Abstract
Acetaminophen-induced liver injury represents the most frequent cause of drug-induced liver failure in the world. Portulaca oleracea L., a widely distributed weed, has been used as a folk medicine in many countries. Previously, we reported that the ethanol extracts of Portulaca oleracea L. (PO) exhibited significant anti-hypoxic activity. In the present study, we investigated the role of PO on acetaminophen (APAP) induced hepatotoxicity. The results demonstrated that PO was an effective anti-oxidative agent, which could, to some extent, reverse APAP-induced hepatotoxicity by regulating the reactive oxygen species (ROS) in the liver of mice. At the same time, PO treatment significantly decreased mice serum levels of IL-6 and TNFα and their mRNA expression in liver tissue IL-α and TNFα play an important role during APAP-induced liver injury. Furthermore, PO inhibited APAP and TNFα-induced activation of JNK, whose activation play an important effect during APAP induced liver injury. These findings suggested that administration of PO may be an effective strategy to prevent or treat liver injury induced by APAP.
Keywords: Acetaminophen, portulaca oleracea L, liver injury, antioxidation, inflammation
Introduction
Liver is important not only for nutrition metabolism but also for degradation of toxic substances and medicine. Therefore, liver makes itself quite susceptible to medicine-induced toxicity. Acetaminophen (APAP) is an analgesic and antipyretic medicine that is frequently used for anti-fever and relieving pain. However, long-term use or overdosage of APAP often causes liver damage. APAP-induced liver damage has been found to be subjected to the loss of glutathione (GSH) that binds to N-acetyl-p-benzoquinone (NAPQI), an intermediate metabolite from APAP [1-3]. The covalent binding of APAP metabolites to macromolecules in the cellular milieu leads to hepatocyte death and liver injury which are characterized by the release of liver alanine aminotransferase (ALT) [1,3,4]. APAP-induced liver toxicity has become a leading cause of both accidental and intentional poisoning [5].
In order to reduce the side effect induced by APAP, N-acetylcysteine (NAC) was given to patients with APAP overdose in the 1970s [6], because NAC promotes hepatic GSH synthesis [7-9]. Although NAC therapy is still the best therapeutic option for the overdose patient [7], the problem is that NAC takes effect only when early administration and declines sharply in the body [9,10]. Thus, it’s urgent to find a new therapeutic agent against APAP-induced liver damage.
In APAP-induced liver injury, it is common to see the increased infiltration and activation of immune cells [11], elevated expression of tumor necrosis factor α (TNF-α and IL-1α [12]. IFN-α deficient mice showed resistance to APAP-induced liver injury indicating that IFN-α may play an important role during the process [13]. APAP mediated oxidative stress stimulates expression of cytokines, whereas inhibition of reactive oxygen species (ROS) reduces liver injury. So, it is the effective way to prevent the liver injury induced by APAP to decrease the levels of inflammation cytokines and oxidative stress.
Portulaca oleracea L. (Portulacaceae) is widely distributed and has been used as a kind of folk medicine in many countries. Portulaca exhibits a wide range of pharmacological effects, including antibacterial, analgesic, anti-inflammatory, skeletal muscle relaxant and wound-healing activities [14-17]. It is an edible plant and is used as vegetable in some place of the world, including United Arab Emirates, Oman and some provinces of China. It is rich in α-linolenic acid and β-carotene [18] and contains flavonoids, coumarin [19] a monoterpene glycoside [20], and alkaloids. In particular, it also contains N-trans-feruloyltyramine [21], dopamine, dopa and a high concentration of noradrenaline [22]. Interestingly, in previous studies we found that the ethanol extracts of Portulaca (PO) exhibited strong anti-hypoxic effect by promoting the activity of the key enzymes in glycolysis and improving the level of ATP in hypoxic mice [23,24]. We speculate if PO is involved in modulating liver function and protecting or improving APAP-induced liver failure. In this paper, we studied the hepatoprotective effects of PO on APAP-induced hepatotoxic mouse model and the regulation mechanism of the protective effects. The results demonstrated that PO could ameliorate APAP-induced liver injury through increasing the anti-oxidative capacity and inhibiting APAP-induced inflammatory response.
Materials and methods
Preparation of ethanol extract from portulaca oleracea L (PO)
Preparation of ethanol extract from Portulaca was described as before [23]. The air dried aerial parts of Portulaca were purchased from the market in Yucheng, He’nan province, China. The plant was identified and authenticated as Portulaca oleracea L. by professor Han-chen Zheng, Department of Pharmacology, Second Military Medical University. A voucher specimen has been deposited in Department of Traditional Chinese Medicine (TCM), Second Military Medical University (20090829). The plant (40 kg) were ground and extracted with 8 times of 80% ethanol for 2 times (1 h/time). The solvent was evaporated under vacuum to get ride of ethanol. The remained liquid were modified with 10% NaOH to the pH6.5~7, then centrifuged at 5000 rpm to get the precipitation and oven dried to get the end extract (241.3 g) which appears black powder, lightly odorless taste.
Animal and liver injury model
All animal treatments and the experiments were carried out with the approval of the Committee of Experimental Animal Administration of the University. Male C57 mice (8 weeks old) were purchased from the Shanghai-BK Ltd. Co., housed at 24±1°C under a 12-h light/12-h dark cycle and had free access to standard pellet diet and tap water. The mice were divided into mock group, PBS+APAP group and PO+APAP group. The PO+APAP group mice were subdivided into 100 mg/kg (l-PO+APAP), 200 mg/kg (m-PO+APAP) and 400 mg/kg group (h-PO+APAP). After adaptation for three days, the mice were orally administrated with 0.5 ml phosphate-buffered saline (0.1M; pH 7.0; PBS) (PBS+APAP group) or indicated dose of PO in 0.5 ml PBS (PO+APAP group) each day for one week. The animals were deprived of food for 15 h before the experiment, with free access to drinking water, then received 300 mg/kg APAP (Sigma, MO) dissolved in warm saline (15 mg/ml intraperitoneally). At selected time after APAP treatment, animals were sacrificed. The plasma and liver were collected for experiment use.
Experimental protocols
After APAP treatment, animals were sacrificed by isoflurane anesthesia. Blood was drawn from the vena cava with heparin containing syringes into a tube and centrifuged. The plasma was collected for determination of aminotransferase activities. Meanwhile, livers were excised and rinsed in saline. A small section from each liver was placed in 10% Formalin in PBS. The remaining liver was frozen in liquid nitrogen and stored at -80°C.
Histology and TUNEL assay
Formalin fixed tissue samples were embedded in paraffin and 5 μm sections were obtained. Sections were stained with hematoxylin-eosin for evaluation of necrosis and with TUNEL assay for determination of cell apoptosis.
Cell cultures and injury of L02 cells and RAW264.7 cells
The L02 cells and RAW264.7 cells were purchased from the Institute of Biochemistry and Cell Biology (Shanghai Institutes for Biological Science) and were cultured in Dulbecco’s modified Eagle’s medium (DMEM, Hycolon) with 10% fetal bovine serum at 37°C, 5% CO2. PO was added to the culture medium 1 h before the start of injury as indicated. After treatment, cells were washed with cold PBS and the protein or RNA were extracted and stored for later use.
RNA isolation and quantitative real-time PCR
Total RNA was isolated from liver tissues with the Trizol reagent. Total RNA (10 μg) was used in a reverse-transcription reaction in a 40 μl of mixture containing 50 U of MMLV-Reverse Transcriptase (New England Biolabs, Beverly, MA) and oligo dT(12-18 mer) primer (GE Healthcare Life Sciences, Piscataway, NJ). Quantitative real-time PCR reactions were performed in 1× Universal Master Mix (Applied Biosystems) with gene-specific primers by the ABI Prism 7300 Sequence Detection System (Applied Biosystems). The primers for gene expression were used as follows: mTNFα forward 5’-AAGCCTGTAGCCCACGTCGTA-3’ and reverse 5’-GGCACCACTAGTTGGTTGTCTTTG-3’; mIL-6, forward 5’-TAGTCCTTCCTACCCCAATTTCC-3’ and reverse 5’-TTGGTCCTTAGCCACTCCTTC-3’; mIFNγ forward 5’-TTACTGCCACGGCACAGTCATTGAA-3’ and reverse 5’-TCGGATGAGCTCATTGAATGCTTGG-3’; hSOD1, forward 5’-AGGGCATCATCAATTTCGAGC-3’ and reverse 5’-GCCCACCGTGTTTTCTGGA-3’; hSOD2, forward 5’-AACCTCAGCCCTAACGGTG-3’ and reverse 5’-AGCAGCAATTTGTAAGTGTCCC-3’; hCatalase, forward 5’-ACTTTGAGGTCACACATGACATT-3’ and reverse 5’-CTGAACCCGATTCTCCAGCA-3’; hGPx1, forward TGCAACCAGTTTGGGCATCA and reverse 5’-ACCGTTCACCTCGCACTTC-3’; hANT1, forward 5’-TCCCCACCCAAGCTCTCAA-3’ and reverse 5’-GTCCAGCGGGTAGACAAAGC-3’.
Assessment of hepatic reactive oxygen species (ROS) content
Hepatic ROS content was measured using 2’-7’-dichlorofluorescein. Briefly, 10 μl of liver homogenates (1 mg/mL) were diluted 100-fold with phosphate-buffered saline (pH7.4), added with 5 μmol/l 2’-7’-dichlorofluorescein and incubated at 37°C for 30 minutes. Fluorescence was measured at excitation wavelength of 485 nm and emission wavelength of 530 nm as previously described [25].
Western blot analysis
The tissues or cells were lysed in RIPA buffer and sonicated 10 seconds for three times. Protein concentrations were measured by BCA kit (Sigma). Proteins were separated by standard SDS-PAGE and then transferred onto polyvinylidene difluoride membranes. The primary antibodies used in these studies were as follows: anti-p-JNK (dilution, 1:1000; Cell Signaling Technology) and anti-β-actin (dilution, 1:1000; Santa Cruz), followed by incubation with an IRDye 800CW-conjugated secondary antibody for 1 h and detection with LI-COR imaging system (LI-COR Biosciences).
Enzyme-linked immunosorbent assay (ELISA)
The concentrations of serum IL-6 and TNF-α were assessed using commercially available ELISA kits (R&D Systems Inc., USA) according to the instruction with the absorbance read on a microplate reader (BIO RAD) at a wavelength of 450 nm.
Statistical analysis
The data were expressed as means ± SD. Statistical analysis was performed by using the Student’s t test. P values < 0.05 were considered to be statistically significant.
Results
PO treatment effectively prevented APAP-induced liver injury
As we knew, overdose APAP (300 mg/kg) induced liver injury. In our animal experiment, APAP resulted in significant elevation of plasma levels of ALT and AST at 6h, while pre-administrated with PO displayed much less of plasma ALT and AST (Figure 1A and 1B). We also found that the protective effects of PO against APAP induced liver injury were in a dose-dependent manner with the different dose of PO (100 mg/kg, 200 mg/kg and 400 mg/kg). In morphological and histological analyses, the liver structure of mock mice was homogenous and red and that of the PBS+APAP group became much darker with the substantial centrilobular necrosis. While the liver structure of the PO+APAP group exhibited crimson with less centrilobular necrosis. There was more DNA fragmentation determined by the TUNEL assay in mice liver of PBS+APAP group than that of the PO+APAP group, indicating that, to some extent, PO prevents APAP-induced liver damage (Figure 1C).
Figure 1.
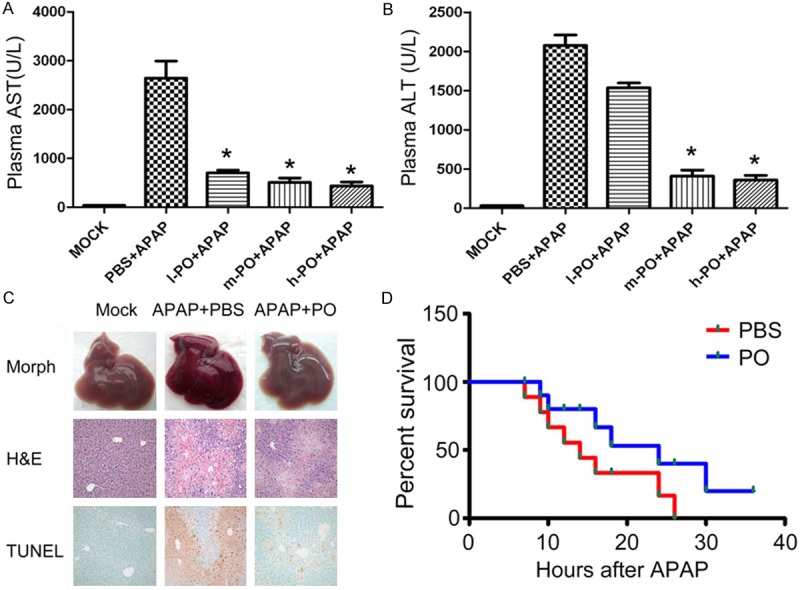
Protective effects of PO against APAP induced mice liver injury. Mice received orally equal volume of PBS and PO one week before APAP intraperitoneal injection. Plasma was taken at 6h after APAP injection. The levels of AST (A) and ALT (B) were detected. l-PO+APAP, m-PO+APAP and h-PO+APAP referred to group administrated PO for 100 mg/kg, 200 mg/kg and 400 mg/kg in 0.5 ml PBS, respectively Data were expressed as the mean ± SD (n=5, **P < 0.01 versus PBS+APAP group). (C) Hematoxylin-Eosin (H&E) staining, and DNA fragmentation (TUNEL assay) were observed in three groups (mock, PBS+APAP or PO+APAP animals, original magnification ×100 for H&E and TUNEL panels). (D) Mice were orally administrated with PBS or PO solution followed by an overnight starvation, and then intraperitoneally received APAP dissolved in warm saline (600 mg/kg). Survival of these animals was monitored over 2 days.
PO reduced the mortality induced by the lethal dosage of APAP
Because PO treatment markedly protected APAP induced acute liver injury in mice, we asked whether PO would survive mice in a lethal dosage of APAP. Administrated with PBS or PO for one week, mice were intraperitoneally received lethal dose of APAP (600 mg/kg). Number of survival animals was counted within 2 days. As shown in Figure 1D, PO reduced the mortality induced by the lethal dosage of APAP.
PO exhibited anti-oxidant effects
As APAP is reportedly to bind GSH, we measured the level of GSH in the livers after APAP administration for 6 hours. We found that PO treatment markedly maintained GSH in a higher level than PBS (Figure 2A). PO also promoted an increase of the total activity of superoxide dismutase (SOD), including copper/zinc superoxide dismutase (SOD1) and manganese superoxide dismutase (SOD2) (Figure 2B). Malondialdehyde (MDA) is an important product of oxidative reaction and its amount reflects the level of ROS. The results showed that MDA amount of PBS/APAP group was near 1.5 times higher than that of PO/APAP group (Figure 2C), indicating that PO may reduce the ROS activities. Another test for hepatic ROS production is detected by dichlorofluorescein (DCF) probe which is from dichlorofluorescein oxidation and emit fluorescence. We found that PO reduced the DCF level compared with PBS control (Figure 2D). Taken together, these results suggested that PO had anti-oxidant effects.
Figure 2.
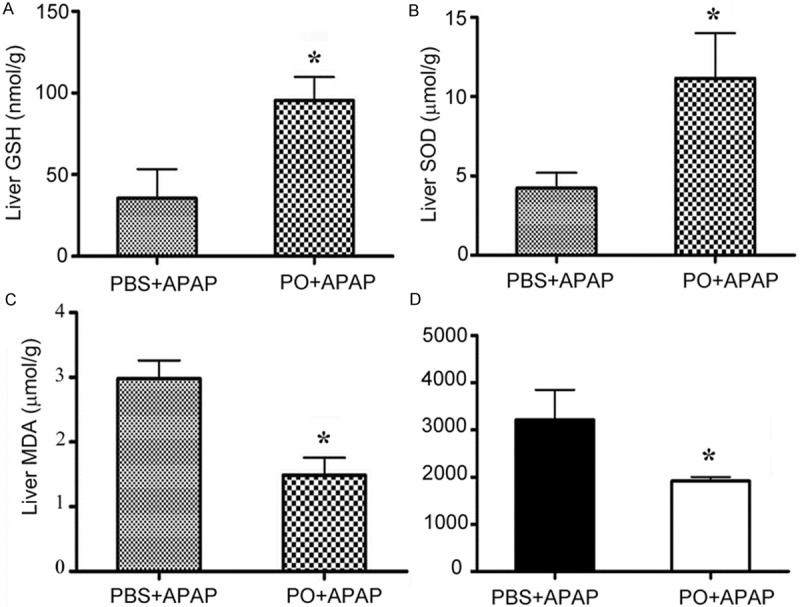
Effects of PO on levels of the GSH, SOD, MDA, and ROS in livers of APAP-treated mice. PBS treated mice and PO pretreated mice were challenged with APAP for 6 h, and the hepatic GSH (A) and SOD (B) activities, the liver MDA (C) and ROS (D) levels were measured. Data were expressed as the means ± SD (n=5 *P < 0.05 versus PBS+APAP group).
PO promoted activities of the ROS defense system in hepatic cells
ROS defense system in mammalian cells functions to detoxify oxidative stress. To further investigate the role of PO in ROS defense system, we stimulated normal embryo hepatocyte L02 cells stimulated with the H2O2 and detected gene expression of SOD1, SOD2, catalase, and glutathione peroxidase (GPx1) (Figure 3). The results showed that PO upregulated these genes compared with DMSO control group (Figure 3A-D). We also detected the expression of the ADP/ATP adenine nucleotide translocator 1 (ANT1) which is generally elevated upon oxidative stress. PO treatment displayed markedly increase of ANT1 mRNA level (Figure 3E). These results indicated that PO reduced liver damage possibly via removal of oxygen radicals by promoting the activities of ROS defense system.
Figure 3.
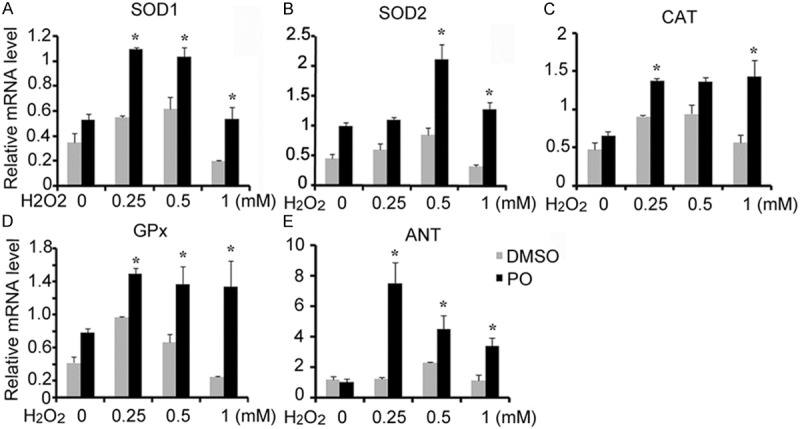
PO regulated genes of ROS defense system in normal liver cells (L02 cells). L02 cells were stimulated by 0.1 mM hydrogen peroxide (H2O2) for 6 h, followed by a recovery period of 2 h. Cells were then harvested for RNA isolation and relative expression of mitochondrial, cytoplasmic, and peroxisomal ROS-detoxifying enzymes was measured by real-time PCR. In all the figures, data were expressed as means ± SD. (n=3. *P < 0.05 versus DMSO group).
PO treatment attenuated the expression of inflammatory cytokines during liver injury
APAP-induced liver injury is thought to be associated with increase of proinflammatory cytokines. We found that proinflammatory cytokines IL-6 and TNFα level in mice serum and their mRNA level of liver tissue were decreased by pre-administration of PO (Figure 4). While the mRNA level of L-6 and TNFα of L02 cells did not affect by PO administration 1 h before TNFα stimulation (Data not shown). Given that mesenchymocytes are also involved in release of the inflammatory cytokines, we detected these cytokines expression in RAW264.7 cells, a macrophage cell lines. PO attenuated TNFα -induced expression of TNFα, IL-6 and IFNα at 8 h compared with PBS treatment (Figure 5). The results indicated that PO ameliorated APAP-induced liver injury possibly through inhibition of cytokines mediated inflammation.
Figure 4.
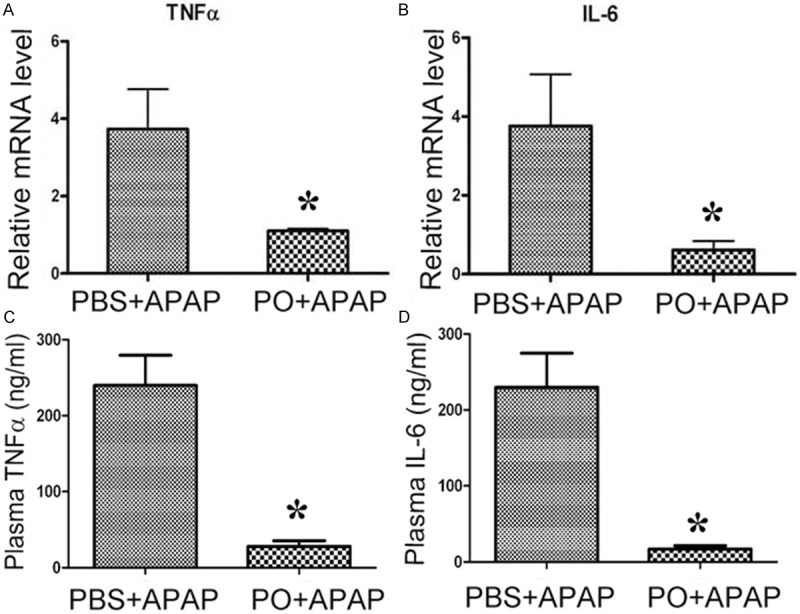
PO inhibited APAP induced IL-6 and TNFα expression in serum and liver of APAP-treated mice. PBS and PO pretreated mice were challenged with APAP for 6 h, then mice plasma were taken. The levels of TNFα (A) and IL-6 (B) were detected and the liver TNFα (C) and Ilα (D) mRNA level were measured by real-time PCR (n=4~5, *P < 0.05 versus PBS+APAP group).
Figure 5.
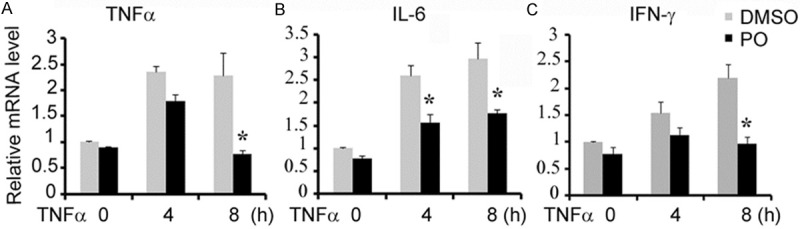
PO treatment reduced the expression of inflammatory cytokines in TNFα stimulated RAW264.7. PO treatment reduced the induction of inflammatory cytokines during liver injury. RAW264.7 cells were pretreated with PO or DMSO, and then stimulated by 20 ng/ml TNFα for 4-8 h. The amounts of TNFα (A) ILα (B) and IFN-α (C) mRNA were then detected. *P < 0.05 versus DMSO group.
PO inhibited APAP and TNFα-induced activation of JNK
JNK is activated during APAP-induced liver injury [26,27], we also found that phosphorylated JNK1 (46 kDa) and JNK2 (54 kDa) were increased at 2 h in the early period after APAP administration. PO treatment reduced the JNK phosphorylation induced by APAP administration (Figure 6A). Furthermore, we found that PO blocked TNFα stimulated JNK phosphorylation (Figure 6B). These results revealed that inhibition of JNK activation by PO might play an important role in protection of APAP-induced liver damage.
Figure 6.
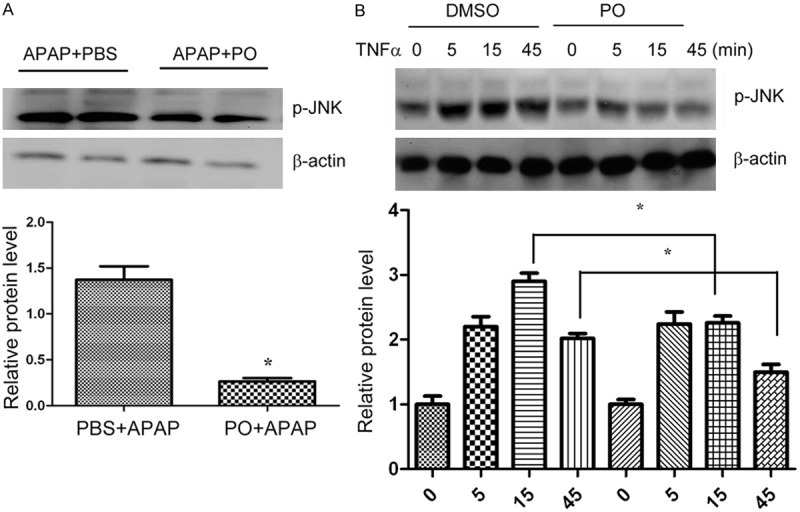
PO inhibited APAP and TNFα-induced activation of JNK. PO inhibited APAP and TNFα-induced activation of JNK. A. PBS and PO pretreated mice were administrated with APAP for 6h, and liver samples were then harvested for analysis of phosphorylation of JNK by western blot. (n=4~5, *p < 0.05 versus PBS+APAP). B. Normal liver cells (L02) were pretreated with PO or DMSO for 1 hour, then stimulated with 20 mg/ml TNFα for 0-45 min. Cells were then harvested and lysed for detection of JNK phosphorylation by western blot. (n=4~5, *p < 0.05 versus DMSO; for at least three different experiments).
Discussion
Overdose of APAP causes severe liver damage [28]. The proportion of cases with acute liver failure attributed to APAP is increasing [29], leading APAP the most frequent cause of drug-induced liver failure in the west world. In our study, we investigated the protective effects of PO on APAP-induced liver injury and demonstrated that PO was potential candidate for treatment of APAP-induced hepatotoxicity.
We found that pretreatment of PO markedly sustained GSH level, promoted the activity of the ROS defense system, i.e. activation of SOD1, SOD2, and ANT1, inhibited JNK activation, reduced APAP-induced cytokines such as IL-6 and TNFα, and extended the mice survival rate after lethal dose of APAP. The results suggested that PO could be served as a novel medicine for prevention and improvement of APAP-induced liver damage.
It is well-known that hepatoprotection by GSH or NAC is through the scavenging capacity for NAPQI in hepatocytes [11,30], as covalent binding of NAPQI to mitochondrial proteins leads to the mitochondrial dysfunction [11,30,31]. ROS activity is potentiated by loss of mitochondrial GSH. ROS induced oxidant stress activated JNK to translocate to the mitochondria and increases the mitochondrial membrane permeability by opening transition pore [32]. In our results, we documented that PO treatment significantly increased GSH product, decreased ROS activity of murine livers (Figure 2), and suppressed the JNK activities in liver after APAP administration (Figure 6A). These results demonstrated the potentially protective role of PO against APAP in liver injury.
SOD is an important enzyme in protection of ROS reaction. The activities of SOD were reported to be low during APAP administration. MDA is another major biomarker of lipid peroxides representing SOD activity [33]. In the present study, we found that PO significantly increased the activities of SOD1 and SOD2, and decreased the MDA level in liver lysates (Figure 2). This indicated that PO, to some extent, ameliorated the oxidative injury induced by APAP. In addition, GPx1 and ANT1 which are involved in reduction of free radial were also elevated by PO upon oxidative stress in L02 cells. So PO might play a role in the regulation of the ROS metabolic gene program.
APAP-induced liver injury is an inflammatory process that is associated with increase of inflammatory cytokines [11]. Our results showed that PO decreased the serum level of TNF-α and IL-6 in mice administrated with APAP. Furthermore PO exhibited not only suppression of APAP-induced mRNA increase of TNF-α and IL-6 in mice liver, but also inhibited expression of the mRNA of these factors in RAW264.7 cells, suggesting that PO protected liver from damage induced by APAP, at least in part, by down regulating of APAP-induced inflammatory cytokines.
JNKs are a family of serine/threonine kinases belonging to the MAPK family and activated through phosphorylation [26,27,34]. JNK plays an important role in stress response and is activated by stresses such as reactive oxygen species (ROS) and UV light and by cytokines such as TNF-α [34,35]. It has been reported that APAP overdose clearly induced prolonged activation of JNK before cell death and JNK inhibitor SP600125 effectively reduced APAP hepatotoxicity. Furthermore, the silencing of JNK gene expression resulted in reduced liver injury after APAP overdose [26,27,32], suggesting inhibition of prolonged JNK activation might be protective for APAP induced liver injury. Our results showed PO decreased the p-JNK expression in mice liver and blocked TNFα stimulated JNK phosphorylation in L02 cells (Figure 6). These results revealed that inhibition of JNK activation by PO might play an important role in protection of APAP-induced liver damage.
It must be mentioned that in this study we did not test individual components of the extract. In order to precisely define which component was responsible for the overall clinical effect, the total plant extract or mixtures of the suggested bioactive phytochemicals in the extract must be tested side by side with the single bioactive compounds to see which one had greater bioactivity. Only then can clear conclusions be made as to whether or not the in vivo effect was a result of one bioactive or a mixture of bioactives that may intensify the potency of a single bioactive product or act synergistically. Further studies regarding the isolation of major bioactive components responsible for the observed effect are the focus of ongoing and planned investigation.
Overall, PO exhibited effective protection for APAP-induced liver injury by preventing the loss of GSH, inhibiting oxidative stress response and JNK activity, reducing levels of inflammatory cytokines, ultimately ameliorating APAP-induced liver injury. These results provided a useful and effective botanical compound from the edible plant Portulaca Oleracea L to be used in reducing APAP side effect in liver.
Acknowledgements
This work was supported by National Natural Science Foundation of China (81102249), Medical Science and Technique projects for young talents of PLA (I3QNP035) and Naval Medicine Research Project of SMMU (2009ZL 01).
Disclosure of conflict of interest
None.
References
- 1.Jaeschke H, Bajt ML. Intracellular signaling mechanisms of acetaminophen-induced liver cell death. Toxicol Sci. 2006;89:31–41. doi: 10.1093/toxsci/kfi336. [DOI] [PubMed] [Google Scholar]
- 2.Larrey D, Pageaux GP. Drug-induced acute liver failure. Eur J Gastroenterol Hepatol. 2005;17:141–143. doi: 10.1097/00042737-200502000-00002. [DOI] [PubMed] [Google Scholar]
- 3.Takemoto K, Hatano E, Iwaisako K, Takeiri M, Noma N, Ohmae S, Toriguchi K, Tanabe K, Tanaka H, Seo S. Necrostatin-1 protects against reactive oxygen species (ROS)-induced hepatotoxicity in acetaminophen-induced acute liver failure. FEBS Open Bio. 2014;4:777–787. doi: 10.1016/j.fob.2014.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kon K, Kim JS, Jaeschke H, Lemasters JJ. Mitochondrial permeability transition in acetaminophen-induced necrosis and apoptosis of cultured mouse hepatocytes. Hepatology. 2004;40:1170–1179. doi: 10.1002/hep.20437. [DOI] [PubMed] [Google Scholar]
- 5.Honmore V, Kandhare A, Zanwar AA, Rojatkar S, Bodhankar S, Natu A. Artemisia pallens alleviates acetaminophen induced toxicity via modulation of endogenous biomarkers. Pharm Biol. 2015;53:571–81. doi: 10.3109/13880209.2014.934382. [DOI] [PubMed] [Google Scholar]
- 6.Prescott LF, Ballantyne A, Proudfoot AT, Park J, Adriaenssens P. Treatment of paracetamol (acetaminophen) poisoning with N-acetylcysteine. Lancet. 1977;310:432–434. doi: 10.1016/s0140-6736(77)90612-2. [DOI] [PubMed] [Google Scholar]
- 7.Polson J, Lee WM. AASLD position paper: the management of acute liver failure. Hepatology. 2005;41:1179–1197. doi: 10.1002/hep.20703. [DOI] [PubMed] [Google Scholar]
- 8.Lauterburg BH, Corcoran GB, Mitchell JR. Mechanism of action of N-acetylcysteine in the protection against the hepatotoxicity of acetaminophen in rats in vivo. J Clin Invest. 1983;71:980. doi: 10.1172/JCI110853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Abdelmegeed MA, Jang S, Banerjee A, Hardwick JP, Song BJ. Robust protein nitration contributes to acetaminophen-induced mitochondrial dysfunction and acute liver injury. Free Radic Biol Med. 2013;60:211–222. doi: 10.1016/j.freeradbiomed.2013.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yang R, Miki K, He X, Killeen ME, Fink MP. Prolonged treatment with N-acetylcystine delays liver recovery from acetaminophen hepatotoxicity. Crit Care. 2009;13:R55. doi: 10.1186/cc7782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jaeschke H. Role of inflammation in the mechanism of acetaminophen-induced hepatotoxicity. Expert Opin Drug Metab Toxicol. 2005;1:389–397. doi: 10.1517/17425255.1.3.389. [DOI] [PubMed] [Google Scholar]
- 12.Blazka ME, Elwell MR, Holladay SD, Wilson RE, Luster MI. Histopathology of acetaminophen-induced liver changes: role of interleukin 1α and tumor necrosis factor α. Toxicol Pathol. 1996;24:181–189. doi: 10.1177/019262339602400206. [DOI] [PubMed] [Google Scholar]
- 13.Ishida Y, Kondo T, Ohshima T, Fujiwara H, Iwakura Y, Mukaida N. A pivotal involvement of IFN-γ in the pathogenesis of acetaminophen-induced acute liver injury. FASEB J. 2002;16:1227–1236. doi: 10.1096/fj.02-0046com. [DOI] [PubMed] [Google Scholar]
- 14.Hu LF, Xu XY, Wang BQ. Research and utilization situation of Portulaca Oleracea L in China. Pract J Med Pharm. 2003;20:315–316. [Google Scholar]
- 15.Xiang L, Xing D, Wang W, Wang R, Ding Y, Du L. Alkaloids from Portulaca oleracea L. Phytochemistry. 2005;66:2595–2601. doi: 10.1016/j.phytochem.2005.08.011. [DOI] [PubMed] [Google Scholar]
- 16.Rashed AN, Afifi FU, Disi AM. Simple evaluation of the wound healing activity of a crude extract of Portulaca oleracea L. (growing in Jordan) in Mus musculus JVI-1. J Ethnopharmacol. 2003;88:131–136. doi: 10.1016/s0378-8741(03)00194-6. [DOI] [PubMed] [Google Scholar]
- 17.Zhang XJ, Ji YB, Qu Z, Xia J, Wang L. Experimental studies on antibiotic functions of Portulaca oleracea L. in vitro. Chin J Microecol. 2002;14:277–280. [Google Scholar]
- 18.Liu LX, Howe P, Zhou YF, Xu ZQ, Hocart C, Zhang R. Fatty acids and b-carotene in Australian purslane (Portulaca oleracea) varieties. J Chromatogr A. 2000;893:207–213. doi: 10.1016/s0021-9673(00)00747-0. [DOI] [PubMed] [Google Scholar]
- 19.Awad NE. Lipid content and antimicrobial activity of phenolic constituents of cultivated Portulaca oleracea L. Bull Facul Pharm Cairo Uni. 1994;32:137–142. [Google Scholar]
- 20.Sakai N, Inada K, Okamoto M, Shizuri Y, Fukuyama Y. Portuloside A, a monoterpene glucoside from Portulaca oleracea. Phytochemistry. 1996;42:1625–1628. [Google Scholar]
- 21.Mizutani M, Hashidoko Y, Tahara S. Factors responsible for inhibiting the motility of zoospores of the phytopathogenic fungus Aphanomyces cochlioides isolated from the non-host plant Portulaca oleracea. FEBS Lett. 1998;438:236–240. doi: 10.1016/s0014-5793(98)01308-8. [DOI] [PubMed] [Google Scholar]
- 22.Feng PC. High concentration of (-)-noradrenaline in Portulaca oleracea L. Nature. 1961;191:1108. doi: 10.1038/1911108a0. [DOI] [PubMed] [Google Scholar]
- 23.Chen CJ, Wang WY, Wang XL, Dong LW, Yue YT, Xin HL, Ling CQ, Li M. Anti-hypoxic activity of the ethanol extract from Portulaca oleracea in mice. J Ethnopharmacol. 2009;124:246–250. doi: 10.1016/j.jep.2009.04.028. [DOI] [PubMed] [Google Scholar]
- 24.Changquan L, Liwei D, Hailiang X, Min L, Wanyin W, Lin J. Ethanol extract of Portulaca oleracea L. protects against hypoxia-induced neuro damage through modulating endogenous erythropoietin expression. J Nutr Biochem. 2014;23:385–391. doi: 10.1016/j.jnutbio.2010.12.015. [DOI] [PubMed] [Google Scholar]
- 25.Llacuna L, Marí M, Lluis JM, García-Ruiz C, Fernández-Checa JC, Morales A. Reactive oxygen species mediate liver injury through parenchymal nuclear factor-κB inactivation in prolonged ischemia/reperfusion. Am J Pathol. 2009;174:1776–1785. doi: 10.2353/ajpath.2009.080857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gunawan BK, Liu ZX, Han D, Hanawa N, Gaarde WA, Kaplowitz N. c-Jun N-terminal kinase plays a major role in murine acetaminophen hepatotoxicity. Gastroenterology. 2006;131:165–178. doi: 10.1053/j.gastro.2006.03.045. [DOI] [PubMed] [Google Scholar]
- 27.Henderson NC, Pollock KJ, Frew J, Mackinnon AC, Flavell RA, Davis RJ, Sethi T, Simpson KJ. Critical role of c-jun (NH2) terminal kinase in paracetamol-induced acute liver failure. Gut. 2007;56:982–990. doi: 10.1136/gut.2006.104372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Martin-Murphy BV, Kominsky DJ, Orlicky DJ, Donohue TM, Ju C. Increased susceptibility of natural killer T-cell-deficient mice to acetaminophen-induced liver injury. Hepatology. 2013;57:1575–1584. doi: 10.1002/hep.26134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Larson AM, Polson J, Fontana RJ, Davern TJ, Lalani E, Hynan LS, Reisch JS, Schiødt FV, Ostapowicz G, Shakil AO. Acetaminophen-induced acute liver failure: results of a United States multicenter, prospective study. Hepatology. 2005;42:1364–1372. doi: 10.1002/hep.20948. [DOI] [PubMed] [Google Scholar]
- 30.Knight TR, Kurtz A, Bajt ML, Hinson JA, Jaeschke H. Vascular and hepatocellular peroxynitrite formation during acetaminophen toxicity: role of mitochondrial oxidant stress. Toxicol Sci. 2001;62:212–220. doi: 10.1093/toxsci/62.2.212. [DOI] [PubMed] [Google Scholar]
- 31.Tirmenstein MA, Nelson SD. Acetaminophen-induced oxidation of protein thiols. Contribution of impaired thiol-metabolizing enzymes and the breakdown of adenine nucleotides. J Bio Chem. 1990;265:3059–3065. [PubMed] [Google Scholar]
- 32.Hanawa N, Shinohara M, Saberi B, Gaarde WA, Han D, Kaplowitz N. Role of JNK translocation to mitochondria leading to inhibition of mitochondria bioenergetics in acetaminophen-induced liver injury. J Bio Chem. 2008;283:13565–13577. doi: 10.1074/jbc.M708916200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rogina B, Helfand SL. Cu, Zn superoxide dismutase deficiencyaccelerates the time course of an age-related markerin Drosophila melanogaster. Biogerontology. 2000;1:163–169. doi: 10.1023/a:1010039813107. [DOI] [PubMed] [Google Scholar]
- 34.Yu Y, Li J, Wan Y, Lu J, Gao J, Huang C. GADD45α Induction by Nickel Negatively Regulates JNKs/p38 Activation via Promoting PP2Cα Expression. PLoS One. 2013;8:e57185. doi: 10.1371/journal.pone.0057185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Han D, Ybanez MD, Ahmadi S, Yeh K, Kaplowitz N. Redox regulation of tumor necrosis factor signaling. Antioxid Redox Signal. 2009;11:2245–2263. doi: 10.1089/ars.2009.2611. [DOI] [PMC free article] [PubMed] [Google Scholar]


