRNAs regulate gene expression and functions in human endothelial cells. Circ Res. 2007;100:1164–73. doi: 10.1161/01.RES.0000265065.26744.17. [DOI] [PubMed] [Google Scholar]
- 5.Dey N, Das F, Ghosh-Choudhury N, Mandal CC, Parekh DJ, Block K, Kasinath BS, Abboud HE, Choudhury GG. microRNA-21 governs TORC1 activation in renal cancer cell proliferation and invasion. PLoS One. 2012;7:e37366. doi: 10.1371/journal.pone.0037366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Song B, Wang C, Liu J, Wang X, Lv L, Wei L, Xie L, Zheng Y, Song X. MicroRNA-21 regulates breast cancer invasion partly by targeting tissue inhibitor of metalloproteinase 3 expression. J Exp Clin Cancer Res. 2010;29:29. doi: 10.1186/1756-9966-29-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen J, Wang DZ. microRNAs in cardiovascular development. J Mol Cell Cardiol. 2012;52:949–57. doi: 10.1016/j.yjmcc.2012.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cheng Y, Zhang C. MicroRNA-21 in cardiovascular disease. J Cardiovasc Transl Res. 2010;3:251–5. doi: 10.1007/s12265-010-9169-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lovis P, Roggli E, Laybutt DR, Gattesco S, Yang JY, Widmann C, Abderrahmani A, Regazzi R. Alterations in microRNA expression contribute to fatty acid-induced pancreatic beta-cell dysfunction. Diabetes. 2008;57:2728–36. doi: 10.2337/db07-1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Du C, Liu C, Kang J, Zhao G, Ye Z, Huang S, Li Z, Wu Z, Pei G. MicroRNA miR-326 regulates TH-17 differentiation and is associated with the pathogenesis of multiple sclerosis. Nat Immunol. 2009;10:1252–9. doi: 10.1038/ni.1798. [DOI] [PubMed] [Google Scholar]
- 11.Liu ZL, Wang H, Liu J, Wang ZX. MicroRNA-21 (miR-21) expression promotes growth, metastasis, and chemo- or radioresistance in non-small cell lung cancer cells by targeting PTEN. Mol Cell Biochem. 2013;372:35–45. doi: 10.1007/s11010-012-1443-3. [DOI] [PubMed] [Google Scholar]
- 12.Uchida Y, Chiyomaru T, Enokida H, Kawakami K, Tatarano S, Kawahara K, Nishiyama K, Seki N, Nakagawa M. MiR-133a induces apoptosis through direct regulation of GSTP1 in bladder cancer cell lines. Urol Oncol. 2013;31:115–23. doi: 10.1016/j.urolonc.2010.09.017. [DOI] [PubMed] [Google Scholar]
- 13.Ambros V, Lee RC, Lavanway A, Williams PT, Jewell D. MicroRNAs and other tiny endogenous RNAs in C. elegans. Curr Biol. 2003;13:807–18. doi: 10.1016/s0960-9822(03)00287-2. [DOI] [PubMed] [Google Scholar]
- 14.Rodriguez A, Griffiths-Jones S, Ashurst JL, Bradley A. Identification of mammalian microRNA host genes and transcription units. Genome Res. 2004;14:1902–10. doi: 10.1101/gr.2722704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ying SY, Lin SL. Intron-derived microRNAs-fine tuning of gene functions. Gene. 2004;342:25–28. doi: 10.1016/j.gene.2004.07.025. [DOI] [PubMed] [Google Scholar]
- 16.Yan L, Hao H, Elton TS, Liu Z, Ou H. Intronic microRNA suppresses endothelial nitric oxide synthase expression and endothelial cell proliferation via inhibition of STAT3 signaling. Mol Cell Biochem. 2011;357:9–19. doi: 10.1007/s11010-011-0870-x. [DOI] [PubMed] [Google Scholar]
- 17.Hobert O. Gene regulation by transcription factors and microRNAs. Science. 2008;319:1785–6. doi: 10.1126/science.1151651. [DOI] [PubMed] [Google Scholar]
- 18.Makeyev EV, Maniatis T. Multilevel regulation of gene expression by microRNAs. Science. 2008;319:1789–90. doi: 10.1126/science.1152326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yeh YM, Chuang CM, Chao KC, Wang LH. MicroRNA-138 suppresses ovarian cancer cell invasion and metastasis by targeting SOX4 and HIF-1α. Int J Cancer. 2013;133:867–78. doi: 10.1002/ijc.28086. [DOI] [PubMed] [Google Scholar]
- 20.Yamashita S, Miyaki S, Kato Y, Yokoyama S, Sato T, Barrionuevo F, Akiyama H, Scherer G, Takada S, Asahara H. L-Sox5 and Sox6 proteins enhance chondrogenic miR-140 microRNA expression by strengthening dimeric Sox9 activity. J Biol Chem. 2012;287:22206–15. doi: 10.1074/jbc.M112.343194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jude JA, Dileepan M, Subramanian S, Solway J, Panettieri RA Jr, Walseth TF, Kannan MS. miR-140-3p regulation of TNF-α-induced CD38 expression in human airway smooth muscle cells. Am J Physiol Lung Cell Mol Physiol. 2012;303:L460–8. doi: 10.1152/ajplung.00041.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yang TQ, Lu XJ, Wu TF, Ding DD, Zhao ZH, Chen GL, Xie XS, Li B, Wei YX, Guo LC, Zhang Y, Huang YL, Zhou YX, Du ZW. MicroRNA-16 inhibits glioma cell growth and invasion through suppression of BCL2 and the nuclear factor-κB1/MMP9 signaling pathway. Cancer Sci. 2014;105:265–71. doi: 10.1111/cas.12351. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 23.Ziche M, Morbidelli L, Masini E, Amerini S, Granger HJ, Maggi CA, Geppetti P, Ledda F. Nitric oxide mediates angiogenesis in vivo and endothelial cell growth and migration in vitro promoted by substance P. J Clin Invest. 1994;94:2036–44. doi: 10.1172/JCI117557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ou H, Shen YH, Utama B, Wang J, Wang X, Coselli J, Wang XL. Effect of nuclear actin on endothelial nitric oxide synthase expression. Arterioscler Thromb Vasc Biol. 2005;25:2509–14. doi: 10.1161/01.ATV.0000189306.99112.4c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang MX, Ou H, Shen YH, Wang J, Wang J, Coselli J, Wang XL. Regulation of endothelial nitric oxide synthase by small RNA. Proc Natl Acad Sci U S A. 2005;102:16967–72. doi: 10.1073/pnas.0503853102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yan L, Kang M, Qin Z, Zhang W, Li Y, Ou H. An intronic miRNA regulates expression of the human endothelial nitric oxide synthase gene and proliferation of endothelial cells by a mechanism related to the transcription factor SP-1. PLoS One. 2013;8:e70658. doi: 10.1371/journal.pone.0070658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Navarro-Antolín J, Rey-Campos J, Lamas S. Transcriptional induction of endothelial nitric oxide gene by cyclosporine A. A role for activator protein-1. J Biol Chem. 2000;275:3075–80. doi: 10.1074/jbc.275.5.3075. [DOI] [PubMed] [Google Scholar]
- 28.Kumar S, Wedgwood S, Black SM. Nordihydroguaiaretic acid increases endothelial nitric oxide synthase expression via the transcription factor AP-1. DNA Cell Biol. 2007;26:853–62. doi: 10.1089/dna.2007.0614. [DOI] [PubMed] [Google Scholar]
- 29.Chen CZ, Li L, Lodish HF, Bartel DP. MicroRNAs modulate hematopoietic lineage differentiation. Science. 2004;303:83–6. doi: 10.1126/science.1091903. [DOI] [PubMed] [Google Scholar]
- 30.Liu F, Zheng S, Liu T, Liu Q, Liang M, Li X, Sheyhidin I, Lu X, Liu W. MicroRNA-21 promotes the proliferation and inhibits apoptosis in Eca109 via activating ERK1/2/MAPK pathway. Mol Cell Biochem. 2013;381:115–25. doi: 10.1007/s11010-013-1693-8. [DOI] [PubMed] [Google Scholar]
- 31.Zhang JG, Wang JJ, Zhao F, Liu Q, Jiang K, Yang GH. MicroRNA-21 (miR-21) represses tumor suppressor PTEN and promotes growth and invasion in non-small cell lung cancer (NSCLC) Clin Chim Acta. 2010;411:846–52. doi: 10.1016/j.cca.2010.02.074. [DOI] [PubMed] [Google Scholar]
- 32.Chen C, Ridzon DA, Broomer AJ, Zhou Z, Lee DH, Nguyen JT, Barbisin M, Xu NL, Mahuvakar VR, Andersen MR, Lao KQ, Livak KJ, Guegler KJ. Real-time quantification of microRNAs by stem-loop RT-PCR. Nucleic Acids Res. 2005;33:e179. doi: 10.1093/nar/gni178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Namba T, Koike H, Murakami K, Aoki M, Makino H, Hashiya N, Ogihara T, Kaneda Y, Kohno M, Morishita R. Angiogenesis induced by endothelial nitric oxide synthase gene through vascular endothelial growth factor expression in a rat hindlimb ischemia model. Circulation. 2003;108:2250–7. doi: 10.1161/01.CIR.0000093190.53478.78. [DOI] [PubMed] [Google Scholar]
- 34.Lee RC, Feinbaum RL, Ambros V. The C. elegans heterochronic gene lin-4 encodes small RNAs with antisense complementarity to lin-14. Cell. 1993;75:843–54. doi: 10.1016/0092-8674(93)90529-y. [DOI] [PubMed] [Google Scholar]
- 35.Lundstrom K. Micro-RNA in disease and gene therapy. Curr Drug Discov Technol. 2011;8:76–86. doi: 10.2174/157016311795563857. [DOI] [PubMed] [Google Scholar]
- 36.Croce CM, Calin GA. miRNAs, cancer, and stem cell division. Cell. 2005;122:6–7. doi: 10.1016/j.cell.2005.06.036. [DOI] [PubMed] [Google Scholar]
- 37.Collares CV, Evangelista AF, Xavier DJ, Rassi DM, Arns T, Foss-Freitas MC, Foss MC, Puthier D, Sakamoto-Hojo ET, Passos GA, Donadi EA. Identifying common and specific microRNAs expressed in peripheral blood mononuclear cell of type 1, type 2, and gestational diabetes mellitus patients. BMC Res Notes. 2013;6:491. doi: 10.1186/1756-0500-6-491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sugihara H, Ishimoto T, Watanabe M, Sawayama H, Iwatsuki M, Baba Y, Komohara Y, Takeya M, Baba H. Identification of miR-30e* regulation of Bmi1 expression mediated by tumor- associated macrophages in gastrointestinal cancer. PLoS One. 2013;8:e81839. doi: 10.1371/journal.pone.0081839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yan F, Liu H, Hao J, Liu Z. Dynamical behaviors of Rb-E2F pathway including negative feedback loops involving miR449. PLoS One. 2012;7:e43908. doi: 10.1371/journal.pone.0043908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Peng C, Wang M, Shen Y, Feng H, Li A. Reconstruction and analysis of transcription factor-miRNA co-regulatory feed-forward loops in human cancers using filter-wrapper feature selection. PLoS One. 2013;8:e78197. doi: 10.1371/journal.pone.0078197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mortuza R, Feng B, Chakrabarti S. miR-195 regulates SIRT1-mediated changes in diabetic retinopathy. Diabetologia. 2014;57:1037–46. doi: 10.1007/s00125-014-3197-9. [DOI] [PubMed] [Google Scholar]
- 42.Liao WT, Ye YP, Zhang NJ, Li TT, Wang SY, Cui YM, Qi L, Wu P, Jiao HL, Xie YJ, Zhang C, Wang JX, Ding YQ. MicroRNA-30b functions as a tumour suppressor in human colorectal cancer by targeting KRAS, PIK3CD and BCL2. J Pathol. 2014;232:415–27. doi: 10.1002/path.4309. [DOI] [PubMed] [Google Scholar]


