Abstract
Context:
Uterine fibroids (UFs) are the most common benign tumors in premenopausal women. In this study, we evaluated the effects of 1,25-dihydroxyvitamin D3 [1,25(OH)2D3] for the treatment of UFs.
Objective:
To determine the role of 1,25(OH)2D3 on the expression of sex steroid receptors in human UF cells.
Design:
Human UFs and their adjacent myometrium were analyzed for expression of estrogen receptor (ER)-α, progesterone receptor (PR)-A, and PR-B, as well as members of the steroid receptor coactivator (SRC) family. Immortalized human uterine fibroid (human uterine leiomyoma [HuLM]) cells were treated with 1,25(OH)2D3 and assayed for the expression and localization of the aforementioned receptors and SRCs using Western blot, immunohistochemistry, immunofluorescence, and immunoprecipitation assays.
Main Outcome Measures:
We discovered a correlation between reduced levels of vitamin D receptor (VDR) and increased levels of ER-α, PR-A, and PR-B in these tissues. We evaluated the effects of 1,25(OH)2D3 on the regulation of the aforementioned sex steroid receptors.
Results:
We observed an inverse correlation between the up-regulated ER-α, PR-A, and PR-B and expression of VDR in UFs. Treatment with 1,25(OH)2D3 significantly decreased levels of ER-α, PR-A, and PR-B, as well as SRCs in HuLM cells (P < .05). In contrast, 1,25(OH)2D3 self-induced its own VDR, which resulted in an induction of VDR-retinoid X receptor-α complex in HuLM cells. Together, these results suggest that 1,25(OH)2D3 functions as an antagonist of sex steroid hormone receptors in HuLM cells.
Conclusions:
1,25(OH)2D3 functions as a potent antiestrogenic/antiprogesteronic agent that may have utility as a novel therapeutic option for UF.
Uterine fibroids (UFs; or leiomyomas) are the leading cause of hysterectomy in women of reproductive age (1–3). Increasing evidence supports the theory that ovarian steroids such as estrogen and progesterone play key roles in the growth of UFs (4–6). UFs are three to four times more likely to occur in African American women, who also suffer from vitamin D deficiency, compared to their Caucasian counterparts (7, 8). The exact cause of this markedly high occurrence of UFs is not yet fully understood, but such association may suggest a role for vitamin D in fibroid biology. We and others have recently reported that women with UFs have lower levels of serum vitamin D3 compared to women who do not have UFs (9–11). Moreover, we also demonstrated a direct association of lower levels of serum vitamin D3 with increased size of UFs within different ethnic groups (9).
1,25-Dihydroxyvitamin D3 [1,25(OH)2D3] is a member of the steroid hormone family and serves as the major regulator of calcium and phosphate homeostasis in the body system (12). Studies have shown that 1,25(OH)2D3 can induce growth arrest, differentiation, and apoptosis in a wide variety of cancer cells (13, 14). Recent studies have demonstrated that 1,25(OH)2D3 or its noncalcemic analog, paricalcitol, inhibits fibroid tumor growth in vivo and can inhibit proliferation of human UF cells in vitro (15–17). Furthermore, we also demonstrated that 1,25(OH)2D3 can inhibit the expression and activities of matrix metalloproteinases and reduce the expression of extracellular matrix proteins in cultured UF cells (18, 19).
In a 2004 report of Andersen et al (20), it is clearly illustrated that fibroid primary cultures have an elevated response to 17β-estradiol compared with myometrial cultures. UFs are responsive to sex steroid hormones, and such responsiveness is enhanced in rodent and human tumors as well as tumor-derived cell lines as compared to their myometrial counterparts (2, 21, 22). The effects of estrogen are mediated primarily via estrogen receptor (ER)-α, the nuclear receptor that belongs to a superfamily of ligand-regulated transcription factors (23). Subsequent to estradiol binding to ER-α, the receptor undergoes a conformational change, dimerizes, and binds either directly to DNA via estrogen response elements or indirectly via interactions with other DNA-bound transcription factors such as Sp1 or AP-1 (23–25). Estrogen exerts its biological effect in estrogen-responsive tissues by binding to ER-α and modulating the transcription of target genes including growth factors and proto-oncogenes, among others (26). Moreover, nongenomic effects of estrogen can be induced by cytoplasmic signaling pathways, which include the activation of the MAPK pathway transduced from growth factor receptors or plasma membrane localized ER (27, 28). Because estrogen functions via binding with ER-α (29), the factors and mechanisms that regulate the level of ER-α are important in determining the amplitude of estrogen-mediated actions in UFs. This knowledge will augment our efforts in the development of new therapeutic approaches for the treatment of UFs.
Recent studies demonstrated that 1,25(OH)2D3 can reduce gene expression of ER-α in human breast cancer cells (30). We have recently reported that lower levels of vitamin D receptor (VDR) are associated with a higher risk of UF pathogenesis (19). Published literature also demonstrates up-regulation of steroid receptors, particularly ER-α in UFs as compared with the adjacent myometrium, yet nothing is known as to whether there is an association between the expression status of these sex steroid receptors and lower levels of VDR. It has also not been determined whether 1,25(OH)2D3 plays an important role in the regulation of sex steroid hormone receptors in UF cells. The aim of this study is to evaluate whether a possible correlation exists between the higher expression of steroid receptors and lower levels of VDR in human UF and to determine whether 1,25(OH)2D3 has the potential to suppress the expression of those receptors in cultured human UF cells, thus implicating a potential nonsurgical therapeutic utility for the treatment of UFs.
Materials and Methods
Cell lines and cultures
Human uterine leiomyoma (HuLM) cells were a kind gift from Dr Darlene Dixon (National Institute of Environmental Health Sciences, Research Triangle Park, North Carolina) (31). These cells were grown in smooth muscle basal medium at 37°C in a humidified atmosphere of 5% CO2 as previously described (32). Primary human UF cells used in this study were described in our prior publications (18).
Reagents and antibodies
1,25(OH)2D3, estrogen, protein G-sepharose, fluorescein isothiocyanate (FITC)-conjugated phalloidin, and anti-β-actin antibodies were purchased from Sigma Biochemicals. NE-PER nuclear and cytoplasmic extraction reagents were purchased from Pierce Biotechnology. Anti-ER-α, anti-PR-A, anti-PR-B, anti-VDR, anti-retinoid X receptor (RXR)-α, anti-poly (ADP-ribose) polymerase (PARP), and anti-rho GDP-dissociation inhibitor (RhoGDI) antibodies were purchased from Santa Cruz Biotechnology. CY3- and FITC-conjugated secondary antibodies were purchased from Jackson ImmunoResearch Laboratories, Inc. Anti-SRC1, anti-SRC2, and anti-SRC3 antibodies were purchased from Thermo Scientific.
Protein extraction from human tissue samples
Human UFs and the adjacent myometrium tissue samples were collected from consenting individuals undergoing surgery (hysterectomy or myomectomy) for UF removal (abdominal, vaginal, and laparoscopic). Tissue samples were collected at various locations within Texas and Tennessee under approved Institutional Review Board protocols (Protocol no. 090630WJR246 11). Methodology for the extraction of proteins from stored tissue samples has been previously described (19). Using Western blot analyses, protein lysates were examined for the expression of ER-α, PR-A, PR-B, VDR, and members of the steroid receptor coactivator (SRC) family.
Cell proliferation assay
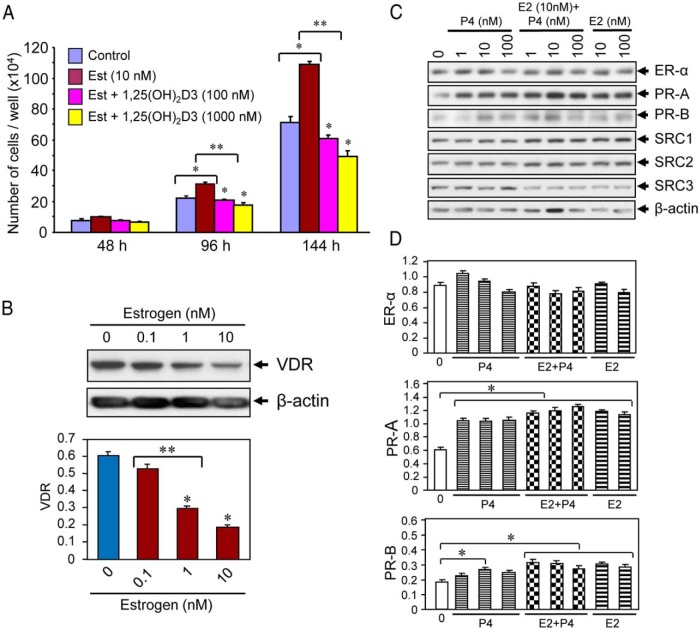
Cell counts were conducted to determine the effect of 1,25(OH)2D3 on estrogen-induced proliferation of HuLM cells. HuLM cells were seeded onto 12-well tissue culture plates (Becton Dickinson) and treated with estrogen in the absence or presence of increasing doses of 1,25(OH)2D3 at different time points; then cells were counted at each time point as described in the figure legend (see Figure 2A). Averaged cell numbers from triplicate wells were used in preparing the graph. Each data point represents the mean ± SD of triplicate wells (n = 3).
Figure 2.
Effect of 1,25(OH)2D3 on estrogen-induced proliferation of cultured human UF cells. A, HuLM cells (5000 cells/well) in 12-well plates were cultured in phenol free smooth muscle basal medium containing 5% fetal bovine serum. Cells were treated with 10 nm estrogen with or without varying doses of 1,25(OH)2D3 (100 and 1000 nm) for 48, 96, and 144 hours. Cultures were replenished every other day with fresh conditioned media. Cells were trypsinized at each time point and counted using cell-counting chamber slides. Each data point is the mean ± SD of triplicate wells (n = 3). *, P < .05 as compared to the corresponding control. **, P < .05 as compared to treatment points. B, Estrogen treatment reduced the levels of VDR in HuLM cells. HuLM cells (0.7 × 106) were cultured in 60-mm dishes, serum starved in phenol free DMEM/F12 medium, and treated with increasing doses of estrogen for 48 hours, as indicated. Equal amounts of each cell lysate were analyzed by Western blot using polyclonal anti-VDR antibody. Western blot with anti-β-actin antibody was used as the loading control. The intensity of each protein band was quantified and normalized to β-actin, and relative values were used to generate graphic data. *, P < .05 compared to corresponding control. **, P < .05 indicates the significance between consecutive doses. C, Estrogen or progesterone treatment induced PRs in HuLM cells. HuLM cells were cultured and serum starved as described above. Cells were treated with different doses of estrogen or progesterone or in combination (estrogen and progesterone together) for 48 hours as indicated. Cell lysates were analyzed by Western blot using anti-ER-α, anti-PR-A, anti-PR-B, anti-SRC1, anti-SRC2, and anti-SRC3 antibodies. Western blot with anti-β-actin antibody was used as the loading control. D, The intensities of above ER-α, PR-A, and PR-B protein bands were quantified and normalized to β-actin, and relative values were used to generate graphic data. *, P < .05 as compared to the corresponding control.
Western blot analyses
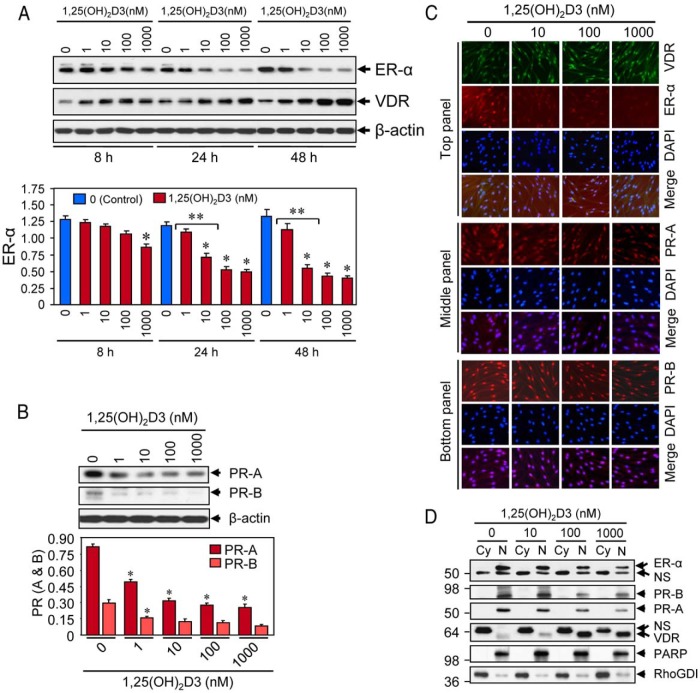
For analyses of protein expression, 0.7 × 106 HuLM cells were cultured in 60-mm tissue culture dishes, serum starved for 20 hours, and subsequently treated with increasing doses of 1,25(OH)2D3, as described in figure legends (see Figures 3, 5, and 6). Preparation of protein lysates from 1,25(OH)2D3-treated and untreated cells as well as Western blot analyses were performed as previously described (19). A 0 nm concentration of 1,25(OH)2D3 in each individual experiment served as the control.
Figure 3.
Effect of 1,25(OH)2D3 on reduction of sex steroid receptors in cultured HuLM cells. A, HuLM cells were serum starved and treated with increasing doses of 1,25(OH)2D3 (0, 1, 10, 100, and 1000 nm) for 8, 24, and 48 hours, and then protein lysates were prepared. Equal amounts of each protein lysate were analyzed by Western blot using anti-ER-α and anti-VDR antibodies. Western blot with anti-β-actin antibody was used as the loading control (top panel). The intensities of ER-α protein bands were quantified and normalized to β-actin, and relative values were used to generate graphic data (bottom panel). *, P < .05, as compared to the corresponding control. **, P < .05 indicates the significance between consecutive doses. VDR and β-actin data have been published previously (19). B, Above HuLM cell lysates were also analyzed for protein expression of PR-A and PR-B. Western blot with anti-β-actin antibody was used as the loading control (top panel). The intensity of each protein band was quantified and normalized to β-actin, as indicated above. *, P < .05 as compared to the corresponding control (bottom panel). C, Immunofluorescence analyses. HuLM cells were cultured on glass cover slips, serum starved for 20 hours, and treated with increasing doses of 1,25(OH)2D3 (0, 10, 100, and 1000 nm) for 48 hours. Cells were fixed in 3.7% formalin solution, permeabilized in 0.2% Triton X-100/PBS, and then incubated with monoclonal anti-ER-α (top panel), or polyclonal anti-VDR (top panel), or anti-PR-A (middle panel), or anti-PR-B (bottom panel) antibodies (1: 50 dilution each) followed by incubation with CY3-conjugated (red) or FITC-conjugated secondary antibodies as appropriate. Nuclei of cells were monitored by 4′,6 diamidino-2-phenylindole (DAPI) (blue). Pictures were captured at ×200 magnification. D, HuLM cells were serum starved and treated with increasing doses of 1,25(OH)2D3 (0, 10, 100, and 1000 nm) for 48 hours. Cytoplasmic (Cy) and nuclear (N) fractions were prepared from 1,25(OH)2D3-treated cells. Equal amounts of each fraction (15 μg) were subjected to Western blot analyses using anti-ER-α, anti-PR-A, anti-PR-B, and anti-VDR antibodies. Western blot with anti-PARP (a nuclear protein) and anti-RhoGDI (a cytoplasmic protein) antibodies were used to ensure the extraction efficiency. The expression of PARP was almost exclusively localized in the nuclear fractions, whereas the expression of RhoGDI was almost exclusively localized in the cytosolic fractions. NS, nonspecific.
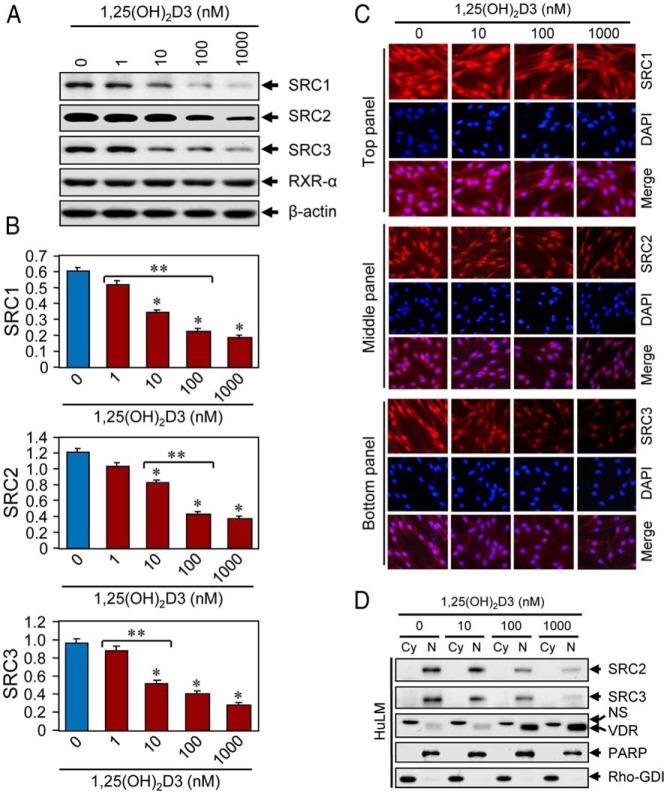
Figure 5.
Effects of 1,25(OH)2D3 on expression of SRC transcriptional coregulators in cultured HuLM cells. A, HuLM cells were serum starved and treated with increasing doses of 1,25(OH)2D3 (0, 1, 10, 100, and 1000 nm) for 48 hours, as described above. Protein lysates prepared from these treated cells were analyzed by Western blot using anti-SRC1, anti-SRC2, anti-SRC3, and anti-RXR-α antibodies. Western blot with anti-β-actin antibody was used as the loading control. B, The intensities of the above SRC1, SRC2, and SRC3 protein bands were quantified and normalized to β-actin, and relative values were used to generate graphic data. *, P < .05, compared to corresponding control. **, P < .05 indicates significance between consecutive doses. C, Immunofluorescence analyses. HuLM cells were cultured on glass cover slips, serum starved for 20 hours, and subsequently treated with increasing doses of 1,25(OH)2D3 (0, 10, 100, and 1000 nm) for 48 hours. Cells were fixed, permeabilized, and then incubated with anti-SRC1 (top panel), anti-SRC2 (middle panel), and anti-SRC3 (bottom panel) antibodies, followed by incubation with CY3-conjugated (red) secondary antibodies as appropriate. Cellular nuclei were monitored by DAPI (blue). Pictures were captured at ×200 magnification. D, HuLM cells were treated with increasing doses of 1,25(OH)2D3 (0, 10, 100, and 1000 nm) for 48 hours. Cytoplasmic (Cy) and nuclear (N) fractions were prepared as described in Figure 3 legend. Equal amounts of each fraction (15 μg) were subjected to Western blot analyses using anti-SRC2, anti-SRC3, and anti-VDR antibodies. Western blot with anti-PARP and anti-RhoGDI antibodies were used to ensure extraction efficiency. NS, nonspecific.
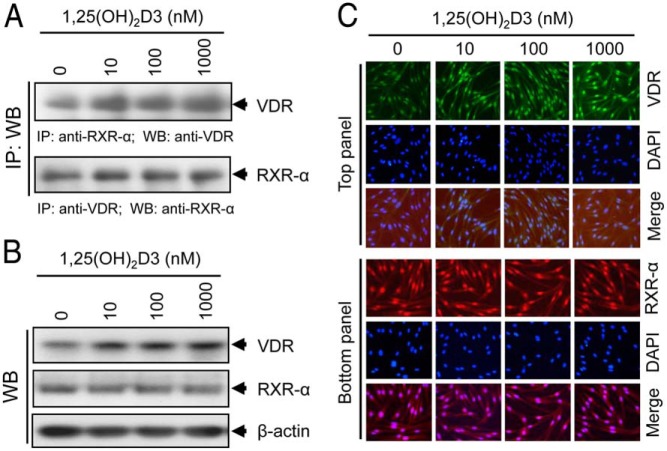
Figure 6.
Effect of 1,25(OH)2D3 on VDR-RXR-α complex formation in cultured HuLM cells. A, HuLM cells were serum starved for 20 hours and treated with increasing doses of 1,25(OH)2D3 (0, 10, 100, and 1000 nm) for 48 hours. Cell lysates prepared from these 1,25(OH)2D3-treated cells were described in Materials and Methods. Cell lysates (1 mg) from each treatment condition were subjected to immunoprecipitation with polyclonal anti-RXR-α (top panel) or anti-VDR (bottom panel) antibodies, and immunoprecipitates were analyzed by Western blot using anti-VDR (top panel) or anti-RXR-α (bottom panel) antibodies. B, The above cell lysates were used to verify the expression of both VDR and RXR-α by Western blot analyses. C, Immunofluorescence analyses were performed using HuLM cells treated with increasing doses of 1,25(OH)2D3 for 48 hours, as described above. Cells were fixed, permeabilized, and then incubated with anti-VDR, and anti-RXR-α antibodies, followed by incubation with CY3-conjugated (red) or FITC-conjugated (green) secondary antibodies, as appropriate. Nuclei of cells were monitored by DAPI (blue). Pictures were captured at ×200 magnification.
Coimmunoprecipitation (Co-IP) analyses
To determine the physical interaction between VDR and RXR-α in HuLM cells, we performed a Co-IP assay as previously described (15). We seeded 2 × 106 HuLM cells in 10-cm tissue culture dishes and incubated them overnight. Cells were preincubated in serum-free medium for 20 hours and treated with increasing doses of 1,25(OH)2D3 for 48 hours; cell lysates were prepared as previously described (15). For Co-IP assay, equal amounts (1 mg) of clear lysates were incubated with 2.5 μg polyclonal anti-RXR-α antibody for 2.5 hours at 4°C, followed by incubation with 25 μL of protein G-Sepharose for an additional hour. Immunoprecipitates were assayed by Western blot analyses using polyclonal anti-VDR antibody. Similarly, Co-IP was performed with anti-VDR antibody, and then Western blot analyses were performed with anti-RXR-α antibody. Western blot analyses were also performed to verify expression of VDR and RXR-α in the above lysates.
Nuclear and cytoplasmic fractions
HuLM cells (2 × 106) were seeded in 10-cm tissue culture dishes and incubated overnight. Cells were preincubated in serum-free medium for 20 hours and treated with increasing doses of 1,25(OH)2D3 for 48 hours. Thereafter, nuclear and cytoplasmic fractions were prepared from pelleted cells using NE-PER nuclear and cytoplasmic extraction reagents; 15 μg of each cytoplasmic and nuclear fraction was subjected to Western blot analyses using antibodies as indicated in figure legends (see Figures 3D and 5D).
Immunofluorescence analyses
Immunofluorescence analyses were conducted as previously described (15). Briefly, HuLM cells were cultured, serum starved, and subsequently treated with 1,25(OH)2D3 for 48 hours. After fixation/permeabilization steps, cells were incubated with either mouse monoclonal or rabbit polyclonal primary antibodies for 1 hour at room temperature, followed by an additional 1-hour incubation with CY3- or FITC-conjugated mouse monoclonal or rabbit polyclonal secondary antibodies. Fluorescent images were taken using an Axiovert 100 M inverted microscope (Nikon TE2000-E fluorescence microscope). Signal intensities were visually compared between untreated control and 1,25(OH)2D3-treated cells.
Statistical analysis
Student's t test was used in assessing significant differences in the expression levels of ER-α, PR-A, and PR-B in human UF vs adjacent myometrium samples. Student's t test was also used to assess any significant differences between untreated control vs 1,25(OH)2D3-treated data points and within the 1,25(OH)2D3-treated data points. Values were considered statistically significant at a 95% confidence level when P < .05. Data were presented as the mean ± SD.
Results
Up-regulation of ER-α and PRs correlated with reduced levels of VDR in human UF tumors
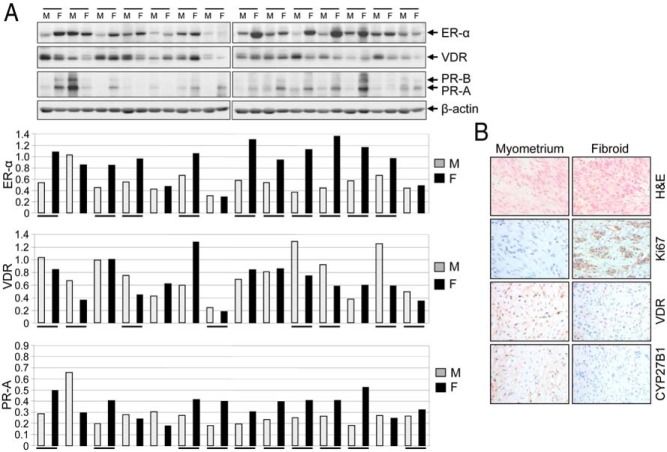
To determine whether up-regulation of sex steroid receptors correlates with lower levels of VDR in human UFs, we performed Western blot analyses using protein lysates prepared from several independent human UF lesions and adjacent myometrium. Our results demonstrated that at least 70% of UF lesions (10 of 14 patients) expressed an induced level of ER-α. Similarly, expression levels of PR-A and PR-B were also up-regulated in UFs (Figure 1A). We observed that VDR expression was lower in 60% of cases (eight of 14) in UFs compared to adjacent myometrium. Correlation between up-regulation of ER-α and reduced expression of VDR was observed in five of 10 cases of UF (Figure 1A). To further verify whether human UF expressed deregulated CYP27B1 (an enzyme responsible for synthesizing the active form of vitamin D3), we applied immunohistochemical analyses. We found lower levels of both CYP27B1 and VDR in UF when compared with the adjacent myometrium (Figure 1B), suggesting that both vitamin D synthesis and signaling apparatus are diminished in UF. Thus, the above results suggest an inverse correlation between higher levels of ER-α, PR-A, and PR-B and lower levels of VDR in human UF.
Figure 1.
An association between higher levels of ER-α, PR-A, and PR-B and reduced levels of VDR in human UFs. A, Protein lysates were prepared from UF (F; n = 14) and adjacent myometrium (M). Equal amounts of each cell lysate (30 μg) were analyzed by Western blot for expression of ER-α, PR-A, PR-B, and VDR (top panel). Specific protein bands were quantified and normalized to β-actin, and relative values were used to generate data graphs (bottom panels). Underlining indicates UF showing up-regulation of ER-α, PR-A, and PR-B and down-regulation of VDR as compared to the adjacent myometrium. VDR and β-actin data have been published previously (19). B, Immunohistochemical analyses of a representative fibroid tumor shows reduced levels of both VDR and CYP27 as compared to adjacent myometrium. Hematoxylin and eosin (H&E) staining of UF shows tumor architecture. UF expressed higher levels of cell proliferation marker Ki67 compared to adjacent myometrium.
1,25(OH)2D3 inhibits estrogen-induced proliferation of cultured HuLM cells
To determine whether estrogen can induce proliferation of HuLM cells, whereas treatment with 1,25(OH)2D3 suppresses estrogen-induced proliferation, we performed a cell proliferation assay. We found that estrogen at 10 nm concentration slightly induced HuLM cell proliferation at 48 hours and that induction was further enhanced in a time-dependent manner. Estrogen significantly induced the proliferation of HuLM cells at 96 hours (1.3-fold; P < .05) and 144 hours (1.5-fold; P < .05) (Figure 2A). On the other hand, 1,25(OH)2D3 significantly inhibited estrogen-induced proliferation of HuLM cells in a dose-dependent manner (Figure 2A; P < .05). To examine whether estrogen induces HuLM cell proliferation by affecting the expression of VDR, we performed Western blot analyses (Figure 2B). We found that estrogen reduced the expression of VDR in a dose-dependent manner in HuLM cells (Figure 2B). We further observed that estrogen or progesterone treatment affects the expression of steroid receptors by performing Western blot analyses. We found that either estrogen or progesterone significantly induced the levels of PR-A and PR-B in HuLM cells, whereas the levels of ER-α did not show significant changes (Figure 2, C and D). Additionally, the levels of SRC1, SRC2, and SRC3 were not affected by estrogen or progesterone (Figure 2C). These results suggest that estrogen induces growth of HuLM cells, whereas 1,25(OH)2D3 function by inhibiting the growth-promoting effects of estrogen on cultured HuLM cells.
1,25(OH)2D3 treatment reduces the expression of ER-α, PR-A, and PR-B proteins in cultured HuLM cells
To examine whether 1,25(OH)2D3 reduces the expression of sex steroid hormone receptors, we performed Western blot analyses. We found that 1,25(OH)2D3 reduced the levels of ER-α in a dose- and time-dependent manner when compared with untreated controls (Figure 3A). 1,25(OH)2D3 at 10 nm concentration significantly reduced the levels of ER-α protein, which was further reduced by higher concentrations of 1,25(OH)2D3 (Figure 3A). On the other hand, 1,25(OH)2D3 induced the levels of VDR in a dose- and time-dependent manner in HuLM cells. In parallel, we also found that 1,25(OH)2D3 at 10 nm concentration significantly reduced levels of PR-A and PR-B in HuLM cells, which was also further reduced by higher concentrations (Figure 3B). Using immunofluorescence analyses, we further observed that HuLM cells showed clear structural smooth muscle F-actin staining, whereas 1,25(OH)2D3 reduced staining in a dose-dependent manner (Supplemental Figure 1). Using immunofluorescence analyses, we found that ER-α, PR-A, and PR-B staining signals (red) were primarily localized in the nuclei of HuLM cells (Figure 3C). Treatment of 1,25(OH)2D3 at 10 nm concentration notably reduced nuclear staining, which was further reduced by higher concentrations (Figure 3C). On the other hand, the staining signals for VDR (green) were also present in the nuclei of HuLM cells, whereas 1,25(OH)2D3 induced nuclear VDR staining in a dose-dependent manner (Figure 3C, top panel). With Western blot analyses using cytoplasmic and nuclear fractions, we further confirmed the presence of ER-α, PR-A, and PR-B in the nuclear fractions, whereas 1,25(OH)2D3 decreased those nuclear receptors in a dose-dependent manner (Figure 3D). On the other hand, 1,25(OH)2D3 induced the levels of VDR in nuclear fractions in a dose-dependent manner in HuLM cells (Figure 3D). To further mimic these findings in human UFs, we performed similar immunofluorescence and Western blot analyses and showed that 1,25(OH)2D3 induced nuclear VDR and reduced nuclear ER-α in primary fibroid cells (Supplemental Figure 2). These results suggest that 1,25(OH)2D3 reduces the expression of nuclear ER-α, PR-A, and PR-B in cultured HuLM cells.
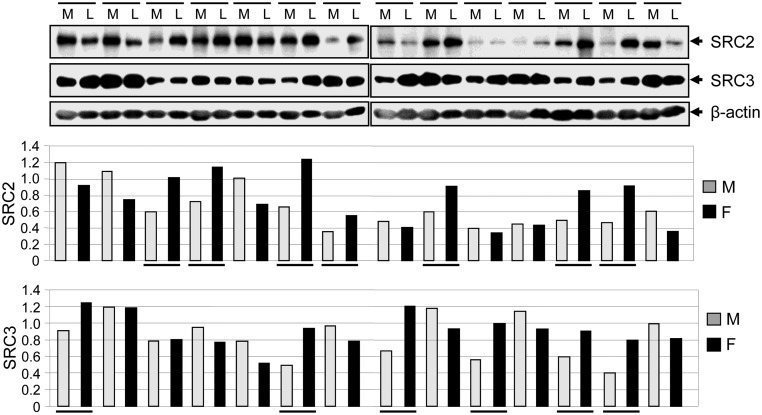
Human UFs express higher levels of sex SRC family proteins, SRC2 and SRC3, when compared with adjacent myometrium
To test whether SRCs are up-regulated in human UFs, we performed Western blot analyses using protein samples isolated from human UFs and their adjacent myometrium. We found that SRC2 was overexpressed in 50% of the cases (seven of 14) of UFs, whereas the SRC3 was overexpressed in 40% of cases (six of 14) as compared with adjacent myometrium (Figure 4). These results indicate that human UFs express higher levels of SRC2 and SRC3 proteins and that up-regulation may have an impact in the tumorigenicity of UFs.
Figure 4.
Human UF expressed higher levels of SRCs as compared with the adjacent myometrium. Protein lysates were prepared from human UF or leiomyoma (L; n = 14) and adjacent myometrium (M). Equal amounts of each protein lysate were assayed for the expression of SRC2 and SRC3 using Western blot analyses (top panel). The intensity of each protein band was quantified and normalized to β-actin, and then relative values were used to generate graphic data (bottom panels). Underlining indicates UF showing up-regulation of SRC2 and SRC3 as compared to adjacent myometrium.
1,25(OH)2D3 reduces the expression of SRC family proteins in cultured HuLM cells
To examine the effect of 1,25(OH)2D3 on the expression of SRCs, we first performed Western blot analyses. We found that at 10 nm concentration, 1,25(OH)2D3 significantly reduced the levels of SRC1, SRC2, and SRC3, which were further reduced in a dose-dependent manner (Figure 5, A and B; P < .05). Treatment of 1,25(OH)2D3 did not affect the expression of RXR-α in HuLM cells (Figure 5A). Next, to examine the effect of 1,25(OH)2D3 on expression and subcellular localization of SRCs in HuLM cells, we performed immunofluorescence analyses. We found that staining signals for both SRC2 and SRC3 (red) were mainly localized in the nuclei of HuLM cells, whereas 1,25(OH)2D3 reduced those staining signals in a dose-dependent manner (Figure 5C, middle and bottom panels). In contrast, staining signals for SRC1 (red) were somewhat localized in both chambers with more intensity in the nuclei and wherein signals were also reduced by 1,25(OH)2D3 in a dose-dependent manner (Figure 5C, top panel). We further quantitatively determined the effects of 1,25(OH)2D3 on nuclear SRC2 and SRC3 by performing Western blot analysis using cytoplasmic and nuclear fractions, as described in Figure 3D. We found that both SRC2 and SRC3 were present in the nuclei of HuLM cells, whereas 1,25(OH)2D3 reduced the levels of those proteins in a dose-dependent manner (Figure 5D). As expected, we noticed that 1,25(OH)2D3 consistently induced the levels of VDR in a dose-dependent manner in HuLM cells (Figure 5D). To further mimic these findings in human UFs, we performed similar immunofluorescence and Western blot analyses and showed that 1,25(OH)2D3 reduced SRC2 and SRC3 in primary fibroid cells (Supplemental Figure 2). These results suggest that 1,25(OH)2D3 reduces the expression levels of nuclear SRC family members in cultured human HuLM cells.
1,25(OH)2D3 treatment induces physical association between VDR and RXR-α in cultured HuLM cells
To determine whether VDR and RXR-α interact to form VDR-RXR-α complex in HuLM cells, we performed a Co-IP assay. Using anti-RXR-α antibody for Co-IP, the presence of VDR in the complex was verified by Western blot with anti-VDR antibody. We found that VDR was present in the complex, whereas 1,25(OH)2D3 induced that interaction as determined by the increased level of VDR in the complex (Figure 6A, top panel). Alternatively, using anti-VDR antibody for Co-IP, the presence of RXR-α protein in the complex was verified by Western blot with anti-RXR-α antibody. Unlike the VDR findings above, we found that similar levels of RXR-α were present in the complex, and such levels were not altered by 1,25(OH)2D3 (Figure 6A, bottom panel). Consistent with Western blot data, our immunofluorescence analyses also confirmed that 1,25(OH)2D3 induced the expression levels of VDR (green, top panel) in HuLM cells, whereas the expression levels of nuclear RXR-α (red, bottom panel) were unaffected by 1,25(OH)2D3 (Figure 6C). These results suggest that VDR and RXR-α physically interact to form the VDR-RXR-α complex, which is further induced by 1,25(OH)2D3 treatment in HuLM cells.
Discussion
The dysregulation of steroid hormones and their receptors is thought to be a primary factor for UF growth (33). Although few reports have shown minimum differences in ER and PR mRNA levels between UFs, the vast majority of previous articles have demonstrated an increased level of ER-α and PR-A/B in fibroid lesions as compared to adjacent myometrium (34–37). These discordant data may reflect intrinsic tumor-to-tumor variations that are unavoidable and, indeed, expected in such a common disease and may reflect some anthropometric factors (weight, race, age, etc), environmental influences (diet, exposure, etc), or genetics, eg, mutation status of MED12, HMGA2, epigenetic, or other factors yet to be discovered (38–40). Our group, along with several others, has previously shown that both 25-hydroxyvitamin D3 deficiency and 1,25(OH)2D3 deficiency are risk factors for the occurrence of UFs (9–11). We have also demonstrated that fibroid tumors express reduced levels of VDR as compared with the adjacent myometrium (19). These findings suggest that both 1,25(OH)2D3 deficiency and reduced expression of VDR, and therefore cumulative attenuation of vitamin D signaling, may be contributing factors in UF pathogenesis.
To date, no study has examined the correlation of ER-α, PR-A, and PR-B with VDR in human UFs. Moreover, the effects of 1,25(OH)2D3 on these nuclear receptors in human UF cells has not yet been determined. Herein, we showed higher levels of ER-α in 70% of cases of UFs as compared with adjacent myometrium (Figure 1). We also observed that these specific fibroid tumors expressed higher levels of ER-α, PR-A, and PR-B; and concurrently expressed reduced levels of VDR. Published studies have demonstrated the up-regulation of progesterone receptors (PR-A and PR-B) in UF as compared to adjacent myometrium (41, 42). Consistent with previous findings, we also observed up-regulation of PR-A and PR-B in UF as compared to adjacent myometrium, and this up-regulation was associated with reduced levels of VDR as shown in Figure 1. A recent study has demonstrated that deregulation of VDR and CYP27B1 occurs during breast cancer development and contributes to abrogation of tumor suppressive effects triggered by vitamin D3 (43). Our results in Figure 1B, show reduced levels of CYP27B1 and VDR in UF and further confirm earlier VDR findings by Western blot analyses, suggesting overall cumulative attenuated vitamin D signaling in fibroid tumor lesions. Therefore, it is possible that higher estrogenic function may suppress 1,25(OH)2D3 function by both reducing the synthesis of bioactive vitamin D3 and limiting available VDR in UF cells.
A number of studies have shown that estrogen is a major player in the growth of UFs. We first confirmed that estrogen induces the proliferation of HuLM cells in a dose- and time-dependent manner (Figure 2A). Interestingly, we found that 1,25(OH)2D3 effectively suppressed estrogen-induced proliferation of HuLM cells (Figure 2A). We also observed that estrogen inhibits VDR, whereas it induced the expression of PRs in HuLM cells (Figure 2B, C, and D). Importantly, these results demonstrate that estrogen induces the proliferation of HuLM cells by inducing PRs and by inhibiting VDR, whereas 1,25(OH)2D3 functions as an antiestrogenic agent in these cells.
We further examined the effect of 1,25(OH)2D3 on the expression of ER-α, PR-A, and PR-B in HuLM cells. Our results demonstrate that 1,25(OH)2D3 has the potential to suppress the expression of ER-α in HuLM cells (Figure 3A). In contrast, we observed that 1,25(OH)2D3 induced the expression of its own VDR in a dose- and time-dependent manner in these cells. Interestingly, we also observed that 1,25(OH)2D3 significantly reduced the expression of PR-A and PR-B in HuLM cells (Figure 3, B–D). These findings further demonstrate that 1,25(OH)2D3 has the potential to suppress nuclear ER-α, PR-A, and PR-B by inducing the nuclear VDR, which seems to function as an antiestrogen in human UF cells. Previous studies demonstrated that 1,25(OH)2D3 is capable of transcriptionally repressing ER-α gene expression, leading to a decreased estrogenic response and reduction of the proliferative stimulus for human breast tumor (44, 45). Thus, the mechanism by which 1,25(OH)2D3 regulates ER-α in HuLM cells appears to be the direct repression of ER-α expression by 1,25(OH)2D3. Our immunofluorescence and Western blot results in Figure 3, C and D, indicate that ER-α, PR-A, and PR-B are mostly localized in the nuclei of HuLM cells, whereas 1,25(OH)2D3 efficiently reduces those nuclear receptors in a dose-dependent manner. Resultant Western blot data in Figure 3D evidenced well-defined bands in the cytoplasmic fractions as well; this could represent a cytoplasmic/cell membrane version of ER-α and VDR, or it could be nonspecific banding. We are unable to differentiate between these two possibilities at this point. VDR has also been shown to present in the cytoplasmic compartment of the cell system (46). Therefore, it is possible that the cytosolic band might represent the VDR that was not affected by 1,25(OH)2D3. We also observed that 1,25(OH)2D3 consistently induced VDR in HuLM cells. These findings suggest that the induction of VDR could be the central mechanism of 1,25(OH)2D3-mediated regulation of sex steroid hormone receptors in HuLM cells, although this approach cannot delineate direct interaction. Further analysis to identify such possible interaction using chromosomal immunoprecipitation (ChIP) and ChIP-seq techniques is in progress in our laboratory.
SRCs are known to play important roles in ER-α-mediated growth of UFs (47). However, a single report has suggested that the levels of SRC mRNAs were up-regulated in 50% of UF cases (48). Similar to this report, we detected up-regulation of SRC2 (50% of cases) and SRC3 (40% of cases) by Western blot analyses in human UFs (Figure 4), suggesting that these coactivators may play important roles in UF pathogenesis. We further determined that 1,25(OH)2D3 significantly reduced SRC1, SRC2, and SRC3 in HuLM cells (Figure 5, A and B), demonstrating the potential of 1,25(OH)2D3 in the regulation of SRC family members. Our immunofluorescence and Western blot results demonstrated that SRCs were primarily localized in the nuclei of HuLM cells, whereas 1,25(OH)2D3 reduced nuclear expression in a dose-dependent manner (Figure 5, C and D). These results further demonstrate that 1,25(OH)2D3 has an important role in reducing the expression of nuclear SRC family members.
In a study conducted by Minghetti and Norman (49), it was shown that 1,25(OH)2D3 functions through its own nuclear VDR, which forms a heterodimeric VDR-RXR-α complex that binds to the vitamin D response element (VDRE) on the promoter of target genes. Studies have also demonstrated a potential negative VDRE in the proximal promoter of the ER-α gene (44, 50). We next performed a Co-IP assay to verify the physical interaction between VDR and RXR-α in HuLM cells. Our results in Figure 6A clearly demonstrate that VDR can physically interact with RXR-α in HuLM cells to form the VDR-RXR-α complex, which is further induced by 1,25(OH)2D3 due to induced levels of VDR. Thus, these results demonstrated that 1,25(OH)2D3 induced nuclear VDR and can form elevated levels of VDR-RXR-α complex, which may further bind to the VDRE on ER-α promoter and that ultimately suppress the gene expression of ER-α.
In summary, we observed an association of higher levels of ER-α, PR-A, or PR-B with reduced levels of VDR in human UFs. Estrogen suppressed the levels of VDR and induced the proliferation of HuLM cells, whereas 1,25(OH)2D3 significantly reduced the estrogen-induced proliferation of HuLM cells. Treatment of 1,25(OH)2D3 reduced the expression of nuclear ER-α, PR-A, PR-B, and the nuclear SRC family members in HuLM cells. In contrast, 1,25(OH)2D3 induced its own VDR in a dose- and time-dependent manner in HuLM cells. Moreover, we showed that VDR can form a heterodimeric VDR-RXR-α complex in HuLM cells, whereas 1,25(OH)2D3 induced that complex due to the induction of VDR, suggesting that vitamin D-mediated reduction of steroid receptor gene expression requires VDR-RXR-α complex in HuLM cells. Future research study designs will seek to determine the detailed molecular mechanisms by which 1,25(OH)2D3 regulates ER-α gene expression in human UF cells.
Acknowledgments
We thank Ms Walidah Walker, MPH, Department of Obstetrics and Gynecology at Georgia Regents University for reviewing of the manuscript.
This study was supported by a Georgia Regents University start-up package, by National Institutes of Health/Research Centers in Minority Institutions Pilot Grant 2G12RR003032-26 (to S.K.H.), and by National Institutes of Health R01 Grant 2R01HD046228-11 (to A.A.-H.).
Disclosure Summary: The authors of this paper have nothing to disclose.
Footnotes
- Co-IP
- coimmunoprecipitation
- DAPI
- 4′,6 diamidino-2-phenylindole
- ER
- estrogen receptor
- FITC
- fluorescein isothiocyanate
- HuLM
- human uterine leiomyoma
- 1,25(OH)2D3
- 1,25-dihydroxyvitamin D3
- PARP
- poly (ADP-ribose) polymerase
- PR
- progesterone receptor
- RhoGDI
- rho GDP-dissociation inhibitor
- RXR
- retinoid X receptor
- SRC
- steroid receptor coactivator
- UF
- uterine fibroid
- VDR
- vitamin D receptor
- VDRE
- vitamin D response element.
References
- 1. Walker CL, Stewart EA. Uterine fibroids: the elephant in the room. Science. 2005;308(5728):1589–1592. [DOI] [PubMed] [Google Scholar]
- 2. Marsh EE, Bulun SE. Steroid hormones and leiomyomas. Obstet Gynecol Clin North Am. 2006;33(1):59–67. [DOI] [PubMed] [Google Scholar]
- 3. Payson M, Leppert P, Segars J. Epidemiology of myomas. Obstet Gynecol Clin North Am. 2006;33(1):1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wilson EA, Yang F, Rees ED. Estradiol and progesterone binding in uterine leiomyomata and in normal uterine tissues. Obstet Gynecol. 1980;55(1):20–24. [PubMed] [Google Scholar]
- 5. Kawaguchi K, Fujii S, Konishi I, Nanbu Y, Nonogaki H, Mori T. Mitotic activity in uterine leiomyomas during the menstrual cycle. Am J Obstet Gynecol. 1989;160(3):637–641. [DOI] [PubMed] [Google Scholar]
- 6. Rein MS, Barbieri RL, Friedman AJ. Progesterone: a critical role in the pathogenesis of uterine myomas. Am J Obstet Gynecol. 1995;172:14–18. [DOI] [PubMed] [Google Scholar]
- 7. Baird DD, Dunson DB. Why is parity protective for uterine fibroids? Epidemiology. 2003;14(2):247–250. [DOI] [PubMed] [Google Scholar]
- 8. Nesby-O'Dell S, Scanlon KS, Cogswell ME, et al. Hypovitaminosis D prevalence and determinants among African American and white women of reproductive age: third National Health and Nutrition Examination Survey, 1988–1994. Am J Clin Nutr. 2002;76(1):187–192. [DOI] [PubMed] [Google Scholar]
- 9. Sabry M, Halder SK, Allah AS, Roshdy E, Rajaratnam V, Al-Hendy A. Serum vitamin D3 level inversely correlates with uterine fibroid volume in different ethnic groups: a cross-sectional observational study. Int J Womens Health. 2013;5:93–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Baird DD, Hill MC, Schectman JM, Hollis BW. Vitamin D and the risk of uterine fibroids. Epidemiology. 2013;24(3):447–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Paffoni A, Somigliana E, Vigano' P, et al. Vitamin D status in women with uterine leiomyomas. J Clin Endocrinol Metab. 2013;98(8):E1374–E1378. [DOI] [PubMed] [Google Scholar]
- 12. Haussler MR, Whitfield GK, Haussler CA, et al. The nuclear vitamin D receptor: biological and molecular regulatory properties revealed. J Bone Miner Res. 1998;13(3):325–349. [DOI] [PubMed] [Google Scholar]
- 13. Skowronski RJ, Peehl DM, Feldman D. Actions of vitamin D3, analogs on human prostate cancer cell lines: comparison with 1,25-dihydroxyvitamin D3. Endocrinology. 1995;136(1):20–26. [DOI] [PubMed] [Google Scholar]
- 14. Campbell MJ, Reddy GS, Koeffler HP. Vitamin D3 analogs and their 24-oxo metabolites equally inhibit clonal proliferation of a variety of cancer cells but have differing molecular effects. J Cell Biochem. 1997;66(3):413–425. [PubMed] [Google Scholar]
- 15. Halder SK, Goodwin JS, Al-Hendy A. 1,25-Dihydroxyvitamin D3 reduces TGF-β3-induced fibrosis-related gene expression in human uterine leiomyoma cells. J Clin Endocrinol Metab. 2011;96(4):E754–E762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Halder SK, Sharan C, Al-Hendy A. 1,25-dihydroxyvitamin D3 treatment shrinks uterine leiomyoma tumors in the Eker rat model. Biol Reprod. 2012;86(4):116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Halder SK, Sharan C, Al-Hendy O, Al-Hendy A. Paricalcitol, a vitamin D receptor activator, inhibits tumor formation in a murine model of uterine fibroids. Reprod Sci. 2014;21(9):1108–1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Halder SK, Osteen KG, Al-Hendy A. Vitamin D3 inhibits expression and activities of matrix metalloproteinase-2 and -9 in human uterine fibroid cells. Hum Reprod. 2013;28(9):2407–2416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Halder SK, Osteen KG, Al-Hendy A. 1,25-Dihydroxyvitamin D3 reduces extracellular matrix-associated protein expression in human uterine fibroid cells. Biol Reprod. 2013;89(6):150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Andersen J, DyReyes VM, Barbieri RL, Coachman DM, Miksicek RJ. Leiomyoma primary cultures have elevated transcriptional response to estrogen compared with autologous myometrial cultures. J Soc Gynecol Investig. 1995;2(3):542–551. [DOI] [PubMed] [Google Scholar]
- 21. Walker CL. Role of hormonal and reproductive factors in the etiology and treatment of uterine leiomyoma. Recent Prog Horm Res. 2002;57:277–294. [DOI] [PubMed] [Google Scholar]
- 22. Maruo T, Ohara N, Wang J, Matsuo H. Sex steroidal regulation of uterine leiomyoma growth and apoptosis. Hum Reprod Update. 2004;10(3):207–220. [DOI] [PubMed] [Google Scholar]
- 23. Nilsson S, Mäkelä S, Treuter E, et al. Mechanisms of estrogen action. Physiol Rev. 2001;81(4):1535–1565. [DOI] [PubMed] [Google Scholar]
- 24. Kushner PJ, Agard DA, Greene GL, et al. Estrogen receptor pathways to AP-1. J Steroid Biochem Mol Biol. 2000;74(5):311–317. [DOI] [PubMed] [Google Scholar]
- 25. Safe S. Transcriptional activation of genes by 17 β-estradiol through estrogen receptor-Sp1 interactions. Vitam Horm. 2001;62:231–252. [DOI] [PubMed] [Google Scholar]
- 26. Curtis SW, Washburn T, Sewall C, et al. Physiological coupling of growth factor and steroid receptor signaling pathways: estrogen receptor knockout mice lack estrogen-like response to epidermal growth factor. Proc Natl Acad Sci USA. 1996;93(22):12626–12630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Segars JH, Driggers PH. Estrogen action and cytoplasmic signaling cascades. Part I: membrane-associated signaling complexes. Trends Endocrinol Metab. 2002;13(8):349–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Levin ER. Integration of the extranuclear and nuclear actions of estrogen. Mol Endocrinol. 2005;19(8):1951–1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Mangelsdorf DJ, Thummel C, Beato M, et al. The nuclear receptor superfamily: the second decade. Cell. 1995;83(6):835–839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Simboli-Campbell M, Narvaez CJ, van Weelden K, Tenniswood M, Welsh J. Comparative effects of 1,25(OH)2D3 and EB1089 on cell cycle kinetics and apoptosis in MCF-7 breast cancer cells. Breast Cancer Res Treat. 1997;42(1):31–41. [DOI] [PubMed] [Google Scholar]
- 31. Carney SA, Tahara H, Swartz CD, et al. Immortalization of human uterine leiomyoma and myometrial cell lines after induction of telomerase activity: molecular and phenotypic characteristics. Lab Invest. 2002;82(6):719–728. [DOI] [PubMed] [Google Scholar]
- 32. Sharan C, Halder SK, Thota C, Jaleel T, Nair S, Al-Hendy A. Vitamin D inhibits proliferation of human uterine leiomyoma cells via catechol-O-methyltransferase. Fertil Steril. 2011;95(1):247–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Wei J, Chiriboga L, Mizuguchi M, Yee H, Mittal K. Expression profile of tuberin and some potential tumorigenic factors in 60 patients with uterine leiomyomata. Mod Pathol. 2005;18(2):179–188. [DOI] [PubMed] [Google Scholar]
- 34. Viville B, Charnock-Jones DS, Sharkey AM, Wetzka B, Smith SK. Distribution of the A and B forms of the progesterone receptor messenger ribonucleic acid and protein in uterine leiomyomata and adjacent myometrium. Hum Reprod. 1997;12(4):815–822. [DOI] [PubMed] [Google Scholar]
- 35. Englund K, Blanck A, Gustavsson I, et al. Sex steroid receptors in human myometrium and fibroids: changes during the menstrual cycle and gonadotropin-releasing hormone treatment. J Clin Endocrinol Metab. 1998;83(11):4092–4096. [DOI] [PubMed] [Google Scholar]
- 36. Wang L, Huang H, Liu D, et al. Evaluation of 14–3–3 protein family levels and associated receptor expression of estrogen and progesterone in human uterine leiomyomas. Gynecol Endocrinol. 2012;28(8):665–668. [DOI] [PubMed] [Google Scholar]
- 37. Shaik NA, Lone WG, Khan IA, Rao KP, Kodati VL, Hasan Q. Enhanced transcription of estrogen receptor α and mitochondrial cytochrome b genes in uterine leiomyomas. Gynecol Endocrinol. 2011;27(12):1094–1098. [DOI] [PubMed] [Google Scholar]
- 38. Bulun SE. Uterine fibroids. N Engl J Med. 2013;369(14):1344–1355. [DOI] [PubMed] [Google Scholar]
- 39. Mas A, Cervello I, Gil-Sanchis C, Simón C. Current understanding of somatic stem cells in leiomyoma formation. Fertil Steril. 2014;102(3):613–620. [DOI] [PubMed] [Google Scholar]
- 40. Mehine M, Mäkinen N, Heinonen HR, Aaltonen LA, Vahteristo P. Genomics of uterine leiomyomas: insights from high-throughput sequencing. Fertil Steril. 2014;102(3):621–629. [DOI] [PubMed] [Google Scholar]
- 41. Kim JJ, Sefton EC. The role of progesterone signaling in the pathogenesis of uterine leiomyoma. Mol Cell Endocrinol. 2012;358(2):223–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Fujimoto J, Hirose R, Ichigo S, Sakaguchi H, Li Y, Tamaya T. Expression of progesterone receptor form A and B mRNAs in uterine leiomyoma. Tumour Biol. 1998;19(2):126–131. [DOI] [PubMed] [Google Scholar]
- 43. Welsh J. Vitamin D metabolism in mammary gland and breast cancer. Mol Cell Endocrinol. 2011;347:55–60. [DOI] [PubMed] [Google Scholar]
- 44. Stoica A, Saceda M, Fakhro A, Solomon HB, Fenster BD, Martin MB. Regulation of estrogen receptor-α gene expression by 1, 25-dihydroxyvitamin D in MCF-7 cells. J Cell Biochem. 1999;75(4):640–651. [PubMed] [Google Scholar]
- 45. Swami S, Krishnan AV, Feldman D. 1α,25-Dihydroxyvitamin D3 down-regulates estrogen receptor abundance and suppresses estrogen actions in MCF-7 human breast cancer cells. Clin Cancer Res. 2000;6(8):3371–3379. [PubMed] [Google Scholar]
- 46. Peleg S, Nguyen CV. The importance of nuclear import in protection of the vitamin D receptor from polyubiquitination and proteasome-mediated degradation. J Cell Biochem. 2010;110(4):926–934. [DOI] [PubMed] [Google Scholar]
- 47. Zhou G, Hashimoto Y, Kwak I, Tsai SY, Tsai MJ. Role of the steroid receptor coactivator SRC-3 in cell growth. Mol Cell Biol. 2003;23(21):7742–7755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Hussein-Fikret S, Fuller PJ, Gargett CE. Expression of steroid receptor coactivators in cultured cells from paired myometrial and fibroid tissues. J Soc Gynecol Investig. 2005;12(6):445–451. [DOI] [PubMed] [Google Scholar]
- 49. Minghetti PP, Norman AW. 1,25(OH)2-vitamin D3 receptors: gene regulation and genetic circuitry. FASEB J. 1988;2(15):3043–3053. [DOI] [PubMed] [Google Scholar]
- 50. Li X, Wong J, Tsai SY, Tsai MJ, O'Malley BW. Progesterone and glucocorticoid receptors recruit distinct coactivator complexes and promote distinct patterns of local chromatin modification. Mol Cell Biol. 2003;23(11):3763–3773. [DOI] [PMC free article] [PubMed] [Google Scholar]