Abstract
Context:
Papillary thyroid cancer (PTC) is the most common endocrine malignancy. The long-term prognosis is generally excellent. Due to a paucity of data, debate exists regarding the benefit of adjuvant radioactive iodine therapy (RAI) for intermediate-risk patients.
Objective:
The objective of the study was to examine the impact of RAI on overall survival in intermediate-risk PTC patients.
Design/Setting:
Adult patients with intermediate-risk PTC who underwent total thyroidectomy with/without RAI in the National Cancer Database, 1998–2006, participated in the study.
Patients:
Intermediate-risk patients, as defined by American Thyroid Association risk and American Joint Commission on Cancer disease stage T3, N0, M0 or Mx, and T1–3, N1, M0, or Mx were included in the study. Patients with aggressive variants and multiple primaries were excluded.
Main Outcome Measures:
Overall survival (OS) for patients treated with and without RAI using univariate and multivariate regression analyses was measured.
Results:
A total of 21 870 patients were included; 15 418 (70.5%) received RAI and 6452 (29.5%) did not. Mean follow-up was 6 years, with the longest follow-up of 14 years. In an unadjusted analysis, RAI was associated with improved OS in all patients (P < .001) as well as in a subgroup analysis among patients younger than 45 years (n = 12 612, P = .002) and 65 years old and older (median OS 140 vs128 mo, n = 2122, P = .008). After a multivariate adjustment for demographic and clinical factors, RAI was associated with a 29% reduction in the risk of death, with a hazard risk 0.71 (95% confidence interval 0.62–0.82, P < .001). For age younger than 45 years, RAI was associated with a 36% reduction in risk of death, with a hazard risk 0.64 (95% confidence interval 0.45- 0.92, P = .016).
Conclusion:
This is the first nationally representative study of intermediate-risk PTC patients and RAI therapy demonstrating an association of RAI with improved overall survival. We recommend that this patient group should be considered for RAI therapy.
Papillary thyroid cancer (PTC) is the most common endocrine malignancy, with a rapidly increasing incidence in the United States over the past 2 decades (1, 2) The long-term prognosis is generally very good with appropriate surgical resection, with or without adjuvant radioactive iodine therapy (RAI) (3). The American Thyroid Association (ATA) currently recommends adjuvant RAI therapy for high-risk patients and select intermediate-risk cases but not for low-risk patients (4). Adjuvant RAI therapy has been debated for intermediate-risk patients because there is a paucity of data regarding the long-term prognosis of patients in this risk group. The ATA classifies thyroid cancer patients into low-, intermediate-, and high-risk groups based on their relative rates of recurrence and mortality (4). Low-risk patients are free of macroscopic disease after surgery, nodal or distant metastases, vascular invasion, or aggressive histologies. Intermediate-risk patients may have microscopic invasion of tumor outside the thyroid into adjoining soft tissue, positive lymph nodes, aggressive histology, vascular invasion, or RAI uptake outside the thyroid gland on whole-body scan after RAI therapy. High-risk patients have macroscopic tumor invasion, distant metastases, and potentially high thyroglobulin levels out of proportion to the post-RAI whole-body scan (4).
In this study, we examined whether adjuvant RAI therapy was associated with a survival advantage among patients with intermediate-risk PTC. Given data from Podnos et al (5) and Jonklaas et al (6)that have suggested RAI may benefit older but not younger patients, we also examined whether RAI impacts survival differently among intermediate-risk patients who are younger (<45 y) vs older (≥65 y).
Materials and Methods
Data
The National Cancer Database (NCDB) is maintained jointly by the National Cancer Society and the American College of Surgeons Commission on Cancer. It captures approximately 85% of all incident thyroid cancer cases in the United States (7). The NCDB Participant User File was used to identify all thyroid cancer cases who underwent thyroid surgery between 1998 and 2006. We limited the analysis to patients diagnosed in 2006 or earlier to allow for the minimum follow-up time of 5 years required to obtain access to survival data in NCDB. International Classification of Diseases for Oncology, third edition, codes 8050/3, 8260/3, 8340/3, 8341/3, 8342/3, and 8343/3 were used to identify patients with papillary thyroid cancer. Data were coded according to the Commission on Cancer Registry Operations and Data Standards Manual, the American Joint Commission on Cancer Manual for Staging of Cancer, and the International Classification of Disease for Oncology. Data were validated at local and NCDB levels. Data were deidentified and submitted to the NCDB in accordance with the Health Insurance Portability and Accountability Act (8). The data analyzed are deidentified, and this study was deemed exempt by our institutional review board.
Intermediate risk definition
The intermediate risk group was defined according to ATA risk criteria and American Joint Commission on Cancer (AJCC) staging. Pathological staging was used to define the T and N stages; pathological and clinical staging were used for identification of distant metastases. Patients with tumor size greater than 4 cm or any size tumor with a minimal extrathyroidal extension and with no evidence of cervical lymph node metastases (T3, N0, M0, or Mx) as well as patients with tumors 4 cm or less who had metastatic cervical lymph nodes (T1–3, N1, M0, or Mx) were selected for inclusion in our analyses.
Inclusion and exclusion criteria
Adults aged 18 years or older diagnosed with papillary thyroid cancer between 1998 and 2006 who underwent total, near-total, or subtotal thyroidectomy were included. Patients were required to have papillary thyroid cancer histology as assessed by the presence of International Classification of Diseases for Oncology, third edition, codes 8050/3, 8260/3, 8340/3, 8341/3, 8342/3, and 8343/3. They were divided into two groups: those who received RAI therapy and those who did not.
Aggressive variants of papillary thyroid cancer, such as tall cell, columnar, sclerosing and insular variants, Hürthle cell, and medullary and other poorly differentiated thyroid cancers were excluded. Patients with more than one primary cancer also were excluded. Additional cases were excluded due to missing data for some variables in the NCDB. For example, cases that remained TxNxMx (Mx were included for those whose T and N stages were known), even after use of the TNM algorithm described above, were excluded.
Study variables
Patient demographic and clinical variables included were age, gender, race, education, insurance status, and year of diagnosis; provider variables included hospital type and location. Pathological and clinical characteristics included in our analyses were tumor size, lymph node status, RAI administration (yes/no), and margin status. Patient comorbidity was represented by the modified Charlson/Deyo scoring system (1992) (9). The NCDB assigns annual income levels by linking a patient's ZIP code to the year 2000 US Census data. The NCDB reports median annual income data within the patient's ZIP code in quartiles. The lower two quartiles represent low median income of less than $35 000, whereas the high median income was $35 000 or greater for the top two quartiles. Similarly, the NCDB assigns the educational level by linking a patient's ZIP code to high school graduation rates from the American Community Survey data. The NCDB reports the median high school graduation rates within the patient's ZIP code in quartiles. The lower two quartiles represent low high school graduation rates of less than 21% for the patient's ZIP code vs the high graduation rates of 21% or greater for the top two quartiles. For the years under study (1998–2006), the comorbidity data were not available in the NCDB before 2003 (38% values missing), and multifocality data were not available before 2004 (52% values missing). Values for these two variables were calculated as percentages of the known values after the years 2003 and 2004, respectively.
Statistical analysis
Patient characteristics were reported as frequencies and proportions for categorical variables and mean and SD for continuous variables. Differences between patients who underwent RAI and those who did not were examined using χ2 tests and Student's t tests for categorical and continuous variables, respectively. Overall survival (OS) was defined as the time from diagnosis to death or last follow-up, whichever came first. For patients who did not die, survival time was censored at the time of last follow-up. Censored patients did not contribute to death events in the Kaplan-Meier curves. Patients with zero months of follow up were excluded. Kaplan-Meier curves were used to visualize OS for those who received RAI vs those who did not, and the log-rank test was used to examine differences in survival. If fewer than 50% of patients died, the median overall survival could not be estimated. To examine the role of RAI by patient age, subgroup analyses for patients younger than 45 years and 65 years or older were performed. A multivariate Cox proportional hazards model was used to examine OS after adjustment for clinical and demographic factors across all ages and in those patients aged younger than 45 years. Significance was prespecified at P = .05 for all statistical tests. All analyses were performed using SPSS version 22 (SPSS, IBM Corp) and SAS version 9.4 (SAS Institute).
Results
A total of 21 870 (31%) of the PTC patients (n = 71 222) met the definition of intermediate risk. Of those, 15 418 (70.5%) received RAI and 6452 (29.5%) did not. Patients who received RAI vs those who did not had a higher proportion of multifocal tumors (51% vs 47%, respectively), lymph node involvement (74% vs 68%), and positive surgical margin status (19% vs 15%), (all P < .01). (Table 1).
Table 1.
Patient Demographic, Clinical, and Pathological Characteristics by RAI Therapy (1998–2006)
| No RAI |
RAI |
P Value | |||
|---|---|---|---|---|---|
| n | (%) | n | (%) | ||
| Intermediate-risk patients (n = 21 870) | 6452 | (29.5) | 15 418 | (70.5) | |
| Age, y | <.001 | ||||
| <45 (n = 12 612) | 3611 | (56.0) | 9001 | (58.0) | |
| 45–64 (n = 7136) | 2133 | (33.0) | 5003 | (32.0) | |
| ≥65 (n = 2112) | 708 | (11.0) | 1414 | (9.0) | |
| Mean (SD) | 44 | (15.3) | 43 | (14.4) | <.001 |
| Female gender | 4584 | (71.0) | 10 851 | (70.4) | NS |
| Race | <.001 | ||||
| White | 5456 | (86.7) | 13 384 | (88.5) | |
| Black | 391 | (6.2) | 762 | (5.0) | |
| Other | 446 | (7.1) | 978 | (6.5) | |
| Year of diagnosis | <.001 | ||||
| 1998–2000 | 1539 | (23.9) | 3105 | (20.1) | |
| 2001–2003 | 1940 | (30.1) | 4389 | (28.5) | |
| 2004–2006 | 2973 | (46.1) | 7924 | (51.4) | |
| Insurance (n = 21 163) | <.001 | ||||
| Insured | 5810 | (95.5) | 14 613 | (96.9) | |
| Income (n = 20 585) | NS | ||||
| High | 4561 | (74.8) | 10 939 | (75.5) | |
| Education level (n = 20 585) | <.001 | ||||
| High | 2562 | (42.0) | 6484 | (44.8) | |
| Facility type | <.001 | ||||
| Community Cancer Program | 541 | (8.4) | 853 | (5.5) | |
| Comprehensive Community Cancer | 3131 | (48.5) | 7734 | (50.2) | |
| Academic | 2521 | (39.1) | 6455 | (41.9) | |
| Other | 259 | (4.0) | 376 | (2.4) | |
| Facility location | <.001 | ||||
| Northeast | 1945 | (30.1) | 3364 | (21.8) | |
| South | 1846 | (28.6) | 4442 | (28.8) | |
| Midwest | 1470 | (22.8) | 3871 | (25.1) | |
| West | 1193 | (18.5) | 3741 | (24.3) | |
| Charlson-Deyo comorbidity score (n = 13 531)a | NS | ||||
| 0 | 3310 | (88.4) | 8743 | (89.3) | |
| 1 | 368 | (9.8) | 922 | (9.4) | |
| 2 | 65 | (1.7) | 123 | (1.3) | |
| Surgical margins (n = 19 786) | <.001 | ||||
| Positive | 861 | (14.7) | 2658 | (19.1) | |
| Multifocality (n = 10 368)b | <.001 | ||||
| Yes | 1293 | (46.6) | 3878 | (51.1) | |
| Lymph nodes status (n = 21 635) | <.001 | ||||
| Not examined | 1380 | (21.6) | 2611 | (17.1) | |
| Negative | 674 | (10.6) | 1434 | (9.4) | |
| Positive | 4334 | (67.9) | 11 209 | (73.5) | |
| Tumor size, cm (n = 20 662) | <.001 | ||||
| ≤1 | 1129 | (19.0) | 2760 | (19.0) | |
| >1–2 | 1760 | (29.0) | 4609 | (32.0) | |
| >2–3 | 1021 | (17.0) | 2775 | (19.0) | |
| >3–4 | 489 | (8.0) | 1239 | (8.0) | |
| >4 | 1675 | (28.0) | 3205 | (22.0) | |
| Mean (SD) | 3 | (3.1) | 2.7 | (2.9) | <.001 |
| Median (IQR) | 2.2 | (1.3–4.4) | 2 | (1.2–3.8) | |
Abbreviations: IQR, interquartile range; NS, not statistically significant. Values are presented as numbers (percentages) of the given sample size unless otherwise indicated.
Variable not collected in NCDB before 2003; values are presented as percentages of the known after 2003.
Variable not collected in NCDB before 2004; values are presented as percentages of the known after 2004.
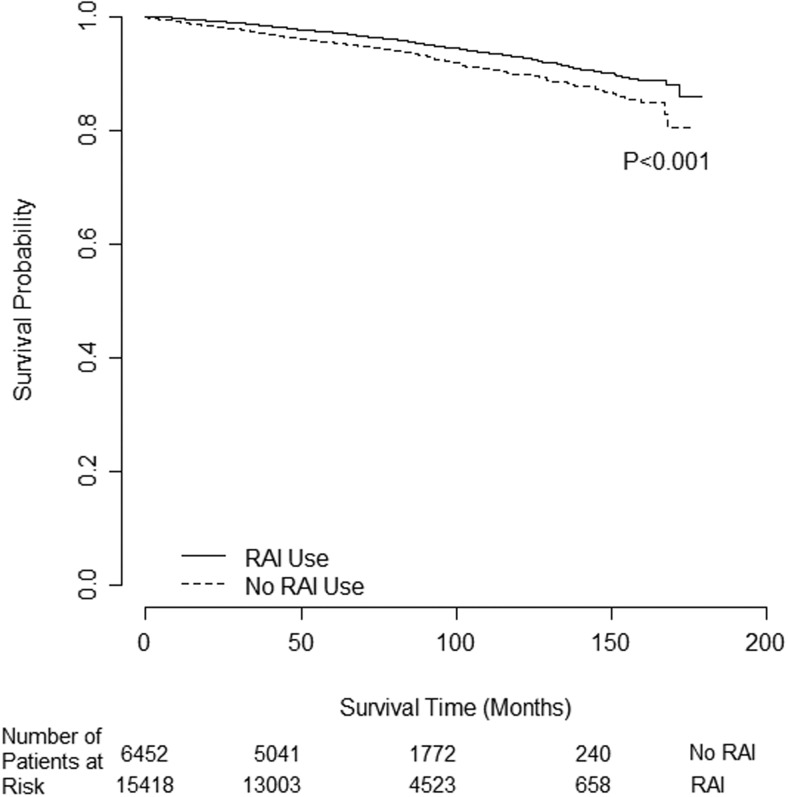
The mean follow-up time was 6.8 years (81 mo; SD 3.1); the longest follow-up time was 14.9 years (179 mo), with median follow-up time of 6.6 years (range 0–14.9, interquartile range 5.1–8.7). There were 730 deaths (5%) in the RAI group vs 424 (7%) in the no RAI group (P < .001). Most patients were alive as of 2006, and a median survival time could not be estimated. RAI administration was associated with improved OS in all patients as demonstrated by Kaplan-Meier survival curves (log-rank P < .001), with a censor rate of 95% (Figure 1).
Figure 1.
Unadjusted overall survival difference for all intermediate-risk PTC patients (n = 21 870) by RAI use.
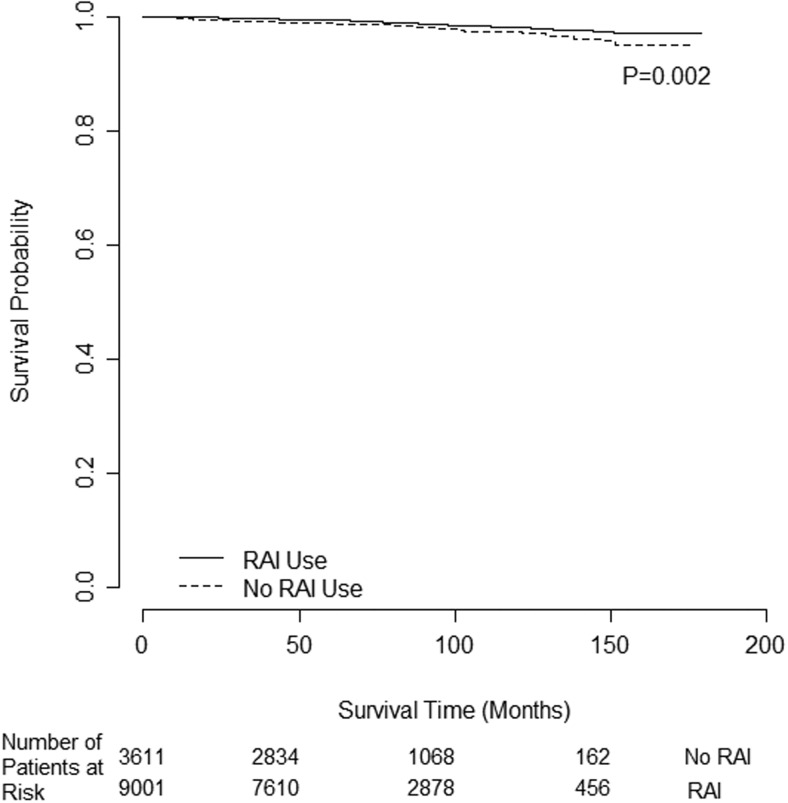
A total of 12 612 patients met criteria for subgroup analysis by age due to age younger than 45 years. Of those, 9001 (71.4%) received RAI. There were 109 deaths (1%) in the RAI group vs 66 deaths (2%) in the no RAI group (P = .007). Most patients were alive as of 2006, and a median survival time could not be estimated. RAI was associated with improved OS in patients younger than 45 years, as demonstrated by Kaplan-Meier survival curves (log-rank P = .002), with a censor rate of 99% (Figure 2).
Figure 2.
Unadjusted OS difference for PTC intermediate-risk patients aged younger than 45 years (n = 12 612) by RAI use.
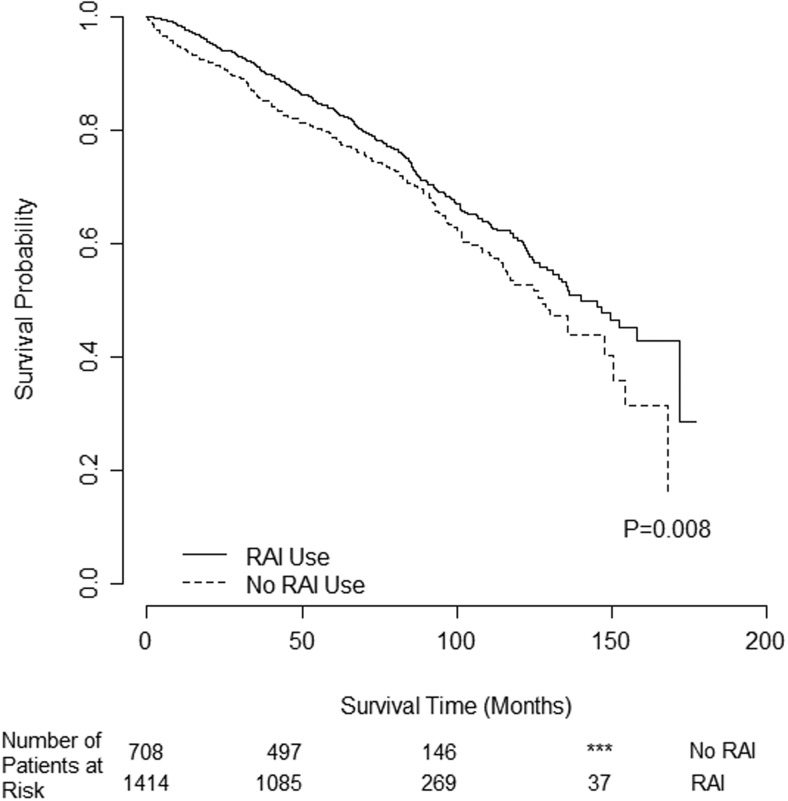
A total of 2122 patients met criteria for subgroup analysis by age due to age 65 years or older. Of those, 1414 (66.6%) received RAI. There were 379 deaths in the RAI group (27%) vs 222 deaths in the no RAI group (31%) (P = .028). RAI was associated with improved OS in patients 65 years old or older as demonstrated by Kaplan-Meier survival curves with a censor rate of 73%, and the median OS was 140 months with RAI vs 128 months without RAI (log-rank P = .008) (Figure 3).
Figure 3.
Unadjusted OS difference for intermediate-risk PTC patients age 65 years or older (n = 2122) by RAI use. ***, Fewer patients than minimum reportable.
After multivariate adjustment for patient demographic and clinical factors, RAI was associated with a 29% reduced risk of death [hazard risk (HR) 0.71 [confidence interval (CI) 0.62–0.82], P < .001]. Patient factors associated with compromised OS included older patient age, male gender, and black race (Table 2). The pathological and clinical factors associated with compromised OS included larger tumor size, presence of lymph node metastases, positive surgical margins, and lack of RAI therapy (all P ≤ .01). Subanalysis for patients age younger than 45 years revealed that RAI was associated with a 36% reduced risk of death [HR 0.64 (CI 0.45–0.92), P = .016]. Patient factors associated with compromised OS for patients younger than 45 years included male gender (Table 3).
Table 2.
Multivariate Cox Proportional Hazards Model of OS for Intermediate-Risk PTC Patients (n = 17 062) for 1998–2006
| HR (95% CI) | P Value | |
|---|---|---|
| Patient age, y | ||
| Increasing age (continuous) | 1.09 (1.09, 1.10) | <.001 |
| Race | ||
| White | 1.00 | |
| Black | 1.62 (1.24, 2.12) | <.001 |
| Other | 0.91 (0.66, 1.25) | .554 |
| Gender | ||
| Male | 1.00 | |
| Female | 0.63 (0.55, 0.72) | <.001 |
| Education level | ||
| High HS graduation rate | 1.00 | |
| Low HS graduation rate | 1.19 (1.03, 1.37) | .018 |
| Insurance | ||
| Not insured | 1.00 | |
| Insured | 1.00 (0.65, 1.56) | .988 |
| Year of diagnosis | ||
| 1998–2000 | 1.00 | |
| 2001–2003 | 1.12 (0.93, 1.35) | .243 |
| 2004–2006 | 1.02 (0.84, 1.24) | .817 |
| Hospital type | ||
| Academic | 1.00 | |
| Community Cancer Program | 0.85 (0.64, 1.13) | .258 |
| Comprehensive Community Cancer Program | 0.99 (0.85, 1.15) | .864 |
| Other specified types of cancer programs | 2.71 (1.60, 4.58) | <.001 |
| Hospital location | ||
| Northeast | 1.00 | |
| Midwest | 1.18 (0.97, 1.43) | .105 |
| South | 1.10 (0.91, 1.33) | .316 |
| West | 0.96 (0.77, 1.21) | .738 |
| Margins | ||
| Negative | 1.00 | |
| Cannot assess | 0.94 (0.58, 1.66) | .822 |
| Positive | 1.37 (1.16, 1.61) | <.001 |
| Lymph nodes status | ||
| Negative | 1.00 | |
| Not examined | 1.03 (0.80, 1.33) | .821 |
| Positive | 1.75 (1.36, 2.24) | <.001 |
| Tumor size, cm | ||
| >4 | 1.00 | |
| >1 to 2 | 0.58 (0.47, 0.72) | <.001 |
| >2 to 3 | 0.64 (0.51, 0.80) | <.001 |
| >3 to 4 | 0.76 (0.58, 0.99) | .040 |
| ≤1 | 0.60 (0.47, 0.75) | <.001 |
| RAI use | ||
| No | 1.00 | |
| Yes | 0.71 (0.62, 0.82) | <.001 |
Abbreviation: HS, high school. HR reference is 1; HR less than 1 is associated with a reduced risk of death; HR greater than 1 is associated with increased risk of death. Statistical significance is P ≤ .05.
Table 3.
Multivariate Cox Proportional Hazards Model of OS for Intermediate-Risk PTC Patients younger than 45 Years Old (n = 9822) for 1998–2006
| HR (95% CI) | P Value | |
|---|---|---|
| Race | ||
| White | 1.00 | |
| Black | 1.74 (0.93, 3.28) | .085 |
| Other | 0.71 (0.26, 1.74) | .448 |
| Gender | ||
| Male | 1.00 | |
| Female | 0.53 (0.37, 0.76) | <.001 |
| Education level | ||
| High HS graduation rate | 1.00 | |
| Low HS graduation rate | 1.22 (0.84, 1.76) | .298 |
| Insurance | ||
| Not Insured | 1.00 | |
| Insured | 1.05 (0.42, 2.58) | .924 |
| Year of diagnosis | ||
| 1998–2000 | 1.00 | |
| 2001–2003 | 0.97 (0.62, 1.51) | .880 |
| 2004–2006 | 1.05 (0.66, 1.66) | .847 |
| Hospital type | ||
| Academic | 1.00 | |
| Community Cancer Program | 1.35 (0.68, 2.68) | .395 |
| Comprehensive Community Cancer Program | 1.16 (0.79, 1.69) | .459 |
| Other specified types of cancer programs | 4.56 (1.79, 11.63) | .002 |
| Hospital location | ||
| Northeast | 1.00 | |
| Midwest | 1.05 (0.64, 1.73) | .855 |
| South | 1.38 (0.86, 2.21) | .179 |
| West | 0.76 (0.42, 1.37) | .361 |
| Margins | ||
| Negative | 1.00 | |
| Cannot assess | 0.90 (0.22, 3.64) | .878 |
| Positive | 0.82 (0.48, 1.41) | .477 |
| Lymph nodes status | ||
| Negative | 1.00 | |
| Not examined | 1.71 (0.69, 4.27) | .248 |
| Positive | 2.27 (0.95, 5.45) | .066 |
| Tumor size, cm | ||
| >4 | 1.00 | |
| >1 to 2 | 0.65 (0.37, 1.13) | .127 |
| >2 to 3 | 0.65 (0.35, 1.21) | .177 |
| >3 to 4 | 0.52 (0.23, 1.18) | .118 |
| ≤1 | 0.80 (0.44, 1.44) | .453 |
| RAI use | ||
| No | 1.00 | |
| Yes | 0.64 (0.45, 0.92) | .016 |
Abbreviation: HS, high school. HR reference is 1; HR less than 1 is associated with a reduced risk of death; HR greater than 1 is associated with an increased risk of death. Statistical significance is P ≤ .05.
To better assess institutional practice patterns, the annual case volume of RAI therapy by hospital (n = 1463 hospitals) was measured. RAI case volume at most hospitals was low, with more than 90% of hospitals treating fewer than 10 patients with RAI annually between the years 1998 and 2006. The mean number of patients treated with RAI per hospital annually was four patients. Only 6% of hospitals (n = 83) treated between 10 and 20 patients, and only 2% of hospitals (n = 36) treated more than 20 patients with RAI annually.
Discussion
This is the first nationally representative study to address the association between RAI and survival for patients with intermediate-risk PTC. Although mortality from PTC is low and the disease is generally associated with an excellent prognosis, we observed a 29% reduced risk of death [HR 0.71 (CI 0.62–0.82)] in our multivariate analyses when RAI was administered in the postoperative setting. Subanalysis of those patients aged younger than 45 years revealed a 36% reduced risk of death on multivariate analysis [HR 0.64 (CI 0.45–0.92)]. This younger age group more closely approximates disease-specific survival. Our results suggest that RAI is associated with improved OS in intermediate-risk patients with papillary thyroid cancer. Our study is adequately powered to detect true differences in overall survival, even with the low mortality reported. Our findings support previous studies suggesting RAI survival benefit in older patients (5, 6). We found that RAI was associated with an increased overall survival in patients younger than 45 years (based on the AJCC system), suggesting that RAI is beneficial, even in younger patients who have the potential for the best prognosis and who may be classified as low risk in alternate stratification systems (6, 10).
The 2009 ATA guidelines recommend RAI use in select cases (4). Due to the indolent nature of differentiated thyroid cancer and long-term survival, there is a scarcity of randomized clinical trials studying papillary thyroid cancer management because a prohibitively large number of patients would be needed, with extended follow-up and associated high cost [median total charges for thyroidectomy have been recently estimated at $27 363 (SD $8530), with median hospital charges of $15 872 (SD $3100) and professional charges $11 170 (SD $6250) based on data from 2011] (11) As a result, there is not a consensus in the literature regarding the potential benefit of RAI for intermediate-risk patients. As outlined by the ATA, the decision to treat is therefore based on clinical judgment, with particular weight placed on patient age, histology, size of tumor, and status of lymph node metastases in combination with other potential risk factors, such as the presence of vascular invasion and tumor multifocality (4).
Adjuvant RAI is used to potentially eliminate a postoperative thyroid remnant, decrease local recurrence, and facilitate follow-up for possible distant metastases through surveillance with serial thyroglobulin levels and whole-body uptake scans (4, 12–14). Although I-131 is widely used, RAI therapy is not without adverse consequences. RAI can impact quality of life and, by extension, medical costs due to side effects such as acute or chronic xerostomia, acute or chronic sialoadentitis, and dental caries, among others (15, 16). Reports of a slightly increased risk of developing secondary primary malignancies after RAI therapy have called for a more selective use of RAI, especially in lower-risk patients (17, 18).
To date, the intermediate-risk group has not been analyzed because other studies used alternate risk and staging definitions in lieu of the ATA definitions, or studies were designed without specifically stratifying patients into an intermediate risk category (5, 6, 19). Smaller studies likely lack the power to analyze this subgroup. Current literature suggests that patients with larger tumors and those who are older may benefit from RAI, although specific tumor size and clinical cutoffs differ (5, 6, 19).
Our finding of a reduced risk of death associated with RAI administration is in contrast to the results of two other large studies, including a nationally representative study using the Surveillance, Epidemiology, and End Results (SEER) database, and a single-institution study from the Mayo Clinic that provided extended patient follow-up (5, 19). Although neither study examined the intermediate-risk group specifically, both studies failed to show a survival benefit for RAI therapy among patients overall (5, 19). Podnos et al (5) failed to show an overall survival benefit associated with RAI on multivariate analysis (median follow-up of 95 mo) among 12 661 PTC patients using the SEER database (P = .9176). Multivariate analysis showed risk ratio (RR) 0.36 for age younger than 45 years,RR0.85 for tumor less than 2 cm, RR 1.28 for positive lymph nodes, RR 2.11 for distant metastasis, all P < .01. However, on subgroup analysis, a survival benefit for older patients was suggested. Specifically, in subgroup analysis, Podnos et al identified improved survival with RAI only among high-risk, older patients who had all of the following risks: patient age older than 45 years, tumor size greater than 2 cm, positive lymph nodes, and distant metastases. Compared with our study, Podnos et al had access to fewer thyroid patients overall (n = 12 661) and included fewer PTC histologies. Notably, the SEER subgroup analysis of high-risk patients was based on just 30 patients (median survival 57 mo with RAI vs 7 mo without RAI, P = .023). In contrast, we analyzed a much larger population (all intermediate risk, n = 21 870, subgroups: age <45 y, n = 12 611 and age ≥65 y, n = 2122), improving our ability to power the study and specifically address survival among the intermediate-risk group.
In a large, single-institution retrospective study (1940–1999) conducted at the Mayo Clinic, Hay et al (19) found no disease specific survival or recurrence benefit when RAI therapy was used among patients with papillary thyroid cancers deemed to be low risk or high risk (n = 2286) over 2 decades of follow-up (1980–1999), despite a significant increase in use of RAI administration since 1975. In the study, a MACIS prognostic score measuring distant metastasis, patient age, completeness of resection, local invasion, and tumor size with a score of less than 6 was deemed to be low risk, and a score of 6 or greater was assessed as high risk (19). Again, due to a difference in risk definitions, the Mayo Clinic study examined low and high risks by measuring distant metastasis, patient age, completeness of resection, local invasion, and tumor size and did not directly evaluate patients at intermediate risk.
A survival benefit with RAI administration has been seen in one study examining a subset of intermediate risk patients, whereas a benefit in disease-free survival was seen in a subset of patients with T2-T4 stage disease in a smaller, single-institution study (6, 20). As part of the National Thyroid Cancer Treatment Cooperative Study Group (NTCTCSG), a prospective multi-institution registry of 11 North American institutions, Jonklaas at al (6) found a survival benefit for adjuvant RAI use in a subset of intermediate-risk patients. They examined 2936 patients with papillary and follicular thyroid cancers between the years 1987 and 2001 (median follow-up 3 y, extending up to 14 y). Using multivariate regression and propensity score analyses, they found that RAI administration was associated with improved overall survival in NTCTSG stages II, III, and IV papillary thyroid cancers. They also found improved disease-specific survival and reduced recurrence rates with RAI use among patients with NTCTSG stages III and IV, but not stage II, disease. In contrast to our study, Jonklaas et al used an alternative NTCTCSG staging system and did not specifically analyze ATA intermediate-risk patients. Depending on patient age, intermediate-risk patients can fall into NTCTSG stages I-III, hampering a direct comparison with our study. For example, NTCTCSG stage II includes some patients who would be considered AJCC stage III (patient aged ≥45 y with minimal extrathyroidal extension as well as patients aged <45 y with tumor size >4 cm). NTCTCSG stage I disease includes patients aged younger than 45 years, even with minimal extrathyroidal extension (AJCC stage III). Although Jonklaas et al found benefit with RAI among a subset of intermediate-risk patients, no benefit was seen in NTCTCSG stage I patients, which includes some intermediate-risk patients younger than 45 years. In contrast, our study found RAI administration was associated with OS benefit in all intermediate-risk patients as defined by ATA criteria, regardless of patient age.
The limitations of this study include those associated with use of large databases and may include NCBD coding errors. However, the NCDB is highly standardized, with numerous quality control measures in place (21). Some NCDB variables such as tumor multifocality and patient comorbidities were incomplete, and vascular invasion was not available for all the years included in our study. Additionally, some variables of interest are not captured in the NCDB. For example, information on post-RAI therapy whole-body scans and patient preference are not available.
Disease recurrence is not captured in the database and could not be analyzed. An important limitation of our study is that the NCDB does not provide disease specific survival information; therefore, we could analyze only overall survival. Although the association of RAI therapy with survival in older patients has been suggested, our study highlights that this association also extends to patients aged younger than 45 years, as demonstrated by our multivariate analysis. The survival benefit found in the younger patients more closely approximates disease-specific survival, mitigating the limitation of using OS as an outcome. The use of the NCDB is itself a major strength of this study, providing the sample size and power that single-institutional studies lack.
Further studies, such as a randomized controlled trial, are warranted; however, the costs of conducting such a study combined with the indolent nature of thyroid cancer have made this prohibitive in the past.
Conclusion
This is the first nationally representative study to address the association of RAI administration with overall survival for intermediate-risk patients, as defined by ATA and AJCC criteria, including subanalyses for patients younger than 45 years and 65 years old and older. Although the event rate for patient mortality is low and reflects the generally favorable prognosis of PTC, we observed a 29% reduced risk of death with RAI therapy [HR 0.71 (CI 0.62–0.82)] in an adjusted analysis for all intermediate-risk patients and a 36% reduced risk of death for patients aged younger than 45 years, [HR 0.64 (CI 0.45–0.92)]. Current ATA guidelines recommend RAI therapy in select intermediate-risk patients. Although the decision to use adjuvant RAI therapy remains centered on individual patient and disease-specific factors, we hope that our findings will inform future guideline recommendations.
Acknowledgments
E.R. acknowledges Paolo Goffredo, MD, and Mohamed Abdelgadir Adam, MD, for their help with National Cancer Database coding and statistics.
The data used in the study are derived from a deidentified National Cancer Data Base file. The American College of Surgeons and the Commission on Cancer have not verified and are not responsible for the analytic or statistical methodology used or the conclusions drawn from these data by the investigators.
This work was supported by National Institutes of Health T-32 Training Grant 2T32DK007012-36A1.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- AJCC
- American Joint Commission on Cancer
- CI
- confidence interval
- HR
- hazard risk
- NCDB
- National Cancer Database
- NTCTCSG
- National Thyroid Cancer Treatment Cooperative Study Group
- OS
- overall survival
- PTC
- papillary thyroid cancer
- RAI
- radioactive iodine
- RR
- risk ratio
- SEER
- Surveillance, Epidemiology, and End Results.
References
- 1. American Cancer Society. Cancer facts, figures. 2013. http://www.cancer.org/acs/groups/content/@epidemiologysurveilance/documents/document/acspc-036845.pdf Accessed November 30, 2014.
- 2. DeSantis CE, Lin CC, Mariotto AB, et al. Cancer treatment and survivorship statistics, 2014. CA Cancer J Clin. 2014;64:252–271. [DOI] [PubMed] [Google Scholar]
- 3. Davies L, Welch HG. Thyroid cancer survival in the United States: observational data from 1973 to 2005. Arch Otolaryngol Head Neck Surg. 2010;136:440–444. [DOI] [PubMed] [Google Scholar]
- 4. Cooper DS, Doherty GM, Haugen BR, et al. Revised American Thyroid Association management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid. 2009;19:1167–1214. [DOI] [PubMed] [Google Scholar]
- 5. Podnos YD, Smith DD, Wagman LD, Ellenhorn JD. Survival in patients with papillary thyroid cancer is not affected by the use of radioactive isotope. J Surg Oncol. 2007;96:3–7. [DOI] [PubMed] [Google Scholar]
- 6. Jonklaas J, Sarlis NJ, Litofsky D, et al. Outcomes of patients with differentiated thyroid carcinoma following initial therapy. Thyroid. 2006;16:1229–1242. [DOI] [PubMed] [Google Scholar]
- 7. Bilimoria KY, Stewart AK, Winchester DP, Ko CY. The National Cancer Database: a powerful initiative to improve cancer care in the United States. Ann Surg Oncol. 2008;15:683–690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Phillips JK, Stewart AK, eds. Facility oncology data standards. Chicago, IL: Commission on Cancer; 2006. [Google Scholar]
- 9. Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45:613–619. [DOI] [PubMed] [Google Scholar]
- 10. Nixon IJ, Patel SG, Palmer FL, et al. Selective use of radioactive iodine in intermediate-risk papillary thyroid cancer. Arch Otolaryngol Head Neck Surg. 2012;138:1141–1146. [DOI] [PubMed] [Google Scholar]
- 11. Morris LF, Romero Arenas MA, et al. Streamlining variability in hospital charges for standard thyroidectomy: developing a strategy to decrease waste. Surgery. 2014;156:1441–1449. [DOI] [PubMed] [Google Scholar]
- 12. Mazzaferri EL, Jhiang SM. Long-term impact of initial surgical and medical therapy on papillary and follicular thyroid cancer. Am J Med. 1994;97:418–428. [DOI] [PubMed] [Google Scholar]
- 13. Sherman SI, Tielens ET, Sostre S, Wharam MD, Jr, Ladenson PW. Clinical utility of posttreatment radioiodine scans in the management of patients with thyroid carcinoma. J Clin Endocrinol Metab. 1994;78:629–634. [DOI] [PubMed] [Google Scholar]
- 14. Tenenbaum F, Corone C, Schlumberger M, Parmentier C. Thyroglobulin measurement and postablative iodine-131 total body scan after total thyroidectomy for differentiated thyroid carcinoma in patients with no evidence of disease. Eur J Cancer. 1996;32a:1262. [DOI] [PubMed] [Google Scholar]
- 15. Mendoza A, Shaffer B, Karakla D, Mason ME, Elkins D, Goffman TE. Quality of life with well-differentiated thyroid cancer: treatment toxicities and their reduction. Thyroid. 2004;14:133–140. [DOI] [PubMed] [Google Scholar]
- 16. Almeida JP, Sanabria AE, Lima EN, Kowalski LP. Late side effects of radioactive iodine on salivary gland function in patients with thyroid cancer. Head Neck. 2011;33:686–690. [DOI] [PubMed] [Google Scholar]
- 17. Sawka AM, Thabane L, Parlea L, et al. Second primary malignancy risk after radioactive iodine treatment for thyroid cancer: a systematic review and meta-analysis. Thyroid. 2009;19:451–457. [DOI] [PubMed] [Google Scholar]
- 18. Iyer NG, Morris LG, Tuttle RM, Shaha AR, Ganly I. Rising incidence of second cancers in patients with low-risk (T1N0) thyroid cancer who receive radioactive iodine therapy. Cancer. 2011;117:4439–4446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hay ID, Thompson GB, Grant CS, et al. Papillary thyroid carcinoma managed at the Mayo Clinic during six decades (1940–1999): temporal trends in initial therapy and long-term outcome in 2444 consecutively treated patients. World J Surg. 2002;26:879–885. [DOI] [PubMed] [Google Scholar]
- 20. Chow SM, Yau S, Kwan CK, Poon PC, Law SC. Local and regional control in patients with papillary thyroid carcinoma: specific indications of external radiotherapy and radioactive iodine according to T and N categories in AJCC 6th edition. Endocr Relat Cancer. 2006;13:1159–1172. [DOI] [PubMed] [Google Scholar]
- 21. Raval MV, Bilimoria KY, Stewart AK, Bentrem DJ, Ko CY. Using the NCDB for cancer care improvement: an introduction to available quality assessment tools. J Surg Oncol. 2009;99:488–490. [DOI] [PubMed] [Google Scholar]