Abstract
Neural precursor cell expressed developmentally down-regulated protein 4 (Nedd4) is the prototypical protein in the Nedd4 ubiquitin ligase (E3) family, which governs ubiquitin-dependent endocytosis and/or degradation of plasma membrane proteins. Loss of Nedd4 results in embryonic or neonatal lethality in mice and reduced insulin/IGF-1 signaling in embryonic fibroblasts. To delineate the roles of Nedd4 in vivo, we examined the phenotypes of heterozygous knockout mice using a high-fat diet-induced obesity (HFDIO) model. We observed that Nedd4+/− mice are moderately insulin resistant but paradoxically protected against HFDIO. After high-fat diet feeding, Nedd4+/− mice showed less body weight gain, less fat mass, and smaller adipocytes vs the wild type. Despite ameliorated HFDIO, Nedd4+/− mice did not manifest improvement in glucose tolerance vs the wild type in both genders. Nedd4+/− male, but not female, mice displayed significantly lower fasting blood glucose levels and serum insulin levels. Under obesogenic conditions, Nedd4+/− mice displayed elevated stimulated lipolytic activity, primarily through a β2-adrenergic receptor. Combined, these data support novel complex roles for Nedd4 in metabolic regulation involving altered insulin and β-adrenergic signaling pathways.
Obesity remains epidemic among developed nations and a major independent risk factor for the development of metabolic syndrome, type 2 diabetes mellitus, cardiovascular disease, and certain types of cancer (1). Overeating and sedentary lifestyles promote obesity, but the molecular bases of obesity are incompletely understood (2). Intracellular ubiquitin-mediated proteolysis is crucial for most biological processes and requires the sequential and cooperative action of ubiquitin-activating enzymes (E1s), ubiquitin-conjugating enzymes (E2s) and ubiquitin ligases (E3s). Although E1s and E2s process ubiquitin, E3s specify substrates and have been substantially studied in diseases associated with abnormal protein accumulation, such as neurodegeneration and cancer (3, 4). Few data exist about the metabolic functions of this enzyme class. Recently several members of the E3 ubiquitin ligase family have been implicated in metabolic regulation, as evident by phenotypes of mice knocked out for mitsugumin 53 (MG53) (5), Itchy homolog E3 ubiquitin protein ligase (Itch) (6), and casitas b-lineage lymphoma (Cbl)-b and c-Cbl (two members of the Cbl family) (7–10). These observations on E3 ligases in obese models suggest potentially important roles for E3 ligases in metabolism.
Rsp5p is the most well-studied yeast E3 ligase and participates in several important cellular processes including lipid and membrane metabolism (11). Its mammalian homologues, a neural precursor cell expressed developmentally down-regulated protein 4 (Nedd4) and Itch are two structurally related E3 ligases belonging to the nine-member Nedd4 family. Nedd4 and Itch both recognize proline-rich consensus sequence motifs (eg, PY and PPLP) and thereby ubiquitinate several overlapping targets in vitro (12), including the aforementioned Cbl proteins (13). Other Nedd4 substrates, such as type 1 insulin-like growth factor receptor (IGF-1R) (14, 15) and fibroblast growth factor receptor 1 (FGFR1) (16), exert central roles in receptor tyrosine kinase signaling associated with metabolic pathways. Because Nedd4 knockout mice have the embryonic/perinatal lethal phenotype, we applied a high-fat diet-induced obesity (HFDIO) model to characterize Nedd4-haploinsufficient (Nedd4+/−) mice. Here we show that partial depletion of Nedd4 reduces insulin signaling in vivo but attenuates HFDIO progression to morbid obesity. High-fat diet (HFD)-fed Nedd4+/− mice display enhanced lipolysis and higher membranous β-2 adrenergic receptor (β2-AR) expression in white adipose tissue (WAT), compared with the wild-type mice on a HFD. These findings suggest that distinct metabolic pathways regulated by Nedd4 may converge to oppose obesity.
Materials and Methods
Animals
Nedd4+/− mice (Nedd4Gt(OST16211)Lex) on a mixed 129S5/SvEvBrd × C57BL/6J background were obtained from the Mutant Mouse Regional Resource Center (University of California, Davis) as previously described (17) and were backcrossed at least 10 times onto the C57BL/6J background. All mice were kept on a 12-hour light, 12-hour dark cycle, with lights on at 6:00 am in a pathogen-free animal facility, with ad libitum access to food and water. Food was removed only when required for an experiment. Starting at 6 weeks of age, mice were fed either a control normal diet (ND; as 8640; Teklad, Harlan Laboratories) with 17% of total calories from fat, 54% from carbohydrate, and 29% from protein, or HFD (TD.06414; Teklad, Harlan Laboratories) with 60.3% of total calories from fat, 21.3% from carbohydrate, and 18.4% from protein. Body weights were monitored weekly with a scale. All animal experiments were approved by the Institutional Animal Care and Use Committee of The University of Tennessee Health Science Center.
Metabolic analyses
Mice were fasted overnight and then weighed prior to a glucose tolerance test (GTT). Glucose was ip injected at 1.5 g/kg body weight. Blood was collected from the lateral saphenous vein. Blood glucose level was measured with a glucometer (OneTouch Ultra; LifeScan) in a drop of blood at time 0 (before ip glucose) and 30, 60, 90, 120, and 180 minutes after glucose injection. For the insulin tolerance test (ITT), mice were fasted for 4 hours before injection of insulin (Eli Lilly) at 0.75 U/kg body weight. Blood glucose concentrations were obtained at time 0 and then 30, 60, and 90 minutes after insulin administration. Sera were separated from clotted blood by centrifugation at 1600 × g for 10 minutes at 4°C and then stored at −80°C until analyses. Serum insulin levels were quantified with a murine ELISA kits (Crystal Chem).
In vivo insulin signaling protein analysis
For the peripheral insulin signaling analyses, after an overnight fast, adult male mice (5 mo old) received regular insulin 5 U/kg ip diluted in sterile normal saline. Mice were euthanized 15 minutes after the injection. Tissues were dissected and snap frozen prior to Western blot analyses performed as previously described (18). The tissues were lysed in radioimmunoprecipitation assay buffer [25 mM Tris-HCl (pH 7.6), 150 mM NaCl, 1% Nonidet P-40, 1% sodium deoxycholate, 0.1% sodium dodecyl sulfate] containing protease and phosphatase inhibitors. Cell debris in the lysates was removed by centrifugation at 4°C.
Quantitative real-time PCR
Total RNA was isolated using Trizol reagent (Invitrogen). One milliliter of Trizol reagent was applied per 50–100 mg of tissue. Cells were lysed by using a homogenizer. A proportional volume of chloroform was added to the samples (0.2 mL per 1 mL of Trizol reagent used) and mixed thoroughly by shaking for 15 seconds. Homogenates were allowed to sit at room temperature for 2–3 minutes before centrifugation at 12 000 × g for 15 minutes. The following steps were performed as described previously (18). Primer sequences were: leptin, forward 5′-CAGGATCAATGACATTTCACACA-3′, reverse 5′-GCTGGTGAGGACCTG; and TTGAT-3′; gapdh, forward 5′-GCAAATTCAACGGCACAG-3′, reverse 5′-CTCGCTCCTGGAAGATGG-3′.
Histology
Mice were perfused with 4% paraformaldehyde and postfixed for 24 hours. Epididymal adipose tissue was dissected and subjected to paraffin embedding. Paraffin-embedded tissue blots were sectioned to 10 μm using a manual rotary microtome (Leica RM 2125). Hematoxylin and eosin (H&E) staining of sections was performed using standard procedures (19). Adipocyte size was measured within micrographs of 300–400 cells per mouse from randomly selected fields using ImageJ software (National Institutes of Health, Bethesda, Maryland; http://imagej.nih.gov/ij/).
Lipolysis assay
Adapted from a previous study (20), the in vivo lipolysis assay used the nonselective β-agonist isoproterenol, which was injected ip at 10 mg/kg body weight after overnight fast. Blood samples were collected at time 0 and then 10, 30, and 60 minutes after injection. For primary adipocyte lipolysis assays (21, 22), epididymal WAT was minced and digested in a Krebs-Ringer bicarbonate (KRB) buffer (pH 7.4), supplemented with 3% fatty acid-free BSA fraction V, 0.5 mM adenosine, and 1 mg/mL type I collagenase at 37°C for 45 minutes. After digestion and three washes with KRB plus 3% fatty acid-free BSA, the dissociated adipocytes were manually counted with a hemocytometer under light microscopy. Approximately 12 000–20 000 adipocytes were resuspended in 400 μL KRB buffer plus 3% fatty acid-free BSA. Isoproterenol was then added to the buffer to a final concentration of 1 μM. After 1 hour of incubation at 37°C with gentle shaking, the samples were centrifuged, and the infranatant was removed for storage at −80°C before use. The glycerol levels were measured using a colorimetric assay kit (Cayman) according to the manufacturer's instructions.
Crude membrane fraction preparation
Purified adipocytes were resuspended in one volume of a buffer of 10 mM Tris-HCl (pH 7.5), 1 mM EDTA (pH 8.0), and 250 mM sucrose prewarmed to 37°C supplemented with protease and phosphatase inhibitors. The adipocytes were homogenized at room temperature by six strokes of a large-bore potter-Elvehjem homogenizer (Arthur H. Thomas Co) fitted with a serrated-edge Teflon pestle. The homogenizer was driven by an Eberbach Con-torque motor operating at maximum speed. The homogenate was then transferred to a round bottom tube and spun at 50 000 × g for 30 minutes at 4°C. The pellet containing the crude fat cell membrane was resuspended in 10 volumes of 50 mM Tris HCl and 10 mM MgCl2 (pH 8.0), containing protease and phosphatase inhibitors at 4°C, and homogenized for 10 strokes. The homogenate was spun at 15 000 × g for 15 minutes at 4°C. The pellet was resuspended in radioimmunoprecipitation assay buffer on ice for at least 1 hour. The lysate was spun again at maximum speed. The supernatant containing the membrane proteins was transferred to a new tube for Western blot analysis.
Chemicals and antibodies
Hematoxylin, eosin, and (−)-isoproterenol hydrochloride were purchased from Sigma-Aldrich. ICI 118551 hydrochloride, CGP-20712 dihydrochloride, xamoterol hemifumarate, and salmeterol xinafoate were from Toris Bioscience. Anti-Nedd4 antibody was purchased from EMD Millipore. Anti-β-actin antibody was from Sigma-Aldrich. Anti-β2-AR (H-20) and anti-glyceraldehyde-3-phosphate dehydrogenase (GAPDH) antibodies were purchased from Santa Cruz Biotechnology. Anti-phospho-IGF-1Rβ (Tyr1135/1136)/insulin receptor (IR)-β (Tyr1150/1151) (19H7), anti-IRβ, anti-IGF-1Rβ, anti-AKT, anti-phospho-AKT (Thr308), and antiphospho-AKT (Ser473) antibodies were purchased from Cell Signaling Technology.
Statistical analysis
Data represent mean ± SEM. Statistical significance was determined by a two-tailed, paired Student's t test between two groups.
Results
Nedd4+/− mice are partially protected against HFDIO
Nedd4+/− mice lack an overt phenotype. They are fertile and viable to at least 18 months of age. We performed genotyping PCR and Western blot analyses to confirm the genotypes (Supplemental Figure 1A and B). In Nedd4+/− mice, at least a 50% reduction in Nedd4 protein levels was identified in all organs tested (Supplemental Figure 1C).
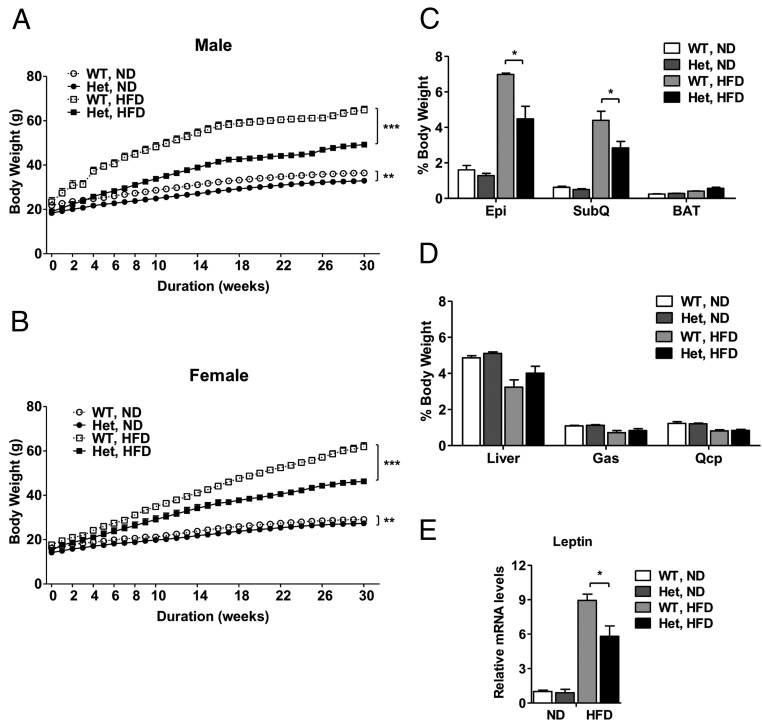
To elucidate the metabolic phenotypes of Nedd4+/− mice, we placed a cohort of Nedd4+/− and wild-type littermates on either normal diet (ND, 17% calories from fat) or HFD (60% calories from fat) starting at age 6 weeks until age 9 months. When fed ND, Nedd4+/− mice of both genders were significantly lighter than their wild-type control (3.66 ± 0.05 g for males, 1.90 ± 0.03 g for females; P < .01). However, in response to HFD, Nedd4+/− mice of both genders demonstrated profoundly less weight gain vs wild type. After 30 weeks of HFD feeding, overall weight gain in Nedd4+/− mice was 30% lower than controls (P < .001) (Figure 1, A and B, and Supplemental Figure 2A). These results were reproduced in at least three generations of mice. Average anus-to-nose length of Nedd4+/− male mice was 0.21 ± 0.05 cm (∼2.4%) shorter than the wild types at age 22 weeks (P < .01) (Supplemental Figure 2B). Postmortem dissection for epididymal, inguinal sc white, and interscapular brown fat depots revealed a significantly reduced accumulation of visceral and sc fat, but not interscapular brown fat, in HFD-fed Nedd4+/− mice compared with controls. Ratios of epididymal and inguinal sc WAT mass vs body mass were approximately 36% lower in Nedd4+/− relative to control mice (P < .05), whereas the ratios of liver and skeletal muscle to body weight were similar between two genotypes (Figure 1, C and D, and Supplemental Figure 2C). In addition, we measured the leptin mRNA levels in epididymal WAT in mice after 4 months of HFD feeding from both genders. The leptin mRNA levels were 32.5% lower in Nedd4+/− mice (P < .05) (Figure 1E), consistent with leptin as an indicator of body fat (23). Physical activity and relative energy intake were unaltered in Nedd4+/− mice vs wild type (Supplemental Figure 3).
Figure 1. Nedd4+/− mice partially resist HFDIO.
A and B, Body weight curves of male (A) and female (B) littermates fed ND or HFD ad libitum over a 30-week period starting at age 6 weeks (n = 8–11). Body weights were monitored weekly. C, Percentages of epididymal (Epi), inguinal sc (SubQ), or brown adipose tissue (BAT) depot weights to total body weight in wild-type (WT) or Nedd4+/− (Het) male mice at age 22 weeks (n = 5–7). D, Percentages of liver, gastrocnemius (Gas), or quadriceps (Qcp) weight to total body weight in WT or Het male mice at age 22 weeks (n = 5–7). E, Leptin mRNA levels in epididymal fat were measured by quantitative real-time PCR and normalized to gapdh mRNA levels (ND, n = 3; HFD, n = 5–6 with both genders). Data represent mean ± SEM. *, P < .05, **; P < .01; ***, P < .001.
Insulin sensitivity and glucose homeostasis in Nedd4+/− mice
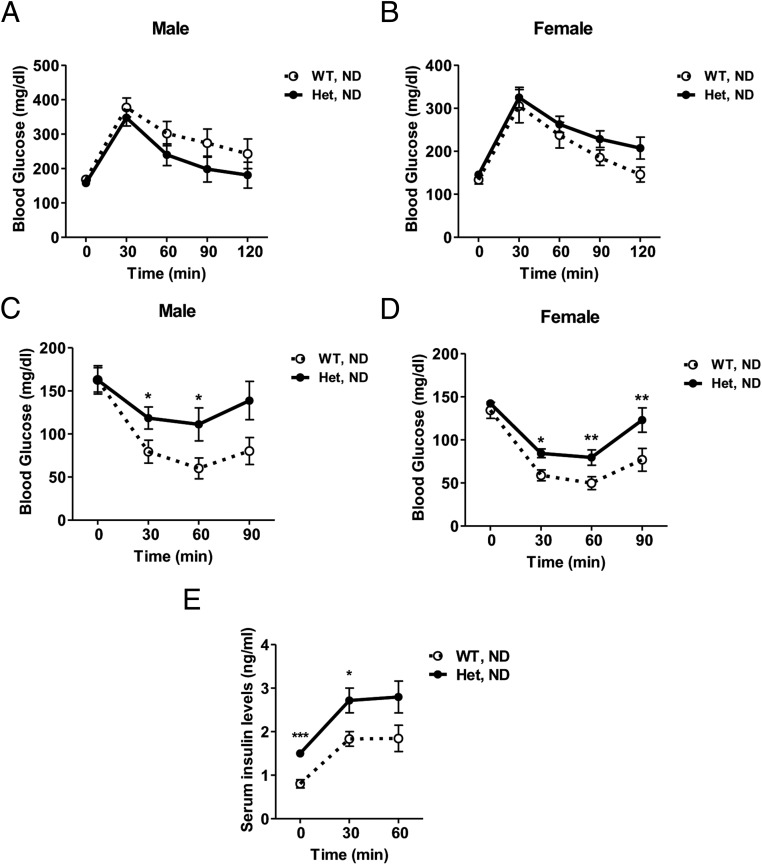
To examine whole-body insulin sensitivity, we performed glucose and insulin tolerance tests (GTT and ITT) in gender-matched littermates. On ND feeding, Nedd4+/− mice were glucose tolerant but showed impaired insulin tolerance during ITT for both genders (Figure 2, A–D), indicating reduced insulin sensitivity. Consistent with these observations, Nedd4+/− mice showed a small but significant 1.5-fold increase in fasting serum insulin levels and circulating insulin during GTT (Figure 2E). To delineate tissue-specific insulin sensitivity in male mice, we analyzed insulin-stimulated signaling in WAT, skeletal muscle, and liver. After a single ip injection of regular insulin, Nedd4+/− mice on ND displayed significantly reduced tyrosine phosphorylation of IR and their downstream protein kinase B/AKT than wild-type controls, consistent with the ITT results (Figure 3, A–C). Previous studies have implicated that Nedd4 regulates protein levels of growth factor receptor-bound protein 10 (Grb10) (24) and phosphatase and tensin homolog deleted from chromosome 10 (PTEN) (25, 26), two negative regulators of insulin signaling. In the present study, we did not observe any increased expression of either Grb10 or PTEN (Supplemental Figure 4, A and B).
Figure 2. Nedd4+/− mice on ND are insulin resistant but glucose tolerant.
A and B, GTT was carried out in wild-type (WT) and Nedd4+/− (Het) mice on ND. Mice fasted overnight were ip injected with glucose at 1.5 g/kg body weight. Blood glucose levels were measured at time 0 and then 30, 60, 90, and 120 minutes after glucose injection. Glucose clearance ability was similar between genotypes for males (A) or females (B) (n = 7–11). C and D, After a 4-hour fast, mice received ip insulin 0.75 U/kg body weight, and then blood glucose was measured at 0, 30, 60, and 90 minutes relative to insulin injection. Het mice were less responsive to insulin (vs WT littermate controls), based on glucose clearance rate in males (A) and females (B) (n = 8–11). E, Serum insulin levels at time 0, 30, and 60 minutes after glucose injection during GTT (n = 8; 4 males and 4 females). Data represent mean ± SEM. *, P < .05; **, P < .01; and ***, P < .001.
Figure 3. Nedd4+/− mice on ND have reduced insulin signaling in WAT, skeletal muscle, and liver.
A–C, ND-fed wild-type (WT) and Nedd4+/− (Het) male mice were fasted overnight and ip injected with saline or insulin 5 U/kg body weight. Insulin-stimulated phosphorylation of IRβ and AKT in WAT (A), skeletal muscle (B), and liver (C) were measured by Western blot analysis (n = 7). Representative Western blots and quantification data were shown. Data represent mean ± SEM. *, P < .05; **, P < .01.
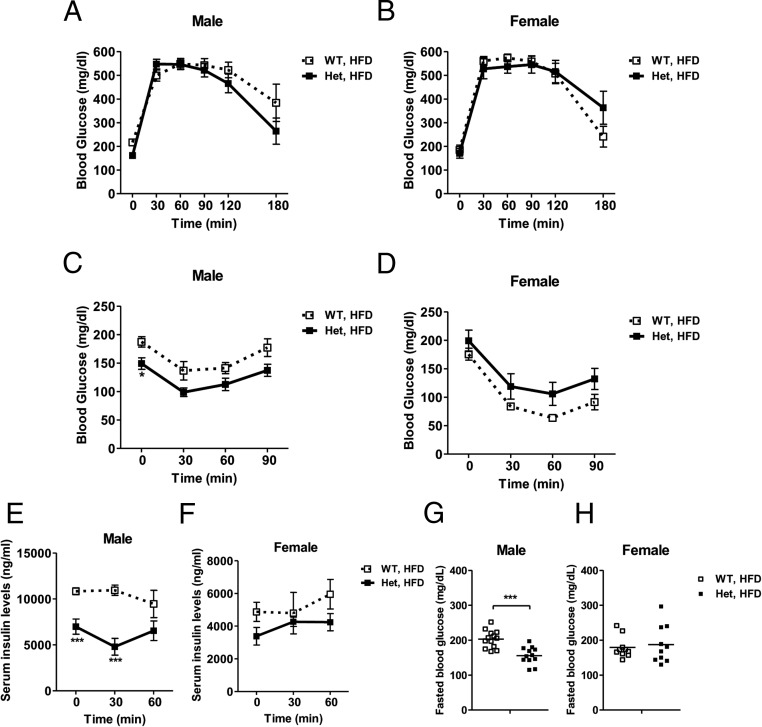
Surprisingly, despite reduced weight gain and fat expansion relative to wild-type controls, both male and female HFD-fed Nedd4+/− mice did not display a manifest improvement in glucose tolerance (Figure 4, A and B). ITT results showed that 16 weeks of HFD feeding induced profound insulin resistance in wild-type males but not females. Glucose clearance of Nedd4+/− mice during the ITT showed slight opposite trends for males and females (Figure 4, C and D). HFD-fed Nedd4+/− males displayed significantly improved fasting blood glucose levels, fasting serum insulin levels, and lower circulating insulin during GTT (Figure 4, E and G). No significant improvement was observed in the females (Figure 4, F and H).
Figure 4. Nedd4+/− male mice on HFD exhibit reduced fasting glucose and insulin levels.
A and B, GTT was carried out in wild-type (WT) and Nedd4+/− (Het) mice on HFD. After an overnight fast, mice received ip glucose 1.5 g/kg body weight, and then blood glucose levels were measured at time 0, 30, 60, 90, 120, and 180 minutes relative to glucose injection. Glucose clearance ability was similar between genotypes in males (A) or females (B) (n = 6–7). C and D, ITT was carried out in WT and Het male (A) and female (B) mice (n = 5–7). After a 4-hour fast, mice received ip insulin 0.75 U/kg body weight, and then blood glucose levels were measured at time 0, 30, 60, and 90 minutes relative to insulin injection. E and F, Serum insulin levels at time 0, 30, and 60 minutes after glucose injection during GTT in males (E) and females (F) (n = 6). G and H, After an overnight fast, blood glucose levels in male (G) and female (H) mice (n = 10–13). Data represent mean ± SEM. *, P < .05; ***, P < .001.
Less lipid accumulation and accelerated adipose lipolysis in Nedd4+/− mice
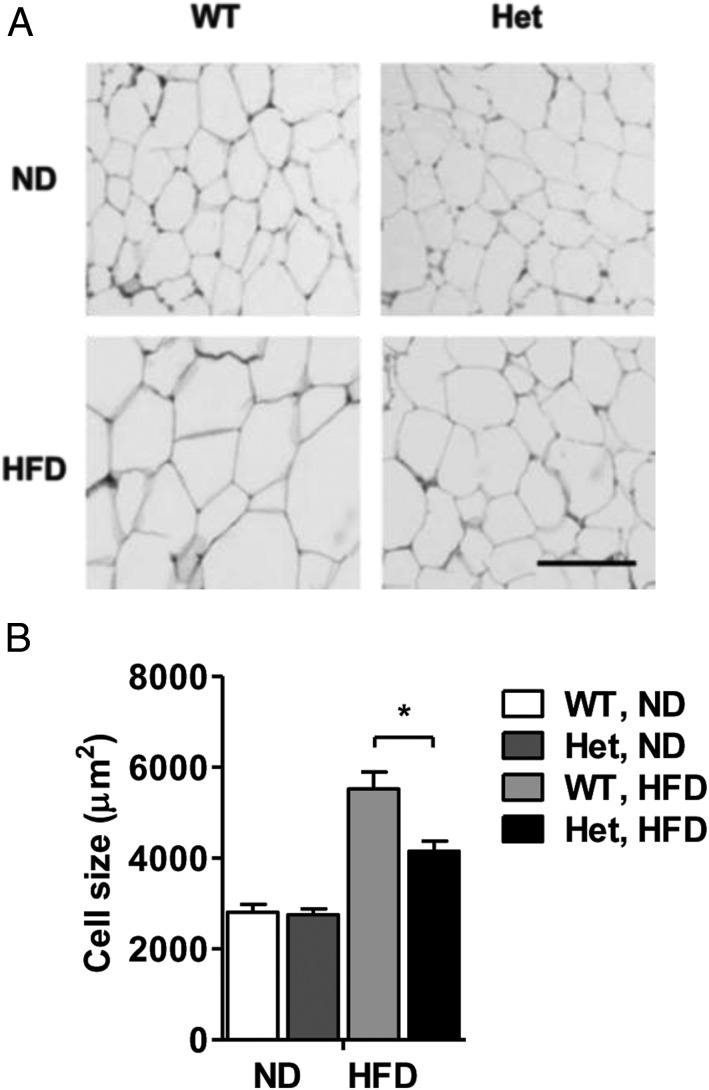
Expansion of WAT in obesity can result from adipocyte hypertrophy (increase in cell size) and/or hyperplasia (increase in cell number) (27, 28). Histology of epididymal WAT revealed 25% smaller average cell size in Nedd4+/− mice on the HFD (P < .05) (Figure 5, A and B). Cell size distribution analysis showed greater frequency of small and midsized adipocytes and lower frequency of large adipocytes in epididymal WAT from HFD-fed Nedd4+/− mice, whereas the distribution curves in ND-fed Nedd4+/− and wild-type mice almost overlapped (Supplemental Figure 5, A and B). In agreement, Nedd4+/− mice on the HFD displayed significantly smaller and fewer lipid droplets than wild types on oil red O stains of hepatic sections (Supplemental Figure 6). Hence, Nedd4 down-regulation in obese mice was associated with reduced lipid accumulation within adipocytes and hepatocytes. Of note, Nedd4 protein levels per se were unaltered by the HFD in WAT, whereas the total IRβ levels were significantly reduced (Supplemental Figure 5C).
Figure 5. Nedd4+/− mice are protected against HFD-induced adipocyte hypertrophy.
A, Representative H&E staining of adipocytes. Scale bar, 100 μm. B, Average adipocyte size in epididymal WAT of littermate wild-type (WT) and Nedd4+/− (Het) mice on ND or HFD (ND, n = 3, two males and one female; HFD, n = 4, two males and two females). Area of adipocytes was measured within 300–400 cells per mouse using ImageJ software (National Institutes of Health). Data represent mean ± SEM. *, P < .05.
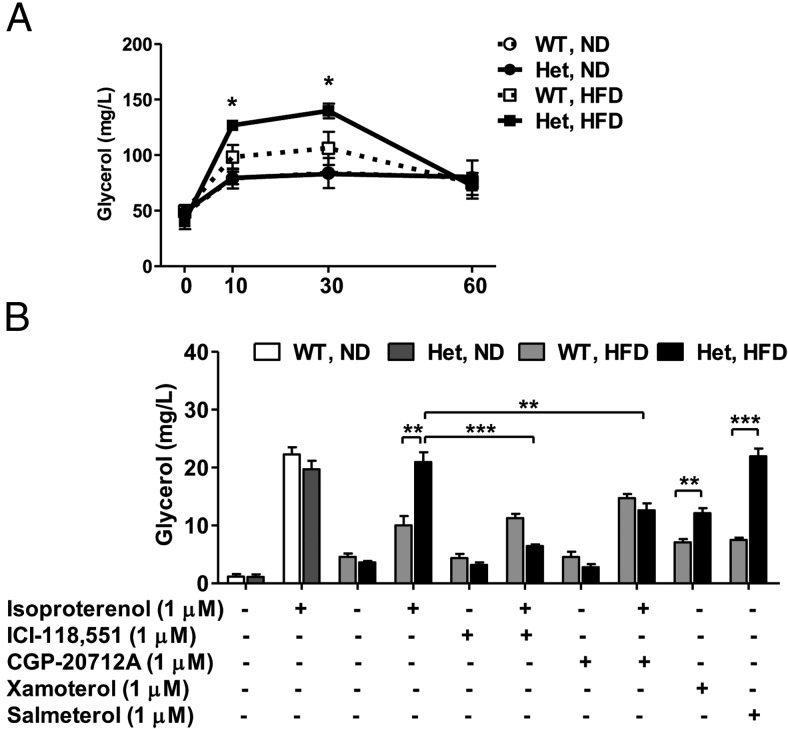
We examined whether lipolytic efficiency was altered in HFD-fed Nedd4+/− mice because they manifested reduced lipid storage. Intriguingly, in response to ip injection of isoproterenol (a nonselective β-AR agonist), the lipolysis rate of HFD-fed Nedd4+/− mice was significantly higher than controls, despite similar lipolytic response between ND-fed Nedd4+/− and wild type (Figure 6A). To confirm this in vivo finding, we assayed lipolysis ex vivo in purified primary adipocytes. Glycerol release from adipocytes from ND-fed animals did not display a difference between genotypes. The HFD greatly inhibited the isoproterenol-stimulated glycerol release in wild-type adipocytes, whereas Nedd4+/− adipocytes remained highly sensitive to β-AR stimulation. To assess whether β1-AR and/or β2-AR mediated this effect, we preincubated the adipocytes with ICI-118551 (a selective β2-AR antagonist) or CGP-20712 (a selective β1-AR antagonist). ICI-118551 completely blocked the lipolytic response elicited by isoproterenol. CGP-20712 only partially inhibited lipolysis, suggesting that β2-AR primarily mediates the enhancement of isoproterenol-induced lipolysis related to the partial loss of Nedd4 function. In addition, treating primary adipocytes with other selective β1-AR or β2-AR agonists (xamoterol and salmeterol, respectively) confirmed β2-AR as the predominant mediator (Figure 6B). Surprisingly, there were no significant changes in serum triglyceride, total cholesterol, and free fatty acid levels in the HFD-fed Nedd4+/− vs the HFD-fed control mice (Supplemental Table 1).
Figure 6. HFD-fed Nedd4+/− mice display enhanced isoproterenol-stimulated lipolysis in vivo and ex vivo.
A, Serum-free glycerol levels were measured in a cohort of 22-week-old wild-type (WT) and Nedd4+/− (Het) mice on ND or HFD at indicated time after ip isoproterenol, 10 mg/kg body weight (n = 5; three males and two females). B, Primary adipocytes purified from epididymal fat of WT and Het mice were incubated with or without 1 μM isoproterenol, xamoterol, or salmeterol as described in Materials and Methods. The β-antagonists ICI-118551 and CGP-20712A were preincubated for 10 minutes, and then glycerol levels in conditioned media were measured (n = 4; two males and two females). Data represent mean ± SEM. *, P < .05; **, P < .01; ***, P < .001.
To test whether the increased lipolytic response is related to β2-AR protein levels, we examined β2-AR expression in HFD-fed Nedd4+/− and control mice. Whole-cell lysates and crude membrane fractions prepared from WAT from HFD-fed Nedd4+/− and wild-type mice were analyzed by immunoblot. Despite only a 1.4-fold increase in total β2-AR protein levels, a dramatic 2.4-fold increase in membrane β2-AR levels was detected (Figure 7, A and B). We failed to detect β1-AR in these experiments, owing to the technical limitations of available antibodies. These observations suggest that the enhanced lipolysis associated with Nedd4 reduction is likely at least due to the increased protein concentration of β2-AR on the adipocyte plasma membrane.
Figure 7. Primary adipocytes from HFD-fed Nedd4+/− mice are associated with increased β2-AR protein concentrations.
A, Representative Western blots for β2-AR expression in total tissue lysates from epididymal WAT in wild-type (WT) and Nedd4+/− (Het) mice after 16 weeks of HFD feeding (n = 4; two males and two females). Quantification is shown in the right panel. B, Representative Western blots for β2-AR expression in crude membrane fraction from primary adipocytes purified from WT and Het male mice after 16 weeks of HFD feeding (n = 3). Data represent mean ± SEM. *, P < .05.
Discussion
To our knowledge, this is the first report that knockdown of Nedd4, a homologous to the E6-AP carboxyl terminus (HECT)-type E3 ubiquitin ligase, exerts a protective effect against HFDIO. We demonstrate that Nedd4 haploinsufficiency affects insulin signaling in vivo and that Nedd4 regulates lipolysis mediated primarily by β2-AR.
Nedd4 was previously reported to control fetal and postnatal growth by positively regulating signaling via the IGF-1R and IR (14, 24). Consistent with a report by Cao et al (24), we observed decreased insulin sensitivity in Nedd4+/− mice at the postnatal stage during the ITT. Insulin signaling was attenuated in several insulin-responsive organs of Nedd4+/− mice. One can speculate about potential PTEN-independent mechanisms to account for this decreased insulin sensitivity. A reduction of Nedd4-mediated, ubiquitin-dependent trafficking of phsophorylated AKT to perinuclear regions, as recently described by Fan et al (29), may partially explain the decreased insulin sensitivity observed in Nedd4+/− mice. An unexpected finding was that even though Nedd4+/− mice at the basal condition already displayed reduced insulin sensitivity, chronically HFD-fed Nedd4+/− mice were partially resistant to obesity but exhibited no apparent improvement in glucose intolerance compared with HFD-fed controls.
The Nedd4+/− phenotype is reminiscent of phenotypes with reduced or depleted expression of peroxisome proliferator-activated receptor-γ (PPAR-γ) (30, 31). PPAR-γ2 knockout mice are insulin resistant but glucose tolerant (30); PPAR-γ+/− mice are insulin resistant but protected against HFDIO (31). Nedd4 and PPAR-γ may regulate overlapping pathways controlling insulin sensitivity. Sexual dimorphism vis-à-vis insulin sensitivity has long been recognized in HFDIO rodent models (32) and under clinical conditions. Observations are assumably attributed to estrogen's effects to improve insulin sensitivity as well as its secretion (33, 34). The sexual difference in insulin sensitivity in Nedd4+/− mice, although beyond the scope of the present study, is likely due to distinct epigenomes and transcriptomes between male and female Nedd4+/− mice, both pre- and postnatally. A deeper understanding of tissue-specific regulation of each of these signaling pathways may provide novel mechanistic insights for Nedd4's actions related to obesity and insulin resistance.
Two types of adaptor protein can recruit Nedd4 to the agonist-activated β2-AR, namely β-arrestin 2 and arrestin-domain-containing proteins (ARRDCs), especially ARRDC2-4, which belong to the α-arrestin family (35–37). ARRDC3-deficient mice also display partial resistance to obesity, presumably via modulating β-adrenergic signaling (38). Our data are consistent with these models in which Nedd4 serves as an E3 ligase for β2-AR internalization and protein degradation. It should be stressed that our work is the first to demonstrate a physiological role of Nedd4-mediated β2-AR signaling in the context of lipolysis. Although agonist-stimulated lipolysis was higher in HFD-fed Nedd4+/− mice, their basal circulating lipid concentrations and ectopic lipid storage did not increase. These data are consistent with phenotypes reported in adipose triglyceride lipase transgenic mice (39), adipocyte phospholipase A2 knockout mice (40), and perilipin knockout mice (41, 42). Reduced adipose mass could have resulted in lower basal free fatty acid release, which might have compensated for the elevated lipolysis within the HFD-fed Nedd4+/− mice. Alternatively, increased β-oxidation at ectopic sites could have maintained circulating lipid homeostasis. We observed enhanced lipolysis in HFD-fed Nedd4+/− mice only under stimulation of β-agonists, whereas basal lipolysis appeared to be unaffected. These observations imply that altered lipolysis in these mice depends upon ligand binding and agree with the ligand-dependent regulation of β2-AR by Nedd4. These data may explain the discrepant lipolytic response (affected only in HFD fed animals) because chronic dietary fat or aging could act as a stressor that stimulates sympathetic nerve activity, releasing catecholamines to facilitate energy expenditure primarily through thermogenesis and β-AR-mediated lipolysis (43).
Prior studies frequently found mutually impedance of Nedd4/Itch and Cbl-b (44, 45). Depletion of Itch in mice has been reported to reverse HFDIO (6). We observed that partial depletion of Nedd4 also attenuates HFDIO progression. Coincidentally, Cbl-b depletion aggravated HFD-induced insulin resistance and macrophage recruitment (8, 46), consistent with its inverse relationship to Nedd4/Itch although cross talk between the molecular pathways of these three E3 ligases, particularly in obesity, deserves further investigation. Our studies provide the first experimental evidence for the involvement of Nedd4 E3 ligase in insulin sensitivity and lipolysis. These data should facilitate our understanding concerning the impact of physiological and pathological contexts on regulating E3 ligase-mediated regulation of the ubiquitin-proteasome system.
Acknowledgments
We gratefully thank Dr Weichun Lin for generously providing the breeding pairs of Nedd4+/− mice; Dr Suzhen Gong for performing the H&E staining for adipose tissue; Professors Aviv Hassid, Edwards Park, and Rajendra Raghow for their helpful discussions of this work.
This work was partially supported by National Institutes of Health Grants R01 AG031893 (to F.-F.L.), R21 AG041934 (to F.-F.L.), R21 NS083908 (to F.-F.L.), R01 HL-085848 (to S.W.B.), R21 HD059292 (to R.J.F.), NIH grant U01 DK085465 (to R.J.F.), R01 HL107500 (to B.X.), and AHA 10SDG3900046 (B.X.); Alzheimer Association Grant IIRG-11-204040 (to F.-F.L.); and Juvenile Diabetes Research Foundation International Grant 1-2011-597 (to R.J.F.).
Disclosure Summary: The authors disclose no conflicts of interest relevant to this work.
Footnotes
- β2-AR
- β-2 adrenergic receptor
- ARRDC
- arrestin-domain-containing protein
- Cbl
- casitas b-lineage lymphoma
- E1
- ubiquitin-activating enzyme
- E2
- ubiquitin-conjugating enzyme
- E3
- ubiquitin ligase
- FGFR1
- fibroblast growth factor receptor 1
- GAPDH
- glyceraldehyde-3-phosphate dehydrogenase
- Grb10
- growth factor receptor-bound protein 10
- GTT
- glucose tolerance test
- H&E
- hematoxylin and eosin
- HECT
- homologous to the E6-AP carboxyl terminus
- HFD
- high-fat diet
- HFDIO
- HFD-induced obesity
- IGF-1R
- IGF type 1 receptor
- IR
- insulin receptor
- Itch
- Itchy homolog E3 ubiquitin protein ligase
- ITT
- insulin tolerance test
- KRB
- Krebs-Ringer bicarbonate
- MG53
- mitsugumin 53
- ND
- normal diet
- Nedd4
- neural precursor cell expressed developmentally down-regulated protein 4
- PPAR-γ
- peroxisome proliferator-activated receptor-γ
- PTEN
- phosphatase and tensin homolog deleted from chromosome 10
- sc
- subcutaneous
- WAT
- white adipose tissue.
References
- 1. Kopelman PG. Obesity as a medical problem. Nature. 2000;404:635–643. [DOI] [PubMed] [Google Scholar]
- 2. Choquet H, Meyre D. Molecular basis of obesity: current status and future prospects. Curr Genomics. 2011;12:154–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Sun Y. Targeting E3 ubiquitin ligases for cancer therapy. Cancer Biol Ther. 2003;2:623–629. [PubMed] [Google Scholar]
- 4. Ardley HC, Robinson PA. The role of ubiquitin-protein ligases in neurodegenerative disease. Neurodegener Dis. 2004;1:71–87. [DOI] [PubMed] [Google Scholar]
- 5. Song R, Peng W, Zhang Y, et al. Central role of E3 ubiquitin ligase MG53 in insulin resistance and metabolic disorders. Nature. 2013;494:375–379. [DOI] [PubMed] [Google Scholar]
- 6. Marino A, Menghini R, Fabrizi M, et al. ITCH deficiency protects from diet-induced obesity. Diabetes. 2014;63:550–561. [DOI] [PubMed] [Google Scholar]
- 7. Hirasaka K, Kohno S, Goto J, et al. Deficiency of Cbl-b gene enhances infiltration and activation of macrophages in adipose tissue and causes peripheral insulin resistance in mice. Diabetes. 2007;56:2511–2522. [DOI] [PubMed] [Google Scholar]
- 8. Abe T, Hirasaka K, Kagawa S, et al. Cbl-b is a critical regulator of macrophage activation associated with obesity-induced insulin resistance in mice. Diabetes. 2013;62:1957–1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Molero JC, Jensen TE, Withers PC, et al. c-Cbl-deficient mice have reduced adiposity, higher energy expenditure, and improved peripheral insulin action. J Clin Invest. 2004;114:1326–1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Molero JC, Waring SG, Cooper A, et al. Casitas b-lineage lymphoma-deficient mice are protected against high-fat diet-induced obesity and insulin resistance. Diabetes. 2006;55:708–715. [DOI] [PubMed] [Google Scholar]
- 11. Hoppe T, Matuschewski K, Rape M, Schlenker S, Ulrich HD, Jentsch S. Activation of a membrane-bound transcription factor by regulated ubiquitin/proteasome-dependent processing. Cell. 2000;102:577–586. [DOI] [PubMed] [Google Scholar]
- 12. Bray SJ. Notch signalling: a simple pathway becomes complex. Nat Rev Mol Cell Biol. 2006;7:678–689. [DOI] [PubMed] [Google Scholar]
- 13. Magnifico A, Ettenberg S, Yang C, et al. WW domain HECT E3s target Cbl RING finger E3s for proteasomal degradation. J Biol Chem. 2003;278:43169–43177. [DOI] [PubMed] [Google Scholar]
- 14. Vecchione A, Marchese A, Henry P, Rotin D, Morrione A. The Grb10/Nedd4 complex regulates ligand-induced ubiquitination and stability of the insulin-like growth factor I receptor. Mol Cell Biol. 2003;23:3363–3372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kwak YD, Wang B, Li JJ, et al. Upregulation of the E3 ligase Nedd4-1 by oxidative stress degrades IGF-1 receptor protein in neurodegeneration. J Neurosci. 2012;32:10971–10981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Persaud A, Alberts P, Hayes M, et al. Nedd4–1 binds and ubiquitylates activated FGFR1 to control its endocytosis and function. EMBO J. 2011;30:3259–3273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Liu Y, Oppenheim RW, Sugiura Y, Lin W. Abnormal development of the neuromuscular junction in Nedd4-deficient mice. Dev Biol. 2009;330:153–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Li JJ, Dolios G, Wang R, Liao FF. Soluble β-amyloid peptides, but not insoluble fibrils, have specific effect on neuronal microRNA expression. PLoS One. 2014;9:e90770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Fischer AH, Jacobson KA, Rose J, Zeller R. Hematoxylin and eosin staining of tissue and cell sections. CSH Protoc. 2008;2008:pdb prot4986. [DOI] [PubMed] [Google Scholar]
- 20. Qiao L, Kinney B, Schaack J, Shao J. Adiponectin inhibits lipolysis in mouse adipocytes. Diabetes. 2011;60:1519–1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Viswanadha S, Londos C. Optimized conditions for measuring lipolysis in murine primary adipocytes. J Lipid Res. 2006;47:1859–1864. [DOI] [PubMed] [Google Scholar]
- 22. Viswanadha S, Londos C. Determination of lipolysis in isolated primary adipocytes. Methods Mol Biol. 2008;456:299–306. [DOI] [PubMed] [Google Scholar]
- 23. Caro JF, Sinha MK, Kolaczynski JW, Zhang PL, Considine RV. Leptin: the tale of an obesity gene. Diabetes. 1996;45:1455–1462. [DOI] [PubMed] [Google Scholar]
- 24. Cao XR, Lill NL, Boase N, et al. Nedd4 controls animal growth by regulating IGF-1 signaling. Sci Signal. 2008;1:ra5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wang X, Trotman LC, Koppie T, et al. Nedd4–1 is a proto-oncogenic ubiquitin ligase for PTEN. Cell. 2007;128:129–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kwak YD, Wang B, Pan W, Xu H, Jiang X, Liao FF. Functional interaction of phosphatase and tensin homologue (PTEN) with the E3 ligase Nedd4-1 during neuronal response to zinc. J Biol Chem. 2010;285:9847–9857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Sun K, Kusminski CM, Scherer PE. Adipose tissue remodeling and obesity. J Clin Invest. 2011;121:2094–2101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. de Ferranti S, Mozaffarian D. The perfect storm: obesity, adipocyte dysfunction, and metabolic consequences. Clin Chem. 2008;54:945–955. [DOI] [PubMed] [Google Scholar]
- 29. Fan CD, Lum MA, Xu C, Black JD, Wang X. Ubiquitin-dependent regulation of phospho-AKT dynamics by the ubiquitin E3 ligase, Nedd4-1, in the insulin-like growth factor-1 response. J Biol Chem. 2013;288:1674–1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Zhang J, Fu M, Cui T, et al. Selective disruption of PPARγ2 impairs the development of adipose tissue and insulin sensitivity. Proc Natl Acad Sci USA. 2004;101:10703–10708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kubota N, Terauchi Y, Miki H, et al. PPAR γ mediates high-fat diet-induced adipocyte hypertrophy and insulin resistance. Mol Cell. 1999;4:597–609. [DOI] [PubMed] [Google Scholar]
- 32. Pettersson US, Walden TB, Carlsson PO, Jansson L, Phillipson M. Female mice are protected against high-fat diet induced metabolic syndrome and increase the regulatory T cell population in adipose tissue. PLoS One. 2012;7:e46057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Louet JF, LeMay C, Mauvais-Jarvis F. Antidiabetic actions of estrogen: insight from human and genetic mouse models. Curr Atheroscler Rep. 2004;6:180–185. [DOI] [PubMed] [Google Scholar]
- 34. Sharma G, Prossnitz ER. Mechanisms of estradiol-induced insulin secretion by the G protein-coupled estrogen receptor GPR30/GPER in pancreatic β-cells. Endocrinology. 2011;152:3030–3039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Nabhan JF, Pan H, Lu Q. Arrestin domain-containing protein 3 recruits the Nedd4 E3 ligase to mediate ubiquitination of the β2-adrenergic receptor. EMBO Rep. 2010;11:605–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Shenoy SK, Xiao K, Venkataramanan V, Snyder PM, Freedman NJ, Weissman AM. Nedd4 mediates agonist-dependent ubiquitination, lysosomal targeting, and degradation of the β2-adrenergic receptor. J Biol Chem. 2008;283:22166–22176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Han SO, Kommaddi RP, Shenoy SK. Distinct roles for β-arrestin2 and arrestin-domain-containing proteins in β2 adrenergic receptor trafficking. EMBO Rep. 2013;14:164–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Patwari P, Emilsson V, Schadt EE, et al. The arrestin domain-containing 3 protein regulates body mass and energy expenditure. Cell Metab. 2011;14:671–683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Ahmadian M, Duncan RE, Varady KA, et al. Adipose overexpression of desnutrin promotes fatty acid use and attenuates diet-induced obesity. Diabetes. 2009;58:855–866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Jaworski K, Ahmadian M, Duncan RE, et al. AdPLA ablation increases lipolysis and prevents obesity induced by high-fat feeding or leptin deficiency. Nat Med. 2009;15:159–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Saha PK, Kojima H, Martinez-Botas J, Sunehag AL, Chan L. Metabolic adaptations in the absence of perilipin: increased β-oxidation and decreased hepatic glucose production associated with peripheral insulin resistance but normal glucose tolerance in perilipin-null mice. J Biol Chem. 2004;279:35150–35158. [DOI] [PubMed] [Google Scholar]
- 42. Tansey JT, Sztalryd C, Gruia-Gray J, et al. Perilipin ablation results in a lean mouse with aberrant adipocyte lipolysis, enhanced leptin production, and resistance to diet-induced obesity. Proc Natl Acad Sci USA. 2001;98:6494–6499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Lowell BB, Bachman ES. β-Adrenergic receptors, diet-induced thermogenesis, and obesity. J Biol Chem. 2003;278:29385–29388. [DOI] [PubMed] [Google Scholar]
- 44. Thien CB, Langdon WY. c-Cbl and Cbl-b ubiquitin ligases: substrate diversity and the negative regulation of signalling responses. Biochem J. 2005;391:153–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Guo H, Qiao G, Ying H, et al. E3 ubiquitin ligase Cbl-b regulates Pten via Nedd4 in T cells independently of its ubiquitin ligase activity. Cell Rep. 2012;1:472–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Abe T, Hirasaka K, Kohno S, et al. Ubiquitin ligase Cbl-b and obesity-induced insulin resistance. Endocr J. 2014;61:529–538. [DOI] [PubMed] [Google Scholar]