Abstract
Background: Cisplatin (CDDP) is employed to enhance radiotherapy’s (RT) effect for various cancers. However, the effects of local RT on chemotherapeutics in the plasma and lymphatic system remain unclear. Here, we evaluated the influence of pelvic irradiation on the pharmacokinetics (PK) of CDDP using rats as an experimental model. Methods and Materials: RT with 2 Gy and 0.5 Gy were delivered to the whole pelvis of Sprague-Dawley rats. CDDP at 5 mg/kg and 10 mg/kg was intravenously infused 24 hours after radiation for the plasma and lymphatic system, respectively. The pharmacokinetics of CDDP in the plasma and lymphatic system were calculated. Results: Compared with sham-irradiated controls, the whole pelvic irradiation increased the area under the concentration versus time curve (AUC) of CDDP (5 mg/kg) in the plasma by 80% at 0.5 Gy and 87% at 2 Gy, respectively. In contrast, the AUC of CDDP decreased in bile by 13% at both dose levels. Intriguingly, RT could also increase the AUC of CDDP (10 mg/kg) in the lymphatic fluid by 87% at 2 Gy. In addition, the AUC in CDDP without and with RT was 2.8-fold and 3.4-fold greater for the lymph system than for the plasma, respectively. Conclusions: A local pelvic RT could modulate the systemic PK of CDDP in both the plasma and lymphatic fluids of the rats. The RT-PK phenomena are worth further investigation.
Keywords: Cisplatin (CDDP), concurrent chemoradiation therapy (CCRT), pharmacokinetics, radiosensitivity, RT-PK
Introduction
Cisplatin (CDDP) is broadly used concurrent with or without radiotherapy (RT) for the treatment of various malignant tumors and increase the relative radiosensitivity [1], inhibits sublethal damage repair [2], arrests the G2 phase of the cell cycle thereby inducing cell death [3], strengthen the killing effect of radiation [4,5] and suppresses tumor neovascularization [6].
Lymph nodes (LN) metastasis and the number of LN metastases are associated with an increased risk of disease spread in gynecologic malignancies [7,8]. Concurrent chemoradiation therapy (CCRT) improves the survival of patients with locally advanced cervical cancer better than chemotherapy or RT alone [9-13] and contributed to a reduction in both local and distant recurrence [11,14,15].
RT is traditionally considered as a local treatment [16]. However, our previous study showed that local pelvic irradiation could significantly modulate the systemic pharmacokinetics (PK) of fluorouracil (5-FU) at both low dose and daily treatment dose [17] through matrix metalloproteinase-8 [18]. RT-PK phenomena have also been noted in head and neck irradiation with 5-FU and CDDP [19].
In the present study, we investigated the pharmacokinetics of CDDP in the plasma and lymphatic systems of rats, using pelvic RT to determine whether pelvic RT plays an important role in modulating the PK of the CDDP for the plasma and lymphatic system potentially contributing to local control and metastatic prevention.
Methods and materials
Materials and reagents
The CDDP and high-performance liquid chromatography (HPLC)-grade methanol were purchased from Sigma Chemicals (St. Louis, MO, USA) and Tedia Company, Inc. (Fairfield, OH, USA), respectively. Milli-Q grade (Millipore, Bedford, MA, USA) water was used for the preparation of the solutions and mobile phases.
Animals and sample preparation
Adult, male Sprague-Dawley rats (300 ± 20 g body weight) were provided by the Laboratory Animal Center at National Yang-Ming University (Taipei, Taiwan). The surgical and experimental protocols involving animals were reviewed and approved by the Institutional Animal Care and Use Committee of National Yang-Ming University. They were housed in a specific pathogen-free environment and had free access to food (Laboratory Rodent Diet 5001, PMI Nutrition International LLC, St. Louis, MO, USA) and water.
Both the animals and samples were prepared according to our previous reports [18,19]. Briefly, the rats were anesthetized with urethane 1 g/ml and α-chloralose 0.1 g/ml (1 ml/ kg, intraperitoneal injection), and were immobilized on a board to undergo computed tomography for simulation of the whole pelvic field [18]. The cranial margin was set at the top of bilateral iliac crest for the whole pelvic field. Conventional radiotherapy was used to deliver the radiation dose with anterior-posterior (AP) - PA portals. The experimental animals were randomized into the control (0 Gy), 0.5, and 2 Gy groups. Each group’s data was collected from 6 rats.
We chose 0.5 and 2 Gy for the rats to simulate the relevant safe and workable dose range for daily treatment of the human torso as described in our previous report [17]. The LD50 of CDDP for rats with oral, intraperitoneal, subcutaneous, and intravenous is 25, 7, 8 and 11 mg/kg, respectively [20]. Thus, we chose 50% of the LD50 of intravenous CDDP, 5 mg/kg, as a feasible dose in rats for examination of the plasma with CDDP initially. Thereafter, intravenous CDDP with 10 mg/kg was used for the lymphatic examination.
For the collection of lymphatic fluid and blood, the rats were given 2 mL of olive oil by oral gavage 30 min before operation, and then anaesthetized with urethane (1 g/kg) intraperitoneally. Surgical sites were shaved and disinfected with 70% ethanol solution, and polyethylene tubes (PE50) were then implanted into the right jugular vein and left carotid artery for intravenous infusion (normal saline, 2 mL/h) and blood sampling, respectively.
The procedure of mesenteric lymph vessel cannulation was performed as previously reported with some modifications [21]. Briefly, a midline laparotomy was performed from the xiphoid, the intestinal mass was displaced with gauze, and the wound was retracted by a 3-0 suture. Mesenteric lymph vessels were easily identified, since they contain white lymph. The mesenteric lymph duct was isolated by teasing away the surrounding tissue with a cotton swab. A small cut was made with a needle, and a silicone tubing (10 cm in length) was inserted into the mesenteric lymph duct. A drop of tissue cement was applied to the hole in the lymph duct to seal it and to fix the cannula in place. The lymph was collected in heparinized Eppendorf tubes at 30-minute intervals.
A 150-μL blood sample was withdrawn from the jugular vein with a fraction collector according to a programmed schedule at 5, 15, 30, 45, and 60 min, and 1.5, 2, 2.5, and 3 h following drug administration. The blood samples were immediately centrifuged at 3300 × g for 10 min. The resulting plasma (50 μL) was added to 1 mL of ethyl acetate in a clean tube, vortexed for 5 min, and centrifuged at 5900 × g for 10 min. After centrifugation, the upper organic layer containing the ethyl acetate was transferred to a new tube and evaporated to dryness under flowing nitrogen. The dried residue was reconstituted with 50 μL of Milli-Q water (Millipore). A 20-μL aliquot of the solution was injected into the high performance liquid chromatography-ultraviolet (HPLC-UV) detection system.
Liquid chromatography
The HPLC system consisted of a chromatographic pump (LC-20AT, Shimadzu, Kyoto, Japan), autosampler (SIL-20AT, Shimadzu), diode array detector (SPD-M20A, Shimadzu), and degasser (DG-240). A reversed-phase C18 column (4.6 × 250 mm, particle size 5 μm, Eclipse XDB, Agilent Technologies, Santa Clara, CA, USA) was used for the HPLC separation. The mobile phase was composed of acetonitrile - 10 mM monosodium phosphate (pH 3.0 adjusted by orthophosphoric acid) (70:30, v/v) at a flow rate of 1.0 ml/min. The chromatographic run time was 13 min and the detection wavelength was set at 254 nm. The mobile phase was filtered through a 0.45 μm Millipore membrane filter and degassed by sonication 2510R-DTH (Branson Ultrasonics, Danbury, CT, USA) before use. The stock solution of CDDP in 50% acetonitrile (500 μg/ml) was diluted with 50% acetonitrile to make serial concentrations of the working standard solutions (1, 5, 10, 50, and 100 μg/ml). The plasma was separated by centrifuging the blood sample at 6000 rpm for 10 min at 4°C. Calibration standards were prepared using 5 μl of the working standard solution spik with 45 μl of blank plasma and bile, and then adding 10 μl of freshly prepared 10% diethyldithiocarbamate in a solution of 0.2 N sodium hydroxide into each sample. These samples were put into a water bath at 45°C for 30 min to form the derivatization of CDDP.
The calibration curves were represented by the peak areas ratio of the CDDP to the internal standard spiked in the blank samples vs. the concentration of CDDP. The limits of detection (LOD) and the limit of quantification (LOQ) were defined as a signal-to-noise ratio of 3 and the lowest concentration of the linear regression, respectively. The 0.01-μg/mL limit of quantification was defined as the lowest concentration on the calibration curve that could be measured routinely with acceptable bias and relative SD.
Pharmacokinetics and data analysis
Pharmacokinetic parameters such as the area under the concentration versus time curve (AUC), terminal elimination phase half-life (t1/2), maximum observed plasma concentration (Cmax), mean residence time (MRT), total plasma clearance (CL), volume of distribution at steady state (Vss), and the elimination constant (Kel) were calculated by the pharmacokinetics calculation software WinNonlin Standard Edition, Version 1.1 (Scientific Consulting, Apex, NC, USA) using a compartmental method.
Statistical methods
The results are presented as means ± standard deviations. Differences in actuarial outcomes between the groups were calculated using one-way analysis of variance (ANOVA), with post hoc multiple comparisons. All analyses were performed using SPSS, version 20.0 (SPSS, IBM, Armonk, NY, USA).
Results
HPLC method validation
There was no interference under the present analytical conditions during the retention time of the CDDP. The peak of the CDDP was well separated and there was no endogenous interference in the rat plasma and lymphatic fluid samples (Figure 1). The selectivity was tested by chromatograms of blank plasma and lymphatic fluid spiked with CDDP standard. Good linearity was achieved in the range of 0.1-10 µg/mL, with all coefficients of correlation greater than 0.995.
Figure 1.
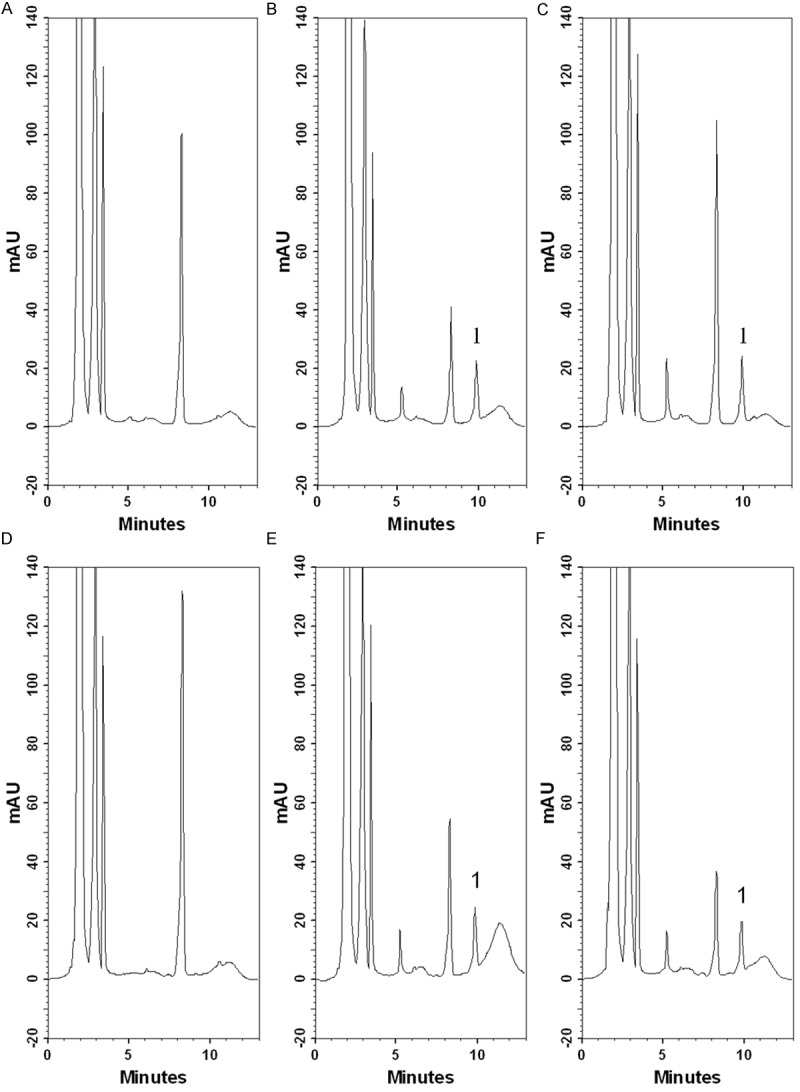
Representative chromatograms of cisplatin in rat plasma and lymphatic fluid. A. blank rat plasma; B. a blank rat plasma sample spiked with cisplatin (10 μg/mL); C. real rat plasma sample containing cisplatin (11 μg/mL) collected at 5 min after cisplatin administration (10 mg/kg, i.v.); D. blank rat lymphatic fluid; E. a blank rat lymphatic fluid sample spiked with cisplatin (10 μg/mL); F. real rat lymphatic fluid sample containing cisplatin (9.87 μg/mL) collected from 60 to 90 min after cisplatin administration (10 mg/kg, i.v.). 1: cisplatin.
Intraday and interday precision (% RSD) and accuracy (% Bias) were determined by repeated analysis of six lots of biological samples spiked with different concentrations of CDDP on the same day and six consecutive days, respectively. Precision and accuracy are presented in Table 1. The ranges of intraday precision and accuracy in the rat plasma vs. the lymphatic fluid were 0.11% to 10.33% and -5.92% to 2.97% vs. 0.51% to 11.71% and -4.02% to 11.61%, respectively. The interday precision and accuracy ranged from 0.12% to 8.57% and -2.71% to 6.47% vs. 0.42% to 13.67% and -3.40% to 8.81%, respectively.
Table 1.
Intra- and Inter-day precision (% RSD) and accuracy (% Bias) of the HPLC-UV method for determination of cisplatin in rat plasma and lymphatic fluid (5 days, 5 replicates per day)
| Intraday | Interday | ||||||
|---|---|---|---|---|---|---|---|
|
|
|||||||
| Matrices | Nominal concentration (µg/mL) | Observed concentration (ng/mL) | Precision (% RSD) | Accuracy (% Bias) | Observed concentration (ng/mL) | Precision (% RSD) | Accuracy (% Bias) |
| Plasma | 0.5 | 0.50 ± 0.05 | 10.33 | -0.68 | 0.53 ± 0.05 | 8.57 | 6.47 |
| 1 | 0.94 ± 0.05 | 5.67 | -5.92 | 0.97 ± 0.04 | 3.73 | -2.71 | |
| 5 | 5.15 ± 0.21 | 4.02 | 2.97 | 5.02 ± 0.21 | 4.27 | 0.37 | |
| 10 | 9.87 ± 0.09 | 0.95 | -1.31 | 9.93 ± 0.15 | 1.52 | -0.67 | |
| 25 | 25.0 ± 0.03 | 0.11 | 0.10 | 25.0 ± 0.03 | 0.12 | 0.10 | |
| Lymphatic fluid | 0.5 | 0.56 ± 0.07 | 11.71 | 11.61 | 0.54 ± 0.07 | 13.67 | 8.81 |
| 1 | 1.07 ± 0.11 | 10.14 | 6.76 | 1.06 ± 0.12 | 10.99 | 5.57 | |
| 5 | 5.04 ± 0.20 | 4.06 | 0.71 | 5.01 ± 0.20 | 3.98 | 0.26 | |
| 10 | 9.60 ± 0.40 | 4.20 | -4.02 | 9.66 ± 0.36 | 3.68 | -3.40 | |
| 25 | 25.2 ± 0.13 | 0.51 | 0.59 | 25.1 ± 0.11 | 0.42 | 0.47 | |
Data expressed as mean ± SD.
Plasma pharmacokinetic parameters of CDDP with pelvic irradiation
Compared with the sham-irradiated controls, the whole pelvic irradiation increased the AUC of CDDP (5 mg/kg) in the plasma by 80% at 0.5 Gy and 87% at 2 Gy, respectively (Figure 2A). As shown in Table 2A, the pelvic irradiation significantly increased the T1/2, MRT, and Cmax, and by contrast, decreased the clearance value of the CDDP by 44.9% in the 0.5-Gy group and 46.6% in the 2-Gy group, respectively. There was no significant difference within the RT groups.
Figure 2.
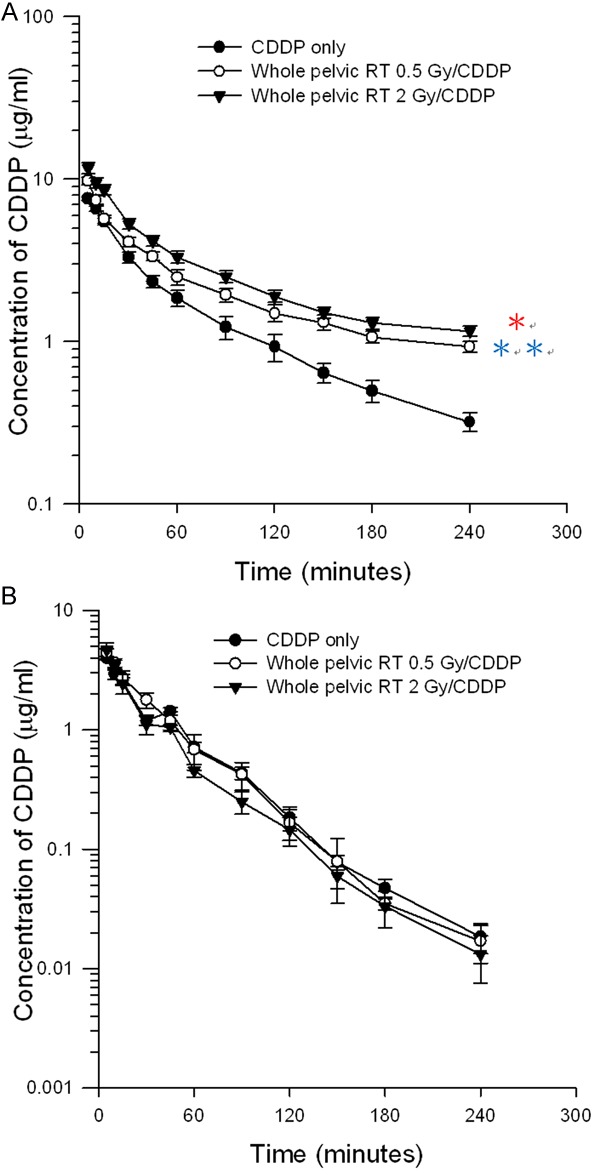
The concentration vs. time curves of cisplatin in rat (A) plasma and (B) bile with or without irradiation therapy (RT) 0.5 Gy and 2 Gy after cisplatin administration (5 mg/kg, i.v.). Data are expressed as mean ± SEM (n = 6).
Table 2.
Estimated pharmacokinetic parameters of cisplatin in rat plasma after cisplatin administration (5 mg/kg, i.v.) after pelvic irradiation with and without 0.5 and 2 Gy irradiation
| A. Pharmacokinetic parameters of cisplatin (CDDP) (5 mg/kg, i.v.) in ratplasma after pelvic irradiation with and without 0.5 and 2 Gy irradiation | ||||
|
| ||||
| Parameters | Control | Whole pelvic RT | Whole pelvic RT | |
|
| ||||
| 0 Gy | 0.5 Gy | 2 Gy | ||
|
| ||||
| AUC (min μg/ml ) | 438 ± 27.5 | 789 ± 31.3* | 819 ± 36.4* | |
| t1/2 (min) | 103 ± 25.0 | 182 ± 33.5* | 173 ± 20.2* | |
| Cmax (μg/ml) | 8.78 ± 0.32 | 11.9 ± 0.43* | 11.6 ± 0.89* | |
| CL (ml/kg/min) | 11.6 ± 0.68 | 6.39 ± 0.19* | 6.19 ± 0.30* | |
| MRT (min) | 102 ± 16.5 | 205 ± 28.4* | 193 ± 11.0* | |
|
| ||||
| B. Pharmacokinetic parameters of cisplatin (CDDP) (5 mg/kg, i.v.) in rat bile after pelvic irradiation with and without 0.5 and 2 Gy | ||||
|
| ||||
| Parameters | Control | Whole pelvic RT | Whole pelvic RT | |
|
| ||||
| 0 Gy | 0.5 Gy | 2 Gy | ||
|
| ||||
| AUC (min μg/ml ) | 141 ± 11.9 | 123 ± 12.5 | 122 ± 10.5 | |
| t1/2 (min) | 42.7 ± 4.7 | 33.6 ± 5.77 | 34.2 ± 3.77 | |
| Cmax (μg/ml) | 4.01 ± 0.25 | 4.43 ± 0.58 | 4.53 ± 0.77 | |
| CL (ml/kg/min) | 36.2 ± 4.67 | 41.0 ± 4.16 | 41.4 ± 3.17 | |
| MRT (min) | 43.5 ± 3.9 | 38.2 ± 4.18 | 36.0 ± 4.05 | |
Data expressed as mean ± standard error of the mean (n = 6). t1/2: half-life; Cmax: the maximum plasma concentration; AUC: area under the concentration-time curve; CL: total plasma clearance; MRT: mean residence time.
p < 0.05: significantly different from the control group.
Bile pharmacokinetic parameters of CDDP and whole pelvic irradiation
Pelvic irradiation decreased the AUC of the CDDP in the bile of the rats by 13% at both dose levels (Figure 2B). There were no statistically significant differences for the AUC, Cmax, CL, T1/2, and MRT, when the RT 0.5-Gy and 2-Gy groups were compared to the sham-irradiated controls (Table 2B).
Plasma and lymphatic fluid pharmacokinetic parameters of CDDP with pelvic irradiation
Intriguingly, RT could also increase the AUC of the CDDP (10 mg/kg) in the lymphatic fluid by 87% at 2 Gy (Figure 3). The AUC in the CDDP (10 mg/kg) without and with RT was 2.8-fold and 3.4-fold greater for the lymph than for the plasma, respectively. In addition, pelvic irradiation significantly increased the Cmax by 67.7% in the plasma and 77.8% in the lymphatic fluid when compared with the non-RT sham group. RT decreased the CL of the CDDP in the plasma by 32.0% and lymphatic system by 46.8% (Table 3).
Figure 3.
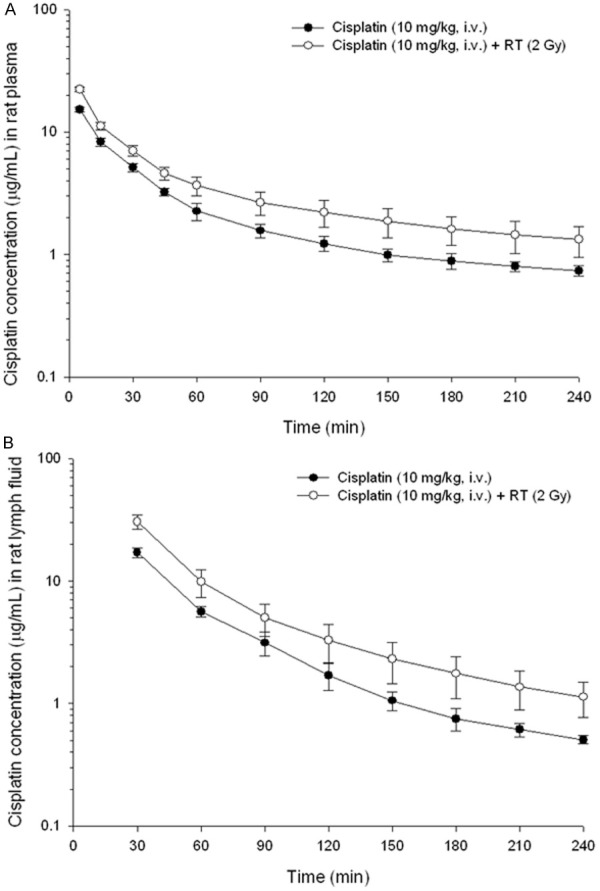
The concentration vs. time curves of cisplatin in rat (A) plasma and (B) lymphatic fluid with or without irradiation therapy (RT) after cisplatin administration (10 mg/kg, i.v.). Data are expressed as mean ± SEM (n = 6).
Table 3.
Estimated pharmacokinetic parameters of cisplatin in rat plasma and lymphatic fluid after cisplatin administration (10 mg/kg, i.v.)
| Parameters | Plasma | Lymphatic fluid | ||
|---|---|---|---|---|
|
|
||||
| Without RT | With RT 2Gy | Without RT | With RT 2Gy | |
| AUC (min µg/mL) | 621 ± 47.7 | 956 ± 125* | 1718 ± 201 | 3214 ± 424* |
| T (min) | 140 ± 28.3 | 131 ± 16.1 | 71.2 ± 15.4 | 93.7 ± 14.1 |
| Cmax (µg/mL) | 15.3 ± 0.62 | 22.3 ± 0.84* | 17.1 ± 1.64 | 30.4 ± 4.05* |
| CL (mL/min/kg) | 13.7 ± 1.79 | 9.31 ± 1.40 | 6.09 ± 0.79 | 3.24 ± 0.40* |
| MRT (min) | 55.8 ± 2.23 | 59.4 ± 6.03 | 32.4 ± 2.9 | 30.2 ± 4.97 |
| Vss (mL/kg) | 1701 ± 162 | 1113 ± 126* | 272 ± 56.4 | 138 ± 23.3 |
Data expressed as mean ± SEM (n = 6). AUC, area under the concentration vs. time curve; t1/2, elimination half-life; Cmax, the peak plasma concentration of a drug after administration; CL, total body clearance; MRT, mean residence time; Vss, volume of distribution.
significantly different from without RT group at p < 0.05.
Discussion
The HPLC-UV detection method with good linearity was achieved and there was no interference under the present analytical conditions of CDDP in the plasma and lymphatic fluid (Figure 1). The accuracy and precision of the concentrations in intra-day and inter-day were all acceptable (Table 1). The results suggested that the analytical method was repeatable and reliable. Further, the original form of CDDP could be detected in the mesenteric lymphatics as in the plasma with or without RT suggesting that intravenous injection of CDDP can pass from the blood into the lymphatic system.
The benefits of concomitant platinum-based regimen chemotherapy with RT have been proved for cervical patients with stage IB to IVA by a number of randomized prospective trials such as the Gynecologic Oncology Group (GOG)-85 [9], GOG-120 [10], GOG-123 [11], Radiation Therapy Oncology Group (RTOG 90-01) [12], and Southwest Oncology Group (SWOG) 8797 [13]. GOG-120 further demonstrated that weekly intravenous cisplatin 40 mg/m2 for 6 weeks in combination with RT is more convenient, equally efficacious, and less toxic than other cisplatin regimens or combinations using 5-FU and/or hydroxyurea [10], and has been established as a new standard for the treatment of locally advanced cervical carcinoma [22].
However, the designs of most clinical trials are according to the hypothesis of the radiosensitization [1] and direct tumor cytotoxicity [5] of the CDDP in the concurrent treatment. In our previous studies, the interactions between local irradiation and 5-FU were not about radiosensitization of chemotherapy agents to RT, but about the RT modulating the functionality of 5-FU [17-19]. Intriguingly, the AUC of the CDDP were also modulated by whole pelvic irradiation, increasing 80% and 87% at 0.5 Gy and 2 Gy, respectively (Figure 2). The modulating function of pelvic RT in the RT-PK phenomena is not only noted in 5-FU [17,18], but also in CDDP. The RT-PK phenomena of CDDP could provide some clues to the better results of platinum-based CCRT for cervical cancer treatment.
GOG-120 showed that weekly CDDP or a combination of a platinum and 5-FU regimen with RT is better than hydroxyurea [10]. However, the possibility of a synergistic effect between cisplatin and fluorouracil is not clear. It has been hypothesized that, as an RT sensitizer, 5-FU interferes with the repair of radiation-induced lesions and potentially increases RT sensitivity [23]. Thomas et al. [24] investigated the combination of radiation and 5-FU infusion and found a high complete response rate. However, a further randomized trial of 5-FU concurrent with RT for cervical cancer found that overall survival and pelvic control was not statistically improved [25]. Recently, a randomized trial compared protracted venous infusion (PVI) 5-FU with weekly cisplatin and found PVI FU does not demonstrate statistical improvement over weekly CDDP as a radiosensitizer [14].
According to the RT-PK phenomena, local pelvic irradiation with 2 Gy reduces the AUC of 5-FU in rats by 21.5% to 31.7% [17,18] and increases the AUC of CDDP by 87%, respectively. There are drug-drug interactions for same drug to different compounds with different results such as Alisporivir, a potent anti-hepatitis C virus molecule. The AUC of Alisporivir could be increased by 9.4-fold and decreased by 86% when Alisporivir is co-administered with ketoconazole or rifampin, respectively [26]. Similarly, in the current study, the interactions for pelvic irradiation to the AUC of CDDP are the opposite of the AUC of 5-FU [17,18]. This may explain why weekly cisplatin or platinum-based regimen concurrent with RT is a better choice for cervical cancer [10] and why 5-FU concurrent with RT for cervical cancer, with its lack of improved overall survival or a better pelvic control rate [25], and PVI FU do not show more benefits than weekly CDDP [14].
The long-term results of RTOG 90-01 showed adding platinum-based regimens to RT could reduce the risk of locoregional failure and distant metastases from cervical carcinoma by 7% and 15%, respectively [15]. In the GOG-123 trial, the risk of recurrence and death was significantly reduced by the lower rate of relapses in the pelvic region in the weekly CDDP combined-therapy group for bulky stage IB cervical cancers in comparison to RT alone [11]. Additionally, distant failure (including abdominal, para-aortic region, bone, liver, and lung) was higher in the PVI FU group (29% vs. 18%) than the CDDP group in the GOG-165 trial [14].
Radiotherapy may cause endothelial cell loss [27] and hypertrophy of the surviving endothelial cells [28] that may impair the vascular and lymphatic systems and enhance vascular permeability. Nevertheless, little is understood about the influence of a daily RT dose for antineoplastic agents in the lymphatic system. Interestingly, RT could also increase the AUC of CDDP in the lymphatic fluid by 87% at 2 Gy (Figure 3). Additionally, the AUC in CDDP was 3.4-fold greater for the lymphatic system than for the plasma in the RT group but was 2.8-fold in the non-RT group, offering evidence that the distribution of CDDP in the lymphatic system could be enhanced by RT (Table 3). This RT-PK phenomena of CDDP in the lymphatic system may have some impact on the clinical practice of CCRT to decrease the regional and distant failure and merits further scientific investigation.
The percentage of adverse effects has been higher in the combined therapy than in the radiotherapy alone group. In GOG-123, the frequencies of transient moderate to severe hematologic and gastrointestinal adverse effects in the CCRT group vs. the RT group were 21% vs. 2% and 14% vs. 5%, respectively [11]. The moderate to severe hematologic and gastrointestinal adverse effects in the CCRT group were 17% as compared to only 1 % in the RT group in the phase III study [29]. In the current study, the AUC of CDDP after whole pelvic irradiation increased 80% and 87% at 0.5 Gy and 2 Gy, respectively. Both the daily practice dose and the off-target dose could increase the AUC of CDDP and provide the one of reasons to explain why the adverse effects look higher in the CCRT than the RT alone group.
There are some limitations in the current study. First, the role of MMP-8 as observed in abdominal and pelvic RT in modulating 5-FU pharmacokinetics is not proved in the current study. Whether this soluble factor has an impact on pelvic RT-modulated pharmacokinetics of CDDP remains to be determined. Second, this study is the effects of irradiation followed by chemotherapy with one-shot design rather than fractionation RT as daily practice. In clinical use of fractionated RT and periodically concurrent chemotherapy, the previous daily fraction of RT may modulate the PK of chemotherapeutics administered in the consecutive day. In the conducting clinical trial, we planned to validate this RT-modulated PK phenomenon in patients receiving CCRT with fractionated RT. Third, this study is the effects of irradiation followed by chemotherapy but concurrent chemotherapy with radiotherapy is the usual way that applied in the clinical practice. However, fluorouracil and cisplatin to radiotherapy within 16 hours after the first radiation fraction was administered that significantly improved the survival rate of women with locally advanced cervical cancer [15]. These data explain the importance of adding chemotherapy to RT but rather than time sequence of drug delivering. The findings for modulation of drug of PK by local RT, as demonstrated in previous and this study, may provide a clue and a research platform to clarify this controversy. Finally, the data of pharmacokinetics for cervical cancer patients during CCRT is not collected in the current study. Thus, the further study for RT-PK phenomena of the CDDP in cervical cancer patients is warranted in the future.
Conclusions
To our best knowledge, this is the first study to prove that pelvic irradiation can significantly modulate the systemic and lymphatic fluid pharmacokinetics of CDDP for both the target (2 Gy) and off-target areas (0.5 Gy). Pelvic RT also increases the distribution of CDDP in the lymphatic system based on our animal model. This study may provide a clue to understanding the unexplained biological enhancement of antineoplastic agents for improving locoregional control of locally advanced gynecologic cancer.
Acknowledgements
This work was supported by the Far Eastern Memorial Hospital grants (FEMH-2014-C-032; FEMH-2014-C-045; FEMH 101-2314-B-418 -010 -MY3) and the National Science Council (MOST 101-2314-B-418 -010 -MY3).
Disclosure of conflict of interest
The authors have declared that no conflict of interest exists.
References
- 1.Pawlik TM, Keyomarsi K. Role of cell cycle in mediating sensitivity to radiotherapy. Int J Radiat Oncol Biol Phys. 2004;59:928–942. doi: 10.1016/j.ijrobp.2004.03.005. [DOI] [PubMed] [Google Scholar]
- 2.Coughlin CT, Richmond RC. Biologic and clinical developments of cisplatin combined with radiation: concepts, utility, projections for new trials, and the emergence of carboplatin. Semin Oncol. 1989;16:31–43. [PubMed] [Google Scholar]
- 3.Sorenson CM, Eastman A. Mechanism of cis-diamminedichloroplatinum(II)-induced cytotoxicity: role of G2 arrest and DNA double-strand breaks. Cancer Res. 1988;48:4484–4488. [PubMed] [Google Scholar]
- 4.Britten RA, Evans AJ, Allalunis-Turner MJ, Pearcey RG. Effect of cisplatin on the clinically relevant radiosensitivity of human cervical carcinoma cell lines. Int J Radiat Oncol Biol Phys. 1996;34:367–374. doi: 10.1016/0360-3016(95)02088-8. [DOI] [PubMed] [Google Scholar]
- 5.Saxena A, Yashar C, Taylor DD, Gercel-Taylor C. Cellular response to chemotherapy and radiation in cervical cancer. Am J Obstet Gynecol. 2005;192:1399–1403. doi: 10.1016/j.ajog.2004.12.045. [DOI] [PubMed] [Google Scholar]
- 6.Yoshikawa A, Saura R, Matsubara T, Mizuno K. A mechanism of cisplatin action: antineoplastic effect through inhibition of neovascularization. Kobe J Med Sci. 1997;43:109–120. [PubMed] [Google Scholar]
- 7.Mundt AJ, McBride R, Rotmensch J, Waggoner SE, Yamada SD, Connell PP. Significant pelvic recurrence in high-risk pathologic stage I--IV endometrial carcinoma patients after adjuvant chemotherapy alone: implications for adjuvant radiation therapy. Int J Radiat Oncol Biol Phys. 2001;50:1145–1153. doi: 10.1016/s0360-3016(01)01566-8. [DOI] [PubMed] [Google Scholar]
- 8.Ditto A, Martinelli F, Lo Vullo S, Reato C, Solima E, Carcangiu M, Haeusler E, Mariani L, Lorusso D, Raspagliesi F. The role of lymphadenectomy in cervical cancer patients: the significance of the number and the status of lymph nodes removed in 526 cases treated in a single institution. Ann Surg Oncol. 2013;20:3948–3954. doi: 10.1245/s10434-013-3067-6. [DOI] [PubMed] [Google Scholar]
- 9.Whitney CW, Sause W, Bundy BN, Malfetano JH, Hannigan EV, Fowler WC Jr, Clarke-Pearson DL, Liao SY. Randomized comparison of fluorouracil plus cisplatin versus hydroxyurea as an adjunct to radiation therapy in stage IIB-IVA carcinoma of the cervix with negative para-aortic lymph nodes: a Gynecologic Oncology Group and Southwest Oncology Group study. J. Clin. Oncol. 1999;17:1339–1348. doi: 10.1200/JCO.1999.17.5.1339. [DOI] [PubMed] [Google Scholar]
- 10.Rose PG, Bundy BN, Watkins EB, Thigpen JT, Deppe G, Maiman MA, Clarke-Pearson DL, Insalaco S. Concurrent cisplatin-based radiotherapy and chemotherapy for locally advanced cervical cancer. N Engl J Med. 1999;340:1144–1153. doi: 10.1056/NEJM199904153401502. [DOI] [PubMed] [Google Scholar]
- 11.Keys HM, Bundy BN, Stehman FB, Muderspach LI, Chafe WE, Suggs CL 3rd, Walker JL, Gersell D. Cisplatin, radiation, and adjuvant hysterectomy compared with radiation and adjuvant hysterectomy for bulky stage IB cervical carcinoma. N Engl J Med. 1999;340:1154–1161. doi: 10.1056/NEJM199904153401503. [DOI] [PubMed] [Google Scholar]
- 12.Morris M, Eifel PJ, Lu J, Grigsby PW, Levenback C, Stevens RE, Rotman M, Gershenson DM, Mutch DG. Pelvic radiation with concurrent chemotherapy compared with pelvic and para-aortic radiation for high-risk cervical cancer. N Engl J Med. 1999;340:1137–1143. doi: 10.1056/NEJM199904153401501. [DOI] [PubMed] [Google Scholar]
- 13.Peters WA 3rd, Liu PY, Barrett RJ 2nd, Stock RJ, Monk BJ, Berek JS, Souhami L, Grigsby P, Gordon W Jr, Alberts DS. Concurrent chemotherapy and pelvic radiation therapy compared with pelvic radiation therapy alone as adjuvant therapy after radical surgery in high-risk early-stage cancer of the cervix. J. Clin. Oncol. 2000;18:1606–1613. doi: 10.1200/JCO.2000.18.8.1606. [DOI] [PubMed] [Google Scholar]
- 14.Lanciano R, Calkins A, Bundy BN, Parham G, Lucci JA 3rd, Moore DH, Monk BJ, O’Connor DM. Randomized comparison of weekly cisplatin or protracted venous infusion of fluorouracil in combination with pelvic radiation in advanced cervix cancer: a gynecologic oncology group study. J. Clin. Oncol. 2005;23:8289–8295. doi: 10.1200/JCO.2004.00.0497. [DOI] [PubMed] [Google Scholar]
- 15.Eifel PJ, Winter K, Morris M, Levenback C, Grigsby PW, Cooper J, Rotman M, Gershenson D, Mutch DG. Pelvic irradiation with concurrent chemotherapy versus pelvic and para-aortic irradiation for high-risk cervical cancer: an update of radiation therapy oncology group trial (RTOG) 90-01. J. Clin. Oncol. 2004;22:872–880. doi: 10.1200/JCO.2004.07.197. [DOI] [PubMed] [Google Scholar]
- 16.Elsasser T, Scholz M. Cluster effects within the local effect model. Radiat Res. 2007;167:319–329. doi: 10.1667/RR0467.1. [DOI] [PubMed] [Google Scholar]
- 17.Hsieh CH, Hsieh YJ, Liu CY, Tai HC, Huang YC, Shueng PW, Wu LJ, Wang LY, Tsai TH, Chen YJ. Abdominal irradiation modulates 5-Fluorouracil pharmacokinetics. J Transl Med. 2010;8:29. doi: 10.1186/1479-5876-8-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hsieh CH, Liu CY, Hsieh YJ, Tai HC, Wang LY, Tsai TH, Chen YJ. Matrix metalloproteinase-8 mediates the unfavorable systemic impact of local irradiation on pharmacokinetics of anti-cancer drug 5-Fluorouracil. PLoS One. 2011;6:e21000. doi: 10.1371/journal.pone.0021000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hsieh CH, Hou ML, Chiang MH, Tai HC, Tien HJ, Wang LY, Tsai TH, Chen YJ. Head and neck irradiation modulates pharmacokinetics of 5-fluorouracil and cisplatin. J Transl Med. 2013;11:231. doi: 10.1186/1479-5876-11-231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Prajda N, Kralovanszky J, Gal F, Kiss F, Kerpel-Fronius S. Evaluation of side effects of platinum complexes (CDDP, CBDCA, CHIP) on rat bone marrow. In Vivo. 1989;3:267–270. [PubMed] [Google Scholar]
- 21.Chen IL, Tsai YJ, Huang CM, Tsai TH. Lymphatic absorption of quercetin and rutin in rat and their pharmacokinetics in systemic plasma. J Agric Food Chem. 2010;58:546–551. doi: 10.1021/jf9026124. [DOI] [PubMed] [Google Scholar]
- 22.Rose PG. Chemoradiotherapy: the new standard care for invasive cervical cancer. Drugs. 2000;60:1239–1244. doi: 10.2165/00003495-200060060-00001. [DOI] [PubMed] [Google Scholar]
- 23.Byfield JE, Calabro-Jones P, Klisak I, Kulhanian F. Pharmacologic requirements for obtaining sensitization of human tumor cells in vitro to combined 5-Fluorouracil or ftorafur and X rays. Int J Radiat Oncol Biol Phys. 1982;8:1923–1933. doi: 10.1016/0360-3016(82)90451-5. [DOI] [PubMed] [Google Scholar]
- 24.Thomas G, Dembo A, Beale F, Bean H, Bush R, Herman J, Pringle J, Rawlings G, Sturgeon J, Fine S, et al. Concurrent radiation, mitomycin C and 5-fluorouracil in poor prognosis carcinoma of cervix: preliminary results of a phase I-II study. Int J Radiat Oncol Biol Phys. 1984;10:1785–1790. doi: 10.1016/0360-3016(84)90550-9. [DOI] [PubMed] [Google Scholar]
- 25.Thomas G, Dembo A, Ackerman I, Franssen E, Balogh J, Fyles A, Levin W. A randomized trial of standard versus partially hyperfractionated radiation with or without concurrent 5-fluorouracil in locally advanced cervical cancer. Gynecol Oncol. 1998;69:137–145. doi: 10.1006/gyno.1998.4990. [DOI] [PubMed] [Google Scholar]
- 26.Xia B, Barve A, Heimbach T, Zhang T, Gu H, Wang L, Einolf H, Alexander N, Hanna I, Ke J, Mangold JB, He H, Sunkara G. Physiologically based pharmacokinetic modeling for assessing the clinical drug-drug interaction of alisporivir. Eur J Pharm Sci. 2014;63:103–112. doi: 10.1016/j.ejps.2014.06.021. [DOI] [PubMed] [Google Scholar]
- 27.Heisel MA, Laug WE, Stowe SM, Jones PA. Effects of X-irradiation on artificial blood vessel wall degradation by invasive tumor cells. Cancer Res. 1984;44:2441–2445. [PubMed] [Google Scholar]
- 28.Rosen EM, Vinter DW, Goldberg ID. Hypertrophy of cultured bovine aortic endothelium following irradiation. Radiat Res. 1989;117:395–408. [PubMed] [Google Scholar]
- 29.Pearcey R, Brundage M, Drouin P, Jeffrey J, Johnston D, Lukka H, MacLean G, Souhami L, Stuart G, Tu D. Phase III trial comparing radical radiotherapy with and without cisplatin chemotherapy in patients with advanced squamous cell cancer of the cervix. J. Clin. Oncol. 2002;20:966–972. doi: 10.1200/JCO.2002.20.4.966. [DOI] [PubMed] [Google Scholar]


