Abstract
CD147 functions as an induction of matrix metalloproteinases and tumor angiogenesis, and is highly expressed in various malignant neoplasms. Recently, CD147 is shown to form a complex with amino acid transporters such as L-type amino acid transporter (LAT1), system ASC amino acid transporter-2 (ASCT2) and 4F2hc as a heavy chain of LAT1. It remains unknown about the existence of these complexes in patients with pancreatic cancer. The aim of this study is to investigate the relationship between CD147 and these amino acid transporters. Ninety-seven patients with pancreatic cancer were evaluated. Tumor sections were stained by immunohistochemistry for LAT1, ASCT2, Ki-67, microvessel density (MVD) determined by CD34, p-AKT, and p-mTOR. CD147 was highly expressed in 23% (22/97) of patients. A high expression of CD147 is significantly associated with N factor, LAT1, ASCT2, Ki-67, VEGF and p-mTOR. A high CD147 expression was identified as a significant prognostic predictor by univariate survival analysis. The coexpression of CD147 and LAT1, and that of CD147 and 4F2hc yielded a significantly worse prognosis than the single expression of LAT1, and that of 4F2hc, respectively. CD147 revealed a significant relationship with the expression level of LAT1 and ASCT2, correlated with tumor proliferation, angiogenesis and mTOR signaling.
Keywords: Pancreatic cancer, CD147, LAT1, ASCT2, amino acid transporter complex
Introduction
Pancreatic cancer is a dismal disease regardless of appropriate treatment, having a short survival time. Surgical resection is a standard therapy in patients with early-stage disease, but, some systemic chemotherapies are recommended as a best practice in advanced disease [1]. However, it is critical to explore the established biomarkers to improve a therapeutic response and patient survival, and further study is warranted to conduct the translational research to investigate a new marker for predicting outcome in patients with pancreatic cancer.
Recently, we had reported that the amino acid transporters such as L-type amino acid transporter 1 (LAT1) and System ASC amino acid transporter-2 (ASCT2) are highly expressed in pancreatic cancer and these transporters play an essential role in the progression and metastases, having a close association with poor outcome [2,3]. As a heavy chain of LAT1, 4F2hc (CD98) is requiring for its functional expression in the plasma membrane [4]. It has been also reported that 4F2hc expression is significantly related to the invasiveness and tumor cell proliferation in several human neoplasms and the role of 4F2hc in cancer is closely associated with the functions of amino acid transporter and integrin signaling, promoting tumor growth and metastases [5-7]. These amino acid transporters are essential for the tumor growth and proliferation, and the expression level of LAT1 and ASCT2 are coordinately increased in cancers [8]. LAT1 provides the essential amino acids to promote tumor cell progression via the mammalian target of rapamycin (mTOR) signaling pathway, and ASCT2 maintains the cytoplasmic amino acid pool necessary to drive the function of LAT1, providing the necessary energy via glutamine delivery [8].
CD147 is a highly glycosylated transmembrane protein of the immunoglobulin superfamily [9]. Previous reports showed that CD147 works as an induction of matrix metalloproteinases and tumor angiogenesis, and is highly expressed in various malignant tumor cells [9]. CD147 is thought to be closely related to worse prognosis [9]. Recently, CD147 form a complex with 4F2hc and monocarboxylate transporters (MCT) on the plasma membrane of malignant neoplasms, and the CD147-4F2hc complex has been reported to significantly contribute to worse outcome of cancer patients through promoting cell proliferation via the PI3K/Akt signaling pathway [7,9]. In the previous reports, CD147 is described to induce tumor cell invasion by stimulating the production of matrix metalloproteinases (MMPs), resulting in tumor invasiveness and widespread [9].
Tsai et al had reported that higher CD147 was associated with shorter survival and correlated with more advanced clinical markers in Chinese patients with pancreatobiliary adenocarcinomas [10]. But, the previous studies including their report [9,10] didn’t focus on the relationship with the expression of amino acid transporters in human cancers. It remains unclear about the clinical significance of CD147 correlated with amino acid transporters such as LAT1, ASCT2 and 4F2hc in various human malignancies. The aim of this study is to investigate the clinicopathological significance of CD147 expression in pancreatic adenocarcinoma, correlated with these amino acid transporters, using the previously reported specimens [2,3].
Materials and methods
Patients
One hundred thirteen consecutive patients with pancreatic ductal adenocarcinoma underwent surgical resection at our institutions, between July 1995 and March 2011. Because of inadequate tissue specimens and induction therapy, 16 patients were not met for inclusion criteria. Thus, 97 patients were eligible for the present study. The authors’ approach to the evaluation and resection of these tumors has been described previously [2,3]. The study protocol was approved by the institutional review board.
All surgical specimens were reviewed and classified according to the WHO classification, and pathologic tumor-node-metastasis (TNM) stages were established using the International System for Staging Pancreatic Cancer adopted by the American Joint Committee on Cancer and the Union Internationale Centre le Cancer [11]. The median age was 67 years (range, 25 to 86 years). Of all patients, 18, 40, 32 and 7 had stage I, II, III and IV tumors, respectively. As postoperative adjuvant chemotherapy, gemcitabine, S-1 (Taiho Pharmaceutical Co., Ltd, Tokyo, Japan) and oral administration of tegafur (a fluorouracil derivative drug) were administered to 31, 15 and 1 patients, respectively. The day of surgery was considered the starting day for measuring postoperative survival. The follow-up duration ranged from 6 to 164 months (median, 14 months).
Immunohistochemical staining
The detailed protocol for immunostaining has been published elsewhere [12-15]. CD147 antibody is an affinity-purified rabbit polyclonal antibody (Santa Cruz Biotechnology, Inc., 1:100 dilution). Immunohistochemical staining was performed on paraffin sections using a polymer peroxidase method [Histofine Simple Stain MAX PO (MULTI) kit (Nichirei Corporation, Tokyo, Japan)]. Briefly, deparaffinized and rehydrated sections were treated with 0.3% hydrogen peroxide in methanol for 30 min to block endogenous peroxidase activity. To expose antigens, sections were autoclaved in citrate buffer (pH 8.0) for 5 min and cooled for 30 min. After rinsing in phosphate-buffered saline (PBS), sections were incubated with affinity-purified anti-CD147 antibody overnight. Thereafter, they were incubated with Histofine Simple Stain MAX PO (MULTI) kit (Nichirei Corporation, Tokyo, Japan). The peroxidase reaction was performed using 0.02% 3,3’-diaminobenzidine tetrahydrochloride and 0.01% hydrogen peroxide in 0.05 M tris-HCl buffer, pH 7.4. Negative control tissue sections were prepared by omitting the primary antibody. LAT1 expression was determined by immunohistochemical staining with a LAT1 antibody (2 mg/mL, anti-human monoclonal mouse antibody, 4A2, provided by Dr H. Endou [J-Pharma, Tokyo, Japan], dilution; 1:3200). The production and characterisation of the LAT1 antibody has previously been described [12]. The 4F2hc antibody is an affinity-purified rabbit polyclonal antibody (Santa Cruz Biotechnology, Inc., 1:100 dilution) raised against a peptide mapping to the carboxy terminus of 4F2hc of human origin. The detailed protocol for immunostaining has been published elsewhere [13]. Immunohistochemical staining of ASCT2 was done using a rabbit anti-ASCT2 polyclonal antibody (1:300 dilution) according to the procedure described previously [14].
CD147, LAT1, 4F2hc, and ASCT2 expression was considered positive only if distinct membrane staining was present.
The expression scores of CD147, LAT1, 4F2hc and ASCT2 were assessed by the extent of staining as follows: 1, ≤10% of tumor area stained; 2, 11-25% stained; 3, 26-50% stained; and 4, ≥51% stained. The staining intensity was not considered when staining was assessed. The tumors in which stained tumor cells were scored as 3 or 4 were defined as high expression.
In order to determine MVD, cellular proliferation, and mTOR signal pathway, immunohistochemical staining was done using the antibodies as follows: mouse monoclonal antibodies against CD34 (Nichirei, Tokyo, Japan; 1:800 dilution) and Ki-67 (Dako, Glostrup, Denmark; 1:40 dilution); a rabbit polyclonal antibody against phosph-Akt (Abcam, Tokyo, Japan; 1:200 dilution); and a rabbit monoclonal antibody against phosph-mTOR (Cell Signaling; 1:80 dilution). The number of CD34-positive vessels was counted in four selected hot spots in a 400x field (0.26 mm2 field area). MVD was defined as the mean count of microvessels per 0.26 mm2 field area, and the tumors in which stained tumor cells represented more than each median value were defined as having high expression. Regarding Ki-67 expression, a highly cellular area of the immunostained sections was evaluated. All epithelial cells with nuclear staining of any intensity were defined as having high expression. Approximately 1000 nuclei were counted on each slide. Proliferative activity was assessed as the percentage of positive Ki-67-stained nuclei (Ki-67 labeling index) in the sample. The median value for the Ki-67 labeling index was evaluated, and the tumor cells with a Ki-67 labeling index greater than the median value were defined as having high expression. Concerning p-AKT and p-mTOR, a semi-quantitative scoring method was used: 1≤10%, 2=10-25%, 3=25-50% and 4≥51% of positive cells. The tumors with staining scores greater than 3 were defined as having high expression [15]. The sections were assessed using a light microscope in a blinded fashion by at least two of the authors.
Statistical analysis
Probability values less than 0.05 indicated a statistically significant difference. Fisher’s exact test was used to examine the association between two categorical variables. The correlation between different variables was analyzed using the nonparametric Spearman’s rank correlation test. The Kaplan-Meier method was used to estimate survival as a function of time, and survival differences were analyzed by the log-rank test. Overall survival (OS) was determined as the time from tumor resection to death from any cause. Progression-free survival (PFS) was defined as the time between tumor resection and the first disease progression or death. Multivariate analyses were performed using a stepwise Cox proportional hazards model to identify independent prognostic factors. Statistical analyses were performed using JMP 8 software (SAS, Institute Inc., Cary, NC, USA) for Windows.
Results
Immunohistochemical finding and patient’s demographics
Ninety-seven primary sites were immunohistochemically analyzed using various biomarkers above described. The expression site of CD147 was predominantly localized on the plasma membrane of the cancer cells (Figure 1). The frequency of CD147 staining was 23% (22/97) of all patients. In the analysis of the other biomarkers, we have previously reported the results of immunohistochemical staining of LAT1, ASCT2, CD98, Ki-67, CD34, p-Akt and p-mTOR [2,3].
Figure 1.
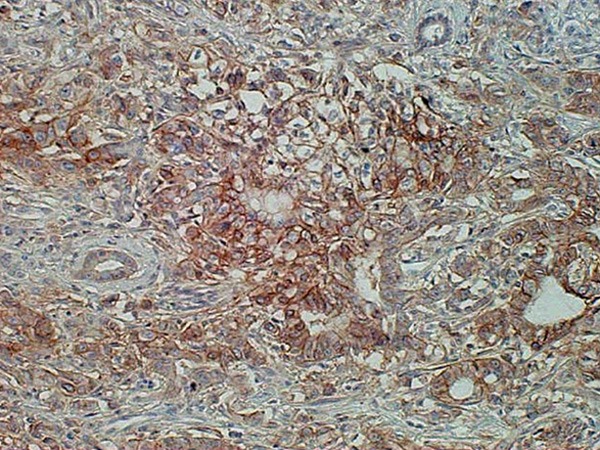
Immunohistochemical staining of CD147 in pancreatic ductal carcinoma. The score of CD147 immunostaining was grade 4 and its immunostaining pattern was membranous.
Table 1 shows patient’s demographics according to the expression of CD147. A high expression of CD147 is significantly associated with N factor, LAT1, ASCT2, Ki-67, VEGF and p-mTOR. Figure 2 shows the percentage of high/positive expression of different markers according to scoring of CD147 expression. The expression rate of these markers is increasing according to high score of CD147 expression. A statistically significant difference of the ASCT2 expression was recognized between scoring of 1 and 2.
Table 1.
Patient’s demographics according to CD147 expression
| Variables | Total | CD147 | |||
|---|---|---|---|---|---|
|
| |||||
| High | Low | P-value | |||
|
| |||||
| (n=97) | (n=22) | (n=75) | |||
| Age | ≤65/>65 yr | 43/54 | 11/11 | 32/43 | 0.628 |
| Sex | Male/female | 48/49 | 10/12 | 38/37 | 0.809 |
| Differentiation | WD or MD/PD | 84/13 | 17/5 | 67/8 | 0.162 |
| T factor | T1-2/T3-4 | 24/73 | 3/19 | 21/54 | 0.261 |
| N factor | No/N1-2 | 40/57 | 17/5 | 23/52 | <0.001 |
| Disease stage | I or II/III or IV | 58/39 | 11/11 | 47/28 | 0.328 |
| Lymphatic permeation | yes/No | 81/16 | 20/2 | 61/14 | 0.513 |
| Vascular invasion | yes/No | 67/30 | 17/5 | 50/25 | 0.436 |
| LAT1 | High/Low | 51/46 | 16/6 | 35/40 | 0.05 |
| CD98 | High/Low | 55/42 | 15/7 | 40/35 | 0.234 |
| ASCT2 | Positive/Negative | 52/45 | 17/5 | 35/40 | 0.014 |
| Ki-67 | High/Low | 48/49 | 17/5 | 31/44 | 0.004 |
| CD34 | High/Low | 48/49 | 14/8 | 34/41 | 0.151 |
| VEGF | High/Low | 42/55 | 16/6 | 26/49 | 0.002 |
| p-AKT | High/Low | 49/48 | 15/7 | 34/41 | 0.088 |
| p-mTOR | High/Low | 41/56 | 16/6 | 25/50 | 0.001 |
High expression of CD147 was defined as scores >2 as shown in the text.
Figure 2.
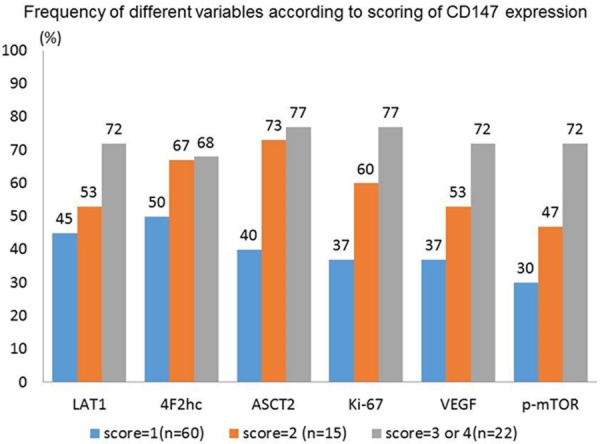
Percentage of high/positive expression of different markers was analyzed according to scoring of CD147 expression. The expression rate of these markers is increasing according to high score of CD147 expression.
Correlation between CD147 and different variables
Spearman’s rank test showed that CD147 was significantly correlated with LAT1 (r=0.205, p=0.043), ASCT2 (r=0.353, p<0.001), Ki-67 (r=0.352, p<0.001), VEGF (r=0.290, p=0.004), p-AKT (r=0.290, p=0.004), and p-mTOR (r=0.411, p<0.001) (Table 2).
Table 2.
Correlation with CD147 expression
| Spearman r | 95% CI | P-value | |
|---|---|---|---|
| LAT1 | 0.205 | 0.004 to 0.394 | 0.043 |
| ASCT2 | 0.353 | 0.159 to 0.520 | <0.001 |
| CD98 | 0.17 | 0.036 to 0.363 | 0.096 |
| Ki-67 | 0.352 | 0.158 to 0.519 | <0.001 |
| CD34 | 0.139 | 0.069 to 0.356 | 0.176 |
| VEGF | 0.343 | 0.148 to 0.512 | <0.001 |
| p-AKT | 0.29 | 0.091 to 0.468 | 0.004 |
| p-mTOR | 0.411 | 0.225 to 0.568 | <0.001 |
Abbreviation: 95% CI, 95% confidence interval.
Survival analysis according to CD147 expression
We had already reported detailed results of the survival analysis on clinicopathological variables, LAT1, ASCT2, 4F2hc, Ki-67, CD34, p-AKT and p-mTOR in the previous articles [2,3]. The survival data was updated in the present study. The five-year survival rate and median survival time for all patients were 33% and 19 months, respectively.
In the present study, the high expression of CD147 was significantly associated with poor OS and PFS by univariate analysis (Figure 3A, 3B). A statistically significant difference in the OS was observed between high and low expression of LAT1, 4F2hc and ASCT2 [2,3]. Based on the results of univariate log-rank test of these amino acid transporters (LAT1, 4F2hc, ASCT2 and CD147), we investigated the prognostic significance of these amino acid transporters using multivariate analysis. Multivariate analysis confirmed that LAT1 (p<0.001) and ASCT2 (p=0.012) were independent prognostic markers for predicting negative outcome, but CD147 (p=0.238) and 4F2hc (p=0.863) were not identified as independent predictors. Moreover, we examined the same analysis of PFS using these markers. By univariate analysis, LAT1 and ASCT2 yielded significant worse predictors with p-value of <0.001 and <0.001, respectively, but, there was no significant difference in the PFS between high and low expression of 4F2hc. Multivariate analysis also identified LAT1 and ASCT2 as independent worse predictors in PFS after surgery.
Figure 3.
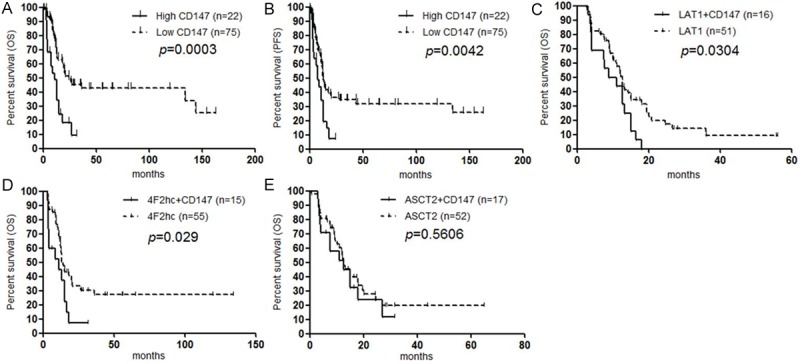
Kaplan-Meier analysis of progression-free survival (PFS) and overall survival (OS) according to CD147 expression. A statistically significant difference in OS (A) and PFS (B) was observed between the patients with high CD147 and those with low CD147 tumor expression. Survival analysis was performed according to the coexpression of CD147 and LAT1 (C), CD147 and 4F2hc (D), and CD147 and ASCT2 (E). Coexpression of CD147 and LAT1 (C), and that of CD147 and 4F2hc (D) yielded a significantly worse prognosis than the single expression of LAT1, and that of 4F2hc, respectively. No significant difference was observed between ASCT2 plus CD147 and ASCT2 alone (E).
Next, we examined the prognostic significance according to the coexpression of CD147 and other amino acid transporters. We found that the coexpression of CD147 and LAT1 (Figure 3C), and that of CD147 and 4F2hc (Figure 3D) yielded significantly worse prognosis than the single expression of LAT1, and that of 4F2hc, respectively. But, no significant difference was observed between ASCT2 plus CD147 and ASCT2 alone (Figure 3E).
Discussion
This is a clinicopathological study to evaluate the prognostic significance of CD147 expression and amino acid transporters (LAT1, ASCT2 and 4F2hc), and the relationship between them. The expression of CD147 was correlated with pathological variables such as cellular proliferation, angiogenesis, and mTOR signaling. In the present study, the expression of CD147 was closely associated with LAT1 and ASCT2, and plays a significant role in the tumor cell proliferation, angiogenesis, metastases and the activation of mTOR signaling pathway. Although the high expression of CD147 was identified as a significant prognostic predictor, the coexpression of CD147 and LAT1 as well as CD147 and 4F2hc was more powerful marker for predicting worse outcome than the single expression of LAT1 or ASCT2. These results suggest that CD147 plays a supportive role for tumor invasiveness and progression to strengthen the function of amino acid transporters in human neoplasms. To our knowledge, this is a first study to investigate the clinical significance of CD147 expression and the amino acid transporters related to malignancies.
Recently, it has been reported that the expression of CD147 is markedly increased in pancreatic cancer cells [16] and the inhibition of CD147 reduces the potential of tumor invasion and metastasis [17]. These experimental studies also indicated that the expression of CD147 plays a crucial role in the tumor progression and survival, suggesting the therapeutic potential as a new target for pancreatic cancer. By experimental research, it was found that the inhibition of CD147 expression significantly reduced angiogenesis and CD147 regulates angiogenesis by possible mechanisms such as cell proliferation, MMP secretion and the activation of PI3K/Akt [9].
The CD147 stimulated the production of VEGF, and inhibition of CD147 reduced the expression of VEGF in vitro, therefore, CD147 was thought to be related to the induction of VEGF [18]. In the present study, we found that there was a significant relationship between CD147 and VEGF in human tissue specimen, but not between CD147 and MVD. To our knowledge, little is known whether CD147 could be associated with the angiogenetic markers such as VEGF or MVD in human pancreatic cancer. Further investigation is required to identify the angiogenic role of CD147 expression in various human neoplasms.
Recently, it was reported that there is a novel monocarboxylate transporter (MCT)-CD147-4F2hc-LAT1 transporter complex, including ASCT2 and EpCAM in three human tumor cell lines using mass spectrometry [19]. Figure 4 shows the modified model of the CD147-4F2hc-LAT1-ASCT2 complexes [19]. There was a direct association between CD147 and 4F2hc, between CD147 and MCT, between EpCAM and 4F2hc, and between LAT1 and 4F2hc. However, no direct cross-linking was observed between CD147 and LAT1, and between CD147 and ASCT2. The complexes of these transporters are thought to play their joint role in cellular energy metabolism, and the presence of ASCT2 and EpCAM is required to transform a transmembrane protein supercomplex. But, it remains unclear whether the existence of these CD147-4F2hc-LAT1-ASCT2 complexes strongly contributes to play an essential role in the development and pathogenesis of human neoplasms. To the best of our knowledge, this is the first investigation to evaluate the clinicopathological significance of the relationship between CD147, and LAT1, ASCT2 and 4F2hc in human tumor specimen. Fei et al identified that the elevated CD147-4F2hc complex was significantly related to an unfavorable prognosis in patients with lung cancer [7]. This is corresponding to the results of our study, suggesting that the coexpression of CD147 and 4F2hc significantly contributed to worse outcome and could be more powerful predictor in both lung cancer and pancreatic cancer. In the previous studies, we found that LAT1, ASCT2 and 4F2hc were identified as a significant predictor for patients with pancreatic cancer [3,13]. Little is known whether the coexpression of CD147 and these amino acid transporters could be a powerful worse predictor compared to the single expression of them. The results of our study suggest that these amino acid transporters need the addition of CD147 expression to accelerate the progression and metastases of cancer cells. Moreover, the overexpression of CD147 caused the activation of mTOR signaling, resulting the tumor cell proliferation and angiogenesis.
Figure 4.
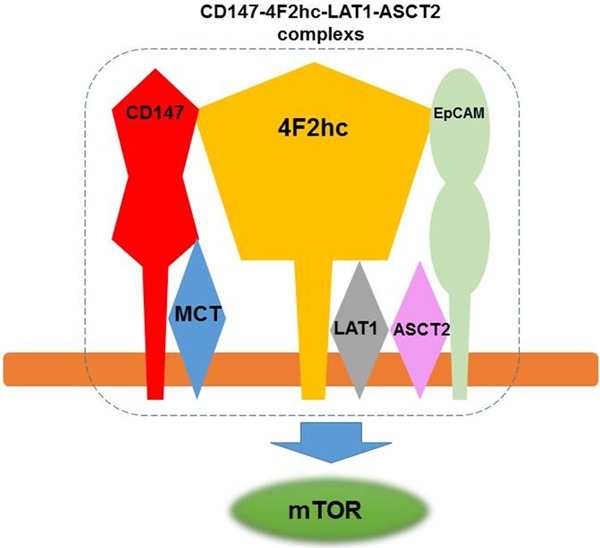
CD147-4F2hc-LAT1-ASCT2 complex. This figure was modified from the figure of reference 19. CD147 directly associated with 4F2hc and MCT, 4F2hc directly associated with LAT1 and EpCAM.
There are several limitations about the present study. We couldn’t evaluate the expression of MCT and EpCAM in the tumor tissues, thus, it remains unclear about the relationship between MCT and CD147, and between EpCAM and 4F2hc in pancreatic cancer. Further study is warranted to add the immunohistochemical staining of MCT and EpCAM expression within tumor tissues.
In conclusion, CD147 showed a significant relationship with the expression level of LAT1 and ASCT2, correlated with tumor proliferation, angiogenesis and mTOR signaling. CD147 was identified as a negative prognostic predictor in pancreatic cancer. Especially, the coexpression of CD147 and LAT1 or CD147 and 4F2hc could be a more powerful marker for predicting poor prognosis after surgery. The inhibition of both CD147 and these amino acid transporters may strongly suppress the tumor growth, and CD147 could be an attractive therapeutic target for pancreatic cancer.
Acknowledgements
This work was supported in part by advanced research for medical products Mining Programme of the National Institute of Biomedical Innovation (NIBIO). We also deeply appreciate Prof. Masahiko Nishiyama of Department of Molecular Pharmacology and Oncology, Prof. Takayuki Asao of Department of Oncology Clinical Development, Gunma University Graduate School of Medicine, for the critical review of this manuscript, and Ms. Yuka Matsui for her technical assistance of manuscript submission.
Disclosure of conflict of interest
We, all authors, have no financial or personal relationships with other people or organizations that could inappropriately influence our work.
References
- 1.Geer RJ, Brennan MF. Prognostic indicators for survival after resection of pancreatic adenocarcinoma. Am J Surg. 1993;165:68–72. doi: 10.1016/s0002-9610(05)80406-4. [DOI] [PubMed] [Google Scholar]
- 2.Kaira K, Sunose Y, Arakawa K, Ogawa T, Sunaga N, Shimizu K, Tominaga H, Oriuchi N, Itoh H, Nagamori S, Kanai Y, Segawa A, Furuya M, Mori M, Oyama T, Takeyoshi I. Prognostic significance of L-type amino-acid transporter 1 expression in surgically resected pancreatic cancer. Br J Cancer. 2012;107:632–638. doi: 10.1038/bjc.2012.310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kaira K, Sunose Y, Arakawa K, Sunaga N, Shimizu K, Tominaga H, Oriuchi N, Nagamori S, Kanai Y, Oyama T, Takeyoshi I. Clinicopathological Significance of ASC-Amino Acid Trans- porter 2 (ASCT2) Expression in Pancreatic Ductal Carcinoma. Histopathology. 2014 doi: 10.1111/his.12464. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 4.Kanai Y, Segawa H, Miyamoto K, Uchino H, Takeda E, Endou H. Expression cloning and characterization of a transporter for large neutral amino acids activated by the heavy chain of 4F2 antigen (CD98) J Biol Chem. 1998;273:23629–23632. doi: 10.1074/jbc.273.37.23629. [DOI] [PubMed] [Google Scholar]
- 5.Kaira K, Oriuchi N, Imai H, Shimizu K, Yanagitani N, Sunaga N, Hisada T, Kawashima O, Kamide Y, Ishizuka T, Kanai Y, Nakajima T, Mori M. CD98 expression is associated with poor prognosis in resected non-small-cell lung cancer with lymph node metastases. Ann Surg Oncol. 2009;16:3473–3481. doi: 10.1245/s10434-009-0685-0. [DOI] [PubMed] [Google Scholar]
- 6.Cantor JM, Ginsberg MH. CD98 at the crossroads of adaptive immunity and cancer. J Cell Sci. 2012;125:1373–1382. doi: 10.1242/jcs.096040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fei F, Li X, Xu L, Li D, Zhang Z, Guo X, Yang H, Chen Z, Xing J. CD147-CD98hc complex contributes to poor prognosis of non-small cell lung cancer patients through promoting cell proliferation via PI3K/Akt signaling pathway. Ann Surg Oncol. 2014;21:4359–4368. doi: 10.1245/s10434-014-3816-1. [DOI] [PubMed] [Google Scholar]
- 8.Fuchs BC, Bode BP. Amino acid transporters ASCT2 and LAT1 in cancer: Partners in crime? Semin Cancer Biol. 2006;15:254–266. doi: 10.1016/j.semcancer.2005.04.005. [DOI] [PubMed] [Google Scholar]
- 9.Weidle UH, Scheuer W, Eggle D, Klostermann S, Stockinger H. Cancer-related issues of CD147. Cancer Genomics Proteomics. 2010;7:157–169. [PubMed] [Google Scholar]
- 10.Tsai WC, Chao YC, Sheu LF, Lin YF, Nieh S, Chen A, Yu CP, Jin JS. EMMPRIN and fascin overexpression associated with clinicopathologic parameters of pancreatobiliary adenocarcinoma in Chinese peple. APMIS. 2007;115:929–938. doi: 10.1111/j.1600-0463.2007.apm_858.x. [DOI] [PubMed] [Google Scholar]
- 11.Sobin LH, Gospodarowicz MK, Wittekind Ch. International Union Against Cancer (UICC) TNM Classification of Malignant Tumors. 7th ed. Oxford, UK: Wiley-Blackwell; 2009. [Google Scholar]
- 12.Sakata T, Ferdous G, Tsuruta T, Satoh T, Baba S, Muto T, Ueno A, Kanai Y, Endou H, Okayasu I. L-type amino acid transporter 1 as a novel biomarker for high-grade malignancy in prostate cancer. Pathol Int. 2009;59:7–18. doi: 10.1111/j.1440-1827.2008.02319.x. [DOI] [PubMed] [Google Scholar]
- 13.Kaira K, Sunose Y, Arakawa K, Ogawa T, Sunaga N, Shimizu K, Tominaga H, Oriuchi N, Itoh H, Nagamori S, Kanai Y, Segawa A, Furuya M, Mori M, Oyama T, Takeyoshi I. Prognostic Sig- nificance of L-type Amino Acid Transporter 1 Expression in Surgically Resected Pancreatic Cancer. Br J Cancer. 2012;107:632–638. doi: 10.1038/bjc.2012.310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shimizu K, Kaira K, Tomizawa Y, Sunaga N, Kawashima O, Oriuchi N, Tominaga H, Nagamori S, Kanai Y, Yamada M, Oyama T, Takeyoshi I. ASC amino-acid transporter 2 (ASCT2) as a novel prognostic marker in non-small cell lung cancer. Br J Cancer. 2014;110:2030–2039. doi: 10.1038/bjc.2014.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kaira K, Oriuchi N, Takahashi T, Nakagawa K, Ohde Y, Okumura T, Murakami H, Shukuya T, Kenmotsu H, Naito T, Kanai Y, Endo M, Kondo H, Nakajima T, Yamamoto N. LAT1 expression is closely associated with hypoxic markers and mTOR in resected non-small cell lung cancer. Am J Transl Res. 2011;3:468–478. [PMC free article] [PubMed] [Google Scholar]
- 16.Li M, Zhai Q, Bharadwaj U, Wang H, Li F, Fisher WE, Chen C, Yao Q. Cyclophilin A is overexpressed in hman pancreatic cancer cells and stimulates cell proliferation through CD147. Cancer. 2006;106:2284–2294. doi: 10.1002/cncr.21862. [DOI] [PubMed] [Google Scholar]
- 17.Pan Y, He B, Song G, Bao Q, Tang Z, Tian F, Wang S. CD147 silencing via RNA interference reduces tumor cell invasion, metastasis and increases chemosensitivity in pancreatic cancer cells. Oncol Rep. 2012;27:2003–2009. doi: 10.3892/or.2012.1729. [DOI] [PubMed] [Google Scholar]
- 18.Voigt H, Vetter-Kauczok CS, Schrama D, Hofmann UB, Becker JC, Houben R. CD147 impacts angiogenesis and metastasis formation. Cancer Invest. 2009;27:329–333. doi: 10.1080/07357900802392675. [DOI] [PubMed] [Google Scholar]
- 19.Xu D, Hemler ME. Metabolic activation-related CD147-CD98 complex. Mol Cell Proteomics. 2005;4:1061–1071. doi: 10.1074/mcp.M400207-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]


