Abstract
The choroid is the most common ocular site for metastatic disease, owing to abundant vascular supply. The primary cancers that most commonly lead to choroidal metastases include breast cancer (40-47%) and lung cancer (21-29%). Bilateral, multifocal metastases are most often secondary to breast cancer, whereas unilateral, unifocal metastasis are more commonly found with lung cancer. The treatment of choroidal metastasis depends on the systemic status of the patient and number, location, and laterality of the choroidal tumors. Treatment options include observation in patients with poor systemic status or those with resolved or asymptomatic disease; systemic chemotherapy, immunotherapy, hormone therapy, or whole eye radiotherapy if the metastases are active, multifocal and bilateral; plaque radiotherapy, transpupillary radiotherapy, or photodynamic therapy for active, solitary metastasis; and enucleation for those with blind painful eye. A database search was performed on PubMed, using the terms “choroidal metastasis,” or “choroidal metastases,” in combination with terms such as “treatment,” “features,” or “diagnosis.” Relevant articles were extracted and reviewed.
Keywords: Choroid, Eye, metastases, metastasis, tumor, uvea
The first documented case of choroidal metastasis was by Perls in 1872.[1] Although once considered a rare entity, choroidal metastases are now considered the most common intraocular malignancy in the adult population. In a review of patients dying from the malignancy, 8% displayed choroidal metastases on autopsy.[2] Choroidal metastases tend to become apparent late in the course of malignancy, and are associated with disseminated disease and poor prognosis.[1,2,3,4,5]
The choroid is the most common ophthalmic site for metastatic disease, and it is postulated that hematogenous dissemination of metastasis from remote major sites typically leads to the high flow choroidal vasculature with metastatic disease.[4,5,6,7,8] The choroid provides a vascular avenue for tumor emboli to sequester and allows an environment receptive to growth.[7] In this article, we will review the common primary tumor origins, the salient features of metastases, and treatment options available for these lesions.
A database search was performed on PubMed, using the terms “choroidal metastasis,” or “choroidal metastases,” in combination with terms such as “treatment,” “features,” or “diagnosis.” Relevant English language articles were extracted, with reference lists of the articles reviewed for applicable articles. In cases of non-English language articles, if the abstract was translated into English, the article was referenced as (abstract) in the citation.
Ophthalmic Features and Origin
The choroid is the most common ocular structure affected by metastases.[5] In a review of 520 patients presenting with 950 metastases to the uvea, the choroid was involved in 88% of cases.[5] Other studies have similarly shown metastases to the choroid in 57-73% of cases.[3,4] Given the predominance of metastases involving the postequatorial region and their frequent development of subretinal fluid, patients typically present with blurred vision (70-81%).[5,9] The other less common manifestations include flashes and floaters (5-12%), and pain (5-14%).[5,10,11] However, 9–11% of patients present with no symptoms and lesions may be found on routine ocular examination [Fig. 1].[5,9]
Figure 1.
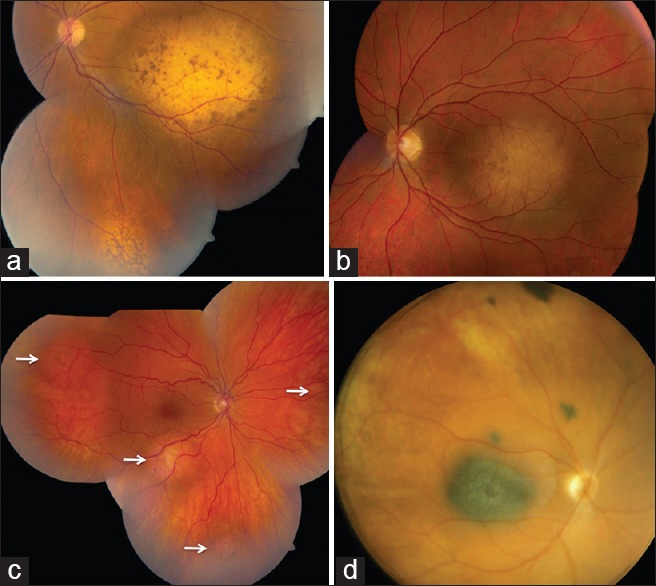
Choroidal metastases. (a) Multifocal amelanotic metastases with overlying brown lipofuscin deposits from breast carcinoma. (b) Unifocal amelanotic metastasis with overlying brown lipofuscin deposits and subretinal fluid from lung carcinoma. (c) Multifocal orange-colored metastases (arrows) with overlying brown lipofuscin deposits from lung carcinoid tumor. (d) Multifocal pigmented metastases from skin malignant melanoma
Based on a review of 479 eyes with choroidal metastasis by Shields et al., choroidal metastases generally appear as a yellow subretinal mass (94%) associated with subretinal fluid (73%).[5] Infrequently, the mass can appear with an orange color, typically with renal cell carcinoma, carcinoid tumor, or thyroid cancer metastases (3%) or brown-gray, usually with metastatic melanoma (3%). The majority of eyes (72%) had one focus, with mean number of tumors per eye being 1.6 (median: 1, range: 1-13).[5] In general, the lesions were located posterior to the equator in 88% of cases. Choroidal metastases were lateral (35%), superior (22%), inferior (17%), nasal (14%) and macular (12%). Measurement of the largest metastases revealed an average base of 9 mm and a thickness of 3 mm.[5] Bilateral, multifocal metastases were most common secondary to breast cancer, while unilateral, unifocal metastasis were more common with lung cancer.[5,12]
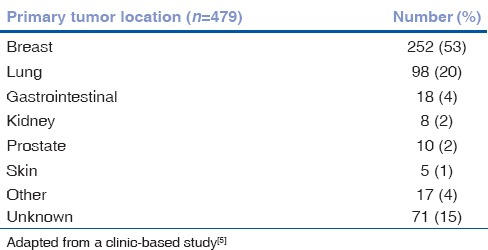
Breast carcinoma is the most common primary source of choroidal metastasis providing origin in 40-53% of cases, with 92% of patients having concurrent systemic metastases [Table 1].[4,5,12,13,14,15] In a study of 349 eyes with choroidal metastasis from breast carcinoma by Demirci et al., the tumors were postequatorial in 89% cases and multifocal in 48% cases.[12] Mean survival time from diagnosis of ocular metastases was 21 months.[12]
Table 1.
Choroidal metastases: Origin, features and therapy. Primary tumor origins
Lung cancer is the second most common source of choroidal metastasis accounting for 20-29% of cases.[4,5] Shah et al. analyzed the relationship of uveal metastases from lung cancer in 194 patients with 374 tumors and found that choroidal metastases from lung cancer tend to be unifocal (77%) and unilateral (82%).[16] Mean survival time following diagnosis of ocular metastases was 12 months.[16]
Less common primary tumors causing choroidal metastases include carcinoma of the gastrointestinal tract (4%), prostate (2%), kidney (2-4%), and skin (2%).[4,5,10] Rare primary carcinomas are metastasizing to choroid include tumors arising from the submandibular gland, thyroid, contralateral choroid, testes, ovaries, urothelial tract, neuroendocrine tumor, and sarcoma.[5,7,8,9,10,17,18,19,20,21,22] Most patients with choroidal metastasis have known systemic cancer at the time of eye diagnosis, but in 34% of cases, the choroidal metastasis precedes diagnosis of systemic cancer. Of those without known cancer, the primary tumor sites included lung cancer (7%), breast cancer (35%), and about 50% of patients with the primary site not found.[5]
Diagnosis
The differential diagnosis of choroidal metastases includes choroidal melanoma, hemangioma, granuloma, osteoma, and sclerochoroidal calcification. In cases without a history of a primary malignancy, diagnosis can be difficult, especially with roughly one-half of cases with no detectable primary tumor.[5] Distinct features on ophthalmoscopy and various imaging modalities distinguish choroidal metastases from other choroidal tumors.
Autofluorescence
Autofluorescence (AF) is a noninvasive imaging technique, which allows assessment of intrinsic AF of ocular tissues. The most common source is lipofuscin, a compound composed of digested outer photoreceptors stored in the retinal pigment epithelium.[23] On ophthalmoscopic examination, choroidal metastases often have overlying subretinal fluid and lipofuscin that typically appear as scattered clumps of brown pigment.[23,24,25,26] AF shows hypoautofluorescence of the tumor with overlying areas of bright 3+ hyperautofluorescence correlating to the deposits of lipofuscin and 2+ hyperautofluorescence of subretinal fluid.[26] These findings not only define tumor surface characteristics, but can also delineate progressing tumor margins.[23,24,25,26]
Ultrasonography
Ultrasonography determines tumor dimensions and echogenicity and allows differentiation of metastases from other intraocular neoplasms, particularly melanomas. In contrast to choroidal melanomas which display medium to low reflectivity on A-scan and are acoustically hollow on B-scan, choroidal metastases have a higher reflectivity on A-scan and appear echo-dense on B-scan, with a significantly lower height to base ratio compared to melanomas.[3,5,27,28] Rarely, cavitary variant has been described with carcinoid metastases, a feature previously only associated with choroidal melanoma.[29] <0.5% of metastases present with a “mushroom” or “collar-button” configuration.[5,30] Thickness tends to correlate to origin of metastases, with a mean thickness of metastases secondary to melanoma measuring 1 mm, breast 2 mm, lung and prostate 3 mm, and gastrointestinal and kidney measuring 4 mm.[5]
Optical Coherence Tomography
Initial studies by Arevalo et al. utilizing spectral domain optical coherence tomography (OCT) characterized the surface overlying choroidal metastasis as undulating, with thickening of the retinal pigment epithelium along with overlying subretinal fluid (86%).[31] Iuliano et al. described similar findings in a case of bilateral choroidal metastases, along with areas of hyperintense irregularities in the photoreceptor layer.[32] Al-Dahmash et al. later used enhanced depth imaging OCT (EDI-OCT) on 14 eyes with choroidal metastases to assess the deeper retinal and choroidal morphology, and found a characteristic “lumpy bumpy” choroidal surface in 9 (63%), with compression of the overlying choriocapillaris in 93% of cases, and irregularities of the outer retinal layer in 11 cases (79%).[33] EDI-OCT also allows detection of micro-metastases often in apparent with fundoscopy.[33]
Fluorescein Angiography
Fluorescein angiography (FA) can provide ancillary data necessary to differentiate choroidal metastases from choroidal melanomas. FA typically displays a hypofluorescent pattern in early arterial phases, with hyperfluoresence in the late venous phases, later than most choroidal melanomas.[3,25,26] Choroidal metastases also contain dilated retinal capillaries with a pinpoint leakage at the tumor border in 73% of cases as compared to melanoma in 16% of cases.[34]
Magnetic Resonance Imaging
Magnetic resonance imaging (MRI) often shows a well-demarcated choroidal mass that appears isointense on T1-weighted images and hypointense on T2-weighted images.[29,35] However, De Potter et al. have documented an unusual presentation of a metastasis on MRI, describing the lesion as a thin, diffuse choroidal mass, more consistent with a choroidal melanoma with optic nerve involvement. The lesion was hyperintense on T1 and hypointense on T2. After enucleation, pathology revealed the mass to be a mucin-secreting adenocarcinoma.[36]
Fine Needle Aspiration Biopsy
In cases of unidentified primary source and indeterminate diagnostic findings, fine needle aspiration biopsy can provide cytological evidence of metastasis versus primary occurrence.[3,37]
For posterior segment lesions with no retinal detachment, biopsy is obtained through a pars plana and transvitreal approach while cases of overlying retinal detachment necessitate a trans-scleral equatorial approach.[37]
Treatment
The treatment of choroidal metastasis depends on the systemic status, number of choroidal tumors, location, and laterality. Observation is preferred in patients with poor systemic status; systemic chemotherapy, immunotherapy, hormone therapy, or whole eye radiotherapy if the metastases are multifocal and bilateral; plaque radiotherapy, transpupillary radiotherapy, or photodynamic therapy (PDT) for solitary metastasis; and enucleation for blind painful eyes.
Systemic Treatment
Systemic chemotherapy, immunotherapy or hormone therapy is the preferred treatment strategy in patients with bilateral, multifocal choroidal metastases. The choice of drug depends on the type of primary cancer. Fenestrated endothelium in the choriocapillaris allows entry of medications into the choroidal tumors to provide effective tumor control.[5]
A large proportion of choroidal metastases from breast carcinomas express estrogen or progesterone receptors, making therapy with tamoxifen and aromatase inhibitors effective.[38,39] Manquez et al. showed tumor regression in 10 out of 17 patients with hormone receptor-positive breast cancer treated with aromatase therapy over a mean follow-up period of 20 months.[39] Regression of choroidal metastasis from breast and lung carcinoma after systemic chemotherapy has been noted.[40,41,42,43] In a study of 4 patients with choroidal metastasis treated with systemic chemotherapy alone, tumor regression occurred in 3 patients.[44]
External Beam Radiotherapy
External beam radiotherapy (EBRT) at a dosage of 40-60 Gy causes tumor regression in 85-93% of patients with vision improvement or stabilization in 56% of eyes.[19,45,46,47,48] However, the extended treatment period of EBRT makes the treatment inconvenient and impractical in critically ill-patients with poor life prognosis. Radiation related complications include cataract (7%), radiation retinopathy (3%), exposure keratopathy (3%), optic neuropathy (2%), and neovascularization of iris (2%).[48]
Gamme Knife Radiosurgery
Gamma knife radiosurgery (GKR) has been used in the treatment of uveal melanomas as well as choroidal metastases.[3] In this technique, multiple gamma rays are focused on the lesion, and usually requires an average dosage of 30 Gy administered over 10 days. Two reports have documented the success of GKR with choroidal metastases, with the larger study comprised of 57 eyes, documenting 100% control in Grade 1 lesions and 63% response in all choroidal metastases overall, over a mean follow-up period of 7 months.[45,46]
Proton Beam Radiotherapy
Proton beam radiotherapy (PBT) allows for more focused irradiation, as compared to GKR, as protons are delivered through beam and travel through tissue depth based on the amount of energy they contain, with less scatter to nearby tissues.[48] Tsina et al. showed regression of choroidal metastases in 84%, and stability of lesion in 14% of eyes treated with PBT over a mean follow-up period of 5 months, with 47% of patients maintaining or improving visual acuity. The average dosage administered was 28 Gy delivered over two treatments.[49] Complications included madarosis (28%), lid burns (17%), iris neovascularization and neovascular glaucoma (8%), cataract (11%), radiation maculopathy (19%) and radiation papillopathy (22%).[49,50]
Plaque Radiotherapy
Plaque radiotherapy provides more focused, targeted radiotherapy than EBRT resulting in good tumor control and fewer ocular complications. Shields et al. treated 36 patients with choroidal metastases with plaque radiotherapy and observed immediate regression in 100% of patients, with lasting regression in 94% over a mean follow-up period of 11 months.[51] Stable or improved vision was achieved in 58%. The average dosage was 69 Gy to the tumor apex, usually, applied over 3 days. Radiation retinopathy and/or papillopathy occurred in three patients (8%).[51] Lim and Petrovich documented tumor shrinkage and subretinal fluid resolution in all 5 eyes treated with plaque radiotherapy, with stable or improved vision in 60% over a mean follow-up period of 10 months. Late radiation complications included optic nerve atrophy (40%) and radiation retinopathy (20%).[51,52]
Transpupillary Thermotherapy
Transpupillary thermotherapy (TTT) uses diode laser to administer heat to the choroid and retinal pigment epithelium, inducing tumor necrosis.[4] The technique was first used as an adjuvant therapy for choroidal melanoma incompletely responsive to plaque radiotherapy.[53] Expansion of the technique into other intraocular tumors has led to the treatment of choroidal metastases.[54,55,56] In a study of 59 eyes with choroidal metastasis managed with TTT as the primary treatment, 71% of eyes showed regression or inhibition of growth, while 7% showed progression over a follow-up period of 15 months.[54]
Photodynamic Therapy
Photodynamic therapy causes tumor necrosis through the production of reactive singlet oxygen, as well intravascular thrombosis and subsequent tumor infarction through verteporfin. PDT has been used in the management of several ocular conditions, including age-related macular degeneration, retinal astrocytic hamartoma, choroidal hemangiomas, and melanomas.[3] PDT has been successfully used as either primary or secondary treatment of choroidal metastases.[57,58,59,60,61,62] Kaliki et al. documented tumor regression in 7 of 9 patients, with stable or improved vision in all but one case over an average follow-up period of 17 months.[62]
Intravitreal Antivascular Endothelial Growth Factor Injection
Intravitreal antivascular endothelial growth factor (VEGF) injections have redirected the management of neovascular ocular conditions. With the dependence of metastases on neovascularization for growth, the use of intravitreal bevacizumab and ranibizumab to prevent angiogenesis is an effective modality of treatment.[63,64,65,66,67,68,69] Successful management of metastases from breast, lung, and colorectal carcinomas have been documented with primary ocular therapy of anti-VEGF injections. Injections are typically given every 1-3 months, depending on tumor response, with regression noted on a follow-up period ranging from 4 to 22 months.[11,70,71,72,73,74]
Conclusion
Choroidal metastasis is the most common intraocular malignancy in the adult population. Choroidal metastases frequently occur in the later stages of disseminated disease and are considered a poor prognostic sign. With increasing treatments available leading to longer survival rates for cancer patients, metastases have the potential to become more prevalent. Effective control of these lesions is imperative. Systemic chemotherapy allows tumor control in some cases, while focal therapy is advised in tumors causing visual loss or is unresponsive to systemic treatment.
Footnotes
Source of Support: Support provided by the Eye Tumor Research Foundation, Philadelphia, PA (CLS), and Hyderabad Eye Research Foundation, Hyderabad, India (SK).
Conflict of Interest: None declared.
References
- 1.Perls M. Beitrage zur geschwulstlehere. Virchows Arch. 1872;1:437, 67. abstract only. [Google Scholar]
- 2.Bloch RS, Gartner S. The incidence of ocular metastatic carcinoma. Arch Ophthalmol. 1971;85:673–5. doi: 10.1001/archopht.1971.00990050675005. [DOI] [PubMed] [Google Scholar]
- 3.Shields JA, Shields CL. In: Intraocular Tumors. An Atlas and Textbook. 2nd ed. Philadelphia, PA: Lippincott Williams and Wilkins; 2008. Metastatic tumors to the uvea, retina, and optic disc; pp. 198–227. [Google Scholar]
- 4.Ferry AP, Font RL. Carcinoma metastatic to the eye and orbit. I. A clinicopathologic study of 227 cases. Arch Ophthalmol. 1974;92:276–86. doi: 10.1001/archopht.1974.01010010286003. [DOI] [PubMed] [Google Scholar]
- 5.Shields CL, Shields JA, Gross NE, Schwartz GP, Lally SE. Survey of 520 eyes with uveal metastases. Ophthalmology. 1997;104:1265–76. doi: 10.1016/s0161-6420(97)30148-1. [DOI] [PubMed] [Google Scholar]
- 6.Eliassi-Rad B, Albert DM, Green WR. Frequency of ocular metastases in patients dying of cancer in eye bank populations. Br J Ophthalmol. 1996;80:125–8. doi: 10.1136/bjo.80.2.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Avram AM, Gielczyk R, Su L, Vine AK, Sisson JC. Choroidal and skin metastases from papillary thyroid cancer: Case and a review of the literature. J Clin Endocrinol Metab. 2004;89:5303–7. doi: 10.1210/jc.2004-0757. [DOI] [PubMed] [Google Scholar]
- 8.Nabi G, Dadeya S, Dogra PN, Lal H. Eye metastasis form urothelial tumours. Int Urol Nephrol. 2002;34:51–4. doi: 10.1023/a:1021350710917. [DOI] [PubMed] [Google Scholar]
- 9.Besic N, Luznik Z. Choroidal and orbital metastases from thyroid cancer. Thyroid. 2013;23:543–51. doi: 10.1089/thy.2012.0021. [DOI] [PubMed] [Google Scholar]
- 10.Harbour JW, De Potter P, Shields CL, Shields JA. Uveal metastasis from carcinoid tumor. Clinical observations in nine cases. Ophthalmology. 1994;101:1084–90. doi: 10.1016/s0161-6420(94)38030-4. [DOI] [PubMed] [Google Scholar]
- 11.Stephens RF, Shields JA. Diagnosis and management of cancer metastatic to the uvea: A study of 70 cases. Ophthalmology. 1979;86:1336–49. doi: 10.1016/s0161-6420(79)35393-3. [DOI] [PubMed] [Google Scholar]
- 12.Demirci H, Shields CL, Chao AN, Shields JA. Uveal metastasis from breast cancer in 264 patients. Am J Ophthalmol. 2003;136:264–71. doi: 10.1016/s0002-9394(03)00192-2. [DOI] [PubMed] [Google Scholar]
- 13.Wiegel T, Kreusel KM, Bornfeld N, Bottke D, Stange M, Foerster MH, et al. Frequency of asymptomatic choroidal metastasis in patients with disseminated breast cancer: Results of a prospective screening programme. Br J Ophthalmol. 1998;82:1159–61. doi: 10.1136/bjo.82.10.1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Merrill CF, Kaufman DI, Dimitrov NV. Breast cancer metastatic to the eye is a common entity. Cancer. 1991;68:623–7. doi: 10.1002/1097-0142(19910801)68:3<623::aid-cncr2820680330>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 15.Thatcher N, Thomas PR. Choroidal metastases from breast carcinoma: A survey of 42 patients and the use of radiation therapy. Clin Radiol. 1975;26:549–53. doi: 10.1016/s0009-9260(75)80120-6. [DOI] [PubMed] [Google Scholar]
- 16.Shah SU, Mashayekhi A, Shields CL, Walia HS, Hubbard GB, 3rd, Zhang J, et al. Uveal metastasis from lung cancer: Clinical features, treatment, and outcome in 194 patients. Ophthalmology. 2014;121:352–7. doi: 10.1016/j.ophtha.2013.07.014. [DOI] [PubMed] [Google Scholar]
- 17.Singh DV, Gupta V, Singh SK. Choroid Metastasis from Testicular Carcinoma: A Rare Entity. Urol Int. 2013 doi: 10.1159/000351925. [DOI] [PubMed] [Google Scholar]
- 18.Kiratli H, Tarlan B, Söylemezoglu F. Papillary thyroid carcinoma: Bilateral choroidal metastases with extrascleral extension. Korean J Ophthalmol. 2013;27:215–8. doi: 10.3341/kjo.2013.27.3.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fernandez-Perez S, Ruiz-Moreno O, Pueyo V, de la Mata G, Pablo L. Bilateral choroidal metastases as presentation of dissemination of cutaneous malignant melanoma. Case Rep Ophthalmol Med 2012. 2012 doi: 10.1155/2012/486167. 486167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shields JA, Carvalho C, Shields CL, Singh AD, Wagner D. Bilateral choroidal metastasis from adenoid cystic carcinoma of the submandibular gland. Retina. 2000;20:406–7. doi: 10.1097/00006982-200004000-00018. [DOI] [PubMed] [Google Scholar]
- 21.De Potter P, Shields CL, Shields JA, Tardio DJ. Uveal metastasis from prostate carcinoma. Cancer. 1993;71:2791–6. doi: 10.1002/1097-0142(19930501)71:9<2791::aid-cncr2820710917>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 22.Eagle RC, Jr, Ehya H, Shields JA, Shields CL. Choroidal metastasis as the initial manifestation of a pigmented neuroendocrine tumor. Arch Ophthalmol. 2000;118:841–5. doi: 10.1001/archopht.118.6.841. [DOI] [PubMed] [Google Scholar]
- 23.Ishida T, Ohno-Matsui K, Kaneko Y, Tobita H, Hayashi K, Shimada N, et al. Autofluorescence of metastatic choroidal tumor. Int Ophthalmol. 2009;29:309–13. doi: 10.1007/s10792-008-9234-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Collet LC, Pulido JS, Gündüz K, Diago T, McCannel C, Blodi C, et al. Fundus autofluorescence in choroidal metastatic lesions: A pilot study. Retina. 2008;28:1251–6. doi: 10.1097/IAE.0b013e318188c7d0. [DOI] [PubMed] [Google Scholar]
- 25.Natesh S, Chin KJ, Finger PT. Choroidal metastases fundus autofluorescence imaging: Correlation to clinical, OCT, and fluorescein angiographic findings. Ophthalmic Surg Lasers Imaging. 2010;41:406–12. doi: 10.3928/15428877-20100426-03. [DOI] [PubMed] [Google Scholar]
- 26.Almeida A, Kaliki S, Shields CL. autofluorescence of intraocular tumours. Curr Opin Ophthalmol. 2013;24:222–32. doi: 10.1097/ICU.0b013e32835f8ba1. [DOI] [PubMed] [Google Scholar]
- 27.Sobottka B, Kreissig I. Ultrasonography of metastases and melanomas of the choroid. Curr Opin Ophthalmol. 1999;10:164–7. doi: 10.1097/00055735-199906000-00002. [DOI] [PubMed] [Google Scholar]
- 28.Verbeek AM. Differential diagnosis of intraocular neoplasms with ultrasonography. Ultrasound Med Biol. 1985;11:163–70. doi: 10.1016/0301-5629(85)90019-5. [DOI] [PubMed] [Google Scholar]
- 29.Shields CL, Say EA, Stanciu NA, Bianciotto C, Danzig CJ, Shields JA. Cavitary choroidal metastasis from lung neuroendocrine tumor: Report of 3 cases. Arch Ophthalmol. 2011;129:102–4. doi: 10.1001/archophthalmol.2010.329. [DOI] [PubMed] [Google Scholar]
- 30.Ward SD, Byrne BJ, Kincaid MC, Mann ES. Ultrasonographic evidence of a mushroom-shaped choroidal metastasis. Am J Ophthalmol. 2000;130:681–2. doi: 10.1016/s0002-9394(00)00604-8. [DOI] [PubMed] [Google Scholar]
- 31.Arevalo JF, Fernandez CF, Garcia RA. Optical coherence tomography characteristics of choroidal metastasis. Ophthalmology. 2005;112:1612–9. doi: 10.1016/j.ophtha.2005.03.030. [DOI] [PubMed] [Google Scholar]
- 32.Iuliano L, Scotti F, Gagliardi M, Bianchi I, Pierro L. SD-OCT patterns of the different stages of choroidal metastases. Ophthalmic Surg Lasers Imaging. 2012;43:e30–4. doi: 10.3928/15428877-20120308-03. Online. [DOI] [PubMed] [Google Scholar]
- 33.Al-Dahmash SA, Shields CL, Kaliki S, Johnson T, Shields JA. Enhanced depth imaging optical coherence tomography of choroidal metastasis in 14 eyes. Retina. 2014;34:1588–93. doi: 10.1097/IAE.0000000000000131. [DOI] [PubMed] [Google Scholar]
- 34.Li L, Wang WJ, Chen RJ, Qian J, Luo CQ, Zhang YJ, et al. Fundus fluorescein angiography in metastatic choroidal carcinomas and differentiating metastatic choroidal carcinomas from primary choroidal melanomas. Zhonghua Yan Ke Za Zhi. 2011;47:27–34. [PubMed] [Google Scholar]
- 35.Peyster RG, Augsburger JJ, Shields JA, Hershey BL, Eagle R, Jr, Haskin ME. Intraocular tumors: Evaluation with MR imaging. Radiology. 1988;168:773–9. doi: 10.1148/radiology.168.3.3406407. [DOI] [PubMed] [Google Scholar]
- 36.De Potter P, Shields JA, Shields CL, Yannuzzi LA, Fisher YE, Rao VM. Unusual MRI findings in metastatic carcinoma to the choroid and optic nerve: A case report. Int Ophthalmol. 1992;16:39–44. doi: 10.1007/BF00917071. [DOI] [PubMed] [Google Scholar]
- 37.Shields JA, Shields CL, Ehya H, Eagle RC, Jr, De Potter P. Fine-needle aspiration biopsy of suspected intraocular tumors. The 1992 Urwick Lecture. Ophthalmology. 1993;100:1677–84. doi: 10.1016/s0161-6420(93)31418-1. [DOI] [PubMed] [Google Scholar]
- 38.Logani S, Gomez H, Jampol LM. Resolution of choroidal metastasis in breast cancer with high estrogen receptors. Arch Ophthalmol. 1992;110:451–2. doi: 10.1001/archopht.1992.01080160029012. [DOI] [PubMed] [Google Scholar]
- 39.Manquez ME, Brown MM, Shields CL, Shields JA. Management of choroidal metastases from breast carcinomas using aromatase inhibitors. Curr Opin Ophthalmol. 2006;17:251–6. doi: 10.1097/01.icu.0000193105.22960.f6. [DOI] [PubMed] [Google Scholar]
- 40.George B, Wirostko WJ, Connor TB, Choong NW. Complete and durable response of choroid metastasis from nonsmall cell lung cancer with systemic bevacizumab and chemotherapy. J Thorac Oncol. 2009;4:661–2. doi: 10.1097/JTO.0b013e31819c9a73. [DOI] [PubMed] [Google Scholar]
- 41.Kosmas C, Malamos NA, Antonopoulos M. Complete regression of choroidal metastases from breast cancer after docetaxel-based systemic chemotherapy. Med Pediatr Oncol. 2000;34:229–30. doi: 10.1002/(sici)1096-911x(200003)34:3<229::aid-mpo16>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 42.Shome D, Jayadev C, Gadgil D, Natarajan S, Jain V. Systemic chemotherapy and tamoxifen induced regression of choroidal metastasis from a breast carcinoma in a male. Indian J Ophthalmol. 2007;55:475–7. doi: 10.4103/0301-4738.36491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Battikh MH, Ben Yahia S, Ben Sayah MM, Maatallah A, Joobeur S, Rouatbi N, et al. Choroid metastases revealing pulmonary adenocarcioma resolved with chemotherapy. Rev Pneumol Clin. 2004;60:353–6. doi: 10.1016/s0761-8417(04)72149-5. [DOI] [PubMed] [Google Scholar]
- 44.Yang CJ, Tsai YM, Tsai MJ, Chang HL, Huang MS. The effect of chemotherapy with cisplatin and pemetrexed for choroidal metastasis of nonsquamous cell carcinoma. Cancer Chemother Pharmacol. 2014;73:199–205. doi: 10.1007/s00280-013-2341-4. [DOI] [PubMed] [Google Scholar]
- 45.Ratanatharathorn V, Powers WE, Grimm J, Steverson N, Han I, Ahmad K, et al. Eye metastasis from carcinoma of the breast: Diagnosis, radiation treatment and results. Cancer Treat Rev. 1991;18:261–76. doi: 10.1016/0305-7372(91)90017-t. [DOI] [PubMed] [Google Scholar]
- 46.Chu FC, Huh SH, Nisce LZ, Simpson LD. Radiation therapy of choroid metastasis from breast cancer. Int J Radiat Oncol Biol Phys. 1977;2:273–9. doi: 10.1016/0360-3016(77)90085-2. [DOI] [PubMed] [Google Scholar]
- 47.Marchini G, Babighian S, Tomazzoli L, Gerosa MA, Nicolato A, Bricolo A, et al. Gamma Knife stereotactic radiosurgery of ocular metastases: A case report. Stereotact Funct Neurosurg. 1995;64(Suppl 1):67–71. doi: 10.1159/000098765. [DOI] [PubMed] [Google Scholar]
- 48.Rudoler SB, Corn BW, Shields CL, De Potter P, Hyslop T, Shields JA, et al. External beam irradiation for choroid metastases: Identification of factors predisposing to long-term sequelae. Int J Radiat Oncol Biol Phys. 1997;38:251–6. doi: 10.1016/s0360-3016(97)00050-3. [DOI] [PubMed] [Google Scholar]
- 49.Tsina EK, Lane AM, Zacks DN, Munzenrider JE, Collier JM, Gragoudas ES. Treatment of metastatic tumors of the choroid with proton beam irradiation. Ophthalmology. 2005;112:337–43. doi: 10.1016/j.ophtha.2004.09.013. [DOI] [PubMed] [Google Scholar]
- 50.Parsons JT, Bova FJ, Fitzgerald CR, Mendenhall WM, Million RR. Radiation retinopathy after external-beam irradiation: Analysis of time-dose factors. Int J Radiat Oncol Biol Phys. 1994;30:765–73. doi: 10.1016/0360-3016(94)90347-6. [DOI] [PubMed] [Google Scholar]
- 51.Shields CL, Shields JA, De Potter P, Quaranta M, Freire J, Brady LW, et al. Plaque radiotherapy for the management of uveal metastasis. Arch Ophthalmol. 1997;115:203–9. doi: 10.1001/archopht.1997.01100150205010. [DOI] [PubMed] [Google Scholar]
- 52.Lim JI, Petrovich Z. Radioactive plaque therapy for metastatic choroidal carcinoma. Ophthalmology. 2000;107:1927–31. doi: 10.1016/s0161-6420(00)00252-9. [DOI] [PubMed] [Google Scholar]
- 53.Oosterhuis JA, Journée-de Korver HG, Kakebeeke-Kemme HM, Bleeker JC. Transpupillary thermotherapy in choroidal melanomas. Arch Ophthalmol. 1995;113:315–21. doi: 10.1001/archopht.1995.01100030071024. [DOI] [PubMed] [Google Scholar]
- 54.Romanowska-Dixon B, Kowal J, Pogrzebielski A, Markiewicz A. Transpupillary thermotherapy (TTT) for intraocular metastases in choroid. Klin Oczna. 2011;113:132–5. [PubMed] [Google Scholar]
- 55.Ardjomand N, Kucharczyk M, Langmann G. Transpupillary thermotherapy for choroidal metastases. Ophthalmologica. 2001;215:241–4. doi: 10.1159/000050867. [DOI] [PubMed] [Google Scholar]
- 56.Vianna RN, Pena R, Muralha A, Muralha L, Muranaka E. Transpupillary thermotherapy in the treatment of choroidal metastasis from breast carcinoma. Int Ophthalmol. 2004;25:23–6. doi: 10.1023/b:inte.0000018525.92811.e4. [DOI] [PubMed] [Google Scholar]
- 57.Harbour JW. Photodynamic therapy for choroidal metastasis from carcinoid tumor. Am J Ophthalmol. 2004;137:1143–5. doi: 10.1016/j.ajo.2004.01.009. [DOI] [PubMed] [Google Scholar]
- 58.Soucek P, Cihelkova I. Photodynamic therapy with verteporfin in subfoveal choroidal metastasis of breast carcinoma (a controlled case) Neuro Endocrinol Lett. 2006;27:725–8. [PubMed] [Google Scholar]
- 59.Isola V, Pece A, Pierro L. Photodynamic therapy with verteporfin of choroidal malignancy from breast cancer. Am J Ophthalmol. 2006;142:885–7. doi: 10.1016/j.ajo.2006.06.008. [DOI] [PubMed] [Google Scholar]
- 60.Mauget-Faÿsse M, Gambrelle J, Quaranta-El Maftouhi M, Moullet I. Photodynamic therapy for choroidal metastasis from lung adenocarcinoma. Acta Ophthalmol Scand. 2006;84:552–4. doi: 10.1111/j.1600-0420.2006.00648.x. [DOI] [PubMed] [Google Scholar]
- 61.Kawakami S, Wakabayashi Y, Goto H. A case of presumed choroidal metastasis from carcinoid tumor treated by Photodynamic therapy with verteporfin. Clin Ophthalmol. 2013;7:2003–6. doi: 10.2147/OPTH.S51196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kaliki S, Shields CL, Al-Dahmash SA, Mashayekhi A, Shields JA. Photodynamic therapy for choroidal metastasis in 8 cases. Ophthalmology. 2012;119:1218–22. doi: 10.1016/j.ophtha.2011.12.024. [DOI] [PubMed] [Google Scholar]
- 63.Detorakis ET, Agorogiannis G, Drakonaki EE, Tsilimbaris MK, Pallikaris IG. Successful management of choroidal metastasis with intravitreal ranibizumab injections. Ophthalmic Surg Lasers Imaging. 2012;43:e47–51. doi: 10.3928/15428877-20120517-03. Online. [DOI] [PubMed] [Google Scholar]
- 64.Amselem L, Cervera E, Díaz-Llopis M, Montero J, Garcia-Pous M, Udaondo P, et al. Intravitreal bevacizumab (Avastin) for choroidal metastasis secondary to breast carcinoma: Short-term follow-up. Eye (Lond) 2007;21:566–7. doi: 10.1038/sj.eye.6702647. [DOI] [PubMed] [Google Scholar]
- 65.Kuo IC, Haller JA, Maffrand R, Sambuelli RH, Reviglio VE. Regression of a subfoveal choroidal metastasis of colorectal carcinoma after intravitreous bevacizumab treatment. Arch Ophthalmol. 2008;126:1311–3. doi: 10.1001/archophthalmol.2008.2. [DOI] [PubMed] [Google Scholar]
- 66.Kim SW, Kim MJ, Huh K, Oh J. Complete regression of choroidal metastasis secondary to nonsmall-cell lung cancer with intravitreal bevacizumab and oral erlotinib combination therapy. Ophthalmologica. 2009;223:411–3. doi: 10.1159/000229307. [DOI] [PubMed] [Google Scholar]
- 67.Mansour AM, Alameddine R. Intravitreal bevacizumab for consecutive multiple choroidal breast metastatic lesions. BMJ Case Rep 2012. 2012 doi: 10.1136/bcr.03.2012.6124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yao HY, Horng CT, Chen JT, Tsai ML. Regression of choroidal metastasis secondary to breast carcinoma with adjuvant intravitreal injection of bevacizumab. Acta Ophthalmol. 2010;88:e282–3. doi: 10.1111/j.1755-3768.2009.01684.x. [DOI] [PubMed] [Google Scholar]
- 69.Lai CL, Fan KS, Lee YH, Chen HC, Fan WH. Intravitreal administration of bevacizumab in the treatment of choroidal metastasis in a patient with erlotinib-failed pulmonary adenocarcinoma. Lung Cancer. 2012;76:496–8. doi: 10.1016/j.lungcan.2012.02.020. [DOI] [PubMed] [Google Scholar]
- 70.Tolentino M. Systemic and ocular safety of intravitreal anti-VEGF therapies for ocular neovascular disease. Surv Ophthalmol. 2011;56:95–113. doi: 10.1016/j.survophthal.2010.08.006. [DOI] [PubMed] [Google Scholar]
- 71.Freedman MI, Folk JC. Metastatic tumors to the eye and orbit. Patient survival and clinical characteristics. Arch Ophthalmol. 1987;105:1215–9. doi: 10.1001/archopht.1987.01060090073031. [DOI] [PubMed] [Google Scholar]
- 72.Albert DM, Rubenstein RA, Scheie HG. Tumor metastasis to the eye. I. Incidence in 213 adult patients with generalized malignancy. Am J Ophthalmol. 1967;63:723–6. [PubMed] [Google Scholar]
- 73.Chen CJ, McCoy AN, Brahmer J, Handa JT. Emerging treatments for choroidal metastases. Surv Ophthalmol. 2011;56:511–21. doi: 10.1016/j.survophthal.2011.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lin CJ, Li KH, Hwang JF, Chen SN. The effect of intravitreal bevacizumab treatment on choroidal metastasis of colon adenocarcinoma - case report. Eye (Lond) 2010;24:1102–3. doi: 10.1038/eye.2009.257. [DOI] [PubMed] [Google Scholar]


