Abstract
Background:
Spectral domain enhanced depth imaging optical coherence tomography (EDI-OCT) can provide anatomic localization of intraocular tumors.
Aims:
The aim was to identify topographical and intrinsic patterns of choroidal tumors on EDI-OCT.
Settings and Design:
Retrospective review.
Materials and Methods:
Analysis of published reports and personal observations using office based EDI-OCT.
Results:
Using EDI-OCT, choroidal nevus displayed a smooth, dome-shaped topography with overlying retinal pigment epithelium alterations, drusen, and occasional subretinal cleft demonstrating photoreceptor loss. Small choroidal melanoma showed smooth, moderately dome-shaped topography, commonly with overlying shallow subretinal fluid that often depicted “shaggy” photoreceptors. Choroidal metastasis showed a minimally “lumpy, bumpy” surface topography and with overlying subretinal fluid and shaggy photoreceptors. Choroidal hemangioma showed a smooth, dome-shaped topography, with expansion of the affected small, medium, and large choroidal vessels. Choroidal lymphoma showed varying topography with increasing tumor thickness as “flat, rippled, or undulating (seasick)” surface. Choroidal osteoma displayed a smooth undulating surface with visible intralesional horizontal lines suggestive of bone lamellae and occasional horizontal and vertical tubules with intralesional “spongy” flecks. Choroidal melanocytosis appeared as uniformly thickened choroid with increased stromal density surrounding the normal choroidal vascular structures.
Conclusions:
Enhanced depth imaging-OCT can depict characteristic patterns that are suggestive of various choroidal tumors.
Keywords: Choroid, enhanced depth imaging optical coherence tomography, hemangioma, lymphoma, melanocytosis, melanoma, metastasis, nevus, osteoma, tumor
Optical coherence tomography (OCT) is an imaging method that provides cross-sectional anatomic depiction of the posterior segment of the eye.[1] There have been several articles written on the OCT features of choroidal tumors using the old-version time-domain OCT (TD-OCT) and the new-version spectral domain OCT (SD-OCT).[2,3,4] Innovations with SD-OCT allows for deeper visualization into the choroid and even the sclera and is termed SD enhanced depth imaging OCT (EDI-OCT).[5] Currently, EDI-OCT provides approximately 3-4 micron resolution of the retina and choroid. In this issue of the journal, we have provided a review of EDI-OCT features of retinal and retinal pigment epithelial tumors. Herein, we provide a review on EDI-OCT of choroidal tumors.
Choroidal Tumors
Choroid nevus
The choroidal nevus is a relatively common, benign intraocular tumor, with potential for visual loss if located under the foveola and with a rare risk for transformation into melanoma.[6,7,8,9,10,11,12] Greenstein et al. reviewed the prevalence of postequatorial choroidal nevus in various ethnic groups and found nevus in 4.1% of whites, 1.2% of Hispanics, 0.7% of blacks, and 0.04% of Chinese.[8] They commented that clinical features were similar among the different ethnic groups. Shields et al. reviewed clinical features of choroidal nevus in 3,422 eyes and noted that three features increase with patient age including nevus thickness, nevus multiplicity, and nevus-related drusen.[6] Transformation of choroidal nevus into melanoma is estimated at 1 in 8845.[12] Clinically-evident risk factors can identify nevus-at-risk, allowing for early intervention for melanoma therapy.[9,11]
Muscat et al. and Espinoza et al. studied TD-OCT of choroidal nevus in a few cases and noted overlying subretinal fluid and occasional intraretinal edema.[13,14] There was little detail on the tumor itself. Shields et al. evaluated a cohort of 120 eyes with choroidal nevus using TD-OCT and identified relatively common overlying retinal alterations of intraretinal edema, subretinal fluid, photoreceptor atrophy, and retinal pigment epithelium (RPE) detachment.[15]
Enhanced depth imaging-OCT of choroidal nevus has provided information on both the retinal and choroidal components. Shah et al. studied 104 eyes with choroidal nevus by EDI-OCT and described nevus as a domed, smooth-surfaced mass with deep choroidal shadowing depending upon tumor pigmentation.[16] Furthermore, they noted that not all patients are candidates for EDI-OCT, particularly if the patient was elderly or uncooperative, or if the eye showed media opacity, or the tumor was peripheral or >5 mm diameter. Specific EDI-OCT features of nevus included choriocapillaris compression overlying the nevus (94%), RPE atrophy (43%), RPE loss (14%), RPE nodularity (8%), photoreceptor loss (43%), ellipsoid irregularity (37%) or loss (6%), and mild inner retinal findings[16,17] [Fig. 1].
Figure 1.
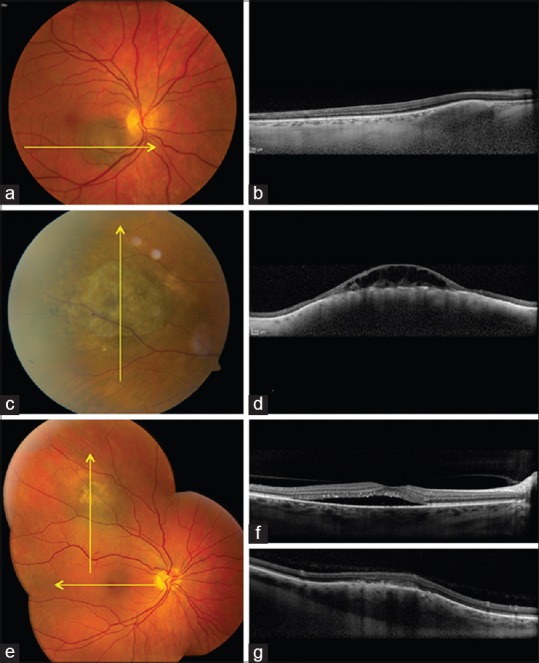
Choroidal tumors (pigmented). (a and b) Choroidal nevus (a) with enhanced depth imaging optical coherence tomography (EDI-OCT) (b) showing slightly elevated mass in outer choroid, compressing choriocapillaris inward. (c and d) Chronic choroidal nevus (c) with central overlying retinoschisis with EDI-OCT (d) showing gentle elevated choroidal mass with retinal pigment epithelial alterations and overlying retinoschisis. (e-g) Small choroidal melanoma (e) with EDI-OCT (f) showing subfoveal fluid with “shaggy” photoreceptors (f) and dome-shaped choroidal mass (g) with overlying outer retinal thickening
In summary, EDI-OCT of choroidal nevus shows smooth-surface topography, chronic overlying RPE and retinal degenerative findings and most prominently, photoreceptor atrophy.
Choroid melanoma
Small choroidal melanoma can be difficult to differentiate from choroidal nevus. Detection of melanoma at small tumor size is important as each millimeter (mm) increase in tumor thickness leads to approximately 5% increased risk for metastatic disease at 10 years.[18]
Differentiation of melanoma from nevus is based on identification of ophthalmoscopically-visible risk factors suggestive of melanoma such as greater tumor thickness, presence of subretinal fluid and overlying orange pigment (lipofuscin), tumor margin near the optic disc, and absence of drusen and halo.[11] TD-OCT has been used to evaluate retinal features over choroidal melanoma, with little detail on the choroidal mass.[13,14]
Enhanced depth imaging-OCT of small choroidal melanoma has revealed improved resolution of the choroidal findings as well as the retinal features. Shields et al. studied 37 eyes with small choroidal melanoma of 3 mm thickness or less and noted that mean tumor thickness was generally measured 50% less on EDI-OCT compared with ultrasonography, related to several factors.[19] These factors include difficulty on ultrasound in pinpointing the precise sclerochoroidal junction, poorer resolution of the overlying retina leading to an inadvertent inclusion with thickness measurement, and gross estimation with ultrasonographic calipers. Mrejen et al. suggested factors that could additionally lead to measurement discrepancy included patient age, globe myopia, diurnal variation of choroidal thickness, variability of scleral thickness, and the difficulty in identification of the posterior choroidal-scleral interface on ultrasonography as well as OCT.[20]
On EDI-OCT, choroidal melanoma shows features similar to nevus with deep optical shadowing and overlying choriocapillaris compression.[19] The most notable difference is that the relative recent melanoma shows subretinal fluid with overlying “shaggy” photoreceptors (49%)[19,21] [Fig. 1]. Other outer and inner retinal degenerative features were noted. A comparison of EDI-OCT of small choroidal melanoma versus similar-size nevus revealed melanoma with increased tumor thickness (P = 0.0001), subretinal fluid (P = 0.0001), subretinal lipofuscin deposition (P = 0.0001), RPE atrophy (P = 0.0002), intraretinal edema (P = 0.0029), photoreceptor shagginess (P = 0.0054), loss of external limiting membrane (P = 0.0082), loss of ellipsoid layer (P = 0.0233), irregularity of inner plexiform layer (P = 0.0385), and irregularity of ganglion cell layer (P = 0.0385).[19] Shaggy photoreceptors were found overlying choroidal melanoma (49%) but not observed in any case of nevus (0%) (P = 0.0001).[19]
In summary, EDI-OCT of choroidal melanoma generally shows gentle domed shaped, smooth-surface topography with relatively fresh subretinal fluid demonstrating shaggy photoreceptors. Shaggy photoreceptors could represent edematous photoreceptors or macrophages with lipofuscin on the posterior surface of the detached retina.
Choroid metastasis
Choroidal metastases appear as a yellow mass deep to the retina, often with overlying subretinal fluid.[22] Most metastases are small to medium size with a mean thickness of 3 mm and are located at the equator or posteriorly, suitable for OCT imaging. Arevalo et al. studied TD-OCT of choroidal metastasis and noted the prominent overlying subretinal fluid but little detail on the tumor itself.[23]
Witkin et al. demonstrated that EDI-OCT can identify subclinical choroidal metastases, barely seen with indirect ophthalmoscopy.[24] Demirci et al. observed EDI-OCT features of choroidal metastasis with plateau surface, shaggy photoreceptors, and subretinal fluid with speckles.[25]
Al-Dahmash et al. imaged 31 eyes with choroidal metastasis using EDI-OCT and found that only 14 (45%) were suitable for study.[26] The unsuitable tumors were generally too peripheral or too large for adequate imaging. The most salient EDI-OCT features included an irregular (“lumpy bumpy”) anterior contour (64%), anterior compression of the overlying choriocapillaris (93%), and posterior shadowing (86%)[26] [Fig. 2]. The “lumpy bumpy” surface is in contrast to melanoma and nevus that shows a smooth domed surface. Other features included overlying RPE abnormalities (78%), and structural loss of the interdigitation of the cone outer segment tips (64%), the ellipsoid portion of photoreceptors (57%), external limiting membrane (29%), outer nuclear layer (7%), and outer plexiform layer (7%). The inner retinal layers (inner nuclear layer to nerve fiber layer) were normal. Subretinal fluid (79%), subretinal lipofuscin (7%), and intraretinal edema (14%) were identified.
Figure 2.
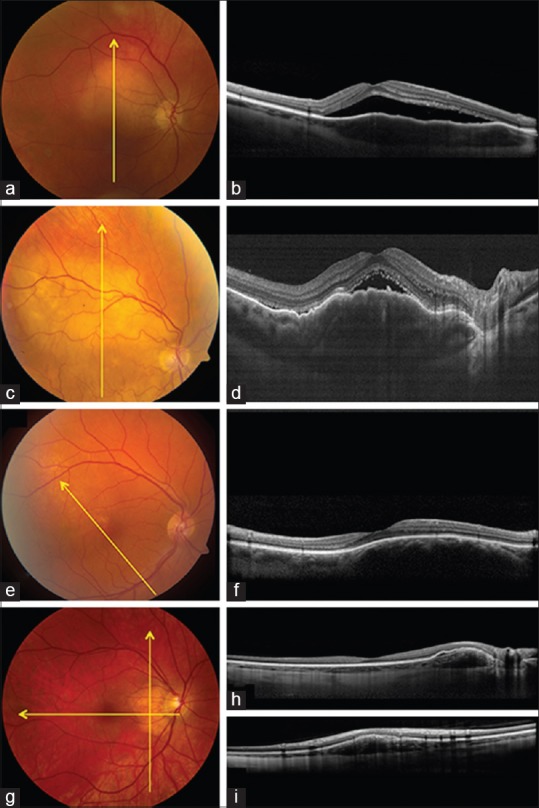
Choroidal tumors (nonpigmented). (a and b) Choroidal metastasis from breast cancer (a) with enhanced depth imaging optical coherence tomography (EDI-OCT) (b) showing subretinal fluid with slightly “shaggy” photoreceptors and slightly “lumpy bumpy” tumor surface. (c and d) Choroidal lymphoma (c) with EDI-OCT (d) showing undulating “seasick” tumor surface with overlying subretinal fluid. (e and f) Choroidal hemangioma (e) with EDI-OCT (f) showing smooth-surface and expanded choroidal vascular spaces. (g-i) Choroidal osteoma (g) with EDI-OCT (h and i) showing overlying retinal pigment epithelial detachment, smooth choroidal elevation and slight intrinsic horizontal lamellae within the choroidal mass
In summary, choroidal metastasis characteristically shows a “lumpy bumpy” topography that can be clinically and ultrasonographically too subtle to appreciate. Relatively fresh overlying subretinal fluid might appear with shaggy photoreceptors.
Choroid hemangioma
Circumscribed choroidal hemangioma is a benign vascular tumor, orange in color and classically located in the macular or paramacular region of the eye.[27] OCT has been found useful for differentiating choroidal hemangioma from central serous chorioretinopathy and for following therapeutic response after treatment.[28,29,30,31,32]
Rojanaporn et al. studied 10 eyes with newly-diagnosed circumscribed choroidal hemangioma, imaged with EDI-OCT, and noted that the tumor characteristically showed a smooth, gently sloping anterior contour (100%) and gradual choroidal expansion without choriocapillaris compression (100%).[32] The lumen of the choroidal vessels in the choriocapillaris, Haller's layer, and Sattler's layer were expanded rather than compressed as is seen with nevus, melanoma, and metastasis[32] [Fig. 2]. They calculated that the Haller's layer of vessels were increased in luminal width by 265% and the Sattler's layer increased by 576%. Other features included partial optical shadowing deep to the hemangioma (90%), subretinal fluid (70%), lipofuscin deposition (10%), and other features related to chronic subretinal fluid.[32]
In summary, EDI-OCT of circumscribed choroidal hemangioma depicted a smooth, gently sloping choroidal mass with expansion of small, medium, and large-size choroidal vessels without compression.
Choroid lymphoma
Choroidal lymphoma is a relatively rare malignancy with amelanotic infiltration of the posterior uvea, classically with low-grade, B-cell lymphoma. In an analysis of 73 eyes with choroidal lymphoma in 59 patients, this condition manifested as a primary (69%) or secondary (31%) infiltration.[33] Choroidal lymphoma can be clinically subtle with thin tumor infiltration that might not be detectable with fundus examination or ultrasonography, or it can be massive with extrascleral extension. By ultrasonography and magnetic resonance imaging, this malignancy shows relatively smooth-surface; however, the findings on EDI-OCT are remarkably different.
Shields et al. described the surface topography of choroidal lymphoma in 14 eyes, comparing the features to the oceanic surface as calm, rippled, or undulating (seasick).[34] With thin lymphoma infiltration (mean: 1.7 mm) the choroidal surface appeared calm, medium infiltration (mean: 2.8 mm) appeared rippled, and thick infiltration (mean: 4.1 mm) as undulating or “seasick”[34] [Fig. 2]. Markedly thickened infiltrates were not measurable on EDI-OCT. In contrast to ultrasound, the features on EDI-OCT were highly suggestive of the diagnosis with remarkable surface undulation. The choroicapillaris was compressed inward and medium, and large choroidal vessels were generally not appreciated.
In summary, EDI-OCT depicts choroidal lymphoma as the ocean surface with a calm, flat infiltration of the choroid if thin, a rippled appearance if thicker, and undulating “seasick” appearance if the tumor is thick.
Choroid osteoma
Choroidal osteoma is a rare tumor comprised of mature bone in the circumpapillary or macular region.[35] Choroidal osteoma usually manifests in young women, appearing as a yellow-orange, sharply marginated calcified plaque. In retrospective reviews choroidal osteoma, tumor growth was found in approximately 50% of cases, poor visual acuity in 50%, and visual acuity loss (>3 lines) in 50% of eyes.[36,37]
Shields et al. evaluated 22 cases of choroidal osteoma with TD-OCT and found the photoreceptor atrophy in all cases that had decalcification compared with intact photoreceptors in those with calcified osteoma.[38] Freton and Finger evaluated choroidal osteoma using EDI-OCT and noted choroidal compression and retinal degenerative changes.[39] Pellegrini et al. described 7 eyes with choroidal osteoma and noted that the inner retina was generally intact, but the outer retina showed anatomic abnormalities in external limiting membrane, myoid zone, ellipsoid zone, and photoreceptors.[40] An important diagnostic feature that they found was an intralesional sponge-like appearance.[40]
Shields et al. evaluated 15 consecutive eyes with choroidal osteoma using EDI-OCT.[41] They recognized an important feature of hyper reflective horizontal lamellar lines in every case, likely representing lamellae of bone [Fig. 2]. These lines can be subtle and are not seen with other choroidal tumors. In 9 cases, there were horizontal tubules (possible bone lamellae or vessels) and in 2 cases there were vertical tubules (presumed Haversion canals). Photoreceptor thinning or loss was noted in eyes with decalcification.[41]
In summary, EDI-OCT of choroidal osteoma reveals subtle horizontal hyper reflective lines (bony lamella), horizontal or vertical tubular channels (possible vessels), and an intrinsic sponge-like appearance.
Choroid melanocytosis
Ocular melanocytosis is a congenital pigmentary condition of the eye and periocular skin, also known as Nevus of Ota. Patients with this pigmentation carry a small risk for the development of uveal melanoma. Choroidal melanocytosis appears as a sectoral or diffuse hyperpigmentation of the posterior segment of the eye, often producing a dark pupillary reflex (melanocoria).[42,43] Affected patients should be carefully monitored for melanoma.
Pellegrini et al. has studied unilateral choroidal melanocytosis in 15 patients with EDI-OCT.[44] They noted several findings including flat surface topography (100%), 23% increased thickness in the affected eye compared to control eye, and perivascular interstitial tissue with 51% increased thickness, likely representing the increased melanocytic concentration. There was minimal effect on the overlying retina. These features allow nearly microscopic confirmation of the increased cellular tissue and emphasize the importance of EDI-OCT in our understanding and monitoring of ocular diseases.
Summary
EDI-OCT is an important tool for the imaging of choroidal tumors. Choroidal nevus, melanoma, and hemangioma have smooth dome-shaped surface but nevus has chronic overlying retinal atrophy, melanoma shows overlying subretinal fluid with “shaggy” photoreceptors, and hemangioma shows expanded luminal size of the choroidal vasculature. Choroidal metastasis has a slightly “lumpy bumpy” surface and often demonstrates overlying subretinal fluid. Choroidal lymphoma appears as “calm, rippled, or undulating” depending on tumor thickness, and choroidal osteoma has slightly domed or undulating surface with intrinsic horizontal and vertical lines and tubules representing bony lamellae and vessels. Choroidal melanocytosis shows a flat surface, but the intrinsic cellular tissue enwrapping the choroidal vessels is increased.
Precis
Spectral domain enhanced depth imaging optical coherence tomography shows characteristic tumor surface topography of dome-shaped for choroidal nevus, melanoma, and hemangioma, “lumpy bumpy” for metastasis, and “placid, rippled, or undulating (seasick)” for lymphoma. Other notable features are intrinsic “lamellae” with vertical or horizontal linear tubules for the osteoma.
Footnotes
Source of Support: Support provided by Eye Tumor Research Foundation, Philadelphia, PA (CLS, JAS), Lift for a Cure, Morrisdale, PA (CLS, JAS), and the Lucille Wiedman Fund for Pediatric Eye Cancer, Philadelphia, PA (JAS, CLS).
Conflict of Interest: None declared.
References
- 1.Huang D, Swanson EA, Lin CP, Schuman JS, Stinson WG, Chang W, et al. Optical coherence tomography. Science. 1991;254:1178–81. doi: 10.1126/science.1957169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shields CL, Materin MA, Shields JA. Review of optical coherence tomography for intraocular tumors. Curr Opin Ophthalmol. 2005;16:141–54. doi: 10.1097/01.icu.0000158258.01681.40. [DOI] [PubMed] [Google Scholar]
- 3.Say EA, Shah SU, Ferenczy S, Shields CL. Optical coherence tomography of retinal and choroidal tumors. J Ophthalmol 2012. 2012 doi: 10.1155/2012/385058. 385058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shields CL, Pellegrini M, Ferenczy SR, Shields JA. Enhanced depth imaging optical coherence tomography of intraocular tumors: From placid to seasick to rock and rolling topography – the 2013 Francesco Orzalesi Lecture. Retina. 2014;34:1495–512. doi: 10.1097/IAE.0000000000000288. [DOI] [PubMed] [Google Scholar]
- 5.Spaide RF, Koizumi H, Pozzoni MC. Enhanced depth imaging spectral-domain optical coherence tomography. Am J Ophthalmol. 2008;146:496–500. doi: 10.1016/j.ajo.2008.05.032. [DOI] [PubMed] [Google Scholar]
- 6.Shields CL, Furuta M, Mashayekhi A, Berman EL, Zahler JD, Hoberman DM, et al. Clinical spectrum of choroidal nevi based on age at presentation in 3422 consecutive eyes. Ophthalmology. 2008;115:546–552. doi: 10.1016/j.ophtha.2007.07.009. [DOI] [PubMed] [Google Scholar]
- 7.Ng CH, Wang JJ, Mitchell P, Amirul Islam FM, Wong TY. Prevalence and characteristics of choroidal nevi in an Asian vs white population. Arch Ophthalmol. 2009;127:314–9. doi: 10.1001/archophthalmol.2008.625. [DOI] [PubMed] [Google Scholar]
- 8.Greenstein MB, Myers CE, Meuer SM, Klein BE, Cotch MF, Wong TY, et al. Prevalence and characteristics of choroidal nevi: The multi-ethnic study of atherosclerosis. Ophthalmology. 2011;118:2468–73. doi: 10.1016/j.ophtha.2011.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shields CL, Cater J, Shields JA, Singh AD, Santos MC, Carvalho C. Combination of clinical factors predictive of growth of small choroidal melanocytic tumors. Arch Ophthalmol. 2000;118:360–4. doi: 10.1001/archopht.118.3.360. [DOI] [PubMed] [Google Scholar]
- 10.Shields CL, Furuta M, Mashayekhi A, Berman EL, Zahler JD, Hoberman DM, et al. Visual acuity in 3422 consecutive eyes with choroidal nevus. Arch Ophthalmol. 2007;125:1501–7. doi: 10.1001/archopht.125.11.1501. [DOI] [PubMed] [Google Scholar]
- 11.Shields CL, Furuta M, Berman EL, Zahler JD, Hoberman DM, Dinh DH, et al. Choroidal nevus transformation into melanoma: Analysis of 2514 consecutive cases. Arch Ophthalmol. 2009;127:981–7. doi: 10.1001/archophthalmol.2009.151. [DOI] [PubMed] [Google Scholar]
- 12.Singh AD, Kalyani P, Topham A. Estimating the risk of malignant transformation of a choroidal nevus. Ophthalmology. 2005;112:1784–9. doi: 10.1016/j.ophtha.2005.06.011. [DOI] [PubMed] [Google Scholar]
- 13.Muscat S, Parks S, Kemp E, Keating D. Secondary retinal changes associated with choroidal naevi and melanomas documented by optical coherence tomography. Br J Ophthalmol. 2004;88:120–4. doi: 10.1136/bjo.88.1.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Espinoza G, Rosenblatt B, Harbour JW. Optical coherence tomography in the evaluation of retinal changes associated with suspicious choroidal melanocytic tumors. Am J Ophthalmol. 2004;137:90–5. doi: 10.1016/s0002-9394(03)00868-7. [DOI] [PubMed] [Google Scholar]
- 15.Shields CL, Mashayekhi A, Materin MA, Luo CK, Marr BP, Demirci H, et al. Optical coherence tomography of choroidal nevus in 120 patients. Retina. 2005;25:243–52. doi: 10.1097/00006982-200504000-00001. [DOI] [PubMed] [Google Scholar]
- 16.Shah SU, Kaliki S, Shields CL, Ferenczy SR, Harmon SA, Shields JA. Enhanced depth imaging optical coherence tomography of choroidal nevus in 104 cases. Ophthalmology. 2012;119:1066–72. doi: 10.1016/j.ophtha.2011.11.001. [DOI] [PubMed] [Google Scholar]
- 17.Sayanagi K, Pelayes DE, Kaiser PK, Singh AD. 3D Spectral domain optical coherence tomography findings in choroidal tumors. Eur J Ophthalmol. 2011;21:271–5. doi: 10.5301/EJO.2010.5848. [DOI] [PubMed] [Google Scholar]
- 18.Shields CL, Furuta M, Thangappan A, Nagori S, Mashayekhi A, Lally DR, et al. Metastasis of uveal melanoma millimeter-by-millimeter in 8033 consecutive eyes. Arch Ophthalmol. 2009;127:989–98. doi: 10.1001/archophthalmol.2009.208. [DOI] [PubMed] [Google Scholar]
- 19.Shields CL, Kaliki S, Rojanaporn D, Ferenczy SR, Shields JA. Enhanced depth imaging optical coherence tomography of small choroidal melanoma: Comparison with choroidal nevus. Arch Ophthalmol. 2012;130:850–6. doi: 10.1001/archophthalmol.2012.1135. [DOI] [PubMed] [Google Scholar]
- 20.Mrejen S, Fung AT, Silverman RH, Kendall C, Freund KB. Potential pitfalls in measuring the thickness of small choroidal melanocytic tumors with ultrasonography. Retina. 2013;33:1293–9. doi: 10.1097/IAE.0b013e318296f681. [DOI] [PubMed] [Google Scholar]
- 21.Repucci M, Rojanaporn D, Shields CL. Shaggy photoreceptors: An EDI-OCT feature to differentiate small choroidal melanoma from choroidal nevus. Retina Today. 2012 Jul-Aug;:45–7. [Google Scholar]
- 22.Shields CL, Shields JA, Gross NE, Schwartz GP, Lally SE. Survey of 520 eyes with uveal metastases. Ophthalmology. 1997;104:1265–76. doi: 10.1016/s0161-6420(97)30148-1. [DOI] [PubMed] [Google Scholar]
- 23.Arevalo JF, Fernandez CF, Garcia RA. Optical coherence tomography characteristics of choroidal metastasis. Ophthalmology. 2005;112:1612–9. doi: 10.1016/j.ophtha.2005.03.030. [DOI] [PubMed] [Google Scholar]
- 24.Witkin AJ, Fischer DH, Shields CL, Reichstein D, Shields JA. Enhanced depth imaging spectral-domain optical coherence tomography of a subtle choroidal metastasis. Eye (Lond) 2012;26:1598–9. doi: 10.1038/eye.2012.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Demirci H, Cullen A, Sundstrom JM. Enhanced depth imaging optical coherence tomography of choroidal metastasis. Retina. 2013;8:303–7. doi: 10.1097/IAE.0000000000000083. [DOI] [PubMed] [Google Scholar]
- 26.Al-Dahmash SA, Shields CL, Kaliki S, Johnson T, Shields JA. Enhanced depth imaging optical coherence tomography of choroidal metastasis in 14 eyes. Retina. 2014;34:1588–93. doi: 10.1097/IAE.0000000000000131. [DOI] [PubMed] [Google Scholar]
- 27.Shields CL, Honavar SG, Shields JA, Cater J, Demirci H. Circumscribed choroidal hemangioma: Clinical manifestations and factors predictive of visual outcome in 200 consecutive cases. Ophthalmology. 2001;108:2237–48. doi: 10.1016/s0161-6420(01)00812-0. [DOI] [PubMed] [Google Scholar]
- 28.Rahman W, Horgan N, Hungerford J. Circumscribed choroidal haemangioma mimicking chronic central serous chorioretinopathy. J Fr Ophtalmol. 2013;36:e37–40. doi: 10.1016/j.jfo.2012.05.006. [DOI] [PubMed] [Google Scholar]
- 29.Blasi MA, Tiberti AC, Scupola A, Balestrazzi A, Colangelo E, Valente P, et al. Photodynamic therapy with verteporfin for symptomatic circumscribed choroidal hemangioma: Five-year outcomes. Ophthalmology. 2010;117:1630–7. doi: 10.1016/j.ophtha.2009.12.033. [DOI] [PubMed] [Google Scholar]
- 30.Liu W, Zhang Y, Xu G, Qian J, Jiang C, Li L. Optical coherence tomography for evaluation of photodynamic therapy in symptomatic circumscribed choroidal hemangioma. Retina. 2011;31:336–43. doi: 10.1097/IAE.0b013e3181e79887. [DOI] [PubMed] [Google Scholar]
- 31.Heimann H, Jmor F, Damato B. Imaging of retinal and choroidal vascular tumours. Eye (Lond) 2013;27:208–16. doi: 10.1038/eye.2012.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rojanaporn D, Kaliki S, Ferenczy SR, Shields CL. Enhanced depth imaging optical coherence tomography of circumscribed choroidal hemangioma in 10 consecutive cases. Submitted for publication. Middle East Afr J Ophthalmol. 2015 doi: 10.4103/0974-9233.150629. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mashayekhi A, Shukla SY, Shields JA, Shields CL. Choroidal lymphoma: Clinical features and association with systemic lymphoma. Ophthalmology. 2014;121:342–51. doi: 10.1016/j.ophtha.2013.06.046. [DOI] [PubMed] [Google Scholar]
- 34.Shields CL, Arepalli S, Pellegrini M, Mashayekhi A, Shields JA. Choroidal lymphoma shows calm, rippled, or undulating topography on enhanced depth imaging optical coherence tomography in 14 eyes. Retina. 2014;34:1347–53. doi: 10.1097/IAE.0000000000000145. [DOI] [PubMed] [Google Scholar]
- 35.Shields CL, Shields JA, Augsburger JJ. Choroidal osteoma. Surv Ophthalmol. 1988;33:17–27. doi: 10.1016/0039-6257(88)90069-0. [DOI] [PubMed] [Google Scholar]
- 36.Aylward GW, Chang TS, Pautler SE, Gass JD. A long-term follow-up of choroidal osteoma. Arch Ophthalmol. 1998;116:1337–41. doi: 10.1001/archopht.116.10.1337. [DOI] [PubMed] [Google Scholar]
- 37.Shields CL, Sun H, Demirci H, Shields JA. Factors predictive of tumor growth, tumor decalcification, choroidal neovascularization, and visual outcome in 74 eyes with choroidal osteoma. Arch Ophthalmol. 2005;123:1658–66. doi: 10.1001/archopht.123.12.1658. [DOI] [PubMed] [Google Scholar]
- 38.Shields CL, Perez B, Materin MA, Mehta S, Shields JA. Optical coherence tomography of choroidal osteoma in 22 cases: Evidence for photoreceptor atrophy over the decalcified portion of the tumor. Ophthalmology. 2007;114:e53–8. doi: 10.1016/j.ophtha.2007.07.037. [DOI] [PubMed] [Google Scholar]
- 39.Freton A, Finger PT. Spectral domain-optical coherence tomography analysis of choroidal osteoma. Br J Ophthalmol. 2012;96:224–8. doi: 10.1136/bjo.2011.202408. [DOI] [PubMed] [Google Scholar]
- 40.Pellegrini M, Invernizzi A, Giani A, Staurenghi G. Enhanced depth imaging optical coherence tomography features of choroidal osteoma. Retina. 2014;34:958–63. doi: 10.1097/IAE.0000000000000017. PMID: 24263470. [DOI] [PubMed] [Google Scholar]
- 41.Shields CL, Arepalli S, Atalay HT, Ferenczy SR, Fulco E, Shields JA. Choroidal osteoma shows bone lamella and vascular channels on enhanced depth imaging optical coherence tomography in 15 cases. Retina. 2014 doi: 10.1097/IAE.0000000000000376. Epub ahead of print PMID: 25296127. [DOI] [PubMed] [Google Scholar]
- 42.Singh AD, De Potter P, Fijal BA, Shields CL, Shields JA, Elston RC. Lifetime prevalence of uveal melanoma in white patients with oculo (dermal) melanocytosis. Ophthalmology. 1998;105:195–8. doi: 10.1016/s0161-6420(98)92205-9. [DOI] [PubMed] [Google Scholar]
- 43.Shields CL, Qureshi A, Mashayekhi A, Park C, Sinha N, Zolotarev F, et al. Sector (partial) oculo (dermal) melanocytosis in 89 eyes. Ophthalmology. 2011;118:2474–9. doi: 10.1016/j.ophtha.2011.05.023. [DOI] [PubMed] [Google Scholar]
- 44.Pellegrini M, Shields CL, Arepalli S, Shields JA. Choroidal melanocytosis evaluation with enhanced depth imaging optical coherence tomography. Ophthalmology. 2014;121:257–61. doi: 10.1016/j.ophtha.2013.08.030. [DOI] [PubMed] [Google Scholar]


