Abstract
Most of the literature related to high altitude medicine is devoted to the short-term effects of high-altitude exposure on human physiology. However, long-term effects of living at high altitudes may be more important in relation to human disease because more than 400 million people worldwide reside above 1500 m. Interestingly, individuals living at higher altitudes have a lower fasting glycemia and better glucose tolerance compared with those who live near sea level. There is also emerging evidence of the lower prevalence of both obesity and diabetes at higher altitudes. The mechanisms underlying improved glucose control at higher altitudes remain unclear. In this review, we present the most current evidence about glucose homeostasis in residents living above 1500 m and discuss possible mechanisms that could explain the lower fasting glycemia and lower prevalence of obesity and diabetes in this population. Understanding the mechanisms that regulate and maintain the lower fasting glycemia in individuals who live at higher altitudes could lead to new therapeutics for impaired glucose homeostasis.
Introduction
Short-term Effects of Altitude on Glycemia
-
Glycemia and Insulinemia Among Highlanders
How reliable are the measurements of plasma glucose at high altitudes?
Does insulin explain the lower glycemia in highlanders?
Is the lower glycemia at higher altitudes acquired?
-
Endogenous Glucose Production at High Altitude: Role of the Liver
Hepatic glucose output (HGO) in lowlanders exposed to high altitude
Role of glucagon among highlanders
Role of hepatic glycogen content
-
Effect of Altitude and Hypoxia on the Skeletal Muscle
Short-term effects of altitude and hypoxia in vivo
Prolonged effects of altitude: evidence in highlanders
Effects of anoxia and hypoxia on glucose uptake in vitro
Role of the Gastrointestinal Tract
Role of the Adipose Tissue
-
Possible Link Between Hypoxia-Inducible Factor (HIF) and Glucose Homeostasis
Genetic adaptation of highlanders to hypoxia
HIF and glucose uptake: evidence in vitro
-
Diabetes and Obesity at High Altitudes
Association between obesity and altitude
Possible factors contributing to the lower prevalence of obesity
Association between diabetes and altitude
Possible factors contributing to the lower prevalence of diabetes
Summary and Conclusions
I. Introduction
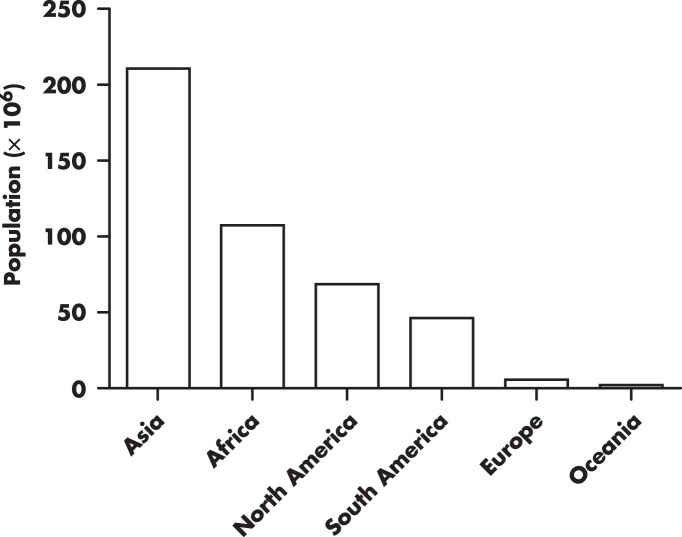
Although there is abundant literature on the short-term effects of exposure to high elevations on human physiology (1–5), including observations documented more than four centuries ago (6), as well as early experimental work performed by Paul Bert in the nineteenth century (7), the impact of prolonged exposure, particularly on blood glucose regulation, has received little attention. This is surprising because approximately 7% of the world's population (ie, ∼440 million people) resides above 1500 m (8), particularly since the number of people living at high elevations has been increasing considerably (by 20%) since 1990 (Figure 1). A plurality of those residing at higher elevations (>4000 m) are primarily from the Andes and Tibet (9). In some Andean countries such as Peru, the percentage of the population living at higher elevations is quite large. Approximately, 25% of Peruvian residents live above 2500 m (www.inei.gob.pe). In the United States, approximately 7.5 million people live above 1500 m (8).
Figure 1. Number of people worldwide living above 1500 m estimated for the year 2000.
Adapted from the Center for International Earth Science Information Network (8).
Many environmental factors differ with prevailing altitude, namely: 1) the partial pressure of oxygen (PaO2) in the breathed air; 2) ambient temperature; 3) relative humidity; and 4) solar and cosmic radiation. Even the composition of bacteria in ambient air and soil varies with altitude (10, 11). However, it is generally accepted that most physiological changes that take place in individuals exposed to high elevation are due to ambient hypoxia (ie, due to a decrease in the PaO2), which occurs as a consequence of a low total atmospheric pressure, although the percentage of oxygen in the air remains the same (20.9%). Whether hypobaria (low atmospheric pressure) induces different physiological changes compared with those produced by hypobaric hypoxia is still in debate (12, 13).
At sea level, the PaO2 and atmospheric pressure values are 149 and 760 mm Hg, respectively. At the top of Mount Everest (8848 m), those values are reduced by two-thirds (1). Not surprisingly, the human body activates numerous adaptive mechanisms in response to an exposure to high elevations (1, 2). Although the PaO2 at 1500 m is approximately 80% of that at sea level (2), the arterial oxygen saturation remains within normal values (≥95%). However, initial adaptive changes become more evident above 1500 m, including increased heart rate, hyperventilation, and subsequently, increased red blood cell mass (3, 4). In healthy subjects, the inverse relationship between oxygen saturation of hemoglobin and altitude is more dramatic over 3000 m (14), which may lead to more profound adaptive mechanisms.
Additional mechanisms are involved in the human adaptation to prolonged exposure to higher altitudes (eg, individuals born and raised at low altitude that migrated to and permanently live at elevated regions). In fact, native inhabitants at altitudes above 3000 m differ in circulatory and hematological features compared with natives of lowlands. For example, children born and residing over 3000 m have a persistent right ventricular predominance compared with children born and residing below 1000 m (15). Young adult men living at 4540 m have considerably higher hemoglobin concentrations (as well as increased hematocrit) compared with men living at 150 m (16). Moreover, individuals residing at very high altitude have a larger chest circumference and cyanosis in their lips and skin (17, 18). What it is unclear is whether the environment, rather than genetic factors, mainly determines the phenotype of a high-altitude individual. These factors have been discussed elsewhere (17–20).
The purpose of the present review is to discuss the most current data about glucose homeostasis in individuals living above 1500 m. We will focus particularly on the possible mechanisms, mainly from an integrative (systemic) point of view, that could explain the lower fasting glycemia and lower prevalence of obesity and diabetes in humans at higher altitudes. In addition, we will briefly discuss the studies examining the effects of simulated altitude (hypobaric hypoxia simulated in a decompression chamber) and experimental hypoxia alone on glucose homeostasis because information on these topics is more limited. Cited references were mainly obtained from PubMed, in either the English or Spanish language.
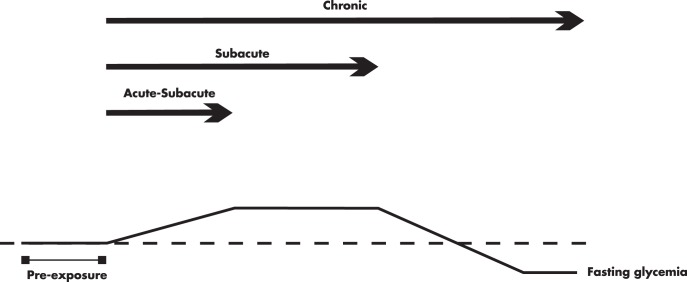
In this review, we considered three altitude environments, as suggested by the International Society for Mountain Medicine (www.ismm.org). High altitude was defined as ≥1500 m and below 3500 m; very high altitude, ≥3500 m and below 5500 m; and extreme altitude, ≥5500 m. Because there is no consensus on the definition of acute and chronic exposure, we arbitrarily defined very acute exposure when the exposure duration is less than 60 minutes; acute exposure, when it lasts 1–24 hours; subacute exposure, when it lasts between 1 day and less than 3 months; and chronic exposure, when it lasts ≥ 3 months. However, for the purposes of this review, very short and acute exposures are considered short-term effects, whereas subacute and chronic are considered long-term effects.
II. Short-term Effects of Altitude on Glycemia
Fasting glycemia of healthy lowlanders exposed to very high altitude has been reported to increase (21–23), decrease (24), or remain unchanged (25, 26). Divergent results may be due to period of exposure. In fact, exposure to very high altitude causes an increase in fasting glycemia in the initial 2–3 days (21–23). This initial increase in glycemia is attenuated as the exposure persists for more than 1 week (25, 26) and may even decrease below pre-exposure values in the second week of a stay at very high altitude (24). Thus, acclimatization to high elevations may allow normalization of glycemia after initial transient hyperglycemia.
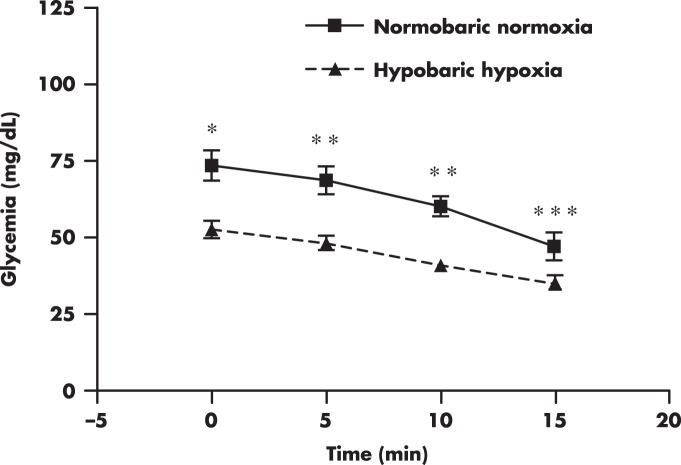
Certainly, other factors may also explain the discrepancies reported in regard to the effect of altitude exposure on fasting glycemia. Physical activity appears to contribute to the susceptibility to hyperglycemia during short-term exposures to high altitude. Exposure to very high altitude induces hyperglycemia in healthy individuals under non-resting conditions (21, 22), but the same exposure does not affect glycemia in individuals remaining at resting conditions (27, 28). One explanation is that physical work could lead to increased sympathetic activity, resulting in hyperglycemia. Numerous studies examining the effect of altitude exposure on urine or plasma catecholamines have reported an increase in catecholamines, mainly norepinephrine, as reviewed by Rostrup (29) and Richalet (30). However, elevation in catecholamines does not always occur after altitude exposure (29), suggesting that altitude-induced hyperglycemia may not be fully explained by catecholamines. Elevation of cortisol during short-term exposure to high altitude (31) could also contribute, at least in part, to the transient hyperglycemia. There is a possibility that prevailing fasting conditions could also mediate the hyperglycemic response after altitude exposure. For example, decreased glycemia has been observed after acute exposure to hypobaric hypoxia in nondiabetic subjects (Figure 2) while remaining in prolonged fasting states (O. Woolcott, unpublished data).
Figure 2. Glycemia profile during an iv insulin tolerance test performed at 150 m (normobaric normoxia) and after acute exposure to simulated altitude (hypobaric hypoxia) at 3200 m.
Male subjects (BMI, 26 ± 3.53 kg/m2 [mean ± SD]) were in an approximately 15-hour fasting state before the commencement of the test (O. Woolcott, unpublished data). *, P < .01; **, P < .001; ***, P < .05, hypobaric hypoxia vs normobaric normoxia.
The previous findings suggest that hyperglycemia may develop in the initial days after exposure to high altitudes, probably secondary to increased stress-related hormones, namely cortisol and norepinephrine. However, it is remarkable that prolonged exposure to high altitudes may instead have potential benefits on glucose homeostasis. Indeed, numerous studies conducted in individuals exposed to high or very high altitude from 10–90 days have not reported hyperglycemia (23, 24, 26, 28, 32–35), regardless of whether they were exposed to simulated (hypobaria and hypoxia) or natural altitude. Moreover, some of these studies show evidence that prolonged exposure to altitude may lower glycemia (24, 28, 32). Figure 3 depicts the different fasting glycemia variations in response to short and prolonged exposure to high altitudes in humans. In fact, acclimatization to high altitude appears to increase the fuel dependence on blood glucose not only in the human skeletal muscle (28, 36, 37) but also in the heart muscle (38). This mechanism may offer a more efficient fuel metabolism (of glucose) in response to hypoxia, as compared with fatty acid oxidation (39).
Figure 3. Differences in fasting venous glycemia in response to short and prolonged exposure to high altitudes in nondiabetic humans.
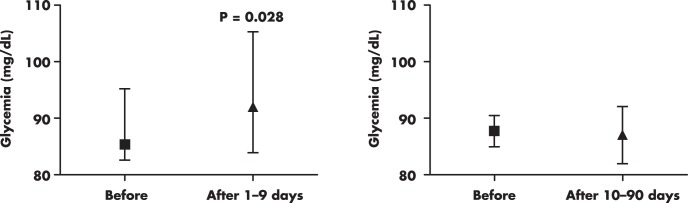
Plots were created by using reported glycemia values from studies conducted in lowlanders exposed for short (1–9 d) (21, 22, 24, 25, 27, 28, 264–266) and prolonged (10–90 d) (23, 24, 26, 28, 32–35) periods to high altitude and very high altitude, under simulated (hypobaria and hypoxia) or natural conditions. Columns and bars are medians and interquartile ranges, respectively.
III. Glycemia and Insulinemia Among Highlanders
There is compelling evidence for lower fasting glycemia in clinically healthy residents living between 3000 and 4500 m, compared with residents living below 500 m. This difference has been reported in men (40–44), nonpregnant women (45–47), pregnant women (47, 48), nondiabetic subjects (40, 45, 48), and also in neonates (48) and appears to be independent of body mass index (BMI) (40, 45, 48). Likewise, individuals living at 3200 m have been shown to have lower 12-hour blood glucose profiles compared with individuals living near sea level (40). From available data (38, 41–43, 49), we have estimated a median fasting plasma glucose concentration of 81.6 mg/dL (interquartile range, 72.2–81.8 mg/dL) for adult males living above 3000 m, whereas in residents from low altitude the estimated value was 91.2 mg/dL (81.9–95.4 mg/dL). In nonpregnant adult females, the median fasting plasma glucose was estimated to be 71.7 mg/dL (68.4–88.7 mg/dL) and 85.9 mg/dL (82.8–93.8 mg/dL), for residents living above 3000 m and those from low altitude, respectively (45–47).
Additional compelling evidence of lower fasting glycemia at higher altitudes comes from a large clinical study conducted in a nationally representative sample from Peru, including adult subjects of both sexes, with or without diabetes (50). This study showed an inverse association between fasting glycemia and altitude: 85 ± 26.6 mg/dL (mean ± SD) at < 1000 m (n = 2425); 83 ± 14.4 mg/dL between 1000–2999 m (n = 808); and 78.8 ± 17.9 mg/dL at > 3000 m (n = 959). It is important to note that although populations from these three altitude bands had comparable mean age, BMI was significantly lower at higher altitudes. However, as pointed out before, independent studies have shown lower glycemia at high altitudes in BMI-matched individuals (40, 45, 48). Nevertheless, it is unclear whether the lower glycemia at higher altitudes reported among Peruvian adult individuals could be explained by differences in diet, physical activity, or family history of diabetes mellitus.
The preponderance of the evidence supports better glycemic control at higher altitudes. However, a few studies have reported no significant differences in fasting glycemia between lowlanders and highlanders (26, 38, 49, 51, 52), although a trend for a lower glycemia at higher altitudes was observed in most of these studies (38, 49, 51, 52). One should note that virtually all studies showing lower glycemia at higher altitudes have been conducted in Andean populations. Therefore, further studies are needed to determine whether differences in glycemia exist between lowlanders and highlanders from other regions of the world. For example, no association between fasting glycemia and altitude has been reported among Indian individuals (26). In that regard, there is good evidence that there are some genetic differences, for example between Andeans and Tibetans (53, 54), including those in pathways known to affect glucose metabolism (55).
A. How reliable are the measurements of plasma glucose at high altitudes?
It has been argued that the lower reported glucose levels observed at higher altitudes are the result of methodological artifacts. There is strong evidence demonstrating the inconsistency of the performance of portable blood glucose meters in the presence of low oxygen tension (56–61) and low ambient temperatures (56, 62). Even glucose-dehydrogenase-based glucose meters, which have been shown to be more accurate at high altitudes (57, 63, 64) due to the insensitivity of glucose dehydrogenase to changes in O2 tension (65, 66), are still prone to error in low ambient temperatures (56). In addition, an increased number of red blood cells may also affect glucose readings in blood glucose meters (67, 68), due to mechanical impairment of the diffusion of plasma into the reagent membrane (69).
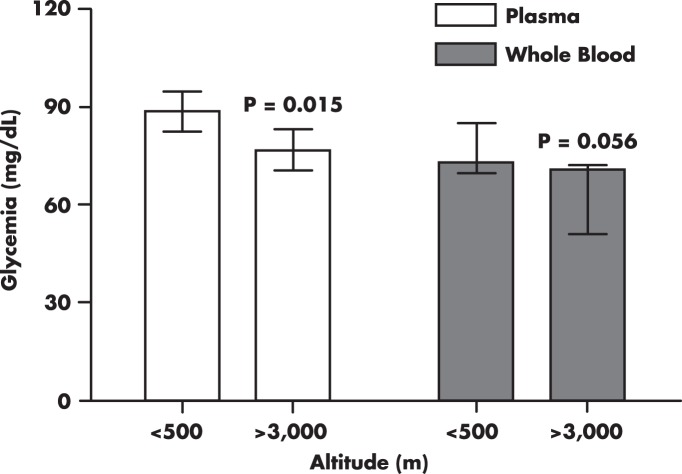
Another potential artifact is an accelerated ex vivo glycolysis (70, 71). Elevated red or white blood cell mass, independent of altitude, may result in pseudohypoglycemia (70, 71). This has also been demonstrated in individuals living at high altitudes (72), explaining the substantial differences in measured glucose concentrations in plasma vs whole blood from the same individual (41, 43) (Figure 4).
Figure 4. Differences in fasting venous glycemia from humans living at low altitudes compared with those living at high altitudes.
Figure was generated using reported values from studies that measured plasma glucose (38, 41–43, 45, 46, 75) and whole-blood glucose (26, 40, 41, 43, 123). Columns and bars are medians and interquartile ranges, respectively.
For the reasons explained before, glucose measurements in plasma samples are strongly recommended (73, 74). Studies comparing fasting glycemia between low and high-altitude populations have used different methodologies (Somogyi-Nelson, Folin-Malmros, Saifer-Gerstenfeld, glucose-oxidase based method, and hexokinase-glucose-6-phosphate dehydrogenase-coupled enzyme assay) (40–45, 48, 75), including methods that are potentially prone to error due to hypoxia, cold temperature, and erythrocytosis (increased red blood cell mass). Almost all of these studies have shown a lower glycemia at higher altitudes. However, the lower fasting glycemia in individuals living at higher altitudes has been confirmed in plasma samples (41, 42, 45, 46, 48, 75, 76). Moreover, these differences have also been found in plasma samples collected at low and high altitudes and later analyzed at sea level (45). Thus, there is little doubt that fasting glycemia is lower at high altitudes. The question that remains is why?
B. Does insulin explain the lower glycemia in highlanders?
Because blood glucose levels are tightly regulated by insulin, it is possible that the lower glycemia among highlanders may be explained by higher plasma insulin concentrations. Lower (45, 47, 48), similar (40, 41), or higher fasting plasma insulin (26) has been reported in highlanders as compared with lowlanders. However, age- and BMI-matched studies do not support hyperinsulinemia as a cause of the lower glycemia (40, 45). Moreover, similar fasting plasma levels of C-peptide, a marker of insulin release (77, 78), and lower plasma insulin levels have been reported in nonpregnant women from high altitude as compared with lowlanders, despite higher BMI (47), suggesting higher hepatic insulin extraction in female highlanders. It is unlikely that the discrepancies on insulin differences between lowlanders and highlanders among studies were explained by the assay method because RIA was used in most of the studies (26, 40, 41, 45, 47).
C. Is the lower glycemia at higher altitudes acquired?
Although numerous studies support the hypothesis that individuals who live at higher altitudes have lower fasting glycemia, little is known as to whether this phenomenon also occurs in individuals who are natives of sea level areas but migrated to or permanently live at high altitudes. There are some studies that support the idea that lower fasting glycemia may be an acquired trait. Lowlanders exposed to very high and extreme altitude for 3–8 weeks showed a significant reduction in fasting glycemia (79, 80). Similar findings have been reported in lowlanders chronically exposed to very high altitude for 24 months, independent of physical activity (44). Whether a decrease in glycemia can be attained in a relatively short time in the general population at moderate altitudes could have potential implications for treating diabetes.
The benefit of living at high altitudes on glucose metabolism appears to dissipate with migration to sea level. These observations were first reported by Carlos Monge-Cassinelli, who found similar fasting glycemia between natives of the coast living at sea level and natives of the Andes who were residing at sea level for at least 1 year (81). Another study reported that highlanders who lived for 2 years at sea level had glycemia similar to native residents of sea level (43). Collectively, these studies indicate that prolonged exposure to high altitude may lower glycemia in lowlanders, whereas highlanders may increase their glycemia when moving to low altitude, suggesting an acquired process.
IV. Endogenous Glucose Production at High Altitude: Role of the Liver
The mechanism(s) that may explain the lower fasting glycemia at higher altitudes is little understood. In the postprandial state, blood glucose concentration depends on glucose entry into the bloodstream from diet and endogenous sources, primarily the liver, and also depends on peripheral glucose disposal (glucose uptake), primarily by the skeletal muscle (82). However, in the fasting state, blood glucose is supplied primarily by the liver (80%) and to a lesser extent by the kidney (20%) (83), with uptake by the central nervous system. Therefore, it is possible that the lower fasting glycemia among highlanders could be determined by a lower glucose production in the liver.
A. Hepatic glucose output (HGO) in lowlanders exposed to high altitude
The widely accepted method to assess HGO is the isotope dilution method (84, 85). To date, no study has assessed HGO in individuals permanently living at high altitude. Intravenous [6,6-2D]glucose infusion experiments in male lowlanders have shown that acute exposure to very high altitude increases HGO, as measured by the rate of glucose appearance, compared with pre-exposure values (1.99 ± 0.11 vs 1.47 ± 0.19 mg·kg−1·min−1, respectively) (86). Conversely, after 7 days of exposure to very high altitude, HGO was similar to pre-exposure values (21), as assessed by the euglycemic hyperinsulinemic clamp technique (87). In both studies, HGO was associated with plasma epinephrine concentrations (21, 86). These results further support the idea that short-term exposure to very high altitude causes a transient hyperglycemia (in which sympathetic activation is likely to be involved) that is attenuated as the exposure continues. However, the question remains as to whether individuals permanently living at high altitudes have lower HGO compared with lowlanders.
B. Role of glucagon among highlanders
HGO regulation is complex, and it involves a close feedback among glucagon, nonesterified fatty acids, insulin (88), and neural input (89). Glucagon, fatty acids, and catecholamines stimulate HGO (90), whereas insulin suppresses HGO (91–93). Studies performed on highlanders using the iv glucagon tolerance test have shown a lower hyperglycemic response to glucagon as compared with lowlanders (94, 95). This may be due to lower endogenous glucose production, primarily the HGO, rather than renal glucose output, because the latter is not affected by glucagon (96, 97) Nevertheless, a major limitation in the interpretation of the iv glucagon tolerance test is that endogenous insulin release will occur as a compensation of the glucagon-induced hyperglycemia (98), unless insulin is experimentally suppressed. Therefore, this approach may confound the contribution of the liver due to concomitant insulin-mediated glucose uptake in the skeletal muscle.
An intriguing finding is lower fasting glycemia and higher fasting glucagonemia in healthy natives from the Andes compared with Australian Caucasians (23), raising the possibility that highlanders could have lower hepatic glucagon sensitivity, which could result in lower HGO.
C. Role of hepatic glycogen content
Studies in rodents chronically exposed (3–8 mo) to natural and simulated very high altitude have shown a considerable reduction (by ∼50%) in hepatic but not muscle glycogen content (99–101), probably as a consequence of reduced activity of gluconeogenic enzymes (100). In fact, the decrease in hepatic glycogen content was accompanied by a significant reduction in glycemia (99). Moreover, HGO is directly related to liver glycogen content in humans (102) and rats (103), suggesting that liver glycogen could contribute to the lower blood glucose among highlanders. However, it cannot be ruled out that low blood glucose may cause a decrease in liver glycogen content. Further studies are needed to elucidate the contribution of the liver to glucose regulation among highlanders.
V. Effect of Altitude and Hypoxia on the Skeletal Muscle
Because fasting plasma glucose levels are determined by a balance between HGO (primarily) and whole-body glucose disposal, the possibility that a higher glucose disposal contributes to the lower fasting glycemia in highlanders has to be considered. In fact, in the fasting state, the noninsulin-mediated glucose disposal accounts for approximately 70–75% of the whole-body glucose disposal in humans (104, 105). Although no study has quantitatively estimated the noninsulin-mediated glucose disposal or the insulin-mediated glucose disposal (insulin sensitivity) in highlanders, there is evidence supporting a higher whole-body glucose disposal in residents of high altitudes.
A. Short-term effects of altitude and hypoxia in vivo
1. Effect of altitude on glucose disposal in lowlanders
Although some human studies have shown improved oral glucose tolerance after exposure to high altitude (106, 107), such findings do not unequivocally indicate a higher glucose disposal because glucose profile changes during oral glucose tolerance test are affected by many factors, including gastrointestinal motility, rate of glucose absorption (108, 109), and incretin hormones (110, 111). In fact, experiments with isotope-labeled glucose have shown a decrease in the rate of glucose disappearance after 2- to 10-day exposure to very high altitude (21, 32). Conversely, more prolonged exposure to very high altitude appears to increase glucose disposal (28). These findings are also consistent with the studies showing a transient hyperglycemia in the lowlander exposed short term to high altitudes.
2. Effect of hypoxia on insulin sensitivity in lowlanders
Because hypoxia is a key factor associated with altitude, examining the short-term effect of hypoxia on insulin sensitivity in vivo may help to explain the glucose regulation in the highlander. Studies in healthy individuals (112, 113) were designed to substantially reduce peripheral oxygen saturation (≤85%) by administration of hypoxic air (∼5% O2). The latter studies showed a decrease in insulin sensitivity as assessed by the glucose infusion rate during euglycemic hyperinsulinemic clamp (87), the “gold standard” method, and the mathematically based “minimal model” (114). Conversely, mild hypoxia (13–15% O2) improved insulin sensitivity in type 2 diabetic subjects (∼2.3 × 10−4/min [mU/L] and ∼1.4 × 10−4/min [mU/L], hypoxia and normoxia, respectively) as assessed by the minimal model (115). Likewise, mild hypoxia has been shown to increase glucose infusion rate by approximately 20% (from ∼8.3 to 9.2 mg/kg/min) in obese subjects (116). Moreover, mild hypoxia (13% O2) has been shown to decrease glycemia in type 2 diabetic subjects (117). These findings suggest that severe hypoxia, associated with a dramatic reduction in peripheral oxygen saturation, may impair insulin sensitivity, whereas mild hypoxia, which is associated with a more moderate reduction in peripheral oxygen saturation, may improve insulin sensitivity.
3. Effect of hypoxia on insulin sensitivity in animals
A study conducted in mice breathing hypoxic air (10% O2) for 4 weeks showed an increase in insulin sensitivity, concomitant with a significant reduction in fasting glycemia (118). Likewise, dogs breathing 8% O2 had an increased rate of whole-body glucose disappearance and leg glucose uptake, although fasting glycemia did not change (119). In addition, perfusion experiments in rat hind limb under anoxic conditions have also shown higher glucose uptake (120, 121). Together, these studies further support an important beneficial effect of prolonged hypoxia on insulin sensitivity. However, the dissociation is still unclear between the changes in insulin sensitivity and the fasting glycemia after acute exposure to hypoxia.
B. Prolonged effects of altitude: evidence in highlanders
Although the evidence presented so far clearly shows that short-term exposure to high altitude and hypoxia may improve glucose disposal in the lowlander, this does not prove a higher glucose disposal in the highlander.
1. Intravenous glucose tolerance
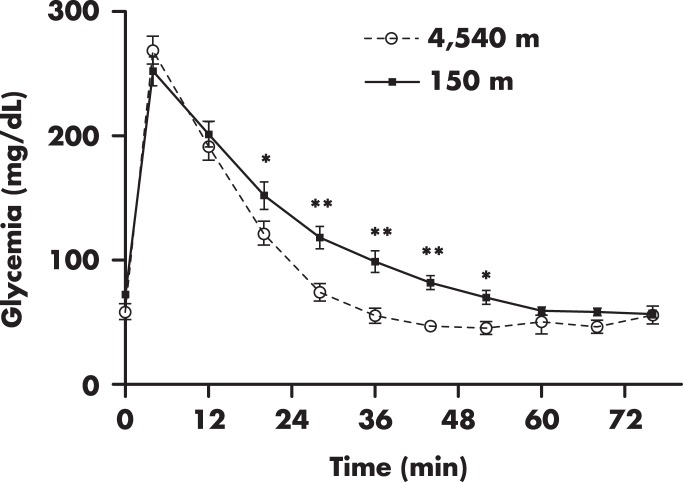
Emilio Picón-Reátegui was one of the pioneer researchers who systematically studied glucose metabolism in Andean residents of very high altitude. One of his most important contributions was to demonstrate a higher glucose disposal in the highlander (122). Male subjects residing at 4540 m showed a more rapid decline of glycemia during the iv glucose tolerance test as compared with lowlanders (122). Although the quantity of glucose administered was not corrected for body weight, reanalysis of available data from this study (122) shows that the differences in glucose profile remained significant when groups were matched for BMI (Figure 5). Using the same test, other investigators have confirmed the previous findings in males and females (42, 46).
Figure 5. Intravenous glucose tolerance test in subjects residing at 4540 m (n = 7) and subjects residing at 150 m (n = 8) above sea level.
Figure was created using available data from Ref. 122, including only subjects with lean BMI (<25 kg/m2). Note the pronounced and faster decrease of glycemia in the higher altitude group after glucose administration (at min 0). Symbols and bars represent mean and SE values, respectively. *, P < .05; **, P < .01.
2. Oral glucose tolerance
Studies in Andean and Indian healthy populations living at very high altitude have shown that these individuals have a better oral glucose tolerance compared with their fellow citizens living at low altitude (44, 123). Among obese individuals, it appears that highlanders living at 3200 m also have a better oral glucose tolerance as compared with sea-level residents (49), although it is not clear whether these differences are explained by differences in BMI between groups. Nevertheless, these findings confirm the results from studies using the iv glucose tolerance test in highlanders.
3. Arterial-venous glucose difference
The difference in arterialized venous-venous (peripheral) glucose concentration, which is proportional to the rate of glucose disposal assessed by tracer-labeled glucose (124), has been reported to be greater in men and pregnant women living at high elevations as compared with lowlanders (48, 123), indicating that highlanders have a higher rate of glucose disposal in peripheral tissues than the rate of endogenous glucose output.
4. Insulin tolerance
Residents of very high altitude appear to have a more sluggish return of glycemia to baseline from 60 to 120 minutes after iv insulin administration during the insulin tolerance test, as compared with lowlanders (125). The major problem in the assessment of whole-body insulin sensitivity with this test (126, 127) is that prolonged hypoglycemia may lead to a counter-regulatory response, confounding the effect of insulin (128).
5. Homeostasis model assessment (HOMA)
A higher insulin sensitivity has been reported in women residing at very high altitude, as compared with lowlanders (45, 47), using the HOMA index (129), a surrogate measure of insulin sensitivity. However, because calculations are based on fasting blood insulin and glucose, the HOMA index could give inaccurate estimations of insulin sensitivity when fasting glycemia is very low (130). Thus, HOMA estimations in high altitude populations should be interpreted with caution.
6. Glucose uptake assessed by positron emission tomography
Noninvasive methods are also useful tools to assess glucose uptake in the human body (131). Regional myocardial uptake of 18F-labeled 2-fluoro-2-deoxy-D-glucose measured by positron emission tomography revealed a higher uptake in Quechua subjects living above 3000 m compared with lowlanders (38). Using the advantages of this technique may help to determine whether specific differences in glucose uptake exist in skeletal muscle, adipose tissue, or the liver between lowlanders and highlanders.
C. Effects of anoxia and hypoxia on glucose uptake in vitro
Previously, we have discussed the evidence in vivo of higher glucose disposal and increased insulin sensitivity in response to high altitude and mild hypoxia (28, 115, 116). In this section, we will discuss additional supporting evidence from in vitro studies in rodent and human skeletal muscle. We will not discuss the mechanisms by which altitude and hypoxia affect glucose metabolism because this topic has been extensively reviewed elsewhere (132–135). However, it should be mentioned that hypoxia favors the rate of glycolysis (132, 134, 136, 137), which may contribute to the elevation of plasma lactate levels seen in individuals exposed to high altitude (138–140). Nevertheless, whether glucose enters the glycolytic pathway or is oxidized to generate ATP, a higher glucose uptake may contribute to the lower glycemia at high altitude.
1. Glucose uptake under anoxia conditions in vitro
Philip Randle was one of the pioneers who examined the role of oxygen tension on mammal cell glucose uptake. His work on rat diaphragm muscle led to the conclusion that anaerobic conditions increase cell glucose uptake (141). These findings were confirmed by Randle and colleagues (142) and by Wheeler (143) in the heart muscle from rats. Later, numerous studies have also reported increased glucose uptake in response to very acute exposure to anoxic conditions (gas mixture, 95% N2/5% CO2/0% O2) in the skeletal muscle of rodents (144–149). Notably, the enhanced glucose uptake induced by anoxia has also been reported in the human skeletal muscle from lean and obese subjects (150) and nonobese type 2 diabetic subjects (151). Moreover, the effects of anoxia on glucose uptake in human and rat skeletal muscle have been shown to be additive to that of insulin (144, 150), but not contractile activity, suggesting that anoxia and contractile activity may stimulate glucose uptake via similar mechanisms (152).
2. Glucose uptake under hypoxia conditions in vitro
Although it is clear that anoxia stimulates glucose uptake in vitro (141–151), few studies have addressed the effect of hypoxia (more clinically relevant) on glucose uptake in the skeletal muscle.
To determine the effect of hypoxia on glucose uptake in vitro, experimental studies have exposed the whole organism to hypoxic air. For example, mice exposed to normobaric hypoxia (10% O2) for 4 weeks showed increased soleus glucose uptake in vitro, but only in the presence of insulin (153), suggesting that hypoxia alone may not be sufficient to promote peripheral glucose uptake. In humans, hypoxic air (∼15% O2) for 10 nights resulted in increased insulin-independent glucose uptake in skeletal muscle (116). Conversely, very short exposure (∼10–22 min) to hypoxic air (11.4–14% O2) did not have an effect on human skeletal muscle glucose uptake (154), including intracellular glucose content (155), suggesting that the effect of hypoxia on glucose uptake is time dependent.
3. Effects of anoxia and hypoxia on glucose transporter 4 (GLUT4) translocation and AMP-activated protein kinase (AMPK) activity
The possible mechanisms by which anoxia/hypoxia may stimulate glucose uptake have been reviewed elsewhere (156, 157). However, it is important to point out that the GLUT4 plays a major role in insulin-dependent muscle glucose transport when translocated from intracellular pools to plasma membrane (158). Increased GLUT4 translocation and protein content of GLUT4 are also likely the main mechanisms by which anoxia stimulates glucose uptake in rodent skeletal muscle and cardiomyocytes (143–145, 159, 160). An increased GLUT4 protein content in human skeletal muscle in response to anoxia has also been reported in human skeletal muscle (151).
Skeletal muscle from rats breathing hypoxic air (9–14% O2) for 4 weeks also showed increased GLUT4 protein content (161). These findings, however, do not answer the question as to whether hypoxia per se has a direct effect on GLUT4 content because many other possible in vivo effects may be activated or inhibited when animals breathe hypoxic air. For example, epinephrine has been shown to stimulate GLUT4 translocation in the absence of insulin (162).
Augmented AMPK activity in the skeletal muscle stimulates glucose transport (157). Recent evidence suggests that hypoxia/anoxia-induced glucose uptake is exclusively coupled to the AMPK-dependent pathway (157, 163), in contrast to muscle contraction, which activates both AMPK-dependent and AMPK-independent pathways (144, 150, 152), challenging the initial concept that anoxia and muscle contraction stimulate glucose uptake under the same mechanism.
In summary, there is strong evidence suggesting that anoxia stimulates glucose uptake by promoting GLUT4 translocation via AMPK-dependent pathway. However, little is known about the direct effect of hypoxia on glucose uptake and the possible mechanisms involved. Moreover, whether GLUT4 and the AMPK pathway contribute to the greater glucose disposal in residents of high altitudes remains unknown.
VI. Role of the Gastrointestinal Tract
Although intestinal carbohydrate absorption in humans may decrease at altitudes over 5700 m (164, 165), this threshold is too high to explain the better oral glucose tolerance in residents living at altitudes below 5000 m (44, 123). Moreover, if exposure to very high altitude causes important intestinal malabsorption, then alteration in the absorption of other nutrients should be expected. However, studies have found no evidence of protein or fat malabsorption in humans exposed to altitudes below 5000 m (166–168).
The evidence to support a possible contribution of gut hormones to the better oral glucose tolerance is limited. In fact, gut hormones have been studied only under short-term exposure to high altitudes. In nonobese individuals passively exposed to high elevations for short periods, fasting plasma ghrelin and cholecystokinin have been shown to decrease below baseline values (169–171), most likely contributing to the transient loss of appetite, a very common symptom seen during short-term altitude exposures. In contrast, plasma levels of peptide YY, an anorexigenic hormone, does not appear to be involved in the anorexic effect of altitude exposure (170, 172). Glucagon-like peptide 1 (GLP-1) and ghrelin are of particular interest because these hormones have opposite effects on insulin secretion and food intake. GLP-1 enhances insulin secretion and suppresses appetite (173, 174). Human studies have shown that plasma fasting GLP-1 levels are not altered after exposure to hypoxia or simulated very high altitude (175, 176), suggesting that the increased oral glucose tolerance in lowlanders after altitude exposure (106, 107) may not be related to incretin effects. Future studies are required to elucidate the mechanisms involved in the greater oral glucose tolerance among highlanders.
VII. Role of the Adipose Tissue
The breakdown of stored lipids in adipose tissue results in the release of fatty acids into the bloodstream. Elevated fatty acid levels may stimulate gluconeogenesis and glycogenolysis, resulting in fasting hyperglycemia (177). Thus, it is possible that the lower fasting glycemia may be explained by lower fatty acids levels. However, studies have reported higher (41), lower (38), or similar (22, 178) plasma fatty acid levels in highlanders as compared with lowlanders. These discrepancies among studies are probably explained by differences in methodology. Fatty acid values are highly influenced by BMI, the type of diet, fasting duration, and lipolysis ex vivo (179–182), and much of this latter information is not described. Careful studies are required to determine whether highlanders have lower fatty acid levels.
Although the adipose tissue secretes numerous hormones (183), of which adiponectin has been strongly associated with improved systemic insulin sensitivity (184), this hormone has not been studied in the highlander. However, it is interesting that adiponectin is increased after subacute exposure to very high altitude (185, 186).
VIII. Possible Link Between Hypoxia-Inducible Factor (HIF) and Glucose Homeostasis
Numerous studies have shown that living above 1500 m may have beneficial effects on glucose homeostasis. However, the mechanisms by which altitude per se signals the cascade of cellular events to produce such physiological effects are little understood. In previous sections, we have discussed studies examining the isolated effect of hypoxia on insulin sensitivity, glucose disposal in vivo (112, 113, 115–119, 154), and muscle glucose uptake in vitro (116, 153), but a direct proof that hypoxia mediates the effects of altitude on glucose homeostasis is still missing.
A. Genetic adaptation of highlanders to hypoxia
Data from genetic studies support the hypothesis that hypoxia could mediate, at least in part, the effects of altitude on human physiology, including glucose homeostasis. Some alleles of genes related to hypoxia have a higher prevalence among highlanders as compared with lowlanders (187, 188). At the cellular level, hypoxia inhibits oxidative phosphorylation and stimulates oxygen signaling pathway through the HIF-1α (156). HIF is a DNA-binding transcription factor associated with specific nuclear cofactors and exists as three isoforms in humans: HIF-1α, HIF-2α, and HIF-3α. Under hypoxic conditions, HIF-1 regulates the expression of several genes that mediate adaptive responses to low oxygen tension in different tissues. Whereas HIF-1α is widely expressed in the body, HIF-2α expression is limited to fewer tissues. However, HIF-2α may be equally essential in the regulation of the hypoxia response (189–192).
It is noteworthy that a higher frequency of a single-nucleotide polymorphism at EPAS1 gene (HIF-2α) has been reported among Tibetans from China living above 4300 m as compared with Beijing residents living below 2000 m (87 and 9% frequency, respectively) (187). Similar increased frequency of single-nucleotide polymorphisms related to the HIF pathway (PPARA) has also been reported in Ethiopian residents at 3200 m compared with Ethiopians living at low altitude (188), suggesting that high altitude populations, regardless of their ethnicity, may display a genetic adaptation to hypoxia via the HIF pathway.
Interestingly, HIF-1α expression has been found to be 60% down-regulated in the skeletal muscle of Tibetan residents of very-high altitude regions as compared with Nepali lowlanders (193). Likewise, genes involved in the glycolytic pathway were down-regulated (193), suggesting a negative feedback mechanism in conditions of chronic exposure to hypoxia. In fact, chronic hypoxia exposure may result in an adaptive (evolutionary) mechanism that could be associated with the “altitude phenotype” in these populations. However, the persistent stimulus (hypoxia) may down-regulate typical responses to hypoxia as a protective mechanism. This phenomenon has been consistently observed in sea-level residents acclimated to high altitude, where the initial hyper-response in pulmonary ventilation and blood lactate accumulation after altitude exposure is followed by a more attenuated response in magnitude if the stimulus continues, as reviewed elsewhere (2, 194, 195). Another possible explanation is that high altitude residents may have increased sensitivity to chronic hypoxia in the skeletal muscle; therefore, increased expression of genes related to hypoxia may not be needed. Further discussion related to the changes over time in gene and protein expression in response to exposure to hypoxia or altitude has been published elsewhere (196–199). Whether hypoxia is the major determinant of the genetic adaptation to high altitude remains to be explored. We believe that hypobaria (low atmospheric pressure) must also play an important role in the genetic adaptation to high altitudes because many functions in the human body are regulated via baroreceptors (200).
B. HIF and glucose uptake: evidence in vitro
Data from in vitro studies may also provide some clues as how hypoxia may mediate the effects of altitude on glucose homeostasis in the highlander. Hypoxia (14.5% O2) increases both HIF-1α and GLUT4 mRNA expression in the human skeletal muscle (201). Moreover, muscle contraction may induce transcriptional activation of SLC2A4 (the gene encoding the protein GLUT4) together with increased expression of HIF-1α in rat skeletal muscle (202). Furthermore, a recent study in skeletal muscle clonal cells has shown that HIF-1α is involved in the regulation of glucose metabolism stimulated by insulin. Knockdown of HIF-1α expression in these cells resulted in a defect in insulin-stimulated glucose uptake due to impaired GLUT4 translocation (203). Together, these findings suggest that HIF may not only signal the effect of hypoxia on glucose uptake but also that HIF may be a constitutive element in the cell signaling of glucose uptake in normoxic conditions. This concept is supported by the findings from a study in individuals with Chuvash polycythemia (204), a congenital disorder manifested by an abnormal elevation in the number of red blood cells secondary to elevated expression of HIF genes due to a mutation in the von Hippel-Lindau gene. These individuals, despite residing at sea level, have lower blood glucose levels and glycated hemoglobin A1c (204).
IX. Diabetes and Obesity at High Altitudes
Type 2 diabetes results from an inadequate regulation of glucose homeostasis. Interestingly, there is growing evidence of a lower prevalence of diabetes at higher altitudes (50, 205–208). Likewise, obesity, a well-established risk factor for type 2 diabetes (209, 210), is less prevalent in populations from high altitudes (211). In this section we will discuss whether the lower prevalence of diabetes and obesity at higher altitudes is independent of potential confounders. In addition, we will argue about the possible explanations for this association.
A. Association between obesity and altitude
1. Obesity in adults
Independent studies have shown an inverse relationship between altitude and prevalence of obesity in representative adult populations from the Andes and Tibet (50, 207, 212, 213). Moreover, a retrospective study has concluded that individuals stationed at higher altitudes appear to have lower hazard rates for obesity (214). Table 1 summarizes the studies conducted in several countries that estimated the prevalence of obesity in representative populations residing at ≥ 1500 m (50, 207, 208, 212, 213, 215–221). It appears that obesity prevalence is inversely proportional to altitude. This relationship has been confirmed in a recent study (see Figure 1 in Ref. 208). Notably, this inverse association between obesity prevalence and altitude has been reported to be independent of lifestyle (208, 213, 222), ethnicity (208, 222), ambient temperature (222), air pollution, health insurance coverage, income, emigration rate, and latitude (208).
Table 1.
Prevalence of Obesity in Adult Populations Living at or Above 1500 m
| First Author, Year (Ref) | Population | Altitude, m | n | Age, y | Diagnosis Criteria | BMI, kg/m2 | Crude Prevalence, % | Age- Adjusted Prevalence, % |
|---|---|---|---|---|---|---|---|---|
| Chen, 2011 (221) | Lhasa, Tibet, China | 3658–4200 | 1289 | ≥18 | N.A. | >28 | 16.7 | |
| Malaga, 2010 (216) | Lari, Peru | 3600 | 74 | >18 | N.A. | ≥30 | 8.7 | |
| Medina, 2006 (215) | Arequipa, Peru | 2390a | 1878 | 20–80 | WHO, 2000 | ≥30 | 17.6 | |
| Mohanna, 2006 (217) | San Pedro de Cajas, Peru | 4100 | 102 | ≥30 | ATP III, 2002 | ≥30 | 10.8 | |
| Negi, 2012 (220) | Spiti Valley, India | 3900 | 242 | ≥20 | Consensus, India | ≥25 | 17.4 | |
| Spiti Valley, India | 3100 | 171 | ≥20 | Consensus, India | ≥25 | 35.7 | ||
| Pajuelo-Ramirez, 2010 (50) | Peru | ≥3000 | 959 | ≥20 | WHO, 1997 | ≥30 | 8.5 | |
| Peru | 1000–2999 | 808 | ≥20 | WHO, 1997 | ≥30 | 11 | ||
| Peru | <1000 | 2425 | ≥20 | WHO, 1997 | ≥30 | 17.5 | ||
| Schargrodsky, 2008 (219) | Quito, Ecuador | 2800 | 1638 | 25–64 | ATP III, 2002 | ≥30 | 16.3 | |
| Bogota, Colombia | 2625 | 1553 | 25–64 | ATP III, 2002 | ≥30 | 18 | ||
| Mexico City, Mexico | 2240 | 1722 | 25–64 | ATP III, 2002 | ≥30 | 31 | ||
| Barquisimeto, Venezuela | 564 | 1848 | 25–64 | ATP III, 2002 | ≥30 | 25.1 | ||
| Santiago, Chile | 520 | 1655 | 25–64 | ATP III, 2002 | ≥30 | 26.6 | ||
| Lima, Peru | 153 | 1652 | 25–64 | ATP III, 2002 | ≥30 | 22.3 | ||
| Buenos Aires, Argentina | 10 | 1482 | 25–64 | ATP III, 2002 | ≥30 | 19.7 | ||
| Seclén, 1999 (207) | Huaraz, Peru | 3052 | 77 | >18 | NIH-US, 1985 | ≥27 | 18.3 | |
| Waiku, Amazon, Peru | 600 | 54 | >18 | NIH-US, 1985 | ≥27 | 0 | ||
| Tarapoto, Amazon, Peru | 333 | 90 | >18 | NIH-US, 1985 | ≥27 | 17 | ||
| Cuñumbuque, Amazon, Peru | 300 | 101 | >18 | NIH-US, 1985 | ≥27 | 10.9 | ||
| Lima, Peru | 123 | 158 | >18 | NIH-US, 1985 | ≥27 | 22.8 | ||
| Castilla, Peru | 30 | 118 | >18 | NIH-US, 1985 | ≥27 | 36.7 | ||
| Segura, 2006 (212) | Andes, Peru | 3493a | N.A. | >18 | N.A. | ≥30 | 9.1 | |
| Andes, Peru | 2351a | N.A. | >18 | N.A. | ≥30 | 8.7 | ||
| Amazon, Peru | 168.5a | 1630 | >18 | N.A. | ≥30 | 11.7 | ||
| Coast, Peru | 31.5a | 7725 | >18 | N.A. | ≥30 | 13.5 | ||
| Shah, 2004 (218) | Ghizer, Pakistan | 1675–1980b | 4203 | ≥18 | WHO, 2000 | ≥30 | 2.3 | |
| Sherpa, 2010 (213) | Lhasa, Tibet, China | 3660 | 371 | 30–70 | WHO, 2000 | ≥30 | 9.7 | |
| Nepal | 2900 | 119 | 30–70 | WHO, 2000 | ≥30 | 11.8 | ||
| Nepal | 1200 | 127 | 30–70 | WHO, 2000 | ≥30 | 19.7 | ||
| Woolcott, 2014 (208) | United States | <1500 | N.A. | ≥20 | ATP II, 1998 | ≥30 | 30.6 | |
| United States | ≥1500 | N.A. | ≥20 | ATP II, 1998 | ≥30 | 23.3 |
Abbreviations: ATP, Adult Treatment Panel; NIH-US, National Institutes of Health-United States; WHO, World Health Organization; N.A., information not available. Studies were selected on the basis that they were conducted in at least one representative population residing at or above 1500 m.
Median altitude of the cities sampled, using altitude values obtained online.
Altitude range is provided elsewhere (268).
2. Obesity in children
Lower prevalence of obesity at higher altitudes has also been reported among children in Argentina (223, 224), with no differences in prevalence between girls and boys (223). Surprisingly, children residing at very high altitude did not show differences in fasting glycemia compared with those residing at low altitude (224). This latter finding suggests that the differences in fasting glycemia between lowlanders and highlanders may vary with age, possibly with a late onset, further supporting the concept of an acquired trait. Intriguingly, a study of a small cohort (nonrepresentative) of Arabian children found a higher prevalence of obesity at high altitude (225), raising the possibility that among children, the inverse association between prevalence of obesity and altitude could depend on ethnicity.
B. Possible factors contributing to the lower prevalence of obesity
The effects of altitude exposure on energy metabolism, weight loss, and obesity have been reviewed elsewhere (198, 226, 227). However, most of the evidence discussed in previous reviews originates from studies examining the effect of short-term exposure to altitude on lowlanders. Here we will mainly focus on studies conducted in highlanders.
1. Energy expenditure and food intake
Although human studies exploring the effect of subacute exposure to high and very high altitude on energy expenditure have shown higher basal metabolic rate (228–231), associated with lower BMI (232), studies comparing basal metabolic rate between lowlanders and highlanders have reported no differences (233, 234). It would be informative to determine whether differences exist in total energy expenditure or nonbasal metabolic rate. For example, one possibility is that unintentional physical activity, which is also an important component of total energy expenditure (235), could be increased among highlanders because living at high altitude may require a higher energy demand to walk in steep topographical terrains. Because ambient temperature is inversely related to geographical elevation (21), cold temperatures at high altitudes could favor increased thermogenesis. This is possible because many communities located at very high altitudes around the world lack the facilities to adjust the temperature of their local environments (eg, home, work).
In a synergistic manner, thyroid hormones and catecholamines induce thermogenesis (236). Although the active forms of T3 (free T3) and T4 (free T4) and basal norepinephrine have been reported to be higher in highlanders (237–239), the interpretation of these findings is difficult because it is not clear whether subjects were matched for BMI or physical activity. A recent study examining the effect of chronic exposure to 4500–4800 m in humans who reside near sea level showed persistent sympathetic hyperactivation, as determined by heart rate variability, for up to 18 months of stay at very high altitude (240). However, subjects had no changes in BMI. The findings from these studies do not conclusively support or refute higher energy expenditure in highlanders. What is unquestionable is the higher energy expenditure after short-term exposure to high altitudes (198).
Acute exposure to altitude induces loss of appetite (241, 242), which may be related to increased leptin, an anorexigenic hormone, and decreased ghrelin and cholecystokinin plasma levels (169–171). However, the association between leptin and altitude appears to be different in individuals living at high altitudes because lower leptin levels have been reported in this population (45, 243, 244), even when leptin values are normalized to body fat mass (45). Because leptin is involved in thermoregulation, the lower leptin values in residents of higher altitudes may represent an adaptive mechanism in response to colder ambient temperatures. However, less clear is whether an association between lower leptin levels and appetite exists in the long term.
2. Poverty and nutrition
It can be argued that populations living at higher altitudes may have lower incomes. Thus, the lower prevalence of obesity at higher altitudes could be associated with poverty. In fact, the lowest BMI values have been reported among populations with the poorest wealth quintiles (245). However, in the United States, for example, the contribution of poverty to the lower prevalence of obesity at higher altitudes is not clear because low income is less frequent at higher altitudes (208). Moreover, the inverse association between obesity and altitude has been reported to be independent of income (208, 222).
As discussed earlier, there is no evidence suggesting intestinal malabsorption below 5000 m (166–168). Therefore, it is unlikely that chronic malabsorption explains differences in BMI between lowlanders and highlanders. However, regional diet may vary considerably among places located at different altitudes. For example, in the Peruvian Andes, cereals constitute a major food ingredient up to 3500 m, whereas at higher altitudes tubers, mainly potatoes, constitute the main component of the diet (246). Although the inverse association between altitude and obesity remains while adjusting for self-reported fruit and vegetable consumption (208, 222), it is possible that differences in the consumption of other food products may account for the lower prevalence of obesity in populations living at higher altitudes.
C. Association between diabetes and altitude
The lower prevalence of diabetes at higher altitudes has been reported in certain groups, including hospital inpatients (205) and soldiers (206), and also in the general population, including large cohorts (207) and nationally representative populations from Peru and the United States (50, 208). Conversely, a small report found no association between diabetes and altitude among Swiss individuals (247). However, 98% of the studied population lived below 1200 m, undermining the power to detect any association.
Table 2 summarizes the studies conducted around the world estimating the prevalence of diabetes in representative populations located at ≥ 1500 m (50, 207, 208, 212, 216, 219–221, 247–250). A trend for an inverse association appears to exist between the prevalence of diabetes and altitude. Indeed, this has been confirmed using county-level data from a nationally representative population of the United States (208). Remarkably, the odds of having obesity and diabetes are approximately 25% and 12% lower, respectively, in individuals living between 1500 and 3500 m than those living below 500 m, while adjusting for multiple factors including self-reported food consumption, physical activity, ethnicity, air pollution, health insurance coverage, income, emigration rate, and latitude (208). Because the latter study utilized data from a telephone-based survey and diabetes was self-reported, whether the inverse association between diabetes and altitude represents cases of type 1 or type 2 diabetes still remains unknown.
Table 2.
Prevalence of Diabetes in Adult Populations Living at or Above 1500 m
| First Author, Year (Ref) | Population | Altitude, m | n | Age, y | Diagnosis Criteria | Glycemia, mg/dL | Crude Prevalence, % | Age- Adjusted Prevalence, % |
|---|---|---|---|---|---|---|---|---|
| Al-Habori, 2004 (249) | Sana, Yemen | 2300 | 498 | 25–65 | WHO, 1999 | ≥126 or 2-h OGTT ≥200 | 4.6 | |
| Chen, 2011 (221) | Lhasa, Tibet, China | 3658–4200 | 1289 | ≥18 | N.A. | ≥126 or 2-h OGTT ≥200 or self-report | 2.9 | |
| Faeh, 2009 (250) | German-speaking Switzerland | 259–300 | N.A. | 40–84 | Self-report | 5.8 | ||
| German-speaking Switzerland | 300–600 | N.A. | 40–84 | Self-report | 3.8 | |||
| German-speaking Switzerland | 600–900 | N.A. | 40–84 | Self-report | 3.4 | |||
| German-speaking Switzerland | 900–1200 | N.A. | 40–84 | Self-report | 4.0 | |||
| German-speaking Switzerland | 1200–1500 | N.A. | 40–84 | Self-report | 4.3 | |||
| German-speaking Switzerland | 1500–1960 | N.A. | 40–84 | Self-report | 5.1 | |||
| Malaga, 2010 (216) | Lari, Peru | 3600 | 74 | >18 | N.A. | ≥126 | 1.3 | |
| Negi, 2012 (220) | Spiti Valley, India | 3900 | 242 | ≥20 | N.A. | ≥126 | 0.4 | |
| Spiti Valley, India | 3100 | 171 | ≥20 | N.A. | ≥126 | 4.1 | ||
| Pajuelo-Ramirez, 2010 (50) | Peru | ≥3000 | 959 | ≥20 | ADA, 1997 | ≥126 | 0.9 | |
| Peru | 1000–2999 | 808 | ≥20 | ADA, 1997 | ≥126 | 1.6 | ||
| Peru | <1000 | 2425 | ≥20 | ADA, 1997 | ≥126 | 2.9 | ||
| Posadas-Romero, 1994 (248) | Mexico City, Mexico | 2255a | 805 | 20–90 | WHO, 1985 | ≥140 or self-report | 8.7 | |
| Schargrodsky, 2008 (219) | Quito, Ecuador | 2800 | 1638 | 25–64 | ADA, 2003 | ≥126 or self-report | 5.9 | |
| Bogota, Colombia | 2625 | 1553 | 25–64 | ADA, 2003 | ≥126 or self-report | 8.1 | ||
| Mexico City, Mexico | 2240 | 1722 | 25–64 | ADA, 2003 | ≥126 or self-report | 8.9 | ||
| Barquisimeto, Venezuela | 564 | 1848 | 25–64 | ADA, 2003 | ≥126 or self-report | 6.0 | ||
| Santiago, Chile | 520 | 1655 | 25–64 | ADA, 2003 | ≥126 or self-report | 7.2 | ||
| Lima, Peru | 153 | 1652 | 25–64 | ADA, 2003 | ≥126 or self-report | 4.4 | ||
| Buenos Aires, Argentina | 10 | 1482 | 25–64 | ADA, 2003 | ≥126 or self-report | 6.2 | ||
| Seclén, 1999 (207) | Huaraz, Peru | 3052 | 77 | >18 | WHO, 1985 | ≥140 or 2-h OGTT ≥200 | 1.3 | |
| Waiku, Amazon, Peru | 600 | 54 | >18 | WHO, 1985 | ≥140 or 2-h OGTT ≥200 | 3.7 | ||
| Tarapoto, Amazon, Peru | 333 | 90 | >18 | WHO, 1985 | ≥140 or 2-h OGTT ≥200 | 4.4 | ||
| Cuñumbuque, Amazon, Peru | 300 | 101 | >18 | WHO, 1985 | ≥140 or 2-h OGTT ≥200 | 2.0 | ||
| Lima, Peru | 123 | 158 | >18 | WHO, 1985 | ≥140 or 2-h OGTT ≥200 | 7.6 | ||
| Castilla, Peru | 30 | 118 | >18 | WHO, 1985 | ≥140 or 2-h OGTT ≥200 | 6.7 | ||
| Segura, 2006 (212) | Andes, Peru | 3493a | N.A. | >18 | - | Self-report | 1.8 | |
| Andes, Peru | 2351a | N.A. | >18 | - | Self-report | 2.4 | ||
| Amazon, Peru | 168.5a | 1630 | >18 | - | Self-report | 3.9 | ||
| Coast, Peru | 31.5a | 7725 | >18 | - | Self-report | 4.3 | ||
| Woolcott, 2014 (208) | United States | <1500 | N.A. | ≥20 | - | Self-report | 9.1 | |
| United States | ≥1500 | N.A. | ≥20 | - | Self-report | 6.4 |
Abbreviations: ADA, American Diabetes Association; OGTT, oral glucose tolerance test; WHO, World Health Organization; N.A., information not available. Studies were selected on the basis that they were conducted in at least one representative population residing at or above 1500 m.
Median altitude of the cities sampled, using altitude values obtained online.
D. Possible factors contributing to the lower prevalence of diabetes
Because obesity is a known risk factor for type 2 diabetes (209, 210), the lower prevalence of obesity at higher altitudes should explain the lower prevalence of diabetes at higher altitudes. However, the lower odds of prevalent diabetes at high altitude remained while adjusting for BMI (208), suggesting that the inverse association between diabetes and altitude may not be accounted for obesity, although it is possible that this association may be mediated by body fat. There is also evidence that ethnicity and access to health care may not account for this association (208, 251).
There are some epidemiological studies that have found a link between environmental factors and diabetes. There is evidence of a possible link between vitamin D (and solar radiation) and diabetes (252). Because solar ultraviolet (UV)-B radiation increases proportionally to altitude (approximately by 7% every kilometer) (253), the possibility that the lower prevalence of diabetes at higher altitudes could be accounted for by solar radiation should be considered. However, this latter possibility seems to be unlikely because administration of vitamin D2 in humans has failed to modify insulin sensitivity (254), a key component in the pathogenesis of diabetes. Moreover, a recent meta-analysis has concluded that administration of vitamin D3 had no effect on incident diabetes (255).
Air pollution, predominantly fine particulate matter, has also been linked to the pathogenesis of type 2 diabetes (256, 257). Although the concentration of fine particulate matter is lower at higher altitudes (258), the association between diabetes and altitude remains after adjustment for air pollution, including particle matter ≤ 2.5 μm and ozone levels (208).
It should be clear that the inverse association between altitude and diabetes does not prove causality. However, the robust evidence in vivo of a lower glycemia (40–43, 45–48) and greater glucose disposal (38, 42, 46, 48, 122, 123) among individuals living at higher altitudes, together with the evidence in vitro of a direct stimulatory effect of anoxia on glucose uptake in the skeletal muscle (144–151), provides some biological explanation for the lower prevalence of diabetes at higher elevations.
X. Summary and Conclusions
Sudden exposure to high or very high altitude may initially lead to fasting hyperglycemia. However, the acclimatization process, as the exposure continues, may attenuate this response. Conversely, chronic or permanent exposure to high altitudes may lower fasting glycemia and glycemia postglucose challenge (40–48, 123), and it is likely to be acquired. The hypothetical variability in fasting glycemia as a function of exposure time to high altitudes is simplified in Figure 6.
Figure 6. Hypothetical variability of fasting glycemia in nondiabetic humans exposed to natural high or very high altitude.
It is possible that the variability of glycemia could also be related to the magnitude of the elevation, with more pronounced changes in glycemia (lowering effect) and faster shifts at higher altitudes. However, this is still speculative. Definitions of acute, subacute, and chronic terms are arbitrary. See Introduction for explanation.
The physiological changes in the human body that determine the lower fasting glycemia among highlanders are not fully understood. However, insulin is unlikely to be involved. Studies showing higher glucagon levels in highlanders and reduced hepatic glycogen content in rodents chronically exposed to hypoxia suggest that the liver may play a role. Less clear is the possible contribution of the gut because most of the studies have focused on the short-term exposure to high altitudes. To date, no study has assessed HGO or peripheral insulin sensitivity in vivo in individuals permanently living at high altitudes. However, there is strong evidence in vivo of a greater glucose disposal during iv glucose administration in permanent residents of high altitudes (42, 46, 122). Moreover, the findings from in vitro studies clearly show that virtual anoxia increases glucose uptake in cultured skeletal muscle from rodents and humans (144–151), most likely linked to increased GLUT4 translocation and GLUT4 content, via the AMPK-dependent pathway and HIF activation. However, the direct effect of hypoxia on glucose uptake in cultured skeletal muscle has not yet been demonstrated.
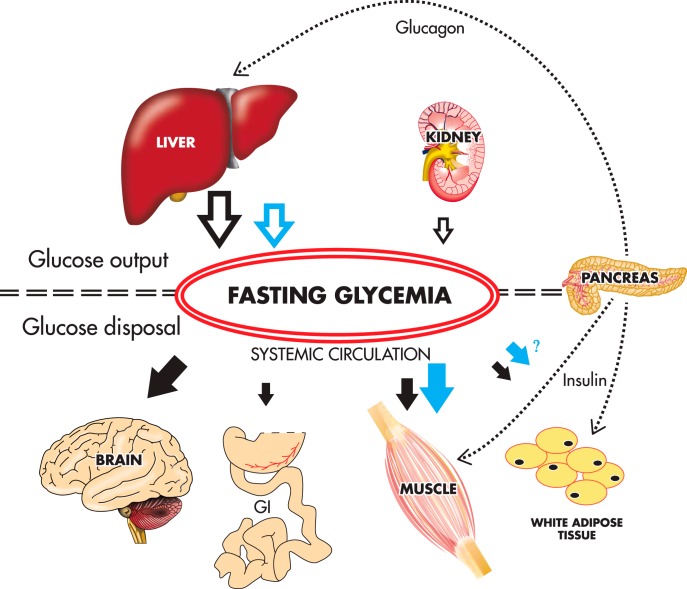
In the postabsorptive state, blood glucose supply depends primarily on the liver (83), whereas glucose disposal occurs primarily in the brain (∼50%) and to a lesser extent in the skeletal muscle (<25%) (259). This widely accepted concept, together with the evidence of higher glucose disposal in vivo in highlanders and increased glucose uptake in the skeletal muscle induced by anoxia and hypoxia in vivo, lead us to hypothesize that the lower fasting glycemia in individuals living at high altitudes is determined by a lower HGO and a higher glucose disposal in the skeletal muscle. This concept is summarized in Figure 7.
Figure 7. Hypothetical schematic to explain the lower fasting glycemia in healthy individuals living at higher altitudes.
The lower fasting glycemia occurs despite similar or lower fasting insulin values as compared to lowlanders (40, 41, 45, 47, 48). In the fasting state, the liver is responsible for maintaining relatively constant the fasting glycemia, contributing up to 80% of the total endogenous glucose production (83). Moreover, there is evidence of a more rapid glucose disposal in highlanders after iv glucose administration (42, 46, 122). Based on these findings, and the evidence of increased skeletal glucose uptake in response to anoxia/hypoxia (116, 144–151, 153), we propose that in the highlander, a lower hepatic glucose output (open blue arrow) and a higher glucose disposal in the skeletal muscle (and probably in the adipose tissue) (solid blue arrows) may account for the lower fasting glycemia as compared with the lowlander (black arrows). For clarity purposes, the substantial fraction of glucose disposal in the liver and kidneys (259, 267) is not represented in the scheme. GI, gastrointestinal tract.
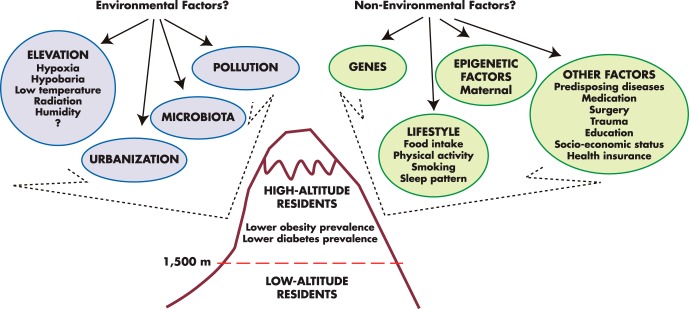
The inverse association between obesity and altitude is well documented, and it remains while adjusting for multiple risk factors (208, 213, 222). Likewise, an inverse association between diabetes and altitude, while adjusting for several factors (including BMI), has also been recently demonstrated (208), but longitudinal clinical studies will be required to determine causality. Figure 8 depicts the complexity of possible factors related to the lower prevalence of obesity and diabetes, including geographical elevation. A recent quasi-experimental study in a military population provides further evidence that altitude could have a direct effect on the pathogenesis of obesity (214).
Figure 8. Factors linked to obesity and diabetes.
Although nonenvironmental factors have been shown to have a stronger association with obesity and diabetes, several studies have confirmed a link between environmental factors and these clinical conditions. Among these factors, geographical elevation has been associated with lower prevalence of obesity and diabetes, independent of several other potential factors (208, 213, 222).
Further experimental studies aiming to determine the altitude threshold and minimum exposure duration needed to influence blood glucose levels may have an important clinical impact. The use of simulated altitude or hypoxia alone would be more practical, but future studies are required to determine whether these approaches lead to similar or better results than those obtained under natural altitude conditions. So far, small clinical studies have shown that hypoxic sessions may improve glucose homeostasis in overweight, obese, and type 2 diabetic subjects (115–117, 260). However, no effects of simulated altitude (whether hypobaric or normobaric hypoxia) have yet been monitored for periods longer than 10 weeks (260). The question also remains as to whether simulated altitude could be widely used as a potential clinical intervention for metabolic disorders. Perhaps hypoxia could be useful as a concomitant therapy rather than as a substitution of conventional approaches.
Concern about the clinical applicability of hypoxia has been raised, given that obstructive sleep apnea (OSA), which is associated with oxygen desaturation, has been linked to impaired insulin sensitivity and higher risk of diabetes (261, 262). However, OSA is a disorder manifested not only by repeated and transitory events of extremely variable oxygen desaturation (oxygen saturation can range from 90 to 50%), but also by blood CO2 retention (263). In addition, OSA is highly associated with obesity (262). Whether the association between sleep apnea and metabolic alterations is independent of CO2 retention or obesity still remains elusive. What appears to be clear is that severe hypoxia (∼5% O2 inspired) per se decreases insulin sensitivity (112, 113), whereas moderate hypoxia (13–15% O2) rather improves insulin sensitivity (115, 116).
Finally, although genetic population studies support the hypothesis that the HIF pathway may play a role in the mechanisms of adaptation to high altitude, whether hypoxia is the only determinant of the genetic adaptation to high altitudes remains to be explored. Understanding the mechanisms that regulate and maintain the lower fasting glycemia in individuals who live at higher altitudes could lead to new therapeutics for impaired glucose homeostasis.
Acknowledgments
This paper is dedicated to the memory of Dr Javier Torres Noriega (1950–2013), who devoted decades to mentoring many pre- and postgraduate students in the field of endocrine and high-altitude physiology at the Universidad Nacional Mayor de San Marcos, Peru.
We thank Mrs Nelly Diaz and Mr Frank Martínez for their valuable help in obtaining historical publications. We also thank Dr Oscar Castillo (Instituto Nacional de Biología Andina, Universidad Nacional Mayor de San Marcos, Peru) for thoughtful discussion on many sections of the present review.
This study was supported by National Institutes of Health Grants DK29867 and DK27619 (to R.N.B.).
Disclosure Summary: The authors disclose no conflict of interest relevant to this work.
Footnotes
- AMPK
- AMP-activated protein kinase
- BMI
- body mass index
- GLUT4
- glucose transporter 4
- GLP-1
- glucagon-like peptide 1
- HGO
- hepatic glucose output
- HIF
- hypoxia-inducible factor
- HOMA
- homeostasis model assessment
- OSA
- obstructive sleep apnea.
References
- 1. West JB. The physiologic basis of high-altitude diseases. Ann Intern Med. 2004;141:789–800. [DOI] [PubMed] [Google Scholar]
- 2. Beall CM. Adaptations to altitude: a current assessment. Annu Rev Anthropol. 2001;30:423–456. [Google Scholar]
- 3. Weil JV, Zwillich CW. Assessment of ventilatory response to hypoxia: methods and interpretation. Chest. 1976;70:124–128. [PubMed] [Google Scholar]
- 4. Peacock AJ. ABC of oxygen: oxygen at high altitude. BMJ. 1998;317:1063–1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Blume FD. Metabolic and endocrine changes at altitude. In: West JB, Lahiri S, eds. High Altitude and Man. Bethesda, MD: American Physiological Society; 1984:37–45. [Google Scholar]
- 6. Kellogg RH. Some high points in high altitude. In: Folinsbee LJ, Wagner JA, Borgia JF, Drinkwater BL, Gliner JA, Bedi JF, eds. Environmental Stress. Individual Human Adaptations. New York, NY: Academic Press, Inc; 1978:317–323. [Google Scholar]
- 7. Bert P. Barometric Pressure: Researches in Experimental Physiology [first published in French in 1878] Columbus, OH: College Book Company; 1943. [Google Scholar]
- 8. Columbia University Center for International Earth Science Information Network. National Aggregates of Geospatial Data Collection: Population, Landscape, and Climate Estimates (PLACE II), version 2 (1990, 2000). http://sedac.ciesin.columbia.edu/data/set/nagdc-population-landscape-climate-estimates-v2 Accessed April 28, 2014.
- 9. Cohen JE, Small C. Hypsographic demography: the distribution of human population by altitude. Proc Natl Acad Sci USA. 1998;95:14009–14014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Burrows SM, Elbert W, Lawrence MG, Poschl U. Bacteria in the global atmosphere – Part 1: review and synthesis of literature data for different ecosystems. Atmos Chem Phys. 2009;9:9263–9280. [Google Scholar]
- 11. Heath D, Williams DR. The environment. In: Heath D, Williams DR, eds. Man at High Altitude: The Pathophysiology of Acclimatization and Adaptation. Edinburgh, UK: Churchill Livingstone; 1977:4–14. [Google Scholar]
- 12. Millet GP, Faiss R, Pialoux V. Last word on Point: Counterpoint: hypobaric hypoxia induces different responses from normobaric hypoxia. J Appl Physiol. 2012;112:1795. [DOI] [PubMed] [Google Scholar]
- 13. Mounier R, Brugniaux JV. Last word on Counterpoint: hypobaric hypoxia does not induce different physiological responses from normobaric hypoxia. J Appl Physiol. 2012;112:1796. [DOI] [PubMed] [Google Scholar]
- 14. Luks AM, Swenson ER. Pulse oximetry at high altitude. High Alt Med Biol. 2011;12:109–119. [DOI] [PubMed] [Google Scholar]
- 15. Arias-Stella J, Recavarren S. Right ventricular hypertrophy in native children living at high altitude. Am J Pathol. 1962;41:55–64. [PMC free article] [PubMed] [Google Scholar]
- 16. Reynafarje C, Lozano R, Valdivieso J. The polycythemia of high altitudes: iron metabolism and related aspects. Blood. 1959;14:433–455. [PubMed] [Google Scholar]
- 17. Rupert JL, Hochachka PW. Genetic approaches to understanding human adaptation to altitude in the Andes. J Exp Biol. 2001;204:3151–3160. [DOI] [PubMed] [Google Scholar]
- 18. Heath D, Williams DR. The physique of high altitude man. In: Heath D, Williams DR, eds. Man at High Altitude: The Pathophysiology of Acclimatization and Adaptation. Edinburgh, UK: Churchill Livingstone; 1977:15–26. [Google Scholar]
- 19. Mirrakhimov MM. Biological and physiological characteristics of the high-altitude natives of Tien Shan and the Pamirs. In: Baker PT, ed. The Biology of High-Altitude Peoples. Cambridge, UK: Cambridge University Press; 1978:299–315. [Google Scholar]
- 20. Frisancho AR. Developmental functional adaptation to high altitude: review. Am J Hum Biol. 2013;25:151–168. [DOI] [PubMed] [Google Scholar]
- 21. Larsen JJ, Hansen JM, Olsen NV, Galbo H, Dela F. The effect of altitude hypoxia on glucose homeostasis in men. J Physiol. 1997;504:241–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Sawhney RC, Malhotra AS, Singh T. Glucoregulatory hormones in man at high altitude. Eur J Appl Physiol Occup Physiol. 1991;62:286–291. [DOI] [PubMed] [Google Scholar]
- 23. Sutton J, Garmendia F. Hormonal variation during physical exertion at high altitude [in Spanish]. Arch Biol Andina. 1977;7:83–93. [PubMed] [Google Scholar]
- 24. Stock MJ, Chapman C, Stirling JL, Campbell IT. Effects of exercise, altitude, and food on blood hormone and metabolite levels. J Appl Physiol. 1978;45:350–354. [DOI] [PubMed] [Google Scholar]
- 25. Consolazio CF, Johnson HL, Krzywicki HJ, Daws TA. Metabolic aspects of acute altitude exposure (4,300 meters) in adequately nourished humans. Am J Clin Nutr. 1972;25:23–29. [DOI] [PubMed] [Google Scholar]
- 26. Sawhney RC, Malhotra AS, Singh T, Rai RM, Sinha KC. Insulin secretion at high altitude in man. Int J Biometeorol. 1986;30:231–238. [DOI] [PubMed] [Google Scholar]
- 27. Johnson HL, Consolazio CF, Burk RF, Daws TA. Glucose-14 C-UL metabolism in man after abrupt altitude exposure (4,300 m). Aerosp Med. 1974;45:849–854. [PubMed] [Google Scholar]
- 28. Brooks GA, Butterfield GE, Wolfe RR, et al. Increased dependence on blood glucose after acclimatization to 4,300 m. J Appl Physiol. 1991;70:919–927. [DOI] [PubMed] [Google Scholar]
- 29. Rostrup M. Catecholamines, hypoxia and high altitude. Acta Physiol Scand. 1998;162:389–399. [DOI] [PubMed] [Google Scholar]
- 30. Richalet JP. The endocrine system. In: Hornbein TF, Schoene RB, eds. High Altitude. An Exploration of Human Adaptation. New York: Marcel Dekker, Inc; 2001;601–644. [Google Scholar]
- 31. Woods DR, Stacey M, Hill N, de Alwis N. Endocrine aspects of high altitude acclimatization and acute mountain sickness. J R Army Med Corps. 2011;157:33–37. [DOI] [PubMed] [Google Scholar]
- 32. Braun B, Mawson JT, Muza SR, et al. Women at altitude: carbohydrate utilization during exercise at 4,300 m. J Appl Physiol (1985). 2000;88:246–256. [DOI] [PubMed] [Google Scholar]
- 33. Brahmachari HD, Malhotra MS, Joseph S, Krishnan UR. Glucose tolerance at high altitude in man. Indian J Med Res. 1973;61:411–415. [PubMed] [Google Scholar]
- 34. Young PM, Sutton JR, Green HJ, et al. Operation Everest II: metabolic and hormonal responses to incremental exercise to exhaustion. J Appl Physiol. 1992;73:2574–2579. [DOI] [PubMed] [Google Scholar]
- 35. Boutwell JH, Cilley JH, Krasno LR, Ivy AC, Farmer CJ. Effect of repeated exposure of human subjects to hypoxia on glucose tolerance, excretion of ascorbic acid, and phenylalanine tolerance. J Appl Physiol. 1950;2:388–392. [Google Scholar]
- 36. Brooks GA, Wolfel EE, Groves BM, et al. Muscle accounts for glucose disposal but not blood lactate appearance during exercise after acclimatization to 4,300 m. J Appl Physiol (1985). 1992;72:2435–2445. [DOI] [PubMed] [Google Scholar]
- 37. Hochachka PW, Stanley C, Matheson GO, McKenzie DC, Allen PS, Parkhouse WS. Metabolic and work efficiencies during exercise in Andean natives. J Appl Physiol (1985). 1991;70:1720–1730. [DOI] [PubMed] [Google Scholar]
- 38. Holden JE, Stone CK, Clark CM, et al. Enhanced cardiac metabolism of plasma glucose in high-altitude natives: adaptation against chronic hypoxia. J Appl Physiol. 1995;79:222–228. [DOI] [PubMed] [Google Scholar]
- 39. Braun B. Effects of high altitude on substrate use and metabolic economy: cause and effect? Med Sci Sports Exerc. 2008;40:1495–1500. [DOI] [PubMed] [Google Scholar]
- 40. Castillo O, Woolcott OO, Gonzales E, et al. Residents at high altitude show a lower glucose profile than sea-level residents throughout 12-hour blood continuous monitoring. High Alt Med Biol. 2007;8:307–311. [DOI] [PubMed] [Google Scholar]
- 41. Garmendia F, Torres J, Tamayo R, Urdanivia E. Contributions to the knowledge of glycemia at high altitude [in Spanish]. Arch Inst Biol Andina. 1972;5:51–56. [PubMed] [Google Scholar]
- 42. Calderón R, Llerena A. Carbohydrate metabolism in people living in chronic hypoxia. Diabetes. 1965;14:100–105. [DOI] [PubMed] [Google Scholar]
- 43. Picon-Reategui E, Buskirk ER, Baker PT. Blood glucose in high-altitude natives and during acclimatization to altitude. J Appl Physiol. 1970;29:560–563. [DOI] [PubMed] [Google Scholar]
- 44. Srivastava KK, Kumria MM, Grover SK, Sridharan K, Malhotra MS. Glucose tolerance of lowlanders during prolonged stay at high altitude and among high altitude natives. Aviat Space Environ Med. 1975;46:144–146. [PubMed] [Google Scholar]
- 45. Lindgärde F, Ercilla MB, Correa LR, Ahrén B. Body adiposity, insulin, and leptin in subgroups of Peruvian Amerindians. High Alt Med. Biol. 2004;5:27–31. [DOI] [PubMed] [Google Scholar]
- 46. Calderón R, Llerena LA, Munive L, Kruger F. Intravenous glucose tolerance test in pregnancy in women living in chronic hypoxia. Diabetes. 1966;15:130–132. [DOI] [PubMed] [Google Scholar]
- 47. Krampl E, Kametas NA, Nowotny P, Roden M, Nicolaides KH. Glucose metabolism in pregnancy at high altitude. Diabetes Care. 2001;24:817–822. [DOI] [PubMed] [Google Scholar]
- 48. Zamudio S, Torricos T, Fik E, et al. Hypoglycemia and the origin of hypoxia-induced reduction in human fetal growth. PLoS One. 2010;5:e8551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Jo N, Garmendia F, Damas G, Pando R, Saavedra S. Lower insulin resistance in obese people from high altitude [in Spanish]. An Fac Med Lima. 1997;58:109–111. [Google Scholar]
- 50. Pajuelo-Ramírez J, Sánchez-Abanto J, Arbañil-Huamán H. Non-transmissible chronic diseases in Peru and their relationship with altitude [in Spanish]. Rev Soc Peru Med Interna. 2010;23:45–52. [Google Scholar]
- 51. Picón-Reátegui E. Effect of chronic hypoxia on the action of epinephrine in carbohydrate metabolism. J Appl Physiol. 1966;21:1181–1184. [DOI] [PubMed] [Google Scholar]
- 52. Garmendia F, Arroyo J, Muro M. Blood sugar of the normal high altitude native [in Spanish]. Arch Inst Biol Andina. 1970;3:209–216. [PubMed] [Google Scholar]
- 53. Foll M, Gaggiotti OE, Daub JT, Vatsiou A, Excoffier L. Widespread signals of convergent adaptation to high altitude in Asia and America. Am J Hum Genet. 2014;95:394–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Bigham AW, Wilson MJ, Julian CG, et al. Andean and Tibetan patterns of adaptation to high altitude. Am J Hum Biol. 2013;25:190–197. [DOI] [PubMed] [Google Scholar]
- 55. Xing G, Qualls C, Huicho L, et al. Adaptation and mal-adaptation to ambient hypoxia; Andean, Ethiopian and Himalayan patterns. PLoS One. 2008;3:e2342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Oberg D, Ostenson CG. Performance of glucose dehydrogenase-and glucose oxidase-based blood glucose meters at high altitude and low temperature. Diabetes Care. 2005;28:1261. [DOI] [PubMed] [Google Scholar]
- 57. de Mol P, Krabbe HG, de Vries ST, et al. Accuracy of handheld blood glucose meters at high altitude. PLoS One. 2010;5:e15485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Gautier JF, Bigard AX, Douce P, Duvallet A, Cathelineau G. Influence of simulated altitude on the performance of five blood glucose meters. Diabetes Care. 1996;19:1430–1433. [DOI] [PubMed] [Google Scholar]
- 59. Olateju T, Begley J, Flanagan D, Kerr D. Effects of simulated altitude on blood glucose meter performance: implications for in-flight blood glucose monitoring. J Diabetes Sci Technol. 2012;6:867–874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Baumstark A, Schmid C, Pleus S, Haug C, Freckmann G. Influence of partial pressure of oxygen in blood samples on measurement performance in glucose-oxidase-based systems for self-monitoring of blood glucose. J Diabetes Sci Technol. 2013;7:1513–1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Pecchio O, Maule S, Migliardi M, Trento M, Veglio M. Effects of exposure at an altitude of 3,000 m on performance of glucose meters. Diabetes Care. 2000;23:129–131. [DOI] [PubMed] [Google Scholar]
- 62. King JM, Eigenmann CA, Colagiuri S. Effect of ambient temperature and humidity on performance of blood glucose meters. Diabet Med. 1995;12:337–340. [DOI] [PubMed] [Google Scholar]
- 63. Chmielewski SA, Kurtock DK, Jennings SA, et al. Precision and accuracy of the Accu-Chek Advantage blood glucose monitoring system at high altitude. Clin Chem. 1996;42:115–117. [PubMed] [Google Scholar]
- 64. Giordano BP, Thrash W, Hollenbaugh L, et al. Performance of seven blood glucose testing systems at high altitude. Diabetes Educ. 1989;15:444–448. [DOI] [PubMed] [Google Scholar]
- 65. Tang Z, Louie RF, Payes M, Chang KC, Kost GJ. Oxygen effects on glucose measurements with a reference analyzer and three handheld meters. Diabetes Technol Ther. 2000;2:349–362. [DOI] [PubMed] [Google Scholar]
- 66. Laurinavicius V, Kurtinaitiene B, Liauksminas V, et al. Oxygen insensitive glucose biosensor based on PQQ-dependent glucose dehydrogenase. Anal Lett. 1999;32:299–316. [Google Scholar]
- 67. Ginsberg BH. Factors affecting blood glucose monitoring: sources of errors in measurement. J Diabetes Sci Technol. 2009;3:903–913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Rao LV, Jakubiak F, Sidwell JS, Winkelman JW, Snyder ML. Accuracy evaluation of a new glucometer with automated hematocrit measurement and correction. Clin Chim Acta. 2005;356:178–183. [DOI] [PubMed] [Google Scholar]
- 69. Tang Z, Lee JH, Louie RF, Kost GJ. Effects of different hematocrit levels on glucose measurements with handheld meters for point-of-care testing. Arch Pathol Lab Med. 2000;124:1135–1140. [DOI] [PubMed] [Google Scholar]
- 70. Billington CJ, Casciato DA, Choquette DL, Morley JE. Artifactual hypoglycemia associated with polycythemia vera. JAMA. 1983;249:774–775. [PubMed] [Google Scholar]
- 71. Field JB, Williams HE. Artifactual hypoglycemia associated with leukemia. N Engl J Med. 1961;265:946–948. [DOI] [PubMed] [Google Scholar]
- 72. Arnaud J, Gutierrez N. Human red cell glycolysis in high altitude chronic hypoxia. Am J Phys Anthropol. 1984;63:307–314. [DOI] [PubMed] [Google Scholar]
- 73. Sacks DB, Arnold M, Bakris GL, et al. Position statement executive summary: guidelines and recommendations for laboratory analysis in the diagnosis and management of diabetes mellitus. Diabetes Care. 2011;34:1419–1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Sacks DB, Bruns DE, Goldstein DE, Maclaren NK, McDonald JM, Parrott M. Guidelines and recommendations for laboratory analysis in the diagnosis and management of diabetes mellitus. Clin Chem. 2002;48:436–472. [PubMed] [Google Scholar]
- 75. Krampl E, Kametas NA, Cacho Zegarra AM, Roden M, Nicolaides KH. Maternal plasma glucose at high altitude. BJOG. 2001;108:254–257. [DOI] [PubMed] [Google Scholar]
- 76. Moret P, Covarrubias E, Coudert J, Duchosal F. Cardiocirculation adaptation to chronic hypoxia. II. Comparative study of myocardial metabolism of glucose, lactate, pyruvate and free fatty acids between sea level and high altitude residents. Acta Cardiol. 1972;27:483–503. [PubMed] [Google Scholar]
- 77. Polonsky KS, Given BD, Hirsch L, et al. Quantitative study of insulin secretion and clearance in normal and obese subjects. J Clin Invest. 1988;81:435–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Caumo A, Florea I, Luzi L. Effect of a variable hepatic insulin clearance on the postprandial insulin profile: insights from a model simulation study. Acta Diabetol. 2007;44:23–29. [DOI] [PubMed] [Google Scholar]
- 79. Roberts AC, Butterfield GE, Cymerman A, Reeves JT, Wolfel EE, Brooks GA. Acclimatization to 4,300-m altitude decreases reliance on fat as a substrate. J Appl Physiol. 1996;81:1762–1771. [DOI] [PubMed] [Google Scholar]
- 80. Benso A, Broglio F, Aimaretti G, et al. Endocrine and metabolic responses to extreme altitude and physical exercise in climbers. Eur J Endocrinol. 2007;157:733–740. [DOI] [PubMed] [Google Scholar]
- 81. Cassinelli M. Glucose, lactic acid, and piruvic acid at sea level and high altitude [in Spanish]. An Fac Med. 1949;32:1–28. [Google Scholar]
- 82. Dinneen S, Gerich J, Rizza R. Carbohydrate metabolism in non-insulin-dependent diabetes mellitus. N Engl J Med. 1992;327:707–713. [DOI] [PubMed] [Google Scholar]
- 83. Gerich JE, Meyer C, Woerle HJ, Stumvoll M. Renal gluconeogenesis: its importance in human glucose homeostasis. Diabetes Care. 2001;24:382–391. [DOI] [PubMed] [Google Scholar]
- 84. Ader M, Bergman RN. Insulin sensitivity in the intact organism. Baillieres Clin Endocrinol Metab. 1987;1:879–910. [DOI] [PubMed] [Google Scholar]
- 85. Cherrington AD. Control of glucose production in vivo by insulin and glucagon. In: Pollock DM, ed. Comprehensive Physiology. Hoboken, NJ: John Wiley, Sons, Inc; 2010:759–785. [Google Scholar]
- 86. Roberts AC, Reeves JT, Butterfield GE, et al. Altitude and β-blockade augment glucose utilization during submaximal exercise. J Appl Physiol. 1996;80:605–615. [DOI] [PubMed] [Google Scholar]
- 87. Andres R, Swerdloff R, Pozefsky T, Coleman D. Manual feedback technique for the control of blood glucose concentration. In: Skeggs LT, ed. Automation in Analytical Chemistry. New York, NY: Mediad, Inc; 1966;486–491. [Google Scholar]
- 88. Lin HV, Accili D. Hormonal regulation of hepatic glucose production in health and disease. Cell Metab. 2011;14:9–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Dufour S, Lebon V, Shulman GI, Petersen KF. Regulation of net hepatic glycogenolysis and gluconeogenesis by epinephrine in humans. Am J Physiol Endocrinol Metab. 2009;297:E231–E235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Vranic M, Miles P, Rastogi K, et al. Effect of stress on glucoregulation in physiology and diabetes. Adv Exp Med Biol. 1991;291:161–183. [DOI] [PubMed] [Google Scholar]
- 91. Jiang G, Zhang BB. Glucagon and regulation of glucose metabolism. Am J Physiol Endocrinol Metab. 2003;284:E671–E678. [DOI] [PubMed] [Google Scholar]
- 92. Bergman RN. Non-esterified fatty acids and the liver: why is insulin secreted into the portal vein? Diabetologia. 2000;43:946–952. [DOI] [PubMed] [Google Scholar]
- 93. Ader M, Bergman RN. Peripheral effects of insulin dominate suppression of fasting hepatic glucose production. Am J Physiol. 1990;258:E1020–E1032. [DOI] [PubMed] [Google Scholar]
- 94. Reynafarje-Hurtado C. Endocrinology. In: Reynafarje-Hurtado C, ed. The adaptation to high altitudes. Peruvian study contribution [in Spanish] Lima, Peru: Concytec; 1990:113–116. [Google Scholar]
- 95. Picon-Reategui E. Effect of glucagon on carbohydrate metabolism in high-altitude residents. Arch Biol Andina. 1981;11:6–15. [Google Scholar]
- 96. Björkman O, Felig P, Wahren J. The contrasting responses of splanchnic and renal glucose output to gluconeogenic substrates and to hypoglucagonemia in 60-h-fasted humans. Diabetes. 1980;29:610–616. [DOI] [PubMed] [Google Scholar]
- 97. Stumvoll M, Meyer C, Kreider M, Perriello G, Gerich J. Effects of glucagon on renal and hepatic glutamine gluconeogenesis in normal postabsorptive humans. Metabolism. 1998;47:1227–1232. [DOI] [PubMed] [Google Scholar]
- 98. Ohneda A, Maruhama Y, Itabashi H, Horigome K, Yanbe A. Diagnostic value of intravenous glucagon test in insulinoma. Tohoku J Exp Med. 1975;116:205–211. [DOI] [PubMed] [Google Scholar]
- 99. Blume FD, Pace N. Effect of translocation to 3,800 m altitude on glycolysis in mice. J Appl Physiol. 1967;23:75–79. [DOI] [PubMed] [Google Scholar]
- 100. Ou LC. Hepatic and renal gluconeogenesis in rats acclimatized to high altitude. J Appl Physiol. 1974;36:303–307. [DOI] [PubMed] [Google Scholar]
- 101. Timiras PS, Hill R, Krum AA, Lis AW. Carbohydrate metabolism in fed and fasted rats exposed to an altitude of 12,470 feet. Am J Physiol. 1958;193:415–424. [DOI] [PubMed] [Google Scholar]
- 102. Wise S, Nielsen M, Rizza R. Effects of hepatic glycogen content on hepatic insulin action in humans: alteration in the relative contributions of glycogenolysis and gluconeogenesis to endogenous glucose production. J Clin Endocrinol Metab. 1997;82:1828–1833. [DOI] [PubMed] [Google Scholar]
- 103. Vissing J, Wallace JL, Galbo H. Effect of liver glycogen content on glucose production in running rats. J Appl Physiol. 1989;66:318–322. [DOI] [PubMed] [Google Scholar]
- 104. Andres R, Cader G, Zierler KL. The quantitatively minor role of carbohydrate in oxidative metabolism by skeletal muscle in intact man in the basal state; measurements of oxygen and glucose uptake and carbon dioxide and lactate production in the forearm. J Clin Invest. 1956;35:671–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Baron AD, Kolterman OG, Bell J, Mandarino LJ, Olefsky JM. Rates of noninsulin-mediated glucose uptake are elevated in type II diabetic subjects. J Clin Invest. 1985;76:1782–1788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Forbes WH. Blood sugar and glucose tolerance at high altitudes. Am J Physiol. 1936;116:309–316. [Google Scholar]
- 107. Chen MT, Lee WC, Chen SC, et al. Effect of a prolonged altitude expedition on glucose tolerance and abdominal fatness. Res Q Exerc Sport. 2010;81:472–477. [DOI] [PubMed] [Google Scholar]
- 108. Anderwald C, Gastaldelli A, Tura A, et al. Mechanism and effects of glucose absorption during an oral glucose tolerance test among females and males. J Clin Endocrinol Metab. 2011;96:515–524. [DOI] [PubMed] [Google Scholar]
- 109. Salinari S, Bertuzzi A, Mingrone G. Intestinal transit of a glucose bolus and incretin kinetics: a mathematical model with application to the oral glucose tolerance test. Am J Physiol Endocrinol Metab. 2011;300:E955–E965. [DOI] [PubMed] [Google Scholar]
- 110. Mari A, Bagger JI, Ferrannini E, Holst JJ, Knop FK, Vilsbøll T. Mechanisms of the incretin effect in subjects with normal glucose tolerance and patients with type 2 diabetes. PLoS One. 2013;8:e73154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Ahrén B, Holst JJ, Mari A. Characterization of GLP-1 effects on β-cell function after meal ingestion in humans. Diabetes Care. 2003;26:2860–2864. [DOI] [PubMed] [Google Scholar]
- 112. Oltmanns KM, Gehring H, Rudolf S, et al. Hypoxia causes glucose intolerance in humans. Am J Respir Crit Care Med. 2004;169:1231–1237. [DOI] [PubMed] [Google Scholar]
- 113. Louis M, Punjabi NM. Effects of acute intermittent hypoxia on glucose metabolism in awake healthy volunteers. J Appl Physiol. 2009;106:1538–1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Bergman RN, Ider YZ, Bowden CR, Cobelli C. Quantitative estimation of insulin sensitivity. Am J Physiol. 1979;236:E667–E677. [DOI] [PubMed] [Google Scholar]
- 115. Mackenzie R, Maxwell N, Castle P, Brickley G, Watt P. Acute hypoxia and exercise improve insulin sensitivity (S(I) (2*)) in individuals with type 2 diabetes. Diabetes Metab Res Rev. 2011;27:94–101. [DOI] [PubMed] [Google Scholar]
- 116. Lecoultre V, Peterson CM, Covington JD, et al. Ten nights of moderate hypoxia improves insulin sensitivity in obese humans. Diabetes Care. 2013;36:e197–e198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Duennwald T, Gatterer H, Groop PH, Burtscher M, Bernardi L. Effects of a single bout of interval hypoxia on cardiorespiratory control and blood glucose in patients with type 2 diabetes. Diabetes Care. 2013;36:2183–2189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Lee EJ, Alonso LC, Stefanovski D, et al. Time-dependent changes in glucose and insulin regulation during intermittent hypoxia and continuous hypoxia. Eur J Appl Physiol. 2013;113:467–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Zinker BA, Namdaran K, Wilson R, Lacy DB, Wasserman DH. Acute adaptation of carbohydrate metabolism to decreased arterial PO2. Am J Physiol. 1994;266:E921–E929. [DOI] [PubMed] [Google Scholar]
- 120. Derave W, Hespel P. Role of adenosine in regulating glucose uptake during contractions and hypoxia in rat skeletal muscle. J Physiol. 1999;515:255–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Wojtaszewski JF, Laustsen JL, Derave W, Richter EA. Hypoxia and contractions do not utilize the same signaling mechanism in stimulating skeletal muscle glucose transport. Biochim Biophys Acta. 1998;1380:396–404. [DOI] [PubMed] [Google Scholar]
- 122. Picon Reategui E. Intravenous glucose tolerance test at sea level and at high altitudes. J Clin Endocrinol Metab. 1963;23:1256–1261. [DOI] [PubMed] [Google Scholar]
- 123. Picon-Reategui E. Studies on the metabolism of carbohydrates at sea level and at high altitudes. Metabolism. 1962;11:1148–1154. [PubMed] [Google Scholar]
- 124. Saccà L, Cicala M, Trimarco B, Ungaro B, Vigorito C. Differential effects of insulin on splanchnic and peripheral glucose disposal after an intravenous glucose load in man. J Clin Invest. 1982;70:117–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Picon-Reategui E. Insulin, epinephrine, and glucagon on the metabolism of carbohydrates at high altitude. Fed Proc. 1966;25:1233–1239. [PubMed] [Google Scholar]
- 126. Grulet H, Durlach V, Hecart AC, Gross A, Leutenegger M. Study of the rate of early glucose disappearance following insulin injection: insulin sensitivity index. Diabetes Res Clin Pract. 1993;20:201–207. [DOI] [PubMed] [Google Scholar]
- 127. Bonora E, Moghetti P, Zancanaro C, et al. Estimates of in vivo insulin action in man: comparison of insulin tolerance tests with euglycemic and hyperglycemic glucose clamp studies. J Clin Endocrinol Metab. 1989;68:374–378. [DOI] [PubMed] [Google Scholar]
- 128. Ferrannini E, Mari A. How to measure insulin sensitivity. J Hypertens. 1998;16:895–906. [DOI] [PubMed] [Google Scholar]
- 129. Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and β-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–419. [DOI] [PubMed] [Google Scholar]
- 130. Wallace TM, Levy JC, Matthews DR. Use and abuse of HOMA modeling. Diabetes Care. 2004;27:1487–1495. [DOI] [PubMed] [Google Scholar]
- 131. Nuutila P. Applications of PET in diabetes research. Horm Metab Res. 1997;29:337–339. [DOI] [PubMed] [Google Scholar]
- 132. Howald H, Pette D, Simoneau JA, Uber A, Hoppeler H, Cerretelli P. Effect of chronic hypoxia on muscle enzyme activities. Int J Sports Med. 1990;11(suppl 1):S10–S14. [DOI] [PubMed] [Google Scholar]
- 133. Cerretelli P, Gelfi C. Energy metabolism in hypoxia: reinterpreting some features of muscle physiology on molecular grounds. Eur J Appl Physiol. 2011;111:421–432. [DOI] [PubMed] [Google Scholar]
- 134. Murray AJ. Metabolic adaptation of skeletal muscle to high altitude hypoxia: how new technologies could resolve the controversies. Genome Med. 2009;1:117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Green HJ, Sutton JR. The effects of altitude on skeletal muscle. In: Hornbein TF, Schoene RB, eds. High Altitude: An Exploration of Human Adaptation. New York, NY: Marcel Dekker, Inc; 2001:443–492. [Google Scholar]
- 136. Clanton TL, Hogan MC, Gladden LB. Regulation of cellular gas exchange, oxygen sensing, and metabolic control. Compr Physiol. 2013;3:1135–1190. [DOI] [PubMed] [Google Scholar]
- 137. Ge RL, Simonson TS, Cooksey RC, et al. Metabolic insight into mechanisms of high-altitude adaptation in Tibetans. Mol Genet Metab. 2012;106:244–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Hogan MC, Cox RH, Welch HG. Lactate accumulation during incremental exercise with varied inspired oxygen fractions. J Appl Physiol Respir Environ Exerc Physiol. 1983;55:1134–1140. [DOI] [PubMed] [Google Scholar]
- 139. Hughson RL, Green HJ, Sharratt MT. Gas exchange, blood lactate, and plasma catecholamines during incremental exercise in hypoxia and normoxia. J Appl Physiol (1985). 1995;79:1134–1141. [DOI] [PubMed] [Google Scholar]
- 140. Brooks GA, Butterfield GE, Wolfe RR, et al. Decreased reliance on lactate during exercise after acclimatization to 4,300 m. J Appl Physiol (1985). 1991;71:333–341. [DOI] [PubMed] [Google Scholar]
- 141. Randle PJ, Smith GH. Regulation of glucose uptake by muscle. 1. The effects of insulin, anaerobiosis and cell poisons on the uptake of glucose and release of potassium by isolated rat diaphragm. Biochem J. 1958;70:490–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Morgan HE, Randle PJ, Regen DM. Regulation of glucose uptake by muscle. 3. The effects of insulin, anoxia, salicylate and 2:4-dinitrophenol on membrane transport and intracellular phosphorylation of glucose in the isolated rat heart. Biochem J. 1959;73:573–579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Wheeler TJ. Translocation of glucose transporters in response to anoxia in heart. J Biol Chem. 1988;263:19447–19454. [PubMed] [Google Scholar]
- 144. Cartee GD, Douen AG, Ramlal T, Klip A, Holloszy JO. Stimulation of glucose transport in skeletal muscle by hypoxia. J Appl Physiol. 1991;70:1593–1600. [DOI] [PubMed] [Google Scholar]
- 145. Reynolds TH, 4th, Brozinick JT, Jr, Rogers MA, Cushman SW. Effects of exercise training on glucose transport and cell surface GLUT-4 in isolated rat epitrochlearis muscle. Am J Physiol. 1997;272:E320–E325. [DOI] [PubMed] [Google Scholar]
- 146. Zierath JR, Tsao TS, Stenbit AE, Ryder JW, Galuska D, Charron MJ. Restoration of hypoxia-stimulated glucose uptake in GLUT4-deficient muscles by muscle-specific GLUT4 transgenic complementation. J Biol Chem. 1998;273:20910–20915. [DOI] [PubMed] [Google Scholar]
- 147. Zhang SJ, Sandström M, Ahlsén M, et al. 2-Methoxyoestradiol inhibits glucose transport in rodent skeletal muscle. Exp Physiol. 2010;95:892–898. [DOI] [PubMed] [Google Scholar]
- 148. Mu J, Brozinick JT, Jr, Valladares O, Bucan M, Birnbaum MJ. A role for AMP-activated protein kinase in contraction- and hypoxia-regulated glucose transport in skeletal muscle. Mol Cell. 2001;7:1085–1094. [DOI] [PubMed] [Google Scholar]
- 149. Lanner JT, Katz A, Tavi P, et al. The role of Ca2+ influx for insulin-mediated glucose uptake in skeletal muscle. Diabetes. 2006;55:2077–2083. [DOI] [PubMed] [Google Scholar]
- 150. Azevedo JL, Jr, Carey JO, Pories WJ, Morris PG, Dohm GL. Hypoxia stimulates glucose transport in insulin-resistant human skeletal muscle. Diabetes. 1995;44:695–698. [DOI] [PubMed] [Google Scholar]
- 151. Ryder JW, Yang J, Galuska D, et al. Use of a novel impermeable biotinylated photolabeling reagent to assess insulin- and hypoxia-stimulated cell surface GLUT4 content in skeletal muscle from type 2 diabetic patients. Diabetes. 2000;49:647–654. [DOI] [PubMed] [Google Scholar]
- 152. Idström JP, Rennie MJ, Scherstén T, Bylund-Fellenius AC. Membrane transport in relation to net uptake of glucose in the perfused rat hindlimb. Stimulatory effect of insulin, hypoxia and contractile activity. Biochem J. 1986;233:131–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Gamboa JL, Garcia-Cazarin ML, Andrade FH. Chronic hypoxia increases insulin-stimulated glucose uptake in mouse soleus muscle. Am J Physiol Regul Integr Comp Physiol. 2011;300:R85–R91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Heinonen I, Kemppainen J, Kaskinoro K, et al. Effects of adenosine, exercise, and moderate acute hypoxia on energy substrate utilization of human skeletal muscle. Am J Physiol Regul Integr Comp Physiol. 2012;302:R385–R390. [DOI] [PubMed] [Google Scholar]
- 155. Katz A, Sahlin K. Effect of hypoxia on glucose metabolism in human skeletal muscle during exercise. Acta Physiol Scand. 1989;136:377–382. [DOI] [PubMed] [Google Scholar]
- 156. Zhang JZ, Behrooz A, Ismail-Beigi F. Regulation of glucose transport by hypoxia. Am J Kidney Dis. 1999;34:189–202. [DOI] [PubMed] [Google Scholar]
- 157. Fujii N, Jessen N, Goodyear LJ. AMP-activated protein kinase and the regulation of glucose transport. Am J Physiol Endocrinol Metab. 2006;291:E867–E877. [DOI] [PubMed] [Google Scholar]
- 158. Zierath JR, Krook A, Wallberg-Henriksson H. Insulin action and insulin resistance in human skeletal muscle. Diabetologia. 2000;43:821–835. [DOI] [PubMed] [Google Scholar]
- 159. Brozinick JT, Jr, Reynolds TH, Dean D, Cartee G, Cushman SW. 1-[N, O-bis-(5-isoquinolinesulphonyl)-N-methyl-L-tyrosyl]-4- phenylpiperazine (KN-62), an inhibitor of calcium-dependent camodulin protein kinase II, inhibits both insulin- and hypoxia-stimulated glucose transport in skeletal muscle. Biochem J. 1999;339:533–540. [PMC free article] [PubMed] [Google Scholar]
- 160. Fazakerley DJ, Lawrence SP, Lizunov VA, Cushman SW, Holman GD. A common trafficking route for GLUT4 in cardiomyocytes in response to insulin, contraction and energy-status signalling. J Cell Sci. 2009;122:727–734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Xia Y, Warshaw JB, Haddad GG. Effect of chronic hypoxia on glucose transporters in heart and skeletal muscle of immature and adult rats. Am J Physiol. 1997;273:R1734–R1741. [DOI] [PubMed] [Google Scholar]
- 162. Han XX, Bonen A. Epinephrine translocates GLUT-4 but inhibits insulin-stimulated glucose transport in rat muscle. Am J Physiol. 1998;274:E700–E707. [DOI] [PubMed] [Google Scholar]
- 163. Nielsen JN, Jørgensen SB, Frøsig C, et al. A possible role for AMP-activated protein kinase in exercise-induced glucose utilization: insights from humans and transgenic animals. Biochem Soc Trans. 2003;31:186–190. [DOI] [PubMed] [Google Scholar]
- 164. Dinmore AJ, Edwards JS, Menzies IS, Travis SP. Intestinal carbohydrate absorption and permeability at high altitude (5,730 m). J Appl Physiol. 1994;76:1903–1907. [DOI] [PubMed] [Google Scholar]
- 165. Boyer SJ, Blume FD. Weight loss and changes in body composition at high altitude. J Appl Physiol. 1984;57:1580–1585. [DOI] [PubMed] [Google Scholar]
- 166. Kayser B, Acheson K, Decombaz J, Fern E, Cerretelli P. Protein absorption and energy digestibility at high altitude. J Appl Physiol. 1992;73:2425–2431. [DOI] [PubMed] [Google Scholar]
- 167. Chesner IM, Small NA, Dykes PW. Intestinal absorption at high altitude. Postgrad Med J. 1987;63:173–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. Sridharan K, Malhotra MS, Upadhayay TN, Grover SK, Dua GL. Changes in gastro-intestinal function in humans at an altitude of 3,500 m. Eur J Appl Physiol. 1982;50:145–154. [Google Scholar]
- 169. Shukla V, Singh SN, Vats P, Singh VK, Singh SB, Banerjee PK. Ghrelin and leptin levels of sojourners and acclimatized lowlanders at high altitude. Nutr Neurosci. 2005;8:161–165. [DOI] [PubMed] [Google Scholar]
- 170. Wasse LK, Sunderland C, King JA, Batterham RL, Stensel DJ. Influence of rest and exercise at a simulated altitude of 4,000 m on appetite, energy intake, and plasma concentrations of acylated ghrelin and peptide YY. J Appl Physiol. 2012;112:552–559. [DOI] [PubMed] [Google Scholar]
- 171. Riepl RL, Fischer R, Hautmann H, et al. Influence of acute exposure to high altitude on basal and postprandial plasma levels of gastroenteropancreatic peptides. PLoS One. 2012;7:e44445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172. Aeberli I, Erb A, Spliethoff K, et al. Disturbed eating at high altitude: influence of food preferences, acute mountain sickness and satiation hormones. Eur J Nutr. 2013;52:625–635. [DOI] [PubMed] [Google Scholar]
- 173. Ahrén B. The future of incretin-based therapy: novel avenues–novel targets. Diabetes Obes Metab. 2011;13(suppl 1):158–166. [DOI] [PubMed] [Google Scholar]
- 174. Cummings DE, Overduin J. Gastrointestinal regulation of food intake. J Clin Invest. 2007;117:13–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175. Snyder EM, Carr RD, Deacon CF, Johnson BD. Overnight hypoxic exposure and glucagon-like peptide-1 and leptin levels in humans. Appl Physiol Nutr Metab. 2008;33:929–935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176. Debevec T, Simpson EJ, Macdonald IA, Eiken O, Mekjavic IB. Exercise training during normobaric hypoxic confinement does not alter hormonal appetite regulation. PLoS One. 2014;9:e98874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177. Staehr P, Hother-Nielsen O, Landau BR, Chandramouli V, Holst JJ, Beck-Nielsen H. Effects of free fatty acids per se on glucose production, gluconeogenesis, and glycogenolysis. Diabetes. 2003;52:260–267. [DOI] [PubMed] [Google Scholar]
- 178. Raynaud J, Drouet L, Martineaud JP, Bordachar J, Coudert J, Durand J. Time course of plasma growth hormone during exercise in humans at altitude. J Appl Physiol. 1981;50:229–233. [DOI] [PubMed] [Google Scholar]
- 179. Bergouignan A, Gozansky WS, Barry DW, et al. Increasing dietary fat elicits similar changes in fat oxidation and markers of muscle oxidative capacity in lean and obese humans. PLoS One. 2012;7:e30164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180. Hojlund K, Wildner-Christensen M, Eshøj O, et al. Reference intervals for glucose, β-cell polypeptides, and counterregulatory factors during prolonged fasting. Am J Physiol Endocrinol Metab. 2001;280:E50–E58. [DOI] [PubMed] [Google Scholar]
- 181. Zambon A, Hashimoto SI, Brunzell JD. Analysis of techniques to obtain plasma for measurement of levels of free fatty acids. J Lipid Res. 1993;34:1021–1028. [PubMed] [Google Scholar]
- 182. Boden G. Obesity, insulin resistance and free fatty acids. Curr Opin Endocrinol Diabetes Obes. 2011;18:139–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183. Ahima RS. Adipose tissue as an endocrine organ. Obesity. 2006;14:242S–249S. [DOI] [PubMed] [Google Scholar]
- 184. Turer AT, Scherer PE. Adiponectin: mechanistic insights and clinical implications. Diabetologia. 2012;55:2319–2326. [DOI] [PubMed] [Google Scholar]
- 185. Smith JD, Cianflone K, Martin J, Poirier P, Broderick TL, Noël M. Plasma adipokine and hormone changes in mountaineers on ascent to 5300 meters. Wilderness Environ Med. 2011;22:107–114. [DOI] [PubMed] [Google Scholar]
- 186. Barnholt KE, Hoffman AR, Rock PB, et al. Endocrine responses to acute and chronic high-altitude exposure (4,300 meters): modulating effects of caloric restriction. Am J Physiol Endocrinol Metab. 2006;290:E1078–E1088. [DOI] [PubMed] [Google Scholar]
- 187. Yi X, Liang Y, Huerta-Sanchez E, et al. Sequencing of 50 human exomes reveals adaptation to high altitude. Science. 2010;329:75–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188. Scheinfeldt LB, Soi S, Thompson S, et al. Genetic adaptation to high altitude in the Ethiopian highlands. Genome Biol. 2012;13:R1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189. Semenza GL, Prabhakar NR. The role of hypoxia-inducible factors in oxygen sensing by the carotid body. Adv Exp Med Biol. 2012;758:1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190. Semenza GL. Oxygen sensing, homeostasis, and disease. N Engl J Med. 2011;365:537–547. [DOI] [PubMed] [Google Scholar]
- 191. Greer SN, Metcalf JL, Wang Y, Ohh M. The updated biology of hypoxia-inducible factor. EMBO J. 2012;31:2448–2460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192. Loboda A, Jozkowicz A, Dulak J. HIF-1 and HIF-2 transcription factors–similar but not identical. Mol Cells. 2010;29:435–442. [DOI] [PubMed] [Google Scholar]
- 193. Gelfi C, De Palma S, Ripamonti M, et al. New aspects of altitude adaptation in Tibetans: a proteomic approach. FASEB J. 2004;18:612–614. [DOI] [PubMed] [Google Scholar]
- 194. Hochachka PW. Mechanism and evolution of hypoxia-tolerance in humans. J Exp Biol. 1998;201:1243–1254. [DOI] [PubMed] [Google Scholar]
- 195. Hochachka PW, Beatty CL, Burelle Y, Trump ME, McKenzie DC, Matheson GO. The lactate paradox in human high-altitude physiological performance. News Physiol Sci. 2002;17:122–126. [DOI] [PubMed] [Google Scholar]
- 196. Viganò A, Ripamonti M, De Palma S, et al. Proteins modulation in human skeletal muscle in the early phase of adaptation to hypobaric hypoxia. Proteomics. 2008;8:4668–4679. [DOI] [PubMed] [Google Scholar]
- 197. Levett DZ, Radford EJ, Menassa DA, et al. Acclimatization of skeletal muscle mitochondria to high-altitude hypoxia during an ascent of Everest. FASEB J. 2012;26:1431–1441. [DOI] [PubMed] [Google Scholar]
- 198. Palmer BF, Clegg DJ. Ascent to altitude as a weight loss method: the good and bad of hypoxia inducible factor activation. Obesity (Silver Spring). 2014;22:311–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199. van Patot MC, Gassmann M. Hypoxia: adapting to high altitude by mutating EPAS-1, the gene encoding HIF-2α. High Alt Med Biol. 2011;12:157–167. [DOI] [PubMed] [Google Scholar]
- 200. Stauss HM. Baroreceptor reflex function. Am J Physiol Regul Integr Comp Physiol. 2002;283:R284–R286. [DOI] [PubMed] [Google Scholar]
- 201. Zoll J, Ponsot E, Dufour S, et al. Exercise training in normobaric hypoxia in endurance runners. III. Muscular adjustments of selected gene transcripts. J Appl Physiol. 2006;100:1258–1266. [DOI] [PubMed] [Google Scholar]
- 202. Lima GA, Anhê GF, Giannocco G, Nunes MT, Correa-Giannella ML, Machado UF. Contractile activity per se induces transcriptional activation of SLC2A4 gene in soleus muscle: involvement of MEF2D, HIF-1a, and TRα transcriptional factors. Am J Physiol Endocrinol Metab. 2009;296:E132–E138. [DOI] [PubMed] [Google Scholar]
- 203. Sakagami H, Makino Y, Mizumoto K, et al. Loss of HIF-1α impairs GLUT4 translocation and glucose uptake by the skeletal muscle cells. Am J Physiol Endocrinol Metab. 2014;306:E1065–E1076. [DOI] [PubMed] [Google Scholar]
- 204. McClain DA, Abuelgasim KA, Nouraie M, et al. Decreased serum glucose and glycosylated hemoglobin levels in patients with Chuvash polycythemia: a role for HIF in glucose metabolism. J Mol Med (Berl). 2013;91:59–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205. Solís J, Guerra-García R. Prevalence of diabetes mellitus in hospitalized patients from highlands [in Spanish]. Arch Biol Andina. 1979;9:21–30. [Google Scholar]
- 206. Singh I, Chohan IS, Lal M, et al. Effects of high altitude stay on the incidence of common diseases in man. Int J Biometeorol. 1977;21:93–122. [DOI] [PubMed] [Google Scholar]
- 207. Seclén S, Leey J, Villena A, et al. Prevalence of obesity, diabetes mellitus, arterial hypertension, and hypercholesterolemia as risk factors for coronary and cerebrovascular diseases in adult populations from the coast, mountain and the forest in Peru [in Spanish]. Acta Méd Per. 1999;17:8–12. [Google Scholar]
- 208. Woolcott OO, Castillo OA, Gutierrez C, Elashoff RM, Stefanovski D, Bergman RN. Inverse association between diabetes and altitude: a cross-sectional study in the adult population of the United States. Obesity (Silver Spring). 2014;22:2080–2090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209. Field AE, Coakley EH, Must A, et al. Impact of overweight on the risk of developing common chronic diseases during a 10-year period. Arch Intern Med. 2001;161:1581–1586. [DOI] [PubMed] [Google Scholar]
- 210. Bonora E, Kiechl S, Willeit J, et al. Population-based incidence rates and risk factors for type 2 diabetes in white individuals: the Bruneck study. Diabetes. 2004;53:1782–1789. [DOI] [PubMed] [Google Scholar]
- 211. Woolcott OO, Castillo OA, Bergman RN. Overweight and obesity in dwellers from highlands [in Spanish]. Rev Peru Epidemiol. 2012;16:5. [Google Scholar]
- 212. Segura-Vega L, Agusti R, Parodi-Ramirez J. Risk factors of cardiovascular diseases in Peru (TORNASOL study)[in Spanish]. Rev Peru Cardiol. 2006;32:82–128. [Google Scholar]
- 213. Sherpa LY, Deji, Stigum H, Chongsuvivatwong V, Thelle DS, Bjertness E. Obesity in Tibetans aged 30–70 living at different altitudes under the north and south faces of Mt. Everest. Int J Environ Res Public Health. 2010;7:1670–1680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214. Voss JD, Allison DB, Webber BJ, Otto JL, Clark LL. Lower obesity rate during residence at high altitude among a military population with frequent migration: a quasi experimental model for investigating spatial causation. PLoS One. 2014;9:e93493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215. Medina J, Morey OL, Zea H, et al. Prevalence of overweight and obesity in an adult population from Greater Arequipa: outcomes from the PREVENCION study [in Spanish]. Rev Peru Cardiol. 2006;32:194–209. [Google Scholar]
- 216. Málaga G, Zevallos-Palacios C, Lazo Mde L, Huayanay C. High frequency of dyslipidemia and impaired fasting glycemia in a high altitude Peruvian population [in Spanish]. Rev Peru Med Exp Salud Publica. 2010;27:557–561. [DOI] [PubMed] [Google Scholar]
- 217. Mohanna S, Baracco R, Seclén S. Lipid profile, waist circumference, and body mass index in a high altitude population. High Alt Med Biol. 2006;7:245–255. [DOI] [PubMed] [Google Scholar]
- 218. Shah SM, Nanan D, Rahbar MH, Rahim M, Nowshad G. Assessing obesity and overweight in a high mountain Pakistani population. Trop Med Int Health. 2004;9:526–532. [DOI] [PubMed] [Google Scholar]
- 219. Schargrodsky H, Hernández-Hernández R, Champagne BM, et al. CARMELA: assessment of cardiovascular risk in seven Latin American cities. Am J Med. 2008;121:58–65. [DOI] [PubMed] [Google Scholar]
- 220. Negi PC, Bhardwaj R, Kandoria A, et al. Epidemiological study of hypertension in natives of Spiti valley in Himalayas and impact of hypobaric hypoxemia; a cross-sectional study. J Assoc Physicians India. 2012;60:21–25. [PubMed] [Google Scholar]
- 221. Chen W, Liu Q, Wang H, et al. Prevalence and risk factors of chronic kidney disease: a population study in the Tibetan population. Nephrol Dial Transplant. 2011;26:1592–1599. [DOI] [PubMed] [Google Scholar]
- 222. Voss JD, Masuoka P, Webber BJ, Scher AI, Atkinson RL. Association of elevation, urbanization and ambient temperature with obesity prevalence in the United States. Int J Obes (Lond). 2013;37:1407–1412. [DOI] [PubMed] [Google Scholar]
- 223. Dipierri JE, Bejarano IF, Alfaro EL, et al. Overweight and obesity prevalence in highlands populations of Jujuy province (Argentina). In: Nieto JL, Obon JA, Baena S, eds. Genes, Environment, and Disease in Human Populations [in Spanish] Zaragoza, Spain: Prensas Universitarias de Zaragoza; 2008:521–530. [Google Scholar]
- 224. Hirschler V, Maccallini G, Aranda C, Molinari C. Dyslipidemia without obesity in indigenous Argentinean children living at high altitude. J Pediatr. 2012;161:646–651.e1. [DOI] [PubMed] [Google Scholar]
- 225. Khalid Mel-H. Is high-altitude environment a risk factor for childhood overweight and obesity in Saudi Arabia? Wilderness Environ Med. 2008;19:157–163. [DOI] [PubMed] [Google Scholar]
- 226. Kayser B, Verges S. Hypoxia, energy balance and obesity: from pathophysiological mechanisms to new treatment strategies. Obes Rev. 2013;14:579–592. [DOI] [PubMed] [Google Scholar]
- 227. Srivastava KK, Kumar R. Human nutrition in cold and high terrestrial altitudes. Int J Biometeorol. 1992;36:10–13. [DOI] [PubMed] [Google Scholar]
- 228. Gill MB, Pugh LG. Basal metabolism and respiration in men living at 5,800 m (19,000 ft). J Appl Physiol. 1964;19:949–954. [DOI] [PubMed] [Google Scholar]
- 229. Stock MJ, Norgan NG, Ferro-Luzzi A, Evans E. Effect of altitude on dietary-induced thermogenesis at rest and during light exercise in man. J Appl Physiol. 1978;45:345–349. [DOI] [PubMed] [Google Scholar]
- 230. Hannon JP, Sudman DM. Basal metabolic and cardiovascular function of women during altitude acclimatization. J Appl Physiol. 1973;34:471–477. [DOI] [PubMed] [Google Scholar]
- 231. Mawson JT, Braun B, Rock PB, Moore LG, Mazzeo R, Butterfield GE. Women at altitude: energy requirement at 4,300 m. J Appl Physiol. 2000;88:272–281. [DOI] [PubMed] [Google Scholar]
- 232. Lippl FJ, Neubauer S, Schipfer S, et al. Hypobaric hypoxia causes body weight reduction in obese subjects. Obesity (Silver Spring). 2010;18:675–681. [DOI] [PubMed] [Google Scholar]
- 233. Leonard WR, Katzmarzyk PT, Stephen MA, Ross AG. Comparison of the heart rate-monitoring and factorial methods: assessment of energy expenditure in highland and coastal Ecuadoreans. Am J Clin Nutr. 1995;61:1146–1152. [DOI] [PubMed] [Google Scholar]
- 234. Kashiwazaki H, Dejima Y, Orias-Rivera J, Coward WA. Energy expenditure determined by the doubly labeled water method in Bolivian Aymara living in a high altitude agropastoral community. Am J Clin Nutr. 1995;62:901–910. [DOI] [PubMed] [Google Scholar]
- 235. Levine JA, Lanningham-Foster LM, McCrady SK, et al. Interindividual variation in posture allocation: possible role in human obesity. Science. 2005;307:584–586. [DOI] [PubMed] [Google Scholar]
- 236. van Marken Lichtenbelt WD, Schrauwen P. Implications of nonshivering thermogenesis for energy balance regulation in humans. Am J Physiol Regul Integr Comp Physiol. 2011;301:R285–R296. [DOI] [PubMed] [Google Scholar]
- 237. Basu M, Pal K, Malhotra AS, Prasad R, Sawhney RC. Free and total thyroid hormones in humans at extreme altitude. Int J Biometeorol. 1995;39:17–21. [DOI] [PubMed] [Google Scholar]
- 238. Ramirez G, Herrera R, Pineda D, Bittle PA, Rabb HA, Bercu BB. The effects of high altitude on hypothalamic-pituitary secretory dynamics in men. Clin Endocrinol (Oxf). 1995;43:11–18. [DOI] [PubMed] [Google Scholar]
- 239. Antezana AM, Richalet JP, Noriega I, Galarza M, Antezana G. Hormonal changes in normal and polycythemic high-altitude natives. J Appl Physiol. 1995;79:795–800. [DOI] [PubMed] [Google Scholar]
- 240. Dhar P, Sharma VK, Hota KB, et al. Autonomic cardiovascular responses in acclimatized lowlanders on prolonged stay at high altitude: a longitudinal follow up study. PLoS One. 2014;9:e84274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241. Tschöp M, Strasburger CJ, Hartmann G, Biollaz J, Bärtsch P. Raised leptin concentrations at high altitude associated with loss of appetite. Lancet. 1998;352:1119–1120. [DOI] [PubMed] [Google Scholar]
- 242. Vats P, Singh VK, Singh SN, Singh SB. High altitude induced anorexia: effect of changes in leptin and oxidative stress levels. Nutr Neurosci. 2007;10:243–249. [DOI] [PubMed] [Google Scholar]
- 243. Woolcott OO, Castillo OA, Torres J, Damas L, Florentini E. Serum leptin levels in dwellers from high altitude lands. High Alt Med Biol. 2002;3:245–246. [DOI] [PubMed] [Google Scholar]
- 244. Santos JL, Pérez-Bravo F, Albala C, Calvillán M, Carrasco E. Plasma leptin and insulin levels in Aymara natives from Chile. Ann Hum Biol. 2000;27:271–279. [DOI] [PubMed] [Google Scholar]
- 245. Subramanian SV, Finlay JE, Neuman M. Global trends in body-mass index. Lancet. 2011;377:1915–1916; author reply 1917–1918. [DOI] [PubMed] [Google Scholar]
- 246. Picon-Reategui E. The food and nutrition of high-altitude populations. In: Baker PT, ed. The Biology of High-Altitude Peoples. Cambridge, UK: Cambridge University Press; 1978:219–249. [Google Scholar]
- 247. Faeh D, Gutzwiller F, Bopp M. Response to letters regarding article, “Lower Mortality from Coronary Heart Disease and Stroke at Higher Altitudes in Switzerland”. Circulation. 2010;121:e378. [DOI] [PubMed] [Google Scholar]
- 248. Posadas-Romero C, Yamamoto-Kimura L, Lerman-Garber I, et al. The prevalence of NIDDM and associated coronary risk factors in Mexico City. Diabetes Care. 1994;17:1441–1448. [DOI] [PubMed] [Google Scholar]
- 249. Al-Habori M, Al-Mamari M, Al-Meeri A. Type II diabetes mellitus and impaired glucose tolerance in Yemen: prevalence, associated metabolic changes and risk factors. Diabetes Res Clin Pract. 2004;65:275–281. [DOI] [PubMed] [Google Scholar]
- 250. Faeh D, Gutzwiller F, Bopp M. Lower mortality from coronary heart disease and stroke at higher altitudes in Switzerland. Circulation. 2009;120:495–501. [DOI] [PubMed] [Google Scholar]
- 251. Burke JP, Williams K, Haffner SM, Villalpando CG, Stern MP. Elevated incidence of type 2 diabetes in San Antonio, Texas, compared with that of Mexico City, Mexico. Diabetes Care. 2001;24:1573–1578. [DOI] [PubMed] [Google Scholar]
- 252. Tai K, Need AG, Horowitz M, Chapman IM. Vitamin D, glucose, insulin, and insulin sensitivity. Nutrition. 2008;24:279–285. [DOI] [PubMed] [Google Scholar]
- 253. Engelsen O. The relationship between ultraviolet radiation exposure and vitamin D status. Nutrients. 2010;2:482–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254. Simha V, Mahmood M, Ansari M, Spellman CW, Shah P. Effect of vitamin D replacement on insulin sensitivity in subjects with vitamin D deficiency. J Investig Med. 2012;60:1214–1218. [DOI] [PubMed] [Google Scholar]
- 255. Seida JC, Mitri J, Colmers IN, et al. Clinical review: effect of vitamin D3 supplementation on improving glucose homeostasis and preventing diabetes: a systematic review and meta-analysis. J Clin Endocrinol Metab. 2014;99:3551–3560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256. Pearson JF, Bachireddy C, Shyamprasad S, Goldfine AB, Brownstein JS. Association between fine particulate matter and diabetes prevalence in the U.S. Diabetes Care. 2010;33:2196–2201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257. Lee DH, Lee IK, Song K, et al. A strong dose-response relation between serum concentrations of persistent organic pollutants and diabetes: results from the National Health and Examination Survey 1999–2002. Diabetes Care. 2006;29:1638–1644. [DOI] [PubMed] [Google Scholar]
- 258. Bravo Alvarez H, Sosa Echeverria R, Sanchez Alvarez P, Krupa S. Air quality standards for particulate matter (PM) at high altitude cities. Environ Pollut. 2013;173:255–256. [DOI] [PubMed] [Google Scholar]
- 259. DeFronzo RA. Pathogenesis of type 2 diabetes mellitus. Med Clin North Am. 2004;88:787–835, ix. [DOI] [PubMed] [Google Scholar]
- 260. Marquez JL, Rubinstein S, Fattor JA, Shah O, Hoffman AR, Friedlander AL. Cyclic hypobaric hypoxia improves markers of glucose metabolism in middle-aged men. High Alt Med Biol. 2013;14:263–272. [DOI] [PubMed] [Google Scholar]
- 261. Aurora RN, Punjabi NM. Obstructive sleep apnoea and type 2 diabetes mellitus: a bidirectional association. Lancet Respir Med. 2013;1:329–338. [DOI] [PubMed] [Google Scholar]
- 262. Tasali E, Mokhlesi B, Van Cauter E. Obstructive sleep apnea and type 2 diabetes: interacting epidemics. Chest. 2008;133:496–506. [DOI] [PubMed] [Google Scholar]
- 263. Niijima M, Kimura H, Edo H, et al. Manifestation of pulmonary hypertension during REM sleep in obstructive sleep apnea syndrome. Am J Respir Crit Care Med. 1999;159:1766–1772. [DOI] [PubMed] [Google Scholar]
- 264. Braun B, Butterfield GE, Dominick SB, et al. Women at altitude: changes in carbohydrate metabolism at 4,300-m elevation and across the menstrual cycle. J Appl Physiol. 1998;85:1966–1973. [DOI] [PubMed] [Google Scholar]
- 265. Kelly KR, Williamson DL, Fealy CE, et al. Acute altitude-induced hypoxia suppresses plasma glucose and leptin in healthy humans. Metabolism. 2010;59:200–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266. Coste O, Van Beers P, Touitou Y. Impact of hypobaric hypoxia in pressurized cabins of simulated long-distance flights on the 24 h patterns of biological variables, fatigue, and clinical status. Chronobiol Int. 2007;24:1139–1157. [DOI] [PubMed] [Google Scholar]
- 267. Meyer C, Nadkarni V, Stumvoll M, Gerich J. Human kidney free fatty acid and glucose uptake: evidence for a renal glucose-fatty acid cycle. Am J Physiol. 1997;273:E650–E654. [DOI] [PubMed] [Google Scholar]
- 268. Khan AJ, Hussain H, Omer SB, et al. High incidence of childhood pneumonia at high altitudes in Pakistan: a longitudinal cohort study. Bull World Health Organ. 2009;87:193–199. [DOI] [PMC free article] [PubMed] [Google Scholar]