Abstract
Targeted therapy in retinoblastoma (RB) is widely accepted as the current management tool with an aim of increasing drug availability at the tumor location. Inevitably the effect is several times higher compared to systemic delivery of chemotherapeutic drugs and carries less systemic toxicity. Despite tremendous advancement in saving life, eye salvage in advanced RB especially with active vitreous seeds remains a challenge. The hypoxic environment of the vitreous and reduced vitreous concentration of the drugs delivered makes these tumor seeds resistant to chemotherapy. Direct delivery of chemotherapeutic drugs into the vitreous cavity aids to overcome these challenges and is progressively being accepted worldwide. However, intraocular procedure in RB was abandoned due to high risk of extraocular tumor dissemination. Recently, the forbidden therapeutic technique was re-explored and modified for safe use. Although eye salvage rate has tremendously improved after intravitreal chemotherapy (IVitC), retinal toxicity, and vision salvage are yet to be validated. In our preliminary report of intravitreal melphalan in 11 eyes, we reported 100% eye salvage and 0% recurrence with an extended 15 months mean follow-up. In this review, we analyzed published reports on IVitC in RB via PubMed, Medline, and conference proceedings citation index, electronic database search, without language restriction that included case series and reports of humans and experimental animal eyes with RB receiving IVitC.
Keywords: Chemotherapy, intravitreal chemotherapy, retina, retinoblastoma, vitreous seeds
Passing through the era of primary enucleation for retinoblastoma (RB), we are now experiencing the benefits of chemotherapy in its various forms.[1,2,3,4,5,6] Targeted treatment in RB management has gained immense popularity recently, including intra-arterial chemotherapy (IAC) and intravitreal chemotherapy (IVitC).[3,4,5,6,7,8,9] These modern techniques allow introduction of chemotherapeutic agents directly at the tumor site, thus attaining maximum concentration locally. There has been a tremendous leap in the management from life salvage to eye and vision salvage. For decades, systemic intravenous chemotherapy has shown promising results.[2] Despite the availability of various protocols, vitreous seeding is the most important limiting factor for globe salvage in advanced intraocular RB.[10]
Treatment of vitreous seeds remains most challenging in RB.[10,11,12] Vitreous seeds are often visualized in endophytic proliferation of large undifferentiated tumors. Pathologically most of the vitreous seeds have been found to be necrotic tumor cells, but they can also exist as intact, viable tumor cells at risk for further tumor growth.[13] Apart from causing tumor implantation on the retina, the seed can also migrate to the anterior segment. Some cases of vitreous seeding are amenable to conventional treatment with systemic intravenous or IAC and radiotherapy, however, massive, diffuse infiltration of the vitreous can be resistant to available measures with unfavorable prognosis leading to enucleation.[10,11,14] Various adjuvant therapeutic modalities have been used in the past to address vitreous seeding in RB including, external beam radiotherapy (EBRT), cryotherapy prior to systemic chemotherapy and periocular delivery of chemotherapeutic agents that increase the therapeutic concentration in vitreous cavity.[11,15,16,17,18,19,20] Globe salvage ranged from as low as 3% with systemic chemotherapy and EBRT, to 77% with high-dose chemotherapy combined with periocular carboplatin and EBRT.[11,17] Reports from the developed countries on the outcome of supra-selective IAC have been encouraging in tumor control and subretinal and vitreous seeds as well. Abramson et al. reported the 2-year probability of eye salvage in advanced RB after IAC with vitreous seeds (64%), subretinal seeds (83%), and both (80%).[14] Shields et al. showed the outcome of IAC in Group D (n = 17) and Group E (n = 14) eyes with overall control of vitreous seeds in 86% (Group D = 91%; Group E = 80%).[4] Recent use of IVitC had shown promising results with 87% to 100% globe salvage in RB with vitreous seeds.[8,9] In this review we describe the indications, technique, complications, and outcome of IVitC for vitreous seeds in RB. Published reports on IVitC for RB both in humans and experimental animals (in vitro and in vivo) were identified and analyzed. The data were collected via PubMed, Medline, and conference proceedings citation Index electronic database search, without language restriction.
History and Background
The principle of IVitC is to achieve maximum drug concentration as close to the tumor for maximum therapeutic effect with the lowest systemic concentration. Introduction of intravitreal chemotherapeutic drugs dates back to early 1960 where Ericson and Rosengren treated six eyes with intravitreal injection of 0.3–0.4 mm3 thiotepa, injected with 0.3 mm fine canula through the posterior part of the ciliary body. It did not draw any definitive conclusion.[21] Further to this case series, Ericson et al. took the experiments to the lab and studied the toxic effects of nitrogen mustard, thiotepa, cyclophosphamide and methotrexate in experimental animals and concluded that except nitrogen mustard, others are devoid of toxicity in rabbit eyes.[22] Later in 1995, Seregard et al. revived the technique and injected 2 mg/0.5 ml of thiotepa in three eyes twice in 2 weeks followed by vitrectomy and continued supplemental thiotepa every week up to a total dose of 10–14 mg. Authors noted that there was absence of clinical response with the injections alone, requiring vitrectomy. Of the three eyes, one eye underwent enucleation, and all were devoid of orbital recurrence and systemic metastasis at a mean follow-up of 54 months. However, one enucleated eye showed proliferating viable tumor cells.[6] Even after these initial reports, there was hesitation to perform intraocular procedures in eyes with RB for many years as it entails risk of extraocular tumor dissemination and high risk of systemic metastasis and death. Karcioglu stressed the risk of needle track tumor dissemination after fine needle aspiration cytology with 25 and 27 gauge needles, instilling awareness and caution in injecting eyes with active RB.[23]
Intravitreal melphalan hydrochloride in retinoblastoma
The use of intravitreal melphalan hydrochloride has recently gained popularity worldwide and various authors consider it as an effective technique to destroy the floating vitreous seeds in RB. This medication is a cytotoxic nitrogen mustard derivative alkylating agent that inhibits both DNA and RNA synthesis, which was originally used for multiple myeloma, malignant melanoma and ovarian cancer. Inomata and Kaneko studied the effect of 12 anti-cancer drugs for eye preservation treatment and found that melphalan was most sensitive.[24] This was not only considered for the popular IAC for RB, but also were subsequently administered intravitreally by Ueda et al. Initial study on albino rabbits showed that a minimum dosage of 10 μg showed no toxic effects to the retina, moderate changes in electroretinogram (ERG) after 20 μg injection and 90 μg had deterioration in retinal function. It was also found that 10 μg injection was equivalent to an intravitreal concentration of 5.9 μg/ml and 4 μg/ml was the concentration required to completely suppress RB cell colony formation in-vivo in albino rabbits.[25] This led to the introduction of intravitreal melphalan as an eye preserving treatment for RB with vitreous seeds. Initially introduced by Suzuki and Kaneko, they have performed 896 melphalan injections in 237 eyes and dosage ranged from 8 μg to 24 μg. They reported extraocular extension in 0.4% (n = 1) following injection with dense vitreous seeds and anterior chamber involvement; yet another one with metastasis where intravitreal melphalan could not be excluded as the cause.[26] Henceforth, well-defined indications, specific contraindications, safe techniques, and dosage were evaluated by various authors.
In 2006, our team in Philadelphia at Wills Eye Hospital treated three patients with intravitreal melphalan using low dose of 8 μg. In each case, we witnessed vitreous seed regression but later recurrence at about 4–6 months point dissuaded us from further pursuing this route. In retrospect, the low dose of 8 μg was insufficient for therapy and in 2010, we adjusted our dose to a higher concentration of 20–30 μg with much-improved results. Currently, intravitreal melphalan is used on a weekly basis for RB in our practice.
Munier et al. recommended guidelines for treating vitreous seeds with intravitreal injection.[8] The indications elaborated were[8] “(i) absence of anterior segment invasion and posterior chamber invasion; (ii) absence of hyaloid detachment; (iii) absence of retinal detachment, tumor, and vitreous seeds at the entry site.” All the eyes underwent ultrasound biomicroscopy examination prior to the injection. They treated 23 eyes with vitreous seeds with 122 intravitreal melphalan injections weekly and documented vitreous seed regression after median of four injections of dose ranging from 20 μg to 30 μg.[8]
Ghassemi and Shields reported an evaluation of proper dose range for IVitC for RB. They explored low dose of 8 μg versus higher dose of 20–30 μg versus highest dose of 50 μg. They found poor control with the lowest dose (8 μg) and local toxicity with the highest dose (50 μg) that included cataract, vitreous and subretinal hemorrhage, and phthisis bulbi.[28] They concluded that a dose of 20–30 μg was ideal.
Most recently, Shields et al. published their study on 11eyes with recurrent vitreous seeds after chemotherapy. They found that after intravitreal injection of 20–30 μg melphalan, there was 100% seed control, 100% eye salvage and no recurrence at 9 months mean follow-up.[9]
Apart from melphalan, other chemotherapeutic agents used were, methotrexate, carboplatin, and topotecan. Kivelä et al. performed intravitreal methotrexate injection (400 μg) in 5 eyes out which two had vitreous seeds. It consisted of frequent injections of 20–27 times for 12 months with induction and maintenance phase with complete response.[29] The problem with methotrexate is the need for numerous injections up to 22 or more per protocol for a 1-year period compared to melphalan with 4–6 injections over 2–3 months.
Smith et al. reported sub-conjunctival carboplatin injection preceded by intravitreal injection of carboplatin (3–6 μg) in two eyes. Vitreous seed failed to respond after a single injection and prompted enucleation, which was histopathologically found to be devoid of any ocular structural toxicity.[30]
Another effective chemotherapy agent for RB is topotecan. Topotecan hydrochloride, a topoisomerase-1 inhibitor is being evaluated as a potential agent for treating vitreous seeds and is still in the experimental phase. Role of periocular topotecan in intraocular RB and intravenous administration for overt extraocular RB has been reported earlier and has limited toxicity.[18,31,32] Buitrago et al. studied the toxicity profile of intravitreal topotecan in rabbit eyes.[33] In their recent case series, Ghassemi et al. explored the effect of intravitreal topotecan combined with melphalan in 9 eyes and found that the combination of both agents was remarkably effective.[34] Similarly, our practice at Wills Eye Hospital has found outstanding vitreous seed control with both agents combined.
In our practice, we follow certain guidelines in evaluation and decision-making. Intravitreal melphalan is indicated in eyes with persistent and or recurrent vitreous seeds and used as adjuvant after maximum chemoreduction of RB either with systemic intravenous chemotherapy or IAC. All eyes undergoing melphalan is examined for contraindications including tumor extension to the ciliary body and/or anterior segment, tumor filling the eye, retinal detachment, and vitreous hemorrhage. Major risk is tumor dissemination breaching the ocular coats and to the anterior segment as well. The injection site is chosen 2 O’ clock hours away from the vitreous seed location in a quadrant free of seeds. The preferred site of injection is pars plana, 3–3.5 mm away from the limbus and the needle pointing towards the center of the vitreous cavity. It is essential to confirm the absence of the tumor in the site selected. Paracentesis prior to injection is preferred by few authors to attain globe hypotony and avoid reflux of vitreous with possible microscopic seed exteriorization. The dosage varied from 20 μg to 30 μg depending upon the distribution and extent of seeds for a maximum of 6 injections. Escalated doses are preferred in the presence of dense vitreous seeds, suboptimal response to prior injection and, recurrence. We observed complete regression of vitreous seeds after a median of four injections (range, 2–6).
Preparation of chemotherapeutic drugs
Melphalan hydrochloride is available as 50 mg lyophilized powder that is reconstituted with preservative-free 0.9% sodium chloride solution in a sterile chamber. Initially 10 ml of 0.9% preservative-free normal saline is added to achieve a concentration of 5 mg/1 ml and vigorously shaken until a clear solution is obtained. Further, 1 ml of melphalan is injected into an evacuated sterile vial to which 24 ml 0.9% sodium chloride is added to yield a solution of 0.2 mg/ml (200 μg/ml). The reconstituted drug (0.3 ml) is then transferred to a 1 ml luer lock syringe through a 5 μ filter. Dosage is adjusted accordingly (20 μg/0.1 ml; 25 μg/0.125 ml; 30 μg/0.15 ml). The major limitation in using melphalan is its short half-life and should be administered within 1 h of reconstitution. Similarly, topotecan hydrochloride is reconstituted to 20 μg/0.1 ml with 0.9% normal saline, but with a longer half-life.
Surgical technique and safety measures
Although the technique of intravitreal injection appears simple, it carries a risk of extraocular tumor dissemination in tumor-filled eyes. However, modified injection techniques can avoid major complications. Prior to the injection, quadrant and site of injection decided according to the distribution of vitreous seeds. Clinical evaluation of 360° pars plana is essential to detect remote tumor foci for safety. Ultrasound biomicroscopy may be used for confirmation. Under aseptic precautions, eye is confirmed, and drug delivered intravitreally via pars plana approach. The needle track size should be kept as small as possible; ideally 32-gauge needle is preferred. Following injection, triple freeze-thaw cryotherapy should be performed at the injection site simultaneously, on withdrawal of the needle [Figs. 1a and b]. The eyeball is gently jiggled with a forceps for the even distribution of the drug injected and reducing local toxicity. Fundus is examined postinjection for acute complications such as retinal detachment, vitreous and/or retinal hemorrhage. Topical balanced salt solution is used to rinse extraocular chemotherapy from the globe surface to minimize toxicity. Topical steroid/antibiotic is applied, and there is no patch or further eye drops prescribed. Family is instructed to not touch the eye for 1-week.
Figure 1.
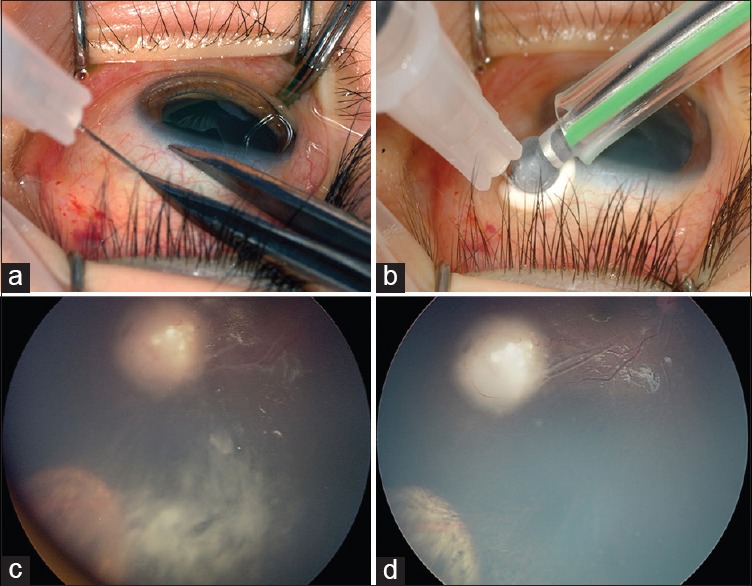
(a and b) Shows the safe technique of intravitreal chemotherapy delivery. Site of injection selected is pars-plana. Cryotherapy is applied simultaneously at the injection site on withdrawal of 32-gauge needle (c and d) fundus photograph of right eye regressed retinoblastoma with persistent vitreous seeds inferotemporally after primary treatment. The vitreous seeds appear completely regressed after 6 cycles of intravitreal melphalan with no recurrence at 9 months follow-up
Outcome and Complications
Since Kaneko et al. reported the efficacy of intravitreal melphalan in RB, it has been popularized as an approach with promising outcome.[7,25,26] Various authors have subsequently reported encouraging results for eye preservation in RB with vitreous seeds[8,9,27,28,29,35] [Table 1]. Munier et al. treated 23 eyes with recurrent and refractory vitreous seeds and achieved vitreous seed control in 91% eyes and the globe salvage in 87%. The Kaplan-Meier 2-year survival analysis estimated 84% ocular survival rate.[8] Shields et al. in their preliminary report of 11 eyes with vitreous seeds, showed 100% globe salvage with melphalan hydrochloride[9] [Figs. 1c and d]. Ghassemi et al. observed 66% eye salvage and rapid control (mean, 1.9; median, 2; range, 1–3 injections) of vitreous seeds with limited toxicity using a combination of melphalan and topotecan at 15 months mean follow-up.
Table 1.
Intravitreal chemotherapy outcome and complications - review
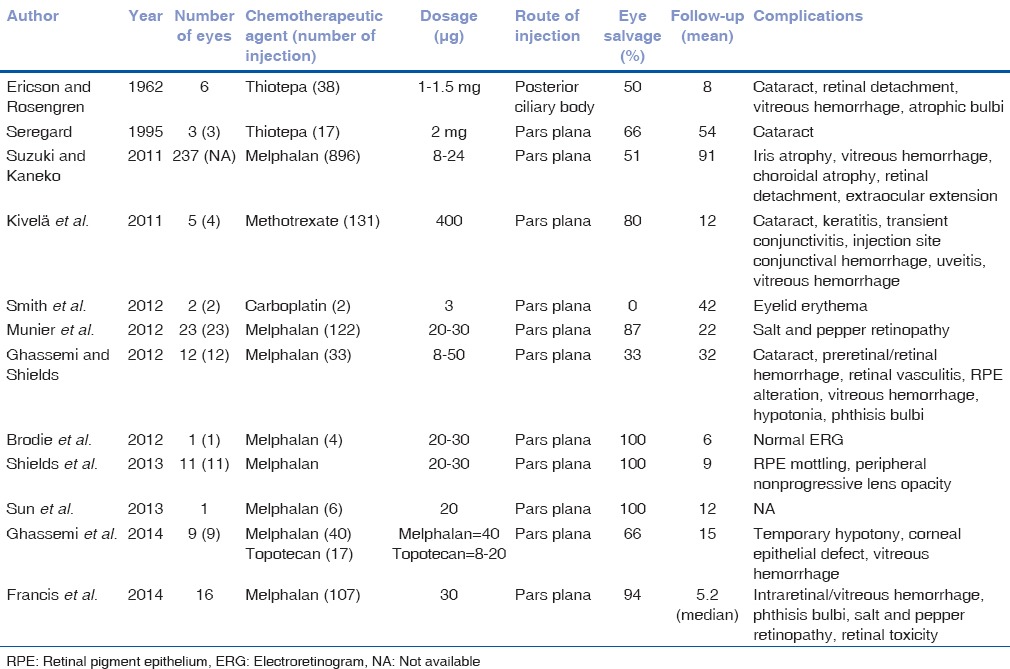
Ocular side effects of IVitC have been studied in detail [Table 1]. Most commonly encountered side effect includes retinal pigment mottling at the site of maximum concentration of the drug and was reported to be 18–43%.[8,9] Brodie et al. reported that photopic ERG was unchanged in an eye following melphalan injection, indicating preservation of retinal function after a dosage of 20–30 μg.[36] Conversely, Francis et al. in their clinical study on 16 patient eyes who received 30 μg, weekly intravitreal melphalan injections (n = 107), showed reduced ERG amplitude in all indicating abrupt permanent retinal toxicity and was found to be nonprogressive on completion of treatment. In the preclinical evaluation of rabbit eyes, which received equivalent dosage, revealed severe retinal damage histopathologically.[37] Smith et al. reviewed the ocular complications of IVitC in RB.[38] Complications reported by Kivelä et al., with methotrexate included transient conjunctivitis, transient keratitis, focal lenticular opacity, and mild vitreous hemorrhage in the series of 6 cases after 131 injections.[29] Associated minor complications of intravitreal melphalan injection include transient injection site erythema, sub-conjunctival hemorrhage and mild vitreous hemorrhage.[38] Ghassemi and Shields reported phthisis bulbi and hypotony with 50 μg leading to enucleation.[27] The author evaluated the enucleated eyes that received one injection of 50 μg and found severe gliosis, vascular occlusion, retinal necrosis, and neovascularization of retina and choroid. These eyes were devoid of viable RB cells. Out of the 7 eyes enucleated, eyes (n = 2) that received low dose (8–10 μg) of melphalan, contained viable tumor cells.[39] Recently, in an interesting meta-analysis by Smith et al., the risk of extraocular spread following intravitreal injection in RB was evaluated. Of the 315 eyes of 304 patients who underwent 1300 injections. The proportion of patients with extraocular spread was found to be 0.003 (1/304; 95% CI: 0.00008–0.0182).[40] From these data, the risk of extraocular dissemination is less and can be prevented with appropriate selection of cases and safety-enhanced techniques.
Conclusion
Current focus in the management of RB is eye salvage and vision salvage after decades-long efforts to save the life.[40] As the role of targeted chemotherapy is substantially gaining popularity, intravitreal injection of chemotherapeutic drugs in a controlled and safe manner is promising and can avoid enucleation and EBRT in advanced RB with vitreous seeds. Nevertheless, there should not be any hesitation in enucleating clinically indicated eyes to prevent systemic metastasis. The emphasis will remain on proper selection of cases, safe technique, and dosage.
Text box 1: Intravitreal chemotherapy in Retinoblastoma Indications:
-
-
Persistent vitreous seeds after systemic intravenous chemotherapy and/or intra-arterial chemotherapy
-
-
Recurrent vitreous seeds after completion of treatment
-
-
Chemoresistant new vitreous seeds
Contraindications:
-
-
Seeds dispersed diffusely in the entire vitreous cavity
-
-
Anterior segment and/or ciliary body invasion
-
-
Secondary glaucoma
-
-
High bullous retinal detachment
-
-
Vitreous hemorrhage obscuring the fundus view
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
References
- 1.Shields CL, Fulco EM, Arias JD, Alarcon C, Pellegrini M, Rishi P, et al. Retinoblastoma frontiers with intravenous, intra-arterial, periocular, and intravitreal chemotherapy. Eye (Lond) 2013;27:253–64. doi: 10.1038/eye.2012.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shields CL, Mashayekhi A, Au AK, Czyz C, Leahey A, Meadows AT, et al. The International Classification of Retinoblastoma predicts chemoreduction success. Ophthalmology. 2006;113:2276–80. doi: 10.1016/j.ophtha.2006.06.018. [DOI] [PubMed] [Google Scholar]
- 3.Abramson DH. Chemosurgery for retinoblastoma: What we know after 5 years. Arch Ophthalmol. 2011;129:1492–4. doi: 10.1001/archophthalmol.2011.354. [DOI] [PubMed] [Google Scholar]
- 4.Shields CL, Manjandavida FP, Lally SE, Pieretti G, Arepalli SA, Caywood EH, et al. Intra-arterial chemotherapy for retinoblastoma in 70 eyes: Outcomes based on the international classification of retinoblastoma. Ophthalmology. 2014;121:1453–60. doi: 10.1016/j.ophtha.2014.01.026. [DOI] [PubMed] [Google Scholar]
- 5.Gobin YP, Dunkel IJ, Marr BP, Brodie SE, Abramson DH. Intra-arterial chemotherapy for the management of retinoblastoma: Four-year experience. Arch Ophthalmol. 2011;129:732–7. doi: 10.1001/archophthalmol.2011.5. [DOI] [PubMed] [Google Scholar]
- 6.Seregard S, Kock E, Af Trampe E. Intravitreal chemotherapy for recurrent retinoblastoma in an only eye. Br J Ophthalmol. 1995;79:194–5. doi: 10.1136/bjo.79.2.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kaneko A, Suzuki S. Eye-preservation treatment of retinoblastoma with vitreous seeding. Jpn J Clin Oncol. 2003;33:601–7. [PubMed] [Google Scholar]
- 8.Munier FL, Gaillard MC, Balmer A, Soliman S, Podilsky G, Moulin AP, et al. Intravitreal chemotherapy for vitreous disease in retinoblastoma revisited: From prohibition to conditional indications. Br J Ophthalmol. 2012;96:1078–83. doi: 10.1136/bjophthalmol-2011-301450. [DOI] [PubMed] [Google Scholar]
- 9.Shields CL, Manjandavida FP, Arepalli S, Kaliki S, Lally SE, Shields JA. Intravitreal melphalan for persistent or recurrent retinoblastoma vitreous seeds: Preliminary results. JAMA Ophthalmol. 2014;132:319–25. doi: 10.1001/jamaophthalmol.2013.7666. [DOI] [PubMed] [Google Scholar]
- 10.Shields CL, Honavar SG, Shields JA, Demirci H, Meadows AT, Naduvilath TJ. Factors predictive of recurrence of retinal tumors, vitreous seeds, and subretinal seeds following chemoreduction for retinoblastoma. Arch Ophthalmol. 2002;120:460–4. [PubMed] [Google Scholar]
- 11.Manjandavida FP, Honavar SG, Reddy VA, Khanna R. Management and outcome of retinoblastoma with vitreous seeds. Ophthalmology. 2014;121:517–24. doi: 10.1016/j.ophtha.2013.09.011. [DOI] [PubMed] [Google Scholar]
- 12.Shields CL, Bianciotto CG, Jabbour P, Ramasubramanian A, Lally SE, Griffin GC, et al. Intra-arterial chemotherapy for retinoblastoma: Report No 1, control of retinal tumors, subretinal seeds, and vitreous seeds. Arch Ophthalmol. 2011;129:1399–406. doi: 10.1001/archophthalmol.2011.150. [DOI] [PubMed] [Google Scholar]
- 13.Amemiya T, Yoshida H, Ishigooka H. Vitreous seeds in retinoblastoma, clinical significance and ultrastructure. Albrecht Von Graefes Arch Klin Exp Ophthalmol. 1979;211:205–13. doi: 10.1007/BF02387425. [DOI] [PubMed] [Google Scholar]
- 14.Abramson DH, Marr BP, Dunkel IJ, Brodie S, Zabor EC, Driscoll SJ, et al. Intra-arterial chemotherapy for retinoblastoma in eyes with vitreous and/or subretinal seeding: 2-year results. Br J Ophthalmol. 2012;96:499–502. doi: 10.1136/bjophthalmol-2011-300498. [DOI] [PubMed] [Google Scholar]
- 15.Wilson TW, Chan HS, Moselhy GM, Heydt DD, Jr, Frey CM, Gallie BL. Penetration of chemotherapy into vitreous is increased by cryotherapy and cyclosporine in rabbits. Arch Ophthalmol. 1996;114:1390–5. doi: 10.1001/archopht.1996.01100140590011. [DOI] [PubMed] [Google Scholar]
- 16.Shields CL, Ramasubramanian A, Thangappan A, Hartzell K, Leahey A, Meadows AT, et al. Chemoreduction for group E retinoblastoma: Comparison of chemoreduction alone versus chemoreduction plus low-dose external radiotherapy in 76 eyes. Ophthalmology. 2009;116:544–51.e1. doi: 10.1016/j.ophtha.2008.10.014. [DOI] [PubMed] [Google Scholar]
- 17.Kingston JE, Hungerford JL, Madreperla SA, Plowman PN. Results of combined chemotherapy and radiotherapy for advanced intraocular retinoblastoma. Arch Ophthalmol. 1996;114:1339–43. doi: 10.1001/archopht.1996.01100140539004. [DOI] [PubMed] [Google Scholar]
- 18.Chantada GL, Fandino AC, Carcaboso AM, Lagomarsino E, de Davila MT, Guitter MR, et al. A phase I study of periocular topotecan in children with intraocular retinoblastoma. Invest Ophthalmol Vis Sci. 2009;50:1492–6. doi: 10.1167/iovs.08-2737. [DOI] [PubMed] [Google Scholar]
- 19.Marr BP, Dunkel IJ, Linker A, Abramson DH. Periocular carboplatin for retinoblastoma: Long-term report (12 years) on efficacy and toxicity. Br J Ophthalmol. 2012;96:881–3. doi: 10.1136/bjophthalmol-2011-300517. [DOI] [PubMed] [Google Scholar]
- 20.Shome D, Poddar N, Sharma V, Sheorey U, Maru GB, Ingle A, et al. Does a nanomolecule of Carboplatin injected periocularly help in attaining higher intravitreal concentrations? Invest Ophthalmol Vis Sci. 2009;50:5896–900. doi: 10.1167/iovs.09-3914. [DOI] [PubMed] [Google Scholar]
- 21.Ericson LA, Rosengren BH. Present therapeutic resources in retinoblastoma. Acta Ophthalmol (Copenh) 1961;39:569–76. doi: 10.1111/j.1755-3768.1961.tb00269.x. [DOI] [PubMed] [Google Scholar]
- 22.Ericson L, Karlberg B, Rosengren BH. Trials of intravitreal injections of chemotherapeutic agents in rabbits. Acta Ophthalmol (Copenh) 1964;42:721–6. doi: 10.1111/j.1755-3768.1964.tb01723.x. [DOI] [PubMed] [Google Scholar]
- 23.Karcioglu ZA, Gordon RA, Karcioglu GL. Tumor seeding in ocular fine needle aspiration biopsy. Ophthalmology. 1985;92:1763–7. doi: 10.1016/s0161-6420(85)34105-2. [DOI] [PubMed] [Google Scholar]
- 24.Inomata M, Kaneko A. In vitro chemosensitivity assays of retinoblastoma cells. Int J Clin Oncol. 2004;9:31–5. doi: 10.1007/s10147-003-0370-4. [DOI] [PubMed] [Google Scholar]
- 25.Ueda M, Tanabe J, Inomata M, Kaneko A, Kimura T. Study on conservative treatment of retinoblastoma – Effect of intravitreal injection of melphalan on the rabbit retina. J Jpn Ophthalmol Soc. 1995;99:1230–35. [PubMed] [Google Scholar]
- 26.Suzuki S, Kaneko A. Buenos Aires, Argentina: 2011. Vitreous injection therapy of melphalan for retinoblastoma (abstract). 15th Biennial Meeting. International Society of Ocular Oncology; November 14-17. [Google Scholar]
- 27.Akiyama M, Yamaoka M, Terao YM. London, UK: 2012. Reduced cycles for systemic chemotherapy with following more focused local treatment for the intraocular retinoblastoma (abstract). 44th Congress of the International Society of Pediatric Oncology (SIOP), 5-8 October. [Google Scholar]
- 28.Ghassemi F, Shields CL. Intravitreal melphalan for refractory or recurrent vitreous seeding from retinoblastoma. Arch Ophthalmol. 2012;130:1268–71. doi: 10.1001/archophthalmol.2012.1983. [DOI] [PubMed] [Google Scholar]
- 29.Kivelä T, Eskelin S, Paloheimo M. Intravitreal methotrexate for retinoblastoma. Ophthalmology. 2011;118:1689.e–6. doi: 10.1016/j.ophtha.2011.02.005. [DOI] [PubMed] [Google Scholar]
- 30.Smith SJ, Pulido JS, Salomão DR, Smith BD, Mohney B. Combined intravitreal and subconjunctival carboplatin for retinoblastoma with vitreous seeds. Br J Ophthalmol. 2012;96:1073–7. doi: 10.1136/bjophthalmol-2011-300829. [DOI] [PubMed] [Google Scholar]
- 31.Carcaboso AM, Bramuglia GF, Chantada GL, Fandiño AC, Chiappetta DA, de Davila MT, et al. Topotecan vitreous levels after periocular or intravenous delivery in rabbits: An alternative for retinoblastoma chemotherapy. Invest Ophthalmol Vis Sci. 2007;48:3761–7. doi: 10.1167/iovs.06-1152. [DOI] [PubMed] [Google Scholar]
- 32.Chantada GL, Fandiño AC, Casak SJ, Mato G, Manzitti J, Schvartzman E. Activity of topotecan in retinoblastoma. Ophthalmic Genet. 2004;25:37–43. doi: 10.1076/opge.25.1.37.28996. [DOI] [PubMed] [Google Scholar]
- 33.Buitrago E, Del Sole MJ, Torbidoni A, Fandino A, Asprea M, Croxatto JO, et al. Ocular and systemic toxicity of intravitreal topotecan in rabbits for potential treatment of retinoblastoma. Exp Eye Res. 2013;108:103–9. doi: 10.1016/j.exer.2013.01.002. [DOI] [PubMed] [Google Scholar]
- 34.Ghassemi F, Shields CL, Ghadimi H, Khodabandeh A, Roohipoor R. Combined intravitreal melphalan and topotecan for refractory or recurrent vitreous seeding from retinoblastoma. JAMA Ophthalmol. 2014;132:936–41. doi: 10.1001/jamaophthalmol.2014.414. [DOI] [PubMed] [Google Scholar]
- 35.Sun YB, Hui P, Punyara K, Bi MC, Li SH, Teng SY, et al. Intravitreal injection of melphalan in the treatment of retinoblastoma with vitreous cavity seeding. Chin Med J (Engl) 2013;126:1587. [PubMed] [Google Scholar]
- 36.Brodie SE, Munier FL, Francis JH, Marr B, Gobin YP, Abramson DH. Persistence of retinal function after intravitreal melphalan injection for retinoblastoma. Doc Ophthalmol. 2013;126:79–84. doi: 10.1007/s10633-012-9358-6. [DOI] [PubMed] [Google Scholar]
- 37.Francis JH, Schaiquevich P, Buitrago E, Del Sole MJ, Zapata G, Croxatto JO, et al. Local and systemic toxicity of intravitreal melphalan for vitreous seeding in retinoblastoma: A preclinical and clinical study. Ophthalmology. 2014;121:1810–7. doi: 10.1016/j.ophtha.2014.03.028. [DOI] [PubMed] [Google Scholar]
- 38.Smith SJ, Smith BD, Mohney BG. Ocular side effects following intravitreal injection therapy for retinoblastoma: A systematic review. Br J Ophthalmol. 2014;98:292–7. doi: 10.1136/bjophthalmol-2013-303885. [DOI] [PubMed] [Google Scholar]
- 39.Ghassemi F, Amoli FA. Pathological findings in enucleated eyes after intravitreal melphalan injection. Int Ophthalmol. 2014;34:533–40. doi: 10.1007/s10792-013-9851-2. [DOI] [PubMed] [Google Scholar]
- 40.Smith SJ, Smith BD. Evaluating the risk of extraocular tumour spread following intravitreal injection therapy for retinoblastoma: A systematic review. Br J Ophthalmol. 2013;97:1231–6. doi: 10.1136/bjophthalmol-2013-303188. [DOI] [PubMed] [Google Scholar]


