Abstract
Context:
Gestational diabetes (GDM) confers a high risk of type 2 diabetes. In the Diabetes Prevention Program (DPP), intensive lifestyle (ILS) and metformin prevented or delayed diabetes in women with a history of GDM.
Objective:
The objective of the study was to evaluate the impact of ILS and metformin intervention over 10 years in women with and without a history of GDM in the DPP/Diabetes Prevention Program Outcomes Study.
Design:
This was a randomized controlled clinical trial with an observational follow-up.
Setting:
The study was conducted at 27 clinical centers.
Participants:
Three hundred fifty women with a history of GDM and 1416 women with previous live births but no history of GDM participated in the study. The participants had an elevated body mass index and fasting glucose and impaired glucose tolerance at study entry.
Interventions:
Interventions included placebo, ILS, or metformin.
Outcomes Measure:
Outcomes measure was diabetes mellitus.
Results:
Over 10 years, women with a history of GDM assigned to placebo had a 48% higher risk of developing diabetes compared with women without a history of GDM. In women with a history of GDM, ILS and metformin reduced progression to diabetes compared with placebo by 35% and 40%, respectively. Among women without a history of GDM, ILS reduced the progression to diabetes by 30%, and metformin did not reduce the progression to diabetes.
Conclusions:
Women with a history of GDM are at an increased risk of developing diabetes. In women with a history of GDM in the DPP/Diabetes Prevention Program Outcomes Study, both lifestyle and metformin were highly effective in reducing progression to diabetes during a 10-year follow-up period. Among women without a history of GDM, lifestyle but not metformin reduced progression to diabetes.
A history of gestational diabetes (GDM) confers an increased risk of developing diabetes (1–3). Recognizing this increased risk, the Diabetes Prevention Program (DPP) sought to include women with a history of GDM and successfully enrolled 350 such participants. In the first 3-year intervention period of the DPP, the rate of progression to diabetes in women with a history of GDM in the placebo group was much higher than in women without a history of GDM (15.2 vs 8.9 cases per 100 person-years, respectively), despite equivalent fasting and 2-hour postglucose load glucose levels at baseline (2). Intensive lifestyle intervention (ILS) reduced the progression to diabetes in the DPP cohort as a whole by 58% and metformin reduced progression by 31%, compared with placebo (4). In women with a history of GDM, ILS reduced the incidence of diabetes by 53% and metformin reduced the incidence by 50%. In women without GDM, ILS had a similar impact on risk reduction (49%), whereas metformin had a nonstatistically significant risk reduction of 14% compared with placebo (2).
The Diabetes Prevention Program Outcomes Study (DPPOS) is a long-term follow-up of the DPP participants to investigate whether the delay in the development of diabetes observed during DPP is sustained and to assess the long-term effects of the interventions on health. DPPOS has followed up participants for an additional 7 years, during which time the lifestyle and metformin groups were encouraged to continue those interventions, and all participants were offered group lifestyle classes (5). Herein we report the 10-year risk of diabetes in women with a history of GDM and in parous women without a history of GDM based on the original randomized interventions during the DPP.
Participants and Methods
Participants
The DPP and DPPOS design, eligibility, and baseline characteristics have been reported elsewhere (4–6). Briefly, the DPP enrolled 3234 participants with impaired glucose tolerance (IGT) and fasting blood glucose 95–125 mg/dL (≤125 mg/dL in the American Indian centers) who were at least 25 years of age and had a body mass index (BMI) of 24 kg/m2 or higher (≥22 kg/m2 in Asian-Americans). Enrollment began in July 1996 and ended in May 1999. The mean age at randomization was 51 years and mean BMI was 34 kg/m2. Sixty-eight percent were women and 45% were members of minority groups (4). The DPP protocol prespecified recruitment targets and planned hypotheses regarding women with a history of GDM. Participants were randomly assigned to one of three treatments: placebo, ILS, aiming for a 7% weight loss and 150 minutes or more of moderate-intensity physical activity per week, or 850 mg metformin twice daily. Participants assigned to placebo or metformin received standard, nonintensive, lifestyle counseling.
Mean follow-up at the end of DPP was 3.2 years. At the end of DPP, in light of the proven benefits of ILS, all participants were offered a group-implemented, 16-session lifestyle intervention during a 1-year bridge period prior to commencement of the DPPOS. In the DPPOS, all participants, including those originally assigned to placebo, were offered a lifestyle session every 3 months to reinforce weight loss and physical activity goals. Participants originally assigned to ILS were offered two additional group programs each year, each comprising four sessions. Those originally assigned to metformin received unmasked metformin at the same prior dose of 850 mg twice daily.
This analysis focuses on the 350 women with a self-reported history of GDM and the 1416 women with at least one live birth without a self-reported history of GDM (Figure 1). Of the 350 women with a history of GDM, 117 (33%) were assigned to ILS, 111 (32%) to metformin, and 122 (35%) to placebo. Among parous women without a history of GDM, 465 (33%) were assigned to ILS, 464 (33%) to metformin, and 487 (34%) to placebo. Two hundred eighty-eight women with a history of GDM (82%) and 1226 women without a history of GDM (87%) (P = .04 for percentage follow-up in each group, χ2 test) enrolled in the observational DPPOS for a median additional follow-up of 6 years.
Figure 1.
Disposition of parous women with at least one live birth, categorized by history of GDM or no history of GDM, in DPP/DPPOS.
Data and measurements
Outcome assessments during the DPPOS continued at the same 6-month intervals as in the DPP, with primary outcome assessments consisting of fasting plasma glucose levels measured every 6 months and 2-hour, 75-g oral glucose tolerance tests conducted yearly. Diagnosis of diabetes was based on the American Diabetes Association criteria of fasting plasma glucose of 126 mg/dL or greater or 2-hour plasma glucose of 200 mg/dL or greater (7) and required confirmation within 6 weeks of the initial test (4). Additional variables assessed for this analysis included standardized weight measurements, BMI, self-reported activity levels using the Modifiable Activity Questionnaire (8), adherence to metformin therapy, glucose, glycated hemoglobin (HbA1c), and insulin levels. Measurement methods for glucose, HbA1c, and insulin have been previously published (6). Insulin secretion was estimated by the corrected insulin response: (100 − 30 min insulin)/[30 min glucose × (30 min glucose − 70 mg/dL)] (9, 10).
Statistical analysis
Baseline characteristics were described using percentages for categorical variables and means ± SD for continuous variables. Comparisons between groups were performed using the χ2 test of independence for categorical variables and the Student's t test for continuous variables.
Cox proportional hazards models were used to assess the effect of the treatment on the development of diabetes before and after adjusting for covariates (11). Analyses stratified by the reported history of GDM were performed, and a test of heterogeneity was used to determine whether the effect of the treatment varied between the subgroups based on GDM history, after adjusting for age at randomization. Because the age ranges of the women with and without a history of GDM were disparate, separate post hoc analyses were completed within age groups, with age groups defined as 25–44 years, 45–59 years, and 60 years of age or older at the time of randomization. Treatments were compared within each GDM and age subgroup using the log-rank test. Similarly, analyses stratified on treatment group were performed comparing the GDM subgroups within each treatment.
Mixed-effects models were used to estimate the mean differences over time in body weight within the different treatment groups in women with and without a history of GDM, adjusted for the baseline values (12). Generalized estimating equations were also used to assess the changes over time in the percentages of participants with adherence to medication in the placebo and metformin groups in the group of women with a history of GDM and the group of parous women with no history of GDM (13).
A value of P < .05 was considered to be statistically significant, and all tests were two sided. The Statistical Analysis Software (SAS) version 9.2 was used for all analyses (SAS Institute, Inc).
Results
Participants
There was a mean 12-year interval from the index GDM pregnancy in women with a history of GDM entering the DPP. As shown in Table 1, women with GDM were significantly younger than parous women without a history of GDM (43.0 ± 7.6 vs 51.1 ± 9.7 y, P < .001). This likely reflects recruitment strategies used for women with a history of GDM, the lack of testing for GDM during pregnancy in older women, and the fact that older women with a history of GDM may have already developed diabetes and were thus ineligible for the DPP. Participants with and without a reported history of GDM were otherwise comparable with respect to BMI, number of live births, and glycemic indices (fasting glucose, 2 h postchallenge glucose, HbA1c, fasting insulin).
Table 1.
Baseline Characteristics of Parous Women in DPP/DPPOS
| History of GDM | No History of GDM | P Value | |
|---|---|---|---|
| n | 350 | 1416 | |
| Age, y | 43.0 ± 7.6 | 51.5 ± 9.7 | <.001 |
| BMI, kg/m2 | 34.2 ± 6.2 | 34.6 ± 6.8 | .379 |
| Live births | 2.61 ± 1.32 | 2.63 ± 1.49 | .794 |
| Fasting glucose, mg/dL | 105.8 ± 8.4 | 105.2 ± 7.9 | .246 |
| Two-hour glucose, mg/dL | 165.8 ± 18.0 | 164.2 ± 17.0 | .118 |
| HbA1c, % | 5.87 ± 0.50 | 5.91 ± 0.49 | .105 |
| Fasting insulin, μU/mL | 26.7 ± 14.5 | 26.3 ± 14.2 | .616 |
| Corrected insulin response | 0.61 ± 0.40 | 0.66 ± 0.42 | .065 |
| Ethnicity, n, (%) | .110 | ||
| Caucasian | 189 (54) | 715 (50) | |
| African American | 63 (18) | 322 (23) | |
| Hispanic | 54 (16) | 238 (17) | |
| American Indian | 36 (10) | 102 (7) | |
| Asian American | 8 (2) | 39 (3) |
Results are presented as mean (SD) or n (percentage).
Participant disposition and treatment exposure are outlined in Figure 1. Of the 350 participants with a history of GDM who enrolled in the DPP, 288 continued in the DPPOS. Of the 1416 parous women without a history of GDM, 1226 participated in the follow-up study. Women who consented to participate in the follow-up study were slightly older (50.3 y vs 46.7, P < .001) and with slightly higher HbA1c values (5.92% vs 5.82% at baseline, P = .006) than those who did not consent to participate in the DPPOS.
Cumulative incidence of diabetes during a 10-year follow-up period
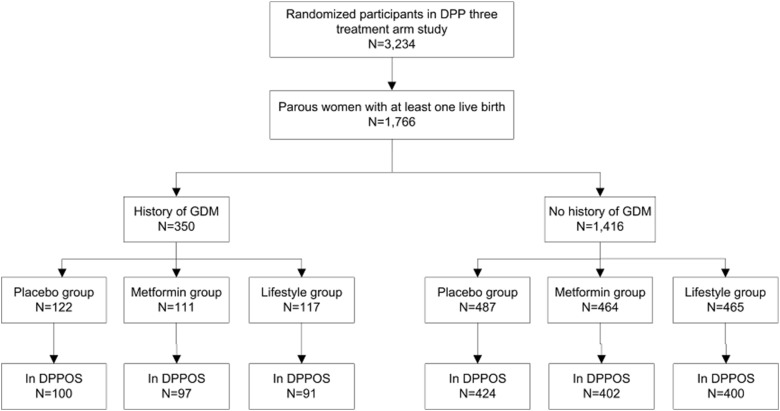
Figure 2 illustrates the cumulative incidence of diabetes by study arm in women with (Figure 2A) and without a history of GDM (Figure 2B) during the study period. Women assigned to placebo demonstrate a significantly greater risk of developing diabetes compared with the other two groups. The incidence of diabetes during the 10-year follow-up period was 11.4 per 100 person-years for women with a history of GDM compared with 6.9 per 100 person-years for women without a history of GDM among placebo participants, reflecting cumulative incidence rates of 64.7% and 49.9%, respectively. Adjusted for age at randomization using a Cox proportional hazards model, women with a history of GDM had a 48% increased risk of developing diabetes compared with women without a history of GDM in the placebo group (P < .05).
Figure 2.
Cumulative incidence of diabetes in women with a history of GDM (n = 350) (A) and parous women without a history of GDM (B) (n = 1416) during the 10-year study period.
Table 2 summarizes the age-adjusted risk reductions among parous women with and without a history of GDM over the 10 years of DPP/DPPOS. Compared with placebo, both ILS and metformin were effective in reducing the progression to diabetes in women with a history of GDM, with ILS demonstrating a 35.2% risk reduction and metformin a 40.4% risk reduction. In parous women without a history of GDM, ILS was similarly effective, reducing the risk of progression to diabetes by 29.7% compared with placebo. In this group of parous women without a history of GDM, metformin produced a nonsignificant risk reduction of only 3.3% compared with placebo. This contrasts with a 40.4% risk reduction with metformin in women with a history of GDM (P = .02 for test of heterogeneity). These findings were confirmed by modeling, which demonstrated a significant interaction between treatment group and history of GDM status (P < .05).
Table 2.
Age-Adjusted Effects of Intensive Lifestyle Intervention and Metformin on Diabetes Progression in Women With or Without a History of Gestational Diabetes Within the DPP/DPPOS
| Placebo |
Metformin |
Lifestyle |
||||
|---|---|---|---|---|---|---|
| GDM | No GDM | GDM | No GDM | GDM | No GDM | |
| Incidence of diabetes (number of cases per 100 person-years)a | 11.4b | 6.9 | 6.8 | 6.7 | 7.6 | 4.9 |
| Reduction in incidence (compared with placebo)a | 40.4c | 3.3 | 35.2c | 29.7c | ||
| Number needed to treat (to prevent one case in 10 y compared with placebo)a | 7.2 | 48.8 | 11.3 | 9.9 | ||
Adjusted for age.
P < .05 compared with non-GDM group.
P < .05 compared with placebo.
Number-needed-to-treat estimations were also performed. Seven and 11 women with a history of GDM and other high-risk DPP characteristics would have to be treated with metformin and lifestyle, respectively, to prevent one case of diabetes over 10 years; similarly, 10 parous women with IGT and no history of GDM would have to be treated with lifestyle to prevent one case of diabetes over 10 years (Table 2).
Post hoc analyses
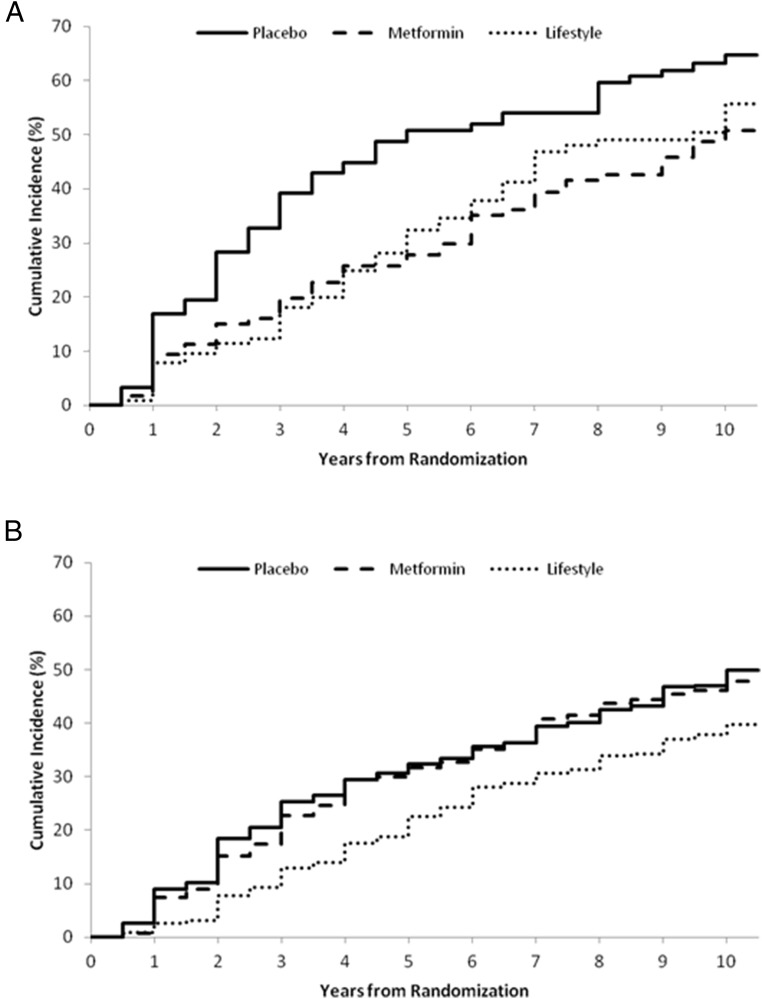
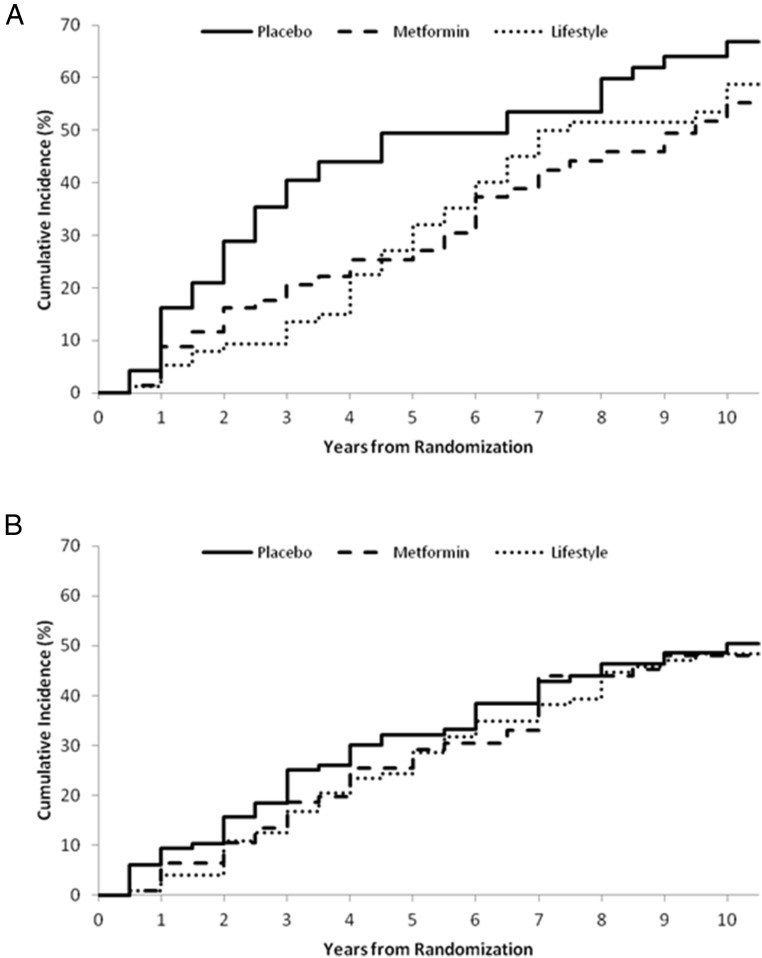
Ages of the two comparator groups, women with vs without a history of GDM, were significantly different and only minimally overlapping (mean ages 43 vs 52 y, respectively, P < .001). Post hoc analyses by age were thus performed to evaluate differences in effects of metformin in these two groups. We hypothesized that the different effects of metformin by age in the DPP cohort as a whole, with metformin being more effective in younger individuals than older individuals, could in part explain the differential effect of metformin in the two comparator groups. In women 25–44 years old at randomization with a history of GDM (n = 218), both ILS and metformin showed a positive nonsignificant pattern of reducing the risk of progression to diabetes compared with placebo [34% (95% confidence interval [CI] −5%, 58%) and 37% (95% CI:−2%, 61%), respectively]. Women 25–44 years of age without a history of GDM (n = 352) showed no pattern of response to either ILS or metformin compared with placebo [8% (95% CI −36%, 38%) vs 11% (95% CI −34%, 41%) risk reduction, respectively] (Figure 3). In women 45–59 years of age at randomization with a history of GDM (n = 121), both ILS and metformin produced similar positive, nonsignificant patterns of reduced risk of progression to diabetes compared with placebo [40% (95% CI −15%, 69%) and 44% (95% CI −7%, 71%), respectively], whereas ILS alone showed a significant reduction [34% (95% CI 13%, 50%)] in women without a history of GDM (n = 791) (Figure 4). There were too few women 60 years of age and older at baseline with a history of GDM (n = 11) to evaluate. In women in this age group without a history of GDM (n = 273), ILS reduced progression to diabetes [41% (95% CI −1%, 66%)]. However, women in this age group responded less well to metformin than placebo [risk increase of 69% (95% CI −171%, −5%)].
Figure 3.
Cumulative incidence of diabetes in women with a history of GDM ( (A) n = 218) and parous women without a history of GDM (B) (n = 352) 25–44 years of age.
Figure 4.
Cumulative incidence of diabetes in women with a history of GDM (A) (n = 121) and parous women without a history of GDM (B) (n = 791) 45–59 years of age.
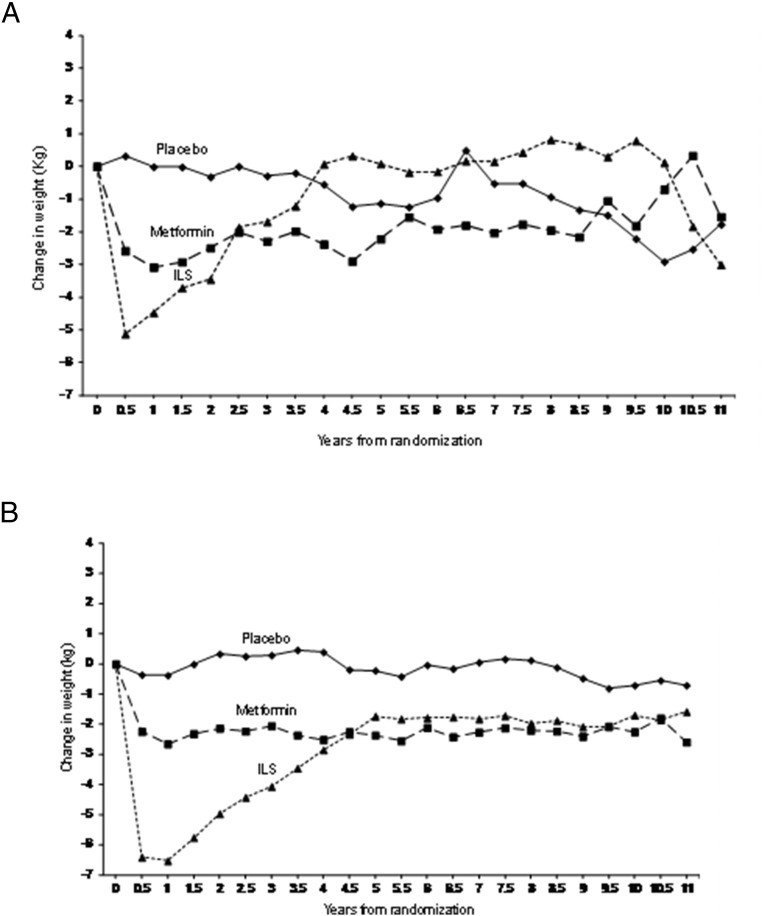
We then evaluated whether the differences in treatment effects between women with a history of GDM and women without a history of GDM were mediated by weight change or adherence to metformin. Patterns of weight change in response to the treatment interventions were similar in the two groups, with the greatest weight change seen with intensive lifestyle intervention, followed by metformin and then placebo (Figure 5). Mean weight change and mean BMI changes over time were not different for placebo or metformin groups but did show a difference in the ILS group, with non-GDM women losing more weight than women with a history of GDM (Figure 5). Adjusted compliance to metformin averaged 77% during the 10-year follow-up period and was comparable between women with or without a history of GDM. Hence, the diminished impact of intervention, particularly of metformin, in women without a history of GDM was not explained by weight change or adherence to metformin by treatment group.
Figure 5.
Change in weight over time in women with a history of GDM (n = 350) (A) and parous women without a history of GDM (B) (n = 1416) by intention to treat.
Finally, we explored corrected insulin response as a marker of endogenous insulin secretion to see whether this could potentially contribute to a difference in response to treatments between women with a history of GDM and those without a history of GDM. Evaluation of corrected insulin response showed that younger women (aged 25–44 y) without a history of GDM had the highest insulin secretion at baseline: corrected insulin response 0.78 (SD 0.50); GDM, age 25–44 years: 0.63 (SD 0.43); GDM, age 45–59 years: 0.58 (SD 0.35); GDM, age 60+ years: 0.53 (SD 0.26); no GDM, age 45–59 years: 0.64 (SD 0.39); and no GDM, age 60+ years: 0.58 (SD 0.38).
Discussion
The high risk of type 2 diabetes has been well documented in women with a history of GDM (1–3, 14). One meta-analysis estimated a relative risk of 4.69 within the first 5 years after delivery and 9.34 after 5 years in developing type 2 diabetes after GDM, compared with those with a normoglycemic pregnancy (15). Another systematic review also found a rapid increase in the cumulative incidence of diabetes occurring in the first 5 years after delivery in women with a history of GDM, with a plateau after 10 years (3).
The cohort of women with GDM enrolled in the DPP had a mean age of 43 years with a mean interval of 12 years from the index GDM pregnancy, suggesting that this study may have excluded the highest risk women with GDM who already progressed to diabetes. Furthermore, it is possible that the non-GDM group included some women with undiagnosed GDM during pregnancy because they may have delivered offspring during a time when universal testing for GDM was not standard of care. The possible exclusion of those at highest risk and the inclusion of those who perhaps had undiagnosed GDM in the non-GDM arm would have biased the results toward the null hypothesis, yet we were still able to see a substantial risk of diabetes in women with a history of GDM over the long term and a remarkable effect of the interventions.
In the initial 3-year intervention period of the DPP, women with a history of GDM had a 71% higher risk of development of diabetes compared with parous women without a history of GDM (2). Here we have shown that in the long-term follow-up of the DPP/DPPOS, in women with a history of GDM, the risk of progression to diabetes remained high (11.4%/y) through 10 years after randomization into DPP/DPPOS, compared with women without GDM whose diabetes incidence rate through 10 years was 6.9%/y. Moreover, this observational follow-up highlights the persistent long-term benefits of ILS in high-risk women with or without a history of GDM, with risk reductions of 35% and 30% seen 10 years after the initial intervention randomization, comparable with the 34% risk reduction seen in the entire DPP/DPPOS cohort at 10 years (5). The effectiveness of metformin in women with a history of GDM seen was also demonstrated in this follow-up study, with a 40% risk reduction compared with placebo. To put this into context, the overall risk reduction with metformin was 18% in the entire DPP/DPPOS cohort at 10 years, suggesting that the GDM population is a particularly high risk population amenable to metformin treatment. In addition, metformin was not effective at reducing progression to diabetes in women without GDM, similar to what was reported in the initial 3-year report of this population (2). Comparably and of note, previous analyses showed no significant effect of metformin on the development of metabolic syndrome in women while showing a significant impact in men (16).
Finally, our finding of the relative lack of effectiveness of metformin in women who did not have a history of GDM was curious. Several possible explanations were explored. First, age-based analyses showed no benefit of metformin in any age group of women without a history of GDM (25–44, 45–59, or ≥ 60 y). In fact, metformin was less effective than placebo in the oldest age group. Second, this finding was unique to women because metformin was effective in young men (17). Evaluation of endogenous insulin secretion at baseline measured using corrected insulin response suggests that younger women (aged 25–44 y) without a history of GDM had the highest insulin secretion at baseline [corrected insulin response 0.78 (SD 0.50)], suggesting potentially lower risk at baseline in this subgroup compared with the other subgroups: GDM, age 25–44 years: 0.63 (SD 0.43); GDM, age 45–59 years: 0.58 (SD 0.35); GDM, age 60+ years: 0.53 (SD 0.26); no GDM, age 45–59 years: 0.64 (SD 0.39); no GDM, age 60+ years: 0.58 (SD 0.38).
In our cohort, the risk of progression to diabetes in younger women without a history of GDM was remarkably lower compared with the study cohort as a whole and the comparison group of women with a history of GDM (Figure 2). To illustrate, the 3-, 5-, and 10-year cumulative incidence of diabetes in the placebo group in the entire DPP/DPPOS cohort was 30.3%, 38.6%, and 55.2%, respectively, and in the age 25–44 years GDM population was 40.5%, 49.4%, and 66.8%, respectively, in contrast to 25.1%, 32.2%, and 50.4%, respectively, in younger women without a history of GDM.
In summary, in the DPP/DPPOS, women with elevated BMI, elevated fasting glucose, impaired glucose tolerance, and a history of GDM had a marked increased risk of short (3 y) and long-term (10 y) development of diabetes compared with parous women without a history of GDM who entered the DPP with a similar baseline degree of glucose intolerance. Intensive lifestyle intervention or metformin was highly effective in reducing progression to diabetes during the 10-year follow-up period of the DPP/DPPOS. Interestingly, among women without a history of GDM, metformin was not effective in reducing progression to diabetes, confirming the trend noted after 3 years of follow-up.
Effects of lifestyle were also sustained during this 10-year follow-up period in women with IGT without history of GDM. We estimate that seven and 11 women with a history of GDM and other high-risk DPP characteristics would have to be treated with metformin and lifestyle, respectively, to prevent one case of diabetes over 10 years; similarly, 10 parous women with IGT and no history of GDM would have to be treated with lifestyle to prevent one case of diabetes over 10 years. We conclude that women with elevated BMI, elevated fasting glucose, impaired glucose tolerance, and a history of GDM are at increased short- and long-term risk of developing diabetes, and this risk can be reduced substantially with either lifestyle intervention or metformin.
Acknowledgments
The study had the clinical trial registration numbers of NCT00004992 (DPP) and NCT00038727 (DPPOS).
This work was supported by the National Institutes of Health, NIDDK, under U01-DK048489.
Disclosure Summary: L.D. has a financial interest in Omada Health, a company that develops online behavior-change programs, with a focus on diabetes. L.D.'s interests were reviewed and are managed by Massachusetts General Hospital and Partners HealthCare in accordance with their conflict of interest policies. The other authors have nothing to declare.
Footnotes
- BMI
- body mass index
- CI
- confidence interval
- DPP
- Diabetes Prevention Program
- DPPOS
- Diabetes Prevention Program Outcomes Study
- GDM
- gestational diabetes
- HbA1c
- glycated hemoglobin
- IGT
- impaired glucose tolerance
- ILS
- intensive lifestyle.
References
- 1. O'Sullivan JB, Mahan CM. Criteria for the oral glucose tolerance test in pregnancy. Diabetes. 1964;13:278–285. [PubMed] [Google Scholar]
- 2. Ratner RE, Christophi CA, Metzger BE, et al. Prevention of diabetes in women with a history of gestational diabetes: effects of metformin and lifestyle interventions. J Clin Endocrinol Metab. 2008;93:4774–4779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kim C, Newton KM, Knopp RH. Gestational diabetes and the incidence of type 2 diabetes: a systematic review. Diabetes Care. 2002;25:1862–1868. [DOI] [PubMed] [Google Scholar]
- 4. Knowler WC, Barrett-Connor E, Fowler SE, et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346:393–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Diabetes Prevention Program Research Group, Knowler WC, Fowler SE, et al. 10-year follow-up of diabetes incidence and weight loss in the Diabetes Prevention Program Outcomes Study. Lancet. 2009;374:1677–1686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. The Diabetes Prevention Program Research Group. The Diabetes Prevention Program: design and methods for a clinical trial in the prevention of type 2 diabetes mellitus. Diabetes Care. 1999;22:623–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Report of the Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Diabetes Care. 1997;20:1183–1197. [DOI] [PubMed] [Google Scholar]
- 8. Kriska AM, Caspersen CJ. Introduction to a collection of physical activity questionnaires. Med Sci Sports Exerc. 1997;29(suppl):S5–S9. [PubMed] [Google Scholar]
- 9. Sluiter WJ, Erkelens DW, Reitsma WD, Doorenbos H. Glucose tolerance and insulin release, a mathematical approach. I. Assay of the B-cell response after oral glucose loading. Diabetes. 1976;25:241–244. [DOI] [PubMed] [Google Scholar]
- 10. Phillips DI, Clark PM, Hales CN, Osmond C. Understanding oral glucose tolerance: comparison of glucose or insulin measurements during the oral glucose tolerance test with specific measurements of insulin resistance and insulin secretion. Diabet Med. 1994;11:286–292. [DOI] [PubMed] [Google Scholar]
- 11. Cox DR. Regression models in life-tables. J R Statistical Soc (B). 1972;34:187–220. [Google Scholar]
- 12. Diggle PJ, Liang KY, Zeger SL. Analysis of longitudinal data. Oxford, UK: Clarendon Press; 1994. [Google Scholar]
- 13. Liang KY, Zeger SL. Longitudinal data analysis using generalized linear models. Biometrika. 1986;73:13–22. [Google Scholar]
- 14. Azen SP, Peters RK, Berkowitz K, Kjos S, Xiang A, Buchanan TA. TRIPOD (TRoglitazone In the Prevention of Diabetes): a randomized, placebo-controlled trial of troglitazone in women with prior gestational diabetes mellitus. Control Clin Trials. 1998;19:217–231. [DOI] [PubMed] [Google Scholar]
- 15. Bellamy L, Casas JP, Hingorani AD, Williams D. Type 2 diabetes mellitus after gestational diabetes: a systematic review and meta-analysis. Lancet. 2009;373:1773–1779. [DOI] [PubMed] [Google Scholar]
- 16. Orchard TJ, Temprosa M, Goldberg R, et al. The effect of metformin and intensive lifestyle intervention on the metabolic syndrome: the Diabetes Prevention Program randomized trial. Ann Intern Med. 2005;142:611–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Diabetes Prevention Program Research Group. HbA1c as a predictor of diabetes and as an outcome in the Diabetes Prevention Program: a randomized clinical trial. Diabetes Care. 2015;38:51–58. [DOI] [PMC free article] [PubMed] [Google Scholar]