Abstract
Context:
Activation of the melanocortin-4 receptor (MC4R) with the synthetic agonist RM-493 decreases body weight and increases energy expenditure (EE) in nonhuman primates. The effects of MC4R agonists on EE in humans have not been examined to date.
Objective, Design, and Setting:
In a randomized, double-blind, placebo-controlled, crossover study, we examined the effects of the MC4R agonist RM-493 on resting energy expenditure (REE) in obese subjects in an inpatient setting.
Study Participants and Methods:
Twelve healthy adults (6 men and 6 women) with body mass index of 35.7 ± 2.9 kg/m2 (mean ± SD) received RM-493 (1 mg/24 h) or placebo by continuous subcutaneous infusion over 72 hours, followed immediately by crossover to the alternate treatment. All subjects received a weight-maintenance diet (50% carbohydrate, 30% fat, and 20% protein) and performed 30 minutes of standardized exercise daily. Continuous EE was measured on the third treatment day in a room calorimeter, and REE in the fasting state was defined as the mean of 2 30-minute resting periods.
Results:
RM-493 increased REE vs placebo by 6.4% (95% confidence interval, 0.68–13.02%), on average by 111 kcal/24 h (95% confidence interval, 15–207 kcal, P = .03). Total daily EE trended higher, whereas the thermic effect of a test meal and exercise EE did not differ significantly. The 23-hour nonexercise respiratory quotient was lower during RM-493 treatment (0.833 ± 0.021 vs 0.848 ± 0.022, P = .02). No adverse effect on heart rate or blood pressure was observed.
Conclusions:
Short-term administration of the MC4R agonist RM-493 increases REE and shifts substrate oxidation to fat in obese individuals.
The central melanocortin system, comprising melanocortins (MCs), agouti, agouti-related proteins, and their receptors (melanocortin receptors [MCRs]), integrates neural, metabolic, and hormonal signals serving a critical role in the maintenance of body weight (1, 2). The MCs are a family of peptide hormones, including α-melanocyte-stimulating hormone (MSH), β-MSH, γ-MSH, and ACTH derived from a common precursor, pro-opiomelanocortin. Activation of MC subtype 4 receptors (MC4Rs) in the hypothalamus in animal models reduces food intake, increases energy expenditure, and chronic activation causes weight loss (3). In humans, the most frequent monogenic etiology of obesity is haploinsufficiency of the MC4R, highlighting a role for the MC4R in energy homeostasis (4). This is exemplified in the Pima Indian population that has a high prevalence of MC4R loss-of-function variants, associated with obesity, type 2 diabetes mellitus, and lower 24-hour resting and sleeping energy expenditure (5). Polymorphisms near MC4R also contribute to common obesity (6). Thus, MC4R is an attractive target for the treatment of obesity (7).
The 5 MCR subtypes have diverse expression and binding profiles, both in the central nervous system and peripherally, and play a role in the regulation of sexual function, pigmentation, inflammation, analgesia, immunomodulation, blood pressure (BP), and steroidogenesis in addition to energy homeostasis (8). Several synthetic MC4R agonists have reached clinical trials, but each had either a lack of efficacy or cardiovascular adverse effects (7). The small peptide MC4R agonist RM-493, administered by subcutaneous infusion for 8 weeks in obese rhesus macaques, decreased food intake, reduced body weight, and improved glucose tolerance (9). The initial decrease in food intake coupled with a sustained increase in EE) caused 13.5% weight loss over 8 weeks, without adverse cardiovascular effects. Whether agonists of the MC4R pathway similarly increase EE in humans is not known. Here we test whether brief administration of RM-493 increases resting energy expenditure (REE) in obese human subjects.
Subjects and Methods
Study design and study subjects
The Institutional Review Board of the National Institute of Diabetes and Digestive and Kidney Diseases approved the study protocol (ClinicalTrials.gov identifier NCT01867437). Written informed consent was obtained from all subjects. Twelve volunteers (6 men and 6 women) in general good health between the ages of 18 and 50 years with a body mass index between 30 and 40 kg/m2 were enrolled. Subjects with diabetes, hypertension, liver enzyme values of >1.5 times the upper limit of normal, thyroid dysfunction, symptomatic sleep apnea, or recent illness, pregnancy, cancer, or surgery were excluded at the initial screening visit.
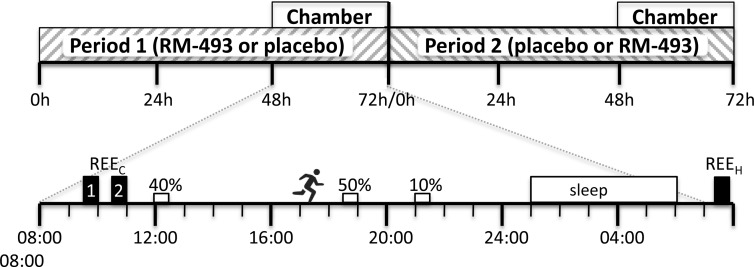
Eligible subjects were admitted to the Metabolic Clinical Research Unit at the National Institutes of Health Clinical Center for an 8-day inpatient stay (Figure 1). On the day of admission, subjects started a weight-maintenance diet (50% carbohydrate, 30% fat, and 20% protein); consumption of caffeine and alcohol and tobacco use were prohibited. Each subject exercised daily for 30 minutes on a treadmill at the same self-selected settings (speed and grade) and was continuously monitored with triaxial accelerometers (Actigraph GT3X+; Actigraph LLC) on the wrist and hip, measuring spontaneous and volitional physical activity. Body weight, BP, and pulse were measured daily in the fasting state. Subjects were randomized to receive RM-493 (1.0 mg/24 h) or placebo for 72 hours by continuous, subcutaneous infusion using an insulin pump (OmniPod; Insulet) starting at 8:00 am on the second day of admission (period 1). At the end of this infusion, the subjects were directly crossed over to a 72-hour infusion with the other treatment (period 2). After completion of the final assessments of period 2, subjects completed the study and were discharged.
Figure 1.
Study design. Subjects received 72-hour infusions of RM-493 or placebo and then immediately were crossed over to the other treatment. In each period, after 48 hours of treatment, subjects entered the metabolic chamber at 8:00 am. REEC was measured at 9:30 to 10:00 and 10:30 to 11:00 am, meals were at noon (40% of kcal), 6:30 pm (50% of kcal), and 9:00 pm (10% of kcal), exercise was at 5:00 to 5:30 pm, and REEH was measured after subjects left the chamber.
Study procedures
EE measurements and substrate oxidation
Each subject underwent continuous recordings of EE and respiratory quotient (RQ) in a whole-room indirect calorimeter (referred to subsequently as a metabolic chamber) at thermoneutrality (24.6 ± 0.7°C) on the third day of each treatment period. The subjects were fasting from 8:00 pm and entered the chamber at 8:00 am for 23 hours, as described previously (10–12). The primary outcome, REEC was defined as the average REE in the metabolic chamber from 9:30 (49.5 hours after the start of treatment) to 10:00 and 10:30 to 11:00 am while the subject was fasting, completely still, and awake in a seated position under the observation of a member of the research team. REE defined in our study and basal metabolic rate are very similar in important aspects (performed after a 10 to 12-hour fast, at thermoneutrality, with the subject awake and at rest without physical and psychological stress) but differ slightly in posture (semirecumbent sitting vs supine). REE is a precise surrogate for basal metabolic rate (10). By allowing the subject to acclimate to the chamber for 90 minutes and then using 2 30-minute measurement periods, we achieve a coefficient of variation of 2.5%, which was similar to previous metabolic chamber REE (REEc) measurements (11), maximizing sensitivity to detect changes in REEC. The 30-minute interval between sessions allows subject movement, improving compliance during the measurement periods. The semirecumbent position of the subject allowed direct observation of the subject to assure that he or she did not fall asleep.
Subjects consumed a standardized test meal containing 40% of their daily energy requirements from 11:30 am to noon. The thermic effect of food (TEF) was assessed by averaging data collected from 11:30 am to 3:00 pm. Exercise EE was assessed during the subject's daily 30 minutes of treadmill walking. Sleeping energy expenditure (SEE) was assessed from midnight to 4:00 am. Data collected during the periods of REEC and SEE were excluded if microwave activity was >2% (24% of the SEE and 3% of the REE data were excluded) (12). Total energy expenditure (TEE) and average RQ of the 23-hour chamber period excluded the 30-minute exercise period.
As a secondary measure, REEH was measured using a hood indirect calorimetry cart system (TrueOne 2400; Parvo Medics) immediately after subjects exited the chamber in fasted and resting state.
Body composition
Total body fat content, total fat mass, and fat free mass were measured using a dual-energy X-ray absorptiometry scanner (Lunar iDXA; GE Healthcare).
MC4R genotyping
The coding regions of MC4R were sequenced at Eurofins Genomics.
Biochemical measurements
Blood was drawn daily after an overnight fast. All clinical chemistry testing was performed by the Department of Laboratory Medicine, Clinical Center, National Institutes of Health. Glucose, insulin, C-peptide, total glucagon-like peptide-1 (GLP-1), free fatty acids (FFAs), triglycerides, TSH, total T4, prolactin, and 24-hour urine cortisol, irisin, total ghrelin, fibroblast growth factor-21, and total peptide YY (PYY) were measured as described in the Supplemental Materials. Total T3, free T3, and free T4 were measured by liquid chromatography-tandem mass spectrometry with electrospray ionization (Agilent 6460 mass spectrophotometer and Agilent 1200 high-performance liquid chromatograph; Agilent Technologies).
Statistical analysis
Descriptive analysis results are reported as means ± SD, if not specified. Comparisons of responses between pre- and posttreatment were performed using a paired t test for normal continuous variables. Normality was checked using Q-Q plots before comparisons, and skewed variables were logarithm transformed. Based on a coefficient of variation of 2.5%, the study had, a priori, 80% power to detect a difference of 2.5% in REEC with 12 subjects. In comparison, REEH (5.4% coefficient of variation for the ParvoMedic cart) requires 39 subjects to detect this difference (13).
Mixed-model regression was used to identify the association between the repeated measurements of response variables with treatment, adjusting for baseline, glucose, insulin, C-peptide, FFAs, triglycerides, total GLP-1, hormones, REEH, and cardiovascular parameters (systolic BP, diastolic BP, and heart rate). For metabolic variables, we included data from fasting daily blood draws corresponding to 24, 48, and 72 hours of drug or placebo. The REEH and vital signs obtained at 72 hours, the maximal duration of treatment, were analyzed. Absence of treatment × period interaction effects were confirmed for all variables studied.
Analyses were performed using SAS (version 9.3; SAS Institute, Cary, NC) and JMP (version 10.0; SAS Institute, Cary, NC) with significance being P < .05. All statistical testing was two-sided and not corrected for multiple comparisons.
Results
Baseline clinical characteristics of study subjects
This study used a crossover design in which each study participant randomly received either placebo or RM-493 and then was crossed over to the alternate treatment. Fifteen subjects were screened, and all of the 12 eligible subjects were randomized and completed the study (Supplemental Figure 1). Baseline clinical characteristics are reported in Table 1. All participants were weight stable, normotensive, and nondiabetic and had normal thyroid function at baseline. Participants were obese (grade I and II, 35.7 ± 2.9 kg/m2, 104 ± 10 kg), with body fat of 49.2 ± 1.5% in the women and 32.5 ± 6.8% in the men. No subjects had exon nucleotide variants of MC4R associated with obesity (Supplemental Materials).
Table 1.
Baseline Clinical Characteristics of 12 Study Subjects
| Characteristic | Value |
|---|---|
| Age, y | 34.9 ± 11.3 |
| Sex (female/male) | 6/6 |
| Race (black/Latino/white) | 9/1/2 |
| Body composition | |
| BMI, kg/m2 | |
| Women | 38.0 ± 1.6 |
| Men | 33.4 ± 1.9 |
| Lean body mass, kg | |
| Women | 48.0 ± 5.6 |
| Men | 67.2 ± 8.5 |
| % body fat | |
| Women | 49.2 ± 1.5 |
| Men | 32.5 ± 6.8 |
| Cardiovascular parameters | |
| SBP, mm Hg | 115 ± 13 |
| DBP, mm Hg | 62 ± 11 |
| HR, beats/min | 64 ± 9.8 |
| Metabolic parameters | |
| Fasting glucose, mg/dL | 91 ± 6 |
| Hemoglobin A1C, % | 5.5 ± 0.3 |
| Fasting insulin, μU/mL | 18 ± 11 |
| Fasting C-peptide, ng/mL | 3.0 ± 1.2 |
| QUICKI | 0.32 ± 0.03 |
| Lipids | |
| Total cholesterol, mg/dL | 163 ± 32 |
| Calculated LDL, mg/dL | 88 ± 32 |
| HDL, mg/dL | 51 ± 15 |
| Triglycerides, mg/dL | 139 ± 69 |
| Free fatty acid, mEq/L | 0.454 ± 0.105 |
Abbreviations: BMI, body mass index; DBP, diastolic blood pressure; HDL, high-density lipoprotein; HR, heart rate; LDL, low-density lipoprotein; QUICKI, quantitative insulin sensitivity check index ; SBP, systolic blood pressure. Values shown are means ± SD.
Administration of placebo and RM-493
Placebo or RM-493 was administered as a continuous subcutaneous infusion during each 72-hour treatment period. Plasma RM-493 levels at 24-hour (5.88 ± 0.59 ng/mL) were similar to those at 72 hours of treatment (5.95 ± 0.88 ng/mL). To confirm drug washout, the RM-493 plasma levels were measured during the second treatment period: in the 6 participants who received RM-493 in the first period, RM-493 levels were 0.50 ± 0.20 ng/mL after 24 hours and undetectable (detection limit of assay < 0.50 ng/mL; Rhythm Pharmaceuticals, personal communication) after 48 hours of placebo treatment, ie, before the studies in the metabolic chamber.
Effects of RM-493 on REE
All participants complied with the metabolic diet and standard exercise routine and maintained their body weight throughout the study. The primary endpoint, REEC, was significantly increased by treatment with RM-493 (1856 ± 369 vs 1745 ± 359 kcal/d with placebo, P = .028), a treatment increase of 110 kcal/d (95% confidence interval [CI], 15–207 kcal/d) (Figure 2). No period effect (P = .32) or treatment × period interaction (P = .30) was observed. REEH was also measured as a secondary outcome after 72 hours of RM-493 administration. REEH trended higher by 79 ± 130 kcal/d (95% CI, −4 to 162 kcal/d, P = .059) compared with that for placebo (Table 2).
Figure 2.
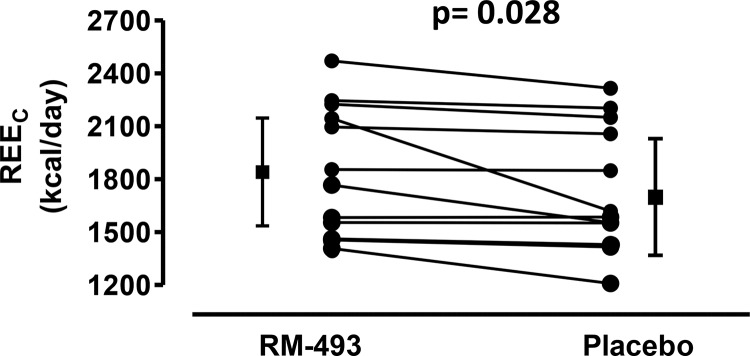
Effect of RM-493 vs placebo on REEC at the end of the treatment period. REEC was measured on the third treatment day of each treatment arm in the metabolic chamber at thermoneutrality with subjects in the resting, postabsorptive state as the mean of 2 30-minute rest periods. Individual data points and aggregate data with error bars are the overall mean ± 95% CI. P values indicate significance of treatment differences using a paired t test.
Table 2.
EE, RQ, and Spontaneous Physical Activity After Placebo and RM-493 Administration in Obese Individuals
| RM-493 | Placebo | P | |
|---|---|---|---|
| REEC (kcal/d) | 1856 ± 369 | 1745 ± 359 | .028 |
| REEH (kcal/d) | 1849 ± 388 | 1769 ± 379 | .059 |
| TEE (kcal/d) | 2509 ± 420 | 2457 ± 399 | .089 |
| Components of TEE | |||
| SEE (kcal/m) | 1.35 ± 0.19 | 1.35 ± 0.18 | .938 |
| Exercise EE (kcal/m)a | 4.49 ± 0.96 | 4.49 ± 1.05 | .998 |
| TEF (kcal/m) | 1.81 ± 0.31 | 1.76 ± 0.33 | .145 |
| 23-hour RQb | 0.83 ± 0.02 | 0.85 ± 0.02 | .017 |
| Spontaneous physical activity | |||
| Hip counts/min | 304 ± 108 | 325 ± 85 | .300 |
| Wrist counts/min | 1307 ± 335 | 1245 ± 465 | .434 |
| Steps/min | 3.22 ± 1.32 | 3.51 ± 1.10 | .333 |
| % sedentary time | 80.7 ± 4.9 | 80.7 ± 4.6 | .940 |
Posttreatment values shown are unadjusted means ± SD. Posttreatment values were adjusted for baseline value and treatment group using a mixed-model regression. P values indicate significance for comparisons between RM-493 and placebo.
n = 10, 2 patients were excluded from analysis because of markedly different exercise efforts during the 2 phases.
Averaged RQ over all nonexercise periods in the chamber.
Effects of RM-493 on TEE and its components, RQ, and physical activity
The metabolic chamber assessments of 23-hour nonexercise, exercise, and SEE and TEF did not differ between the treatment arms (Table 2). The 23-hour nonexercise RQ was significantly lower during RM-493 treatment vs placebo (0.833 ± 0.021 vs 0.848 ± 0.022, P = .02), indicating a shift toward fat oxidation. Spontaneous and volitional physical activities as measured by hip and wrist accelerometers were similar in both treatment phases (Table 2).
Effects of RM-493 on metabolic parameters and hormonal profile
RM-493 treatment was associated with small increases in plasma fasting glucose, insulin, C-peptide, triglyceride, FFA, and total GLP-1 and PYY levels (Table 3). TSH levels were higher, albeit in the normal range after RM-493 administration, although there was no effect on total or free T3 and T4 levels (Table 3). Similarly, there were no differences in urinary cortisol adjusted for creatinine excretion and plasma levels of prolactin, ghrelin, irisin, and fibroblast growth factor-21 between the treatment groups (Supplemental Table 1).
Table 3.
Metabolic Parameters and Hormone Levels After Placebo and RM-493 Administration in Obese Individuals
| RM-493 | Placebo | P | |
|---|---|---|---|
| Metabolic parameters | |||
| Fasting glucose, mg/dL | 95 ± 5.8 | 91 ± 3.6 | .003 |
| Fasting insulin, μU/mL | 26.2 ± 16 | 18.4 ± 12 | .008 |
| Fasting C-peptide, ng/mL | 3.6 ± 1.2 | 3.2 ± 1.2 | .043 |
| Triglycerides, mg/dL | 150 ± 70 | 129 ± 54 | .035 |
| FFA, mEq/L | 0.442 ± 0.1 | 0.334 ± 0.1 | .009 |
| Total GLP-1, pmol/L | 3.24 ± 1.15 | 2.27 ± 0.96 | .003 |
| Total PYY, pg/mL | 53.0 ± 19.1 | 38.7 ± 16.6 | .037 |
| Thyroid parameters | |||
| TSH, μU/mL | 1.86 ± 1.0 | 1.51 ± 0.73 | .009 |
| T3, ng/dL | 119 ± 40 | 112 ± 36 | .324 |
| T4, μg/dL | 8.9 ± 1.3 | 8.7 ± 1.7 | .382 |
| Free T3, pg/mL | 2.6 ± 0.8 | 2.5 ± 0.7 | .312 |
| Free T4, ng/dL | 2.2 ± 0.4 | 2.1 ± 0.3 | .308 |
Posttreatment values shown are unadjusted means ± SD. P values indicate significance for comparisons between RM-493 and placebo.
Adverse effects associated with RM-493 and placebo administration
MC4R agonists have been previously reported to increase BP and heart rate (9, 14). No differences were observed in systolic (118 ± 10 vs 118 ± 9 mm Hg, P = .69) or diastolic (68 ± 8 vs 69 ± 10 mm Hg, P = .56) BP or in heart rate (67 ± 9 vs 69 ± 11 bpm, P = .11) by 72 hour of treatment. During the RM-493 treatment phase, headache (n = 3), arthralgia (n = 2), nausea (n = 2), spontaneous penile erections (n = 1), and female genital sensitivity (n = 2) were reported symptoms that were characterized as mild, transient, and resolved without sequelae. At the end of the study, 10 of 12 participants correctly identified their RM-493 treatment order assignment.
Discussion
Safe pharmacotherapeutic options are needed to treat obesity. The role of the MC system in maintaining the balance between caloric intake and EE makes it an attractive target for therapeutic development (7, 8). We report the first clinical study of the effect of a MC4R agonist on EE. RM-493 administration increased REE measured in the metabolic chamber by 6.4% (110 kcal/d). Consistent with these findings, RM-493 increased REE measured by hood indirect calorimetry, a less robust method, by 4.7% (∼79 kcal/d, P = .059). These results are concordant with those of studies demonstrating increases in EE by MC agonists in wild-type rodents (15, 16) but not in MC4R-deficient mice (17). The increase in REE is comparable to the increase in EE caused by single doses of sibutramine (18) or caffeine (19).
In our human study, RM-493 administration increased fat oxidation and circulating FFA levels. In rodents, MC4R agonists also increased fat oxidation, which was inhibited by the MCR antagonist SHU9119 (15) and was lost in MC4R knockout mice (20). Note that in our study, there was no confounding reduction in food intake to account for the lower RQ. Considering that low fatty acid oxidation is associated with an increased risk of obesity (21), MC4R agonists may aid in weight loss by initiating a shift in substrate oxidation.
RM-493 (formerly BIM-22493), is a cyclic peptide (Acetyl-L-arginyl-cyclo[L-cysteinyl-D-alanyl-L-histidinyl-D-phenylalanyl-L-arginyl-L-tryptophanyl-L-cysteinyl]-amide) full agonist (EC50 = 0.27 nM) of human MC4R and binds with a Ki of 2.1 nM and ∼10-fold selectivity over human MC3R (7). We administered RM-493 at 1 mg/24 h, producing steady-state plasma concentrations of ∼5 nM. Because RM-493 is not efficiently bound by plasma proteins, this concentration should activate MC4Rs, assuming it reaches the relevant tissue sites. Thus, the increase in REE by RM-493 is probably via agonism at MC4R.
In this small study, no significant effect of RM-493 on TEE, SEE, or TEF was found, possibly due to insufficient power. Further clinical studies are required to determine whether the increase in REE is sustained and leads to weight loss in humans. However, in nonhuman primates, 8-week administration of RM-493 was associated with an increase (∼14%) in TEE as measured by double-labeled water, and weight loss continued even after animals normalized food intake (9).
Chronic administration of RM-493 in rhesus monkeys improved glucose tolerance and reduced serum triglycerides in the setting of weight loss (9). In contrast, we observed during short-term treatment with stable caloric intake that RM-493 administration increased plasma triglyceride and FFA levels, probably via adipose tissue lipolysis. In rodents, central administration of MTII triggers lipid mobilization, increases sympathetic drive to white adipose tissue (22) and increases skeletal muscle AMP-activated protein kinase activity, which increases skeletal muscle FFA β-oxidation (23). The lower 23-hour nonexercise RQ after RM-493 administration in the current study reflects this increased fatty acid oxidation.
In contrast to the acute lowering of plasma insulin levels with MC4R agonists, in this study we observed statistically significant, but very small, increases in fasting plasma glucose, insulin, and C-peptide levels. The previously tested MC4R agonist LY2112688 (acetyl-d-arginyl-[l-cysteinyl-l-glutamyl-l-histidinyl-d-phenylalanyl-l-arginyl-l-tryptophanyl-l-cysteine]-amide) did not affect glucose and insulin levels (14). Consistent with this finding, in ongoing clinical studies of RM-493 (up to 12 weeks), no potentially adverse and/or significant changes in glucose, insulin, and triglyceride parameters have been observed (K.M.G. and L.H.T.V.d.P., unpublished results). Longer term clinical studies with RM-493 on insulin sensitivity are needed to put these observations in perspective. Similar to our findings, prior rodent studies showed that central MCR stimulation can enhance hepatic glucose production, accompanied by a nonsignificant increase in fasting glucose levels (24). RM-493 treatment had no significant effect on urinary free cortisol levels or thyroid hormone levels. There was a small but significant increase in TSH levels, which may be explained by MC4R activation of hypothalamic TRH expression (25).
Recently, MC4R receptor activation in intestinal epithelial L-cells was shown to acutely increase GLP-1 and PYY secretion in vivo in mice and in colon explants from both mice and humans (26). Similar to the rodent data, fasting total GLP-1 and PYY levels were slightly, but significantly, increased during RM-493 administration in our study. Thus, it is possible that MC4R-induced GLP-1 production may contribute to eventual beneficial effects on insulin and glucose, whereas PYY (assumed to be PYY3–36) can positively affect energy metabolism during obesity treatment. It is possible that increases in both peptides may contribute to the weight loss effect of RM-493. Given the small number of patients evaluated, these studies require follow-up in larger populations.
Unlike that of LY2112688, short-term administration of RM-493 was not associated with increased BP and heart rate (14). In an acute nonhuman primate study, both LY2112688 and RM-493 reduced food intake, whereas only LY2112688 increased heart rate and BP (9). The current study and additional phase 1 studies in which RM-493 was dosed for up to 28 days (K.M.G. and L.H.T.V.d.P., unpublished results) did not elicit adverse cardiovascular effects. In our study, nausea, penile erections, and female genital sensitivity were observed, similar to findings in other studies of MC agonists in humans (14, 27–29). Larger, long-term clinical trials will be required to evaluate the overall safety and tolerability of RM-493 for the treatment of obesity, including cardiovascular effects.
Our study has several limitations. First, the treatment duration in this proof-of-concept study was 72 hours; longer studies are needed to see whether the REE increase and changes in other analytes are sustained. Second, the study was small, powered to detect changes in REE measured in a sensitive metabolic chamber and was not powered to detect changes in TEE, SEE, or TEF. Third, 75% of study participants were African American. Although, there is no evidence to suggest racial or ethnic differences in response to MC4R agonists, studies with a more diverse population would increase the generality of our findings. Fourth, although not detected, the possibility of carryover effects cannot be completely ruled out, because patients entered the second crossover period without an extended washout.
In summary, we report that a 3-day administration of an MC4R agonist, RM-493, significantly increased REE and preferentially increased fat oxidation in obese individuals. RM-493 exhibited minimal side effects and no pressor effects as observed with prior MC4R agonists in development. Our study provides a mechanistic rationale for the use of selective MC4R agonists in the treatment of obesity and the metabolic syndrome. Longer-term studies in obese individuals are necessary to determine whether MC4R agonists are effective therapeutic agents for weight loss.
Acknowledgments
We thank Steven Soldin, Verena Gounden, Edward Cowen, Amber Courville, the National Institute of Diabetes and Digestive and Kidney Diseases Clinical Core (Mary Walter, Anula Bhusry, Darhlene Banks, and Yuhai Dai), the Clinical Center Nutrition Department, and the nursing staff of the Metabolic Clinical Research Unit and Pat Noonan for pharmacokinetic support and Hillori Conners for supporting the initial study design.
This work was supported by the Intramural Research Program of the National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health, and by Rhythm Pharmaceuticals.
This study is registered at ClinicalTrials.gov under identifier NCT01867437.
K.Y.C. participated in the design of the study, collection of data, data analysis, and writing of the manuscript and approved the final manuscript. R.M. participated in the collection of data, data analysis, and writing of the manuscript and approved the final manuscript. B.S.A, K.P.M, P.S., R.J.B., and T.L.P. participated in the conduct of the study and collection of data and approved the final manuscript. X.Z. and M.R. participated in data management and analysis and approved the final manuscript. R.D.C. and B.L.P. participated in the study design, interpretation of data, and writing of the manuscript and approved the final manuscript. K.G., L.V.P, M.L.R., and M.C.S. participated in the design, interpretation of data, and writing of the manuscript and approved the final manuscript. M.C.S. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Disclosure Summary: K.G. and L.V.d.P. are employees of Rhythm Pharmaceuticals, a privately held biotechnology company that is developing RM-493 for the treatment of obesity. The other authors have nothing to disclose.
Footnotes
- BP
- blood pressure
- CI
- confidence interval
- EE
- energy expenditure
- FFA
- free fatty acid
- GLP-1
- glucagon-like peptide 1
- MC
- melanocortin
- MCR
- melanocortin receptor
- MC4R
- melanocortin-4 receptor
- MSH
- melanocyte-stimulating hormone
- PYY
- peptide YY
- REE
- resting energy expenditure
- RQ
- respiratory quotient
- SEE
- sleeping energy expenditure
- TEE
- total energy expenditure
- TEF
- thermic effect of food.
References
- 1. Cone RD. The central melanocortin system and energy homeostasis. Trends Endocrinol Metab. 1999;10:211–216. [DOI] [PubMed] [Google Scholar]
- 2. Cone RD. Studies on the physiological functions of the melanocortin system. Endocr Rev. 2006;27:736–749. [DOI] [PubMed] [Google Scholar]
- 3. Fan W, Boston BA, Kesterson RA, Hruby VJ, Cone RD. Role of melanocortinergic neurons in feeding and the agouti obesity syndrome. Nature. 1997;385:165–168. [DOI] [PubMed] [Google Scholar]
- 4. Vaisse C, Clement K, Durand E, Hercberg S, Guy-Grand B, Froguel P. Melanocortin-4 receptor mutations are a frequent and heterogeneous cause of morbid obesity. J Clin Invest. 2000;106:253–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Krakoff J, Ma L, Kobes S, et al. Lower metabolic rate in individuals heterozygous for either a frameshift or a functional missense MC4R variant. Diabetes. 2008;57:3267–3272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hinney A, Volckmar AL, Knoll N. Melanocortin-4 receptor in energy homeostasis and obesity pathogenesis. Prog Mol Biol Transl Sci. 2013;114:147–191. [DOI] [PubMed] [Google Scholar]
- 7. Fani L, Bak S, Delhanty P, van Rossum EF, van den Akker EL. The melanocortin-4 receptor as target for obesity treatment: a systematic review of emerging pharmacological therapeutic options. Int J Obes (Lond). 2014;38:163–169. [DOI] [PubMed] [Google Scholar]
- 8. Tao YX. The melanocortin-4 receptor: physiology, pharmacology, and pathophysiology. Endocr Rev. 2010;31:506–543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kievit P, Halem H, Marks DL, et al. Chronic treatment with a melanocortin-4 receptor agonist causes weight loss, reduces insulin resistance, and improves cardiovascular function in diet-induced obese rhesus macaques. Diabetes. 2013;62:490–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Schutz Y, Jequier E. Resting Energy Expenditure, Thermic Effect of Food and Total Energy Expenditure. New York, NY: Marcel Dekker; 1998. [Google Scholar]
- 11. Rumpler WV, Seale JL, Conway JM, Moe PW. Repeatability of 24-h energy expenditure measurements in humans by indirect calorimetry. Am J Clin Nutr. 1990;51:147–152. [DOI] [PubMed] [Google Scholar]
- 12. Ravussin E, Lillioja S, Anderson TE, Christin L, Bogardus C. Determinants of 24-hour energy expenditure in man. Methods and results using a respiratory chamber. J Clin Invest. 1986;78:1568–1578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Cooper JA, Watras AC, O'Brien MJ, et al. Assessing validity and reliability of resting metabolic rate in six gas analysis systems. J Am Diet Assoc. 2009;109:128–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Greenfield JR, Miller JW, Keogh JM, et al. Modulation of blood pressure by central melanocortinergic pathways. N Engl J Med. 2009;360:44–52. [DOI] [PubMed] [Google Scholar]
- 15. Hwa JJ, Ghibaudi L, Gao J, Parker EM. Central melanocortin system modulates energy intake and expenditure of obese and lean Zucker rats. Am J Physiol Regul Integr Comp Physiol. 2001;281:R444–R451. [DOI] [PubMed] [Google Scholar]
- 16. Cowley MA, Pronchuk N, Fan W, Dinulescu DM, Colmers WF, Cone RD. Integration of NPY, AGRP, and melanocortin signals in the hypothalamic paraventricular nucleus: evidence of a cellular basis for the adipostat. Neuron. 1999;24:155–163. [DOI] [PubMed] [Google Scholar]
- 17. Hsiung HM, Hertel J, Zhang XY, et al. A novel and selective beta-melanocyte-stimulating hormone-derived peptide agonist for melanocortin 4 receptor potently decreased food intake and body weight gain in diet-induced obese rats. Endocrinology. 2005;146:5257–5266. [DOI] [PubMed] [Google Scholar]
- 18. Hansen DL, Toubro S, Stock MJ, Macdonald IA, Astrup A. Thermogenic effects of sibutramine in humans. Am J Clin Nutr. 1998;68:1180–1186. [DOI] [PubMed] [Google Scholar]
- 19. Astrup A, Toubro S, Cannon S, Hein P, Breum L, Madsen J. Caffeine: a double-blind, placebo-controlled study of its thermogenic, metabolic, and cardiovascular effects in healthy volunteers. Am J Clin Nutr. 1990;51:759–767. [DOI] [PubMed] [Google Scholar]
- 20. Butler AA, Marks DL, Fan W, Kuhn CM, Bartolome M, Cone RD. Melanocortin-4 receptor is required for acute homeostatic responses to increased dietary fat. Nat Neurosci. 2001;4:605–611. [DOI] [PubMed] [Google Scholar]
- 21. Zurlo F, Lillioja S, Esposito-Del Puente A, et al. Low ratio of fat to carbohydrate oxidation as predictor of weight gain: study of 24-h RQ. Am J Physiol. 1990;259:E650–E657. [DOI] [PubMed] [Google Scholar]
- 22. Fan W, Dinulescu DM, Butler AA, Zhou J, Marks DL, Cone RD. The central melanocortin system can directly regulate serum insulin levels. Endocrinology. 2000;141:3072–3079. [DOI] [PubMed] [Google Scholar]
- 23. Tanaka T, Masuzaki H, Yasue S, et al. Central melanocortin signaling restores skeletal muscle AMP-activated protein kinase phosphorylation in mice fed a high-fat diet. Cell Metab. 2007;5:395–402. [DOI] [PubMed] [Google Scholar]
- 24. Heijboer AC, van den Hoek AM, Pijl H, et al. Intracerebroventricular administration of melanotan II increases insulin sensitivity of glucose disposal in mice. Diabetologia. 2005;48:1621–1626. [DOI] [PubMed] [Google Scholar]
- 25. Harris M, Aschkenasi C, Elias CF, et al. Transcriptional regulation of the thyrotropin-releasing hormone gene by leptin and melanocortin signaling. J Clin Invest. 2001;107:111–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Panaro BL, Tough IR, Engelstoft MS, et al. 2014 The melanocortin-4 receptor is expressed in enteroendocrine L cells and regulates the release of peptide YY and glucagon-like peptide 1 in vivo. Cell Metab. 2014;20:1018–1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Royalty JE, Konradsen G, Eskerod O, Wulff BS, Hansen BS. Investigation of safety, tolerability, pharmacokinetics, and pharmacodynamics of single and multiple doses of a long-acting α-MSH analog in healthy overweight and obese subjects. J Clin Pharmacol. 2014;54:394–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Safarinejad MR. Evaluation of the safety and efficacy of bremelanotide, a melanocortin receptor agonist, in female subjects with arousal disorder: a double-blind placebo-controlled, fixed dose, randomized study. J Sex Med. 2008;5:887–897. [DOI] [PubMed] [Google Scholar]
- 29. Diamond LE, Earle DC, Rosen RC, Willett MS, Molinoff PB. Double-blind, placebo-controlled evaluation of the safety, pharmacokinetic properties and pharmacodynamic effects of intranasal PT-141, a melanocortin receptor agonist, in healthy males and patients with mild-to-moderate erectile dysfunction. Int J Impot Res. 2004;16:51–59. [DOI] [PubMed] [Google Scholar]