Abstract
The management of retinoblastoma (RB) has dramatically changed over the past two decades from previous radiotherapy methods to current chemotherapy strategies. RB is a remarkably chemotherapy-sensitive tumor. Chemotherapy is currently used as a first-line approach for children with this malignancy and can be delivered by intravenous, intra-arterial, periocular, and intravitreal routes. The choice of route for chemotherapy administration depends upon the tumor laterality and tumor staging. Intravenous chemotherapy (IVC) is used most often in bilateral cases, orbital RB, and as an adjuvant treatment in high-risk RB. Intra-arterial chemotherapy (IAC) is used in cases with group C or D RB and selected cases of group E tumor. Periocular chemotherapy is used as an adjunct treatment in eyes with group D and E RB and those with persistent/recurrent vitreous seeds. Intravitreal chemotherapy is reserved for eyes with persistent/recurrent vitreous seeds. In this review, we describe the various forms of chemotherapy used in the management of RB. A database search was performed on PubMed, using the terms “RB,” and “treatment,” “chemotherapy,” “systemic chemotherapy,” “IVC,” “IAC,” “periocular chemotherapy,” or “intravitreal chemotherapy.” Relevant English language articles were extracted, reviewed, and referenced appropriately.
Keywords: Chemotherapy, intravitreal chemotherapy, intra-arterial chemotherapy, intravenous chemotherapy, periocular chemotherapy, retinoblastoma, Subtenon's chemotherapy, treatment
Retinoblastoma (RB) has emerged as a malignancy with one of the highest survival rates among all pediatric cancers, owing to improving treatment methods over the years.[1,2,3] There is a major shift from globe-sacrificing methods to globe-saving alternatives in the treatment of most cases, using novel techniques of chemotherapy. Chemotherapy can be delivered by intravenous, intra-arterial, periocular, and intravitreal routes. The choice of route of chemotherapy administration depends upon the tumor laterality and tumor staging. In this review, we will discuss the methods of chemotherapy.
A database search was performed on PubMed, using the terms “RB,” and “treatment,” “chemotherapy,” “systemic chemotherapy,” “intravenous chemotherapy (IVC),” “intra-arterial chemotherapy (IAC),” “periocular chemotherapy,” or “intravitreal chemotherapy.” Relevant English language articles were extracted, reviewed, and referenced appropriately.
Intravenous Chemotherapy
Intravenous chemotherapy is used for chemoreduction along with focal therapy for intraocular RB to facilitate globe and vision salvage, In addition, IVC is employed for orbital RB to control the malignancy prior to enucleation, for chemoprophylaxis in eyes with high-risk RB to prevent systemic metastasis, and for treatment of metastatic RB[4,5,6,7,8,9,10] [Table 1]. The advantages of IVC include:[9,11,12,13,14,15,16]
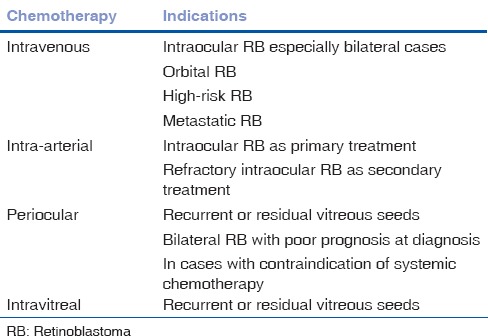
Table 1.
Indications of chemotherapy for RB
Control of intraocular tumor
Resolution of retinal detachment (RD)
Globe salvage
Vision salvage
Prevention of pinealoblastoma
Prevention of systemic metastasis in high-risk RB, and
Reduction of long-term second nonocular cancers.
Chemoreduction of intraocular retinoblastoma
In 1953, Carl Kupfer first introduced IVC for the treatment of RB.[17] Kupfer used nitrogen mustard along with X-ray treatment, with an aim to decrease the dose of radiation.[17] Similar technique was adopted by Reese et al. but ultimately the technique was abandoned owing to side-effects.[18] However, the use of IVC for chemoreduction of intraocular RB gained popularity only in 1996 following reports of successful treatment of RB from four major oncology centers.[4,5,6,7] Since then, the use of chemotherapeutic regimen vincristine sulfate, etoposide phosphate, and carboplatin (VEC) for 6 cycles every 3-4 weeks has become widely acceptable.[19]
Intravenous chemotherapy causes a mean decrease of 35-48% in tumor base and 49-58% in tumor thickness [Fig. 1].[4,20] Increased penetration of carboplatin greater than 15 fold is achieved with single freeze-thaw cryotherapy delivered 24 hours before IVC.[21] Standard dose VEC is used in most cases, while high-dose VEC with intensified dose of carboplatin and etoposide combined with standard dose of vincristine is preferred in patients with bilateral groups D and E RB[3,4,16,19,22] [Table 2]. The International Classification of RB (ICoR) can predict the success of chemoreduction in cases with intraocular RB.[11] In an analysis of 249 eyes with RB based on ICoR by Shields et al., tumor control (avoidance of external beam radiotherapy (EBRT) and enucleation) with standard dose VEC was recorded in 100% of group A, 93% of group B, 90% of group C, and 47% of group D eyes.[11] Subtenon's carboplatin with systemic high dose VEC achieves tumor control with the globe salvage in 60% group D eyes.[22] Group E eyes are the most difficult to treat as the tumor burden is often extreme and recurrence is problematic, but investigators found that combination of VEC followed by prophylactic low-dose EBRT achieves tumor control with globe salvage in 83% group E eyes.[23] Despite encouraging results with VEC for group E eyes, enucleation is still preferred for unilateral group E eyes since preenucleation chemotherapy can downstage the disease and increase the risk of metastastic death.[24]
Figure 1.
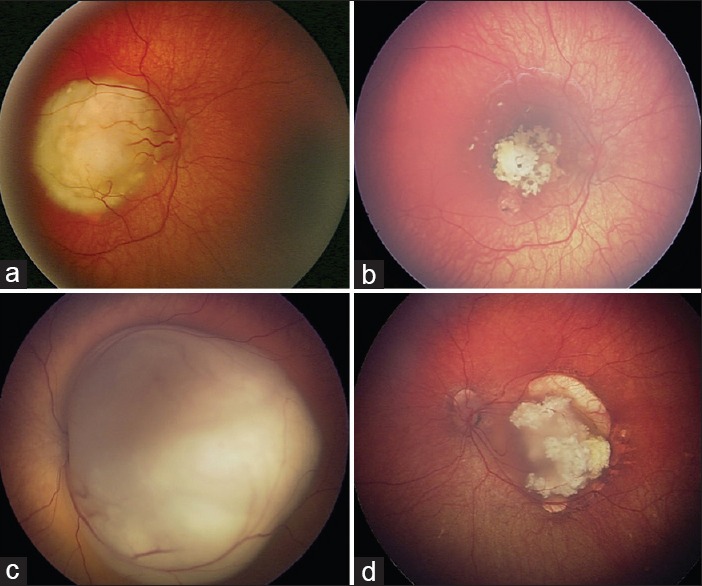
Treatment of intraocular retinoblastoma with intravenous chemotherapy (a) group B retinoblastoma of the right eye showed (b) complete tumor control with 6 cycles of intravenous chemotherapy (IVC). (c) Group C retinoblastoma of the left eye showed (d) complete tumor control with 6 cycles of IVC
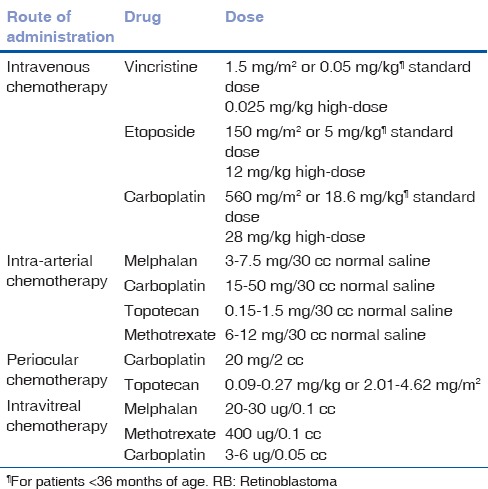
Table 2.
Routes and dosages of chemotherapy for RB
Intravenous chemotherapy can cause dramatic resolution of subretinal fluid associated with RB.[12] In an analysis of 17 eyes with total RD from RB, complete resolution of RD occurred in 76% eyes and partial resolution in 24%.[12] Visual outcome in RB cases following IVC depends on tumor location and presence or absence of RD. Good visual outcome is achieved with extramacular tumors not associated with RD. Visual acuity of 20/200 or better is achieved in 67-71% eyes and 20/40 or better in 37-50% eyes.[13,20]
Intravenous chemotherapy is the treatment of choice in bilateral cases of RB. In these cases with germline mutation, in addition to intraocular tumor control, IVC also prevents the occurrence of pinealoblastoma and reduces the incidence of long-term second nonocular cancers to 4% at 11 years.[14,15,19] These additional benefits could be a direct effect of IVC or indirect benefit by avoidance of EBRT.
Chemoreduction of the orbital retinoblastoma
The incidence of orbital RB varies from 6% to 8% in developed countries and 5-44% in developing countries.[25,26,27,28] The presence of orbital invasion increases the risk of systemic metastasis by 10-27 fold and is associated with poor prognosis.[29]
The purpose of IVC in orbital RB is to facilitate enucleation/extended enucleation/exenteration as appropriate [Fig. 2]. Multimodal treatment protocol by combination of IVC, EBRT, and surgery is recommended.[8,27,30,31] IVC using 3 to 9 cycles of high-dose VEC allows resolution of orbital component and facilitates enucleation/extended enucleation/exenteration. This is followed by 40-50 Gy EBRT over 20-25 fractions and extended high dose chemotherapy for a total of 12 cycles.[8,27] Using this treatment protocol, Honavar and Singh studied 16 cases with unilateral orbital RB without intracranial extension and systemic metastasis, and reported good clinical outcome.[8,27] All affected eyes became phthisical after 3-6 cycles of high-dose VEC facilitating enucleation, and 88% were free of local recurrence or systemic metastasis at 3-year follow-up.[8,27]
Figure 2.
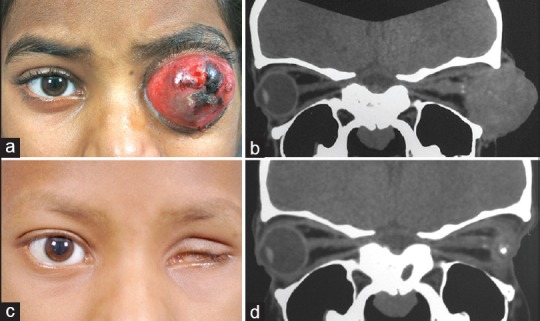
Treatment of the orbital retinoblastoma with intravenous chemotherapy (a) Orbital retinoblastoma of the left eye confirmed on (b) computed tomography scan of the orbit. (c and d) Nine cycles of high-dose chemotherapy resulted in phthisis bulbi facilitating enucleation. No residual tumor was noted on histopathology. The patient subsequently underwent left orbit external beam radiotherapy and further three cycles of high-dose chemotherapy
Chemoprophylaxis in high-risk retinoblastoma
High-risk RB identified from histopathologic factors from enucleated eyes predicts the development of metastatic disease and related mortality.[32,33,34,35] Patients demonstrating such risk factors are given postenucleation adjuvant IVC as chemoprophylaxis for protection from presumed systemic micrometastases and related death.
Four to six cycles of adjuvant IVC is administered in cases with high-risk RB.[9,36] In cases with extrascleral extension and/or tumor extension to optic nerve transection additional orbital EBRT (40-50 Gy) is recommended.[9,36] In a study of 80 patients with high-risk RB by Honavar et al. a significant difference was found in the incidence of metastasis between the group that had received adjuvant therapy (4%) and the group that had not (24%), indicating the importance of postenucleation adjuvant IVC.[36] In a recent study of 52 patients with high-risk RB, Kaliki et al. found that the use of VEC as postenucleation adjuvant IVC further reduced the incidence of systemic metastasis. In that series, no child with high-risk RB developed metastatic disease, signifying the importance of IVC as chemo-prevention.[9]
Metastatic retinoblastoma
Metastatic RB is more common in developing countries (9-11%) than developed countries (5-6%).[10,37,38,39] The higher incidence of metastatic disease in developing countries is due to late referral and delayed diagnosis. Metastatic RB is associated with poor prognosis.[10,37,38,39]
High-dose chemotherapy with autologous stem cell rescue (ASCR) may be beneficial for some cases of metastatic RB.[40,41] In a study of 13 patients with trilateral RB by Dunkel et al. induction chemotherapy with VEC and cyclophosphamide in addition to high-dose chemotherapy with thiotepa-based or melphalan and cyclophosphamide with ASCR resulted in prevention of death in 38% cases without the need of EBRT at 6 years follow-up.[40] Beneficial effects were seen in cases with central nervous system (CNS) metastatic disease with high-dose chemotherapy and ASCR with survival in 40% cases (2 of 5 cases) at a mean follow-up period of 6 years.[40] Similar encouraging results were noted with high-dose chemotherapy and ASCR in cases with extra-CNS metastatic disease with survival in 67% (10 of 15 cases) cases at 9 years follow-up.[42] The use of EBRT to the metastatic sites is controversial.[40,41,42,43]
Complications of intravenous chemotherapy
The predictable complications of IVC include febrile episodes, temporary alopecia, and bone marrow suppression.[19] Cytopenias requiring platelet and/or packed red cells is common with high-dose chemotherapy and uncommon with standard-dose chemotherapy. Vincristine and etoposide can cause peripheral neuropathy, and allergic reactions to etoposide and carboplatin can occur.[19]
Cisplatin-induced ototoxicity is reported in 0-17% cases.[44,45] Properly dosed carboplatin in children with RB result in rare occurrences of hearing deficit.[44] Etoposide-induced secondary acute myelogenous leukemia has been reported in the literature; however, the incidence of IVC induced secondary acute myeloid leukemia is very low.[46,47] Though IVC-induced infertility has been speculated, there is no evidence supporting this with our protocol of VEC. Our personal experience using proper dosing of IVC reveals excellent tumor control with minimal toxicities and no fertility issues.
Intra-arterial Chemotherapy
Over the past few years, there has been increasing enthusiasm regarding IAC in the treatment of RB. Reese et al. were the first to investigate IAC for RB by intracarotid administration of triethylene melamine in 1958.[48] Subsequently in 1990's, Kaneko described the selective ophthalmic arterial injection of melphalan by distal occlusion of internal carotid artery with a balloon catheter.[49] This technique was further modified by Abramson et al. by supraselective IAC involving injection of melphalan directly into the proximal portion of the ophthalmic artery.[50] Shields et al. independently studied IAC and found excellent tumor control with low ophthalmic risks.[51] Various studies have shown that IAC can be used as primary treatment or secondary treatment in eyes with recurrent/residual RB.[50,51,52,53,54,55,56,57,58] [Table 1]. The various agents used for IAC in RB include melphalan, carboplatin, topotecan, and methotrexate[50,51,52,53,54,55,56,57,58] [Table 2]. The advantages of IAC include:[50,51,52,53,54,55,56,57]
Control of intraocular tumor
Resolution of RD
Globe salvage
Minimal systemic side-effects.
In a study of selective IAC with melphalan in 408 eyes of 343 patients by Suzuki et al. globe salvage was achieved in 100% group A, 88% group B, 65% group C, 45% group D, and 30% group E eyes.[52] Visual acuity of 20/40 or better was achieved in 51% eyes.[52] Gobin et al. reported their experience in 95 eyes with supraselective IAC, with 82% globe salvage when used as primary treatment and 58% globe salvage when used as secondary treatment.[53] Shields et al. reported their experience in 70 consecutive patients treated with supraselective IAC, with 72% globe salvage when used as primary treatment and 62% globe salvage when used as secondary treatment [Fig. 3].[55] Based on ICoR, globe salvage was achieved in 100% group B, 100% group C, 94% group D, and 36% group E eyes.[55] Based on various studies, IAC allows the globe salvage in 58-100% cases when used as primary treatment, and 50-75% cases as secondary treatment.[51,52,53,54,55,56,57] Globe salvage can be achieved in 100% group B, 100% group C, 75-100% group D, and 30-36% group E eyes.[51,52,53,54,55,56,57] However, IAC for group E RB should be used with caution due to the increased prevalence of high-risk RB in these eyes, necessitating enucleation and adjuvant IVC rather than IAC.[59,60]
Figure 3.
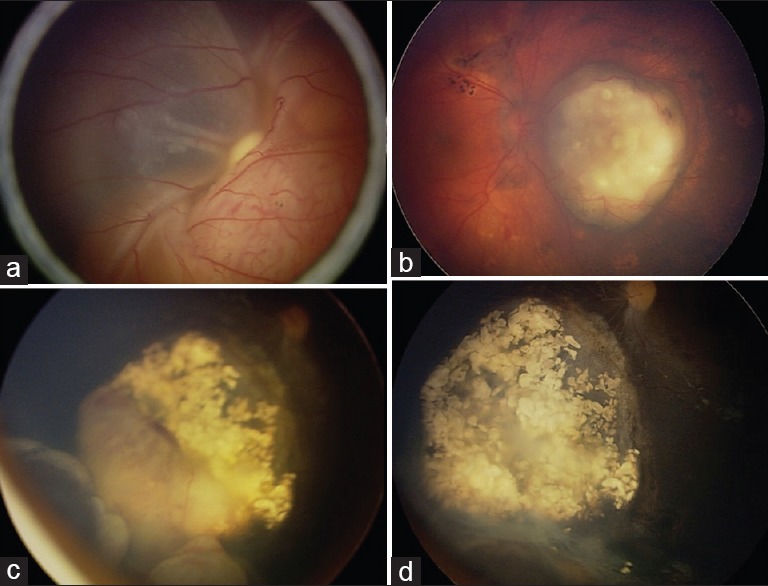
Treatment of intraocular retinoblastoma with intra-arterial chemotherapy (a) group E retinoblastoma of the left eye showed (b) complete tumor control with primary intra-arterial chemotherapy (IAC). Surrounding retinal pigment epithelial changes is also noted. (c) Tumor recurrence following intravenous chemotherapy showed (d) complete tumor control with secondary IAC
Similar to IVC, IAC causes resolution of subretinal fluid associated with RB.[61] In a study of 15 patients with RB-induced RD, resolution of RD was noted in 43% cases with complete RD and 100% cases with partial RD.[61]
Complications of Intra-arterial Chemotherapy
Intra-arterial chemotherapy causes minimal systemic side-effects. Transient neutropenia develops in 11% patients after IAC that does not require transfusion.[53,55] Owing to minimal systemic absorption of drugs, IAC offers no protection against systemic metastasis, pinealoblastoma, and second cancers. Of 78 patients treated with IAC, two children developed systemic metastasis.[53] Local side-effects at the injection site and carotid spasm can also occur.
Ocular complications with IAC are well-documented.[50,52,53,54,55,56,62,63,64,65,66,67] Less severe, temporary side-effects include periorbital edema, periocular hyperemia, madarosis, and ocular dysmotility. More severe complications include vitreous hemorrhage in 13-27%, RD in 15 to 27%, retinal pigment epithelial changes occur in 5-53%, retinal ischemia in 4-24%, and chorioretinal atrophy in <1 to 29% cases.[50,52,53,54,55,56,62,63,64,65,66,67] These changes occur either secondary to drug toxicity or competency of ophthalmic artery catheterization resulting in structural and vascular damage of the retina causing visual loss.[67]
Periocular chemotherapy
Periocular administration of chemotherapy allows delivery of higher concentrations of chemotherapy drugs to the posterior segments of the eye. In 1996, Harbour et al. explored the use of periocular carboplatin in the treatment of RB in animal models.[68] Subsequent study by Mendelsohn et al. demonstrated that administration of periocular carboplatin is safe and results in higher vitreous concentration of carboplatin by 8-10-fold compared to intravenous administration.[69] Similarly, Mao et al. showed that the concentrations in the aqueous and vitreous humor after subconjunctival administration of etoposide were 2-4 times higher than those obtained after intravenous administration.[70] A study by Carcabaso et al. revealed that comparable vitreous concentrations of topotecan were achieved when administered through periocular or systemic routes.[71]
The available data does not support the use of periocular chemotherapy as monotherapy. Periocular carboplatin is used as an adjunct treatment for eyes with persistent vitreous seeding despite other forms of treatment, in patients with bilateral RB with poor prognosis at diagnosis, or in those in whom IVC is contraindicated[72] [Tables 1 and 2, Fig. 4] Periocular carboplatin is effective for noncalcified vitreous seeds, and not effective against solid tumor or subretinal seeds.[73] Periocular topotecan is effective against group A and B tumors.[74] The various delivery systems of periocular chemotherapy being investigated include episcleral implants, fibrin sealants, and nanomolecular composition.[72]
Figure 4.
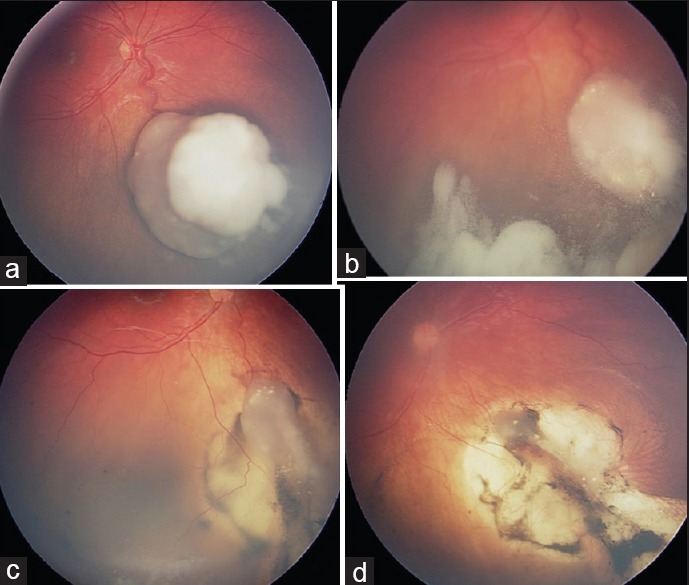
Treatment of vitreous seeds with periocular chemotherapy (a) group C retinoblastoma of the right eye showed (b) dispersion of vitreous seeds during intravenous chemotherapy. (c) Reduction in vitreous seeds following one dose of 20 mg/2 cc periocular carboplatin and (d) complete tumor control following the second dose of periocular carboplatin
Complications of periocular chemotherapy
Periocular chemotherapy is devoid of systemic side-effects. The most common side-effect is periorbital edema and erythema. The other side-effects include ocular motility changes, orbital fibrosis, optic atrophy, pseudo-preseptal cellulitis, and rarely ophthalmic arterial alterations.[72]
Intravitreal chemotherapy
Ericson and Rosengren first investigated the use of intravitreal chemotherapy for RB in 1960's.[75] However, this technique did not gain popularity due to the risk of tumor seeding after any intraocular intervention in RB. In 1995, Seregard et al. demonstrated the absence of local recurrence and metastatic disease following administration of intravitreal thiotepa.[76]
Inomata and Kaneko found melphalan to be the most sensitive chemotherapeutic agent against RB based on in vitro testing of 12 agents, and a dose of 4 ug/ml achieved complete tumor suppression [Fig. 5].[77] In the rabbit model, the concentration of 5.9 μg/ml showed no retinal toxicity, and this correlates to human vitreous doses of 20-30 ug.[16] These findings have prompted 20-30 ug melphalan as the drug of choice for intravitreal chemotherapy, with minimal ocular complications and no significant electroretinogram changes at this dose.[78,79] Dose greater than 50 ug is associated with severe ocular complications.[79] Satisfactory results have also been reported with an intravitreal methotrexate, carboplatin and topotecan[80,81,82] [Table 2]. In the recent times, the use of intravitreal chemotherapy through pars plana route for recurrent/residual vitreous seeds has shown promising results[78,79,80,81,82,83,84,85,86,87,88][Table 1].
Figure 5.
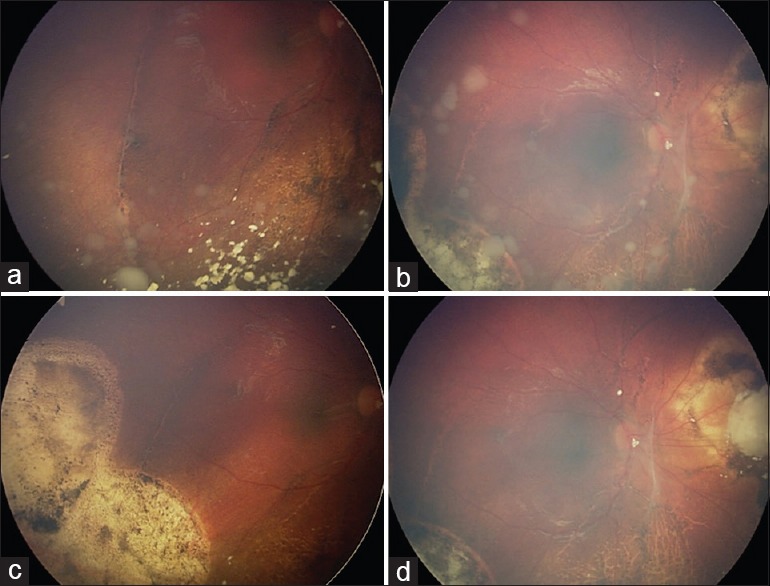
Treatment of vitreous seeds with intravitreal chemotherapy (a and b). Recurrent vitreous seeds in the right eye following 3 cycles of intra-arterial chemotherapy (c and d). Complete tumor control was achieved with 6 monthly doses of 20 ug melphalan
Kaneko and Suzuki treated 41 eyes with 8ug intravitreal melphalan and reported the globe salvage in 51% cases.[88] In a study of 23 eyes with active vitreous seeds, Munier et al. reported the globe salvage in 87% eyes with complete remission of vitreous seeds over a mean follow-up period of 2 years.[85] Intravitreal melphalan 20-30 ug was injected every 7-10 days via the parsplana approach.[85] Shields et al. studied 11 eyes treated with intravitreal melphalan and reported the globe salvage in 100% eyes at a mean follow-up of 9 months.[83] Intravitreal melphalan 20-30 ug was injected every month via the pars plana approach with concomitant triple freeze-thaw cryotherapy to the injection site.[83] Kivelä et al. adapted the intraocular lymphoma protocol to inject intravitreal methotrexate and reported complete remission of vitreous seeds in 100% cases (2 of 2 eyes), subretinal seeds in 67% (2 of 3 eyes), and small retinal foci in 50% (2 of 4 eyes).[80]
Omplications of intravitreal chemotherapy
The most concerning side-effect of intravitreal chemotherapy is extraocular tumor spread. Extraocular tumor spread from intravitreal chemotherapy can occur per-operatively due to spilling of tumor cells from surgical instruments while removing from the eye or reflux of contaminated vitreous at the injection site; or postoperatively due to tumor growth along the needle tract.[87] Extraocular tumor spread can be prevented by ultrasound biomicroscopic guided selection of injection site to avoid injection directly into the contaminated posterior chamber and subsequent tumor growth along the needle tract; anterior chamber paracentesis to create a transient hypotony and avoid reflux of contaminated vitreous at the injection site secondary to variations in intraocular pressure; triple freeze-thaw cryotherapy at the injection site; and injecting a bleb of subconjunctival chemotherapy (carboplatin) prior to intravitreal chemotherapy.[81,83,85,87]
The other serious side-effects include iris atrophy, vitreous hemorrhage, RD, and chorioretinal atrophy. Minor side-effects include transient conjunctivitis, transient keratitis, corneal edema, cataract, ocular inflammation, vitreous condensation and banding, and retinal pigment epithelial changes.[89]
Conclusion
The management of RB has evolved over the years with improving methods of treatment. The combination of various treatment modalities is likely to increase the rate of tumor control and globe salvage. The treatment of intraocular RB is likely heading in the direction of a combination of IVC and IAC along with focal treatments such as cryotherapy and transpupillary thermotherapy. Periocular chemotherapy and intravitreal chemotherapy are promising adjunct therapies in refractory cases.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
References
- 1.Young JL, Smith MA, Roffers SD, Liff JM, Bunin GR. Retinoblastoma. In: Ries LA, Smith MA, Gurney JG, Linet M, Tamra T, Young JL, et al., editors. Cancer Incidence and Survival among Children and Adolescents: United States SEER Program 1975-1995. Maryland: National Cancer Institute, SEER Program; 2012. [Google Scholar]
- 2.Ramasubramanian A, Shields CL, editors. New Delhi, India: Jaypee Brothers Medical Publishers; 2012. Epidemiology and magnitude of the problem. Retinoblastoma; pp. 10–5. [Google Scholar]
- 3.Shields JA, Shields CL, editors. An Atlas and Textbook. 2nd ed. Philadelphia, PA: Lippincott Williams Wilkins; 2008. Retinoblastoma. Intraocular Tumors; pp. 293–365. [Google Scholar]
- 4.Shields CL, De Potter P, Himelstein BP, Shields JA, Meadows AT, Maris JM. Chemoreduction in the initial management of intraocular retinoblastoma. Arch Ophthalmol. 1996;114:1330–8. doi: 10.1001/archopht.1996.01100140530002. [DOI] [PubMed] [Google Scholar]
- 5.Murphree AL, Villablanca JG, Deegan WF, 3rd, Sato JK, Malogolowkin M, Fisher A, et al. Chemotherapy plus local treatment in the management of intraocular retinoblastoma. Arch Ophthalmol. 1996;114:1348–56. doi: 10.1001/archopht.1996.01100140548005. [DOI] [PubMed] [Google Scholar]
- 6.Gallie BL, Budning A, DeBoer G, Thiessen JJ, Koren G, Verjee Z, et al. Chemotherapy with focal therapy can cure intraocular retinoblastoma without radiotherapy. Arch Ophthalmol. 1996;114:1321–8. doi: 10.1001/archopht.1996.01100140521001. [DOI] [PubMed] [Google Scholar]
- 7.Kingston JE, Hungerford JL, Madreperla SA, Plowman PN. Results of combined chemotherapy and radiotherapy for advanced intraocular retinoblastoma. Arch Ophthalmol. 1996;114:1339–43. doi: 10.1001/archopht.1996.01100140539004. [DOI] [PubMed] [Google Scholar]
- 8.Honavar SG, Singh AD. Management of advanced retinoblastoma. Ophthalmol Clin North Am. 2005;18:65–73. doi: 10.1016/j.ohc.2004.09.001. viii. [DOI] [PubMed] [Google Scholar]
- 9.Kaliki S, Shields CL, Shah SU, Eagle RC, Jr, Shields JA, Leahey A. Postenucleation adjuvant chemotherapy with vincristine, etoposide, and carboplatin for the treatment of high-risk retinoblastoma. Arch Ophthalmol. 2011;129:1422–7. doi: 10.1001/archophthalmol.2011.289. [DOI] [PubMed] [Google Scholar]
- 10.Leal-Leal CA, Rivera-Luna R, Flores-Rojo M, Juárez-Echenique JC, Ordaz JC, Amador-Zarco J. Survival in extra-orbital metastatic retinoblastoma: Treatment results. Clin Transl Oncol. 2006;8:39–44. doi: 10.1007/s12094-006-0093-x. [DOI] [PubMed] [Google Scholar]
- 11.Shields CL, Mashayekhi A, Au AK, Czyz C, Leahey A, Meadows AT, et al. The International Classification of Retinoblastoma predicts chemoreduction success. Ophthalmology. 2006;113:2276–80. doi: 10.1016/j.ophtha.2006.06.018. [DOI] [PubMed] [Google Scholar]
- 12.Shields CL, Shields JA, DePotter P, Himelstein BP, Meadows AT. The effect of chemoreduction on retinoblastoma-induced retinal detachment. J Pediatr Ophthalmol Strabismus. 1997;34:165–9. doi: 10.3928/0191-3913-19970501-07. [DOI] [PubMed] [Google Scholar]
- 13.Demirci H, Shields CL, Meadows AT, Shields JA. Long-term visual outcome following chemoreduction for retinoblastoma. Arch Ophthalmol. 2005;123:1525–30. doi: 10.1001/archopht.123.11.1525. [DOI] [PubMed] [Google Scholar]
- 14.Shields CL, Meadows AT, Shields JA, Carvalho C, Smith AF. Chemoreduction for retinoblastoma may prevent intracranial neuroblastic malignancy (trilateral retinoblastoma) Arch Ophthalmol. 2001;119:1269–72. doi: 10.1001/archopht.119.9.1269. [DOI] [PubMed] [Google Scholar]
- 15.Turaka K, Shields CL, Leahey A, Meadows AT. Second malignant neoplasms following chemoreduction for retinoblastoma in 272 patients. Pediatr Blood Cancer. 2012;59:121–5. doi: 10.1002/pbc.23278. [DOI] [PubMed] [Google Scholar]
- 16.Shields CL, Fulco EM, Arias JD, Alarcon C, Pellegrini M, Rishi P, et al. Retinoblastoma frontiers with intravenous, intra-arterial, periocular, and intravitreal chemotherapy. Eye (Lond) 2013;27:253–64. doi: 10.1038/eye.2012.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kupfer C. Retinoblastoma treated with intravenous nitrogen mustard. Am J Ophthalmol. 1953;36:1721–3. doi: 10.1016/0002-9394(53)90009-9. [DOI] [PubMed] [Google Scholar]
- 18.Reese AB, Hyman GA, Tapley ND, Forrest AW. The treatment of retinoblastoma by x-ray and triethylene melamine. AMA Arch Ophthalmol. 1958;60:897–906. doi: 10.1001/archopht.1958.00940080917010. [DOI] [PubMed] [Google Scholar]
- 19.Leahey AM. Systemic chemotherapy: A pediatric oncology perspective. In: Ramasubramanian A, Shields CL, editors. Retinoblastoma. New Delhi, India: Jaypee Brothers Medical Publishers; 2012. pp. 81–5. [Google Scholar]
- 20.Narang S, Mashayekhi A, Rudich D, Shields CL. Predictors of long-term visual outcome after chemoreduction for management of intraocular retinoblastoma. Clin Experiment Ophthalmol. 2012;40:736–42. doi: 10.1111/j.1442-9071.2012.02757.x. [DOI] [PubMed] [Google Scholar]
- 21.Wilson TW, Chan HS, Moselhy GM, Heydt DD, Jr, Frey CM, Gallie BL. Penetration of chemotherapy into vitreous is increased by cryotherapy and cyclosporine in rabbits. Arch Ophthalmol. 1996;114:1390–5. doi: 10.1001/archopht.1996.01100140590011. [DOI] [PubMed] [Google Scholar]
- 22.Villablanca JG, Jubran R, Murphree AL. Phase I Study of Subtenon Carboplatin with Systemic High dose Carboplatin/Etoposide/ Vincristine (CEV) for Eyes with Disseminated Intraocular Retinoblastoma (RB). [Abstract] Proceedings of XIII Biannual Meeting of ISGED and the X International Symposium on Retinoblastoma, Fort Lauderdale, Fl, USA; May 4. 2001 [Google Scholar]
- 23.Shields CL, Ramasubramanian A, Thangappan A, Hartzell K, Leahey A, Meadows AT, et al. Chemoreduction for group E retinoblastoma: Comparison of chemoreduction alone versus chemoreduction plus low-dose external radiotherapy in 76 eyes. Ophthalmology. 2009;116:544–51.e1. doi: 10.1016/j.ophtha.2008.10.014. [DOI] [PubMed] [Google Scholar]
- 24.Zhao J, Dimaras H, Massey C, Xu X, Huang D, Li B, et al. Pre-enucleation chemotherapy for eyes severely affected by retinoblastoma masks risk of tumor extension and increases death from metastasis. J Clin Oncol. 2011;29:845–51. doi: 10.1200/JCO.2010.32.5332. [DOI] [PubMed] [Google Scholar]
- 25.Ellsworth RM. Orbital retinoblastoma. Trans Am Ophthalmol Soc. 1974;72:79–88. [PMC free article] [PubMed] [Google Scholar]
- 26.Grabowski EF, Abramson DH. Intraocular and extraocular retinoblastoma. Hematol Oncol Clin North Am. 1987;1:721–35. [PubMed] [Google Scholar]
- 27.Honavar SG. Orbital Retinoblastoma. In: Ramasubramanian A, Shields CL, editors. Retinoblastoma. New Delhi, India: Jaypee Brothers Medical Publishers; 2012. pp. 167–78. [Google Scholar]
- 28.Badhu B, Sah SP, Thakur SK, Dulal S, Kumar S, Sood A, et al. Clinical presentation of retinoblastoma in Eastern Nepal. Clin Experiment Ophthalmol. 2005;33:386–9. doi: 10.1111/j.1442-9071.2005.01010.x. [DOI] [PubMed] [Google Scholar]
- 29.Singh AD, Shields CL, Shields JA. Prognostic factors in retinoblastoma. J Pediatr Ophthalmol Strabismus. 2000;37:134–41. doi: 10.3928/0191-3913-20000501-04. [DOI] [PubMed] [Google Scholar]
- 30.Goble RR, McKenzie J, Kingston JE, Plowman PN, Hungerford JL. Orbital recurrence of retinoblastoma successfully treated by combined therapy. Br J Ophthalmol. 1990;74:97–8. doi: 10.1136/bjo.74.2.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Doz F, Khelfaoui F, Mosseri V, Validire P, Quintana E, Michon J, et al. The role of chemotherapy in orbital involvement of retinoblastoma. The experience of a single institution with 33 patients. Cancer. 1994;74:722–32. doi: 10.1002/1097-0142(19940715)74:2<722::aid-cncr2820740228>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 32.Shields CL, Shields JA, Baez K, Cater JR, De Potter P. Optic nerve invasion of retinoblastoma. Metastatic potential and clinical risk factors. Cancer. 1994;73:692–8. doi: 10.1002/1097-0142(19940201)73:3<692::aid-cncr2820730331>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 33.Shields CL, Shields JA, Baez KA, Cater J, De Potter PV. Choroidal invasion of retinoblastoma: Metastatic potential and clinical risk factors. Br J Ophthalmol. 1993;77:544–8. doi: 10.1136/bjo.77.9.544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Messmer EP, Heinrich T, Höpping W, de Sutter E, Havers W, Sauerwein W. Risk factors for metastases in patients with retinoblastoma. Ophthalmology. 1991;98:136–41. doi: 10.1016/s0161-6420(91)32325-x. [DOI] [PubMed] [Google Scholar]
- 35.Khelfaoui F, Validire P, Auperin A, Quintana E, Michon J, Pacquement H, et al. Histopathologic risk factors in retinoblastoma: A retrospective study of 172 patients treated in a single institution. Cancer. 1996;77:1206–13. [PubMed] [Google Scholar]
- 36.Honavar SG, Singh AD, Shields CL, Meadows AT, Demirci H, Cater J, et al. Postenucleation adjuvant therapy in high-risk retinoblastoma. Arch Ophthalmol. 2002;120:923–31. doi: 10.1001/archopht.120.7.923. [DOI] [PubMed] [Google Scholar]
- 37.Jubran RF, Erdreich-Epstein A, Butturini A, Murphree AL, Villablanca JG. Approaches to treatment for extraocular retinoblastoma: Children's Hospital Los Angeles experience. J Pediatr Hematol Oncol. 2004;26:31–4. doi: 10.1097/00043426-200401000-00011. [DOI] [PubMed] [Google Scholar]
- 38.Cozza R, De Ioris MA, Ilari I, Devito R, Fidani P, De Sio L, et al. Metastatic retinoblastoma: Single institution experience over two decades. Br J Ophthalmol. 2009;93:1163–6. doi: 10.1136/bjo.2008.148932. [DOI] [PubMed] [Google Scholar]
- 39.Günalp I, Gündüz K, Arslan Y. Retinoblastoma in Turkey: Diagnosis and clinical characteristics. Ophthalmic Genet. 1996;17:21–7. doi: 10.3109/13816819609057865. [DOI] [PubMed] [Google Scholar]
- 40.Dunkel IJ, Jubran RF, Gururangan S, Chantada GL, Finlay JL, Goldman S, et al. Trilateral retinoblastoma: Potentially curable with intensive chemotherapy. Pediatr Blood Cancer. 2010;54:384–7. doi: 10.1002/pbc.22336. [DOI] [PubMed] [Google Scholar]
- 41.Dunkel IJ, Chan HS, Jubran R, Chantada GL, Goldman S, Chintagumpala M, et al. High-dose chemotherapy with autologous hematopoietic stem cell rescue for stage 4B retinoblastoma. Pediatr Blood Cancer. 2010;55:149–52. doi: 10.1002/pbc.22491. [DOI] [PubMed] [Google Scholar]
- 42.Dunkel IJ, Khakoo Y, Kernan NA, Gershon T, Gilheeney S, Lyden DC, et al. Intensive multimodality therapy for patients with stage 4a metastatic retinoblastoma. Pediatr Blood Cancer. 2010;55:55–9. doi: 10.1002/pbc.22504. [DOI] [PubMed] [Google Scholar]
- 43.Chantada GL, Leal-Leal CA, Dunkel IJ. Metastatic retinoblastoma. In: Ramasubramanian A, Shields CL, editors. Retinoblastoma. New Delhi, India: Jaypee Brothers Medical Publishers; 2012. pp. 186–90. [Google Scholar]
- 44.Lambert MP, Shields C, Meadows AT. A retrospective review of hearing in children with retinoblastoma treated with carboplatin-based chemotherapy. Pediatr Blood Cancer. 2008;50:223–6. doi: 10.1002/pbc.21155. [DOI] [PubMed] [Google Scholar]
- 45.Qaddoumi I, Bass JK, Wu J, Billups CA, Wozniak AW, Merchant TE, et al. Carboplatin-associated ototoxicity in children with retinoblastoma. J Clin Oncol. 2012;30:1034–41. doi: 10.1200/JCO.2011.36.9744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gombos DS, Hungerford J, Abramson DH, Kingston J, Chantada G, Dunkel IJ, et al. Secondary acute myelogenous leukemia in patients with retinoblastoma: Is chemotherapy a factor? Ophthalmology. 2007;114:1378–83. doi: 10.1016/j.ophtha.2007.03.074. [DOI] [PubMed] [Google Scholar]
- 47.Turaka K, Shields CL, Meadows AT, Leahey A. Second malignant neoplasms following chemoreduction with carboplatin, etoposide, and vincristine in 245 patients with intraocular retinoblastoma. Pediatr Blood Cancer. 2012;59:121–5. doi: 10.1002/pbc.23278. [DOI] [PubMed] [Google Scholar]
- 48.Reese AB, Hyman GA, Tapley ND, Forrest AW. The treatment of retinoblastoma by x-ray and triethylene melamine. AMA Arch Ophthalmol. 1958;60:897–906. doi: 10.1001/archopht.1958.00940080917010. [DOI] [PubMed] [Google Scholar]
- 49.Kaneko A. Japanese contributions to ocular oncology. Int J Clin Oncol. 1999;4:321–6. [Google Scholar]
- 50.Abramson DH, Dunkel IJ, Brodie SE, Kim JW, Gobin YP. A phase I/II study of direct intraarterial (ophthalmic artery) chemotherapy with melphalan for intraocular retinoblastoma initial results. Ophthalmology. 2008;115:1398–404.e1. doi: 10.1016/j.ophtha.2007.12.014. [DOI] [PubMed] [Google Scholar]
- 51.Shields CL, Bianciotto CG, Jabbour P, Ramasubramanian A, Lally SE, Griffin GC, et al. Intra-arterial chemotherapy for retinoblastoma: Report No 1, control of retinal tumors, subretinal seeds, and vitreous seeds. Arch Ophthalmol. 2011;129:1399–406. doi: 10.1001/archophthalmol.2011.150. [DOI] [PubMed] [Google Scholar]
- 52.Suzuki S, Yamane T, Mohri M, Kaneko A. Selective ophthalmic arterial injection therapy for intraocular retinoblastoma: The long-term prognosis. Ophthalmology. 2011;118:2081–7. doi: 10.1016/j.ophtha.2011.03.013. [DOI] [PubMed] [Google Scholar]
- 53.Gobin YP, Dunkel IJ, Marr BP, Brodie SE, Abramson DH. Intra-arterial chemotherapy for the management of retinoblastoma: Four-year experience. Arch Ophthalmol. 2011;129:732–7. doi: 10.1001/archophthalmol.2011.5. [DOI] [PubMed] [Google Scholar]
- 54.Peterson EC, Elhammady MS, Quintero-Wolfe S, Murray TG, Aziz-Sultan MA. Selective ophthalmic artery infusion of chemotherapy for advanced intraocular retinoblastoma: Initial experience with 17 tumors. J Neurosurg. 2011;114:1603–8. doi: 10.3171/2011.1.JNS10466. [DOI] [PubMed] [Google Scholar]
- 55.Shields CL, Manjandavida FP, Pieretti G, Arepalli SA, Jabbour P, Shields JA. Intra-arterial chemotherapy for retinoblastoma: Use as primary or secondary therapy in 70 eyes. Ophthalmology. 2014;121:1453–60. doi: 10.1016/j.ophtha.2014.01.026. [DOI] [PubMed] [Google Scholar]
- 56.Munier FL, Beck-Popovic M, Balmer A, Gaillard MC, Bovey E, Binaghi S. Occurrence of sectoral choroidal occlusive vasculopathy and retinal arteriolar embolization after superselective ophthalmic artery chemotherapy for advanced intraocular retinoblastoma. Retina. 2011;31:566–73. doi: 10.1097/IAE.0b013e318203c101. [DOI] [PubMed] [Google Scholar]
- 57.Shields CL, Kaliki S, Al-Dahmash S, Rojanaporn D, Leahey A, Griffin G, et al. Management of advanced retinoblastoma with intravenous chemotherapy then intra-arterial chemotherapy as alternative to enucleation. Retina. 2013;33:2103–9. doi: 10.1097/IAE.0b013e318295f783. [DOI] [PubMed] [Google Scholar]
- 58.Francis JH, Gobin YP, Dunkel IJ, Marr BP, Brodie SE, Jonna G, et al. Carboplatin +/-topotecan ophthalmic artery chemosurgery for intraocular retinoblastoma. PLoS One. 2013;8:e72441. doi: 10.1371/journal.pone.0072441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wilson MW, Qaddoumi I, Billups C, Haik BG, Rodriguez-Galindo C. A clinicopathological correlation of 67 eyes primarily enucleated for advanced intraocular retinoblastoma. Br J Ophthalmol. 2011;95:553–8. doi: 10.1136/bjo.2009.177444. [DOI] [PubMed] [Google Scholar]
- 60.Kaliki S, Shields CL, Rojanaporn D, Al-Dahmash S, McLaughlin JP, Shields JA, et al. High-risk retinoblastoma based on international classification of retinoblastoma: Analysis of 519 enucleated eyes. Ophthalmology. 2013;120:997–1003. doi: 10.1016/j.ophtha.2012.10.044. [DOI] [PubMed] [Google Scholar]
- 61.Shields CL, Kaliki S, Shah SU, Bianciotto CG, Jabbour P, Shields JA. Effect of intraarterial chemotherapy on retinoblastoma-induced retinal detachment. Retina. 2012;32:799–804. doi: 10.1097/IAE.0b013e31823d8e1e. [DOI] [PubMed] [Google Scholar]
- 62.Muen WJ, Kingston JE, Robertson F, Brew S, Sagoo MS, Reddy MA. Efficacy and complications of super-selective intra-ophthalmic artery melphalan for the treatment of refractory retinoblastoma. Ophthalmology. 2012;119:611–6. doi: 10.1016/j.ophtha.2011.08.045. [DOI] [PubMed] [Google Scholar]
- 63.Vajzovic LM, Murray TG, Aziz-Sultan MA, Schefler AC, Wolfe SQ, Hess D, et al. Supraselective intra-arterial chemotherapy: Evaluation of treatment-related complications in advanced retinoblastoma. Clin Ophthalmol. 2011;5:171–6. doi: 10.2147/OPTH.S12665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Shields CL, Bianciotto CG, Jabbour P, Griffin GC, Ramasubramanian A, Rosenwasser R, et al. Intra-arterial chemotherapy for retinoblastoma: Report No 2, treatment complications. Arch Ophthalmol. 2011;129:1407–15. doi: 10.1001/archophthalmol.2011.151. [DOI] [PubMed] [Google Scholar]
- 65.Venturi C, Bracco S, Cerase A, Cioni S, Galluzzi P, Gennari P, et al. Superselective ophthalmic artery infusion of melphalan for intraocular retinoblastoma: Preliminary results from 140 treatments. Acta Ophthalmol. 2013;91:335–42. doi: 10.1111/j.1755-3768.2011.02296.x. [DOI] [PubMed] [Google Scholar]
- 66.Bianciotto C, Shields CL, Iturralde JC, Sarici A, Jabbour P, Shields JA. Fluorescein angiographic findings after intra-arterial chemotherapy for retinoblastoma. Ophthalmology. 2012;119:843–9. doi: 10.1016/j.ophtha.2011.09.040. [DOI] [PubMed] [Google Scholar]
- 67.Francis JH, Abramson DH, Gobin YP, Marr BP, Dunkel IJ, Riedel ER, et al. Electroretinogram monitoring of dose-dependent toxicity after ophthalmic artery chemosurgery in retinoblastoma eyes: Six year review. PLoS One. 2014;9:e84247. doi: 10.1371/journal.pone.0084247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Harbour JW, Murray TG, Hamasaki D, Cicciarelli N, Hernández E, Smith B, et al. Local carboplatin therapy in transgenic murine retinoblastoma. Invest Ophthalmol Vis Sci. 1996;37:1892–8. [PubMed] [Google Scholar]
- 69.Mendelsohn ME, Abramson DH, Madden T, Tong W, Tran HT, Dunkel IJ. Intraocular concentrations of chemotherapeutic agents after systemic or local administration. Arch Ophthalmol. 1998;116:1209–12. doi: 10.1001/archopht.116.9.1209. [DOI] [PubMed] [Google Scholar]
- 70.Mao Y, Wu Z, Yang H, Lin S, Zheng J. Measurement of intraocular concentrations of etoposide after systemic and local administration. Yan Ke Xue Bao. 2004;20:178–80. 186. [PubMed] [Google Scholar]
- 71.Carcaboso AM, Bramuglia GF, Chantada GL, Fandiño AC, Chiappetta DA, de Davila MT, et al. Topotecan vitreous levels after periocular or intravenous delivery in rabbits: An alternative for retinoblastoma chemotherapy. Invest Ophthalmol Vis Sci. 2007;48:3761–7. doi: 10.1167/iovs.06-1152. [DOI] [PubMed] [Google Scholar]
- 72.Schefler AC, Braich PS. Local chemotherapy. In: Ramasubramanian A, Shields CL, editors. Retinoblastoma. New Delhi, India: Jaypee Brothers Medical Publishers; 2012. pp. 99–108. [Google Scholar]
- 73.Marr BP, Dunkel IJ, Linker A, Abramson DH. Periocular carboplatin for retinoblastoma: Long-term report (12 years) on efficacy and toxicity. Br J Ophthalmol. 2012;96:881–3. doi: 10.1136/bjophthalmol-2011-300517. [DOI] [PubMed] [Google Scholar]
- 74.Mallipatna AC, Dimaras H, Chan HS, Héon E, Gallie BL. Periocular topotecan for intraocular retinoblastoma. Arch Ophthalmol. 2011;129:738–45. doi: 10.1001/archophthalmol.2011.130. [DOI] [PubMed] [Google Scholar]
- 75.Ericson LA, Rosengren BH. Present therapeutic resources in retinoblastoma. Acta Ophthalmol (Copenh) 1961;39:569–76. doi: 10.1111/j.1755-3768.1961.tb00269.x. [DOI] [PubMed] [Google Scholar]
- 76.Seregard S, Kock E, Af Trampe E. Intravitreal chemotherapy for recurrent retinoblastoma in an only eye. Br J Ophthalmol. 1995;79:194–5. doi: 10.1136/bjo.79.2.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Inomata M, Kaneko A. Chemosensitivity profiles of primary and cultured human retinoblastoma cells in a human tumor clonogenic assay. Jpn J Cancer Res. 1987;78:858–68. [PubMed] [Google Scholar]
- 78.Brodie SE, Munier FL, Francis JH, Marr B, Gobin YP, Abramson DH. Persistence of retinal function after intravitreal melphalan injection for retinoblastoma. Doc Ophthalmol. 2013;126:79–84. doi: 10.1007/s10633-012-9358-6. [DOI] [PubMed] [Google Scholar]
- 79.Ghassemi F, Shields CL. Intravitreal melphalan for refractory or recurrent vitreous seeding from retinoblastoma. Arch Ophthalmol. 2012;130:1268–71. doi: 10.1001/archophthalmol.2012.1983. [DOI] [PubMed] [Google Scholar]
- 80.Kivelä T, Eskelin S, Paloheimo M. Intravitreal methotrexate for retinoblastoma. Ophthalmology. 2011;118:1689.e1–6. doi: 10.1016/j.ophtha.2011.02.005. [DOI] [PubMed] [Google Scholar]
- 81.Smith SJ, Pulido JS, Salomão DR, Smith BD, Mohney B. Combined intravitreal and subconjunctival carboplatin for retinoblastoma with vitreous seeds. Br J Ophthalmol. 2012;96:1073–7. doi: 10.1136/bjophthalmol-2011-300829. [DOI] [PubMed] [Google Scholar]
- 82.Buitrago E, Del Sole MJ, Torbidoni A, Fandino A, Asprea M, Croxatto JO, et al. Ocular and systemic toxicity of intravitreal topotecan in rabbits for potential treatment of retinoblastoma. Exp Eye Res. 2013;108:103–9. doi: 10.1016/j.exer.2013.01.002. [DOI] [PubMed] [Google Scholar]
- 83.Shields CL, Manjandavida FP, Arepalli S, Kaliki S, Lally SE, Shields JA. Intravitreal melphalan for persistent or recurrent retinoblastoma vitreous seeds: Preliminary results. JAMA Ophthalmol. 2014;132:319–25. doi: 10.1001/jamaophthalmol.2013.7666. [DOI] [PubMed] [Google Scholar]
- 84.Brodie SE, Munier FL, Francis JH, Marr B, Gobin YP, Abramson DH. Persistence of retinal function after intravitreal melphalan injection for retinoblastoma. Doc Ophthalmol. 2013;126:79–84. doi: 10.1007/s10633-012-9358-6. [DOI] [PubMed] [Google Scholar]
- 85.Munier FL, Gaillard MC, Balmer A, Soliman S, Podilsky G, Moulin AP, et al. Intravitreal chemotherapy for vitreous disease in retinoblastoma revisited: From prohibition to conditional indications. Br J Ophthalmol. 2012;96:1078–83. doi: 10.1136/bjophthalmol-2011-301450. [DOI] [PubMed] [Google Scholar]
- 86.Seregard S, Singh AD. Retinoblastoma: Direct chemotherapeutic drug delivery into the vitreous cavity. Br J Ophthalmol. 2012;96:473–4. doi: 10.1136/bjophthalmol-2012-301528. [DOI] [PubMed] [Google Scholar]
- 87.Munier FL, Gaillard MC, Balmer A, Beck-Popovic M. Intravitreal chemotherapy for vitreous seeding in retinoblastoma: Recent advances and perspectives. Saudi J Ophthalmol. 2013;27:147–50. doi: 10.1016/j.sjopt.2013.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kaneko A, Suzuki S. Eye-preservation treatment of retinoblastoma with vitreous seeding. Jpn J Clin Oncol. 2003;33:601–7. [PubMed] [Google Scholar]
- 89.Smith SJ, Smith BD, Mohney BG. Ocular side effects following intravitreal injection therapy for retinoblastoma: A systematic review. Br J Ophthalmol. 2014;98:292–7. doi: 10.1136/bjophthalmol-2013-303885. [DOI] [PubMed] [Google Scholar]


