Abstract
Background: Betel quid dependence (BQD) patients have a cluster of cognitive, behavioral, and physiological symptoms which are associated with structural abnormalities in brain gray matter. However, so far there have neither been brain structural studies investigating the alterations related to BQD, nor studies assessing the brain structural changes with clinical indexes. Methods: 65 subjects were recruited including 33 ‘pure’ BQD patients and another 32 gender and age matched in the control group. T1 structural voxel-based morphometry (VBM) was performed to investigate the gray matter (GM) volume alterations. In BQD patients, Pearson correlation analysis was performed to investigate the association between GM segmentations and clinical indexes, including BQD scores, illness duration, SAS and SDS. Results: Compared with that of the control group, the VBM of GM in BQD patients exhibited a significant decrease in volume (All P values > 0.05, AlphaSim correction) in the midbrain, right anterior cingulate cortex (rACC), bilateral dorsolateral prefrontal cortex (dlPFC) and right superior temporal gyrus (STG), and also there was an increased volume in right hippocampal and right precuneus. GM volumes of the left DLPFC and right rACC showed negative correlation with the duration of BQD, meanwhile, midbrain volumes were negative correlating with BQD scores (All P values > 0.05). Conclusions: Our findings suggested that brain structural changes were present in BQD patients, and those may be a neurobiological basis for BQD patients. These findings may provide a new insight into the pathogenesis of BQD. Also, VBM is an effective tool for in vivo investigation of gray matter alterations in patients with BQD.
Keywords: Betel quid, drug dependence, gray matter, voxel-based morphometry, betel quid dependence
Introduction
Betel quid (BQ) ranked the fourth most frequently consumed psychoactive substance around the globe, following only alcohol, nicotine, and caffeine in prevalence [1]. Although BQ is chewed by approximately 600 million people globally, its use is concentrated in South Asia, Southeast Asia, and Pacific islands [2]. The term “betel quid” refers to a mixture of ingredients that most typically includes areca nut, piper betel leaf (a common vine), slaked lime (calcium hydroxide), and tobacco, though the ingredients of quid vary considerably by region, country, ethnicity, and personal preference [3,4]. The fruit of Areca catechu tree is commonly known as Areca nut (AN) (synonym betel nut). In Hainan provinces of China, BQ is h ombination of fresh AN and slaked lime (i.e. aqueous calcium hydroxide paste) wrapped in a betel leaf without tobacco and other ngredients (Figure 1).
Figure 1.

Betel quid in Hainan provinces of China includes fresh areca nut, betel leaf, slaked lime (white calcium hydroxide on the surface of betel leaf).
BQ chewing is an important behavior from a public health perspective because it is associated with a variety of health issues, most notably oral cancer and precancerous conditions such as leukoplakia and oral submucous fibrosis [5]. Consequently, BQ has been categorized into a Group 1 carcinogen by the International Agency for Research on Cancer [3,6]. However, to date, most research on BQ chewing has been limited to epidemiological and biological investigations [7,8]. Limited research has been conducted to understand the behavioral and psychological factors that lead individuals to initiate and/or maintain BQ chewing.
BQ use is associated with a dependency syndrome, which comprises increased concentration, mild euphoria, relaxation, postprandial satisfaction and a withdrawal syndrome associated with insomnia, mood swings, irritability and anxiety, the severity of which can be compared with that of amphetamine use [9]. A few studies [8,10-12] have assessed betel quid dependence using approaches based upon the Diagnostic and Statistical Manual of Mental Disorders (DSM; American Psychiatric Association, 2000) and the ICD-10 (World Health Organization, 1992). Alternatively, a few studies have assessed BQ dependence using adapted versions of dependence scales that were de-signed for substances other than BQ such as opioids [4] or tobacco [13]. Lee and his colleagues [14] recently developed, and provided initial validation for, the first instrument de-signed specifically for measuring BQ dependence: the Betel Quid Dependence Scale (BQDS).
Thus far, the reasons for BQ chewing are poorly understood. Social learning theories [15,16] posit that substance use behaviors are motivated by expectancy reasons: that is, individuals use a substance because of the positive outcomes associated with the use. For example, individuals may continue to use an addictive substance because they like the way the substance makes them feel. Thus there may be several social, cultural, behavioral, and physiological reasons that influence substance use initiation and maintenance [17]. From prevention and treatment point of views, it is important to understand such reasons so that strategies may be developed to help individuals overcome an addiction. Dopamine neurons have been proved to play an important role in the acquisition and sustention of a large number of these reward-dependent activities. It has been put forward that the evolving brain networks which reward survival-improving behavior are prone to be interrupted by habits. Generally, habitual and addictive action is connected with fronto-striatal network, which include prefrontal brain areas that have top-down effect on the ventral striatum involving in reward mechanism [18]. It has been indicated by neuroscientific literature that a lot of conventional behaviors can lead to the structural alteration of brain, e.g. learning the way to drive a taxi [19], learning to become a professional instrument player [20] and studying the way of juggling [21]. On account of the previous study, structural alterations in the brain network of individuals are predicted, which indicate trends to dependency including tolerance, withdrawal, concentration, desire and reduced management of BQ addiction and continued chewing in spite of convincing indications of negative effects. Determining such neural mechanism would help design prevention and treatment programs aiming to reduce the prevalence of BQ chewing.
Methods
Ethics statement
This study was approved by our research ethics review board of the the People’s hospital of Hainan Province, Haikou, China according to the Declaration of Helsinki. The consent form has been read and signed by each subject before being included in the study.
Inclusion and exclusion criteria
Persons with exclusive usage of BQs were included. Persons with usage of BQ without tobacco at least one day at a time for no less than 5 year were categorized as “individuals using BQ without tobacco”. Persons without usage of BQ, areca nut and tobacco (in all forms) were defined as “control individuals”.
During the first session we administered the BQD Scale (BQDS). BQDS was been recently developed by Lee and his colleagues [14], which is the first instrument designed specifically for measuring BQ dependence. The BQDS is comprised of three factors: “physical and psychological urgent need”, “increasing dose”, and “maladaptive use” [22]. The BQDS have assessed BQ dependence using approaches based upon the Diagnostic and Statistical Manual of Mental Disorders (DSM) and the ICD-10. The BQDS was found to have good internal consistency (α=0.92) and construct validity [14]. Moreover, in order to assess the emotional status of every subject, the Self-Rating Anxiety Scale (SAS) and the Self-Rating Depression Scale (SDS) were adopted on the scanning day. BQD participants conformed to the criteria for present BQ addiction, as diagnosed by the BQDS > 4 [14], SAS > 50 and SDS > 50.
The exclusion criteria were as follows: (1) tobacco smokers; (2) persons with usage of different forms of tobacco without smoke e.g. gutka and/or paan masala; (3) persons with self-claimed systemic diseases such as neurological disorder, cardiovascular disease, diabetes mellitus, epilepsy, thyroid and renal disorders; (4) persons with present or recent history of any Axis I psychiatric and/or substance use diseases; (5) present use of psychotropic drugs; (6) left-handers; and (7) not able to read and write Chinese.
Study participants
At last, 33 BQD volunteers and 32 control individuals recruited from a residential area of Wanning City of Hainan province, China were included.
Questionnaire
A questionnaire in simple Chinese acquiring information including age and gender as well as monthly income, educational status, duration of BQ chewing habit, daily dosage of BQ and duration time of quid placement in mouth was distributed to all participants (n=65). Because wine has played an important role in Chinese social aspects of life, all participants were also assessed for alcohol use in the past 30 days, including the average frequency of drinking and number of drinks per occasion.
MRI data acquisition
MRI data were obtained on a Siemens Verio3T MRI scanner using a standard 6-channel head coil (Erlangen, Germany) in the Department of Radiology, People’s hospital of Hainan Province. All participants were required to keep their heads motionless, their eyes closed and their brains think of nothing systematically or fall asleep. A routine MR scanning was performed for purpose of excluding gross cerebral pathology. Anatomical images in terms of the functional slice locations using spin-echo imaging in the axial plan which was parallel to the Anterior Commissure-Posterior Commissure (AC-PC) line were then acquired. A T1-weighted structural image with high resolution was obtained with a MPRAGE sequence (repetition time=2300 ms, echo time=2.9 ms, TI=900 ms, field of view=256×256 mm, flip angle=9, in-plane matrix=256×256, slice thickness=1 mm, no gap, and voxel dimension=1×1×1.33 mm3). All participants finished with no report of discomfort during or after the scanning and without falling asleep during the procedure. And under the examination of two skilled radiologists, there was no sign of significant structural injury in all participants on the basis of traditional MRI scans.
MRI data post-processing
The VBM8 tool-box (http://dbm.neuro.uni-jena.de/vbm) with default parameters running in the SPM8 (statistical parametric mapping, http://www.fil.ion.ucl.ac.uk/spm) software was used to conduct data analysis. Specifically, structural imaging data was deviation corrected, categorized according to tissue, and changed into standard Montreal Neurological Institute (MNI) space within a unified model, which proved to be a 12-parameter affine-only nonlinear alteration. Subsequently, gray matter (GM) was analyzed. The non-linear constituents originated from the normalization matrix multiplied the analysis for the purpose of sustaining actual GM values locally (coordinated GM volumes). Lastly, a Gaussian kernel of 4 mm full width at half maximum (FWHM) was adapted to smooth the coordinated volumes. The resultant images were used for statistical analysis.
Statistical analysis
With the use of the SPM8 software package, statistical analysis of structural imaging data was conducted. In order to evaluate the changes in the brain volume related to BQD of participants, dependent sample t tests were used to analyze smoothed GM, with gender and age as nuisance covariates. The statistical threshold for the data analysis was set at P < 0.05 (after AlphaSim correction).
The GM values on average revealed by VBM for all voxels in abnormal regions were extracted and correlation was made with BQDS, BQD duration, SAS, SDS in BQD subjects with Pearson correlation analysis, in order to detect any connection between structural abnormalities and characteristics of addiction. The age, education years, monthly income, alcohol last 30 days, SAS, SDS, data were studied with the software SPSS version 16.0 (SPSS Inc. Chicago, IL, USA), while Chi-square test was performed for the purpose of discovering the importance of gender in both groups. P values less than 0.05 were considered as statistically significant.
Results
Characteristics of the study population
Sixty-five persons (33 BQD users and 32 controls) were included in the study. The average age of the BQD sample was 46.7±9.4 years; the average age of the healthy controls (HC) was 45.8±9.3 years. The BQD sample was 72.7% male, and had completed 12.3±2.7 year’s education; The HC sample was 62.5% male, and had completed 12.6±2.4 years education. The average monthly income of subjects in BQD was $423.5±73.7; the average monthly income of subjects in HC was $413.1±73.0. Individuals indicated that they had been chewing BQ with dependency syndrome for a mean duration of 20.6±6.9 years (range 7 to 31 years), a mean BQDS of 10±3.4 (range 5 to 16) and consumed an average of 342±106 g/day BQ (range 200 to 500 g/day) daily. BQD chewers placed BQ in their mouth for an average of 7.6±2.4 minutes (range 3 to 12) before spitting-out the remnants. In order to eliminate the interference of depression and degrees of anxiety, all participants were evaluated with the SAS and SDS, and the results failed to reach cut-off for clinical significance on average. The dosage of alcohol in the past 30 days in BQD and HC group, were 200.2±34.8 g and 189.0±33.4 g respectively, which indicated they were not alcoholics, therefore there were no alcohol-caused effects on BQD usage. There was no difference for the age, sex, education years, monthly income, alcohol last 30 days and SAS between the BQD patients and HC (P values > 0.05); however, the BQD patients had a lower SDS score than HC (P values > 0.05). Table 1 summarizes the demographics of BQD and Healthy control participants.
Table 1.
Demographics and clinical characteristics of participants
| Betel Quid Dependence (BQD) | Healthy control (HC) | Statistics | P value | |
|---|---|---|---|---|
| Age | 46.7±9.4 | 45.8±9.3 | 0.446 | 0.657 |
| Gender (male/female) | 24/9 | 20/12 | 0.777 | 0.378 |
| Education (years) | 12.3±2.7 | 12.6±2.4 | 0.512 | 0.610 |
| Monthly income (US $) | 423.5±73.7 | 413.1±73.0 | 0.799 | 0.427 |
| Betel Quid Dependence Scale (BQDS) | 10±3.4 | N/A | ||
| Dosage of Betel Quid (g/day) | 342±106 | N/A | ||
| Duration of placement of Betal quid in the mouth (min) | 7.6±2.4 | N/A | ||
| Duration of Betel Quid (years) | 20.6±6.9 | N/A | ||
| Alcohol last 30 days (g) | 200.2±34.8 | 189.0±33.4 | 1.025 | 0.309 |
| Handedness (right/left) | 33/0 | 32/0 | > 0.99a | |
| Self-Rating Anxiety Scale | 27.2±5.6 | 28.3±6.1 | 0.759 | 0.451 |
| Self-Rating Depression Scale | 28.6±6.6 | 32.8±7.5 | 2.385 | 0.020* |
Note: unless otherwise indicated, data are means ± standard deviations. N/A=not applicable. Values are expressed as mean ± SD. aThe P value for gender distribution in the two groups was obtained bychi-square test. bcThe P value for age, Education, Alcohol last 30 days, SAS, SDS difference between the two patient groups was obtained by independent-samples t test.
P < 0.05.
Voxel-based morphometry (VBM)
Compared with HC, BQD patients exhibited declined GM volume in midbrain, right rostral anterior cingulate cortex (rACC), bilateral dorsolateral prefrontal cortex (dlPFC), right superior temporal gyrus (STG), and greater volume in right hippocampal and right precuneus (Figure 2; All P values > 0.05, AlphSim corrected). These results are shown in Figure 2 and Table 2.
Figure 2.
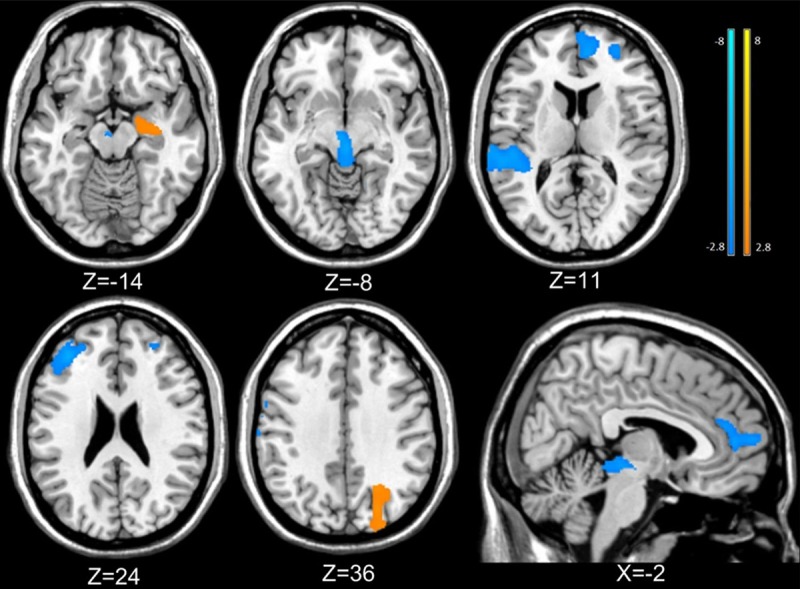
Gray matter changes in BQD patients and healthy controls. Compared with healthy controls, BQD patients exhibited decreased volume in midbrain, right rostral anterior cingulate cortex (rACC), bilateral dorsolateral prefrontal cortex (dlPFC), right superior temporal gyrus (STG), and increased volume in right hippocampal and right precuneus (BQD=Betel quid dependence; All P < 0.05, AlphSim corrected).
Table 2.
Regions showing GM volume differences between BQD and HC
| Regions | BA | Cluster size (voxel) | T scores of peak voxel | Coordinates of peak voxel in MNI space (x, y, z) |
|---|---|---|---|---|
| BQD > HC | ||||
| Midbrain | 466 | -4.60 | 0 -27 -4.5 | |
| L.dlPFC | 46 | 867 | -4.49 | -39 40.5 28.5 |
| R.dlPFC | 108 | -3.98 | 33 54 7.5 | |
| R.ACC | 24 | 219 | -4.08 | -4.5 33 15 |
| R.STG | 41 | 1356 | -5.04 | -49.5 -33 7.5 |
| BQD > HC | ||||
| R.Hippocampal | 521 | 4.82 | 33 -16.5 -24 | |
| R.Precuneus | 19 | 557 | 4.63 | 22 -68 34 |
Abbreviations: L, left; R, right; dlPFC= dorsolateral prefrontal cortex; ACC= anterior cingulate cortex; STG=superior temporal gyrus; BA: Brodmann’s area. T: statistical value of peak voxel showing gray matter volume differences between the two groups (negative values: BQD > HCs; positive values: BQD > HCs). MNI: Montreal Neurological Institute Coordinate System or Template; x, y, z: coordinates of primary peak locations in the MNI space. a The regions survived the height but not the extent threshold.
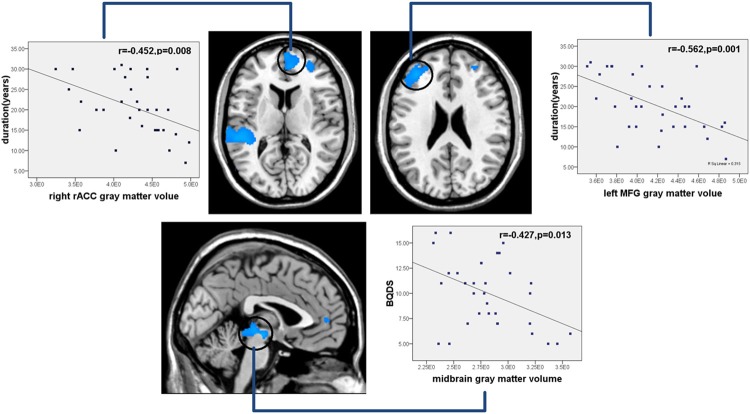
Gray matter volumes of the left DLPFC, the right rACC indicated a negative association with duration of BQD (r1=-0.452, 1=0.008; r2=-0.562, ?P2=0.001); when finding association between the BQD score and GM segmentations, a negative correlation in the midbrain volume in BQD patients was found (r3=-0.427, 3=0.013), as shown in Figure 3. No correlation was found between other gray matter volume and clinical indexes, including BQD scores, illness duration, SAS, SDS in BQD patients.
Figure 3.
Correlation results between gray matter alteration and duration and BQDS. Pearson correlation analyses reveals that gray matter volumes of the left DLPFC, the right rACC showed a negative correlation with duration of BQD, midbrain volume were negatively correlated with BQD scores in BQD patients. (BQD=Betel quid dependence; BQDS=Betel quid dependence score; rACC=rostral anterior cingulate cortex; MFG=Medial frontal gyrus; P < 0.05).
Discussion
We assumed that the brain structure may have some particular alterations which have relation to the issues in terms of behavior and emotion of BQD patients. This is the first report about the brain structural changes in gray matter volume in a sample of BQD. Alterations of structure in GM in some psychiatric diseases have been detected due to the use of VBM [23]. Gray matter volume is thought to represent the density of neuronal and glial cell bodies in addition to the number of dendritic branches and short range axons [24], the reported alterations in brain structure were reflection of the possible disorder pathology. Therefore, gray matter volume can be thought as a measure of local network integrity.
Consistent with previous drug dependent VBM studies [25], atrophy was shown in regional GM volume within BQD patients in the midbrain, bilateral DLPFC, the right rACC. Moreover, atrophy of the left DLPFC, the right rACC had negative association with the duration time of BQD, which consistent with the previous internet addiction study by Yuan K and his colleagues [26]. These outcomes indicated that as BQD remained, atrophy of brain in DLPFC and ACC was severer. Decreased volume of midbrain was negatively correlation with BQDS, which demonstrated that the more serious of BQD extent, the volume of midbrain is decreased. We also found right hippocampus and right precuneus demonstrating larger amount of GM volume in BQD patients, which were not investigated in other drug dependence studies. Currently, the possible confounding influences of age, sex and the whole brain volume were all imported as covariates.
The areas of gray matter alterations detected between the BQD and healthy controls were connected conceptually by the regions in charge of regulating cognitive, reward and emotional behaviors. It have been indicated by various neurocognitive researches that cognitive management is correlated with a special cortico-subcortical network which includes the rACC and the DLPFC [27]. Higher activation was demonstrated in medial frontal gyrus (BA 10) during result selection with uncertainty in persons with psychostimulant [28]. Based on a prominent hypothesis which controls conflict [29], rACC marks the appearance of reaction conflict which leads to aggregation of the DLPFC for greater cognitive management for succeeding behaviors. Also, it has been suggested that latest discoveries on fMRI connectivity study support the idea that neural networks linking the medial frontal cortex with paralimbic and temporal areas have much fewer effects on cocaine takers [30]. Our outcomes of the decreased GM volume in the DLPFC and rACC, may at least in some degree, be in correlation with cognitive management and behavioral dysfunctions directed by goal in BQ addiction [31,32].
As shown in Figure 3, the longer the time of an individual’s BQ chewing, the more significant the decline in GM of the middle frontal gyri and rACC. Taking into consideration the crucial roles of the frontal cortex and the limbic system during addiction, it seems reasonable to connect the structural alterations and the duration alterations of BQD. Nevertheless, the discussion of these alterations would still require further methods in view of the chicken and egg question.
Midbrain dopamine (DA) neurons play a central role in a wide range of behaviors, from attention and motivation to motor control and reinforcement. The release of DA is modulated by a number of factors, and its deregulation has been implicated in addiction. Dopamine in the reward circuit is increased due to drugs abuse of individuals which is considered the basis of rewarding influences. Hence the majority of clinical researches in terms of addiction have concentrated on the midbrain dopamine regions regarded to be participated in reward, regulation and habit formation [33]. Although there is a long history of research implicating the midbrain dopamine system in reward processing, there has been little research results about the gray volumes of midbrain. Carlson J. M and his colleagues [34] had a research into the possibility that structural variability in the midbrain had link with the functional variability in reward reactivity, which found that as midbrain volumes increased, fMRI reward reactivity in the VS and mPFC also increased, presumably greater integrity in dopamine-producing neurons in the midbrain should coincide with greater dopamine production and availability in target regions such as the VS and mPFC. A series of indications suggest that the impulsive activities are regulated by frontostriatal systems and thus lead to the psychostimulant dependence. However, it can be acknowledged that such problems need to be inspected by genetic imaging or longitudinal imaging researches in persons prone to psychostimulant addiction [35,36].
Like other drug dependence [37,38], we also found reduced GM volume in BQD individuals in the superior temporal gyrus (STG) which has been implicated as a critical structure in social cognition [39,40] and shared certain functional repetition [41] especially connected to emotion [42]. In view of the STG proximity to the insula, their correlations and the remarkable volume decrease in the insula discovered by previous research, the outcome may not be surprising [43]. The discoveries during VBM study also offer evidence for the idea that neural networks linking the medial frontal cortex with temporal areas are greatly destroyed in BQD patients, which is consistent with previous study on cocaine users of fMRI connectivity analyses [30].
Right hippocampus volume was increased in our study, whereas most addiction studies showing decreased volume of hippocampus. BQ study has been reported to inhibit MAO-A activity [44] and elevate 5-HT and NA levels [45] in rats favoring anti-depressant-like properties. A. catechu alkaloids have been the focus of investigation, particularly arecoline which has been regarded as an active constituent that remains the basis of the majority of the biological behaviors caused by nut [46]. Thus, the BQ has great potential as lead for antidepressants drug discovery. Hippocampus is the key structures of emotion behaviors, especially in depression, so the increased volume of hippocampus may be underline the mechanism of the BQ anti-depression effects, however, this need further study.
Limitation
To our understanding, this research is the first VBM-investigation demonstrating an association of BQ dependence behavior with GM volume. The sufficient sample size, the reliable clinical characterization and the consistency between outcomes of various analytical methods (differences between groups and correlation regressions monitoring the connection between GM and clinical indexes, including BQD scores, illness duration) are all considered advantages of this research. From another aspect, we should also recognize several drawbacks. First of all, although it has been demonstrated in our outcomes that the GM alterations are possibly the result of BQ dependence, we cannot neglect the possible reason for the excessive use of BQ, which manages the difference in structure between the normal controls and BQ dependence. These cognitive brain areas relating to management in certain subjects have abnormal features and thus are relatively prone to develop BQ addiction. Therefore it is necessary to further consider the cause and effect issues with an all-inclusive experimental design in the future work. Nevertheless, we proposed that the current research results were the outcome of BQ dependence. Second, as for the connection between the volume alterations and duration time of BQ dependence, the years of BQ dependence is a general characterization in accordance with the memory of the BQD individuals. And such connections indicated that the declined GM volume of the left DLPFC, the right rACC had cumulative influences. Moreover, although depression and degrees of anxiety which failed to reach the cut-off for clinical significance had quite small influence on regression models if included, their effect can’t be fully excluded. Lastly, while the outcomes are discussed specific to BQD, excessive alcohol consumption is still extremely prevalent among BQD subjects. However, the carefully chose sample in the present study insured relative specificity of BQD correlative issues and low use of other medications. In the future, we will combine these discoveries in structure with resting state fMRI in BQD individuals.
Conclusion
It is suggested in our study that the alterations of brain structure exist in BQD patients and dysfunction of the reward system, cognitive system and emotion system may be a neurobiological basis for BQD patients. Overall, the GM volume alterations in current research demonstrated a microstructural change in the brain, which further promoted our understanding of BQD.
Acknowledgements
This study was supported by the grants from the Natural Science Foundation of China (Grant No.81260218 for Jianjun Li), Natural Science Foundation of Hainan Province (Grant No.812149 for Feng Chen, Grant No.813201 for Tao Liu), Key science and technology project of Hainan Province (Grant No.ZDXM20120047 for Jianjun Li), The Social Science development Foundation of Hainan province (Grant No.SF201312 for Feng Chen), Hainan Health Institution Project (Grant No.2012PT-06 for Tao Liu) and National clinical key subject construction project.
Disclosure of conflict of interest
All authors have agreed to the submission of this article in this form, and we do not have any conflict of interests that might be interpreted as influencing its content. The authors reported no biomedical financial interests or potential conflicts of interest.
References
- 1.Boucher BJ, Mannan N. Metabolic effects of the consumption of Areca catechu. Addict Biol. 2002;7:103–110. doi: 10.1080/13556210120091464. [DOI] [PubMed] [Google Scholar]
- 2.Gupta PC, Warnakulasuriya S. Global epidemiology of areca nut usage. Addict Biol. 2002;7:77–83. doi: 10.1080/13556210020091437. [DOI] [PubMed] [Google Scholar]
- 3.IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Betel-quid and areca-nut chewing and some areca-nut derived nitrosamines. IARC Monogr Eval Carcinog Risks Hum. 2004;85:1–334. [PMC free article] [PubMed] [Google Scholar]
- 4.Winstock A. Areca nut-abuse liability, dependence and public health. Addict Biol. 2002;7:133–138. doi: 10.1080/13556210120091509. [DOI] [PubMed] [Google Scholar]
- 5.Trivedy CR, Craig G, Warnakulasuriya S. The oral health consequences of chewing areca nut. Addict Biol. 2002;7:115–125. doi: 10.1080/13556210120091482. [DOI] [PubMed] [Google Scholar]
- 6.Lin CF, Wang JD, Chen PH, Chang SJ, Yang YH, Ko YC. Predictors of betel quid chewing behavior and cessation patterns in Taiwan aborigines. BMC Public Health. 2006;6:271. doi: 10.1186/1471-2458-6-271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ghani WM, Razak IA, Yang YH, Talib NA, Ikeda N, Axell T, Gupta PC, Handa Y, Abdullah N, Zain RB. Factors affecting commencement and cessation of betel quid chewing behaviour in Malaysian adults. BMC Public Health. 2011;11:82. doi: 10.1186/1471-2458-11-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee CH, Ko AM, Warnakulasuriya S, Ling TY, Sunarjo , Rajapakse PS, Zain RB, Ibrahim SO, Zhang SS, Wu HJ, Liu L, Kuntoro , Utomo B, Warusavithana SA, Razak IA, Abdullah N, Shrestha P, Shieh TY, Yen CY, Ko YC. Population burden of betel quid abuse and its relation to oral premalignant disorders in South, Southeast, and East Asia: an Asian Betel-quid Consortium Study. Am J Public Health. 2012;102:e17–24. doi: 10.2105/AJPH.2011.300521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Giri S, Idle JR, Chen C, Zabriskie TM, Krausz KW, Gonzalez FJ. A metabolomic approach to the metabolism of the areca nut alkaloids arecoline and arecaidine in the mouse. Chem Res Toxicol. 2006;19:818–827. doi: 10.1021/tx0600402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Benegal V, Rajkumar RP, Muralidharan K. Does areca nut use lead to dependence? Drug Alcohol Depend. 2008;97:114–121. doi: 10.1016/j.drugalcdep.2008.03.016. [DOI] [PubMed] [Google Scholar]
- 11.Chandra PS, Carey MP, Carey KB, Jairam KR. Prevalence and correlates of areca nut use among psychiatric patients in India. Drug Alcohol Depend. 2003;69:311–316. doi: 10.1016/s0376-8716(02)00329-0. [DOI] [PubMed] [Google Scholar]
- 12.Mubeen K, Kumar CN, Puja R, Jigna VR, Chandrashekar H. Psychiatric morbidity among patients with oral sub-mucous fibrosis: a preliminary study. J Oral Pathol Med. 2010;39:761–764. doi: 10.1111/j.1600-0714.2010.00948.x. [DOI] [PubMed] [Google Scholar]
- 13.Bhat SJ, Blank MD, Balster RL, Nichter M. Areca nut dependence among chewers in a South Indian community who do not also use tobacco. Addiction. 2010;105:1303–1310. doi: 10.1111/j.1360-0443.2010.02952.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee CY, Chang CS, Shieh TY, Chang YY. Development and validation of a self-rating scale for betel quid chewers based on a male-prisoner population in Taiwan: the Betel Quid Dependence Scale. Drug Alcohol Depend. 2012;121:18–22. doi: 10.1016/j.drugalcdep.2011.07.027. [DOI] [PubMed] [Google Scholar]
- 15.Abrams DB, Niaura RS. Social learning theory. In: Blane HT, Leonard KE, editors. Psychological theories of drinking and alcoholism. New York: Guilford Press; 1987. pp. 181–226. [Google Scholar]
- 16.Brandon TH, Juliano LM, Copeland AL. Expectancies for tobacco smoking. In: Kirsch I, editor. How expectancies shape experience. Washington, DC: American Psychological Association; 1999. pp. 263–299. [Google Scholar]
- 17.Sussman SY. In: Drug abuse: concepts, prevention, and cessation. Ames SL, editor. Cambridge: Cambridge University Press; 2008. [Google Scholar]
- 18.Ashby FG, Turner BO, Horvitz JC. Cortical and basal ganglia contributions to habit learning and automaticity. Trends Cogn Sci. 2010;14:208–215. doi: 10.1016/j.tics.2010.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Woollett K, Maguire EA. Acquiring “the Knowledge” of London’s layout drives structural brain changes. Curr Biol. 2011;21:2109–2114. doi: 10.1016/j.cub.2011.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gaser C, Schlaug G. Brain structures differ between musicians and non-musicians. J Neurosci. 2003;23:9240–9245. doi: 10.1523/JNEUROSCI.23-27-09240.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Draganski B, Gaser C, Busch V, Schuierer G, Bogdahn U, May A. Neuroplasticity: changes in grey matter induced by training. Nature. 2004;427:311–312. doi: 10.1038/427311a. [DOI] [PubMed] [Google Scholar]
- 22.Herzog TA, Murphy KL, Little MA, Suguitan GS, Pokhrel P, Kawamoto CT. The Betel Quid Dependence Scale: replication and extension in a Guamanian sample. Drug Alcohol Depend. 2014;138:154–160. doi: 10.1016/j.drugalcdep.2014.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Drevets WC, Price JL, Furey ML. Brain structural and functional abnormalities in mood disorders: implications for neurocircuitry models of depression. Brain Struct Funct. 2008;213:93–118. doi: 10.1007/s00429-008-0189-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zatorre RJ, Fields RD, Johansen-Berg H. Plasticity in gray and white: neuroimaging changes in brain structure during learning. Nat Neurosci. 2012;15:528–536. doi: 10.1038/nn.3045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhou Y, Lin FC, Du YS, Qin LD, Zhao ZM, Xu JR, Lei H. Gray matter abnormalities in Internet addiction: a voxel-based morphometry study. Eur J Radiol. 2011;79:92–95. doi: 10.1016/j.ejrad.2009.10.025. [DOI] [PubMed] [Google Scholar]
- 26.Yuan K, Qin W, Wang G, Zeng F, Zhao L, Yang X, Liu P, Liu J, Sun J, von Deneen KM, Gong Q, Liu Y, Tian J. Microstructure abnormalities in adolescents with internet addiction disorder. PLoS One. 2011;6:e20708. doi: 10.1371/journal.pone.0020708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Barber AD, Carter CS. Cognitive control involved in overcoming prepotent response tendencies and switching between tasks. Cereb Cortex. 2005;15:899–912. doi: 10.1093/cercor/bhh189. [DOI] [PubMed] [Google Scholar]
- 28.Leland DS, Arce E, Feinstein JS, Paulus MP. Young adult stimulant users’ increased striatal activation during uncertainty is related to impulsivity. Neuroimage. 2006;33:725–731. doi: 10.1016/j.neuroimage.2006.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Botvinick MM, Braver TS, Barch DM, Carter CS, Cohen JD. Conflict monitoring and cognitive control. Psychol Rev. 2001;108:624–652. doi: 10.1037/0033-295x.108.3.624. [DOI] [PubMed] [Google Scholar]
- 30.Gu H, Salmeron BJ, Ross TJ, Geng X, Zhan W, Stein EA, Yang Y. Mesocorticolimbic circuits are impaired in chronic cocaine users as demonstrated by resting-state functional connectivity. Neuroimage. 2010;53:593–601. doi: 10.1016/j.neuroimage.2010.06.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ko CH, Liu GC, Hsiao S, Yen JY, Yang MJ, Lin WC, Yen CF, Chen CS. Brain activities associated with gaming urge of online gaming addiction. J Psychiatr Res. 2009;43:739–747. doi: 10.1016/j.jpsychires.2008.09.012. [DOI] [PubMed] [Google Scholar]
- 32.Ko CH, Hsiao S, Liu GC, Yen JY, Yang MJ, Yen CF. The characteristics of decision making, potential to take risks, and personality of college students with Internet addiction. Psychiatry Res. 2010;175:121–125. doi: 10.1016/j.psychres.2008.10.004. [DOI] [PubMed] [Google Scholar]
- 33.Everitt BJ, Dickinson A, Robbins TW. The neuropsychological basis of addictive behaviour. Brain Res Brain Res Rev. 2001;36:129–138. doi: 10.1016/s0165-0173(01)00088-1. [DOI] [PubMed] [Google Scholar]
- 34.Carlson JM, Foti D, Harmon-Jones E, Proudfit GH. Midbrain volume predicts fMRI and ERP measures of reward reactivity. Brain Struct Funct. 2014 doi: 10.1007/s00429-014-0725-9. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 35.Kreek MJ, Bart G, Lilly C, LaForge KS, Nielsen DA. Pharmacogenetics and human molecular genetics of opiate and cocaine addictions and their treatments. Pharmacol Rev. 2005;57:1–26. doi: 10.1124/pr.57.1.1. [DOI] [PubMed] [Google Scholar]
- 36.Verdejo-Garcia A, Lawrence AJ, Clark L. Impulsivity as a vulnerability marker for substance-use disorders: review of findings from high-risk research, problem gamblers and genetic association studies. Neurosci Biobehav Rev. 2008;32:777–810. doi: 10.1016/j.neubiorev.2007.11.003. [DOI] [PubMed] [Google Scholar]
- 37.Franklin TR, Acton PD, Maldjian JA, Gray JD, Croft JR, Dackis CA, O’Brien CP, Childress AR. Decreased gray matter concentration in the insular, orbitofrontal, cingulate, and temporal cortices of cocaine patients. Biol Psychiatry. 2002;51:134–142. doi: 10.1016/s0006-3223(01)01269-0. [DOI] [PubMed] [Google Scholar]
- 38.Sim ME, Lyoo IK, Streeter CC, Covell J, Sarid-Segal O, Ciraulo DA, Kim MJ, Kaufman MJ, Yurgelun-Todd DA, Renshaw PF. Cerebellar gray matter volume correlates with duration of cocaine use in cocaine-dependent subjects. Neuropsychopharmacology. 2007;32:2229–2237. doi: 10.1038/sj.npp.1301346. [DOI] [PubMed] [Google Scholar]
- 39.Bigler ED, Mortensen S, Neeley ES, Ozonoff S, Krasny L, Johnson M, Lu J, Provencal SL, McMahon W, Lainhart JE. Superior temporal gyrus, language function, and autism. Dev Neuropsychol. 2007;31:217–238. doi: 10.1080/87565640701190841. [DOI] [PubMed] [Google Scholar]
- 40.Jou RJ, Minshew NJ, Keshavan MS, Vitale MP, Hardan AY. Enlarged right superior temporal gyrus in children and adolescents with autism. Brain Res. 2010;1360:205–212. doi: 10.1016/j.brainres.2010.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Paulus MP, Feinstein JS, Leland D, Simmons AN. Superior temporal gyrus and insula provide response and outcome-dependent information during assessment and action selection in a decision-making situation. Neuroimage. 2005;25:607–615. doi: 10.1016/j.neuroimage.2004.12.055. [DOI] [PubMed] [Google Scholar]
- 42.Critchley HD, Wiens S, Rotshtein P, Ohman A, Dolan RJ. Neural systems supporting interoceptive awareness. Nat Neurosci. 2004;7:189–195. doi: 10.1038/nn1176. [DOI] [PubMed] [Google Scholar]
- 43.Weller RE, Stoeckel LE, Milby JB, Bolding M, Twieg DB, Knowlton RC, Avison MJ, Ding Z. Smaller regional gray matter volume in homeless african american cocaine-dependent men: a preliminary report. Open Neuroimag J. 2011;5:57–64. doi: 10.2174/1874440001105010057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dar A, Khatoon S, Rahman G, Atta Ur R. Anti-depressant activities of Areca catechu fruit extract. Phytomedicine. 1997;4:41–45. doi: 10.1016/S0944-7113(97)80026-8. [DOI] [PubMed] [Google Scholar]
- 45.Abbas G, Naqvi S, Erum S, Ahmed S, Atta ur R, Dar A. Potential antidepressant activity of Areca catechu nut via elevation of serotonin and noradrenaline in the hippocampus of rats. Phytother Res. 2013;27:39–45. doi: 10.1002/ptr.4674. [DOI] [PubMed] [Google Scholar]
- 46.Nelson BS, Heischober B. Betel nut: a common drug used by naturalized citizens from India, Far East Asia, and the South Pacific Islands. Ann Emerg Med. 1999;34:238–243. doi: 10.1016/s0196-0644(99)70239-8. [DOI] [PubMed] [Google Scholar]