Abstract
Genetic variants in pre-microRNA (miRNA) genes or the 3’UTR of miRNA target genes could influence miRNA-mediated regulation of gene expression and thus contribute to the susceptibility and prognosis of human diseases. This study aimed to investigate the effect of 6 miRNA-related polymorphisms (miR-149 rs71428439, miR-146a rs2910164, miR-499 rs3746444, miR-423 rs6505162, miR-4513 rs2168518, and FABP2 rs11724758) on prognosis in 1004 patients with angiographic coronary artery disease (CAD). We found that miR-4513 rs2168518 was associated with blood pressure, triglycerides, total cholesterol, and fasting glucose levels, and risk of diabetes mellitus. miR-499 rs3746444 and miR-423 rs6505162 were associated with blood pressure and HDL levels, respectively. Both miR-4513 rs2168518 and miR-499 rs3746444 had significant impact on even-free survival. Furthermore, miR-4513 rs2168518 was associated with higher morality in CAD patients. In conclusion, miR-4513 rs2168518 and miR-499 rs3746444 might be potential biomarkers for the clinical prognosis of CAD.
Keywords: Coronary artery disease, microRNA, polymorphism, lipid, cardiovascular event, mortality
Introduction
Despite major recent advances in prevention, diagnosis and treatment, cardiovascular disease remains the single leading cause of death in both developing and developed countries. Coronary artery disease (CAD) is a complex cardiovascular disease resulting from many risk factors, including genetic and environmental factors. Although the prognosis of CAD is good, some patients will suffer cardiovascular event or die in the following months or years. Identification of biomarkers that could discriminate CAD patients with high risk of cardiovascular event or die will improved patient outcomes and prolong survival.
MicroRNAs (miRNAs) are a class of conservative, small, single-strand, non-coding RNAs that regulate gene expression through degradation of target mRNAs or inhibition of translation [1,2]. There are 1881 miRNA precursors in human genome (miRBase database 21), which are further cleaved by Dicer ribonuclease to generate 2588 different mature miRNA [3]. Since miRNAs lack perfect complementarity to their mRNA targets, a particular miRNA can target hundreds of target genes [4]. It is estimated that miRNAs influence the expression of approximately 60% of human genes [5], and thereby these small RNAs have been suggested to play important roles in almost every biological process [6]. Numerous studies have clearly demonstrated that miRNAs are associated with a large variety of human diseases such as cancer [6,7] and cardiovascular diseases [8]. miRNAs are not only intimately involved in cardiac development, but also regulate cardiac regeneration, hypertrophy and remodeling [8,9]. Given the important role of miRNAs in cardiovascular diseases, they are being exploited for diagnosis, prevention, and treatment of cardiovascular diseases.
Genetic variants located in pre-miRNA genes and miRNA-binding sites in target genes may disrupt specific miRNA-mRNA target site interaction and thus result in the deregulation of target gene expression [10-12]. Accordingly, the corresponding phenotypes are likely to be affected. Given the central role of miRNAs as regulators of translation, genetic variants in miRNA genes and miRNA-binding sites may contribute to a wide range of phenotypic variation and disease susceptibility [10,12-15]. However, so far only very few genetic studies have attempted to identify single nucleotide polymorphisms (SNPs) in miRNA genes and binding sites which are associated with the prognosis of coronary artery disease (CAD). In the present study, we aimed to investigate the effect of miRNA-related SNPs on the prognosis of CAD. In addition, we also conducted a survey the association of these miRNA-related SNPs with cardiometabolic phenotypes.
Materials and methods
Patients
We included 1004 patients (710 men and 294 women) with angiographic CAD in the study for whom genomic DNA was available. Patients were recruited from hospitalized patients in Union Hospital (Fuzhou, China). To reduce the potential confounding from ethnic backgrounds, only subjects with self-reported origin of Chinese Han in Fujian province were enrolled. Patients with malignant disease, or sever hepatic or renal dysfunction were excluded from the study on the basis of clinical examinations. Diabetes mellitus (DM) was defined as a self-reported doctor’s diagnosis, fasting plasma glucose (FPG) ≥ 7.0 mmol/l, or use of antidiabetic medications. Hypertension (HP) was defined as systolic blood pressure (SBP) of 140 mmHg or higher, a diastolic blood pressure (DBP) of 90 mmHg or higher, or use of prescription anti-hypertensive medication. Written informed consent was obtained from all study participants, and the ethics committee of Union Hospital approved the study protocol.
Genotyping
Five miRNA (miR-149 rs71428439, miR-146a rs2910164, miR-499 rs3746444, miR-4513 rs2168518, and miR-423 rs6505162), and one binding site SNPs (FABP2 rs11724758) were selected from the literature. Genomic DNA was extracted from peripheral blood samples using the Universal Genomic DNA Extraction Kit (Takara, Dalian, China) according to manufacturer’s introductions. All patients were genotyped for these 5 miRNA-related SNPs. The genotypes of all 5 SNPs were determined using a polymerase chain reaction-based method, as previously described [14,16].
Study endpoint
The primary endpoint of the study was the first major cardiovascular event [myocardial infarction (MI), stroke, heart failure, recanalization, coronary artery bypass grafting, resuscitated cardiac arrest, and cardiovascular death]. Second endpoint was all-cause mortality.
Statistical analyses
All statistical analyses were performed using SPSS version 17.0 (SPSS, IL, USA). All analyses were performed using two-tailed tests for significance. The Hardy-Weinberg equilibrium was assessed by a chi-square test. All quantitative variables were expressed as mean ± standard deviation (SD), and qualitative variables were expressed as percentages. One-way ANOVA was conducted to compare the means of plasma lipids, blood pressure, and fasting plasma glucose by SNPs. Chi-square test was used to examine the potential difference across groups in case of categorical data. Logistic regression model was performed to calculate odds ratios (ORs) and 95% confidence intervals (CIs). Survival analysis was performed using the Kaplan-Meier method and the log-rank test. Cox regression analyses were performed to determine whether miRNA-related SNPs were independently associated with cardiovascular event or all-cause mortality. Dominant, codominant, recessive and additive models of inheritance were used for survival analyses. A two-sided P < 0.05 was considered statistically significant.
Results
Baseline clinical characteristics according to genotypes
The genotype distributions of 5 SNPs (miR-149 rs71428439, miR-146a rs2910164, miR-499 rs3746444, miR-423 rs6505162, and miR-4513 rs2168518) were in agreement with Hardy-Weinberg equilibrium (P > 0.05), whereas FABP2 rs11724758 deviated from Hardy-Weinberg equilibrium (P < 0.05), which was excluded from further analysis. As shown in Table 1, miR-4513 rs2168518 was significantly associated with DM (P = 0.014). The T allele and TT genotype of miR-4513 rs2168518 were associated with increased risk of DM under dominant (OR = 1.372, 95% CI: 1.023-1.839, P = 0.034), additive (OR = 1.370, 95% CI: 1.092-1.718, P = 0.006), and codominant (OR = 2.114, 95% CI: 1.251-3.571, P = 0.005) and recessive models (OR = 1.925, 95% CI: 1.160-3.195, P = 0.011).
Table 1.
Baseline clinical characteristics according to polymorphisms
| Variables | rs71428439 | P value | rs2910164 | P value | rs3746444 | P value | rs6505162 | P value | rs2168518 | P value | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|
|
||||||||||||||||
| AA | AG | GG | CC | CG | GG | AA | AG | GG | AA | AC | CC | CC | CT | TT | ||||||
| Age, years | 63.9±10.6 | 63.9±10.8 | 64.0±11.0 | 0.995 | 63.4±10.9 | 64.0±10.9 | 64.8±10.1 | 0.378 | 64.5±12.0 | 63.7±10.7 | 64.0±10.7 | 0.876 | 65.7±11.0 | 63.6±10.9 | 63.9±10.6 | 0.477 | 64.2±10.7 | 63.4±10.8 | 64.6±10.6 | 0.488 |
| Male, % | 70.3 | 70.4 | 72.9 | 0.783 | 67.4 | 71.1 | 72.2 | 0.539 | 71.4 | 69.4 | 68.9 | 0.793 | 66.7 | 68.6 | 72.0 | 0.457 | 72.0 | 70.7 | 62.0 | 0.215 |
| HP, % | 69.9 | 65.5 | 65.7 | 0.370 | 66.9 | 68.0 | 66.1 | 0.836 | 66.6 | 67.5 | 74.4 | 0.564 | 63.4 | 68.1 | 67.0 | 0.826 | 66.1 | 68.1 | 70.4 | 0.684 |
| MI, % | 33.4 | 26.4 | 27.1 | 0.069 | 29.3 | 29.5 | 28.7 | 0.965 | 29.5 | 29.2 | 24.4 | 0.771 | 31.0 | 30.5 | 28.4 | 0.782 | 27.3 | 31.6 | 31.0 | 0.335 |
| DM, % | 23.6 | 26.5 | 18.6 | 0.118 | 21.5 | 26.7 | 21.6 | 0.175 | 24.8 | 22.3 | 24.4 | 0.725 | 22.5 | 23.4 | 24.5 | 0.906 | 21.5 | 25.5 | 36.6 | 0.014 |
| SBP, mmHg | 136.3±20.9 | 134.3±19.4 | 132.7±18.4 | 0.115 | 133.1±19.3 | 135.7±19.8 | 135.7±20.7 | 0.163 | 134.3±19.8 | 134.9±19.6 | 142.5±20.4 | 0.031 | 135.9±21.4 | 135.2±19.6 | 134.5±19.9 | 0.831 | 133.4±19.4 | 135.8±20.0 | 140.9±21.5 | 0.006 |
| DBP, mmHg | 81.3±11.3 | 80.6±11.1 | 80.4±10.6 | 0.603 | 80.4±11.3 | 80.8±11.2 | 81.6±10.5 | 0.485 | 80.3±11.1 | 81.5±11.1 | 84.6±10.9 | 0.023 | 79.7±10.9 | 81.4±11.4 | 80.6±11.0 | 0.513 | 80.1±10.8 | 81.5±11.5 | 83.1±11.1 | 0.036 |
| TC, mmol/L | 4.7±1.1 | 4.7±1.0 | 4.7±1.2 | 0.720 | 4.7±1.1 | 4.7±1.0 | 4.6±1.1 | 0.582 | 4.8±0.8 | 4.7±1.1 | 4.7±1.1 | 0.902 | 4.9±1.2 | 4.7±1.0 | 4.7±1.1 | 0.473 | 4.6±1.1 | 4.7±1.0 | 5.0±1.4 | 0.038 |
| TG, mmol/L | 1.9±1.3 | 2.0±1.2 | 1.8±1.1 | 0.877 | 1.9±1.4 | 2.1±1.2 | 1.7±0.9 | 0.074 | 2.1±1.1 | 1.9±1.2 | 1.8±1.2 | 0.404 | 1.9±1.1 | 1.8±1.1 | 1.9±1.3 | 0.859 | 1.9±1.4 | 1.8±1.1 | 1.9±1.1 | 0.387 |
| HDL, mmol/L | 1.1±0.4 | 1.2±0.4 | 1.2±0.5 | 0.380 | 1.2±0.4 | 1.2±0.4 | 1.2±0.3 | 0.749 | 1.1±0.3 | 1.1±0.4 | 1.2±0.4 | 0.637 | 1.3±0.8 | 1.2±0.4 | 1.2±0.4 | 0.034 | 1.1±0.4 | 1.2±0.4 | 1.2±0.3 | 0.155 |
| LDL, mmol/L | 2.7±0.9 | 2.8±0.9 | 2.8±1.0 | 0.515 | 2.8±0.9 | 2.7±0.8 | 2.7±0.9 | 0.569 | 2.7±0.7 | 2.7±0.9 | 2.8±0.9 | 0.729 | 2.9±1.1 | 2.7±0.9 | 2.7±0.9 | 0.417 | 2.7±0.9 | 2.7±0.8 | 3.0±1.0 | 0.106 |
| FPG, mmol/L | 1.3±0.2 | 1.3±0.2 | 1.3±0.2 | 0.293 | 1.3±0.2 | 1.3±0.2 | 1.3±0.2 | 0.125 | 1.3±0.2 | 1.3±0.2 | 1.3±0.2 | 0.138 | 1.4±0.2 | 1.3±0.2 | 1.3±0.2 | 0.293 | 1.3±0.2 | 1.3±0.2 | 1.3±0.2 | 0.935 |
TG, triglycerides. TC, total cholesterol. HDL, high density lipoprotein. LDL, low density lipoprotein.
We next investigated whether these 5 SNPs were associated with lipid traits. Furthermore, we also investigated the association of these SNPs with blood pressure and FPG. Two of 5 SNPs (miR-499 rs3746444 and miR-4513 rs2168518) were significantly associated with both SBP and DBP (P < 0.05, Table 1). Among lipid traits, the TT genotype of miR-4513 rs2168518 was significantly associated with higher plasma triglyceride level (P = 0.038), while the AA genotype of miR-423 rs6505162 conferred higher level of HDL (P = 0.034). Additionally, miR-4513 rs2168518 also showed significant associated with fasting plasma glucose (P = 0.024) with each copy of the minor allele showing 0.327 mmol/L higher FPG.
Relationship of SNPs to even-free survival (EFS)
During the five-year follow-up, there were 244 cardiovascular events. Table 2 showed the estimated HRs, 95% CIs, and P values of the independent predictors of EFS. Two SNPs (miR-499 rs3746444 and miR-4513 rs2168518) showed an effect on the primary endpoint (Figure 1A and 1B). The GG genotype of miR-499 rs3746444 had a significant influence on the primary endpoint under codominant [adjusted hazard ratio (HR) = 1.913, 95% CI: 1.162-3.152, P = 0.011] and recessive models (adjusted HR = 1.954, 95% CI: 1.197-3.192, P = 007). While miR-4513 rs2168518 showed significant association with the primary endpoint under codominant (CT vs TT, adjusted HR = 1.345, 95% CI: 1.022-1.770, P = 0.034; TT vs CC, adjusted HR = 1.714, 95% CI: 1.077-2.729, P = 0.023), dominant (adjusted HR = 1.401, 95% CI: 1.079-1.819, P = 0.011) and additive models (adjusted HR = 1.323, 95% CI: 1.084-1.615, P = 0.006).
Table 2.
Associations between 5 SNPs and event-free survival in CAD patients
| SNP | Genetic model | Crude HR (95% CI) | P value | Adjusted HR (95% CI)a | P value |
|---|---|---|---|---|---|
| rs71428439 | AA | 1 | 1 | ||
| AG | 1.197 (0.904-1.584) | 0.209 | 1.168 (0.874-1.560) | 0.295 | |
| GG | 1.203 (0.833-1.737) | 0.324 | 1.146 (0.784-1.676) | 0.482 | |
| Dominant | 1.198 (0.920-1.561) | 0.179 | 1.163 (0.884-1.529) | 0.280 | |
| Recessive | 1.088 (0.783-1.512) | 0.616 | 1.048 (0.747-1.472) | 0.785 | |
| Additive | 1.112 (0.933-1.325) | 0.235 | 1.085 (0.905-1.301) | 0.377 | |
| rs2910164 | GG | 1 | 1 | ||
| CG | 1.156 (0.804-1.661) | 0.434 | 1.164 (0.799-1.696) | 0.430 | |
| CC | 1.069 (0.803-1.423) | 0.649 | 1.106 (0.824-1.148) | 0.504 | |
| Dominant | 1.092 (0.8833-1.430) | 0.524 | 1.119 (0.848-1.478) | 0.427 | |
| Recessive | 1.111 (0.808-1.527) | 0.518 | 1.095 (0.787-1.524) | 0.591 | |
| Additive | 1.074 (0.898-1.285) | 0.433 | 1.081 (0.899-1.300) | 0.406 | |
| rs3746444 | AA | 1 | 1 | ||
| AG | 0.885 (0.662-1.183) | 0.409 | 0.932 (0.691-1.256) | 0.643 | |
| GG | 1.818 (1.130-2.925) | 0.014 | 1.913 (1.162-3.152) | 0.011 | |
| Dominant | 1.003 (0.770-1.307) | 0.982 | 1.050 (0.798-1.381) | 0.728 | |
| Recessive | 1.885 (1.180-3.011) | 0.008 | 1.954 (1.197-3.192) | 0.007 | |
| Additive | 1.113 (0.899-1.378) | 0.326 | 0.867 (0.695-1.082) | 0.207 | |
| rs6505162 | CC | 1 | |||
| AC | 1.007 (0.767-1.323) | 0.959 | 0.983 (0.741-1.304) | 0.904 | |
| AA | 1.001 (0.528-1.897) | 0.998 | 1.036 (0.522-2.057) | 0.920 | |
| Dominant | 1.007 (0.775-1.308) | 0.961 | 0.987 (0.752-1.296) | 0.927 | |
| Recessive | 0.999 (0.530-1.880) | 0.997 | 1.041 (0.527-2.055) | 0.908 | |
| Additive | 1.004 (0.805-1.253) | 0.969 | 0.995 (0788-1.257) | 0.968 | |
| rs2168518 | CC | 1 | 1 | ||
| CT | 1.287 (0.988-1.677) | 0.061 | 1.345 (1.022-1.770) | 0.034 | |
| TT | 1.721 (1.101-2.689) | 0.017 | 1.714 (1.077-2.729) | 0.023 | |
| Dominant | 1.350 (1.051-1.736) | 0.019 | 1.401 (1.079-1.819) | 0.011 | |
| Recessive | 1.545 (1.006-2.374) | 0.047 | 1.503 (0.962-2.349) | 0.074 | |
| Additive | 1.302 (1.074-1.579) | 0.007 | 1.323 (1.084-1.615) | 0.006 |
adjusted for age, sex, DM, MI, SBP, DBP, TC, TG, HDL, LDL and FPG.
Figure 1.
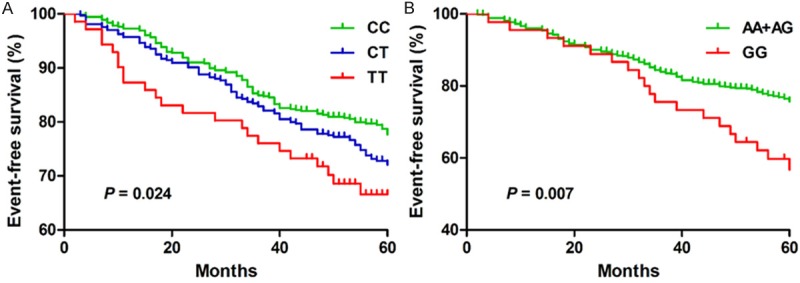
Survival analysis with distribution of patients according to rs2168518 (A) and rs3746444 genotypes (B).
Association of SNPs with death
We then evaluated whether any of the 5 SNPs were related to death (Table 3). A total of 86 (91.4%) of 1004 patients died during the five-year follow up. Survival analysis revealed that miR-4513 rs2168518 was significantly associated with mortality (P < 0.001, Figure 2). Univariate Cox regression analysis showed that miR-4513 rs2168518 was a significant prognostic biomarker of CAD (dominant model, adjusted HR = 2.307, 95% CI: 1.486-3.584, P < 0.001; recessive model, adjusted HR = 2.121, 95% CI: 1.126-3.955, P = 0.020; additive model, adjusted HR = 1.855, 95% CI: 1.367-2.517, P < 0.001; Table 3). The association remained significant after adjustment for adjusted for age, sex, DM, MI, SBP, DBP, TC, TG, HDL, LDL and FPG (dominant model, adjusted HR = 2.349, 95% CI: 1.483-3.721, P < 0.001; recessive model, adjusted HR = 2.101, 95% CI: 1.063-4.152, P = 0.033; additive model, adjusted HR = 1.879, 95% CI: 1.360-2.959, P < 0.001). In addition, the GG genotype of miR-149 rs71428439 showed a non-significant trend in univariate analysis (P = 0.086). The difference reached significance when adjusted for age, sex, DM, MI, SBP, DBP, TC, TG, HDL, LDL and FPG (adjusted HR = 1.821, 95% CI: 1.008-3.290, P = 0.047).
Table 3.
Associations between 5 SNPs and overall survival in CAD patients
| SNP | Genetic model | Crude HR (95% CI) | P value | Adjusted HR (95% CI)a | P value |
|---|---|---|---|---|---|
| rs71428439 | AA | 1 | 1 | ||
| AG | 1.156 (0.710-1.884) | 0.560 | 1.207 (0.724-2.011) | 0.470 | |
| GG | 1.652 (0.931-2.933) | 0.086 | 1.821 (1.008-3.290) | 0.047 | |
| Dominant | 1.290 (0.822-2.025) | 0.269 | 1.375 (0.858-2.201) | 0.185 | |
| Recessive | 1.524 (0.924-2.513) | 0.099 | 1.637 (0.981-2.731) | 0.059 | |
| Additive | 1.275 (0.952-1.707) | 0.103 | 1.340 (0.991-1.812) | 0.057 | |
| rs2910164 | GG | 1 | 1 | ||
| CG | 0.850 (0.537-1.344) | 0.487 | 0.797 (0.495-1.282) | 0.350 | |
| CC | 0.666 (0.344-1.290) | 0.229 | 0.664 (0.331-1.331) | 0.664 | |
| Dominant | 0.800 (0.518-1.236) | 0.314 | 0.762 (0.485-1.197) | 0.238 | |
| Recessive | 0.732 (0.398-1.347) | 0.316 | 0.757 (0.398-1.439) | 0.396 | |
| Additive | 0.825 (0.606-1.121) | 0.219 | 0.809 (0.583-1.121) | 0.203 | |
| rs3746444 | AA | 1 | 1 | ||
| AG | 0.726 (0.438-1.204) | 0.215 | 0.752 (0.446-1.267) | 0.284 | |
| GG | 1.245 (0.500-3.098) | 0.638 | 1.470 (0.582-3.713) | 0.415 | |
| Dominant | 0.792 (0.497-1.262) | 0.327 | 0.835 (0.517-1.350) | 0.462 | |
| Recessive | 1.361 (0.551-3.357) | 0.504 | 1.590 (0.635-3.978) | 0.322 | |
| Additive | 0.892 (0.606-1.313) | 0.563 | 0.947 (0.636-1.410) | 0.787 | |
| rs6505162 | CC | 1 | 1 | ||
| AC | 0.991 (0.626-1.569) | 0.970 | 0.953 (0.593-1.532) | 0.843 | |
| AA | 0.814 (0.255-2.600) | 0.728 | 0.541 (0.131-2.236) | 0.397 | |
| Dominant | 0.970 (0.623-1.512) | 0.894 | 0.902 (0.568-1.432) | 0.661 | |
| Recessive | 0.816 (0.258-2.583) | 0.730 | 0.549 (0.134-2.249) | 0.405 | |
| Additive | 0.956 (0.656-1.393) | 0.814 | 0.873 (0.586-1.300) | 0.504 | |
| rs2168518 | CC | 1 | 1 | ||
| CT | 2.169 (1.370-3.434) | 0.001 | 3.122 (1.495-6.521) | 0.002 | |
| TT | 3.102 (1.559-6.715) | 0.001 | 2.215 (1.372-3.575) | 0.001 | |
| Dominant | 2.307 (1.486-3.584) | < 0.001 | 2.349 (1.483-3.721) | < 0.001 | |
| Recessive | 2.121 (1.126-3.955) | 0.020 | 2.101 (1.063-4.152) | 0.033 | |
| Additive | 1.855 (1.367-2.517) | < 0.001 | 1.879 (1.360-2.959) | < 0.001 |
adjusted for age, sex, DM, MI, SBP, DBP, TC, TG, HDL, LDL and FPG.
Figure 2.
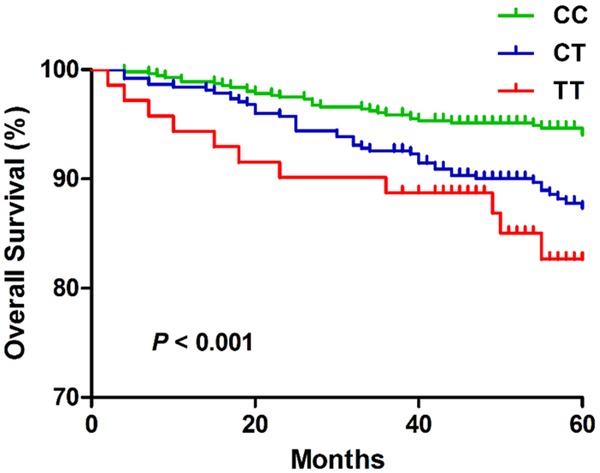
Kaplan-Meier curves for overall survival according to rs2168518 genotypes.
Discussion
This study assessed the influence of miRNA-related SNPs on the risk of incident cardiovascular events and the prognosis of CAD in a Chinese Han population. miR-4513 rs2168518 and mir-499 rs3746444 influence the probability of undergoing subsequent cardiovascular events after the diagnosis of CAD. In addition, we observed that miR-4513 rs2168518 was associated with higher morality in CAD patients. These data could have significant clinical implications on evaluating the risk of cardiovascular events or the possibility of intensive treatment interventions in CAD patients.
miR-499, located in intron 19 of Myh7b, is specifically expressed in skeletal muscle, cardiac cells and a subset of cells in the brain [17,18]. It has been shown that miR-499 play critical roles in both cardiac and skeletal muscle biology. miR-499 is involved in cardiac differentiation [19], and regulates the cardiac response to stress [20]. Elevated miR-499 level prevents cardiomyocyte apoptosis via inhibiting mitochondrial apoptosis pathway [20,21]. miR-499 rs3746444 has been implicated in a variety of human diseases, such as cardiovascular disease and cancer [14,22-24]. Chen et al. [14] found an association of the GG genotype rs3746444 with increased risk of MI. Zhi et al. [23] found that GG genotype of rs3746444 conferred increased risk for CAD, but did not influence on the prognosis in CAD patients. In this study, we found that patients with the GG genotype of rs3746444 had higher risk of cardiovascular event, which was inconsistent with result of previous study [23]. There are several potential explanations for this inconsistency. The first is that genetic differences exist between two Han Chinese populations. Previous studies have demonstrated that there exist genetic heterogeneity within the Han Chinese population [25,26]. Another possible explanation for the observed difference between our study and Zhi et al.’s study may be that we used a different definition of cardiovascular event than did Zhi et al. The third is that there are differences in sample size and follow-up time between our study and Zhi et al.’s study. Our study with larger samples and longer follow up might yield more robust data on the role of rs3746444 in prognosis of CAD. Furthermore, we found weak effect of the GG genotype of rs3746444 on SBP and DBP, whereas hypertension is poor prognostic factor for CAD. Therefore, rs3746444 may contribute to impaired function of miR-499 in anti-apoptosis in cardiomyocytes and blood pressure regulation.
GOSR2 is a Golgi-associated soluble N-ethylmaleimide-sensitive factor attachment receptor (SNARE) protein that is expressed in multiple tissues and organs [27]. GOSR2 is involved in transport of proteins, such as angiotensinogen, insulin, and leptin, between Golgi compartments. Variants of GOSR2 are found to be associated with risk of some diseases, including hypertension, MI, and CAD [12,28,29]. rs2168518 is a seed region variant of miR-4513. The T allele of rs2168518 causes a reduced miR-4513 activity, leading to increased level of GOSR2 [12]. Ghanbari et al. reported that rs2168518 was associated not only with levels of TC, LDL, FPG, SBP, and DBP, but also with CAD risk [12]. In the present study, we found that rs2168518 influenced not only risk for DM, but also for poor prognosis in CAD patients. EFS and overall survival were both worse for patients with TT genotype. Although the mechanism underlying the influence of rs2168518 on EFS and mortality remains unknown, it can be surmised that this genetic variant affects not only the development and progression of coronary atherosclerosis, but also plaque instability or coronary thrombosis due to its pleiotropic effects on blood pressure, lipid, and glucose levels.
In summary, the findings provide first evidence that mir-499 rs3746444 and miR-4513 rs2168518 are potential prognostic biomarkers for CAD patients. Further replication and validation in larger cohorts is necessary to validate these results.
Acknowledgements
The project was supported by Key Clinical Specialty Discipline Construction Program of Chinese and Fujian province, China, and the fund for State key disciplines of China (grant No. 2010302).
Disclosure of conflict of interest
None.
References
- 1.Makeyev EV, Maniatis T. Multilevel regulation of gene expression by microRNAs. Science. 2008;319:1789–1790. doi: 10.1126/science.1152326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shukla GC, Singh J, Barik S. MicroRNAs: Processing, Maturation, Target Recognition and Regulatory Functions. Mol Cell Pharmacol. 2011;3:83–92. [PMC free article] [PubMed] [Google Scholar]
- 3.Kozomara A, Griffiths-Jones S. miRBase: annotating high confidence microRNAs using deep sequencing data. Nucleic Acids Res. 2014;42:D68–73. doi: 10.1093/nar/gkt1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thomson DW, Bracken CP, Goodall GJ. Experimental strategies for microRNA target identification. Nucleic Acids Res. 2011;39:6845–6853. doi: 10.1093/nar/gkr330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Noren Hooten N, Fitzpatrick M, Wood WH 3rd, De S, Ejiogu N, Zhang Y, Mattison JA, Becker KG, Zonderman AB, Evans MK. Age-related changes in microRNA levels in serum. Aging (Albany NY) 2013;5:725–740. doi: 10.18632/aging.100603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tao K, Yang J, Guo Z, Hu Y, Sheng H, Gao H, Yu H. Prognostic value of miR-221-3p, miR-342-3p and miR-491-5p expression in colon cancer. Am J Transl Res. 2014;6:391–401. [PMC free article] [PubMed] [Google Scholar]
- 7.Yu H, Li H, Qian H, Jiao X, Zhu X, Jiang X, Dai G, Huang J. Upregulation of miR-301a correlates with poor prognosis in triple-negative breast cancer. Med Oncol. 2014;31:283. doi: 10.1007/s12032-014-0283-2. [DOI] [PubMed] [Google Scholar]
- 8.Condorelli G, Latronico MV, Cavarretta E. microRNAs in cardiovascular diseases: current knowledge and the road ahead. J Am Coll Cardiol. 2014;63:2177–2187. doi: 10.1016/j.jacc.2014.01.050. [DOI] [PubMed] [Google Scholar]
- 9.Kataoka M, Wang DZ. Non-Coding RNAs Including miRNAs and lncRNAs in Cardiovascular Biology and Disease. Cells. 2014;3:883–898. doi: 10.3390/cells3030883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hu Z, Chen J, Tian T, Zhou X, Gu H, Xu L, Zeng Y, Miao R, Jin G, Ma H, Chen Y, Shen H. Genetic variants of miRNA sequences and non-small cell lung cancer survival. J Clin Invest. 2008;118:2600–2608. doi: 10.1172/JCI34934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sun Q, Gu H, Zeng Y, Xia Y, Wang Y, Jing Y, Yang L, Wang B. Hsa-mir-27a genetic variant contributes to gastric cancer susceptibility through affecting miR-27a and target gene expression. Cancer Sci. 2010;101:2241–2247. doi: 10.1111/j.1349-7006.2010.01667.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ghanbari M, de Vries PS, de Looper H, Peters MJ, Schurmann C, Yaghootkar H, Dorr M, Frayling TM, Uitterlinden AG, Hofman A, van Meurs JB, Erkeland SJ, Franco OH, De- hghan A. A Genetic Variant in the Seed Region of Mir-4513 Shows Pleiotropic Effects on Lipid and Glucose Homeostasis, Blood Pressure and Coronary Artery Disease. Hum Mutat. 2014;35:1524–31. doi: 10.1002/humu.22706. [DOI] [PubMed] [Google Scholar]
- 13.Lin J, Huang Y, Zhang X, Chen J, Sheng H. Association of miR-146a rs2910164 with childhood IgA nephropathy. Pediatr Nephrol. 2014;29:1979–1986. doi: 10.1007/s00467-014-2818-3. [DOI] [PubMed] [Google Scholar]
- 14.Chen C, Hong H, Chen L, Shi X, Chen Y, Weng Q. Association of microRNA polymorphisms with the risk of myocardial infarction in a Chinese population. Tohoku J Exp Med. 2014;233:89–94. doi: 10.1620/tjem.233.89. [DOI] [PubMed] [Google Scholar]
- 15.Wu C, Li M, Hu C, Duan H. Prognostic role of microRNA polymorphisms in patients with advanced esophageal squamous cell carcinoma receiving platinum-based chemotherapy. Cancer Chemother Pharmacol. 2014;73:335–341. doi: 10.1007/s00280-013-2364-x. [DOI] [PubMed] [Google Scholar]
- 16.Xu G, You Q, Pickerill S, Zhong H, Wang H, Shi J, Luo Y, You P, Kong H, Lu F, Hu L. Application of PCR-LDR-nucleic acid detection strip in detection of YMDD mutation in hepatitis B patients treated with lamivudine. J Med Virol. 2010;82:1143–1149. doi: 10.1002/jmv.21811. [DOI] [PubMed] [Google Scholar]
- 17.van Rooij E, Quiat D, Johnson BA, Sutherland LB, Qi X, Richardson JA, Kelm RJ Jr, Olson EN. A family of microRNAs encoded by myosin genes governs myosin expression and muscle performance. Dev Cell. 2009;17:662–673. doi: 10.1016/j.devcel.2009.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bell ML, Buvoli M, Leinwand LA. Uncoupling of expression of an intronic microRNA and its myosin host gene by exon skipping. Mol Cell Biol. 2010;30:1937–1945. doi: 10.1128/MCB.01370-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wilson KD, Hu SJ, Venkatasubrahmanyam S, Fu JD, Sun N, Abilez OJ, Baugh JJA, Jia FJ, Ghosh Z, Li RA, Butte AJ, Wu JC. Dynamic MicroRNA Expression Programs During Cardiac Differentiation of Human Embryonic Stem Cells Role for miR-499. Circulation-Cardiovascular Genetics. 2010;3:426–U497. doi: 10.1161/CIRCGENETICS.109.934281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang JJ, Jia ZQ, Zhang CG, Sun M, Wang WP, Chen P, Ma KT, Zhang YY, Li XH, Zhou CY. miR-499 protects cardiomyocytes from H2O2-induced apoptosis via its effects on Pdcd4 and Pacs2. Rna Biology. 2014;11:339–350. doi: 10.4161/rna.28300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang JX, Jiao JQ, Li QA, Long B, Wang K, Liu JP, Li YR, Li PF. miR-499 regulates mitochondrial dynamics by targeting calcineurin and dynamin-related protein-1. Nat Med. 2011;17:71–8. doi: 10.1038/nm.2282. [DOI] [PubMed] [Google Scholar]
- 22.Sun H, Li Q, Yang T, Wang W. Quantitative assessment of the association between microRNA-499 rs3746444 A/G polymorphism and cancer risk. Tumour Biol. 2014;35:2351–2358. doi: 10.1007/s13277-013-1407-6. [DOI] [PubMed] [Google Scholar]
- 23.Zhi H, Wang L, Ma G, Ye X, Yu X, Zhu Y, Zhang Y, Zhang J, Wang B. Polymorphisms of miRNAs genes are associated with the risk and prognosis of coronary artery disease. Clin Res Cardiol. 2012;101:289–296. doi: 10.1007/s00392-011-0391-3. [DOI] [PubMed] [Google Scholar]
- 24.Liu Y, Ma Y, Zhang B, Wang SX, Wang XM, Yu JM. Genetic polymorphisms in pre-microRNAs and risk of ischemic stroke in a Chinese population. J Mol Neurosci. 2014;52:473–480. doi: 10.1007/s12031-013-0152-z. [DOI] [PubMed] [Google Scholar]
- 25.Xu S, Yin X, Li S, Jin W, Lou H, Yang L, Gong X, Wang H, Shen Y, Pan X, He Y, Yang Y, Wang Y, Fu W, An Y, Wang J, Tan J, Qian J, Chen X, Zhang X, Sun Y, Zhang X, Wu B, Jin L. Genomic dissection of population substructure of Han Chinese and its implication in association studies. Am J Hum Genet. 2009;85:762–774. doi: 10.1016/j.ajhg.2009.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen J, Zheng H, Bei JX, Sun L, Jia WH, Li T, Zhang F, Seielstad M, Zeng YX, Zhang X, Liu J. Genetic structure of the Han Chinese population revealed by genome-wide SNP variation. Am J Hum Genet. 2009;85:775–785. doi: 10.1016/j.ajhg.2009.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lowe SL, Peter F, Subramaniam VN, Wong SH, Hong W. A SNARE involved in protein transport through the Golgi apparatus. Nature. 1997;389:881–884. doi: 10.1038/39923. [DOI] [PubMed] [Google Scholar]
- 28.Pan S, Nakayama T, Sato N, Izumi Y, Soma M, Aoi N, Ma Y, Hinohara S, Doba N. A haplotype of the GOSR2 gene is associated with myocardial infarction in Japanese men. Genet Test Mol Biomarkers. 2013;17:481–488. doi: 10.1089/gtmb.2012.0379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.International Consortium for Blood Pressure Genome-Wide Association Studies. Genetic variants in novel pathways influence blood pressure and cardiovascular disease risk. Nature. 2011;478:103–109. doi: 10.1038/nature10405. [DOI] [PMC free article] [PubMed] [Google Scholar]


