Abstract
Context:
Uterine leiomyoma is the most common benign tumor in reproductive-age women. Using a dye-exclusion technique, we previously identified a side population of leiomyoma cells exhibiting stem cell characteristics. However, unless mixed with mature myometrial cells, these leiomyoma side population cells did not survive or grow well in vitro or in vivo.
Objective:
The objective of this study was to identify cell surface markers to isolate leiomyoma stem/progenitor cells.
Design:
Real-time PCR screening was used to identify cell surface markers preferentially expressed in leiomyoma side population cells. In vitro colony-formation assay and in vivo tumor-regeneration assay were used to demonstrate functions of leiomyoma stem/progenitor cells.
Results:
We found significantly elevated CD49b and CD34 gene expression in side population cells compared with main population cells. Leiomyoma cells were sorted into three populations based on the expression of CD34 and CD49b: CD34+/CD49b+, CD34+/CD49b−, and CD34−/CD49b− cells, with the majority of the side population cells residing in the CD34+/CD49b+ fraction. Of these populations, CD34+/CD49b+ cells expressed the lowest levels of estrogen receptor-α, progesterone receptor, and α-smooth muscle actin, but the highest levels of KLF4, NANOG, SOX2, and OCT4, confirming their more undifferentiated status. The stemness of CD34+/CD49b+ cells was also demonstrated by their strongest in vitro colony-formation capacity and in vivo tumor-regeneration ability.
Conclusions:
CD34 and CD49b are cell surface markers that can be used to enrich a subpopulation of leiomyoma cells possessing stem/progenitor cell properties; this technique will accelerate efforts to develop new therapies for uterine leiomyoma.
Uterine leiomyomas occur in approximately 77% of women in the US and can cause severe morbidity and infertility (1). No stand-alone therapy currently exists, and all approved agents have limited efficacy and significant side effects. Consequently, leiomyomas are the leading cause of hysterectomy in the US, with an estimated total annual cost of $5.9–34.4 billion (2). The discovery of novel treatments is largely hindered by our inability to study and develop therapies targeting the unique tumor stem/progenitor cells from which leiomyoma originates.
Complex chromosomal rearrangements or single gene mutations correlate with the clonal expansion of a progenitor cell that initiates a leiomyoma (3, 4). The side population (SP) cells with stem cell-like characteristics derived from leiomyoma tissue, but not from the adjacent normal myometrium, carry MED12 mutations (5). In vivo models demonstrate that estrogen- and progesterone-dependent progression of these tumors requires multipotent somatic stem cells (5–7). Unfortunately, many fundamental questions remain unanswered regarding the properties of these SP cells due to technical difficulties in isolating them, such as high sensitivity to slight changes in staining conditions (8).
Specific cell surface markers (CSMs) could be used as an alternative approach to isolate stem/progenitor cells from normal tissues or tumors, and these markers represent potential candidate targets for novel treatments, particularly for antibody-based therapeutics (9). Stem cells isolated by antibody-based cell sorting may be used directly to test their in vivo tumorigenicity. Here, we investigated CSMs for enrichment and isolation of viable populations of leiomyoma stem cells.
Materials and Methods
Only tissue collection procedures are described here. Detailed protocols can be found in Supplemental Text. Antibodies and primer sets used in this study are listed in Supplemental Tables 1 and 2, respectively.
Tissue collection
Uterine leiomyoma tissues were obtained at surgery from premenopausal women (mean age 40 years, range 33–48) following receipt of informed consent. Northwestern University's Institutional Review Board approved the protocol for the acquisition of surgical specimens. No subject received hormonal treatment during the six months prior to surgery. The tissues were dissociated as previously described (10). All experiments were repeated using cells isolated from at least three patient samples and the exact sample numbers used in each experiment are described in the figure legends.
Results
Cell surface marker characterization of leiomyoma side and main population cells
Leiomyoma SP (LMSP) and main population (LMMP) cells were isolated as described previously (5). To screen for differentially expressed CSMs that could be used to enrich LMSP cells, we performed a CSM PCR array comparing CSM gene expression between LMSP and LMMP cells (Supplemental Table 3). Of the differentially expressed markers identified by the array, we further characterized the protein expression of blood cell lineage specific markers (CD2, CD3, CD8, CD20), recently found novel stem CSM CD49b, and mesenchymal stem CSM CD73 in LMSP and LMMP cells by flow cytometric analysis. We also checked the protein levels of the known stem cell marker CD34 and the mesenchymal lineage marker CD90, which were not included in the array. We did not detect any significant expression of the typical hematopoietic markers CD2, CD3, CD8, or CD20 in either LMSP or LMMP cells (Supplemental Figure 1A). Neither CD73 nor CD90 were differentially expressed between LMMP and LMSP cells. Interestingly, 76.7 ± 13.7% of LMSP cells expressed CD49b, compared to only 14.3 ± 1.5% of LMMP cells. CD34 was also differentially expressed: 81.3 ± 25.3% of LMSP cells vs 32.6 ± 27.3% of LMMP cells. Most importantly, most LMSP cells (91 ± 1.7%) expressed both CD34 and CD49b, compared with only 9.7 ± 2% of LMMP cells (Supplemental Figure 1B). These data suggested that CD34 and CD49b would be appropriate candidate markers to enrich LMSP cells.
CD34 and CD49b fractionate leiomyoma cells into three populations with distinct gene expression profiles
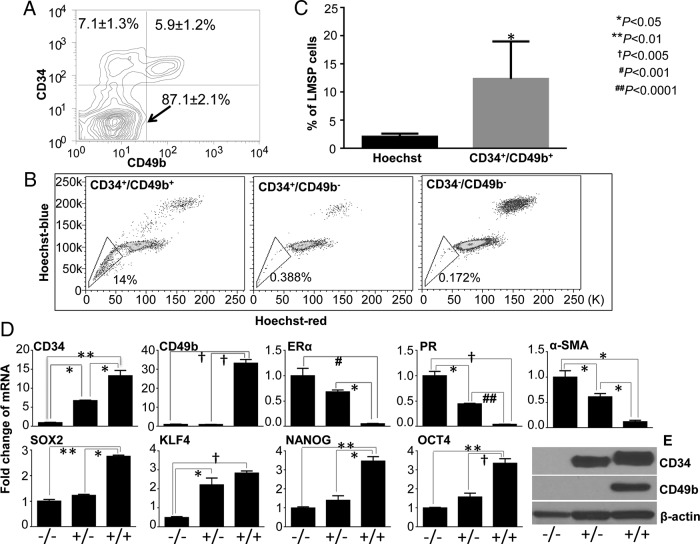
To verify whether CD34 and CD49b could be used to enrich for LMSP cells, the total leiomyoma cells were stained with anti-CD34, anti-CD49b, and Hoechst, followed by flow cytometric analysis. The differential expression of CD34 and CD49b identified three distinct cell populations: CD34+/CD49b+ (5.9 ± 1.2%), CD34+/CD49b− (7.1 ± 1.3%), and CD34−/CD49b− (87.1 ± 2.1%) (Figure 1A). We discovered that LMSP cells were almost exclusively found in the CD34+/CD49b+ population (Figure 1B), accounting for 11.85 ± 4.37% of the CD34+/CD49b+ cells (Figure 1C). Compared with the total leiomyoma cell population containing 1.66 ± 0.62% LMSP cells based on the Hoechst dye technique, CD34+/CD49b+ cells significantly enriched LMSP cells by approximately eight-fold (Figure 1C).
Figure 1.
Identification and characterization of three populations of leiomyoma cells based on the expression of cell surface markers CD34 and CD49b. (A) Differential expression of CD34 and CD49b fractionates the total leiomyoma cells into three subpopulations: CD34−/CD49b−, CD34+/CD49b−, and CD34+/CD49b+ cells. Contour plot is a representative of experiments run with cells from 8 independent patient samples. Values are expressed as mean ± SEM. (B) CD34+/CD49b+ cells were selectively enriched for LMSP cells (left). LMSP cells were rarely detected in the CD34+/CD49b− (middle) or CD34−/CD49b− (right) subpopulations. Dot plot shows one representative experiment repeated with cells from three independent subjects. (C) Percentage of LMSP cells detected by the Hoechst dye exclusion method in total leiomyoma cells and in the CD34+/CD49b+ cell population. Values are expressed as mean ± SEM for three independent subjects. (D) Quantitative real-time RT-PCR was performed to determine the mRNA levels of cell surface markers (CD34 and CD49b), stem cell factors (KLF4, NANOG, SOX2, and OCT4), steroid receptors (ERα and PR), and smooth muscle cell marker α-SMA in the three FACS-sorted subpopulations of leiomyoma cells. Graphs are means ± SEM of representative triplicate experiments, repeated using cells from 3 to 5 independent patient samples. (E) Representative western blots show differential protein expression of CD34 and CD49b between three leiomyoma cell populations. Experiments were repeated with cells from three patient samples.
Quantitative PCR confirmed that CD34+/CD49b+ cells expressed significantly higher mRNA levels of CD34 and CD49b than CD34−/CD49b− cells (Figure 1D). Interestingly, CD34 expression was markedly higher in CD34+/CD49b+ than in CD34+/CD49b− cells, although both populations were CD34-positive. These differences were recapitulated by immunoblotting analysis: the highest CD34 protein levels were observed in CD34+/CD49b+ cells and CD49b protein expression could be detected exclusively in these cells (Figure 1E). Furthermore, CD34+/CD49b+ cells expressed the lowest levels of estrogen receptor-α (ERα), progesterone receptor (PR), and α-smooth muscle actin (α-SMA), but the highest levels of stem cell factors OCT4, KLF4, SOX2, and NANOG, suggesting that this population has an immature status.
In vitro colony formation by CD34+/CD49b+ cells
Because progenitor cells are generally characterized in vitro by their colony-forming potential (11), we assessed the in vitro colony-formation capacities of the three leiomyoma cell populations. We observed that 100 cells/well produced well-separated individual colony (Supplemental Figure 2A, top row), therefore we quantified the colony formation efficiency (CFE) at this seeding density. As shown in Supplemental Figure 2B, CD34+/CD49b+ cells had the highest colony-forming capacity, with an average CFE of 8 ± 1.6%, suggesting a pronounced progenitor identity.
In vivo human leiomyoma-like tumor reconstitution from CD34+/CD49b+ cells
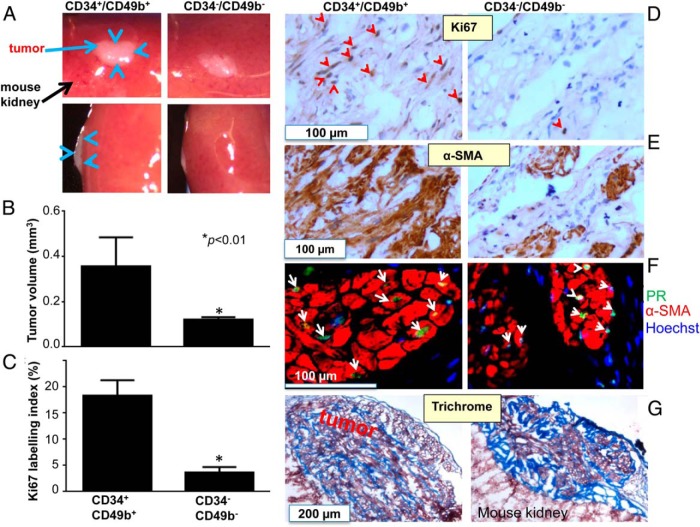
Transplantation assays are fundamental for evaluating stemness (11, 12). While most leiomyoma cells were CD34−/CD49b−, LMSP cells resided mostly in the more clonogenic, immature CD34+/CD49b+ population; thus, we focused our in vivo xenograft experiments on these two populations. As shown in Figure 2, A and B, CD34+/CD49b+ cells produced large cellular tumors (0.45 ± 0.05 mm3), while CD34−/CD49b− cells did not give rise to cellular tumors and remained as small fibrotic lesions (0.12 ± 0.01 mm3). The Ki67 index was significantly higher (6.1-fold) in tumors derived from CD34+/CD49b+ cells than those from CD34−/CD49b− cells (Figure 2, C and D). The increased Ki67 expression in the CD34+/CD49b+-reconstituted tumors was confirmed using an alternative antibody (DAKO) (Supplemental Figure 3). We also observed that most CD34+/CD49b+ regenerated tumor cells expressed PR and α-SMA at similar levels as those derived from CD34−/CD49b− cells (Figure 2, E and F). Finally, the presence of extracellular matrix was confirmed by positive Trichrome staining (Figure 2G). These data establish the functional tumor stem/progenitor characteristics of CD34+/CD49b+ cells in vivo.
Figure 2.
Regeneration of human leiomyoma-like tumors by CD34+/CD49b+ cells. (A) Macroscopic visualization of the regenerated tumor (blue arrows) 8 weeks after engrafting. (B) Quantification of tumor volume. Data shown are mean ± SEM of representative triplicate experiments, repeated using cells from three independent patient samples. (C) Quantification of the percentage of Ki67 positive cells (Ki67 labeling index) in tumors regenerated from CD34+/CD49b+ and CD34−/CD49b− cells. Values are expressed as means ± SEM of representative triplicate experiments, repeated using cells from three patient samples. (D) and (E) Representative images of immunostaining of CD34+/CD49b+ or CD34−/CD49b− cell-reconstituted leiomyoma tissues with antibodies against proliferation marker Ki67 (D) and α-SMA (E). Tissues were counterstained with hematoxylin. Red arrowheads indicate Ki67-positive cells. (F) Immunofluorescent staining of CD34+/CD49b+ or CD34−/CD49b− cell-reconstituted leiomyoma tissues with antibodies against PR (green) and α-SMA (red). Nuclei were stained with Hoechst dye (blue) and white arrowheads indicate PR-positive cells. (G) Trichrome staining to characterize expression of typical extracellular matrix proteins in regenerated tissues.
Discussion
The novel and major finding of this study was the identification of two CSMs, CD34 and CD49b, which enabled the isolation of stem/progenitor cells from human uterine leiomyoma tissue. The CD34+/CD49b+ cells expressed the highest levels of key stem cell factors, possessed the strongest in vitro colony-forming capacity, and were substantially enriched for LMSP cells, which have been reported to be tumor-initiating cells in leiomyoma (5, 6). Importantly, CD34+/CD49b+ cells had potent in vivo self-renewal and proliferative capacities, generating tumors that phenocopied patient tumors following engraftment.
This is the first report to use CD34 and CD49b in combination to isolate leiomyoma stem/progenitor cells. CD34 is a well-known marker of activated progenitor cells (13, 14). Recently, integrins garnered attention in the field of stem cell research when CD49f and CD49a were identified as novel stem cell markers (15, 16). Functionally, CD49f enhances multipotency and maintains stemness through the direct regulation of OCT4 and SOX2 (17). Moreover, CD49b has recently been discovered as a CSM to improve isolation of the primitive human hematopoietic stem cell (HSC) (18). These findings suggest that CD34 and CD49b are valid markers for identifying leiomyoma stem cells.
Because most CD49b-positive cells were also CD34-positive, adding the CD34 marker did not change much of the LMSP cells selected. Nevertheless, we suggest that the combination of CD34 and CD49b markers sorts leiomyoma cells into three populations with distinct gene expression profiles that are predictive of their differentiation status. Consistent with the known gene expression signature of LMSP cells, molecular characterization of the CD34+/CD49b+ cells indicated that they are immature cells, given the significantly reduced expression levels of the typical smooth muscle marker α-SMA and the steroid receptors ERα and PR, as well as their abundant expression of key stem cell factors OCT 4, NANOG, SOX2, and KLF4 (11, 12). Mas et al failed to find CD34-positive cells in their established LMSP cell lines. This discrepancy may be explained by the fact that they characterized CD34 expression after the LMSP cells were in vitro cultured and passaged (6).
A minimal operational definition of tumor-initiating cells is that they have tumor reconstitution ability (9). Indeed, CD34+/CD49b+ cells, without the presence of myometrial cells, could reconstitute leiomyomas in mouse kidney capsules with histological and molecular features of original tumors. One interesting conundrum we draw from this analysis is that tumor-initiating CD34+/CD49b+ cells, like previous descriptions of LMSP cells, are important and sufficient for estrogen- and progesterone-dependent tumor growth, yet they express the lowest levels of the steroid hormone receptors relative to CD34−/CD49b− or LMMP cells (5, 6). We have recently described a paracrine mode of cell-to-cell interactions within the tumors or with myometrial cells which may allow this growth (10). Alternatively, we cannot exclude potential nongenomic effects of steroid hormones on leiomyoma growth (19). We hypothesize that the ability to isolate pure and healthy populations of CD34+/CD49b+ cells will allow us to more efficiently explore the mechanisms of this paradox. Intriguingly, as CD34+/CD49b+ cells matured (or just grew) during the tumor formation assay they acquired strikingly higher levels of α-SMA and PR to levels similar to seen in CD34−/CD49b− cells. These findings suggest that the engrafted CD34+/CD49b+ cells may differentiate, initially to CD34+/CD49b− and then eventually to CD34−/CD49b− cells that express α-SMA and PR. Leiomyomas are thought to be monoclonal tumors arising from the myometrium (20). Our data suggests that the cells composing such solid tumors are not simply transformed myometrium smooth muscle cell clones, but are instead complex structures composed of the three populations based on CD34 and CD49b expression. Detailed studies are underway to investigate the potential functional hierarchy among leiomyoma cells as they progress from CD34+/CD49b+ to CD34+/CD49b− to CD34−/CD49b− cells.
Conclusion
Uterine leiomyoma CD34+/CD49b+ cells exhibit characteristics of somatic stem/progenitor cells. Understanding how to block the function of these tumor-initiating cells will reveal new and more tractable approaches to treat or even prevent uterine leiomyoma.
Acknowledgments
We thank Dr. Takeshi Kurita for technical help and discussion and Dr. Chunfa Jie for statistical analysis.
We acknowledge the Northwestern University Flow Cytometry Facility, Cell Imaging Facility, and Mouse Histology and Phenotyping Laboratory, which are co-funded by National Cancer Institute Cancer Center Grant No. CA060553.This work was supported by The National Institutes of Health Grants No. P01-057 877 (to Bulun SE), RO1HD064402 (to Kurita T), and Northwestern Memorial Foundation (to Yin P).
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- α-SMA
- α-smooth muscle actin
- CFE
- colony formation efficiency
- CSM
- cell surface markers
- HSC
- hematopoietic stem cell
- LMMP
- Leiomyoma main population
- LMSP
- Leiomyoma side population
- PR
- progesterone receptor
- SP
- side population.
References
- 1. Walker CL, Stewart EA. Uterine fibroids: the elephant in the room. Science. 2005;308:1589–1592. [DOI] [PubMed] [Google Scholar]
- 2. Cardozo ER, Clark AD, Banks NK, Henne MB, Stegmann BJ, Segars JH. The estimated annual cost of uterine leiomyomata in the United States. Am J Obstet Gynecol. 2012;206:211.e1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Mäkinen N, Mehine M, Tolvanen J, et al. MED12, the mediator complex subunit 12 gene, is mutated at high frequency in uterine leiomyomas. Science. 2011;334:252–255. [DOI] [PubMed] [Google Scholar]
- 4. Hodge JC, Morton CC. Genetic heterogeneity among uterine leiomyomata: insights into malignant progression. Hum Mol Genet. 2007;16:R7–R13. [DOI] [PubMed] [Google Scholar]
- 5. Ono M, Qiang W, Serna VA, et al. Role of stem cells in human uterine leiomyoma growth. PLoS One. 2012;7:e36935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Mas A, Cervelló I, Gil-Sanchis C, et al. Identification and characterization of the human leiomyoma side population as putative tumor-initiating cells. Fertil Steril. 2012;98:741–751.e6. [DOI] [PubMed] [Google Scholar]
- 7. Ishikawa H, Ishi K, Serna VA, Kakazu R, Bulun SE, Kurita T. Progesterone is essential for maintenance and growth of uterine leiomyoma. Endocrinology. 2010;151:2433–2442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Golebiewska A, Brons NH, Bjerkvig R, Niclou SP. Critical appraisal of the side population assay in stem cell and cancer stem cell research. Cell Stem Cell. 2011;8:136–147. [DOI] [PubMed] [Google Scholar]
- 9. Zhou BB, Zhang H, Damelin M, Geles KG, Grindley JC, Dirks PB. Tumour-initiating cells: challenges and opportunities for anticancer drug discovery. Nat Rev Drug Discov. 2009;8:806–823. [DOI] [PubMed] [Google Scholar]
- 10. Ono M, Yin P, Navarro A, et al. Paracrine activation of WNT/β-catenin pathway in uterine leiomyoma stem cells promotes tumor growth. Proc Natl Acad Sci U S A. 2013;110:17053–17058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Stingl J, Eirew P, Ricketson I, et al. Purification and unique properties of mammary epithelial stem cells. Nature. 2006;439:993–997. [DOI] [PubMed] [Google Scholar]
- 12. Shackleton M, Vaillant F, Simpson KJ, et al. Generation of a functional mammary gland from a single stem cell. Nature. 2006;439:84–88. [DOI] [PubMed] [Google Scholar]
- 13. Beauchamp JR, Heslop L, Yu DS, et al. Expression of CD34 and Myf5 defines the majority of quiescent adult skeletal muscle satellite cells. J Cell Biol. 2000;151:1221–1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Schober M, Fuchs E. Tumor-initiating stem cells of squamous cell carcinomas and their control by TGF-β and integrin/focal adhesion kinase (FAK) signaling. Proc Natl Acad Sci U S A. 2011;108:10544–10549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Notta F, Doulatov S, Laurenti E, Poeppl A, Jurisica I, Dick JE. Isolation of single human hematopoietic stem cells capable of long-term multilineage engraftment. Science. 2011;333:218–221. [DOI] [PubMed] [Google Scholar]
- 16. Deschaseaux F, Charbord P. Human marrow stromal precursors are alpha 1 integrin subunit-positive. J Cell Physiol. 2000;184:319–325. [DOI] [PubMed] [Google Scholar]
- 17. Yu KR, Yang SR, Jung JW, et al. CD49f enhances multipotency and maintains stemness through the direct regulation of OCT4 and SOX2. Stem Cells. 2012;30:876–887. [DOI] [PubMed] [Google Scholar]
- 18. Wong WM, Sigvardsson M, Astrand-Grundstrom I, et al. Expression of integrin α2 receptor in human cord blood CD34+CD38−CD90+ stem cells engrafting long-term in NOD/SCID-IL2Rγ(c) null mice. Stem Cells. 2013;31:360–371. [DOI] [PubMed] [Google Scholar]
- 19. Hoekstra AV, Sefton EC, Berry E, et al. Progestins activate the AKT pathway in leiomyoma cells and promote survival. J Clin Endocrinol Metab. 2009;94:1768–1774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Zhang P, Zhang C, Hao J, et al. Use of X-chromosome inactivation pattern to determine the clonal origins of uterine leiomyoma and leiomyosarcoma. Hum Pathol. 2006;37:1350–1356. [DOI] [PubMed] [Google Scholar]