Abstract
Context:
Short sleep duration is associated with an increased risk of type 2 diabetes. Subchronic sleep restriction (SR) causes insulin resistance, but the mechanisms and roles of specific tissues are unclear.
Objective:
The purpose of this article was to determine whether subchronic SR altered (1) hepatic insulin sensitivity, (2) peripheral insulin sensitivity, and (3) substrate utilization.
Design:
This was a randomized crossover study in which 14 subjects underwent 2 admissions separated by a washout period. Each admission had 2 acclimatization nights followed by 5 nights of either SR (4 hours time in bed) or normal sleep (8 hours time in bed).
Main Outcome Measure/Methods:
Insulin sensitivity (measured by hyperinsulinemic-euglycemic clamp) and hepatic insulin sensitivity (measured by stable isotope techniques) were measured. In addition, we assayed stress hormone (24-hour urine free cortisol, metanephrine, and normetanephrine), nonesterified fatty acid (NEFA), and β-hydroxybutyrate (β-OH butyrate) levels. Resting energy expenditure (REE) and respiratory quotient (RQ) were measured by indirect calorimetry.
Results:
Compared to normal sleep, whole-body insulin sensitivity decreased by 25% (P = .008) with SR and peripheral insulin sensitivity decreased by 29% (P = .003). Whereas hepatic insulin sensitivity (endogenous glucose production) did not change significantly, percent gluconeogenesis increased (P = .03). Stress hormones increased modestly (cortisol by 21%, P = .04; metanephrine by 8%, P = .014; normetanephrine by 18%, P = .002). Fasting NEFA and β-OH butyrate levels increased substantially (62% and 55%, respectively). REE did not change (P = 0.98), but RQ decreased (0.81±.02 vs 0.75±0.02, P = .045).
Conclusion:
Subchronic SR causes unique metabolic disturbances characterized by peripheral, but not hepatic, insulin resistance; this was associated with a robust increase in fasting NEFA levels (indicative of increased lipolysis), decreased RQ, and increased β-OH butyrate levels (indicative of whole-body and hepatic fat oxidation, respectively). We postulate that elevated NEFA levels are partially responsible for the decrease in peripheral sensitivity and modulation of hepatic metabolism (ie, increase in gluconeogenesis without increase in endogenous glucose production). Elevated cortisol and metanephrine levels may contribute to insulin resistance by increasing lipolysis and NEFA levels.
Epidemiologic studies have shown that short sleep duration is associated with an increased risk of type 2 diabetes mellitus (1). Furthermore, studies in small numbers of healthy volunteers have demonstrated that subchronic (ie, 2–8 nights) sleep restriction decreases glucose tolerance (2) and impairs insulin sensitivity (3, 4). However, the tissue-specific effects of sleep loss remain poorly understood. In addition, the role of substrate utilization and lipid metabolism in altering insulin sensitivity during sleep loss has not been well studied.
Insulin resistance affects fat, skeletal muscle, and liver. In peripheral tissues (adipose and skeletal muscle), insulin resistance is characterized by decreased insulin-mediated glucose uptake. In the liver, it is manifested as either increased endogenous glucose production (EGP) or attenuation in the ability of insulin to suppress EGP. Insulin resistance is also associated with blunted suppression of lipolysis in adipose tissue, increased nonesterified fatty acid (NEFA) levels (5, 6), and altered fatty acid oxidation (7, 8). At present, it is not known whether subchronic sleep restriction alters insulin sensitivity in both hepatic and peripheral tissues or whether it directly affects substrate utilization.
Mechanisms that have been postulated to explain the effects of sleep restriction on glucose metabolism include sympathetic activation (2), inflammation (9), and alterations in the 24-hour profile of stress hormones (eg, cortisol and catecholamines) (10). A few clinical studies have indicated that adipose tissue may play a role. Specifically, Broussard et al (11) found that subchronic sleep restriction altered insulin signaling in adipose tissue by decreasing the phosphorylation of Akt. Two other studies assessed changes in NEFAs that occur with sleep restriction; however, in one of these studies, subjects were fed a hypocaloric diet (which promotes lipolysis) (12), and in the other, food intake was not controlled (13). Despite these data, there are no published studies looking at concomitant changes in skeletal muscle, liver, and adipose tissue in response to subchronic sleep restriction. Therefore, we assessed the effects of subchronic sleep restriction on both peripheral and hepatic insulin sensitivity, lipid metabolism, and substrate utilization coupled with integrated measures of cortisol and catecholamine excretion and plasma fatty acids in the setting of a controlled diet.
Materials and Methods
Subjects and study design
We performed a randomized, crossover study in healthy volunteers. Each subject underwent 2 inpatient admissions to the Clinical Research Center (CRC) at San Francisco General Hospital, each lasting 1 week and separated by a washout period (lasting 4–10 weeks). Admissions were rigorously controlled with respect to diet, activity, and sleep/wake patterns; detailed assessments of metabolism and hormones were performed at the end of each study condition. The Committee on Human Research at the University of California, San Francisco, approved the protocol. Subjects were recruited from the community using website postings, and informed consent was obtained. Sixteen healthy men and women, between the ages of 18 and 45 years, were enrolled; of these, 1 subject was excluded after failing to remain in the CRC during acclimatization and a second subject was unable to complete the second CRC admission. Thus, we have evaluable data for 14 subjects.
Exclusion criteria were a history of a chronic medical or psychiatric condition, use of any medications, obesity (body mass index of ≥30 kg/m2), use of any tobacco products or illicit drugs within the past year, any history of heavy alcohol use, a first-degree relative with type 2 diabetes, night-shift work, a history of sleep problems, or regular daytime naps. Fasting blood samples were obtained to ensure that insulin levels and results for a comprehensive metabolic panel, thyroid function, insulin levels, complete blood count, and liver function were within normal limits; women underwent a urine pregnancy screen. Subjects scoring >5 on the Pittsburgh Sleep Quality Index questionnaire were excluded (14). A wrist actigraph (Octagonal Basic Motionlogger-L; Ambulatory Monitoring, Inc) was worn for 3 consecutive days and nights, in conjunction with a sleep diary, to estimate habitual sleep duration. Subjects with nightly sleep duration of <6 hours or >9 hours were excluded.
Assignment to the initial study condition was randomized using a computerized random number generator. Of the 14 evaluable subjects, 7 underwent normal sleep first. The first 2 days and nights (nights 1 and 2) were acclimatization, and the subsequent 5 nights (nights 3–7) were the experimental sleep condition. On day 3, a constant, isocaloric metabolic diet was started consisting of fixed caloric content and proportions of carbohydrate (55%), protein (15%), and fat (30%), to maintain body weight and minimize dietary influences on metabolism (15). Caffeine intake was restricted to 100 mg (1 cup of coffee) at breakfast. Meals and snacks were served at the same time of the day, and subjects were required to consume all food provided. An oral glucose tolerance test (OGTT) was performed on day 7 and a hyperinsulinemic-euglycemic clamp with stable isotope tracers on day 8 (after 5 nights of the sleep condition). Two consecutive 24-hour urine collections were obtained for hormone measurements starting at 7:00 am on day 6; the results reported are the average of values from these 2 days. Subjects remained in the CRC and were monitored throughout each admission; they walked outside the CRC daily for 30 minutes, accompanied by a research coordinator. Waking hours were spent watching television, reading, working on a computer, talking to staff, or engaging in arts and crafts.
Sleep conditions and sleep monitoring
On the first 2 acclimatization nights, subjects spent 9 hours time in bed (TIB) (10:00 pm–7:00 am). During normal sleep, subjects spent 8 hours TIB (11:00 pm–7:00 am) for 5 consecutive nights. During sleep restriction, they spent 4 hours TIB (1:00–5:00 am). In both conditions, TIB was centered around 3 am. Daytime naps were not allowed. Subjects were monitored by the CRC staff continuously to confirm wakefulness as per the study condition.
Subjects wore an actigraph throughout the admissions, and actigraphic sleep duration was used to confirm sleep/wake times. Data were collected in 3 modes and were analyzed in the zero-crossing mode using the Cole-Kripke algorithm with Action W-2 software (version 7) (16).
Total sleep time and sleep stages were recorded with an ambulatory polysomnogram (titanium; Embla/Natus Neurology). The signals recorded were as follows: electroencephalogram sites F3-M2, F4-M1, C3-M2, C4-M1, O1-M2, and O2-M1; left and right electrooculograms; 3 submental electromyograms; bilateral anterior tibialis electromyograms; and an electrocardiogram in accordance with standardized guidelines (17). Digitized data were imported for visual scoring using 30-second epochs according to American Academy of Sleep Medicine guidelines (17). Sleep scoring was done by a sleep technician using RemLogic sleep analysis software (Embla/Natus Neurology). The apnea-hypopnea index was ≤10 in all subjects, indicating that none had sleep-disordered breathing.
Body composition
Fat and lean body mass (LBM) were measured by whole-body dual-energy X-ray absorptiometry (Discovery Wi; Hologic) with regional analyses as described previously (18).
Indirect calorimetry
Resting energy expenditure (REE) and respiratory quotient (RQ) were determined by indirect calorimetry (DeltaTrac metabolic monitor), under fasting conditions on day 8.
OGTT and hyperinsulinemic-euglycemic clamp
An OGTT was performed on day 7 of each admission after an overnight fast. Subjects drank 75 g of glucose (in 250 mL of water); blood samples were obtained at 0, 30, 60, 90, and 120 minutes for determination of plasma glucose and serum insulin levels. Integrated area under the curve (AUC) for glucose and insulin concentrations was calculated by the trapezoidal method.
A hyperinsulinemic-euglycemic clamp was performed by the method of DeFronzo et al (19), as used by us previously (18). After an overnight fast, insulin (Humulin; Eli Lilly) was infused at a rate of 40 mU/m2/min for 180 minutes. Blood samples were collected from a retrograde intravenous line placed in a hand that was warmed in a heated box at 50 to 55°C. Glucose concentrations were measured at 5-minute intervals, and the rate of infusion of 20% dextrose (labeled with [U-13C]glucose) was adjusted to maintain the blood glucose level at 90 mg/dL. Blood samples were collected for serum insulin levels during the clamp. Whole-body insulin sensitivity (M/I value) was calculated using the steady-state glucose infusion rates during the last 30 minutes of the clamp, adjusted for insulin levels. Peripheral insulin sensitivity was calculated by adjusting M/I for changes in EGP. The clamp was performed in 13 of the 14 evaluable subjects; in 1 woman, a retrograde intravenous line could not be inserted successfully.
Stable isotope tracer methods
EGP and gluconeogenesis were measured under both fasting and hyperinsulinemic conditions. Starting at 7:00 am on day 8, [U-13C]glucose and [2-13C]glycerol were infused intravenously as a bolus (72 and 90 mg/kg LBM) followed by continuous infusion (1.2 and 15 mg/kg LBM/h). Steady-state blood sampling was performed at 10-minute intervals between 10:00 and 10:30 am (for fasting measurements) and during the last 30 minutes of the clamp.
Isotopic enrichments were determined using gas chromatography-mass spectrometry. EGP and gluconeogenesis were determined using dilution techniques and mass isotopomer distribution analysis as described previously (15, 20). Glycogenolysis was calculated as described previously (20). Stable isotope studies were completed in 11 of the 14 subjects included in this analysis. One woman who did not undergo the clamp also did not have stable isotope infusions, and in 2 other subjects, paired data were not available for technical reasons.
Assays
Triglyceride (TG) and total and high-density lipoprotein (HDL) cholesterol concentrations were measured using reagents from Thermo Scientific, NEFAs were measured using Wako kits, and β-hydroxybutyrate (β-OH butyrate) was measured using the Wako Autokit 3-HB. Low-density lipoprotein (LDL) cholesterol was calculated using the Friedewald equation (21). Whole-blood and plasma glucose levels were measured by the glucose oxidase method (YSI 2300 STAT Plus Glucose Analyzer; YSI Inc). Serum insulin was measured by RIA (EMD Millipore Corporation). Twenty-four-hour urine free cortisol and fractionated metanephrines with simultaneous urine creatinine (to confirm accuracy of collection) were quantified by ARUP Laboratories; the former was measured using liquid chromatography-tandem mass spectrometry and the latter by HPLC-tandem mass spectrometry.
Statistical analysis
Analyses were performed using STATA (version 13.0; StataCorp LP). Baseline characteristics are summarized as means ± SD for continuous data and as counts and percentages for categorical data. For comparisons of subjects between the normal and sleep-restricted conditions, data are presented as means ± SEM. Because of the skewed distribution of outcome variables, we used the Wilcoxon signed rank test (a nonparametric test) to compare differences between normal sleep and sleep restriction. A two-tailed P value of <.05 was considered statistically significant.
Results
Of the 14 evaluable subjects, 8 were men, the mean age was 27 years, and there was a wide range of ethnic groups (Table 1). Mean fasting glucose at the time of screening was 81 mg/dL, and average nightly sleep duration at home was 7.7 hours. Subjects did not have a significant change in weight between the 2 study conditions (P = .90).
Table 1.
Baseline Characteristics
| Variable | Value (n = 140) |
|---|---|
| Age, y, mean ± SD | 27 ± 5 |
| Male sex, n (%) | 8 (57) |
| Race/ethnicity, n (%) | |
| Caucasian | 5 (36) |
| Hispanic | 4 (29) |
| Asian | 4 (29) |
| African-American | 1 (7) |
| Fasting glucose, mg/dL, mean ± SD | 81 ± 7 |
| HOMA-IR, mean ± SD | 2.0 ± 0.6 |
| PSQI score, median | 3.5 |
| Body composition, mean ± SD | |
| BMI, kg/m2 | 24.1 ± 4.1 |
| Total body fat, % total body mass | 24.9 ± 8.1 |
| Truncal fat, % total body fat | 24.8 ± 7.2 |
| LBM, kg | 47.4 ± 10.5 |
| Lipids, mean ± SD | |
| Total cholesterol, mg/dL | 159 ± 21 |
| LDL cholesterol, mg/dL | 89 ± 20 |
| HDL cholesterol, mg/dL | 50 ± 12 |
| TGs, mg/dL | 96 ± 43 |
| Actigraphic sleep duration, mean ± SDa | |
| Minutes | 459 ± 47 |
| Hours | 7.7 ± 0.8 |
Abbreviations: BMI, body mass index; HOMA-IR, homeostasis model assessment of insulin resistance (calculated as fasting insulin [microunits per milliliter] × fasting glucose [millimoles per liter]/22.5); PSQI, Pittsburgh Sleep Quality Index.
Nightly sleep duration at home, as measured by actigraph, during screening.
Sleep
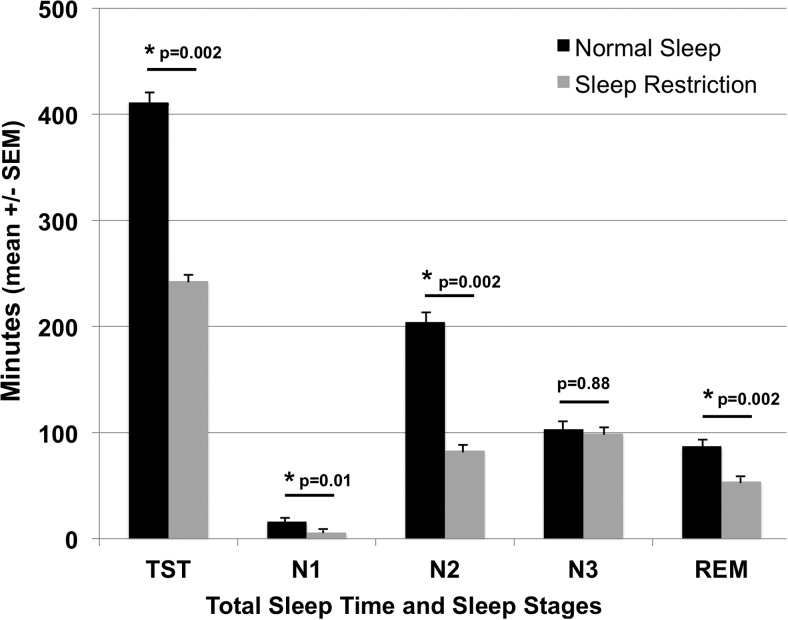
Total sleep time, as determined by polysomnography, averaged 411 ± 14 minutes during normal sleep and 242 ± 6 minutes during sleep restriction (P = .002) (Figure 1). The decreased sleep time during sleep restriction was primarily in the amount of rapid eye movement (REM) sleep (87 ± 8 vs 54 ± 3 minutes, P = .002), N2 stage sleep (204 ± 16 vs 83 ± 6 minutes, P = .002), and N1 stage sleep (16 ± 3 vs 6 ± 2 minutes, P = .01). Minutes of N3 stage sleep (which index slow wave sleep) were not significantly different between the sleep conditions (103 ± 11 vs 99 ± 6 minutes, P = .88); thus, N3 stage sleep as a percentage of total sleep time increased during sleep restriction (25 ± 2% vs 41 ± 2%, P = .002).
Figure 1.
Sleep duration and architecture during normal sleep and sleep restriction. Total sleep time (TST) and sleep stages (N1, N2, N3, and REM), as determined by polysomnography, on the last night of the sleep condition are shown. Mean ± SEM TST was lower during the sleep-restricted condition than during the normal sleep condition, as were minutes of N1 stage sleep, N2 stage sleep, and REM sleep. Minutes of N3 stage sleep were not significantly different between the 2 study conditions.
Glucose metabolism
OGTT
Fasting plasma glucose (87 ± 0.9 vs 87 ± 1.4 mg/dL, P = .4) and insulin (13.9 ± 1.3 vs 13.4 ± 1.1 μIU/mL, P = .55) levels were similar during normal sleep and sleep restriction. During the OGTT, glucose levels (120 minute: 127 ± 7 vs 123 ± 7 mg/dL, P = .77), glucose AUC (374 ± 14 vs 366 ± 13, P = .68), and insulin levels (120 minute: 77.8 ± 12.5 vs 92.7 ± 17.5 μIU/mL, P = .17) were also not significantly different between the 2 study conditions. However, insulin AUC, an integrated measure of insulin secretion, increased by 20% with sleep restriction (215 ± 27 vs 259 ± 40, P = .04).
Hyperinsulinemic-euglycemic clamp
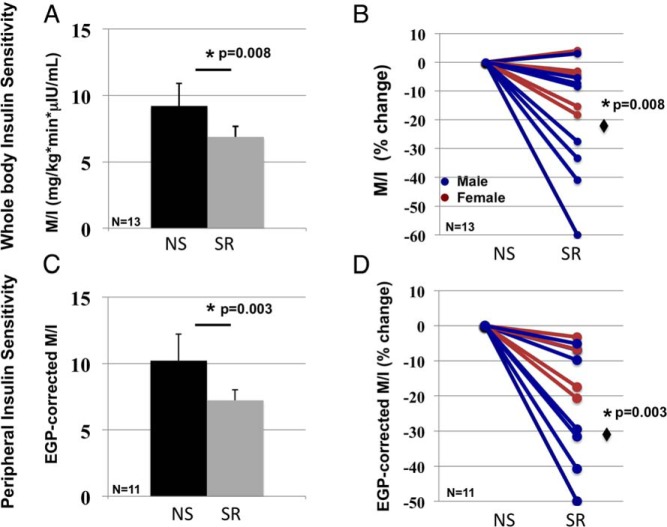
The clamp conditions were met as shown by similar levels of steady-state insulin (79 ± 3.6 vs 82 ± 3.4 μIU/mL, P = .27) and glucose (79.3 ± 0.3 vs 79.0 ± 0.3 mg/dL, P = .25) concentrations during normal sleep and sleep restriction. Whole-body insulin sensitivity decreased by 25% (9.20 ± 1.77 to 6.88 ± 0.80 mg/kg · min · μIU/mL, P = .008) (Figure 2) and peripheral insulin sensitivity by 29% (10.21 ± 2.06 to 7.23 ± 0.94 mg/kg · min · μIU/mL, P = .003) with sleep restriction. Whole-body insulin sensitivity did not differ significantly in men vs women (P = .20); however, men had the most robust decrease in insulin sensitivity with sleep restriction (ie, ≥30% decrease in M/I).
Figure 2.
Effects of sleep restriction on whole-body and peripheral insulin sensitivity. Whole-body insulin sensitivity (ie, insulin-mediated glucose uptake [M/I]) decreased by an average of 25% (P = .008) with sleep restriction (SR) compared with that for normal sleep (NS). A, Mean ± SEM values. B, Subject level data (color coded by sex) as a percentage. C and D, Peripheral insulin sensitivity (ie, EGP-corrected M/I), determined in 11 subjects, was 29% lower with sleep restriction (P = .003).
Stable isotope studies
EGP did not differ under fasting conditions (P = .68) (Table 2). In contrast to the findings with peripheral insulin sensitivity, EGP during hyperinsulinemia was not worsened by sleep restriction. Indeed, during the clamp, EGP was modestly lower during sleep restriction (P = .04), reflecting a significantly lower rate of glycogenolysis (P = .007). In contrast, gluconeogenesis accounted for a greater percentage of EGP during sleep restriction than during normal sleep, under both fasting (P = .03) and hyperinsulinemic (P = .006) conditions.
Table 2.
Effects of Sleep Restriction on Hepatic Insulin Sensitivity
| Normal Sleep | Sleep Restriction | Pa | |
|---|---|---|---|
| EGP, mg/kg · min | |||
| Fasting | 2.59 ± 0.16 | 2.46 ± 0.13 | .680 |
| Hyperinsulinemic clamp | 0.30 ± 0.03 | 0.19 ± 0.03 | .040 |
| Suppression, % | 88 ± 2 | 92 ± 2 | .053 |
| GNG, % EGP | |||
| Fasting | 26 ± 1.9 | 29 ± 1.8 | .030 |
| Hyperinsulinemic clamp | 3.9 ± 0.06 | 4.6 ± 0.06 | .006 |
| Glycogenolysis, % EGP | |||
| Fasting | 74 ± 1.9 | 71 ± 1.8 | .039 |
| Hyperinsulinemic clamp | 96 ± 0.6 | 95 ± 0.6 | .007 |
Abbreviations: EGP, endogenous glucose production; GNG, gluconeogenesis. n = 11. Data are means ± SEM.
P values were calculated by the Wilcoxon signed rank test; values in bold indicate statistical significance (ie, <.05).
Stress hormones, lipids, and indirect calorimetry
Stress hormones
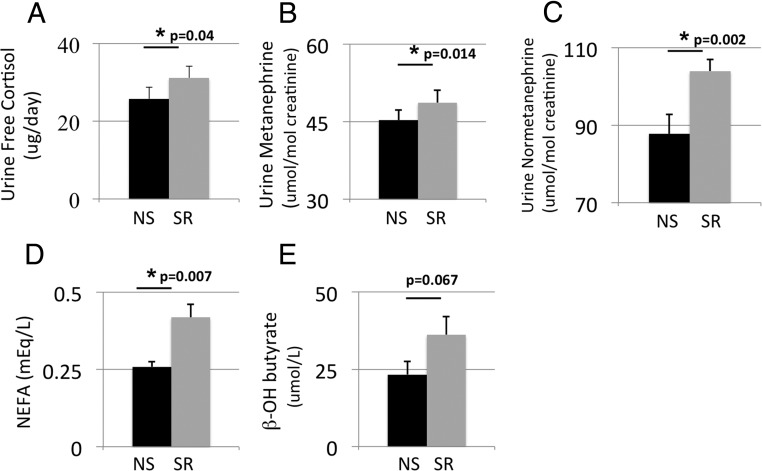
The 24-hour urine free cortisol secretion increased by 21% (P = .04), urine metanephrine by 8% (P = .014), and urine normetanephrine by 18% (P = .002) during sleep restriction (Figure 3).
Figure 3.
Changes in hormones, NEFAs and β-OH butyrate with sleep restriction. Mean ± SEM levels of 24-hour urine free cortisol were higher with sleep restriction (SR) that with normal sleep (NS), as were urine metanephrine and normetanephrine, serum NEFA, and β-hydroxybutyrate levels. P values were calculated by the Wilcoxon signed rank test.
Lipids and indirect calorimetry
Circulating levels of NEFAs were 62% higher during sleep restriction (P = .007) (Figure 3). NEFAs measured during the hyperinsulinemic-euglycemic clamp were suppressed (to levels below those of the standard curve) during both sleep conditions. Although REE was almost identical during the 2 sleep conditions, RQ decreased significantly during sleep restriction, indicating increased fat oxidation. β-OH butyrate, a measure of hepatic fat oxidation, was 55% higher with sleep restriction, but this did not reach statistical significance (P = .067). Consistent with increased fat oxidation, TGs decreased by 24% with sleep restriction (Table 3). Total cholesterol, LDL cholesterol, and HDL cholesterol did not change.
Table 3.
Lipids and Indirect Calorimetry After Normal Sleep and Sleep Restriction
| Normal Sleep | Sleep Restriction | Pa | |
|---|---|---|---|
| Indirect calorimetry | |||
| REE, kcal/d | 1598 ± 71 | 1597 ± 75 | .98 |
| RQ | 0.81 ± 0.02 | 0.75 ± 0.02 | .045 |
| Lipids | |||
| Total cholesterol, mg/dL | 145 ± 7 | 140 ± 9 | .20 |
| LDL cholesterol, mg/dL | 80 ± 7 | 79 ± 7 | .69 |
| HDL cholesterol, mg/dL | 43 ± 4 | 44 ± 3 | .44 |
| TGs, mg/dL | 113 ± 16 | 87 ± 14 | .004 |
n = 11. Data are means ± SEM.
P values were calculated by the Wilcoxon signed rank test; values in bold indicate statistical significance (ie, <.05).
Discussion
In this randomized, crossover study of healthy men and women, we found that subchronic sleep restriction for 5 nights caused a selective form of insulin resistance: peripheral insulin sensitivity decreased, but hepatic insulin sensitivity did not. These changes were observed despite no significant differences in minutes of slow-wave sleep (stage N3). Furthermore, sleep restriction caused (1) a modest increase in cortisol and catecholamines, (2) a robust increase in NEFA levels, (3) a decrease in RQ with no change in REE (indicating increased whole-body fatty acid oxidation and reduced carbohydrate oxidation), and (4) elevated β-OH butyrate levels (indicating an increase in hepatic fatty acid oxidation). Concomitant with the increased fatty acid oxidation, fasting TG levels decreased. Despite the lack of change in EGP, sleep restriction led to an interesting modulation of hepatic metabolism including a modest increase in gluconeogenesis (during both fasting and hyperinsulinemic conditions), a decrease in glycogenolysis, and an increase in hepatic fat oxidation. Although some of these changes are subtle, they may be important for understanding the complex interaction between various tissues in response to subchronic sleep restriction.
Decreased whole-body insulin sensitivity with subchronic sleep restriction has been shown in other studies (3, 4). Our study confirms the development of insulin resistance and indicates that it is driven by changes in extrahepatic tissues; overall hepatic insulin sensitivity is not significantly altered. However, this is the first study to determine the effects of subchronic sleep restriction on liver metabolism and hepatic insulin sensitivity and the only study to assess gluconeogenesis in response to sleep loss. One previous study had shown that acute sleep restriction (for 1 night) increased hepatic insulin resistance (22). However, the authors used an unusual study design of fragmented sleep loss (ie, 2 hours TIB, 4 hours awake, and 2 hours TIB again), and they did not observe any changes in cortisol levels with sleep loss. In contrast, we used a paradigm of subchronic sleep restriction (for 5 nights), with a continuous 4 hours of TIB (from 1:00 to 5:00 am). Of note, a discrepancy between peripheral and hepatic insulin sensitivity is seen in other conditions, including caloric restriction and aging (23, 24).
To probe potential mechanisms, we assessed stress hormones and lipid metabolism. We found that subchronic sleep restriction modestly increased cortisol levels, confirming the results of prior studies (3, 25). Substantial increases in cortisol are known to decrease insulin sensitivity in both liver and peripheral tissues (26), whereas modest elevations of cortisol (because of exogenous administration) may cause hepatic insulin resistance without altering peripheral insulin sensitivity (27). The pattern that we observed with sleep restriction (ie, peripheral insulin resistance but not hepatic) could be due to differences in the means by which cortisol is increased (ie, exogenous vs endogenous). Alternatively, in the setting of sleep loss, cortisol may affect insulin sensitivity by indirect mechanisms (ie, elevation of NEFAs). Previous studies have demonstrated that increases in catecholamines and acute or subacute increases in cortisol promote lipolysis (28–31). In our study, the modest increases in cortisol and catecholamines may be responsible for the higher rate of lipolysis as suggested by increased circulating NEFAs. NEFAs are well known to cause peripheral insulin resistance by decreasing skeletal muscle uptake of glucose (32). We postulate that elevated NEFAs are partially responsible for the peripheral insulin resistance observed with subchronic sleep restriction. Of note, in our small study, as with others (3), changes in peripheral insulin sensitivity did not correlate in a statistically significant manner with changes in cortisol or catecholamines; however, it is difficult to demonstrate correlations when the incremental changes are small in a study of this size.
With respect to hepatic glucose metabolism, we found that sleep restriction caused an increase in fractional gluconeogenesis with a reciprocal decrease in glycogenolysis, resulting in no net change in fasting EGP. Elevated NEFA levels may explain this subtle pattern of changes in the liver. Studies in humans have demonstrated that NEFAs increase gluconeogenesis and concomitantly decrease glycogenolysis, with no overall change in EGP (33, 34). The higher rate of gluconeogenesis may be sustained by the increased hepatic fatty acid oxidation. Consistent with these findings, the increased β-OH butyrate in our study suggests increased hepatic fatty acid oxidation with sleep restriction, whereas the decreased RQ indicates increased whole-body fatty acid oxidation. Further studies are needed to explore the molecular mechanisms involved. For example, peroxisome proliferator-activated receptor α (PPARα) is a nuclear receptor that promotes hepatic fat oxidation and ketogenesis; its expression in the liver exhibits circadian rhythmicity (35). Rodent studies have shown that sleep deprivation increases expression of PPARα (36). Alteration in PPARα expression is one potential mechanism by which sleep restriction may lead to changes in hepatic metabolism such as those we observed in this study.
With respect to lipid metabolism, sleep restriction decreased TG levels by 24% without any effect on total or LDL cholesterol levels. This decrease in TG levels may be attributed to lower very low-density lipoprotein (VLDL) production or increased clearance. We favor an effect on VLDL production because increased hepatic fat oxidation (as supported by the elevated β-OH butyrate levels) would be consistent with (1) decreased NEFA reesterification for VLDL production and (2) decreased hepatic de novo lipogenesis (DNL). We have previously shown that gluconeogenic and lipogenic substrates can be channeled to either pathway (20); it is likely that the higher gluconeogenic flux in sleep restriction is associated with lower DNL flux. We hypothesize that this combination of findings observed with sleep restriction (ie, increase in NEFAs along with decreased TG and elevated RQ) indicates that substrates (such as glycerol) entering the liver are being preferentially shunted to the gluconeogenesis pathway and away from the DNL pathway during sleep loss.
Finally, we observed changes in whole-body and peripheral insulin sensitivity despite preservation of minutes of N3 sleep, which is hypothesized to be the most “restorative” stage; the decreased total sleep time in our study reflects a decrease in N1, N2, and REM sleep. Other studies have found that a selective decrease in N3 sleep without any change in total sleep time (37) or circadian misalignment independent of sleep loss (38) alters insulin sensitivity. Our results indicate that other stages of sleep or total sleep time may be just as important as N3 sleep with respect to metabolic outcomes.
Limitations of this study include a small sample size; however, our sample had both men and women and a wide range of ethnic groups. Second, our methodology did not allow us to determine whether the increase in NEFAs was due to increased lipolysis or decreased clearance. Given that these were young, healthy, lean subjects and fatty acid oxidation is increased, we propose that decreased clearance of NEFAs is unlikely. Third, we do not have measurements of GH secretory patterns or thyroid hormone levels in this study. Both of these hormone axes affect metabolism and may be altered by sleep loss. Last, we do not have data from indirect calorimetry performed during the hyperinsulinemic-euglycemic clamp, which could help further elucidate the effects of sleep restriction on glucose oxidation and nonoxidative glucose disposal.
In conclusion, we have described a unique pattern of insulin resistance and metabolic disturbance that occurs with subchronic sleep restriction. These results may have implications for the treatment of patients with diabetes who have disturbed sleep (ie, those with obstructive sleep apnea). In addition, these results suggest areas for future research, especially with respect to the molecular mechanisms involved.
Acknowledgments
We gratefully acknowledge the contributions of Alaisa Emery (data collection), Viva Tai (designed and coordinated metabolic diet), Michael Wen (analyzed stable isotope data and aided in collection of hyperinsulinemic-euglycemic clamp data), and Leslie Ruoff (acquisition and analysis of polysomnographic data) to this study.
This work was supported by funding from the National Institutes of Health (National Heart, Lung, and Blood Institute Grant K23HL096832, National Center for Research Resources Grant UL 1 TR000004) and with resources and the use of the facilities of the San Francisco Veterans' Affairs Medical Center
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- AUC
- area under the curve
- β-OH butyrate
- β-hydroxybutyrate
- CRC
- Clinical Research Center
- DNL
- de novo lipolysis
- EGP
- endogenous glucose production
- HDL
- high-density lipoprotein
- LBM
- lean body mass
- LDL
- low-density lipoprotein
- NEFA
- nonesterified fatty acid
- OGTT
- oral glucose tolerance test
- PPARα
- peroxisome proliferator–activated receptor α
- REE
- resting energy expenditure
- RQ
- respiratory quotient
- TG
- triglyceride
- TIB
- time in bed
- VLDL
- very low-density lipoprotein.
References
- 1. Cappuccio FP, D'Elia L, Strazzullo P, Miller MA. Quantity and quality of sleep and incidence of type 2 diabetes: A systematic review and meta-analysis. Diabetes Care. 2010;33:414–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Spiegel K, Leproult R, Van Cauter E. Impact of sleep debt on metabolic and endocrine function. Lancet. 1999;354:1435–1439. [DOI] [PubMed] [Google Scholar]
- 3. Buxton OM, Pavlova M, Reid EW, Wang W, Simonson DC, Adler GK. Sleep restriction for 1 week reduces insulin sensitivity in healthy men. Diabetes. 2010;59:2126–2133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Nedeltcheva AV, Kessler L, Imperial J, Penev PD. Exposure to recurrent sleep restriction in the setting of high caloric intake and physical inactivity results in increased insulin resistance and reduced glucose tolerance. J Clin Endocrinol Metab. 2009;94:3242–3250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. McGarry JD. What if Minkowski had been ageusic? An alternative angle on diabetes. Science. 1992;258:766–770. [DOI] [PubMed] [Google Scholar]
- 6. Eckel RH, Alberti KG, Grundy SM, Zimmet P. The metabolic syndrome. Lancet. 2010;375:181–183. [DOI] [PubMed] [Google Scholar]
- 7. Groop LC, Bonadonna RC, DelPrato S, et al. Glucose and free fatty acid metabolism in non-insulin-dependent diabetes mellitus. Evidence for multiple sites of insulin resistance. J Clin Invest. 1989;84:205–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lillioja S, Bogardus C, Mott DM, Kennedy AL, Knowler WC, Howard BV. Relationship between insulin-mediated glucose disposal and lipid metabolism in man. J Clin Invest. 1985;75:1106–1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Mullington JM, Simpson NS, Meier-Ewert HK, Haack M. Sleep loss and inflammation. Best Prac Res Clin Endocrinol Metab. 2010;24:775–784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Schmid SM, Hallschmid M, Schultes B. The metabolic burden of sleep loss. Lancet Diabetes Endocrinol. 2015;3:52–62. [DOI] [PubMed] [Google Scholar]
- 11. Broussard JL, Ehrmann DA, Van Cauter E, Tasali E, Brady MJ. Impaired insulin signaling in human adipocytes after experimental sleep restriction: a randomized, crossover study. Ann Intern Med. 2012;157:549–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Nedeltcheva AV, Imperial JG, Penev PD. Effects of sleep restriction on glucose control and insulin secretion during diet-induced weight loss. Obesity. 2012;20:1379–1386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Schmid SM, Hallschmid M, Jauch-Chara K, et al. Disturbed glucoregulatory response to food intake after moderate sleep restriction. Sleep. 2011;34:371–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Buysse DJ, Reynolds CF, 3rd, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28:193–213. [DOI] [PubMed] [Google Scholar]
- 15. Schwarz JM, Neese RA, Turner S, Dare D, Hellerstein MK. Short-term alterations in carbohydrate energy intake in humans. Striking effects on hepatic glucose production, de novo lipogenesis, lipolysis, and whole-body fuel selection. J Clin Invest. 1995;96:2735–2743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Jean-Louis G, Kripke DF, Mason WJ, Elliott JA, Youngstedt SD. Sleep estimation from wrist movement quantified by different actigraphic modalities. J Neurosci Methods. 2001;105:185–191. [DOI] [PubMed] [Google Scholar]
- 17. Iber C, Ancoli-Israel S, Chesson AL, Quan SF, American Academy of Sleep Medicine. The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology and Technical Specifications. Westchester, IL: American Academy of Sleep Medicine; 2007. [Google Scholar]
- 18. Rao MN, Mulligan K, Tai V, et al. Effects of insulin-like growth factor (IGF)-I/IGF-binding protein-3 treatment on glucose metabolism and fat distribution in human immunodeficiency virus-infected patients with abdominal obesity and insulin resistance. J Clin Endocrinol Metab. 2010;95:4361–4366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. DeFronzo RA, Tobin JD, Andres R. Glucose clamp technique: a method for quantifying insulin secretion and resistance. Am J Physiol. 1979;237:E214–E223. [DOI] [PubMed] [Google Scholar]
- 20. Schwarz JM, Mulligan K, Lee J, et al. Effects of recombinant human growth hormone on hepatic lipid and carbohydrate metabolism in HIV-infected patients with fat accumulation. J Clin Endocrinol Metab. 2002;87:942–945. [DOI] [PubMed] [Google Scholar]
- 21. Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18:499–502. [PubMed] [Google Scholar]
- 22. Donga E, van Dijk M, van Dijk JG, et al. A single night of partial sleep deprivation induces insulin resistance in multiple metabolic pathways in healthy subjects. J Clin Endocrinol Metab. 2010;95:2963–2968. [DOI] [PubMed] [Google Scholar]
- 23. Barzilai N, Banerjee S, Hawkins M, Chen W, Rossetti L. Caloric restriction reverses hepatic insulin resistance in aging rats by decreasing visceral fat. J Clin Invest. 1998;101:1353–1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Broughton DL, James OW, Alberti KG, Taylor R. Peripheral and hepatic insulin sensitivity in healthy elderly human subjects. Eur J Clin Invest. 1991;21:13–21. [DOI] [PubMed] [Google Scholar]
- 25. Reynolds AC, Dorrian J, Liu PY, et al. Impact of five nights of sleep restriction on glucose metabolism, leptin and testosterone in young adult men. PLoS One. 2012;7:e41218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Rizza RA, Mandarino LJ, Gerich JE. Cortisol-induced insulin resistance in man: impaired suppression of glucose production and stimulation of glucose utilization due to a postreceptor defect of insulin action. J Clin Endocrinol Metab. 1982;54:131–138. [DOI] [PubMed] [Google Scholar]
- 27. van Raalte DH, Brands M, van der Zijl NJ, et al. Low-dose glucocorticoid treatment affects multiple aspects of intermediary metabolism in healthy humans: a randomised controlled trial. Diabetologia. 2011;54:2103–2112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Dinneen S, Alzaid A, Miles J, Rizza R. Metabolic effects of the nocturnal rise in cortisol on carbohydrate metabolism in normal humans. J Clin Invest. 1993;92:2283–2290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Divertie GD, Jensen MD, Miles JM. Stimulation of lipolysis in humans by physiological hypercortisolemia. Diabetes. 1991;40:1228–1232. [DOI] [PubMed] [Google Scholar]
- 30. Arner P. Control of lipolysis and its relevance to development of obesity in man. Diabetes Metab Rev. 1988;4:507–515. [PubMed] [Google Scholar]
- 31. Macfarlane DP, Forbes S, Walker BR. Glucocorticoids and fatty acid metabolism in humans: fuelling fat redistribution in the metabolic syndrome. J Endocrinol. 2008;197:189–204. [DOI] [PubMed] [Google Scholar]
- 32. Shulman GI. Cellular mechanisms of insulin resistance. J Clin Invest. 2000;106:171–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Chen X, Iqbal N, Boden G. The effects of free fatty acids on gluconeogenesis and glycogenolysis in normal subjects. J Clin Invest. 1999;103:365–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ferrannini E, Barrett EJ, Bevilacqua S. Effect of fatty acids on glucose production and utilization in man. J Clin Invest. 1983;72:1737–1747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Yang X, Downes M, Yu RT, et al. Nuclear receptor expression links the circadian clock to metabolism. Cell. 2006;126:801–810. [DOI] [PubMed] [Google Scholar]
- 36. Chikahisa S, Shimizu N, Shiuchi T, Séi H. Ketone body metabolism and sleep homeostasis in mice. Neuropharmacology. 2014;79:399–404. [DOI] [PubMed] [Google Scholar]
- 37. Tasali E, Leproult R, Ehrmann DA, Van Cauter E. Slow-wave sleep and the risk of type 2 diabetes in humans. Proc Natl Acad Sci USA. 2008;105:1044–1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Leproult R, Holmbäck U, Van Cauter E. Circadian misalignment augments markers of insulin resistance and inflammation, independently of sleep loss. Diabetes. 2014;63:1860–1869. [DOI] [PMC free article] [PubMed] [Google Scholar]