Abstract
Context:
Hypothyroidism is a common condition that disproportionately affects hemodialysis patients. In the general population, hypothyroidism is associated with higher mortality, particularly in populations with underlying cardiovascular risk. Despite their heightened cardiovascular mortality, the impact of hypothyroidism on the survival of hemodialysis patients remains uncertain.
Objective:
To examine whether hypothyroidism is independently associated with higher mortality in hemodialysis patients.
Design, Setting, and Patients:
Among 8840 incident hemodialysis patients receiving care from a large national dialysis provider from January 2007 to December 2011, we examined the association of hypothyroidism (TSH >5.0 mIU/L) with mortality.
Main Outcome Measures:
Associations between baseline and time-dependent hypothyroidism with all-cause mortality were determined using case-mix adjusted Cox models. In secondary analyses, we examined the impact of low-normal, upper-normal, subclinical range, and overt range TSH levels (TSH ≥0.5–3.0, >3.0–5.0, >5.0–10.0, and >10.0 mIU/L, respectively) on mortality risk.
Results:
The study population consisted of 1928 (22%) hypothyroid and 6912 (78%) euthyroid patients. Baseline and time-dependent hypothyroidism were associated with higher mortality: adjusted hazard ratios (95% confidence intervals) were 1.47 (1.34–1.61) and 1.62 (1.45–1.80), respectively. Compared to low-normal TSH, upper-normal, subclinical hypothyroid, and overt hypothyroid TSH levels were associated with incrementally higher adjusted death risk in baseline and time-dependent analyses. In time-dependent analyses, the hypothyroidism-mortality association was increasingly stronger across higher body mass index strata.
Conclusions:
Hypothyroidism as well as upper-normal TSH levels are associated with higher mortality in hemodialysis patients. Further studies are needed to determine whether restoration of TSH to low-normal levels with thyroid hormone replacement therapy ameliorates adverse outcomes in hemodialysis patients.
Data spanning over three decades show that hypothyroidism is highly prevalent in predialysis and dialysis-dependent chronic kidney disease patients (1–10). For example, data from the Third National Health and Nutrition Examination Survey (NHANES III) demonstrate an increasing prevalence of hypothyroidism with incrementally impaired kidney function: 5.4, 10.9, 20.4, 23.0, and 23.1% with estimated glomerular filtration rates of ≥90, 60–89, 45–59, 30–44, and <30 mL/min/1.73 m2, respectively (6). Although prevalence estimates vary across populations, epidemiological data show a disproportionately higher burden of hypothyroidism in hemodialysis (HD) patients (4, 5, 8, 9). Indeed, many cases may remain latent or undiagnosed due to symptom overlap with uremia and coexisting comorbidities in this population.
In the general population, hypothyroidism has been associated with adverse cardiovascular (CV) measures (eg, endothelial dysfunction, dyslipidemia, accelerated atherosclerosis, impaired systolic and diastolic function) (11–15), greater frequency of coronary heart disease and congestive heart failure (CHF) events (16, 17), and higher mortality particularly in high CV risk populations (18–20). In dialysis patients, CV disease is the leading cause of mortality (45% of deaths), with many of these fatalities related to sudden cardiac death, CHF, and coronary heart disease (21, 22). These data have prompted an increasing interest in hypothyroidism as a novel risk factor for mortality in this population (23).
However, data examining the association between hypothyroidism and mortality in dialysis patients have been sparse and inconsistent. To date, very few studies have directly examined the association of hypothyroidism, defined by elevated TSH, as the most sensitive and specific single measure of hypothyroidism (24–26) with mortality in this population (8, 27). In the first of these studies, hypothyroidism was associated with greater all-cause death risk in a dialysis cohort from two tertiary care centers in the northeastern United States (8). Yet in a subsequent study, subclinical hypothyroidism examined neither separately nor in combination with overt hypothyroidism was associated with mortality in a prospective cohort of diabetic HD patients in Germany (27). However, interpretation of these data is made difficult due to: 1) their focus upon baseline thyroid function only (ie, they did not account for variations in thyroid function over time); 2) limited ability to comprehensively adjust for comorbidity, laboratory, and medication confounders; and 3) inclusion of both incident and prevalent dialysis patients.
To address these limitations, we examined the association of baseline and repeated measures of TSH with mortality in incident HD patients from a large dialysis provider in the United States. We hypothesized that hypothyroidism was independently associated with higher risk for mortality in this nationally representative HD population.
Subjects and Methods
Source cohort
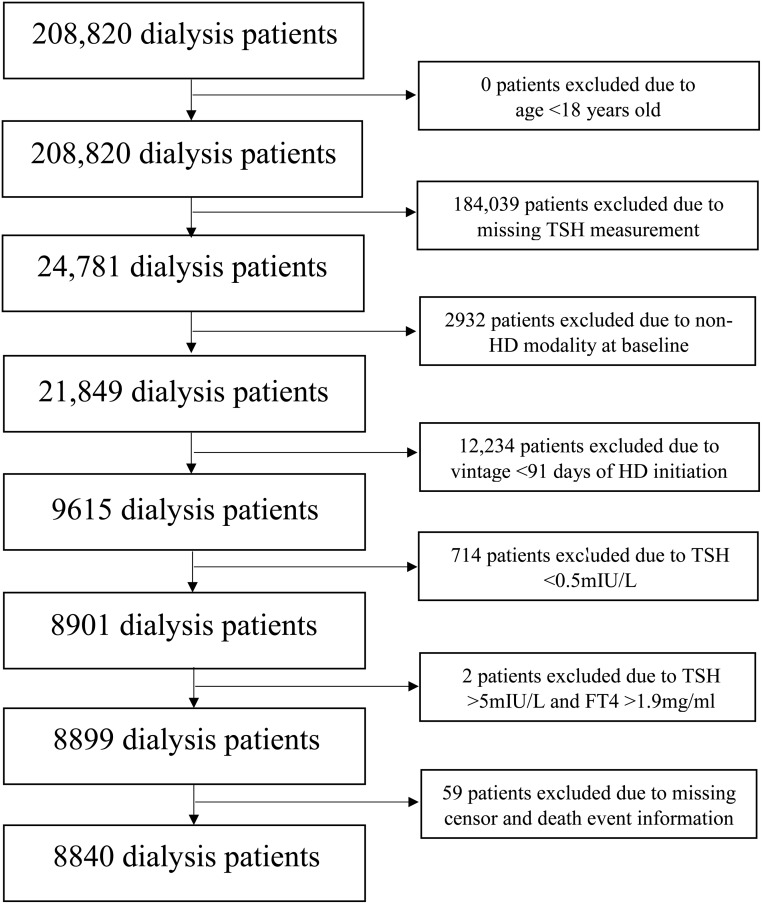
We conducted a historical cohort study using data from a large US dialysis organization (LDO) with comprehensive capture of longitudinal data on patients' sociodemographics, comorbidities (ascertained from ICD-9 codes), laboratory tests, dialysis treatment characteristics, clinical events, and vital status. The original source population was a cohort of 208 820 incident (newly initiated) dialysis patients receiving care in one of the LDO outpatient facilities over a 5-year period. Patients were included provided that at study entry (defined as the time of baseline TSH measurement) they were ≥18 years old, had a dialysis vintage of ≤91 days, were undergoing thrice-weekly in-center HD, had ≥ one TSH measure(s) in their baseline (first) calendar quarter, and received care within a LDO dialysis unit from January 1, 2007 to December 30, 2011. Patients were excluded if at study entry they were receiving peritoneal dialysis or home HD or they had missing censor/death date information. Given that the comparison of interest was between euthyroidism and hypothyroidism, patients with primary hyperthyroidism (TSH level <0.5 mIU/L) or a pattern of secondary hyperthyroidism (TSH level >5.0 mIU/L and free T4 [FT4] >1.9 ng/dL) at the time of study entry were excluded (Figure 1). The study was approved by the Institutional Review Committees of the Los Angeles Biomedical Research Institute at Harbor-UCLA, the University of California Irvine Medical Center, and the University of Washington.
Figure 1.
Algorithm for the study cohort creation.
Exposure ascertainment
The exposure of interest was thyroid functional status defined according to TSH level. In primary analyses, we examined thyroid functional status dichotomized as: hypothyroidism (TSH > 5.0 mIU/L) vs euthyroidism (TSH ≥0.5–5.0 mIU/L). Given heterogeneous recommendations regarding the appropriate threshold for the upper limit of normal of TSH in the general population (28–31), we conducted secondary analyses in which we categorized thyroid functional status as low-normal, high-normal, subclinical range, and overt range TSH levels (TSH ≥0.5–3.0, >3.0–5.0, >5.0–10.0, and >10.0 mIU/L, respectively) (8). Although subclinical and overt hypothyroidism are typically ascertained using concomitant TSH and FT4 values, we classified TSH levels as those commonly associated with subclinical and overt hypothyroidism (TSH >5.0–10.0 and >10.0 mIU/L, respectively), given the sparse measurement of FT4 in the cohort. We also conducted sensitivity analyses in which we examined finer categories of TSH divided into deciles.
Outcome ascertainment
The primary outcome of interest was all-cause death. At-risk time began the day after the baseline TSH measurement. Patients were censored for kidney transplantation, transfer to a non-LDO dialysis unit, transfer to an alternative dialysis modality other than in-center thrice-weekly HD, or at the end of the study (December 31, 2011).
Statistical analysis
The association between thyroid function and mortality was estimated using two methods. 1) Baseline thyroid function–mortality associations were assessed in which thyroid function and covariates were determined at baseline and their association with subsequent mortality was estimated. 2) Time-dependent thyroid function–mortality associations were examined in which thyroid functional status and time-dependent covariates were time-updated. In time-dependent models, patients who were found to have a change in thyroid function during TSH testing immediately crossed over to the new exposure category, with the rationale that change in thyroid function had been ongoing during the prior exposure period. Subsequent time intervals in which patients were hyperthyroid (TSH <0.5 mIU/L) were excluded.
We estimated the association between thyroid functional status and mortality using Cox proportional hazard models with three levels of covariate adjustment:
Model 1: adjusted for patients' calendar quarter of study entry.
Model 2: adjusted for covariates in model 1, as well as age, sex, race/ethnicity, cause of end-stage renal disease (ESRD), vascular access type, dialysis vintage, body mass index (BMI), and history of the following comorbidities: diabetes, CHF, cerebrovascular disease, myocardial infarction (MI), other cardiac disease, hypertension, and peripheral vascular disease (PVD). In time-dependent analyses, vascular access type and BMI were examined as time-dependent covariates.
Model 3: adjusted for covariates in model 2, as well as serum creatinine, albumin, ferritin, iron saturation, total iron binding capacity, bicarbonate, hemoglobin, calcium, phosphate, parathyroid hormone (PTH), dialysis dose (single pool Kt/V), cholesterol, weekly median erythropoietin stimulating agent (ESA) dose, and cumulative quarterly iv iron dose. In time-dependent analyses, laboratory and in-center dialysis medication covariates were examined as time-dependent variables summarized over 91-day periods (ie, mean or median values over the quarter for each patient).
We a priori defined model 2 as our preferred model, which included core sociodemographic measures and other confounders of the association between hypothyroidism and outcomes. Model 3 was designated as an exploratory model, which included confounders as well as potential causal pathway intermediates of the hypothyroidism–mortality association.
We also conducted subgroup analyses in which we examined the thyroid function–mortality association across strata of baseline age (>60 vs ≤60 y), sex, and race/ethnicity (non-Hispanic White, non-Hispanic Black, Hispanic, and other race/ethnicity). To explore the hypothesis that hypothyroidism may be a physiological adaptation in HD patients prone to protein-energy wasting (32), we also examined thyroid function–mortality associations across strata of baseline BMI (categorized as ≤20, >20 to 25, >25 to 30, and >30 kg/m2).
Missing covariate values were addressed using multiple imputation methods (33). Proportional hazards assumptions were checked by graphical and formal testing including Schoenfeld residuals. Analyses and figures were conducted using R version 3.1.1 (R Foundation for Statistical Computing), Stata version 12.0 (Stata Corp), and SigmaPlot version 12.5 (Systat Software).
Results
Study population description
Among 8840 patients meeting eligibility criteria (Figure 1), we observed that 22% (n = 1928) had hypothyroidism and 78% (n = 6912) had euthyroidism based on baseline TSH levels. In the overall cohort, the mean ± SD, median (interquartile range), and minimum-maximum values of observed TSH levels were 5.4 ± 12.9, 2.5 (1.5, 4.5), and 0.5 to 190.8 mIU/L, respectively. We compared baseline characteristics among included vs excluded patients in the study, and we found that those who were included were more likely to be older, female, and non-Hispanic white; more likely to have an arteriovenous fistula (AVF), arteriovenous graft (AVG), or catheter and less likely to have an unknown vascular access status; more likely to have a history of diabetes, CHF, MI, other cardiac disease, hypertension, and PVD; and less likely to be non-Hispanic black; and they had a greater proportion of death events compared to excluded patients (Supplemental Table 1).
Comparison of baseline characteristics among hypothyroid vs euthyroid patients is shown in Table 1. Hypothyroid and euthyroid patients were similar in terms of the cause of ESRD, mean BMI, and prevalence of diabetes, CHF, hypertension, PVD, and cerebrovascular disease. Compared to euthyroid patients, hypothyroid patients tended to be older and female, were more likely to be non-Hispanic white and less likely to be non-Hispanic black or Hispanic, were less likely to have an AVF/AVG, were more likely to have a history of MI and other cardiac disease, and had a higher weekly median ESA dose (data not shown).
Table 1.
Baseline Characteristics of Incident HD Patients According to Baseline Thyroid Functional Status Defined by Serum TSH Level
| Overall | Euthyroid, TSH 0.5–5.0 mIU/L | Hypothyroid, TSH >5.0 mIU/L | P Value | |
|---|---|---|---|---|
| n | 8840 | 6912 | 1928 | N/A |
| TSH level, mIU/L | 5.4 ± 12.9 | 2.2 ± 1.13 | 16.8 ± 24.2 | <.001 |
| Age, y | 65 ± 15 | 64 ± 15 | 67 ± 14 | <.001 |
| Female, % | 49 | 47 | 57 | <.001 |
| Race/ethnicity, % | ||||
| Non-Hispanic white | 62 | 59 | 71 | |
| Non-Hispanic black | 19 | 21 | 12 | |
| Hispanics | 12 | 13 | 11 | |
| Other | 6 | 7 | 6 | <.001 |
| Vintage, d | 24 ± 25 | 24 ± 25 | 23 ± 24 | .4 |
| Vascular access, % | ||||
| AVF/AVG | 18 | 19 | 14 | |
| Catheter | 63 | 63 | 63 | |
| Unknown | 19 | 18 | 23 | <.001 |
| Cause of ESRD, % | ||||
| Diabetes | 46 | 46 | 45 | |
| Glomerulonephritis | 10 | 10 | 11 | |
| Hypertension | 25 | 26 | 24 | |
| Cystic disease | 2 | 2 | 1 | |
| Other | 17 | 16 | 20 | <.001 |
| BMI, kg/m2 | 28.6 ± 7.9 | 28.7 ± 7.8 | 28.6 ± 8.1 | .8 |
| Comorbidities, % | ||||
| Diabetes | 64 | 64 | 63 | .2 |
| CHF | 16 | 16 | 17 | .2 |
| MI | 12 | 12 | 14 | .05 |
| Other cardiac | 12 | 12 | 14 | .002 |
| Hypertension | 30 | 31 | 30 | .7 |
| PVD | 9 | 9 | 9 | .9 |
| Cerebrovascular disease | 2 | 1 | 2 | .1 |
| Laboratory variables | ||||
| Serum creatinine, mg/dL | 5.0 (3.8, 6.5) | 5.1 (3.9, 6.6) | 4.7 (3.6, 6.0) | <.001 |
| Serum albumin, g/dL | 3.5 (3.2, 3.8) | 3.6 (3.2, 3.9) | 3.4 (3.0, 3.7) | <.001 |
| Ferritin, ng/mL | 294 (172, 494) | 291 (172, 488) | 305 (171, 507) | .2 |
| Iron saturation, % | 21 (17, 27) | 21 (17, 27) | 21 (16, 27) | .2 |
| TIBC, μg/dL | 224 (192, 257) | 226 (195, 258) | 214 (181, 251) | <.001 |
| Bicarbonate, mEq/L | 24 (22, 26) | 24 (22, 26) | 24 (22, 26) | .001 |
| Hemoglobin, g/dL | 11.1 (10.3, 11.9) | 11.2 (10.4, 11.9) | 11.0 (10.1, 11.8) | <.001 |
| Calcium, mg/dL | 9.1 (8.8, 9.4) | 9.1 (8.8, 9.4) | 9.1 (8.8, 9.4) | .7 |
| Phosphate, mg/dL | 4.7 (4.0, 5.5) | 4.7 (4.0, 5.5) | 4.6 (3.9, 5.4) | <.001 |
| PTH, pg/mL | 283 (175, 448) | 294 (182, 463) | 252 (152, 394) | <.001 |
| spKt/V | 1.5 (1.3, 1.7) | 1.5 (1.3, 1.7) | 1.4 (1.2, 1.7) | .006 |
| Total cholesterol, mg/dL | 143 (118, 176) | 144 (119, 176) | 141 (114, 176) | .01 |
Abbreviations: N/A, not applicable; AVF, arteriovenous fistula; AVG, arteriovenous graft; TIBC, total iron binding capacity; spKt/V, single pool Kt/V. Data are expressed as mean ± SD or median (interquartile range), unless otherwise specified. P values were calculated by ANOVA, chi-square, or Kruskal Wallis tests.
Hypothyroidism and mortality
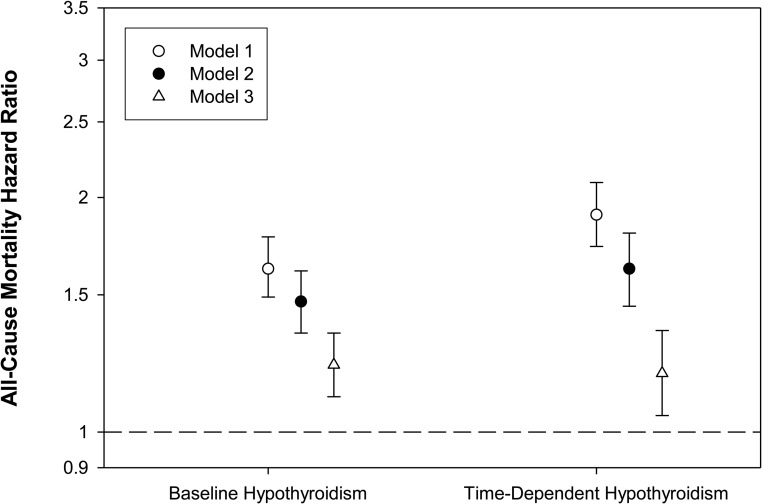
In the primary survival analyses, patients contributed a total of 12 330 years of follow-up, during which time 2420 deaths were observed. Median at-risk time was 1.0 year. In our primary model (model 2), baseline hypothyroidism was associated with increased mortality risk compared to euthyroidism: adjusted hazard ratio (aHR) (95% confidence interval [CI]), 1.47 (1.34–1.61); P < .001. These estimates were attenuated but remained potent and statistically significant after further adjustment in model 3: aHR (95% CI), 1.22 (1.11–1.34); P < .001 (Figure 2 and Supplemental Table 2).
Figure 2.
The association between baseline and time-dependent hypothyroidism and all-cause mortality (reference group: euthyroidism). Model 1 is adjusted for entry calendar quarter. Model 2 is adjusted for covariates in model 1, plus age, sex, race/ethnicity, cause of ESRD, vascular access, dialysis vintage, BMI, diabetes, CHF, cerebrovascular disease, MI, other cardiac disease, hypertension, and PVD. Model 3 is adjusted for covariates in model 2, plus serum creatinine, albumin, ferritin, iron saturation, TIBC, bicarbonate, hemoglobin, calcium, phosphate, PTH, spKt/V, cholesterol, weekly ESA dose, and cumulative quarterly iv iron dose. Error bars represent 95% CIs.
In time-dependent survival models, the association between time-dependent hypothyroidism and mortality was even stronger in model 2: aHR (95% CI), 1.62 (1.45–1.80); P < .001. These estimates were attenuated but remained potent and statistically significant after further adjustment in model 3: aHR (95% CI), 1.19 (1.05–1.35); P = .006 (Figure 2 and Supplemental Table 2).
TSH gradations and mortality
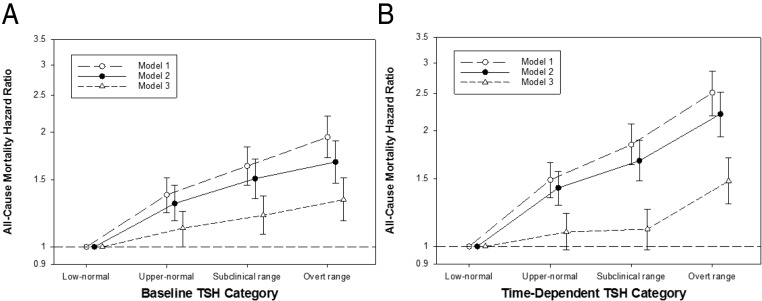
When examining granular categories of baseline TSH level, we observed a graded association between higher TSH level and mortality risk. In model 2, baseline upper-normal, subclinical range, and overt range TSH levels were associated with an incrementally higher mortality risk (reference: low-normal TSH): aHR (95% CI) 1.30 (1.17–1.45), 1.51 (1.34–1.70), and 1.67 (1.47–1.90), respectively; and P < .001 for all (Figure 3 and Supplemental Table 3). These estimates were mildly attenuated after further adjustment in model 3, but remained statistically significant.
Figure 3.
The association between baseline (A) and time-dependent (B) TSH categories and all-cause mortality (reference group: low-normal TSH). Thyroid functional status is categorized as low-normal, upper-normal, subclinical range, and overt range TSH levels (TSH ≥0.5–3.0, >3.0–5.0, >5.0–10.0, and >10.0 mIU/L, respectively). Model 1 is adjusted for entry calendar quarter. Model 2 is adjusted for covariates in model 1, plus age, sex, race/ethnicity, cause of ESRD, vascular access, dialysis vintage, BMI, diabetes, CHF, cerebrovascular disease, MI, other cardiac disease, hypertension, and PVD. Model 3 is adjusted for covariates in model 2, plus serum creatinine, albumin, ferritin, iron saturation, TIBC, bicarbonate, hemoglobin, calcium, phosphate, PTH, spKt/V, cholesterol, weekly ESA dose, and cumulative quarterly iv iron dose. Error bars represent 95% CIs.
In time-dependent analyses, we observed an analogous pattern of association between higher TSH level and mortality risk. In model 2, time-dependent upper-normal, subclinical range, and overt range TSH levels were associated with an incrementally higher mortality risk (reference: low-normal TSH): aHR (95% CI) 1.42 (1.28–1.57), 1.67 (1.48–1.89), and 2.21 (1.93–2.52), respectively; and P < .001 for all (Figure 3 and Supplemental Table 3). In model 3, estimates for high-normal and subclinical range TSH levels were attenuated toward the null, whereas the association between overt range TSH levels and mortality remained statistically significant: aHR (95% CI) 1.09 (0.98–1.22), P = .1; 1.11 (0.98–1.25), P = .1; and 1.48 (1.29–1.70), P < .001, respectively.
TSH deciles and mortality
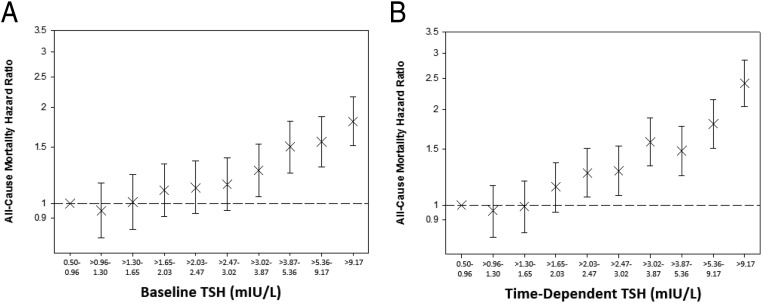
When examining deciles of baseline TSH, model 2 showed that TSH categories above a threshold of 3.0 mIU/L were consistently associated with greater death risk (Figure 4 and Supplemental Table 4). In model 3, this threshold was >9.2 mIU/L. When examining deciles of time-dependent TSH, we observed that TSH categories above a comparatively lower TSH threshold of 2.0 mIU/L were associated with higher mortality risk in model 2. However, this threshold was >9.2 mIU/L in model 3 (Figure 4 and Supplemental Table 4).
Figure 4.
The association between baseline (A) and time-dependent (B) TSH deciles and all-cause mortality. Analyses were adjusted for covariates in model 2: entry calendar quarter, age, sex, race/ethnicity, cause of ESRD, vascular access, dialysis vintage, BMI, diabetes, CHF, cerebrovascular disease, MI, other cardiac disease, hypertension, and PVD. Error bars represent 95% CIs.
Secondary subgroup analyses
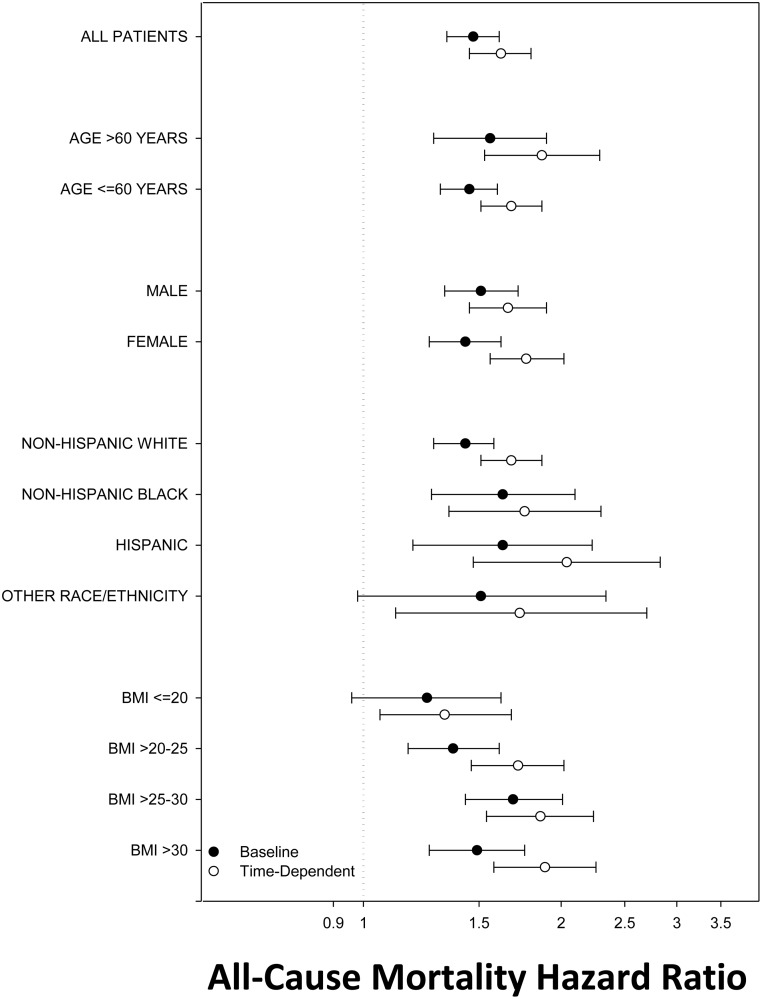
We then examined the association between hypothyroidism and mortality across clinically relevant subgroups in analyses adjusted for covariates in model 2 (Figure 5 and Supplemental Table 5). In analyses stratified by BMI level, there was an incrementally stronger association between time-dependent hypothyroidism and mortality across higher BMI categories. Similarly, in baseline analyses, hypothyroidism was associated with higher death risk in BMI categories >20 kg/m2 but not in the BMI category of ≤20 kg/m2. In analyses categorized by race/ethnicity, baseline and time-dependent hypothyroidism were each associated with higher mortality risk in non-Hispanic White, non-Hispanic Black, and Hispanic patients. Similarly, baseline and time-dependent hypothyroidism were each associated with higher mortality risk across all subcategories of age and sex.
Figure 5.
Subgroup analyses of the association between baseline and time-dependent hypothyroidism and all-cause mortality (reference group: euthyroidism). Analyses were adjusted for covariates in model 2: entry calendar quarter, age, sex, race/ethnicity, cause of ESRD, vascular access, dialysis vintage, BMI, diabetes, CHF, cerebrovascular disease, MI, other cardiac disease, hypertension, and PVD. Error bars represent 95% CIs.
Discussion
In this nationally representative cohort of incident HD patients, we found that baseline hypothyroidism was associated with a 47% higher death risk using multivariable models that accounted for differences in case-mix covariates across exposure groups. These associations were even stronger in time-dependent models that examined short-term thyroid function–mortality associations (ie, 62% increased death risk). When we examined finer gradations of thyroid function, we observed that higher TSH levels within the normal range (ie, above 2–3 mIU/L) were also associated with increased mortality risk in these patients.
In the general population, abundant data suggest that hypothyroidism adversely impacts CV health through multiple pathways. First, hypothyroidism may accelerate atherosclerosis through effects on endothelial dysfunction due to impaired vasodilator (eg, nitric oxide, adrenomedullin) synthesis and activity (34); increased systemic vascular resistance and diastolic hypertension (13); dyslipidemia due to reduced low-density lipoprotein receptor number and clearance, and decreased catabolism of cholesterol into bile (35); and hyperhomocysteinemia (34). Second, hypothyroidism engenders systolic and diastolic dysfunction via genomic (ie, altered myocyte contractility and relaxation gene transcription) and nongenomic effects (ie, membrane channel modulation) (13). Third, hypothyroidism may promote ventricular irritability and malignant arrhythmias due to changes in cardiac channel expression and subsequent prolongation of the cardiac action potential and QT interval (13). Fourth, hypothyroidism may exacerbate anemia (due to decreased erythropoietin production, iron deficiency, vitamin B12 deficiency, and blood loss associated with impaired hemostasis) (36–40), leading to greater ESA requirements (41), a known CV risk factor in HD patients (42, 43). Epidemiological data have also shown that hypothyroidism is associated with higher mortality, particularly in populations with underlying CV risk (18–20).
These data have prompted interest in hypothyroidism as a novel and potentially modifiable risk factor for CV morbidity and mortality in HD patients, in whom various thyroid functional derangement patterns have been observed (23). For example, recent data suggest that low T3 is highly prevalent in dialysis patients (44), and it is associated with atherosclerosis, coronary/vascular calcification, endothelial dysfunction, arterial stiffness, altered flow-mediated vasodilation, impaired left ventricular systolic function, increased left ventricular mass, and up to a 2.7- and 4-fold higher all-cause and CV death risk, respectively, in this population (45–51). However, the peripheral conversion of T4 to T3 by type 1 deiodinase enzyme (the source of 20% of T3) (52, 53) is highly sensitive to protein-energy wasting, inflammation, and uremia (53–56), which in and of themselves are independent predictors of CV disease and death (57–59). Hence, a solitary low T3 level is more likely to be a marker of nonthyroidal illness in the general and dialysis populations (54, 60). In contrast, TSH is considered the most sensitive and specific single measure of hypothyroidism in the general population, owing to its inverse logarithmic association with circulating thyroid hormones (ie, T3 and T4), and it is the preferred metric for the screening, diagnosis, and treatment monitoring of primary hypothyroidism (24–26). Although some TSH alterations may be observed in uremia in the absence of underlying thyroid disease (ie, altered clearance, response to TRH, pulsatility, half-life, glycosylation, and bioactivity) (44, 55), levels are typically normal in nonthyroidal illness (54).
Prior studies that have directly examined the association between hypothyroidism defined by TSH and mortality in the dialysis population have shown conflicting findings (8, 27). In a cohort of 2715 prevalent plus incident HD and peritoneal dialysis patients receiving care at two tertiary hospitals in Boston, hypothyroidism ascertained at baseline was associated with a 27% higher mortality risk independent of age, sex, diabetes, and noncardiovascular hospitalization in the preceding year (8). However, in a recent study comprised of 1000 German diabetic HD patients receiving dialysis ≤2 years and enrolled in the 4D Study (Die Deutsche Diabetes Dialyze Studie), subclinical hypothyroidism ascertained by elevated TSH and normal FT4 levels did not predict sudden cardiac death, CV events, or all-cause mortality; in sensitivity analyses that combined subclinical and overt hypothyroidism (elevated TSH and decreased FT4) into a single category, a null association was also observed (27). However, this lack of association may be explained by the small number of subclinical and overt hypothyroid patients (18 patients, or 1.8% of the cohort). In addition, this study was conducted among a select group of patients from a clinical trial who were more likely to be healthier and hence more robust to hypothyroid-related complications as compared to the general dialysis population. Moreover, whereas it is well known that thyroid hormone levels fluctuate over time and are influenced by comorbidities, nutritional status, and medications (46, 53, 54), both studies measured thyroid function at a single point in time and had limited ability to comprehensively adjust for plausible confounders of the hypothyroidism–mortality association.
To our knowledge, ours is the largest study of hypothyroidism and mortality in dialysis patients, as well as the first study to examine serial measurements of TSH that may change over time (ie, time-dependent TSH) in its relationship to subsequent death risk in a nationally representative incident HD population. In analyses of both baseline and time-dependent thyroid function, we observed that hypothyroidism was independently associated with higher mortality risk. Although baseline thyroid function–mortality analyses examine the impact of hypothyroidism on long-term survival, time-dependent thyroid function–mortality analyses assess short-term risk. In these time-dependent analyses, each patient's follow-up period was divided into multiple discrete time windows (determined by serial TSH measurements), and for each time window a separate Cox regression analysis was conducted using the TSH value at the start of the respective time window. A weighted average of all time window hazard ratios was then estimated as a single hazard ratio representing the short-term risk of TSH upon mortality (61). Thus, our findings suggest that hypothyroidism adversely impacts both short- and long-term survival in HD patients, which may be explained by the aforementioned diverse pathways by which thyroid hormone deficiency influences CV health.
At present, the optimal TSH range in dialysis patients remains unknown. In the general population, some experts have suggested that the upper limit of normal be considered to be 2.5–3.0 mIU/L instead of 4.0–5.0 mIU/L, whereas others have recommended using age- and race/ethnicity-based normal ranges (28–31). Our study found that higher TSH levels, even in the normal range, were associated with higher death risk in analyses adjusted for case-mix covariates. In time-dependent analyses, we observed an attenuation of these associations toward the null after adjustment for laboratory and medication covariates; however, these latter findings should be tempered by the realization that there may have been overadjustment for potential causal pathway intermediates of the hypothyroidism–mortality association (eg, hemoglobin, iron indices).
Another novel finding in our study was the differential relationship between hypothyroidism and mortality across subgroups of BMI. In time-dependent analyses, these associations were incrementally stronger across higher levels of BMI. Similarly, in baseline analyses, we observed a significant association between hypothyroidism and mortality in BMI categories >20 kg/m2 but not in the BMI category ≤20 kg/m2. A possible explanation for the null findings in the lowest BMI category may be that the ill CV effects of hypothyroidism are abrogated in underweight dialysis patients among whom reduced thyroid function is a means to conserve metabolism. Indeed, early studies suggested that thyroid hormone deficiency (ie, low T3) may be a physiological adaptation in dialysis patients among whom hypercatabolism, protein-energy wasting, and dialytic protein losses are frequently observed and are potent predictors of mortality (32). Additional study is warranted to elaborate the relationship between thyroid function, nutritional status, body mass, and mortality in dialysis patients.
The strengths of our study include its examination of a large, nationally representative cohort of dialysis patients; focus upon an incident HD cohort whose characteristics are not confounded by survivor bias and whose mortality risk may differ from that of prevalent patients (62); comprehensive availability of detailed, longitudinal patient-level comorbidity, laboratory, and dialysis-treatment data; and laboratory data collected in the outpatient setting and uniformly measured at a single laboratory facility. Several limitations should be acknowledged. First, included patients were required to have one or more TSH measurements, which were conducted at the discretion of health care providers. Although the indications for TSH testing are unknown, these patients may have had a higher pretest probability of thyroid functional disease, and Supplemental Table 1 suggests that included patients had a higher baseline comorbidity burden compared to excluded patients, which may impact generalizability. Notwithstanding potential confounding by indication, the mortality-predictability of incrementally higher TSH levels was robust, independent of sociodemographics and comorbid conditions. Second, due to their sparse measurement, we were unable to distinguish between subclinical and overt hypothyroidism using FT4 levels. However, it should be noted that FT4 assays routinely used in the clinical setting (eg, FT4 analog assay or FT4 index) are hormone-protein binding dependent and may result in spurious results in conditions where serum protein levels are low (eg, malnutrition) or circulating substances impair hormone-protein binding (eg, uremia) (55, 63). Third, due to data limitations, we were unable to determine which patients were receiving thyroid hormone replacement therapy or the impact of treatment on outcomes. Fourth, we had limited ability to ascertain cause of death (eg, CV). Lastly, as with all observational studies, our findings do not confirm a causal association between hypothyroidism and mortality.
In conclusion, our study suggests that hypothyroidism is associated with heightened death risk in incident HD patients. Additionally, higher TSH levels, even within the normal range, predict mortality risk in this population. At this time, further studies are needed to investigate mechanistic pathways by which hypothyroidism adversely impacts survival and to determine whether correction of hypothyroidism with thyroid hormone replacement therapy (eg, levothyroxine) (64) leads to improved outcomes in dialysis patients.
Acknowledgments
We thank DaVita Clinical Research for providing the clinical data, analysis, and review for this research project and for advancing the knowledge and practice of kidney care.
The authors are supported by research grants from the National Institutes of Health/National Institute of Diabetes and Digestive and Kidney Diseases, including K23-DK102903 (to C.M.R.), K24-DK091419 (to K.K.-Z.), R01DK09568 (to R.M. and K.K.-Z.), R01-DK078106 (to K.K.-Z.), R01-DK096920 (to C.P.K. and K.K.-Z.), U01-DK102163 (to K.K.-Z. and C.P.K.), and UL1 TR000153 (to D.V.N.), and philanthropist grants from Mr. Harold Simmons and Mr. Louis Chang.
Portions of these data have been presented as an oral abstract at the American Society of Nephrology Kidney Week Conference, November 11–16, 2014, Philadelphia, PA.
Disclosure Summary: None of the authors declare any relevant conflicts of interest.
Footnotes
- aHR
- adjusted hazard ratio
- AVF
- arteriovenous fistula
- AVG
- arteriovenous fistula
- BMI
- body mass index
- CHF
- congestive heart failure
- CI
- confidence interval
- CV
- cardiovascular
- ESA
- erythropoietin stimulating agent
- ESRD
- end-stage renal disease
- FT4
- free T4
- HD
- hemodialysis
- LDO
- large dialysis organization
- MI
- myocardial infarction
- PVD
- peripheral vascular disease.
References
- 1. Bando Y, Ushiogi Y, Okafuji K, Toya D, Tanaka N, Miura S. Non-autoimmune primary hypothyroidism in diabetic and non-diabetic chronic renal dysfunction. Exp Clin Endocrinol Diabetes. 2002;110(8):408–415. [DOI] [PubMed] [Google Scholar]
- 2. Chonchol M, Lippi G, Salvagno G, Zoppini G, Muggeo M, Targher G. Prevalence of subclinical hypothyroidism in patients with chronic kidney disease. Clin J Am Soc Nephrol. 2008;3(5):1296–1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kaptein EM, Quion-Verde H, Chooljian CJ, et al. The thyroid in end-stage renal disease. Medicine. 1988;67(3):187–197. [DOI] [PubMed] [Google Scholar]
- 4. Kutlay S, Atli T, Koseogullari O, Nergizoglu G, Duman N, Gullu S. Thyroid disorders in hemodialysis patients in an iodine-deficient community. Artif Organs. 2005;29(4):329–332. [DOI] [PubMed] [Google Scholar]
- 5. Lin CC, Chen TW, Ng YY, Chou YH, Yang WC. Thyroid dysfunction and nodular goiter in hemodialysis and peritoneal dialysis patients. Perit Dial Int. 1998;18(5):516–521. [PubMed] [Google Scholar]
- 6. Lo JC, Chertow GM, Go AS, Hsu CY. Increased prevalence of subclinical and clinical hypothyroidism in persons with chronic kidney disease. Kidney Int. 2005;67(3):1047–1052. [DOI] [PubMed] [Google Scholar]
- 7. Ng YY, Wu SC, Lin HD, et al. Prevalence of clinical and subclinical thyroid disease in a peritoneal dialysis population. Perit Dial Int. 2012;32(1):86–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Rhee CM, Alexander EK, Bhan I, Brunelli SM. Hypothyroidism and mortality among dialysis patients. Clin J Am Soc Nephrol. 2013;8(4):593–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Shantha GP, Kumar AA, Bhise V, Khanna R, Sivagnanam K, Subramanian KK. Prevalence of subclinical hypothyroidism in patients with end-stage renal disease and the role of serum albumin: a cross-sectional study from south India. Cardiorenal Med. 2011;1(4):255–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Targher G, Chonchol M, Zoppini G, et al. Prevalence of thyroid autoimmunity and subclinical hypothyroidism in persons with chronic kidney disease not requiring chronic dialysis. Clin Chem Lab Med. 2009;47(11):1367–1371. [DOI] [PubMed] [Google Scholar]
- 11. Cappola AR, Ladenson PW. Hypothyroidism and atherosclerosis. J Clin Endocrinol Metab. 2003;88(6):2438–2444. [DOI] [PubMed] [Google Scholar]
- 12. Hak AE, Pols HA, Visser TJ, Drexhage HA, Hofman A, Witteman JC. Subclinical hypothyroidism is an independent risk factor for atherosclerosis and myocardial infarction in elderly women: the Rotterdam Study. Ann Intern Med. 2000;132(4):270–278. [DOI] [PubMed] [Google Scholar]
- 13. Klein I, Ojamaa K. Thyroid hormone and the cardiovascular system. N Engl J Med. 2001;344(7):501–509. [DOI] [PubMed] [Google Scholar]
- 14. Owen PJ, Sabit R, Lazarus JH. Thyroid disease and vascular function. Thyroid. 2007;17(6):519–524. [DOI] [PubMed] [Google Scholar]
- 15. Pearce EN. Update in lipid alterations in subclinical hypothyroidism. J Clin Endocrinol Metab. 2012;97(2):326–333. [DOI] [PubMed] [Google Scholar]
- 16. Gencer B, Collet TH, Virgini V, et al. Subclinical thyroid dysfunction and the risk of heart failure events: an individual participant data analysis from 6 prospective cohorts. Circulation. 2012;126(9):1040–1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Rodondi N, den Elzen WP, Bauer DC, et al. Subclinical hypothyroidism and the risk of coronary heart disease and mortality. JAMA. 2010;304(12):1365–1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Iervasi G, Molinaro S, Landi P, et al. Association between increased mortality and mild thyroid dysfunction in cardiac patients. Arch Intern Med. 2007;167(14):1526–1532. [DOI] [PubMed] [Google Scholar]
- 19. McQuade C, Skugor M, Brennan DM, Hoar B, Stevenson C, Hoogwerf BJ. Hypothyroidism and moderate subclinical hypothyroidism are associated with increased all-cause mortality independent of coronary heart disease risk factors: a PreCIS database study. Thyroid. 2011;21(8):837–843. [DOI] [PubMed] [Google Scholar]
- 20. Molinaro S, Iervasi G, Lorenzoni V, et al. Persistence of mortality risk in patients with acute cardiac diseases and mild thyroid dysfunction. Am J Med Sci. 2012;343(1):65–70. [DOI] [PubMed] [Google Scholar]
- 21. Wheeler DC, Haynes R, Landray MJ, Baigent C. Cardiovascular aspects of kidney disease. In: Taal MW, Chertow GM, Marsden PA, Skorecki K, Yu AS, Brenner BM, eds. Brenner and Rector's The Kidney. 9th ed Philadelphia, PA: Elsevier Saunders; 2011:2059–2080. [Google Scholar]
- 22. US Renal Data System. USRDS 2013 Annual Data Report: Atlas of Chronic Kidney Disease and End-Stage Renal Disease in the United States. Bethesda, MD: National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; 2013. [Google Scholar]
- 23. Rhee CM, Brent GA, Kovesdy CP, et al. Thyroid functional disease: an under-recognized cardiovascular risk factor in kidney disease patients [published online February 25, 2014]. Nephrol Dial Transplant. doi:10.1093/ndt/gfu024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ladenson PW. Diagnosis of hypothyroidism. In: Braverman LE, Cooper DS, eds. Werner and Ingbar's The Thyroid. 10th ed Chap 45 Philadelphia, PA: Lippincott Williams and Wilkins; 2013:606–611. [Google Scholar]
- 25. Jonklaas J. Treatment of hypothyroidism. In: Braverman LE, Cooper DS, eds. Werner and Ingbar's The Thyroid. 10th ed Chap 46 Philadelphia, PA: Lippincott Williams and Wilkins; 2013:611–628. [Google Scholar]
- 26. Larsen PR. Thyroid-pituitary interaction: feedback regulation of thyrotropin secretion by thyroid hormones. N Engl J Med. 1982;306(1):23–32. [DOI] [PubMed] [Google Scholar]
- 27. Drechsler C, Schneider A, Gutjahr-Lengsfeld L, et al. Thyroid function, cardiovascular events, and mortality in diabetic hemodialysis patients. Am J Kidney Dis. 2014;63(6):988–996. [DOI] [PubMed] [Google Scholar]
- 28. Baloch Z, Carayon P, Conte-Devolx B, et al. Laboratory medicine practice guidelines. Laboratory support for the diagnosis and monitoring of thyroid disease. Thyroid. 2003;13(1):3–126. [DOI] [PubMed] [Google Scholar]
- 29. Baskin HJ, Cobin RH, Duick DS, et al. American Association of Clinical Endocrinologists medical guidelines for clinical practice for the evaluation and treatment of hyperthyroidism and hypothyroidism. Endocr Pract. 2002; 8(6):457–469. [PubMed] [Google Scholar]
- 30. Surks MI, Boucai L. Age- and race-based serum thyrotropin reference limits. J Clin Endocrinol Metab. 2010;95(2):496–502. [DOI] [PubMed] [Google Scholar]
- 31. Wartofsky L, Dickey RA. The evidence for a narrower thyrotropin reference range is compelling. J Clin Endocrinol Metab. 2005;90(9):5483–5488. [DOI] [PubMed] [Google Scholar]
- 32. Lim VS. Thyroid function in patients with chronic renal failure. Am J Kidney Dis. 2001;38(4 suppl 1):S80–S84. [DOI] [PubMed] [Google Scholar]
- 33. Little RJ, Rubin DB. Statistical Analysis with Missing Data. Hoboken, NJ: John Wiley and Sons; 2002. [Google Scholar]
- 34. Klein I, Danzi S. Thyroid disease and the heart. Circulation. 2007;116(15):1725–1735. [DOI] [PubMed] [Google Scholar]
- 35. Klein I. Endocrine disorders and cardiovascular disease. In: Bonow RO, Mann DL, Zipes DP, Libby P, eds. Braunwald's Heart Disease. 9th ed Chap 86 Philadelphia, PA: Elsevier Saunders; 2011:1829–1843. [Google Scholar]
- 36. Chadarevian R, Bruckert E, Leenhardt L, Giral P, Ankri A, Turpin G. Components of the fibrinolytic system are differently altered in moderate and severe hypothyroidism. J Clin Endocrinol Metab. 2001;86(2):732–737. [DOI] [PubMed] [Google Scholar]
- 37. Cinemre H, Bilir C, Gokosmanoglu F, Bahcebasi T. Hematologic effects of levothyroxine in iron-deficient subclinical hypothyroid patients: a randomized, double-blind, controlled study. J Clin Endocrinol Metab. 2009;94(1):151–156. [DOI] [PubMed] [Google Scholar]
- 38. Donati RM, Fletcher JW, Warnecke MA, Gallagher NI. Erythropoiesis in hypothyroidism. Proc Soc Exp Biol Med. 1973;144(1):78–82. [DOI] [PubMed] [Google Scholar]
- 39. Erdogan M, Kösenli A, Ganidagli S, Kulaksizoglu M. Characteristics of anemia in subclinical and overt hypothyroid patients. Endocr J. 2012;59(3):213–220. [DOI] [PubMed] [Google Scholar]
- 40. Fein HG, Rivlin RS. Anemia in thyroid diseases. Med Clin North Am. 1975;59(5):1133–1145. [DOI] [PubMed] [Google Scholar]
- 41. Nassar A, Saqi A, Baloch Z, LiVolsi V. Carcinoma showing thymuslike element of the thyroid. Acta Cytol. 2003;47(2):313–315. [PubMed] [Google Scholar]
- 42. Singh AK. What is causing the mortality in treating the anemia of chronic kidney disease: erythropoietin dose or hemoglobin level? Curr Opin Nephrol Hypertens. 2010;19(5):420–424. [DOI] [PubMed] [Google Scholar]
- 43. Szczech LA, Barnhart HX, Inrig JK, et al. Secondary analysis of the CHOIR trial epoetin-α dose and achieved hemoglobin outcomes. Kidney Int. 2008;74(6):791–798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Carrero JJ, Stenvinkel P, Lindholm B. Endocrine aspects of chronic kidney disease. In: Taal MW, Chertow GM, Marsden PA, Skorecki K, Yu AS, Brenner BM, eds. Brenner and Rector's The Kidney. 9th ed Chap 57 Philadelphia, PA: Elsevier Saunders; 2011:2122–2137. [Google Scholar]
- 45. Meuwese CL, Carrero JJ, Cabezas-Rodríguez I, et al. Nonthyroidal illness: a risk factor for coronary calcification and arterial stiffness in patients undergoing peritoneal dialysis? J Intern Med. 2013;274(6):584–593. [DOI] [PubMed] [Google Scholar]
- 46. Meuwese CL, Dekker FW, Lindholm B, et al. Baseline levels and trimestral variation of triiodothyronine and thyroxine and their association with mortality in maintenance hemodialysis patients. Clin J Am Soc Nephrol. 2012;7(1):131–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Tatar E, Kircelli F, Asci G, et al. Associations of triiodothyronine levels with carotid atherosclerosis and arterial stiffness in hemodialysis patients. Clin J Am Soc Nephrol. 2011;6(9):2240–2246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Tatar E, Sezis Demirci M, Kircelli F, et al. The association between thyroid hormones and arterial stiffness in peritoneal dialysis patients. Int Urol Nephrol. 2012;44(2):601–606. [DOI] [PubMed] [Google Scholar]
- 49. Yilmaz MI, Sonmez A, Karaman M, et al. Low triiodothyronine alters flow-mediated vasodilatation in advanced nondiabetic kidney disease. Am J Nephrol. 2011;33(1):25–32. [DOI] [PubMed] [Google Scholar]
- 50. Zoccali C, Benedetto F, Mallamaci F, et al. Low triiodothyronine and cardiomyopathy in patients with end-stage renal disease. J Hypertens. 2006;24(10):2039–2046. [DOI] [PubMed] [Google Scholar]
- 51. Zoccali C, Mallamaci F, Tripepi G, Cutrupi S, Pizzini P. Low triiodothyronine and survival in end-stage renal disease. Kidney Int. 2006;70(3):523–528. [DOI] [PubMed] [Google Scholar]
- 52. Brent GA. Mechanisms of thyroid hormone action. J Clin Invest. 2012;122(9):3035–3043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Langton JE, Brent GA. Nonthyroidal illness syndrome: evaluation of thyroid function in sick patients. Endocrinol Metab Clin North Am. 2002;31(1):159–172. [DOI] [PubMed] [Google Scholar]
- 54. Wiersinga WM, Van den Berghe G. Nonthyroidal illness syndrome. In: Braverman LE, Cooper DS, eds. Werner and Ingbar's The Thyroid. 10th ed Chap 11C Philadelphia, PA: Lippincott Williams and Wilkins; 2012:203–216. [Google Scholar]
- 55. Kaptein EM. Thyroid hormone metabolism and thyroid diseases in chronic renal failure. Endocr Rev. 1996;17(1):45–63. [DOI] [PubMed] [Google Scholar]
- 56. Mariani LH, Berns JS. The renal manifestations of thyroid disease. J Am Soc Nephrol. 2012;23(1):22–26. [DOI] [PubMed] [Google Scholar]
- 57. Himmelfarb J, Stenvinkel P, Ikizler TA, Hakim RM. The elephant in uremia: oxidant stress as a unifying concept of cardiovascular disease in uremia. Kidney Int. 2002;62(5):1524–1538. [DOI] [PubMed] [Google Scholar]
- 58. Kalantar-Zadeh K, Ikizler TA, Block G, Avram MM, Kopple JD. Malnutrition-inflammation complex syndrome in dialysis patients: causes and consequences. Am J Kidney Dis. 2003;42(5):864–881. [DOI] [PubMed] [Google Scholar]
- 59. Kalantar-Zadeh K, Kopple JD. Relative contributions of nutrition and inflammation to clinical outcome in dialysis patients. Am J Kidney Dis. 2001;38(6):1343–1350. [DOI] [PubMed] [Google Scholar]
- 60. Meuwese CL, Dekkers OM, Stenvinkel P, Dekker FW, Carrero JJ. Nonthyroidal illness and the cardiorenal syndrome. Nat Rev Nephrol. 2013;9(10):599–609. [DOI] [PubMed] [Google Scholar]
- 61. Dekker FW, de Mutsert R, van Dijk PC, Zoccali C, Jager KJ. Survival analysis: time-dependent effects and time-varying risk factors. Kidney Int. 2008;74(8):994–997. [DOI] [PubMed] [Google Scholar]
- 62. Van Biesen W, Vanholder R, Debacquer D, De Backer G, Lameire N. Comparison of survival on CAPD and haemodialysis: statistical pitfalls. Nephrol Dial Transplant. 2000;15(3):307–311. [DOI] [PubMed] [Google Scholar]
- 63. Soldin OP. Measuring serum thyroid-stimulating hormone, thyroid hormones, thyroid-directed antibodies, and transport proteins. In: Braverman LE, Cooper DS, eds. Werner and Ingbar's The Thyroid. 10th ed Chap 13A Philadelphia, PA: Lippincott Williams and Wilkins; 2012:279–296. [Google Scholar]
- 64. Jonklaas J, Bianco AC, Bauer AJ, et al. Guidelines for the treatment of hypothyroidism: prepared by the American Thyroid Association Task Force on thyroid hormone replacement. Thyroid. 2014;24(12):1670–751. [DOI] [PMC free article] [PubMed] [Google Scholar]