Abstract
Hypothalamic inflammation, involving microglia activation in the arcuate nucleus (ARC), is proposed as a novel underlying mechanism in obesity, insulin and leptin resistance. However, whether activated microglia affects ARC neuronal activity, and consequently basal and hormonal-induced food intake, is unknown. We show that lipopolysaccharide, an agonist of the toll-like receptor-4 (TLR4), which we found to be expressed in ARC microglia, inhibited the firing activity of the majority of orexigenic agouti gene-related protein/neuropeptide Y neurons, whereas it increased the activity of the majority of anorexigenic proopiomelanocortin neurons. Lipopolysaccharide effects in agouti gene-related protein/neuropeptide Y (but not in proopiomelanocortin) neurons were occluded by inhibiting microglia function or by blocking TLR4 receptors. Finally, we report that inhibition of hypothalamic microglia altered basal food intake, also preventing central orexigenic responses to ghrelin. Our studies support a major role for a TLR4-mediated microglia signaling pathway in the control of ARC neuronal activity and feeding behavior.
Reciprocal interactions between the central nervous system (CNS) and the immune system enable the coordination of immune responses to other physiological processes, maximizing in turn the organism's adaptability to complex environments (1). It is now recognized that in addition to adaptive immunity, complex neuroimmune interactions implicate the innate immune system (2), whose activation evoke varied behavioral changes, including reduced food intake, sickness, and depression (2, 3). Still, the precise brain circuitry and signals underlying innate immunity-brain interactions in the context of specific behaviors remain elusive.
In addition to sensing peripheral innate immune responses (3), the brain possesses its own innate immune system, in which microglia constitute the main innate immunity effector cells. Thus, microglia are the first responders to injury and infections, releasing proinflammatory signals including cytokines and chemokines (4).
The hypothalamic arcuate nucleus (ARC) plays a pivotal role in food intake and body weight regulation (5, 6), and it is directly implicated in metabolic diseases including obesity and diabetes. The two major ARC neuronal types include the orexigenic agouti gene-related protein (AgRP) neurons [which coexpress the neuropeptide Y (NPY)] and the anorexigenic proopiomelanocortin (POMC) neurons. These neurons are the targets of circulating leptin and ghrelin, and their activation results in opposing effects on feeding and metabolism (7, 8).
Evidence for hypothalamic ARC inflammation, including activated microglial cells, microglia IgG accumulation, and enhanced inflammatory cytokines, has been reported in experimental models of obesity and diabetes, supporting a pathophysiological link between microglia-mediated inflammation and obesity, insulin, and leptin resistance (9–14). Nevertheless, the precise cellular mechanisms linking activated microglia and altered ARC function, ultimately reflected in an altered ARC neuronal output, remain unknown. Furthermore, and even at a more fundamental level, whether activated microglia results in changes in ARC neuronal activity has not been explored thus far.
Lipopolysaccharide (LPS) is a commonly used inflammogen that activates microglia (15). The toll-like receptor-4 (TLR4), a member of the large family of pattern recognition receptors that plays a critical role in innate immune responses (16), is the primary LPS receptor (17, 18). TLR4 is predominantly expressed in brain microglia (19, 20), mediating microglia responses to LPS (21–23). Importantly, hypothalamic TLR4 activation triggered production of proinflammatory cytokines, disrupting leptin and insulin signaling (19, 24).
In this study, we dissected the functional consequences of microglia activation in the ARC both at the single neuronal and whole system levels. Using whole-cell patch-clamp recordings from identified ARC neurons in rats and mice, we investigated the consequences of TLR4-mediated microglia activation on the firing activity of POMC and AgRP/NPY neurons. Moreover, we tested whether hypothalamic loss of microglia function in vivo affected the CNS control of basal and ghrelin-induced food intake.
Materials and Methods
Animals
Data were obtained from male Wistar rats (200–250 g), purchased from Harlan Laboratories, and from heterozygous transgenic NPY-green fluorescent protein (GFP) male/female mice (20–35 g) (backcrossed seven generations onto C57BL [Horvath et al (31)], kindly provided by Dr Matthias Tschöp (Institute for Diabetes and Obesity, Helmholtz Centre Munich, Munich, Germany). The animals were housed in a temperature-controlled environment with 12-hour light, 12-hour dark cycle (lights on at 7:00 am) and given ad libitum access to food and water. All the procedures used in this study were carried out in agreement with Georgia Regents University Institutional Animal Care and Use Committee guidelines.
Electrophysiology
Conventional whole-cell patch-clamp recordings were obtained, as previously described (25). Briefly, male rats (n = 29) or GFP-NPY mice (n = 16, male = 8 and female = 8) were anesthetized and the brains harvested. Coronal hypothalamic slices containing the arcuate nucleus were cut (230 μm) using a vibroslicer. For electrophysiological recordings, a slice was transferred to a submersion-type recording chamber, continuously perfused (∼2 mL/min−1 at 30–32°C) with a standard artificial cerebrospinal fluid (ACSF) bubbled with a gas mixture of 95% O2 and 5% CO2. The patch pipette internal solution contained (millimoles): K-gluconic acid 130, KCl 20, HEPES 10, EGTA 0.5, Mg-ATP 0.9, and Na-GTP 0.3. The pH was slowly titrated to 7.25–7.30. Firing activity was recorded in the current-clamp mode, before and during TLR4 activation with lipopolysaccharide (LPS-EK, 10 μg/mL; catalog number tlrl-eklps; InvivoGen) in slices pretreated or not (1–2 h) with minocycline hydrochloride (Minoc, 100 μM; Sigma-Aldrich). Firing rate (defined as the number of spikes, sec−1) was measured during a control period (2 min period before drug application) and during a period (8–9 min) that started 1 minute after the LPS administration. Neurons were considered responsive if a change in firing rate of at least 10% was observed between control and LPS administration. Detailed methods are described in the Supplemental Materials and Methods.
Fluorescence Immunohistochemistry
To study the distribution of microglia within the ARC, male rats (n = 4) were deeply anesthetized and perfused transcardially with 0.01 M PBS (150 mL at pH 7.4) followed by 4% paraformaldehyde (350 mL). Brain were removed and postfixed in 4% paraformaldehyde for 3 hours at 4°C. Sequential coronal sections (25 μm) through the ARC nucleus between bregma −2.12 and −2.8 (26) were cut with a cryostat and incubated for 24 hours in primary antibodies (Table 1) of rabbit microglia marker antiionized calcium binding adaptor molecule 1 (IBA1) antibody (019–19 741, 1:200; Wako) for IBA1 single staining, guinea pig anti-AgRP polyclonal antibody (ab181493, 1:500; Abcam), and goat-anti-POMC antibody (ab32893, 1:300; Abcam) for AgRP and POMC costaining. To determine whether TLR4 is expressed in ARC microglia, a different set of sections were incubated in a cocktail of a goat anti-IBA1 antibody (ab5076, 1:1000; Abcam) along with rabbit anti-TLR4 antibody (ab13556, 1:200; Abcam). Reactions with primary antibodies were followed by 4 hours of incubation in the presence of (a) fluorescently labeled secondary antibody (Jackson Immunoresearch Laboratories) donkey antirabbit Cy5 (1:250, for IBA1), donkey anti-guinea pig Cy3 (1:250, for AgRP), and horse antigoat fluorescein isothiocyanate (for POMC) or horse antigoat Cy5 (for IBA1) and horse antirabbit Cy3 (for TLR4). Sections were also counterstained with TOTO3 (1:10 000, 15 min; Molecular Probes) and then mounted and visualized using confocal microscopy. Detailed methods are described in the Supplemental Materials and Methods.
Table 1.
Antibody Information
| Peptide/Protein Target | Antigen Sequence (if Known) | Name of Antibody | Manufacturer, Catalog Number, and/or Name of Individual Providing the Antibody | Species Raised (Monoclonal or Polyclonal) | Dilution Used |
|---|---|---|---|---|---|
| IBA1 | 17-kDa EF hand protein | Anti-Iba1 | Wago; 019-19741 | Rabbit, polyclonal | 1:200 |
| AgRP | Synthetic peptide within mouse AGRP (C terminal) | Anti-AgRP | Abcam; ab181493 | Guinea pig, polyclonal | 1:500 |
| POMC | C-terminal amino acids 256–267 of human POMC | Anti-POMC | Abcam; ab32893 | Goat, polyclonal | 1:300 |
| IBA1 | Amino acids 135–147 of human Iba1 | Anti-Iba1 | Abcam; ab5076 | Goat, polyclonal | 1:1000 |
| TLR4 | Synthetic peptide corresponding to human TLR4 amino acids 420–435 | Anti-TLR4 | Abcam, ab13556 | Rabbit, polyclonal | 1:200 |
| TLR4 | Clone MTS510 | MAb mTLR4/MD2 | Invivogen; mab-mtlr4md2 | Mouse, monoclonal | 0.1 μg/mL |
Confocal Imaging
Immunofluorescence intensity was quantified using a threshold paradigm, as previously described (27). The ARC nucleus was manually traced using POMC and AgRP immunoreactivities as anatomical landmarks to delineate two main regions of interest in ARC areas enriched with POMC (lateral ARC aspects) and AgRP (medial ARC aspects) neuronal populations (28, 29). Subsequently the channel containing the target signal (IBA1) was used. Background fluorescence was subtracted from the images from areas lacking immunoreactive signal, and a threshold was set to pass intensities 1.5 times above background fluorescence. The density of IBA1 threshold signal within the traced regions of interest was obtained using the same demarcated areas and expressed as a percentage of threshold area (ie, a percentage of total area containing threshold immunoreactive signal). The mean immunoreactivity intensity was also obtained from the same regions and expressed as arbitrary units (AU) (27). Detailed methods are described in the Supplemental Materials and Methods.
Intracerebroventricular administration of Minoc and ghrelin
For inhibition of the microglial activity and administration of ghrelin, lateral intracerebroventricular (ICV) infusion probes (guiding probe) were placed into the lateral ventricle (n = 29 male rats). ICV coordinates were adapted from previous studies (30). An acute ICV infusion of Minoc (100 μg per 5 μL vehicle) and/or ghrelin (10 μg per 5 μL vehicle) was performed 10 days after rats recovered from surgery and compounds were injected at 9:00 am (2 h after lights on) via insertion of injection needles into the guiding probe. Food intake was measured 2 and 4 hours after injection. For a more long-term study, ICV Minoc infusion was performed for 5 days. On day 5, ICV ghrelin was injected at 9:00 am, and food intake was measured after 6 hours.
Statistical analysis
Results are expressed as mean ± SEM. Electrophysiological data were analyzed using a χ2 test or paired t tests, as indicated. One- or two-way ANOVA was used for food intake studies, as indicated. Correlation coefficients were done using Pearson correlation test. Statistical analysis was performed using GraphPad Prism 5.0 (GraphPad Software, Inc). Differences were considered significant at P < .05.
Results
TLR4 signaling differentially regulates activity of neuronal subpopulation in the arcuate nucleus from rats
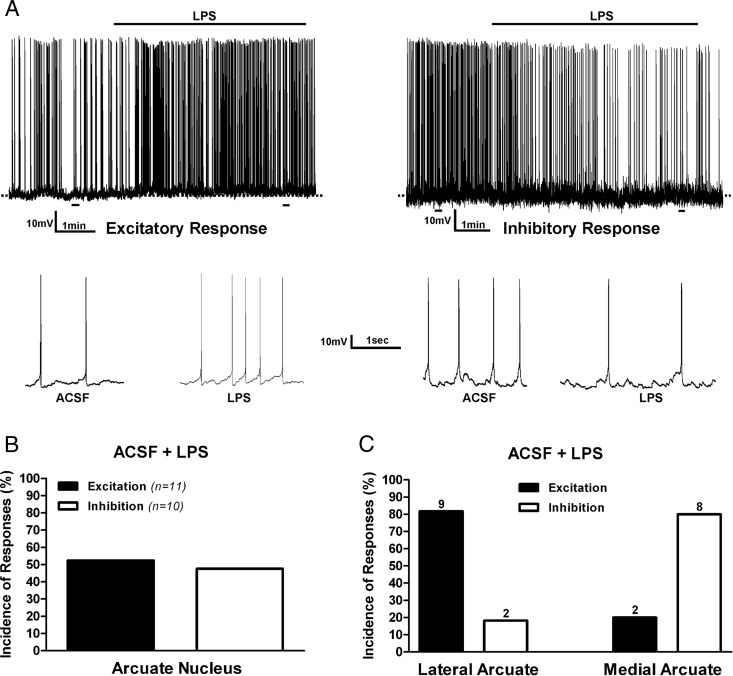
To evaluate the effect of LPS on arcuate nucleus neuronal activity, we activated TLR4 using bath-applied agonist LPS in hypothalamic brain slices containing the ARC and measured changes in firing rate and membrane potential in recorded neurons. ARC neurons responsive to TLR4 activation were either stimulated (11 of 21) or inhibited (10 of 21) (Figure 1, A and B). To determine whether these differential responses to TLR4 activation could be due to distinct neurochemical phenotypes within the ARC, we separately recorded from biocytin-filled neurons and compared their responsiveness according to their mediolateral distribution within the nucleus. Neurons were arbitrarily categorized as medial ARC if located within 0–140 μm from the wall of the third ventricle, whereas those located between 260 and 300 μm from the third ventricle were categorized as lateral ARC. We found that the majority of neurons located in the lateral ARC (9 of 11, 82%) (including the POMC immunoreactive neurons) showed excitatory responses to TLR4 activation, whereas the majority of neurons located in the medial ARC (8 of 10, 80%) (including POMC immune negative), showed an inhibitory response to TLR4 activation (Figure 1C). The incidence of the response types between the lateral and medial ARC neurons were significantly different (P < .02, χ2 test).
Figure 1. Effects of TLR4 activation on ARC neuronal activity.
A, Examples of electrophysiological recordings obtained from ARC neurons showing either an excitatory (left panel) or inhibitory (right panel) response to bath-applied LPS (10 μg/mL). The lower panels show action potentials at an expanded time scale, taken from the underlined areas from the upper panels. Summary of the incidence of excitatory and inhibitory neuronal responses observed in pooled ARC neurons (B) or according to neuronal distribution in lateral or medial ARC aspects (C). Numbers on top of bars are the number of neurons per group.
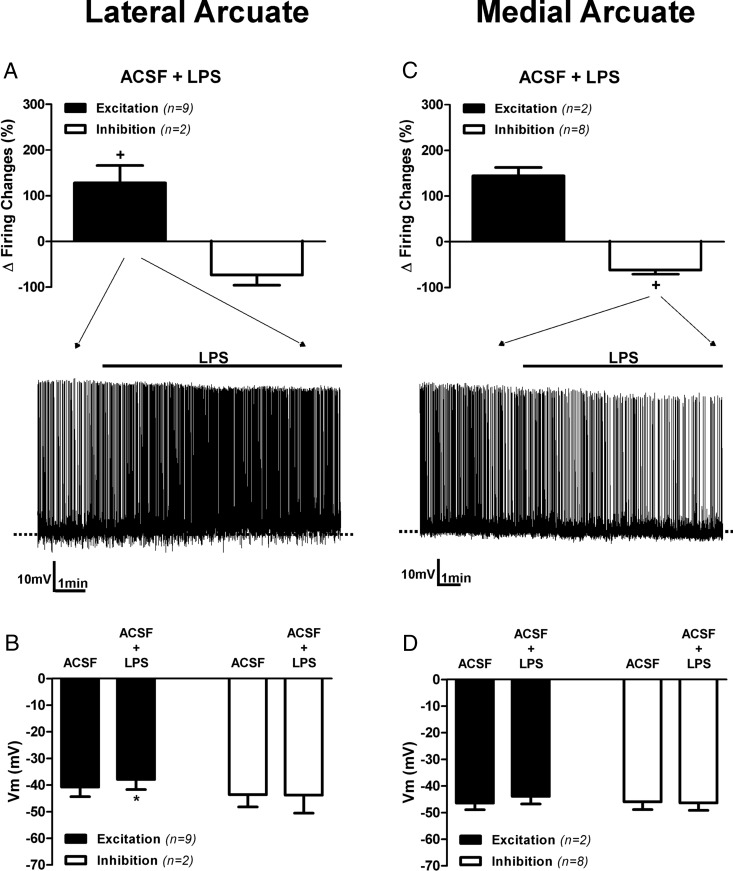
A summary of changes in membrane potential and firing activity after TLR4 activation in the lateral and medial ARC neurons is shown in Figure 2. Most lateral ARC neurons showed a significant increase in firing rate (baseline 1.40 ± 0.33 Hz; LPS: 2.57 ± 0.63 Hz; n = 9) along with a membrane depolarization (baseline, −40.7 ± 3.6 mV; LPS, −37.9 ± 3.8 mV; n = 9) in response to LPS (P < .05 for both variables, paired t tests) (Figure 2, A and B). The number of neurons showing an inhibitory response in this group precluded meaningful statistical analysis. Conversely, most medial ARC neurons (8 of 10, 80%) showed a significant decrease in firing rate after LPS (baseline, 0.59 ± 0.06 Hz; LPS, 0.22 ± 0.06 Hz; n = 8, P< .05, paired t test), which was not accompanied by a change in membrane potential (baseline, −43.6 ± 4.7 mV; LPS, −43.8 ± 6.7 mV; n = 8) (Figure 2, C and D). The low number of neurons showing an excitatory response in this group also precluded relevant statistical analysis. A plot of percentage changes in firing rate vs changes in membrane potential from all neurons submitted to TLR4 activation failed to revealed a significant correlation between these two parameters (r2 = 0.36, P = .11).
Figure 2. Contrasting effects of TLR4 activation on laterally and medially located ARC neurons.
Mean changes in firing activity (A) and mean changes membrane potential in neurons (B) located in lateral aspects of the ARC after LPS (10 μg/mL) application are shown. Mean changes in firing activity (C) and mean changes in membrane potential in neurons (D) located in medial aspects of the ARC after LPS (10 μg/mL) application are also shown. Representative examples of the most predominant response in each group is shown in the traces in panels A and C. Note that LPS increased and decreased the degree of firing activity in most neurons located in the lateral and medial ARC, respectively. +, P < .05 (paired t test within its own group). Data are represented as mean ± SEM. *, P < .05 compared with ACSF group (paired t test).
In select cases, we were also able to identify recorded biocyted-filled neurons as either POMC immunoreactive (n = 7) (Supplemental Figure 1) or POMC immune negative (n = 6). Most ARC neurons recorded in rats (85%, including POMC+, POMC−, and nonimmunoidentified neurons) displayed large low-threshold depolarization (LTD) when subjected to depolarizing steps of increasing magnitude from a hyperpolarized holding potential (−90 mV) (Supplemental Figure 1). Largely linear changes in membrane potential, but no evident LTDs, were observed in the rest of the cells (not shown). It was therefore not possible to differentiate ARC neuronal phenotypes based on basic intrinsic membrane properties. Taken together, our results suggest that POMC neurons exhibit excitatory responses, whereas AgRP neurons show inhibitory responses to TLR4 activation.
TLR4 activation evokes inhibitory responses in mouse AgRP/NPY neurons
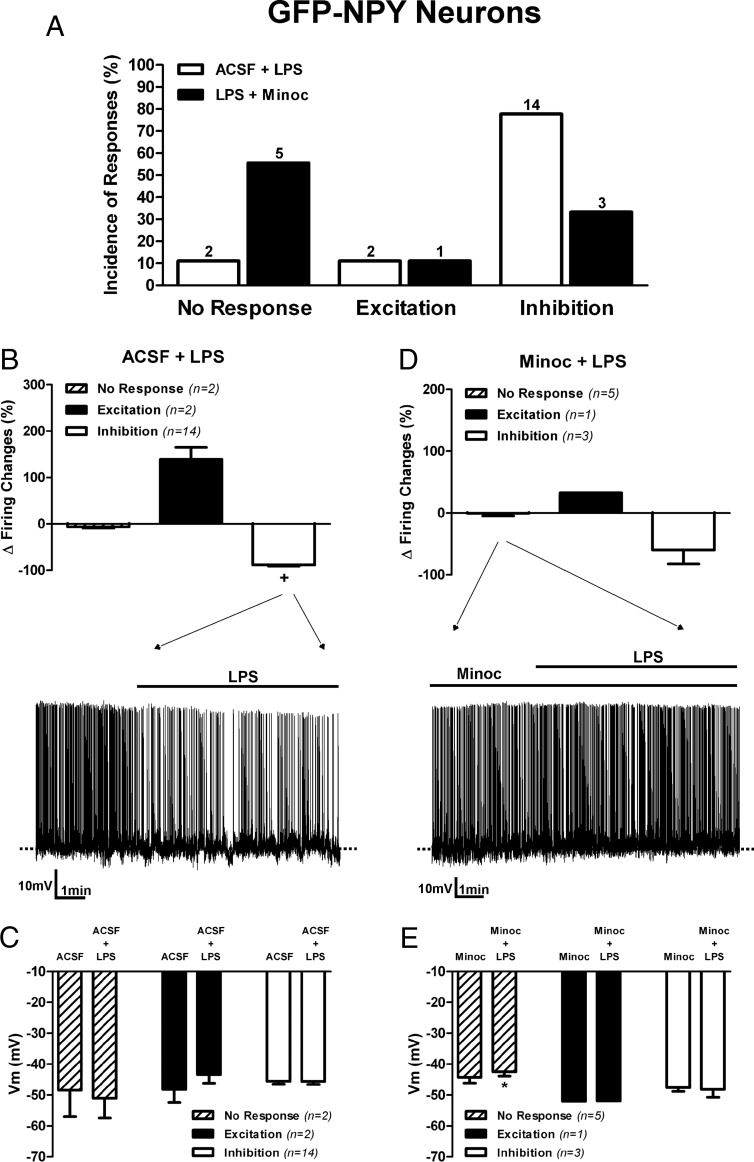
To more conclusively address cell type-dependent ARC responses to TLR4 activation, we preformed recordings in transgenic GFP-NPY mice in which AgRP/NPY neurons are readily identified based on the expression of GFP (31). A representative example of a recorded GFP-NPY neuron in a mouse hypothalamic slice is shown in Supplemental Figure 2. Similar to rat ARC neurons, most GFP-NPY and non-GFP-NPY ARC neurons in mice displayed large LTD when subjected to depolarizing steps of increasing magnitude from a hyperpolarized holding potential (−90 mV) (Supplemental Figure 2). In response to LPS, most responsive GFP-NPY neurons (14 of 16, 87.5%) displayed a decrease in firing discharge (baseline, 0.63 ± 0.12 Hz; LPS, 0.09 ± 0.03 Hz, P > .05, paired t test) (Figure 3, A and B). This inhibition, however, was not accompanied by membrane hyperpolarization (Figure 3C). The rest of the responsive neurons (2 of 16, 12.5%) showed an increase in firing activity. In these mice, only two of the total AgRP/NPY neurons tested (18) were nonresponsive. Similar LPS-mediated decreases in firing activity were observed when data were compared between male and female mice (Supplemental Figure 3). Finally, and similar to recordings obtained from ARC neurons in rats, we failed to reveal a strong and significant correlation between percentage changes in the firing rate vs changes in the membrane potential from all GFP-NPY neurons submitted to TLR4 activation (r2 = 0.45, P = .06).
Figure 3. TLR4 activation inhibits most GFP-NPY neurons in mice.
A, Summary of the incidence of ARC GFP-NPY neuronal responses to LPS (10 μg/mL) in the absence or presence of Minoc (100 μM). B, Bar graphs and representative example of the effects LPS on GFP-NPY mean firing activity. C, Mean changes in membrane potential. D, Bar graphs and representative example of the effects LPS in the presence of Minoc on GFP-NPY mean firing activity. E, Mean changes in membrane potential (n = number of neurons). Data are represented as mean ± SEM. +, P < .05 (paired t test within its own group). *, P < .05 compared with Minoc (no LPS) group (paired t test).
Conversely, in most non-GFP-NPY neurons (four of seven, 57.1%), TLR4 activation evoked an increase in firing activity (not shown). These data are in agreement with our results obtained in rat medial ARC AgRP/NPY neurons (see Figures 1C and 2A) and support the general view that TLR4 evokes predominantly decreased activity in AgRP/NPY and increased activity in POMC ARC neurons.
Inhibition of activated microglia prevents TLR4-mediated effects in AgRP/NPY but not in POMC neurons
It has been shown that TLR4s are present in microglial cell of the ARC, and its expression is increased during metabolic diseases (10, 12, 14, 19). However, whether activation of microglial cells in the ARC affects local neuronal activity has not yet been investigated. Thus, to test whether TLR4-mediated effects on ARC neuronal activity were mediated indirectly via microglia activation, we tested the effects of Minoc, a tetracycline derivative that inhibits microglia activation (32–35). In recordings obtained from identified GFP-NPY neurons in mice (Figure 3, A, D, and E), we found that in the presence of Minoc, most GFP-NPY neurons failed to show a change in firing activity in response to TLR4 activation (five of nine, 55.6%). However, a small (<2 mV) thought significant membrane depolarization was still observed (Figure 3E). In this treatment, only three of nine neurons (33.3%) still showed a decreased in firing rate (baseline, 0.41 ± 0.13 Hz; LPS, 0.42 ± 0.13 Hz), which was not accompanied by membrane hyperpolarization (Figure 3, D and E). In summary, most TLR4-mediated inhibitory responses in GFP-NPY neurons were blunted in the presence of minocycline, resulting in an overall difference in the incidence of response types when comparing TLR4 responses in ACSF vs Minoc in these neurons (P > .05, χ2 test).
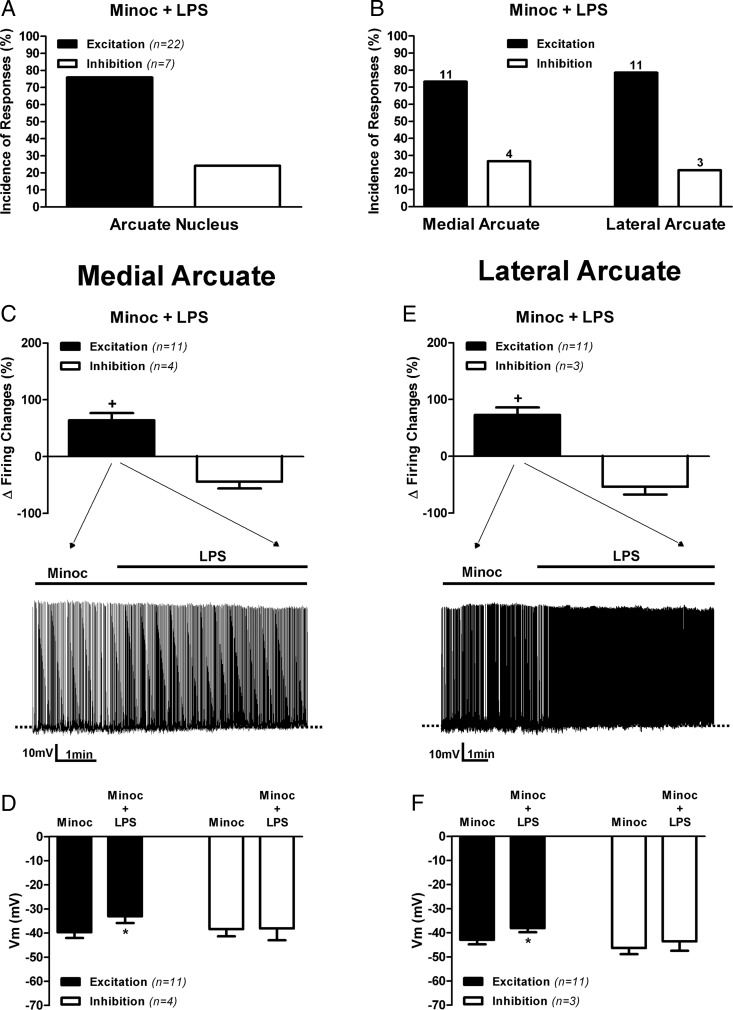
Differently from what we observed in control conditions in rat ARC neurons (ie, about equal proportions of excited and inhibited responsive neurons) (Figure 1B), TLR4 activation in rat slices pretreated with Minoc for 1–2 hours (see Materials and Methods) resulted in a majority of responsive neurons showing an increased firing activity (22 of 29, ∼76%), whereas the minority showed a decreased activity (7 of 29, ∼24%) (Figure 4A). When we subsequently analyzed rat ARC neurons according to their topographical distribution, we found a complete shift in the response pattern of rat medial ARC neurons in the presence of Minoc (Figure 4, B and C). Thus, differently from what we observed in control conditions (see Figures 1C and 2C), most medial ARC neurons (11 of 15, ∼73%) showed an excitatory response to TLR4 activation, characterized also by membrane depolarization (baseline, −39.7 ± 2.35 mV; LPS, −33.1 ± 2.77 mV) and an increase in firing discharge (baseline, 1.31 ± 0.17 Hz; LPS, 2.07 ± 0.22 Hz, P < .05 for both variables, paired t test) (Figure 4, C and D), whereas an inhibitory response was observed only in the remainder (4 of 15, ∼27%) (Figure 4B). Consequently, the overall incidence of response types in medial ARC neurons in control ACSF and in the presence of Minoc was significantly different (P > .05, χ2 test).
Figure 4. Inhibition of activated microglia cells prevented TLR4-mediated inhibition of medial, but no lateral, ARC neuronal activity.
Summary of the incidence of excitatory and inhibitory neuronal responses to LPS (10 μg/mL) in slices preincubated with Minoc (100 μM) observed in pooled ARC neurons (A) or according to neuronal distribution in lateral or medial ARC aspects (B). Numbers on top of bars are the number of neurons per group. Mean changes in firing activity (C) and mean changes membrane potential (D) in neurons located in medial aspects of the ARC after LPS application (10 μg/mL) in slices preincubated with Minoc (100 μM). Mean changes in firing activity (E) and mean changes in membrane potential (F) in neurons located in lateral aspects of the ARC after LPS application (10 μg/mL) in slices preincubated with Minoc (100 μM). Representative examples of the most predominant response in each group are shown in the traces in panels C and E. Data are represented as mean ± SEM. +, P < .05 (paired t test within its own group); *, P < .05 compared with Minoc (no LPS) group (paired t test).
Conversely, Minoc did not affect the response to LPS in neurons in the lateral ARC (Figure 4, B and E). Thus, similar to what we observed in control conditions, TLR4 activation induced excitatory responses in 11 of 14 (∼79%), characterized by a significant membrane depolarization (baseline, −42.89 ± 1.91 mV; LPS, −38.06 ± 1.68 mV) and increased firing discharge (baseline, 0.92 ± 0.20 Hz; LPS, 1.50 ± 0.29 Hz, P < .05 for both variables, paired t test) (Figure 4, B, E, and F). Accordingly, the overall incidence of response types in lateral ARC neurons in control ACSF and in the presence of Minoc was not significantly different (P > .9, χ2 test). Taken together, our results indicate that inhibitory (but not excitatory) responses evoked by TLR4 activation in AgRP/NPY neurons are largely blunted in the presence of a microglia cell inhibitor, supporting a cell type-dependent contribution of microglial cells to neuronal regulation in the ARC.
TLR4 mediates LPS-inhibitory actions in mouse arcuate neuronal activity
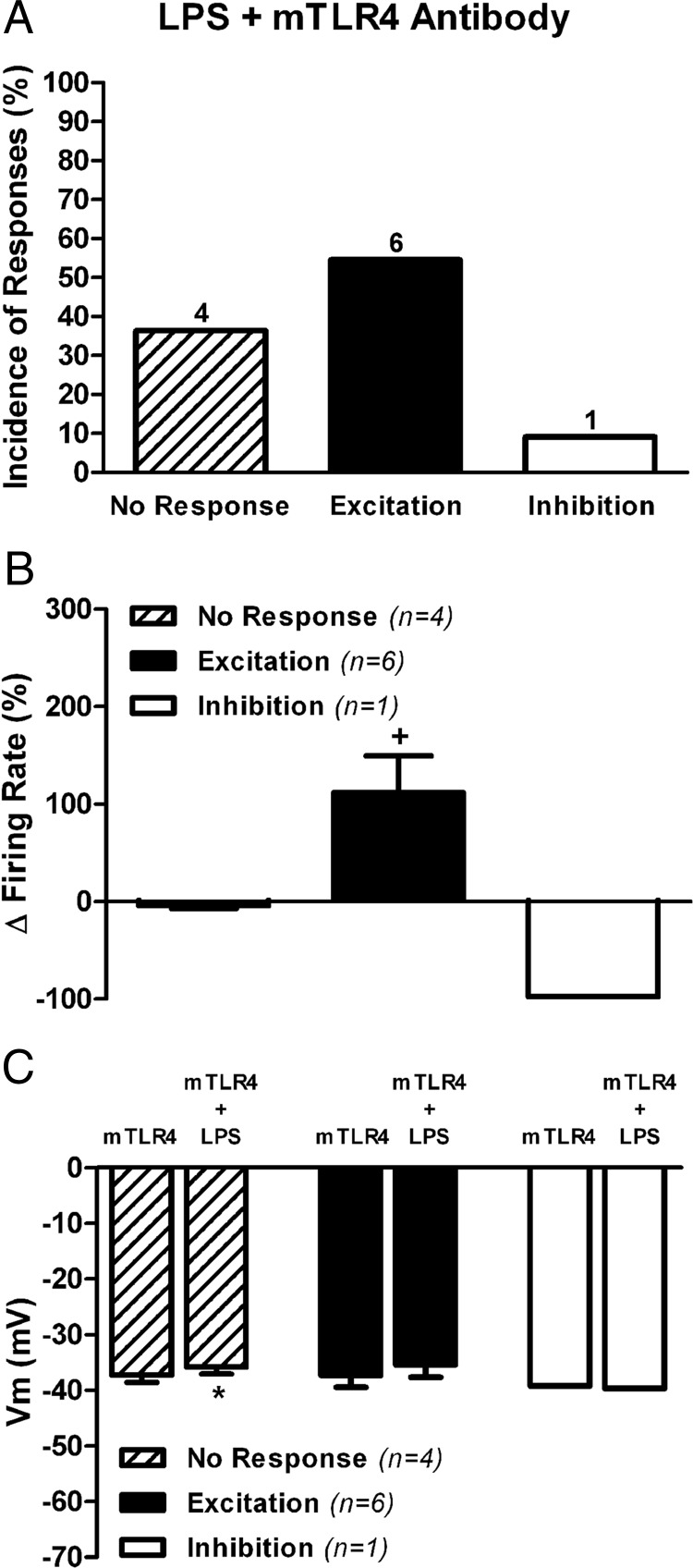
TLR4, the primary LPS receptor, is predominantly expressed in microglia and mediates microglia responses to LPS (19, 20). Given, however, the disparate cell type-dependent effects of LPS on ARC neuronal activity as well as the cell type-dependent contribution of microglia to these effects, we investigated the exact contribution of TLR4 to the reported LPS-mediated effects. To this end, we used a purified monoclonal antibody against mouse TLR4 (Table 1), previously shown to efficiently neutralize TLR4 responses to LPS (36). Because this antibody reacts only with mouse TLR4, experiments were performed in ARC brain slices obtained from male C57BL mice (n = 4). Slices were preincubated for approximately 3.5 hours in ACSF containing 0.1 μg/mL mTLR4 antibody and then subjected to LPS application as above. As shown in Figure 5, inhibitory responses to LPS in the presence of the TLR4 antibody were abrogated (1 of 11), whereas excitatory responses still persisted (6 of 11) (Figure 5A). Mean changes in firing rate in this condition are shown in Figure 5B (excitatory response values: baseline, 0.46 ± 0.19 Hz; LPS, 0.82 ± 0.29 Hz, P < .05, paired t test), which was not accompanied by a change in membrane potential (Figure 5C). These results are in general agreement with results obtained in Minoc (both in rats and mice) in which inhibitory, but not excitatory responses, were blunted.
Figure 5. TLR4 blockade abrogated LPS-evoked inhibitory responses in mice ARC neurons.
A, Summary of the incidence of mice ARC neuronal responses to LPS (10 μg/mL) in slices preincubated in the presence of a purified monoclonal antibody against mouse (mTLR4 antibody, 0.1 μg/mL). B, Bar graphs summarizing the effects LPS on mean firing activity. C, Mean changes in membrane potential in the presence of the mTLR4 antibody (n = number of neurons). Data are represented as mean ± SEM. +, P < .05 (paired t test within its own group); *, P < .05 compared with mTLT4 (no LPS) group (paired t test).
Topographical distribution of microglia and TLR4 expression in the ARC
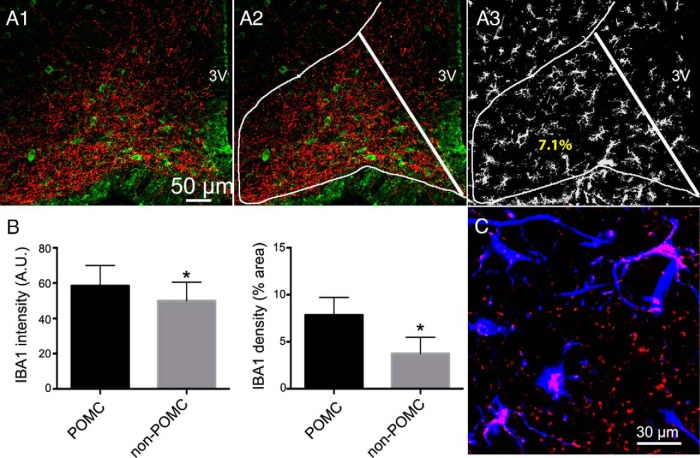
Immunostaining of microglial cells with the microglia marker IBA1 (37) was used to assess the distribution of microglial cells within the ARC, which was further delineated by POMC and AgRP immunostaining. Although our results showed robust POMC somata staining, AgRP labeled only thin processes, which as previously described, which were enriched in lateral aspects of the ARC-containing POMC neurons (see Figure 6A) (28, 29). Thus, for quantitative purposes, we compared IBA1 staining in lateral POMC enriched areas vs medial POMC-negative areas (see Figure 6, A1–A3). Interestingly, although IBA1-stained microglial cells were observed throughout the ARC, they appeared to be heterogeneously distributed, with a significantly higher predominance, as shown by a higher IBA1 density and intensity signal (POMC, 58.5 ± 4.1 AU; non-POMC, 49.9 ± 3.7 AU, P < .05, paired t test) in lateral POMC-enriched aspects of the ARC compared with more medial, POMC-lacking areas (see Figure 6B).
Figure 6. Topographical distribution of microglial cells and microglial TLR4 expression in the rat ARC.
A1, Representative confocal photomicrograph showing immunofluorescence staining for AgRP (red) and POMC (green) in the ARC. A2, The same image is shown with lines delimiting the POMC (lateral) and non-POMC (medial) areas used for quantification. A3, immunofluorescence staining for IBA1-positive (white) microglia cells. Numbers in A3 represent the IBA1 density values for each subregion of the arcuate displayed. B, Summary data showing mean differences in IBA1 staining intensity (left panel) and density (right panel) between POMC and non-POMC ARC regions. C, Confocal photomicrograph showing TLR4 (red) and IBA1 (blue) staining in the ARC. Note the presence of TLR4 staining in microglia cells (purple). Data are represented as mean ± SEM. *, P < .05 compared with POMC group (paired t test). 3V, third ventricle.
To determine whether ARC microglia expressed TLR4, we used mouse brain tissue because the TLR4 antibody we tested reacts with mouse tissue (it also reacts with human tissue but was not applicable in the present study). As shown in the representative example in Figure 6C, we found a considerable degree of positive TLR4 staining within IBA1-stained microglia cells within the ARC.
Microglia inhibition alters feeding responses to ghrelin
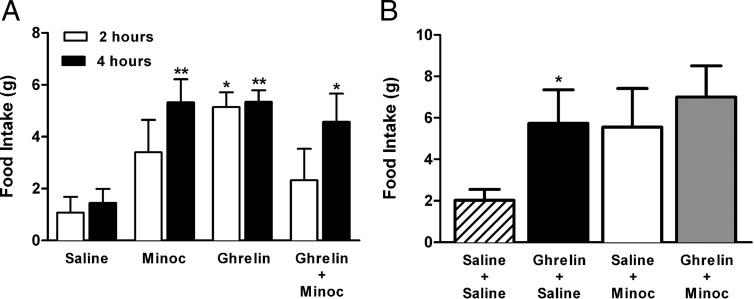
The results mentioned above indicate that microglia activation influences ARC neuronal excitability, inhibiting primarily AgRP/NPY neurons. We next investigated whether neuronal-mediated microglia actions themselves affected food intake as well as orexigenic effects of centrally applied ghrelin. ICV administration of ghrelin (10 μg/per 5 μL vehicle), an orexigenic concentration based on previous studies (38), increased food intake at 2 and 4 hours after the injection (P < .05 and P < .01, respectively, Bonferroni's post hoc test, two way ANOVA) (Figure 7A). ICV administration of Minoc alone (100 μg per 5 μL vehicle) affected food intake. A strong tendency for an increased food intake was already observed 2 hours after the injection, which became statistically significant at 4 hours (P < .01, Bonferroni's post hoc test, two way ANOVA) (Figure 7A). Importantly, coadministration of Minoc + ghrelin failed to increase food intake 2 hours after the injection, and although an increased food intake was observed at 4 hours (when compared with control) (P < .05, Bonferroni's post hoc test one way ANOVA), this effect was not different from that observed by either Minoc or ghrelin alone (Figure 7A). These results suggest that microglia inhibition per se increased food intake, interfering with and/or occluding the orexigenic effect of ghrelin. To further test whether this was the case, we performed another set of experiments, in which microglia activity was inhibited prior (ICV Minoc for 5 d) to ghrelin administration. As shown in Figure 7B, mice receiving ICV saline for 5 days showed a significant increase in food intake in response to an acute ICV infusion of ghrelin (P < .05, Bonferroni's post hoc test one way ANOVA). Conversely, in mice receiving ICV Minoc for 5 days, a tendency for a higher baseline food intake was already observed, and ICV ghrelin failed to evoke a further increase in food intake (P = NS, Bonferroni's post hoc test one way ANOVA (Figure 7B).
Figure 7. Effects of ICV minocycline on basal food intake and ghrelin-mediated orexigenic actions.
A, Summary showing mean effects of acute ICV administration of saline (control), Minoc, ghrelin, or the combination of minocycline and ghrelin (n = 4 for each group). Food intake was measured 2 and 4 hours after acute administration. *, P< .05, **, P< .01 compared with respective time control groups (Bonferroni's post hoc test). B, Summary effects on food intake of an acute ICV administration of saline or ghrelin in rats that previously received ICV injections saline or ICV Minoc. Groups included (acute-chronic) saline-saline (n = 6), ghrelin-saline (n = 6), saline-Minoc (n = 4), and ghrelin-Minoc (n = 5). Food intake was measured 6 hours after acute saline/ghrelin infusions. Data are represented as mean ± SEM. *, P < .05 vs saline-saline group (Bonferroni's post hoc test).
Discussion
Using patch-clamp electrophysiology and immunohistochemistry in rats and GFP-NPY transgenic mice, we report the following: 1) acute exposure to LPS evoked excitatory responses in most POMC neurons and inhibitory responses in AgRP/NPY neurons; 2) LPS effects in AgRP/NPY (but not in POMC) neurons were blocked when microglia function was inhibited or when TLR4 receptors were blocked; 3) TLR4 receptors are expressed in ARC microglia, and although microglia cells were present throughout the ARC, a predominant distribution was observed in POMC-enriched regions; and 4) inhibition of hypothalamic microglia function affected orexigenic responses to ghrelin. Taken together, our results indicate that activation of ARC microglial TLR4 evokes cell type-selective neuronal responses, leading to a predominant inhibition of AgRP/NPY activity. Although previous studies evaluated the effects of LPS/TLR4-mediated inflammation on ARC c-FOS and neuropeptide mRNA measurements (39–42), this is, to the best of our knowledge, the first study to demonstrate the consequences of microglial cell activation on firing activity within identified ARC neuronal populations. Finally, our results support an active role of microglia in the modulation of basal food intake and its regulation by ghrelin.
A major finding from our study is that TLR4 activation evoked contrasting responses in the firing activity of ARC neurons. To determine whether these represented differential effects within selective ARC neuronal populations, we used a combination of complementary approaches. First, we differentiated neurons according to their topographical location within the ARC, based on the well-established fact that mediobasal aspects of the ARC are enriched in AgRP/NPY neurons, whereas lateral aspects are enriched in POMC neurons (43). Given that this topographical segregation is not absolute, we also identified post hoc the neurochemical phenotype of a subgroup of recorded neurons (44). A limitation of this approach is that cell dialysis during whole-cell patch clamp recordings diminish cytoplasmic immunoreactivity, resulting in a low-throughput. Moreover, although the POMC antibody labeled neuronal somata, the AgRP antibody predominantly labeled axonal processes (eg, Supplemental Figure 1 and Figure 6), thus precluding immunochemical identification of AgRP/NPY immunoreactive neurons. Despite these technical limitations, we were still able to positively identify a subset of recorded neurons as POMC immunoreactive, whose response pattern to TLR4 activation was similar to that reported in most the population of lateral ARC neurons (ie, increased firing activity). To further circumscribe these limitations, we also obtained recordings from GFP-NPY ARC neurons in transgenic mice. Taking into account all these combined approaches, our results strongly suggest that LPS-mediated excitatory responses were predominantly observed in POMC neurons, whereas inhibitory responses were predominantly observed in AgRP/NPY neurons. Our results are in agreement with previous studies showing that LPS treatment reduced the number of fasting-induced c-FOS expression in NPY ARC neurons (39) while increasing ARC POMC and cocaine- and amphetamine-regulated transcript mRNA levels (42).
Moreover, an important observation was that in several cases, LPS-mediated changes in firing activity did not occur concomitantly with changes in membrane potential. In fact, a very weak correlation between these two parameters was observed, suggesting that a mechanism other than membrane depolarization/hyperpolarization contributed to LPS effects. As most ARC neurons in this study were spontaneously active, changes in any conductance that affected the action potential waveform, as well as changes in the frequency or randomness of postsynaptic potentials, could affect the degree of firing discharge without necessarily evoking a sustained change in membrane potential.
In addition to the differential net effect of LPS on neuronal activity between POMC and AgRP/NPY neurons, our results also suggest a differential cellular substrate and signaling mechanism underlying these effects. We found that microglial cell inhibition with Minoc (33) largely blunted TLR4-mediated inhibitory effects in AgRP/NPY neurons, without substantially affecting excitatory responses in POMC neurons. An important caveat to consider, however, is that although Minoc is widely used as a microglia cell inhibitor (32–35), other signaling pathways can also be targeted (32, 45, 46). Further supporting the involvement of microglia, we found that blockade of TLR4 also largely blunted inhibitory (but not excitatory) responses to LPS. As stated above, previous studies showed that TLR4 are predominantly expressed in microglial cells (19, 20), a fact we corroborated in our study in IBA1-stained ARC microglial cells (Figure 6). Thus, taken together, our results strongly support that activation of microglial TLR4 receptors in the ARC leads to AgRP/NPY neuronal inhibition.
In contrast to these actions, we found that LPS-mediated increase in ARC firing activity, the predominant response observed in POMC neurons, was not dependent on activated microglial cells (ie, were not blocked by Minoc or the TLR4 antibody). A noteworthy difference between the Minoc effect in ARC neurons in rats and mice emerged from our studies. In rat AgRP/NPY neurons, Minoc not only blunted inhibitory responses but also unveiled an excitatory response to TLR4 activation (ie, the predominant response switched from 80% inhibition to 73% excitation before and after Minoc, respectively). Thus, although AgRP/NPY neurons in the rat ARC are amenable to both inhibition (via a TLR4-microglia dependent mechanism) and stimulation (via a TLR4-microglia independent mechanism) by LPS, the inhibitory effect predominates. Conversely, in mice AgRP/NPY neurons, Minoc treatment largely blocked inhibitory responses to TLR4 activation, resulting in more nonresponsive neurons, without altering the incidence of excitatory responses. We currently do not have an explanation for these species-dependent differences in Minoc effects on ARC neurons.
Although the present results strongly support cell type-differential responses to microglia TLR4 activation in the ARC, important questions remain to be answered. For example, in contrast to a predominant TLR4, microglia-mediated functional effect on AgRP/NPY neurons, we found a predominant distribution of microglia in lateral, POMC-enriched ARC regions. These results suggest that mechanisms downstream to the activated microglial cells, eg, differential neuronal sensitivity to microglial-derived signals, likely contributed to such cell type-selective responses. Given that a plethora of molecules are known to be released by activated microglia, including cytokines, chemokines, and a variety of neuroactive substances, such as nitric oxide and reactive oxygen species, it is beyond the scope of this work to address their relative contribution to the effects reported here. Based on recent study by Borner et al (47), however, showing that LPS inhibited, in an inducible nitric oxide synthase-dependent manner, orexigenic ghrelin-sensitive ARC neurons, it is reasonable to speculate a contribution of inducible N nitric oxide O synthase-nitric oxide to the TLR4-mediated microglia inhibition of AgRP/NPY neurons.
It is also important to acknowledge the fact that LPS excitatory responses persisted after Minoc and TLR4 receptor blockade. These results suggest that a microglia, TLR4-independent mechanism is involved in mediating these actions. Evidence for TLR4-independent responses to LPS has been reported, including activation of nicotinamide adenine dinucleotide phosphate oxidase (48) and ceramide-activated protein kinase (49). Clearly, future studies are warranted to investigate the precise signaling mechanisms underlying TLR4-mediated effects on ARC neuronal activity.
Based on our results showing that TLR4-mediated microglia activation resulted in a predominantly inhibitory effect on AgRP/NPY neurons, a proanorectic effect would be anticipated after microglia activation. This is in agreement with the well-established anorectic effect mediated by LPS-induced inflammation (50), as well as the notion that these effects result from interactions with the central melanocortin system (51, 52).
Finally, to gain more insights into the functional implications of microglia effects on ARC neuronal activity, we performed in vivo studies in which we evaluated the role of microglia on basal food intake as well as on orexigenic effects of ghrelin. Our results showed that microglia inhibition with Minoc (32–35) per se for a period of 2–4 hours, increased food intake and interfered with and/or occluded orexigenic effects of centrally administered ghrelin. Based on these results, along with our in vitro studies showing an inhibitory action of microglia activation on AgRP/NPY neurons, it is reasonable to speculate that microglia in situ tonically inhibit AgRP/NPY activity. Thus, microglia inhibition by Minoc would lead to an increased AgRP/NPY activity along with an orexigenic output from the ARC, which could be sufficient to occlude a subsequent orexigenic stimulus by ghrelin. Unfortunately, due to the limited time that a neuron can be recorded in the whole-cell patch clamp technique, we were unable to test in vitro whether Minoc per se affected the firing activity of ARC neurons within the time frame that changes in food intake were observed. Although additional studies are needed to thoroughly elucidate the underlying mechanisms and players mediating microglia effects on food intake, our combined in vitro and in vivo approaches do support a major role of a microglia TLR4-mediated pathway in the regulation of ARC neuronal activity and, consequently, food intake.
Finally, it is important to take into consideration that in this work we evaluated the acute effects of microglia activation on ARC neuronal activity. Thus, whether the reported responses persist after sustained microglia activation, such as during the established phase of a local inflammatory process, remains to be determined. This is relevant within the context of a recent study that showed that during high-fat diet-induced ARC inflammation, the microglia activation profile at the initial stages served as a neuroprotective mechanism, whereas at chronic stages it contributed to neuronal injury (11). Thus, the profile of microglia-mediated effects on ARC neuronal activity may also be dependent on the time course of the inflammatory process.
In summary, our results show that acute TLR4-mediated microglia activation in the ARC results in an inhibitory effect on the firing activity of AgRP/NPY neurons. Moreover, we showed that microglia inhibition in vivo leads to increased food intake, interfering with ghrelin orexigenic actions. Thus, taken together, our studies support a major role for a TLR4-mediated microglia signaling pathway in the control of ARC neuronal activity and feeding behavior.
Acknowledgments
This work was supported in part by National Heart, Lung, and Blood Institute National Institutes of Health Grant HL112225 (to J.E.S.) and by the Helmholtz Alliance ICEMED (Imaging and Curing Environmental Metabolic Diseases), through the Initiative and Networking Fund of the Helmholtz Association.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- ACSF
- artificial cerebrospinal fluid
- AgRP
- agouti gene-related protein
- ARC
- arcuate nucleus
- AU
- arbitrary unit
- CNS
- central nervous system
- GFP
- green fluorescent protein
- IBA1
- ionized calcium binding adaptor molecule 1
- ICV
- intracerebroventricular
- LPS
- lipopolysaccharide
- LTD
- low-threshold depolarization
- Minoc
- minocycline
- NPY
- neuropeptide Y
- POMC
- proopiomelanocortin
- TLR4
- toll-like receptor-4.
References
- 1. McEwen BS. Physiology and neurobiology of stress and adaptation: central role of the brain. Physiol Rev. 2007;87(3):873–904. [DOI] [PubMed] [Google Scholar]
- 2. Irwin MR, Cole SW. Reciprocal regulation of the neural and innate immune systems. Nat Rev Immunol. 2011;11(9):625–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Dantzer R, O'Connor JC, Freund GG, Johnson RW, Kelley KW. From inflammation to sickness and depression: when the immune system subjugates the brain. Nat Rev Neurosci. 2008;9(1):46–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Saijo K, Glass CK. Microglial cell origin and phenotypes in health and disease. Nat Rev Immunol. 2011;11(11):775–787. [DOI] [PubMed] [Google Scholar]
- 5. Sawchenko PE. Toward a new neurobiology of energy balance, appetite, and obesity: the anatomists weigh in. J Comp Neurol. 1998;402(4):435–441. [PubMed] [Google Scholar]
- 6. Schwartz MW, Woods SC, Porte D, Jr, Seeley RJ, Baskin DG. Central nervous system control of food intake. Nature. 2000;404(6778):661–671. [DOI] [PubMed] [Google Scholar]
- 7. Kalra SP, Dube MG, Pu S, Xu B, Horvath TL, Kalra PS. Interacting appetite-regulating pathways in the hypothalamic regulation of body weight. Endocr Rev. 1999;20(1):68–100. [DOI] [PubMed] [Google Scholar]
- 8. Nakazato M, Murakami N, Date Y, et al. A role for ghrelin in the central regulation of feeding. Nature. 2001;409(6817):194–198. [DOI] [PubMed] [Google Scholar]
- 9. Gao Y, Ottaway N, Schriever SC, et al. Hormones and diet, but not body weight, control hypothalamic microglial activity. Glia. 2014;62(1):17–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Garcia-Caceres C, Yi CX, Tschop MH. Hypothalamic astrocytes in obesity. Endocrinol Metab Clin North Am. 2013;42(1):57–66. [DOI] [PubMed] [Google Scholar]
- 11. Thaler JP, Yi CX, Schur EA, et al. Obesity is associated with hypothalamic injury in rodents and humans. J Clin Invest. 2012;122(1):153–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Yi CX, Gericke M, Kruger M, et al. High calorie diet triggers hypothalamic angiopathy. Mol Metab. 2012;1(1–2):95–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Yi CX, Habegger KM, Chowen JA, Stern J, Tschop MH. A role for astrocytes in the central control of metabolism. Neuroendocrinology. 2011;93(3):143–149. [DOI] [PubMed] [Google Scholar]
- 14. Yi CX, Tschop MH, Woods SC, Hofmann SM. High-fat-diet exposure induces IgG accumulation in hypothalamic microglia. Disease Models Mech. 2012;5(5):686–690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hanisch UK. Microglia as a source and target of cytokines. Glia. 2002;40(2):140–155. [DOI] [PubMed] [Google Scholar]
- 16. Sirisinha S. Insight into the mechanisms regulating immune homeostasis in health and disease. Asian Pac J Allergy Immunol. 2011;29(1):1–14. [PubMed] [Google Scholar]
- 17. Gay NJ, Gangloff M, Weber AN. Toll-like receptors as molecular switches. Nat Rev Immunol. 2006;6(9):693–698. [DOI] [PubMed] [Google Scholar]
- 18. Lien E, Means TK, Heine H, et al. Toll-like receptor 4 imparts ligand-specific recognition of bacterial lipopolysaccharide. J Clin Invest. 2000;105(4):497–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Milanski M, Degasperi G, Coope A, et al. Saturated fatty acids produce an inflammatory response predominantly through the activation of TLR4 signaling in hypothalamus: implications for the pathogenesis of obesity. J Neurosci. 2009;29(2):359–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Olson JK, Miller SD. Microglia initiate central nervous system innate and adaptive immune responses through multiple TLRs. J Immunol. 2004;173(6):3916–3924. [DOI] [PubMed] [Google Scholar]
- 21. Chakravarty S, Herkenham M. Toll-like receptor 4 on nonhematopoietic cells sustains CNS inflammation during endotoxemia, independent of systemic cytokines. J Neurosci. 2005;25(7):1788–1796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lehnardt S, Lachance C, Patrizi S, et al. The toll-like receptor TLR4 is necessary for lipopolysaccharide-induced oligodendrocyte injury in the CNS. J Neurosci. 2002;22(7):2478–2486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lund S, Christensen KV, Hedtjarn M, et al. The dynamics of the LPS triggered inflammatory response of murine microglia under different culture and in vivo conditions. J Neuroimmunol. 2006;180(1–2):71–87. [DOI] [PubMed] [Google Scholar]
- 24. Moraes JC, Coope A, Morari J, et al. High-fat diet induces apoptosis of hypothalamic neurons. PloS One. 2009;4(4):e5045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Park JB, Skalska S, Son S, Stern JE. Dual GABAA receptor-mediated inhibition in rat presympathetic paraventricular nucleus neurons. J Physiol. 2007;582(Pt 2):539–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. 3rd ed San Diego, CA: Academic Press; 1997. [Google Scholar]
- 27. Biancardi VC, Campos RR, Stern JE. Altered balance of gamma-aminobutyric acidergic and glutamatergic afferent inputs in rostral ventrolateral medulla-projecting neurons in the paraventricular nucleus of the hypothalamus of renovascular hypertensive rats. J Comp Neurol. 2010;518(5):567–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Broberger C, Landry M, Wong H, Walsh JN, Hokfelt T. Subtypes Y1 and Y2 of the neuropeptide Y receptor are respectively expressed in pro-opiomelanocortin- and neuropeptide-Y-containing neurons of the rat hypothalamic arcuate nucleus. Neuroendocrinology. 1997;66(6):393–408. [DOI] [PubMed] [Google Scholar]
- 29. Elias CF, Saper CB, Maratos-Flier E, et al. Chemically defined projections linking the mediobasal hypothalamus and the lateral hypothalamic area. J Comp Neurol. 1998;402(4):442–459. [PubMed] [Google Scholar]
- 30. Yi CX, Serlie MJ, Ackermans MT, et al. A major role for perifornical orexin neurons in the control of glucose metabolism in rats. Diabetes. 2009;58(9):1998–2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Horvath TL, Sarman B, Garcia-Caceres C, et al. Synaptic input organization of the melanocortin system predicts diet-induced hypothalamic reactive gliosis and obesity. Proc Natl Acad Sci USA. 2010;107(33):14875–14880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Chen M, Ona VO, Li M, et al. Minocycline inhibits caspase-1 and caspase-3 expression and delays mortality in a transgenic mouse model of Huntington disease. Nat Med. 2000;6(7):797–801. [DOI] [PubMed] [Google Scholar]
- 33. Tikka T, Fiebich BL, Goldsteins G, Keinanen R, Koistinaho J. Minocycline, a tetracycline derivative, is neuroprotective against excitotoxicity by inhibiting activation and proliferation of microglia. J Neurosci. 2001;21(8):2580–2588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Yrjanheikki J, Keinanen R, Pellikka M, Hokfelt T, Koistinaho J. Tetracyclines inhibit microglial activation and are neuroprotective in global brain ischemia. Proc Natl Acad Sci USA. 1998;95(26):15769–15774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Yrjanheikki J, Tikka T, Keinanen R, Goldsteins G, Chan PH, Koistinaho J. A tetracycline derivative, minocycline, reduces inflammation and protects against focal cerebral ischemia with a wide therapeutic window. Proc Natl Acad Sci USA. 1999;96(23):13496–13500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Blanco AM, Valles SL, Pascual M, Guerri C. Involvement of TLR4/type I IL-1 receptor signaling in the induction of inflammatory mediators and cell death induced by ethanol in cultured astrocytes. J Immunol. 2005;175(10):6893–6899. [DOI] [PubMed] [Google Scholar]
- 37. Ito D, Imai Y, Ohsawa K, Nakajima K, Fukuuchi Y, Kohsaka S. Microglia-specific localisation of a novel calcium binding protein, Iba1. Brain Res Mol Brain Res. 1998;57(1):1–9. [DOI] [PubMed] [Google Scholar]
- 38. Tang-Christensen M, Vrang N, Ortmann S, Bidlingmaier M, Horvath TL, Tschop M. Central administration of ghrelin and agouti-related protein (83-132) increases food intake and decreases spontaneous locomotor activity in rats. Endocrinology. 2004;145(10):4645–4652. [DOI] [PubMed] [Google Scholar]
- 39. Becskei C, Riediger T, Hernadfalvy N, Arsenijevic D, Lutz TA, Langhans W. Inhibitory effects of lipopolysaccharide on hypothalamic nuclei implicated in the control of food intake. Brain Behav Immun. 2008;22(1):56–64. [DOI] [PubMed] [Google Scholar]
- 40. Rivest S, Laflamme N, Nappi RE. Immune challenge and immobilization stress induce transcription of the gene encoding the CRF receptor in selective nuclei of the rat hypothalamus. J Neurosci. 1995;15(4):2680–2695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Sagar SM, Price KJ, Kasting NW, Sharp FR. Anatomic patterns of Fos immunostaining in rat brain following systemic endotoxin administration. Brain Res Bull. 1995;36(4):381–392. [DOI] [PubMed] [Google Scholar]
- 42. Sergeyev V, Broberger C, Hokfelt T. Effect of LPS administration on the expression of POMC, NPY, galanin, CART and MCH mRNAs in the rat hypothalamus. Brain Res Mol Brain Res. 2001;90(2):93–100. [DOI] [PubMed] [Google Scholar]
- 43. Hahn TM, Breininger JF, Baskin DG, Schwartz MW. Coexpression of Agrp and NPY in fasting-activated hypothalamic neurons. Nat Neurosci. 1998;1(4):271–272. [DOI] [PubMed] [Google Scholar]
- 44. Stern JE, Armstrong WE. Electrophysiological differences between oxytocin and vasopressin neurones recorded from female rats in vitro. J Physiol. 1995;488( Pt 3):701–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Garrido-Mesa N, Zarzuelo A, Galvez J. Minocycline: far beyond an antibiotic. Br J Pharmacol. 2013;169(2):337–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Kim TH, Kim HI, Kim J, Park M, Song JH. Effects of minocycline on Na+ currents in rat dorsal root ganglion neurons. Brain Res. 2011;1370:34–42. [DOI] [PubMed] [Google Scholar]
- 47. Borner T, Pinkernell S, Lutz TA, Riediger T. Lipopolysaccharide inhibits ghrelin-excited neurons of the arcuate nucleus and reduces food intake via central nitric oxide signaling. Brain Behav Immun. 2012;26(6):867–879. [DOI] [PubMed] [Google Scholar]
- 48. Qin L, Li G, Qian X, et al. Interactive role of the toll-like receptor 4 and reactive oxygen species in LPS-induced microglia activation. Glia. 2005;52(1):78–84. [DOI] [PubMed] [Google Scholar]
- 49. Wright SD, Kolesnick RN. Does endotoxin stimulate cells by mimicking ceramide? Immunol Today. 1995;16(6):297–302. [DOI] [PubMed] [Google Scholar]
- 50. Riediger T, Eisele N, Scheel C, Lutz TA. Effects of glucagon-like peptide 1 and oxyntomodulin on neuronal activity of ghrelin-sensitive neurons in the hypothalamic arcuate nucleus. Am J Physiol Regul Integr Comp Physiol. 2010;298(4):R1061–R1067. [DOI] [PubMed] [Google Scholar]
- 51. Madison LD, Scarlett JM, Levasseur P, et al. Prostacyclin signaling regulates circulating ghrelin during acute inflammation. J Endocrinol. 2008;196(2):263–273. [DOI] [PubMed] [Google Scholar]
- 52. Sartin JL, Marks DL, McMahon CD, et al. Central role of the melanocortin-4 receptors in appetite regulation after endotoxin. J Anim Sci. 2008;86(10):2557–2567. [DOI] [PubMed] [Google Scholar]