Abstract
Context:
Higher dietary net acid loads have been associated with increased bone resorption, reduced bone mineral density (BMD), and increased fracture risk.
Objective:
The objective was to compare bicarbonate (HCO3) measured in arterialized venous blood samples to skeletal outcomes.
Design:
Arterialized venous samples collected from participants in the Health, Aging and Body Composition (Health ABC) Study were compared to BMD and rate of bone loss.
Setting:
The setting was a community-based observational cohort.
Participants:
A total of 2287 men and women age 74 ± 3 years participated.
Intervention:
Arterialized venous blood was obtained at the year 3 study visit and analyzed for pH and pCO2. HCO3 was determined using the Henderson-Hasselbalch equation.
Main Outcome Measure:
BMD was measured at the hip by dual-energy x-ray absorptiometry at the year 1 (baseline) and year 3 study visits.
Results:
Plasma HCO3 was positively associated with BMD at both year 1 (P = .001) and year 3 (P = .001) in models adjusted for age, race, sex, clinic site, smoking, weight, and estimated glomerular filtration rate. Plasma HCO3 was inversely associated with rate of bone loss at the total hip over the 2.1 ± 0.3 (mean ± SD) years between the two bone density measurements (P < .001). Across quartiles of plasma HCO3, the rate of change in BMD over the 2.1 years ranged from a loss of 0.72%/y in the lowest quartile to a gain of 0.15%/y in the highest quartile of HCO3.
Conclusions:
Arterialized plasma HCO3 was associated positively with cross-sectional BMD and inversely with the rate of bone loss, implying that systemic acid-base status is an important determinant of skeletal health during aging. Ongoing bone loss was linearly related to arterialized HCO3, even after adjustment for age and renal function. Further research in this area may have major public health implications because reducing dietary net acid load is possible through dietary intervention or through supplementation with alkaline potassium compounds.
The skeletal effects of chronically ingesting a diet imbalanced in acid and base precursors remain in contention, although higher estimated dietary net acid loads have been associated with increased bone resorption, reduced bone mineral density (BMD), and increased fracture risk (1–4). The metabolism of dietary protein delivers noncarbonic acid to the systemic circulation from the oxidation of organic sulfur in sulfur-containing proteins, creating sulfuric acid (5–7). Dietary base in the form of bicarbonate results from the metabolism of organic potassium salts (eg, citrate, malate, gluconate), found predominantly in fruit and nongrain vegetable foods (8). Diets rich in protein and cereal grains but limited in vegetable/fruit intake, common among inhabitants of industrialized nations, tend to be rich in acid precursors and low in base precursors. Metabolism of such diets creates sufficient excess noncarbonic acid to induce a low-level chronic metabolic acidosis that correlates in severity with the dietary net acid load (5, 7, 9–11).
Renal acid excretory capacity becomes progressively impaired as renal function declines with aging (12–15). Consequently, individuals who habitually eat diets rich in protein/cereal and limited in vegetable/fruit develop a progressive increase in blood acidity and decrease in plasma bicarbonate with age (14, 16), even without increases in the dietary net acid load. Even with normal renal function, the kidneys do not excrete the entire daily acid load generated by a net acid-producing diet (10, 11). Nevertheless, arterial pH is maintained within a very narrow range inversely related to the magnitude of the daily dietary net acid load (11, 17).
A considerable body of data support the hypothesis that the skeleton functions as a continual base reservoir, providing for the titration of metabolic acid over a lifetime of dietary net acid loading (18), due predominantly to mobilization of alkali from bone (11, 19–21) that is used to titrate the persistent dietary acid load. Bone releases base, in the form of alkaline salts of calcium (phosphates, carbonates, hydroxides), in response to the extracellular acidity and hypobicarbonatemia that are induced by the net acid-producing diet and amplified by age-related decline in renal acid-base regulatory ability (17, 22–26). As base release persists, the liberated minerals accompanying the base are excreted in the urine, and bone mass progressively declines (27–29). In addition, extracellular acidification and hypobicarbonatemia in vitro increase the activity of osteoclasts (30–34) and suppress the activity of osteoblasts (32), suggesting that acidosis-induced loss of bone mass may be, in part, a cell-mediated process.
Whereas accumulating evidence suggests that the habitual ingestion of net acid-producing diets has adverse consequences on the skeleton, the degree of the attendant extracellular acidification has not been directly linked to the degree of bone loss. To investigate the relationship between systemic acid-base status and the skeleton, we used pCO2-adjusted plasma bicarbonate measured in arterialized venous blood samples as an index of dietary net acid-induced systemic acid-base status collected from men and women participating in the Heath, Aging and Body Composition (Health ABC) study and examined the relationship with BMD and interval rate of bone loss.
Subjects and Methods
Health ABC is a prospective observational cohort study examining the relationship between changes in body composition and disability, morbidity, and mortality. The cohort is comprised of 3075 men and women who were between ages 70 and 79 years at recruitment in 1997–1998 at the University of Pittsburgh and the University of Tennessee at Memphis. Participants were recruited from all age-eligible Blacks residing in Pittsburgh, Pennsylvania, and Memphis, Tennessee, and a random sample of White Medicare beneficiaries. The cohort is approximately 40% Black and evenly divided between male and female participants. Potential participants were excluded if they reported the inability to independently perform activities of daily living, walk up 10 steps without resting, or walk a quarter of a mile. The appropriate institutional review boards approved the study procedures, and all participants provided informed consent.
Arterialized venous blood gas measurements
Arterialized venous blood was obtained at the year 3 clinic visit by inserting a butterfly catheter into a superficial vein on the dorsum of the participant's hand. The butterfly tubing was flushed with 3 mL heparin flush solution, and the forearm and hand were wrapped snugly in a water circulating heating pad at 42°C with the end of the butterfly tubing extending beyond the heating pad. After a minimum of 15 minutes, 3–5 mL of blood/heparin flush mixture was withdrawn from the butterfly; then 2–3 mL of blood were withdrawn in a preheparinized blood gas syringe, any air was expelled, the syringe was capped, and the sample was placed on ice immediately. The whole-blood samples were promptly analyzed in triplicate for pH, pCO2, and pO2 on a Radiometer ABL5 blood gas analyzer (Radiometer America, Inc). Standards were analyzed daily as well as immediately before and after each participant sample.
A two-point calibration using manufacturer standards was performed each morning and every 4 hours during machine operation. A quality control (QC) standard ampule was analyzed in duplicate before each participant's blood gas sample was analyzed, and a second, different QC ampule was analyzed in duplicate after each participant's sample was analyzed. On the rare occasions when the QC ampule values were not in the expected range, the machine underwent trouble shooting until the QC procedures were passed.
The pH and pCO2 in arterialized venous samples correlate to arterial samples with r = 0.97 to r = 0.99 (35). Bicarbonate was determined using the Henderson-Hasselbalch equation by the blood gas analyzer from directly measured pH and pCO2. To isolate the metabolic component of acid-base status, the resulting bicarbonate values were adjusted to the mean pCO2 for the cohort according to the methods of Kurtz et al (11). After adjustment for pCO2, the triplicate bicarbonate values for each individual were averaged to provide a single bicarbonate value for each participant.
Bone mineral density
BMD was measured at the proximal femur by dual-energy x-ray absorptiometry (DXA) on a Hologic QDR 4500A densitometer using software version 9.03 (Hologic, Inc). DXA quality assurance included daily phantom scanning and circulation between the two sites of a cross-calibration phantom. BMD of the proximal femur was obtained at year 1 and year 3 visits; there were 2.1 ± 0.3 (mean ± SD) years between the two DXA measurements. Annualized percentage change in bone density was calculated as the difference between the second bone density measurement and the baseline value divided by the baseline value, multiplied by 100, and then divided by the number of years between measurements.
Covariates
Covariates included in all models were assessed at year 1. Height was measured using a Harpenden stadiometer. Weight was measured with a calibrated balance beam scale. Demographic characteristics and health habits such as smoking were assessed by an interviewer-administered standardized questionnaire. Smoking was quantified as ever smoker yes/no. Serum was analyzed for creatinine using standard methods at the Laboratory of Clinical Biochemistry at the University of Vermont. Estimated glomerular filtration rate (eGFR) was calculated using the formula from the Modification of Diet in Renal Disease (MDRD) study (36). Protein and potassium intakes were measured using the Block dietary food frequency questionnaire (37).
Statistical analyses
χ2 tests were used for categorical variables, and ANOVA was used for continuous variables to test for differences between individuals in whom bicarbonate measurements had been obtained and those in whom measurements could not be obtained because they had either withdrawn from the study or had technical issues such as difficult venous access. Linear regression was used to relate year 3 arterialized venous bicarbonate to year 1 and year 3 femoral neck and total hip BMD and annualized rate of bone loss at the femur between the two visits. Results for the femoral neck and total hip were similar; results for total hip are presented. Analyses were adjusted for age, race, sex, clinic site, weight, and smoking. Other covariates potentially related to bone density or acid-base balance, including protein intake, renal function, dietary potassium intake, calcium intake, angiotensin-converting enzyme inhibitor use, and angiotensin II receptor blocker use were added to the model using backward stepwise regression, retaining variables associated with the outcome variable at P < .05.
To examine for possible nonlinear effects, plasma bicarbonate was divided into quartiles, and BMD and rate of bone loss were examined across quartiles with adjustment for the same potential confounders.
Results
Arterialized venous plasma bicarbonate measurements were available in 2287 participants, representing 75% of the participants who had hip BMD measurements at year 1 and 89% of those who had hip BMD measurements at year 3. Participants in whom blood gas measurements could not be obtained (n = 788) were more likely to be Black (52 vs 38%; P < .001), slightly older (73.9 vs 73.5 y; P = .01), and have a smoking history (59 vs 55%; P = .04), but they did not differ in weight, proportion from each sex, estimated protein intake, or eGFR (Table 1). BMD was significantly higher in those with a blood gas measurement (P = .05).
Table 1.
Baseline (Year 1) Characteristics of the Health, Aging and Body Composition Cohort in Whom Arterialized Venous Blood Gas Measurements Were Obtained at the Year 3 Visit and Those in Whom Measurements Could Not Be Obtained
| Characteristic | Have Bicarbonate Measurement | No Bicarbonate Measurement | P Value |
|---|---|---|---|
| n | 2287 | 788 | |
| Age, y | 73.5 ± 2.8 | 73.9 ± 3.0 | .001 |
| Race, % | .001 | ||
| Black | 38 | 52 | |
| White | 62 | 48 | |
| Sex, % | .20 | ||
| Male | 49 | 47 | |
| Female | 51 | 53 | |
| Weight, kg | 76.0 ± 15.0 | 75.2 ± 15.3 | .20 |
| eGFR, mL/min | 73.0 ± 15.8 | 71.7 ± 18.9 | .07 |
| Total hip BMD, g/cm2 | 0.890 ± 0.17 | 0.876 ± 0.18 | .05 |
| Total protein intake, g/d | 66.9 ± 28.8 | 68.2 ± 31.2 | .40 |
| Ever smoked, % | 55 | 59 | .04 |
Values are expressed as mean ± SD or percentage.
The pCO2-adjusted bicarbonate averaged 25.1 ± 1.4 mmol/L (mean ± SD) in the whole cohort and differed between the sexes and two races. White women had the highest values, averaging 25.5 ± 1.3 mmol/L whereas Black men had the lowest values, averaging 24.7 ± 1.5 mmol/L. In the whole cohort, plasma bicarbonate was positively associated with BMD at both the year 1 (r = 0.56; P = .001) and year 3 (r = 0.54; P = .001) measurements in models adjusted for age, race, sex, clinic site, weight, smoking, and eGFR. eGFR was significantly related to BMD at year 1 and at year 3; however, the addition of eGFR to the bone density models did not change the relationship between arterialized venous bicarbonate and bone density. Dietary intakes including total protein intake, potassium intake, and calcium intake were not significantly related to bone density in these models and were not retained in the final model. Models were further adjusted for angiotensin-converting enzyme inhibitor use and angiotensin II receptor blocker use; these medication variables were not significantly related to bone outcomes and did not change the relationship between bicarbonate measures and skeletal outcomes. Because fewer bicarbonate measurements were available in Black participants (n = 869) compared to White participants (n = 1418), models were also stratified by race. Within each race strata, the relationships between bicarbonate, BMD, and bone loss were the same as in the overall models adjusted for race. Subanalyses by sex revealed similar relationships in both subgroups. Each mmol/L higher level of bicarbonate was associated with 0.006 g/cm2 (95% confidence interval [CI], 0.002–0.01) greater bone density at year 1 and 0.007 g/cm2 (95% CI, 0.003–0.011) higher bone density at year 3 (Table 2).
Table 2.
Coefficients and β (Standardized Regression) Coefficients for Variables in Multivariate Model Relating Arterialized Venous Bicarbonate at Year 3 to BMD at Baseline (Year 1) and Year 3 Among the 2287 Members of the Health, Aging and Body Composition Cohort in Whom Arterialized Venous Blood Gas Measurements Were Obtained at the Year 3 Visit
| Variable | Baseline (Year 1) |
Year 3 |
||||||
|---|---|---|---|---|---|---|---|---|
| Coefficient | β Coefficient | SE | P Value | Coefficient | β Coefficient | SE | P Value | |
| Arterialized venous bicarbonate, mmol/L | 0.006 | 0.051 | 0.002 | .001 | 0.007 | 0.057 | 0.002 | .001 |
| Race, Black/White | 0.064 | 0.185 | 0.006 | <.001 | 0.057 | 0.163 | 0.006 | <.001 |
| Gender, male/female | −0.124 | −0.371 | 0.006 | <.001 | −0.114 | −0.333 | 0.006 | <.001 |
| Clinic site, Memphis/Pittsburgh | 0.028 | 0.084 | 0.005 | <.001 | 0.031 | 0.092 | 0.005 | <.001 |
| Age, y | −0.003 | −0.056 | 0.001 | <.001 | −0.004 | −0.067 | 0.001 | <.001 |
| Weight, kg | 0.005 | 0.418 | 0.0001 | <.001 | 0.005 | 0.437 | 0.0002 | <.001 |
| Smoking status, yes/no | −0.002 | −0.009 | 0.003 | .56 | −0.001 | −0.003 | 0.003 | .85 |
| eGFR (MDRD), mL/min/1.73 m2 | −0.001 | −0.072 | 0.0002 | <.001 | −0.001 | −0.067 | 0.0002 | <.001 |
Abbreviation: MDRD, Modification of Diet in Renal Disease equation.
Plasma bicarbonate was inversely associated with rate of bone loss at the total hip over the 2.1 ± 0.3 (mean ± SD) years between BMD measurements in models adjusted for age, race, sex, clinic site, weight, and smoking (P < .001). The lower the plasma bicarbonate, the greater the rate of annual bone loss. Bone loss was 0.2%/y (95% CI, 0.09–0.3) higher for every 1 mmol/L lower level of bicarbonate (Table 3). MDRD-eGFR, baseline (year 1) hip bone density, total protein intake, potassium intake, total calcium intake, angiotensin-converting enzyme inhibitor use, and angiotensin II receptor blocker use were not related to bone loss and did not alter the relationship between bicarbonate and bone loss.
Table 3.
Coefficients and β (Standardized Regression) Coefficients for Variables in Multivariate Model Relating Arterialized Venous Bicarbonate Obtained at Year 3 to Percentage Change in BMD Over the 2.1 ± 0.3 (mean ± SD) Years Between Baseline (Year 1) and Year 3 Among the 2287 Members of the Health, Aging and Body Composition Cohort in Whom Arterialized Venous Blood Gas Measurements Were Obtained at the Year 3 Clinic Visit
| Coefficient | β Coefficient | SE | P Value | |
|---|---|---|---|---|
| Arterialized venous bicarbonate, mmol/L | 0.194 | 0.079 | 0.053 | <.001 |
| Race, Black/White | −0.729 | −0.107 | 0.152 | <.001 |
| Gender, male/female | 1.046 | 0.158 | 0.161 | <.001 |
| Clinic site, Memphis/Pittsburgh | 0.160 | 0.024 | 0.139 | .25 |
| Age, y | −0.069 | −0.059 | 0.025 | .005 |
| Weight, kg | 0.032 | 0.142 | 0.005 | <.001 |
| Smoking status, yes/no | 0.137 | 0.040 | 0.076 | .07 |
| eGFR (MDRD), mL/min/1.73 m2 | 0.003 | 0.013 | 0.005 | .54 |
Abbreviation: MDRD, Modification of Diet in Renal Disease equation.
Across quartiles of plasma bicarbonate, the rate of change in bone density over the 2.1 years between year 1 and year 3 ranged from a loss of 0.72%/y in the lowest quartile to a gain of 0.15%/y in the highest quartile of bicarbonate (Figure 1).
Figure 1.
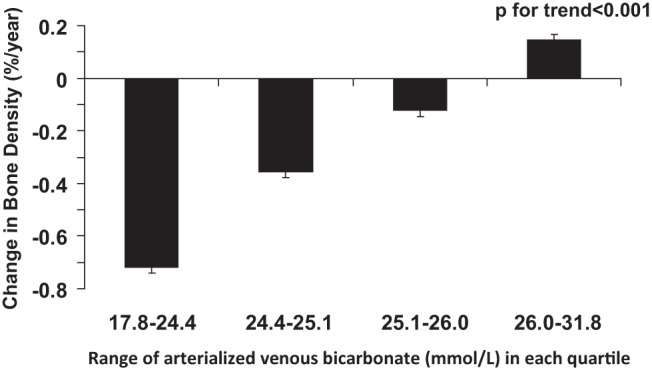
Annualized rate of change in BMD (percent per year) by quartile of arterialized venous bicarbonate adjusted for age, sex, race, clinic site, smoking, and eGFR.
Discussion
Our finding that arterialized plasma bicarbonate is associated positively with cross-sectional BMD and also negatively with the rate of bone loss in generally well-functioning men and women in their seventies supports the hypothesis that systemic acid-base status is an important determinant of skeletal health in the elderly. Our observation that ongoing bone loss was linearly related to arterialized bicarbonate, even after adjustments for age and renal function, suggests that the skeleton, at least in older individuals, functions as a base reservoir with potentially negative consequences for the skeleton. Further research and confirmation of these findings may have major public health implications because reducing dietary net acid load is possible through dietary intervention, by increasing dietary intake of fruit and vegetables while maintaining adequate protein intake, or through base supplementation with alkaline compounds such as potassium citrate or potassium bicarbonate.
Previous analyses have examined the relationship between the skeleton and acid-base metabolism using estimates of dietary net acid load from dietary intake data (1, 2, 38–41). Although dietary intake data provide useful estimates of dietary acid exposure (42, 43) and are practical for the large cohorts and long-term follow-up necessary for skeletal outcomes, direct physiological measurements of acid-base balance have been scarce. Although arterial blood provides the most direct measure of acid load, arterialized venous samples provide nearly identical values. Therefore, arterialized venous samples provide an accurate assessment of the acid load presented to the skeleton by the arterial system (35). This is the first study to compare in vivo arterialized venous plasma bicarbonate measurements to cross-sectional BMD and longitudinal changes in BMD.
The limitations of this study include the potential presence of unmeasured confounders, residual influence of confounders measured with misclassification, and uncertainties in the intra-individual variability of the arterialized bicarbonate measure. However, variability in the measurement, assuming it is nondifferential with respect to the outcome, would be most likely to attenuate any real association rather than introduce a spurious association. This was an observational study, and direct inferences about cause and effect cannot be made. Participants were recruited from just two centers, and only well-functioning older adults were recruited, which limits the generalizability of the observations. Additionally, it is possible that other causes of metabolic acidosis contributed to the plasma bicarbonate values among our participants in addition to their typical dietary acid load. We do not have data that would allow us to examine for aldosterone deficiency, pseudohypoaldosteronism type II, or other causes of metabolic acidosis. However, angiotensin-converting enzyme inhibitor or angiotensin II receptor blocker use was not significantly related to BMD or bone loss in our models and did not significantly affect the coefficients or level of statistical significance for the relationship between bicarbonate and BMD or bone loss. In metabolic acidosis, increased serum chloride levels occur when the rate of production or excretion of other anions (that might otherwise manifest as an anion gap) is sufficiently slow or rapid, respectively. Thus, in otherwise healthy participants, when dietary acid load is the chief cause of metabolic acidosis, it is usually accompanied by increased blood levels of chloride. We do not have data on chloride levels in our study population.
We found that BMD was highest and bone loss was nonexistent in persons in the highest quartile of plasma bicarbonate concentration (mean [HCO3], 26.8 ± 0.8 mmol/L) with no evidence of a threshold or plateau over the range of concentrations observed in our study. Bicarbonate concentrations in the highest quartile were well within the typical physiological range for adults. Based on published equations relating pCO2-adjusted plasma bicarbonate concentration to dietary net acid load (11, 14), the latter might have ranged from as high as 150 mEq/d at the lowest quartile of plasma bicarbonate to slightly negative values (ie, a dietary net base load) at the highest quartile of plasma bicarbonate. These findings raise the possibility that even higher plasma bicarbonate concentrations, as would occur with habitual ingestion of net base-producing diets, could have even larger effects, potentially shifting the typical age-related bone mass decline to positive bone mass gain.
These data are supported by intervention results using intermediary markers and dietary manipulations. Previous short-term studies have demonstrated decreased urine calcium excretion and/or lower markers of bone resorption on diets supplemented with vegetables or alkaline potassium salts (3, 44–49). Longer-term data demonstrate sustained improvement in calcium balance over 6 months of supplementation with potassium citrate (50). In addition, supplementation with 60 mmol/d of potassium citrate was shown to improve bone density compared to placebo in older individuals with normal bone density and fairly low baseline net acid excretion (51).
The range of bicarbonate values observed in this cohort was relatively narrow, yet a significant association with both bone density and bone loss was evident. These results suggest that sufficient base supplementation to beneficially affect the skeleton could be achieved through dietary modifications or supplements of bicarbonate or bicarbonate precursors (eg, potassium citrate). Interventions to provide increased dietary base through dietary modification and/or specific base supplementation are inexpensive and have minimal risk. Sufficient dietary base precursors to neutralize the dietary acid generated by the consumption of an adequate daily protein intake could be achieved by concurrently consuming six or seven servings per day of potassium-rich fruit and vegetables, an intervention consistent with current recommended dietary guidelines. As such, this could be implemented on a population-wide basis and provide substantial protection against skeletal loss in the older population.
Our data provide direct evidence for the important role of acid-base status in skeletal health. We demonstrated that the degree of systemic acidity, as measured by arterialized blood gas samples, is associated with bone density and bone loss, further supporting a role for interventions that can alter acid-base status as a strategy to combat age-related declines in bone density.
Acknowledgments
This research was supported by National Institute on Aging (NIA) Contracts N01-AG-6-2101, N01-AG-6-2103, and N01-AG-6-2106; NIA Grant R01-AG028050; and National Institute of Nursing Research Grant R01-NR012459. This research was also supported in part by the Intramural Research Program of the National Institutes of Health, NIA.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- BMD
- bone mineral density
- CI
- confidence interval
- DXA
- dual-energy x-ray absorptiometry
- eGFR
- estimated glomerular filtration rate.
References
- 1. New SA, MacDonald HM, Campbell MK, et al. Lower estimates of net endogenous non-carbonic acid production are positively associated with indexes of bone health in premenopausal and perimenopausal women. Am J Clin Nutr. 2004;79(1):131–138. [DOI] [PubMed] [Google Scholar]
- 2. Sellmeyer DE, Stone KL, Sebastian A, Cummings SR. A high ratio of dietary animal to vegetable protein increases the rate of bone loss and the risk of fracture in postmenopausal women. Study of Osteoporotic Fractures Research Group. Am J Clin Nutr. 2001;73(1):118–122. [DOI] [PubMed] [Google Scholar]
- 3. Sebastian A, Morris RC., Jr Improved mineral balance and skeletal metabolism in postmenopausal women treated with potassium bicarbonate. N Engl J Med. 1994;331(4):279. [DOI] [PubMed] [Google Scholar]
- 4. Lemann J, Jr, Bushinsky DA, Hamm LL. Bone buffering of acid and base in humans. Am J Physiol Renal Physiol. 2003;285(5):F811–F832. [DOI] [PubMed] [Google Scholar]
- 5. Sherman HC, Sinclair JE. The balance of acid-forming and base-forming elements in foods. J Biol Chem. 1907(3):307–309. [Google Scholar]
- 6. Abelow B. Dietary protein and acid production. Calcif Tissue Int. 1990;46(5):348–350. [DOI] [PubMed] [Google Scholar]
- 7. Lennon EJ, Lemann J., Jr Influence of diet composition on endogenous fixed acid production. Am J Clin Nutr. 1968;21(5):451–456. [DOI] [PubMed] [Google Scholar]
- 8. Breslau NA, Brinkley L, Hill KD, Pak CY. Relationship of animal protein-rich diet to kidney stone formation and calcium metabolism. J Clin Endocrinol Metab. 1988;66(1):140–146. [DOI] [PubMed] [Google Scholar]
- 9. Bernstein D, Wachman A, Hattner R. Acid base balance in metabolic bone disease. Chap. 18. In: Barzel US, ed. Osteoporosis. New York, NY: Grune & Stratton; 1970;207–216. [Google Scholar]
- 10. Lennon EJ, Lemann J, Jr, Litzow JR. The effects of diet and stool composition on the net external acid balance of normal subjects. J Clin Invest. 1966;45(10):1601–1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kurtz I, Maher T, Hulter HN, Schambelan M, Sebastian A. Effect of diet on plasma acid-base composition in normal humans. Kidney Int. 1983;24(5):670–680. [DOI] [PubMed] [Google Scholar]
- 12. Adler S, Lindeman RD, Yiengst MJ, Beard E, Shock NW. Effect of acute acid loading on urinary acid excretion by the aging human kidney. J Lab Clin Med. 1968;72(2):278–289. [PubMed] [Google Scholar]
- 13. Agarwal BN, Cabebe FG. Renal acidification in elderly subjects. Nephron. 1980;26(6):291–295. [DOI] [PubMed] [Google Scholar]
- 14. Frassetto LA, Morris RC, Jr, Sebastian A. Effect of age on blood acid-base composition in adult humans: role of age-related renal functional decline. Am J Physiol. 1996;271:F1114–F1122. [DOI] [PubMed] [Google Scholar]
- 15. Hilton JG, Goodbody MF, Jr, Kruesi OR. The effect of prolonged administration of ammonium chloride on the blood acid-base equilibrium of geriatric subjects. J Am Geriatr Soc. 1955;3(9):697–703. [DOI] [PubMed] [Google Scholar]
- 16. Frassetto L, Sebastian A. Age and systemic acid-base equilibrium: analysis of published data. J Gerontol A Biol Sci Med Sci. 1996;51(1):B91–B99. [DOI] [PubMed] [Google Scholar]
- 17. Kleinman J, Lemann JJ. Acid production. Chap 9. In: Maxwell M, Kleeman C, Narins RG, eds. Clinical Disorders of Fluid and Electrolyte Metabolism. 4th ed New York, NY: McGraw-Hill Inc; 1987:159–173. [Google Scholar]
- 18. Green J, Kleeman CR. Role of bone in regulation of systemic acid-base balance. Kidney Int. 1991;39(1):9–26. [DOI] [PubMed] [Google Scholar]
- 19. Madias NE, Adrogué HJ, Horowitz GL, Cohen JJ, Schwartz WB. A redefinition of normal acid-base equilibrium in man: Carbon dioxide tension as a key determinant of normal plasma bicarbonate concentration. Kidney Int. 1979;16(5):612–618. [DOI] [PubMed] [Google Scholar]
- 20. Moller B. The hydrogen ion concentration of arterial blood. Acta Med Scand. 1959;165:1–68.13800809 [Google Scholar]
- 21. Shock NW, Hastings AB. Studies of the acid-base balance of the blood. III. Variation in the acid-base balance of the blood in normal individuals. J Biol Chem. 1934;104:585–600. [Google Scholar]
- 22. Lemann J, Jr, Litzow JR, Lennon EJ. The effects of chronic acid loads in normal man: further evidence for the participation of bone mineral in the defense against chronic metabolic acidosis. J Clin Invest. 1966;45(10):1608–1614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bushinsky DA. Internal exchanges of hydrogen ions: bone. Chap 4. In: Seldin DW, Giebisch G, eds. The Regulation of Acid-Base Balance. Philadelphia, PA: Raven Press; 1989;69–88. [Google Scholar]
- 24. Yoshimura H, Fujimoto M, Okumura O, Sugimoto J, Kuwada T. Three-step-regulation of acid-base balance in body fluid after acid load. Jpn J Physiol. 1961;11:109–125. [DOI] [PubMed] [Google Scholar]
- 25. Lemann J, Jr, Lennon EJ, Goodman AD, Litzow JR, Relman AS. The net balance of acid in subjects given large loads of acid or alkali. J Clin Invest. 1965;44:507–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lemann J, Litzow JR, Lennon EJ. Studies of the mechanism by which chronic metabolic acidosis augments urinary calcium excretion in man. J Clin Invest. 1967;46(8):1318–1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Barzel US. The effect of excessive acid feeding on bone. Calcif. Tissue Res. 1969(4):94–100. [DOI] [PubMed] [Google Scholar]
- 28. Barzel US. Acid-induced osteoporosis: An experimental model of human osteoporosis. Calcif Tissue Res. 1976;21(suppl):417–422. [PubMed] [Google Scholar]
- 29. Barzel US, Jowsey J. The effects of chronic acid and alkali administration on bone turnover in adult rats. Clin Sci. 1969;36:517–524. [PubMed] [Google Scholar]
- 30. Delling G, Donath K. Morphometric, electron microscopic and physico-chemical studies in experimental osteoporosis induced by chronic acidosis in the rat [in German]. Virchows Arch A Pathol Pathol Anat. 1973;358(4):321–330. [PubMed] [Google Scholar]
- 31. Goldhaber P, Rabadjija L. H+ stimulation of cell-mediated bone resorption in tissue culture. Am J Physiol. 1987;253:E90–E98. [DOI] [PubMed] [Google Scholar]
- 32. Krieger NS, Sessler NE, Bushinsky DA. Acidosis inhibits osteoblastic and stimulates osteoclastic activity in vitro. Am J Physiol. 1992;262:F442–F448. [DOI] [PubMed] [Google Scholar]
- 33. Arnett TR, Dempster DW. Effect of pH on bone resorption by rat osteoclasts in vitro. Endocrinology. 1986;119(1):119–124. [DOI] [PubMed] [Google Scholar]
- 34. Teti A, Blair HC, Schlesinger P, et al. Extracellular protons acidify osteoclasts, reduce cytosolic calcium, and promote expression of cell-matrix attachment structures. J Clin Invest. 1989;84(3):773–780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Forster HV, Dempsey JA, Thomson J, Vidruk E, DoPico GA. Estimation of arterial PO2, PCO2, pH, and lactate from arterialized venous blood. J Appl Physiol. 1972;32(1):134–137. [DOI] [PubMed] [Google Scholar]
- 36. Levey AS, Coresh J, Balk E, et al. National Kidney Foundation practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Ann Intern Med. 2003;139(2):137–147. [DOI] [PubMed] [Google Scholar]
- 37. Block G, Woods M, Potosky A, Clifford C. Validation of a self-administered diet history questionnaire using multiple diet records. J Clin Epidemiol. 1990;43(12):1327–1335. [DOI] [PubMed] [Google Scholar]
- 38. New SA, Bolton-Smith C, Grubb DA, Reid DM. Influence of nutritional factors on bone mass. Osteoporos Int. 1996;6(suppl):128. [Google Scholar]
- 39. New SA, Millward DJ. Calcium, protein, and fruit and vegetables as dietary determinants of bone health. Am J Clin Nutr. 2003;77(5):1340–1341; author reply 1341. [DOI] [PubMed] [Google Scholar]
- 40. Abelow BJ, Holford TR, Insogna KL. Cross-cultural association between dietary animal protein and hip fracture: A hypothesis. Calcif Tissue Int. 1992;50(1):14–18. [DOI] [PubMed] [Google Scholar]
- 41. Frassetto L, Todd KM, Morris RC, Jr, Sebastian A. Role of diet net acid load on hip fracture incidence worldwide. Am Soc Nephrol. 1997;8:551 (Abstract). [Google Scholar]
- 42. Frassetto LA, Todd KM, Morris RC, Jr, Sebastian A. Estimation of net endogenous noncarbonic acid production in humans from diet potassium and protein contents. Am J Clin Nutr. 1998;68(3):576–583. [DOI] [PubMed] [Google Scholar]
- 43. Remer T, Manz F. Estimation of the renal net acid excretion by adults consuming diets containing variable amounts of protein. Am J Clin Nutr. 1994;59(6):1356–1361. [DOI] [PubMed] [Google Scholar]
- 44. Lemann J., Jr Relationship between urinary calcium and net acid excretion as determined by dietary protein and potassium: a review. Nephron. 1999;81(suppl 1):18–25. [DOI] [PubMed] [Google Scholar]
- 45. Lemann J, Jr, Gray RW, Pleuss JA. Potassium bicarbonate, but not sodium bicarbonate, reduces urinary calcium excretion and improves calcium balance in healthy men. Kidney Int. 1989;35(2):688–695. [DOI] [PubMed] [Google Scholar]
- 46. Lemann J, Jr, Pleuss JA, Gray RW, Hoffmann RG. Potassium administration reduces and potassium deprivation increases urinary calcium excretion in healthy adults [corrected]. Kidney Int. 1991;39(5):973–983. [DOI] [PubMed] [Google Scholar]
- 47. Sakhaee K, Maalouf NM, Abrams SA, Pak CY. Effects of potassium alkali and calcium supplementation on bone turnover in postmenopausal women. J Clin Endocrinol Metab. 2005;90(6):3528–3533. [DOI] [PubMed] [Google Scholar]
- 48. Marangella M, Di Stefano M, Casalis S, Berutti S, D'Amelio P, Isaia GC. Effects of potassium citrate supplementation on bone metabolism. Calcif Tissue Int. 2004;74(4):330–335. [DOI] [PubMed] [Google Scholar]
- 49. Lin PH, Ginty F, Appel LJ, et al. The DASH diet and sodium reduction improve markers of bone turnover and calcium metabolism in adults. J Nutr. 2003;133(10):3130–3136. [DOI] [PubMed] [Google Scholar]
- 50. Moseley KF, Weaver CM, Appel L, Sebastian A, Sellmeyer DE. Potassium citrate supplementation results in sustained improvement in calcium balance in older men and women. J Bone Miner Res. 2013;28(3):497–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Jehle S, Hulter HN, Krapf R. Effect of potassium citrate on bone density, microarchitecture, and fracture risk in healthy older adults without osteoporosis: a randomized controlled trial. J Clin Endocrinol Metab. 2013;98(1):207–217. [DOI] [PubMed] [Google Scholar]


