Abstract
Micronutrients are required in small proportions in a diet to carry out key metabolic roles for biomass and energy production. Humans receive micronutrients either directly from their diet or from gut microbiota that metabolize other nutrients. The nematode Caenorhabditis elegans and its bacterial diet provide a relatively simple and genetically tractable model to study both direct and microbe-mediated effects of micronutrients. Recently, this model has been used to gain insight into the relationship between micronutrients, physiology and metabolism. In particular, two B-type vitamins, vitamin B12 and folate, have been studied in detail. Here we review how C. elegans and its bacterial diet provide a powerful interspecies systems biology model that facilitates the precise delineation of micronutrient effects and the mechanisms involved.
Keywords: C. elegans, micronutrients, vitamin B, folate, gut microbiota, metabolism
Diet and micronutrients
An organism can be conceptualized as a living system that receives dietary inputs and converts these into biomass for reproduction, cell renewal or wound healing, and into energy to sustain daily tasks at the cellular and organismal levels. The diet consists of macronutrients that are consumed in large amounts and micronutrients that are much less abundant. Macronutrients, such as carbohydrates, fats and proteins are the main providers of carbon, nitrogen, and electrons/reducing power and are either incorporated into biomass or converted to energy. By contrast, the contribution of micronutrients such as vitamins, cofactors and most minerals to biomass and energy generation is more indirect. Generally, micronutrients are not metabolized except for minor modifications to acquire active molecular forms. However, they do play crucial roles in biomass synthesis and energy generation by activating metabolic enzymes, preventing oxidative damage as antioxidants, or by the hormonal regulation of biological processes.
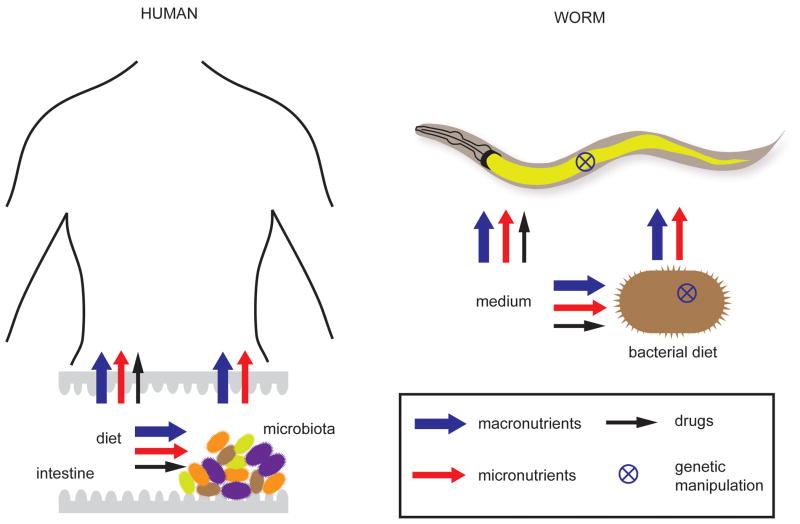
Diet can affect the human body directly or indirectly (Figure 1). Direct effects involve the absorbance and utilization of nutrients from the diet itself. Indirect dietary effects are mediated by the microbial community in the digestive track, known as the gut microbiota (hereafter referred to as the microbiota) (Figure 1). The importance of such indirect effects on human health and disease is increasingly appreciated [1]. First, microbiota metabolize dietary compounds that cannot be metabolized by humans. For instance, gut bacteria break down plant-derived dietary fibers resulting in the production of the short chain fatty acids (Box 1) propionate and butyrate [2]. Second, the microbiota can provide micronutrients such as vitamin B12 and vitamin K [3]. Third, the breakdown of xenobiotics by microbial metabolism can contribute to the nutrient pool in the gut during medical treatment [4]. It is therefore perhaps not surprising that the microbiota is increasingly associated with a wide variety of human diseases, including type-2 diabetes [5], cardiovascular disease [6], obesity [7], immunity and inflammatory disease [8] and even autism [9]. Although the role of the microbiota on human health and disease is widely acknowledged, the underlying mechanisms are mostly not resolved.
Figure 1.
C. elegans as a model organism to study the direct and microbiota-mediated effects of diet.
Box 1.
Glossary
- Life history traits
Measurable physiological and phenotypic properties that affect health of an animal and the population. Examples include brood size, developmental rate and lifespan.
- Postembryonic developmental rate
The rate at which C. elegans proceeds through the larval stages (L1, L2, L3, and L4) to the young adult stage and then to adulthood.
- RNAi
RNA interference to inhibit gene expression. In C. elegans, double stranded RNA (dsRNA) complementary to the targeted mRNA on one strand is given to worms by feeding a bacterial strain that carries the dsRNA in a plasmid vector.
- Short chain fatty acids (SCFA)
Fatty acids with five or fewer carbons: including formic- (1 carbon), acetic- (2 carbon), propionic- (3 carbon), butyric- (4 carbon) and valeric- (5 carbon) acid. SCFA are products of fatty acid beta oxidation as well bacterial fermentation, for example in human colon.
- Vitamin B12 (cobalamin)
One of the water-soluble B vitamins that is important for blood formation and normal functioning of nerves. It is the most complicated vitamin, with a cobalt atom placed at the center of a tetra-pyrrole ring. Depending on the group (R) attached to this cobalt atom, it takes different forms, which include hydroxocobalamin (R=OH), adenosylcobalamin (R=adenosyl group), and methylcobalamin (R=methyl group).
- Vitamin B9 (Folic acid)
Another water-soluble B vitamin that is important for cell division and growth. Folic acid is activated when converted to dihydrofolate, which is transformed into tetrahydrofolate, the active form in one carbon pool by folate (also known as the folate cycle; Figure 3). Different derivatives of tetrahydrofolate exist including 10-formyltetrahydrofolate, 5,10-methenyltetrahydrofolate, 5,10-methylenetetrahydrofolate, and 5-methyltetrahydrofolate.
Systematically studying the effects and mechanisms of individual micronutrients is difficult in humans and other mammals, including mice. This is not only because of the long lifespans and internal complexity of these organisms, but also because genome-scale whole organism genetics is not feasible for ethical reasons or is prohibitively expensive. Further, gut microbiota are highly complex communities including many species that are yet to be cultured in the laboratory. Here, we discuss how the nematode Caenorhabditis elegans and its bacterial diet have been used as a genetically tractable model to gain insights into the physiological effects of micronutrients, with a focus on B-type vitamins.
C. elegans as a model organism
C. elegans is a metazoan that lives in temperate soil environments and mainly feeds on bacteria growing on rotting vegetation [10]. Since it was introduced as a genetic model organism [11], “the worm” has proven to be a powerful model that has served to provide seminal insights into a myriad of biological processes including development [12, 13], cancer [14], and aging [15]. C. elegans offers multiple unique advantages as a model organism, such as physiological simplicity (~1000 somatic cells), a short lifespan (~2 weeks), an early and short reproductive period, and hermaphroditic reproduction.
In the last few years the worm has also been developed as a suitable model to study the effects of diet on metabolism, physiology and gene expression. An interesting aspect of the C. elegans model is that bacteria serve as food, which means that bacterial biomass is the main source of macronutrients. Thus, the relationship of C. elegans with bacteria is different from the synergistic relationship between mammals and microbiota. However, live bacteria can also provide sufficient supplies of micronutrients to the worm as a by-product of their metabolism, absorb compounds from the environment and deliver them to the worm, or convert compounds into other molecules that are useful for the worm. Thus, the bacterial component of the C. elegans model can represent both direct and indirect aspects of a diet (Figure 1). In addition, C. elegans is able to directly absorb micronutrients and drugs from its environment or the growth medium, representing direct effects by itself.
The standard laboratory diet of C. elegans has been OP50, a B strain of Escherichia coli, although it has been fed many different types of bacteria in controlled experiments [16]. Typically, a co-culture of C. elegans and a bacterium is grown on solid media supplemented with peptone to promote bacterial proliferation, and this mode of growth is referred to as monoxenic. However, C. elegans can also be cultured in liquid with bacteria, or in an axenic medium with a well-defined set of macro- and micronutrients [17]. Thus, the direct effects of specific nutrients on C. elegans can be tested by the addition or omission of these factors either in axenic or monoxenic growth conditions. However, postembryonic developmental rate and fecundity are compromised in axenic medium and in monoxenic conditions with dead bacteria [18, 19]. This implies that metabolically active bacteria support optimal worm health, analogous to the microbiota that grows in the nutritionally rich environment of the human gut and supports human health by metabolic by-products. Because C. elegans and its bacterial diets are genetically tractable, they can both be manipulated to systematically dissect the underlying mechanisms using an “interspecies systems biology” approach.
Different bacterial diets cause significant changes in C. elegans physiology and gene expression when compared to the standard OP50 diet [19–22]. Changes elicited by one particular bacterial diet, Comamonas aquatica (hereafter referred to as Comamonas), were attributed to a dilutable compound, because this species can affect worm development and gene expression even when dramatically diluted with the OP50 diet [19]. Combined genetic analyses of E. coli, Comamonas and the worm itself unveiled that this dilutable compound is the micronutrient vitamin B12 [23] (see below). This provides a powerful example of how monoxenic growth of C. elegans can be used as a model system to derive connections between micronutrients, gene expression and life-history traits (Box 1). We will first review studies of vitamin B12 and folate using the worm model, and then briefly discuss additional studies that focused on other micronutrients.
Vitamin B12 profoundly affects C. elegans physiology and metabolism
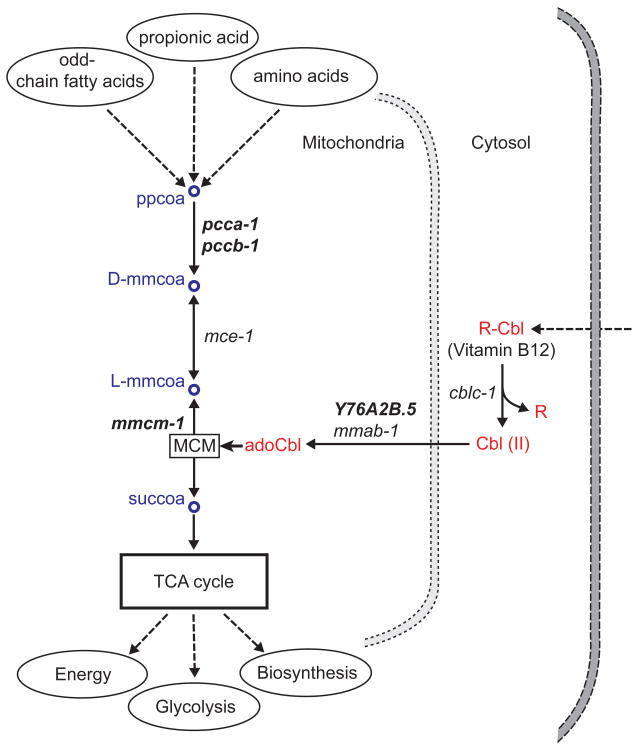
Vitamin B12, or cobalamin, occurs in different forms (Box 1) and is an essential cofactor for two enzymes in two distinct pathways. The first enzyme is methylmalonyl coenzyme A (CoA) mutase (MCM), which uses adenosylcobalamin as a cofactor and converts methylmalonyl-CoA to succinyl-CoA as part of the mitochondrial propionic acid breakdown pathway (Figure 2). This pathway receives carbon from the catabolism of propionic acid, branched chain amino acids, odd-chain fatty acids, and cholesterol. The end product succinyl-CoA then enters the tricarboxylic acid (TCA) cycle to generate energy and recycle carbon. The interruption of this pathway in humans, either due to recessive mutations in the gene encoding MCM or because of vitamin B12 deficiency, causes the devastating disease methylmalonic aciduria, which manifests itself as organ failure as a result of the accumulation of methylmalonic acid in tissues and body fluids [24–26].
Figure 2.
Propionic acid breakdown pathway and Vitamin B12. C. elegans genes that are associated with each reaction are shown in italics. Human orthologs of selected genes (bold italics) are provided in Table 1. Dashed arrows indicate pathways not shown in detail. MCM, Methylmalonyl-CoA mutase; ppcoa, propionyl CoA; mmcoa, methylmalonyl CoA; succoa, succinyl CoA; Cbl, cobalamin; adoCbl, adonesylcobalamine.
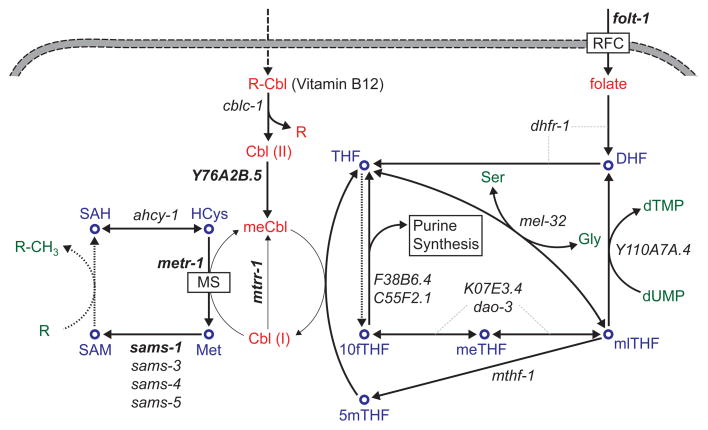
The second vitamin B12-dependent enzyme, methionine synthase (MS), uses methylcobalamin to convert homocysteine (HCys) to methionine in the cytosolic methionine/SAM cycle (Figure 3). Because of this reaction, vitamin B12 deficiency is associated with the metabolic syndrome homocysteinemia, which results from the toxic build-up of HCys [27, 28]. MS activity is connected to DNA synthesis and amino acid interconversions via the folic acid cycle (Figure 3). Some of the methionine synthesized by MS is used for protein production, while the rest is recycled back to HCys by the generation and subsequent conversion of S-adenosylmethionine (SAM) into S-adenosylhomocysteine (SAH). SAM acts as the major methyl donor for the methylation of nucleic acids, proteins (e.g., histones), lipids (e.g., phosphatidylcholine), and other metabolites such as neurotransmitters, creatine, carnitine and polyamines. Thus, MS activity can be linked to important cellular processes involving biomass synthesis as well as regulation. In accordance with these broad impacts of MS (Figure 3), vitamin B12 deficiency has also been associated with megaloblastic anemia [29], neuropathy [30], fetal development defects [31] and pregnancy loss [32]. Overall, vitamin B12 is a crucial micronutrient for human health because of its role in both pathways (Figures 2 and 3).
Figure 3.
Methionine and folic acid cycles. C. elegans genes that are associated with each reaction are shown in italics. Human orthologs of selected genes (bold italics) are provided in Table 1. Dotted arrows indicate multiple reactions with multiple genes. MS, methionine synthase; RFC, reduced folate carrier; DHF, dihydrofolate; THF, tetrahydrofolate; 10fTHF, 10-formyltetrahydrofolate, meTHF, 5,10-methenyltetrahydrofolate; mlTHF, 5,10-methylenetetrahydrofolate; 5mTHF, 5-methyltetrahydrofolate; HCys, homocysteine; Met, methionine; SAM, S-adenosyl methionine; SAH, S-adenosyl homocysteine; Cbl, cobalamin; meCbl, methylcobalamine; Ser, serine; Gly, glycine.
Whereas many insects and plants do not need vitamin B12, C. elegans, like humans, must obtain vitamin B12 from its diet. Remarkably, vitamin B12 is uniquely synthesized by bacteria and archaea [33], with the better defined bacterial pathway comprising more than 20 enzymes. It is estimated that about 1/3 of bacterial species can synthesize vitamin B12 [34]. As E. coli does not have this pathway, the source of vitamin B12 in the standard C. elegans diet is the vitamin absorbed by bacteria from culturing media.
The requirement for exogenously provided vitamin B12 for C. elegans was recently demonstrated. Bito et al. propagated worms for several generations on peptone-free medium with bacteria grown in the absence of vitamin B12 to establish truly vitamin B12 deficient conditions [35]. They then measured various physiological variables in the worm, as well as enzymatic activity of MS and MCM, and the concentration of key metabolites. In agreement with human and mouse studies showing fetal loss and infertility under vitamin B12 deplete conditions [32, 36, 37], the brood size of vitamin B12-deficient worms was significantly reduced. In addition, the average lifespan was reduced and animals were shorter. Not surprisingly, these observations were correlated with loss of activity in MS and MCM and a corresponding increase in methylmalonic acid and HCys levels. Because the latter can inhibit collagen biosynthesis [38], the authors hypothesized that the adverse effects of vitamin B12 deficiency on C. elegans morphology were due to increased HCys levels. Furthermore, the authors indicated that the SAM/SAH ratio is altered during vitamin B12 deficiency in the worm (see Figure 3). Although DNA methylation does not occur in worms, histone methylation does [39], and therefore, vitamin B12 deficiency can potentially affect gene expression via this pathway. Moreover, because SAM is a highly anabolic molecule, a reduction in vitamin B12 can also affect biomass production directly by decreasing the synthesis of essential components such as phosphatidylcholine in cellular membranes [40]. Likely because of the regulatory and metabolic roles of SAM, sams genes in C. elegans (Figure 3) have been associated with fertility [41] and aging [42].
Watson et al [23] recently discovered that the standard E. coli diet does not represent ample vitamin B12 conditions, although animals may not be truly vitamin B12 deficient [35]. They found that the acceleration of worm development and reduction in brood size elicited by a Comamonas diet could be partly explained by this bacterium’s ability to provide higher levels of vitamin B12. Previously, they had generated a C. elegans dietary sensor strain that expresses the green fluorescent protein (GFP) under the control of the promoter of the most differentially expressed gene, the acyl-CoA dehydrogenase acdh-1, which serves as a proxy for the induced gene expression changes [19]. On animals fed E. coli, GFP expression is high, whereas on Comamonas it is barely detectable. By transposon-based mutagenesis in Comamonas, Watson et al. identified mutations in the vitamin B12 biosynthesis pathway that lead to a failure of C. elegans to repress GFP expression [23]. In addition, a genome-scale screen of the E. coli deletion collection [43] showed that a deletion in the tonB gene further activates GFP expression in the worm. This gene encodes an outermembrane siderophore transporter that is important for the uptake of vitamin B12 by E. coli. Furthermore, RNAi silencing of two worm genes involved in the conversion of cobalamin to its active forms, mttr-1 and Y76A2B.5 (Figure 2 and Table 1), repressed the response of the dietary sensor. Also, genes encoding the enzymes that directly use vitamin B12 as a cofactor, metr-1 and mmcm-1, were both uncovered during prior genome-scale genetic screens in the worm using the dietary sensor [44]. Consistent with all the genetic evidence, direct supplementation of the standard E. coli diet with extra vitamin B12 both repressed the dietary sensor and, importantly, mimicked the effects of the Comamonas diet on developmental rate and egg laying. However, the negative effect of Comamonas on C. elegans lifespan [19] was not reproduced by vitamin B12, demonstrating that other bacterial factors are responsible for this effect.
Table 1.
Selected genes in Vitamin B12- and folic acid-related pathwaysa.
| Pathway Figure | Protein Name | H. sapiens | C. elegans |
|---|---|---|---|
| Figure 2 | Propionyl-CoA carboxylase alpha subunit | PCCA | pcca-1 |
| Propionyl-CoA carboxylase beta subunit | PCCB | pccb-1 | |
| Methylmalonyl-CoA epimerase | MCEE | mce-1 | |
| Methylmalonyl-CoA mutase | MCM | mmcm-1 | |
|
| |||
| Figure 3 | Methionine synthase reductase | MTRR | mtrr-1 |
| Methionine synthase | MTR | metr-1 | |
| S-adenosylmethionine synthetase | MAT1A | sams-1 | |
| Reduced folate carrier | SLC19A1 | folt-1 | |
|
| |||
| Both | Methylmalonic aciduria and homocystinuria type D protein | MMADHC | Y76A2B.5 |
To determine the mechanism of developmental acceleration, Watson et al. tested the effect of vitamin B12 on animals that harbor mutations in either of the two pathways that require this vitamin (Figures 2 and 3). While the developmental rate of animals harboring mutations in mce-1 or pcca-1 (propionic acid breakdown, Table 1, Figure 2) could be accelerated by vitamin B12 supplementation, mutations in sams-1 or metr-1 (methionine/SAM cycle, Table 1, Figure 3) rendered the animal unresponsive to vitamin B12. The effects of these mutations on egg laying were similar. Thus, the vitamin B12 phenotypes related to biomass production occur via the methionine/SAM cycle, which is in agreement with the central anabolic role of this pathway (Figure 3).
A major function of the propionic acid breakdown pathway (Figure 2) is to prevent buildup of this toxic metabolite, which can be produced by central metabolism or obtained from the diet. In propionic acid toxicity assays [23], vitamin B12 supplementation significantly improved the animal’s ability to withstand high doses of propionic acid, presumably by enhancing the catabolism of propionyl-CoA (Figure 2). Interestingly, a Δmetr-1 mutant (Table 1, Figure 3) had improved survival rates under vitamin B12-limiting conditions as compared to wild type animals, implicating that the two vitamin B12 reactions may compete with each other for this cofactor, and that blockage of one pathway may increase the flux in the other. In humans, propionic acid is produced by the microbiota from the digestion of dietary fibers [2] as well as by endogenous metabolism from propionyl-CoA. Although propionic acid has positive roles in human metabolism such as serving as an energy source [2], high levels of propionate have been associated with toxicity, leading to neurological disorders and gingival inflammation [45]. The inborn metabolic disorder propionic aciduria/acidemia is an extreme case of toxicity where mutations in the PCCA gene (Table 1) render the enzyme inactive thereby causing the build-up of propionate and other toxic compounds resulting in damage to various organs and eventually death [46]. The worm results indicate that this animal is also a good model to study propionic acid toxicity [23].
Vitamin B9, or folate, greatly affects C. elegans biology by direct or microbe-mediated mechanisms
Folic acid (or folate) is another water-soluble B vitamin (Box 1). The active forms of folate act as donors or acceptors of one-carbon groups in amino acid and nucleic acid metabolism (Figure 3). Due to its role in the biosynthesis of thymine and purines, as well as in amino acid conversions, folate levels are critical for fast-dividing cells. Accordingly, folate deficiency in humans has been associated with anemia [47], fetal growth retardation [48], and neural tube defects during embryonic development [49]. Folate and vitamin B12 metabolism intersect in the methionine/SAM cycle (Figure 3). Likely because of this intersection these two vitamins can have similar effects [50]. For instance, vitamin B12 deficiency can mimic folate deficiency because of the so-called methyl trap [51], where 5-methyltetrahydrofolate (5mTHF) cannot be converted back to tetrahydrofolate (THF) due to lowered activity of MS, and therefore, active folate forms cannot be replenished.
Similar to humans, C. elegans obtains folate from its diet. Earlier studies with the worm focused on folate transport: the folt-1 gene was found to encode the major folate uptake system [52]. This resembles the corresponding protein in mammals, the reduced folate carrier (RFC; Figure 3, Table 1), in multiple ways. First, both proteins are able to transport not only folate but also its derivatives. Secondly, FOLT-1 and mammalian RFC are both developmentally regulated. The expression of folt-1 is highest in earlier larval stages [52]. Similarly, in rats, RFC is expressed at higher levels during earlier stages of postembryonic development [53]. Finally, drugs that inhibit folate uptake by targeting RFC in humans also do so in the worm [52].
Because studies with RFC in mammals had been limited to the cellular level, Balamurugan et al. proposed that C. elegans may provide a suitable model to study unknown aspects of folate uptake at the whole animal level [52]. Austin et al. [54] found that loss of folt-1 reduces C. elegans lifespan by 44%, and greatly reduces fertility by attenuating spermatogenesis and decreasing oogenesis likely due to a reduction in germline nuclei [54]. In addition, the defecation rate of Δfolt-1 animals is lower than that of wild type animals, which suggests an overall reduced metabolic rate. Thus folate uptake seems to play an important role in both reproduction and metabolism of C. elegans. However, since the deletion allele used in this study was not outcrossed, in the absence of complementation tests to rescue the mutation and additional RNAi tests, there is also the possibility that unknown linked mutations explain the observed phenotypes.
Other recent studies on C. elegans folate metabolism emphasized the indirect effects of diet (Figure 1). For instance, Virk et al. serendipitously discovered that impairment of folate synthesis in E. coli extends C. elegans lifespan by more than 30% [55]. This can occur genetically by mutating a key bacterial gene (aroD) that is involved in the biosynthesis of aromatic compounds such as precursors of folate, or by inhibiting folate synthesis with bacteriostatic antibiotic sulfamethoxazole. Supplementation with folate precursors overrides the effect of aroD mutants and restores lifespan. Analytical measurements showed that the folate cycle in the worm was functional even when bacterial biosynthesis of folate is blocked. While reduction of bacterial folate synthesis delays aging, lifespan is reduced in folt-1 mutant animals [54] (see above). Thus, there must be an optimal level of folate in the worm to support long lifespan as both limited and excess folate can reduce lifespan.
Although bacterial growth was not affected by drug treatment under the conditions tested [55], it is still not clear if the observed changes are due to broader impacts of folate metabolism in the bacterium, or simply to a decrease in the amount of dietary folate [56]. Experiments with axenic medium where bacterial metabolism is eliminated will be helpful to directly test the effect of dietary folate on the worm. An interesting observation in support of bacterial folate synthesis being the main effector is that sulfo drugs that inhibit microbial folate biosynthesis also extend lifespan in rats [57]. Taken together, C. elegans is a suitable model to derive hypotheses for the effects of folate metabolism status in microbiota on human health [55, 56].
A surprising microbe-mediated effect on worm physiology and metabolism was recently discovered [58] during the studies with the anti-diabetic drug metformin, which had been known to extend lifespan in C. elegans [59] and mice [60]. Cabreiro et al. showed that this occurs through bacterial folate and methionine metabolism [58]. Remarkably, metformin increases the lifespan of C. elegans when fed live bacteria, but reduces it when animals are fed dead bacteria or are grown in axenic medium. Analysis of active bacteria showed that metformin changed the relative abundance of folate derivatives: meTHF was increased, while methionine was decreased, which is consistent with a possible inhibition of MS (Figure 3). The authors proposed that decreased methionine levels in the diet reduced SAM/SAH ratio in the worm (as can be inferred from Figure 3), which mimics dietary restriction (DR). Glucose supplementation suppressed the effects of metformin in C. elegans, suggesting that a high sugar diet might offset the benefits of metformin [58]. Taken together, bacteria may mediate some of the effects of metformin, which has implications for human treatment as well since it may affect or be metabolized by the microbiota. This is supported by the observation that the composition of microbiota is sensitive to metformin treatment in rats [61, 62] and type-2 diabetes patients [63].
Other micronutrients
Micronutrients other than B vitamins have also been studied for their direct or bacteria-mediated effects on C. elegans, particularly on aging. Based on the mitochondrial theory of aging [64], antioxidants such as vitamin C and vitamin E may be expected to increase lifespan, but studies with these compounds disagree on their effects on humans as well as C. elegans [65, 66]. Because the bacterial diet of C. elegans is not a source of these vitamins, they are to be tested by dietary supplementation. However, worms cannot efficiently uptake these compounds [65, 66]. Recently, Nishikawa and colleagues developed methods to orally deliver to C. elegans both hydrophilic compounds such as vitamin C derivatives [67] and hydrophobic compounds such as vitamin E derivatives [68] by vehicles that act as bacteria-like particles. For both families of vitamins, they were able to demonstrate an increase in lifespan, thus demonstrating the direct effect of these antioxidants for the worm model.
Another micronutrient that may affect aging in C. elegans is Coenzyme Q (Q), which has diverse functions including its major role in the electron transport chain and as an antioxidant [69]. C. elegans produces its own specific form (Q9) [70], but can also use Q8 from its E. coli diet [71]. When fed mutant E. coli that do not produce Q8, the lifespan of the worm increases [72]. Remarkably, this effect was the result of altered bacterial metabolism, and not due to direct dietary contribution [73], as supplemented Q10 did not reverse the lifespan extension. Further, the lifespan extension with Q-less E. coli was not due to lowered caloric intake because of compromised bacterial growth. One possibility for the mechanism is the production of fermentation metabolites such as ethanol and lactic acid that may be the actual causes of lifespan extension in the worm [73]. However, more recently, Gomez et al. [74] showed that lactic acid produced by Q-less E. coli was not responsible for the life-span extension. Instead, they suggested that the survival rate of worms may be improved due to the delay in the colonization of the worm gut by respiratory-deficient bacteria as compared to the wild type bacteria.
Finally, a surprising compound that was recently shown to delay aging in C. elegans is alpha-ketoglutarate (AKG) [75], an intermediate in the TCA cycle. Direct supplementation of AKG extended lifespan by more than 25% irrespective of bacterial metabolic activity. Rather, AKG acts by binding and suppressing ATP synthase to reduce energy generation in the worm. This effect is dependent on the target of rapamycin pathway, whose inactivation delays aging [76]. Overall, AKG supplementation creates DR-like conditions. Interestingly, AKG concentrations also increase during starvation, suggesting the presence of a feedback mechanism responding to nutrient availability [75]. Thus the common metabolite AKG also acts as a signaling molecule.
Concluding remarks
Altogether, the studies we discussed firmly establish the nematode C. elegans and its bacterial diet as a model for both direct and microbe-mediated effects of diet on health and longevity. Since micronutrients obtained from the diet or supplied by the microbiota affect human health by complicated mechanisms that are difficult to systematically characterize, it is likely that studies using the interspecies worm system will greatly illuminate basic physiological responses and underlying mechanisms.
The worm/bacteria system will not be a suitable model for all aspects of human diet and metabolic diseases. First, C. elegans and the co-cultured bacteria do not represent a case of mutualism as in human and microbiota, but have a trophic relationship. Thus, the C. elegans/bacteria model is limited to a simplified conceptual model of direct and indirect effects as in Figure 1. Second, whereas human microbiota is a complex community, C. elegans interacts with a pure culture during monoxenic growth in the laboratory. However, monoxenically grown C. elegans does offer the possibility of testing different bacterial species individually for their role on indirect dietary effects, as long as they can be cultured in the laboratory. In any case, pathway-level mechanisms discovered in the worm can serve as hypotheses for further testing in mammalian model systems or humans.
HIGHLIGHTS.
Micronutrients affect human health either directly or indirectly via gut microbiota
Caenorhabditis elegans and its bacterial diet can be used as a model for both effects
The worm and its bacterial diet provide a powerful interspecies systems biology model
The worm/bacteria model connects vitamins B9 and B12 to animal physiology and metabolism
Acknowledgments
This work was supported by the National Institute of Health (grant DK068429 to A.J.M.W.). We thank members of the Walhout laboratory, notably Lesley MacNeil and Emma Watson for helpful discussions. We are also grateful to the anonymous reviewers for their valuable input.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES CITED
- 1.Sommer F, Backhed F. The gut microbiota - masters of host development and physiology. Nat Rev Microbiol. 2013;11:227–238. doi: 10.1038/nrmicro2974. [DOI] [PubMed] [Google Scholar]
- 2.Tremaroli V, Backhed F. Functional interactions between the gut microbiota and host metabolism. Nature. 2012;489:242–249. doi: 10.1038/nature11552. [DOI] [PubMed] [Google Scholar]
- 3.LeBlanc JG, et al. Bacteria as vitamin suppliers to their host: a gut microbiota perspective. Current opinion in biotechnology. 2013;24:160–168. doi: 10.1016/j.copbio.2012.08.005. [DOI] [PubMed] [Google Scholar]
- 4.Ursell LK, et al. The Intestinal Metabolome: An Intersection Between Microbiota and Host. Gastroenterology. 2014;146:1470–1476. doi: 10.1053/j.gastro.2014.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tilg H, Moschen AR. Microbiota and diabetes: an evolving relationship. Gut. 2014 doi: 10.1136/gutjnl-2014-306928. [DOI] [PubMed] [Google Scholar]
- 6.Wang ZN, et al. Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature. 2011;472:57–U82. doi: 10.1038/nature09922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Million M, et al. Gut bacterial microbiota and obesity. Clinical microbiology and infection: the official publication of the European Society of Clinical Microbiology and Infectious Diseases. 2013;19:305–313. doi: 10.1111/1469-0691.12172. [DOI] [PubMed] [Google Scholar]
- 8.Kamada N, et al. Role of the gut microbiota in immunity and inflammatory disease. Nature Reviews Immunology. 2013;13:321–335. doi: 10.1038/nri3430. [DOI] [PubMed] [Google Scholar]
- 9.Hsiao EY, et al. Microbiota Modulate Behavioral and Physiological Abnormalities Associated with Neurodevelopmental Disorders. Cell. 2013;155:1451–1463. doi: 10.1016/j.cell.2013.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Felix MA, Braendle C. The natural history of Caenorhabditis elegans. Curr Biol. 2010;20:R965–R969. doi: 10.1016/j.cub.2010.09.050. [DOI] [PubMed] [Google Scholar]
- 11.Brenner S. The genetics of Caenorhabditis elegans. Genetics. 1974;77:71–94. doi: 10.1093/genetics/77.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Emmons SW. Male development. WormBook: the online review of C. elegans biology. 2005:1–22. doi: 10.1895/wormbook.1.33.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sternberg PW. Vulval development. WormBook: the online review of C. elegans biology. 2005:1–28. doi: 10.1895/wormbook.1.6.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kirienko NV, et al. Cancer models in Caenorhabditis elegans. Developmental dynamics: an official publication of the American Association of Anatomists. 2010;239:1413–1448. doi: 10.1002/dvdy.22247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kenyon CJ. The genetics of ageing. Nature. 2010;464:504–512. doi: 10.1038/nature08980. [DOI] [PubMed] [Google Scholar]
- 16.Avery L, You YJ. C. elegans feeding. WormBook: the online review of C. elegans biology. 2012:1–23. doi: 10.1895/wormbook.1.150.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lu NC, Goetsch KM. Carbohydrate Requirement of Caenorhabditis-Elegans and the Final Development of a Chemically-Defined Medium. Nematologica. 1993;39:303–311. [Google Scholar]
- 18.Lenaerts I, et al. Dietary restriction of Caenorhabditis elegans by axenic culture reflects nutritional requirement for constituents provided by metabolically active microbes. Journals of Gerontology Series a-Biological Sciences and Medical Sciences. 2008;63:242–252. doi: 10.1093/gerona/63.3.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.MacNeil LT, et al. Diet-Induced Developmental Acceleration Independent of TOR and Insulin in C. elegans. Cell. 2013;153:240–252. doi: 10.1016/j.cell.2013.02.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Coolon JD, et al. Caenorhabditis elegans genomic response to soil bacteria predicts environment-specific genetic effects on life history traits. Plos Genet. 2009;5:e1000503. doi: 10.1371/journal.pgen.1000503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shtonda BB, Avery L. Dietary choice behavior in Caenorhabditis elegans. J Exp Biol. 2006;209:89–102. doi: 10.1242/jeb.01955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Soukas AA, et al. Rictor/TORC2 regulates fat metabolism, feeding, growth, and life span in Caenorhabditis elegans. Genes Dev. 2009;23:496–511. doi: 10.1101/gad.1775409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Watson E, et al. Interspecies Systems Biology Uncovers Metabolites Affecting C. elegans Gene Expression and Life History Traits (vol 156, pg 759, 2014) Cell. 2014;156:1336–1337. doi: 10.1016/j.cell.2014.01.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hauser NS, et al. Variable dietary management of methylmalonic acidemia: metabolic and energetic correlations. Am J Clin Nutr. 2011;93:47–56. doi: 10.3945/ajcn.110.004341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Horster F, et al. Prediction of outcome in isolated methylmalonic acidurias: combined use of clinical and biochemical parameters. J Inherit Metab Dis. 2009;32:630–639. doi: 10.1007/s10545-009-1189-6. [DOI] [PubMed] [Google Scholar]
- 26.Weisfeld-Adams JD, et al. Newborn screening and early biochemical follow-up in combined methylmalonic aciduria and homocystinuria, cblC type, and utility of methionine as a secondary screening analyte. Molecular genetics and metabolism. 2010;99:116–123. doi: 10.1016/j.ymgme.2009.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Deshmukh US, et al. Effect of physiological doses of oral vitamin B-12 on plasma homocysteine: a randomized, placebo-controlled, double-blind trial in India. Eur J Clin Nutr. 2010;64:495–502. doi: 10.1038/ejcn.2010.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Remacha AF, et al. Vitamin B12 deficiency, hyperhomocysteinemia and thrombosis: a case and control study. Int J Hematol. 2011;93:458–464. doi: 10.1007/s12185-011-0825-8. [DOI] [PubMed] [Google Scholar]
- 29.Hoffbrand AV. Megaloblastic Anemias. In: Longo DL, et al., editors. Harrison’s Principles of Internal Medicine. 18. McGraw-Hill Companies, Inc; 2012. [Google Scholar]
- 30.Kumar N. Neurologic aspects of cobalamin (B12) deficiency. Handbook of clinical neurology. 2014;120:915–926. doi: 10.1016/B978-0-7020-4087-0.00060-7. [DOI] [PubMed] [Google Scholar]
- 31.Pepper MR, Black MM. B12 in fetal development. Seminars in cell & developmental biology. 2011;22:619–623. doi: 10.1016/j.semcdb.2011.05.005. [DOI] [PubMed] [Google Scholar]
- 32.Candito M, et al. Clinical B12 deficiency in one case of recurrent spontaneous pregnancy loss. Clinical chemistry and laboratory medicine: CCLM/FESCC. 2003;41:1026–1027. doi: 10.1515/CCLM.2003.157. [DOI] [PubMed] [Google Scholar]
- 33.Rodionov DA, et al. Comparative genomics of the vitamin B12 metabolism and regulation in prokaryotes. The Journal of biological chemistry. 2003;278:41148–41159. doi: 10.1074/jbc.M305837200. [DOI] [PubMed] [Google Scholar]
- 34.Sanudo-Wilhelmy SA, et al. The role of B vitamins in marine biogeochemistry. Annual review of marine science. 2014;6:339–367. doi: 10.1146/annurev-marine-120710-100912. [DOI] [PubMed] [Google Scholar]
- 35.Bito T, et al. Vitamin B12 deficiency in Caenorhabditis elegans results in loss of fertility, extended life cycle, and reduced lifespan. FEBS Open Bio. 2013;3:112–117. doi: 10.1016/j.fob.2013.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pront R, et al. Prevalence of low serum cobalamin in infertile couples. Andrologia. 2009;41:46–50. doi: 10.1111/j.1439-0272.2008.00895.x. [DOI] [PubMed] [Google Scholar]
- 37.Watanabe T, et al. The effects of dietary vitamin B12 deficiency on sperm maturation in developing and growing male rats. Congenital anomalies. 2003;43:57–64. doi: 10.1111/j.1741-4520.2003.tb01027.x. [DOI] [PubMed] [Google Scholar]
- 38.Thaler R, et al. Homocysteine suppresses the expression of the collagen cross-linker lysyl oxidase involving IL-6, Fli1, and epigenetic DNA methylation. The Journal of biological chemistry. 2011;286:5578–5588. doi: 10.1074/jbc.M110.166181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Greer EL, et al. A histone methylation network regulates transgenerational epigenetic memory in C. elegans. Cell reports. 2014;7:113–126. doi: 10.1016/j.celrep.2014.02.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Walker AK, et al. A conserved SREBP-1/phosphatidylcholine feedback circuit regulates lipogenesis in metazoans. Cell. 2011;147:840–852. doi: 10.1016/j.cell.2011.09.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tamiya H, et al. Conserved SAMS function in regulating egg-laying in C. elegans. J Recept Sig Transd. 2013;33:56–62. doi: 10.3109/10799893.2012.756896. [DOI] [PubMed] [Google Scholar]
- 42.Hansen M, et al. New genes tied to endocrine, metabolic, and dietary regulation of lifespan from a Caenorhabditis elegans genomic RNAi screen. Plos Genet. 2005;1:119–128. doi: 10.1371/journal.pgen.0010017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Baba T, et al. Construction of Escherichia coli K-12 in-frame, single–gene knockout mutants: the Keio collection. Molecular systems biology. 2006;2:2006. doi: 10.1038/msb4100050. 0008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Watson E, et al. Integration of Metabolic and Gene Regulatory Networks Modulates the C. elegans Dietary Response (vol 153, pg 253, 2013) Cell. 2013;153:1406–1407. doi: 10.1016/j.cell.2013.02.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Al-Lahham SH, et al. Biological effects of propionic acid in humans; metabolism, potential applications and underlying mechanisms. Biochimica et biophysica acta. 2010;1801:1175–1183. doi: 10.1016/j.bbalip.2010.07.007. [DOI] [PubMed] [Google Scholar]
- 46.Pena L, et al. Natural history of propionic acidemia. Molecular genetics and metabolism. 2012;105:5–9. doi: 10.1016/j.ymgme.2011.09.022. [DOI] [PubMed] [Google Scholar]
- 47.Odewole OA, et al. Near-elimination of folate-deficiency anemia by mandatory folic acid fortification in older US adults: Reasons for Geographic and Racial Differences in Stroke study 2003–2007. Am J Clin Nutr. 2013;98:1042–1047. doi: 10.3945/ajcn.113.059683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.van Uitert EM, Steegers-Theunissen RP. Influence of maternal folate status on human fetal growth parameters. Molecular nutrition & food research. 2013;57:582–595. doi: 10.1002/mnfr.201200084. [DOI] [PubMed] [Google Scholar]
- 49.Osterhues A, et al. The role of folic acid fortification in neural tube defects: a review. Critical reviews in food science and nutrition. 2013;53:1180–1190. doi: 10.1080/10408398.2011.575966. [DOI] [PubMed] [Google Scholar]
- 50.Shane B, Stokstad EL. Vitamin B12-folate interrelationships. Annual review of nutrition. 1985;5:115–141. doi: 10.1146/annurev.nu.05.070185.000555. [DOI] [PubMed] [Google Scholar]
- 51.Mato JM, et al. Methionine metabolism and liver disease. Annual review of nutrition. 2008;28:273–293. doi: 10.1146/annurev.nutr.28.061807.155438. [DOI] [PubMed] [Google Scholar]
- 52.Balamurugan K, et al. Cloning and functional characterization of a folate transporter from the nematode Caenorhabditis elegans. Am J Physiol Cell Physiol. 2007;293:C670–681. doi: 10.1152/ajpcell.00516.2006. [DOI] [PubMed] [Google Scholar]
- 53.Balamurugan K, Said HM. Ontogenic regulation of folate transport across rat jejunal brush-border membrane. Am J Physiol-Gastr L. 2003;285:G1068–G1073. doi: 10.1152/ajpgi.00188.2003. [DOI] [PubMed] [Google Scholar]
- 54.Austin MU, et al. Knockout of the folate transporter folt-1 causes germline and somatic defects in C. elegans. BMC Dev Biol. 2010;10:46. doi: 10.1186/1471-213X-10-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Virk B, et al. Excessive folate synthesis limits lifespan in the C. elegans: E. coli aging model. BMC Biol. 2012;10:67. doi: 10.1186/1741-7007-10-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nguyen TP, Clarke CF. Folate status of gut microbiome affects Caenorhabditis elegans lifespan. BMC Biol. 2012;10:66. doi: 10.1186/1741-7007-10-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Martin GM. The biology of aging: 1985–2010 and beyond. FASEB J. 2011;25:3756–3762. doi: 10.1096/fj.11-1102.ufm. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cabreiro F, et al. Metformin retards aging in C. elegans by altering microbial folate and methionine metabolism. Cell. 2013;153:228–239. doi: 10.1016/j.cell.2013.02.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Onken B, Driscoll M. Metformin induces a dietary restriction-like state and the oxidative stress response to extend C. elegans Healthspan via AMPK, LKB1, and SKN-1. PLoS One. 2010;5:e8758. doi: 10.1371/journal.pone.0008758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Martin-Montalvo A, et al. Metformin improves healthspan and lifespan in mice. Nat Commun. 2013:4. doi: 10.1038/ncomms3192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Pyra KA, et al. Prebiotic fiber increases hepatic acetyl CoA carboxylase phosphorylation and suppresses glucose-dependent insulinotropic polypeptide secretion more effectively when used with metformin in obese rats. J Nutr. 2012;142:213–220. doi: 10.3945/jn.111.147132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Shin NR, et al. An increase in the Akkermansia spp. population induced by metformin treatment improves glucose homeostasis in diet-induced obese mice. Gut. 2014;63:727–735. doi: 10.1136/gutjnl-2012-303839. [DOI] [PubMed] [Google Scholar]
- 63.Karlsson FH, et al. Gut metagenome in European women with normal, impaired and diabetic glucose control. Nature. 2013;498:99. doi: 10.1038/nature12198. [DOI] [PubMed] [Google Scholar]
- 64.Barja G. Updating the mitochondrial free radical theory of aging: an integrated view, key aspects, and confounding concepts. Antioxidants & redox signaling. 2013;19:1420–1445. doi: 10.1089/ars.2012.5148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ernst IMA, et al. Vitamin E supplementation and lifespan in model organisms. Ageing Research Reviews. 2013;12:365–375. doi: 10.1016/j.arr.2012.10.002. [DOI] [PubMed] [Google Scholar]
- 66.Pallauf K, et al. Vitamin C and lifespan in model organisms. Food and Chemical Toxicology. 2013;58:255–263. doi: 10.1016/j.fct.2013.04.046. [DOI] [PubMed] [Google Scholar]
- 67.Shibamura A, et al. A method for oral administration of hydrophilic substances to Caenorhabditis elegans: Effects of oral supplementation with antioxidants on the nematode lifespan. Mechanisms of Ageing and Development. 2009;130:652–655. doi: 10.1016/j.mad.2009.06.008. [DOI] [PubMed] [Google Scholar]
- 68.Kashima N, et al. Development of a method for oral administration of hydrophobic substances to Caenorhabditis elegans: pro-longevity effects of oral supplementation with lipid-soluble antioxidants. Biogerontology. 2012;13:337–344. doi: 10.1007/s10522-012-9378-3. [DOI] [PubMed] [Google Scholar]
- 69.Bentinger M, et al. Coenzyme Q--biosynthesis and functions. Biochemical and biophysical research communications. 2010;396:74–79. doi: 10.1016/j.bbrc.2010.02.147. [DOI] [PubMed] [Google Scholar]
- 70.Vasta V, et al. Altered redox status of coenzyme Q9 reflects mitochondrial electron transport chain deficiencies in Caenorhabditis elegans. Mitochondrion. 2011;11:136–138. doi: 10.1016/j.mito.2010.09.002. [DOI] [PubMed] [Google Scholar]
- 71.Jonassen T, et al. Development and fertility in Caenorhabditis elegans clk-1 mutants depend upon transport of dietary coenzyme Q8 to mitochondria. The Journal of biological chemistry. 2002;277:45020–45027. doi: 10.1074/jbc.M204758200. [DOI] [PubMed] [Google Scholar]
- 72.Larsen PL, Clarke CF. Extension of life-span in Caenorhabditis elegans by a diet lacking coenzyme Q. Science. 2002;295:120–123. doi: 10.1126/science.1064653. [DOI] [PubMed] [Google Scholar]
- 73.Saiki R, et al. Altered bacterial metabolism, not coenzyme Q content, is responsible for the lifespan extension in Caenorhabditis elegans fed an Escherichia coli diet lacking coenzyme Q. Aging Cell. 2008;7:291–304. doi: 10.1111/j.1474-9726.2008.00378.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Gomez F, et al. Delayed accumulation of intestinal coliform bacteria enhances life span and stress resistance in Caenorhabditis elegans fed respiratory deficient E. coli. Bmc Microbiol. 2012:12. doi: 10.1186/1471-2180-12-300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Chin RM, et al. The metabolite alpha-ketoglutarate extends lifespan by inhibiting ATP synthase and TOR. Nature. 2014 doi: 10.1038/nature13264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Stanfel MN, et al. The TOR pathway comes of age. Biochimica et biophysica acta. 2009;1790:1067–1074. doi: 10.1016/j.bbagen.2009.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]