Abstract
Context:
Sex hormones may influence adipose tissue deposition, possibly contributing to sex disparities in cardiovascular disease risk.
Objective:
We hypothesized that associations of sex hormone levels with visceral and subcutaneous fat differ by sex.
Design, Setting, and Participants:
Participants were from the Multi-Ethnic Study of Atherosclerosis with sex hormone levels at baseline and visceral and subcutaneous fat measurements from computed tomography at visit 2 or 3 (n = 1835).
Main Outcome Measures:
Multivariable linear regression was used to investigate the relationships between sex hormones and adiposity. Testing for interaction by sex, race/ethnicity, and age was conducted.
Results:
In adjusted models, there was a modest significant positive association between estradiol and visceral fat in both sexes (percent difference in visceral fat for 1% difference in hormone [95% confidence interval] in women, 5.44 [1.82, 9.09]; and in men, 8.22 [0.61, 16.18]). Higher bioavailable T was significantly associated with higher visceral and subcutaneous fat in women and with the reverse in men (women, 14.38 [10.23, 18.69]; men, −7.69 [−13.06, −1.00]). Higher dehydroepiandrosterone was associated with higher visceral fat in women (7.57 [1.71, 13.88]), but not in men (−2.47 [−8.88, 4.29]). Higher SHBG was associated with significantly lower levels of adiposity in both sexes (women, −24.42 [−28.11, −20.55]; men, −27.39 [−32.97, −21.34]). There was no significant interaction by race/ethnicity or age.
Conclusion:
Sex hormones are significantly associated with adiposity, and the associations of androgens differ qualitatively by sex. This heterogeneity may help explain the complexity of the contribution of sex hormones to sex differences in cardiovascular disease.
Sexual dimorphism in body composition has been a topic of interest for more than 50 years (1) and has been suggested to explain some of the sex differences in cardiovascular disease and mortality (2). Despite this interest, it remains unclear why women have more subcutaneous fat but less visceral fat than men (2). One suggestion is that sex hormones play a large role in determining healthy body composition (3). Estrogen has been proposed to explain the healthier fat distributions of women (3), and it has been suggested that in men testosterone (T) has a significant role in determining fat distribution and maintaining lean mass (4, 5). There are few data about the effect of estradiol on visceral adiposity in men (6) or of free T on adiposity in women (7). One possible reason for this complexity is that the relationships between sex hormones and body composition are different, and potentially even reversed, for women and men. Although there is increasing evidence that fat distribution may differ by race (8), there is sparse evidence about sex hormone differences by race. Using the context provided above, the aim of this study was to determine whether sex hormones are cross-sectionally associated with computed tomography (CT)-derived measures of visceral and subcutaneous fat by sex and race/ethnicity in the Multi-Ethnic Study of Atherosclerosis (MESA).
Subjects and Methods
Study population
MESA is a longitudinal multisite study with baseline data collection on 6814 participants who were aged 45 to 84 years in 2000 to 2002 and free of known cardiovascular disease (9). MESA's Abdominal Body Composition, Inflammation, and Cardiovascular Disease Ancillary Study (n = 1947) consists of a 30% random sample of the MESA cohort that received CT scans divided between visits 2 and 3 (2002–2005). Excluding 24 participants with uncodeable scans and 88 women with unknown menopausal status at visit 1 gave a final sample size of 1835. For this analysis, we combined sex hormone data and covariates collected at baseline and body composition data from scans conducted at visits 2 and 3. Institutional Review Board (IRB) approval for the MESA study was provided by multiple institutional IRBs, and all participants provided informed consent.
Measurement of body composition and sex hormones
CT scans of the abdomen were used to measure visceral and subcutaneous fat mass by semiautomated segmentation of the body compartments using the Medical Image Processing, Analysis, and Visualization (MIPAV) software program from the National Institutes of Health. Total visceral and subcutaneous fat areas (separately) were defined as the average of two CT slices obtained at the L4/L5 vertebrae. Additional information on the measurement of visceral and subcutaneous fat is available in the Supplemental Data. Sex hormone levels were measured at baseline for all male and all postmenopausal female participants with morning fasting serum samples, as previously described (10).
Statistical analysis
We calculated means and standard deviations (SD) or medians and interquartile ranges for baseline characteristics by sex. We calculated the means and 95% confidence intervals of visceral fat and subcutaneous fat, respectively, for sex-specific quintiles of each sex hormone for women and men separately. We then used the Cuzick nonparametric test for trend across ordered groups to assess the significance of trends in body composition by increasing sex hormone quintile.
We used multivariable linear regression to determine whether higher sex hormone levels at baseline were associated with CT measures of adiposity at visits 2 and 3. Based on the understanding that the hormone distributions might not overlap for women and men, we decided a priori to create separate models for women and men. All body composition and sex hormone values were natural log transformed, and results are reported as back-transformed percent differences. We formally tested for interaction by race/ethnicity using interaction terms in the model. We included models with different levels of adjustment for confounding, including adjustment for other sex hormones. We then created more parsimonious final models by retaining only those variables that were significant at the P < .05 level in the full models for either men or women, in order to test for heterogeneity using the same model for both sexes. We also formally tested for interactions by sex using Wald tests. We performed sensitivity analyses to ensure that the estimates from the final regression model were robust to multiple conditions. Finally, we estimated the association between total T and adiposity, in addition to the main models using bioavailable T. Analyses were performed using Stata 11 (StataCorp).
Results
The final sample included 855 postmenopausal women and 936 men aged 44 to 84 years at baseline. Women had significantly lower estradiol, bioavailable T, dehydroepiandrosterone (DHEA), and visceral fat than men, but higher sex hormone binding globulin (SHBG) and subcutaneous fat (Supplemental Table 1). There were no differences by race/ethnicity.
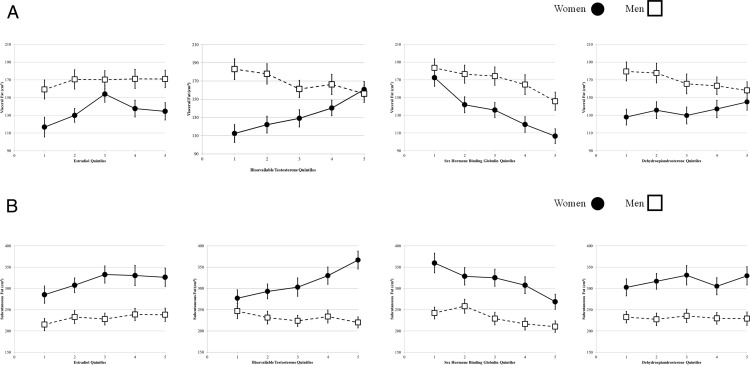
Figure 1 shows the unadjusted means and 95% confidence intervals of visceral fat and subcutaneous fat by sex-specific quintiles of estradiol, bioavailable T, SHBG, and DHEA for women and men. Sex-specific hormone quintiles are given in Supplemental Table 2. For visceral fat (Figure 1A), there was a significant positive trend for estradiol, bioavailable T, and DHEA, and a significant negative trend for SHBG in women. In men, higher quintiles of bioavailable T, DHEA, and SHBG were significantly associated with lower mean visceral fat. All trends between visceral fat and sex hormones were significant at the P < .05 level, except for the estradiol relationship in men. For subcutaneous fat (Figure 1B), results were similar to those for visceral fat, except that the relationship with estradiol in men was significantly positive, and no significant trend was seen for DHEA in either women or men.
Figure 1.
A, Unadjusted mean visceral fat area by sex-specific sex hormone quintiles. B, Unadjusted mean subcutaneous fat area by sex-specific hormone quintiles.
Table 1 and Supplemental Table 3 show results from the adjusted models, which gave broadly similar but attenuated results to those shown in Figure 1. We detected no interactions by race/ethnicity. In the final models, we found a significant interaction by sex for bioavailable T and DHEA, but not for estradiol or SHBG for both visceral and subcutaneous fat models. Estimates remained similar when adjusting for other hormones. We observed few changes in the estimates with subgroup analysis, with estimates in the same direction as those from the main analysis (Supplemental Table 4). Finally, visceral fat estimates for total T were similar to those for bioavailable T, even after adjusting for SHBG (data not shown).
Table 1.
Percent Difference in Back-Transformed Visceral Fat (ln cm2) for Every Percent Difference in Back-Transformed Sex Hormone (ln nmol/L) in 1835 Participants Aged 45–84 Years in the MESA Body Composition Ancillary Study
| Visceral Fat | Women | Men | P Value for Heterogeneity by Sex |
|---|---|---|---|
| Unadjusted model | |||
| Estradiol | 5.02 (1.01, 9.42) | 6.08 (−1.98, 15.03) | .83 |
| Bioavailable T | 17.25 (12.75, 22.14) | −9.51 (−15.63, −2.96) | <.001 |
| SHBG | −25.92 (−29.53, −22.12) | −23.66 (−28.82, −17.30) | .53 |
| DHEA | 6.72 (0.60, 12.75) | −10.42 (−16.47, −4.69) | <.001 |
| Adjusted for race/ethnicity + age + income + education | |||
| Estradiol | 8.22 (4.39, 11.63) | 9.75 (1.92, 18.53) | .75 |
| Bioavailable T | 17.35 (12.75, 22.14) | −8.61 (−13.93, −1.98) | <.001 |
| SHBG | −27.39 (−30.93, −23.66) | −30.93 (−35.60, −25.17) | .30 |
| DHEA | 10.52 (4.08, 17.35) | −3.34 (−9.52, 3.56) | .003 |
| Final model: Adjusted for race/ethnicity + age + income + exercise + SBP + DBP + BPmeds + glucose | |||
| Estradiol | 5.44 (1.82, 9.09) | 8.22 (0.61, 16.18) | .53 |
| Bioavailable T | 14.38 (10.23, 18.69) | −7.69 (−13.06, −1.00) | <.001 |
| SHBG | −24.42 (−28.11, −20.55) | −27.39 (−32.97, −21.34) | .43 |
| DHEA | 7.57 (1.71, 13.88) | −2.47 (−8.88, 4.29) | .020 |
| Final model adjusted for SHBG | |||
| Estradiol | 13.88 (10.3, 17.35) | 8.76 (1.41, 17.35) | .27 |
| Bioavailable T | 2.02 (−2.96, 6.18) | −9.52 (−15.63, −3.92) | .0032 |
| Final model adjusted for estradiol and bioavailable T | |||
| SHBG | −28.11 (−32.63, −23.71) | −28.82 (−34.14, −23.34) | .89 |
| Final model adjusted for estradiol and bioavailable T as mediators | |||
| DHEA | −2.96 (−8.66, 7.52) | −2.69 (−9.15, 4.22) | .95 |
Abbreviations: SBP, systolic blood pressure; DBP, diastolic blood pressure; BPmeds, blood pressure medications; glucose, fasting glucose. Boldface data indicate significant difference from zero at P < .05 level.
Discussion
In the MESA study, significant qualitative differences by sex, but not race/ethnicity, exist in the relationship between selected sex hormones and CT-measured body composition. In men, androgens were negatively related in varying degrees to fat in the visceral and subcutaneous compartments. This association was reversed for women. In contrast, SHBG was inversely associated with fat from both compartments for both women and men. In women, there was a significant, but modest, positive association between estradiol and visceral fat, whereas for men this association was also positive, but not significant.
Studies in men have shown the importance of T in maintaining healthy body composition (11). Although the evidence for T is strongest for its association with lean muscle mass, T has been shown to play a role in the deposition of fat to different body compartments (5). Most of the previous studies are consistent with our findings. Studies in men generally found an inverse association between T and adiposity, as well as an inverse association between SHBG and adiposity (4, 12–16). Studies in women found a positive association between T and adiposity (6, 7, 17–20) but an inverse association between SHBG and adiposity (17, 19–21). Studies investigating the association between estradiol and adiposity have produced inconsistent findings (4, 6, 7, 12, 20); however, the two studies that investigated these relationships using CT or magnetic resonance imaging to measure body composition found results somewhat similar to ours (4, 7).
Despite increasing interest in the role that sex hormones play in body composition, studies that simultaneously investigate the associations of both estrogen and androgen are rare (6, 7, 12, 20). Similarly, studies on sex hormones have predominantly focused on the health of either women or men, but not both, limiting inference about differences by sex in the same population. The need for such comprehensive investigations of sex hormones is exemplified by prior work suggesting sexual dimorphism in the concentration of sex hormone receptors in different fat depots (3). Differing levels of sex hormones, in combination with different concentrations of receptors for those hormones, may help explain the inverse associations we found between bioavailable T and fat mass in women and men.
This study has a number of limitations. First, there are differentially missing data at the upper ranges of visceral and subcutaneous fat measurements. This may limit the validity of the inference of this study at the extreme upper range of body composition values, particularly for subcutaneous fat. Second, whereas the baseline sex hormone measures are not contemporaneous with the body composition measures, for a number of reasons we expect that the hypothesized associations will still hold. Body composition is generally slow to change, the time between baseline sex hormone collection and CT scans is relatively short (2–4 y), and sex hormone measurement temporally precedes the body composition measurements. Third, the use of immunoassays may limit our ability to measure low levels of estradiol in men or T in women, and the lack of free T measurements may limit the comparability of our results. Fourth, the measurement of data at only a single time point means that we cannot investigate the temporality of the relationship between hormones and adiposity. This is particularly challenging because adipose tissue is known to be hormonally active and at high levels gives rise to relatively higher levels of estradiol, specifically in postmenopausal women. Similarly, a bidirectional association (5), or one that includes the aromatization of T into estradiol, has also been suggested for obesity and T in men (22).
The primary strength of this study is that the large diverse study population allows for investigation of differences by sex and race/ethnicity. Another strength is that we used the “gold standard” measurement of visceral and subcutaneous fat to allow for more precise and valid estimates of body composition. Finally, such a comprehensive investigation of the role of sex hormones on adiposity has been rare in the literature. Most studies have looked at only one sex or class of hormones in isolation. By investigating the influence of both estradiol and T on both women and men in the same population, this study provides rare evidence about the ways in which the relationship with body composition may differ in women and men.
In the MESA study, the relationship between androgens and CT-measured adiposity was qualitatively inverse for women and men, but SHBG was negatively associated with adiposity in both women and men. The relationship between sex hormones and adiposity did not differ by race/ethnicity. Heterogeneity by sex in the relationship between hormones and body composition may indicate biological differences in the mechanisms controlling fat distribution that may help explain sex differences in cardiovascular disease risk and guide future hormonal intervention studies to favorably influence body composition.
Acknowledgments
The authors thank the other investigators of the MESA study, the staff, and the participants for their valuable contributions. A full list of participating MESA investigators and institutions can be found at http://www.mesa-nhlbi.org.
This research was supported by Contracts N01-HC-95159, N01-HC-95160, N01-HC-95161, N01-HC-95162, N01-HC-95163, N01-HC-95164, N01-HC-95165, N01-HC-95166, N01-HC-95167, N01-HC-95168, and N01-HC-95169 from the National Heart, Lung, and Blood Institute (NHLBI) and by Grants UL1-TR-000040 and UL1-RR-025005 from the National Center for Research Resources.
M.L.M.-C. was supported by National Institutes of Health (NIH)/NHLBI Grant T32 HL007024, NIH/National Institute of Diabetes and Digestive and Kidney Diseases Grant T32 DK062707, and NIH/NHLBI Grant T32HL079891. The MESA Abdominal Body Composition, Inflammation and Cardiovascular Disease ancillary study is supported by NIH/NHLBI Grant R01 HL088451.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- CT
- computed tomography
- DHEA
- dehydroepiandrosterone.
References
- 1. Vague J. The degree of masculine differentiation of obesities: a factor determining predisposition to diabetes, atherosclerosis, gout, and uric calculous disease. Am J Clin Nutr. 1956;4:20–34. [DOI] [PubMed] [Google Scholar]
- 2. Vitale C, Fini M, Speziale G, Chierchia S. Gender differences in the cardiovascular effects of sex hormones. Fundam Clin Pharmacol. 2010;24:675–685. [DOI] [PubMed] [Google Scholar]
- 3. Nedungadi TP, Clegg DJ. Sexual dimorphism in body fat distribution and risk for cardiovascular diseases. J Cardiovasc Transl Res. 2009;2:321–327. [DOI] [PubMed] [Google Scholar]
- 4. Nielsen TL, Hagen C, Wraae K, et al. Visceral and subcutaneous adipose tissue assessed by magnetic resonance imaging in relation to circulating androgens, sex hormone-binding globulin, and luteinizing hormone in young men. J Clin Endocrinol Metab. 2007;92:2696–2705. [DOI] [PubMed] [Google Scholar]
- 5. Allan CA, McLachlan RI. Androgens and obesity. Curr Opin Endocrinol Diabetes Obes. 2010;17:224–232. [DOI] [PubMed] [Google Scholar]
- 6. Oh JY, Barrett-Connor E, Wedick NM, Wingard DL. Endogenous sex hormones and the development of type 2 diabetes in older men and women: the Rancho Bernardo study. Diabetes Care. 2002;25:55–60. [DOI] [PubMed] [Google Scholar]
- 7. Janssen I, Powell LH, Kazlauskaite R, Dugan SA. Testosterone and visceral fat in midlife women: the Study of Women's Health Across the Nation (SWAN) fat patterning study. Obesity (Silver Spring). 2010;18:604–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wagner DR, Heyward VH. Measures of body composition in blacks and whites: a comparative review. Am J Clin Nutr. 2000;71:1392–1402. [DOI] [PubMed] [Google Scholar]
- 9. Bild DE, Bluemke DA, Burke GL, et al. Multi-ethnic study of atherosclerosis: objectives and design. Am J Epidemiol. 2002;156:871–881. [DOI] [PubMed] [Google Scholar]
- 10. Golden SH, Dobs AS, Vaidya D, et al. Endogenous sex hormones and glucose tolerance status in postmenopausal women. J Clin Endocrinol Metab. 2007;92:1289–1295. [DOI] [PubMed] [Google Scholar]
- 11. Dhindsa S, Miller MG, McWhirter CL, et al. Testosterone concentrations in diabetic and nondiabetic obese men. Diabetes Care. 2010;33:1186–1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Vermeulen A, Kaufman JM, Deslypere JP, Thomas G. Attenuated luteinizing hormone (LH) pulse amplitude but normal LH pulse frequency, and its relation to plasma androgens in hypogonadism of obese men. J Clin Endocrinol Metab. 1993;76:1140–1146. [DOI] [PubMed] [Google Scholar]
- 13. Tsai EC, Matsumoto AM, Fujimoto WY, Boyko EJ. Association of bioavailable, free, and total testosterone with insulin resistance: influence of sex hormone-binding globulin and body fat. Diabetes Care. 2004;27:861–868. [DOI] [PubMed] [Google Scholar]
- 14. Kapoor D, Aldred H, Clark S, Channer KS, Jones TH. Clinical and biochemical assessment of hypogonadism in men with type 2 diabetes: correlations with bioavailable testosterone and visceral adiposity. Diabetes Care. 2007;30:911–917. [DOI] [PubMed] [Google Scholar]
- 15. Osuna JA, Gómez-Pérez R, Arata-Bellabarba G, Villaroel V. Relationship between BMI, total testosterone, sex hormone-binding-globulin, leptin, insulin and insulin resistance in obese men. Arch Androl. 2006;52:355–361. [DOI] [PubMed] [Google Scholar]
- 16. Derby CA, Zilber S, Brambilla D, Morales KH, McKinlay JB. Body mass index, waist circumference and waist to hip ratio and change in sex steroid hormones: the Massachusetts Male Ageing Study. Clin Endocrinol (Oxf). 2006;65:125–131. [DOI] [PubMed] [Google Scholar]
- 17. Sutton-Tyrrell K, Wildman RP, Matthews KA, et al. Sex-hormone-binding globulin and the free androgen index are related to cardiovascular risk factors in multiethnic premenopausal and perimenopausal women enrolled in the Study of Women Across the Nation (SWAN). Circulation. 2005;111:1242–1249. [DOI] [PubMed] [Google Scholar]
- 18. Goodman-Gruen D, Barrett-Connor E. Sex differences in the association of endogenous sex hormone levels and glucose tolerance status in older men and women. Diabetes Care. 2000;23:912–918. [DOI] [PubMed] [Google Scholar]
- 19. Randolph JF, Jr, Sowers M, Gold EB, et al. Reproductive hormones in the early menopausal transition: relationship to ethnicity, body size, and menopausal status. J Clin Endocrinol Metab. 2003;88:1516–1522. [DOI] [PubMed] [Google Scholar]
- 20. Kalish GM, Barrett-Connor E, Laughlin GA, Gulanski BI. Association of endogenous sex hormones and insulin resistance among postmenopausal women: results from the Postmenopausal Estrogen/Progestin Intervention Trial. J Clin Endocrinol Metab. 2003;88:1646–1652. [DOI] [PubMed] [Google Scholar]
- 21. Andersson B, Mårin P, Lissner L, Vermeulen A, Björntorp P. Testosterone concentrations in women and men with NIDDM. Diabetes Care. 1994;17:405–411. [DOI] [PubMed] [Google Scholar]
- 22. Finkelstein JS, Lee H, Burnett-Bowie SA, et al. Gonadal steroids and body composition, strength, and sexual function in men. N Engl J Med. 2013;369:1011–1022. [DOI] [PMC free article] [PubMed] [Google Scholar]