Abstract
Kisspeptin, encoded by the Kiss1 gene, binds to a specific G protein-coupled receptor (kisspeptin1 receptor) to regulate the central reproductive axis. Kisspeptin has also been reported to be expressed in peripheral tissues, including the testes. However, factors regulating testicular kisspeptin and its role in reproduction are unknown. Our objective herein was to begin to address kisspeptin function in the testis. In particular, we sought to determine the level of kisspeptin in the testis in comparison with the brain and other tissues, how these levels change from the prepubertal period through sexual maturation, and the factors involved in kisspeptin regulation in the testis. Immunohistochemical analysis of testis sections using a validated kisspeptin antibody localized kisspeptin to the Leydig cells. Kisspeptin was not detected in germ cells or Sertoli cells within the seminiferous tubules at any developmental time period studied, from prepuberty to sexual maturation. A developmental time course of testicular kisspeptin revealed that its mRNA and protein levels increased during development, reaching robust levels at postnatal day 28, correlating with pubertal onset. In vitro studies of primary mouse Leydig cells, as well as in vivo studies, indicated clearly that LH is involved in regulating levels of Leydig cell kisspeptin. Interestingly, gonadectomy resulted in elevated LH but reduced serum kisspeptin levels, suggesting that testicular kisspeptin may be secreted. These data document kisspeptin expression in mouse Leydig cells, its secretion into peripheral serum, and its regulation by changes in reproductive neuroendocrine function.
It is well established that kisspeptin and its G protein-coupled receptor (kisspeptin1 receptor [KISS1R], formerly G protein-coupled receptor 54 [GPR54]) play critical roles in regulating the hypothalamic-pituitary-gonadal axis (1). Kisspeptin neurons, located in the arcuate nucleus of the hypothalamus (ARC) and anteroventral periventricular nucleus of the hypothalamus (AVPV), secrete kisspeptin proteins that bind to KISS1R on GnRH neurons and stimulate GnRH secretion (2). Kisspeptin signaling contributes to the initiation of puberty and plays a leading role in adult reproduction.
Mouse kisspeptin is translated into a 130-amino acid peptide with an N-terminal secretory signal sequence and amidated C terminus. Kisspeptin-130 undergoes proteolytic cleavage, which produces a 54-amino acid peptide, also known as metastin. Shorter cleaved peptides are also found in different tissues, but all share the C-terminal 10 amino acids (kisspeptin-10 (kp-10)) required for biological activity (3). In addition to its expression in the hypothalamus, kisspeptin has also been localized to peripheral tissues, including liver, fat, gonads, intestine, and placenta (4–7). However, its role in these tissues is unclear.
Human spermatozoa have been found to express kisspeptin and KISS1R. Kisspeptin and its receptor are present mainly in the sperm head around the neck and in the flagellum midpiece (8). Kisspeptin has recently been reported to be present in spermatocytes and spermatids of adult rhesus monkeys (9). Two recently published studies reported the presence of kisspeptin in the mouse testis. Anjum et al (10) reported the immunolocalization of kisspeptin along with GnRH, GnRH receptor, and gonadotropin-inhibiting hormone in mouse testis Leydig cells. Mei et al (11) used transgenic mice with Kiss1 alleles targeted with a LacZ reporter gene to define the kisspeptin1 (Kiss1) testicular cell expression profile. In this case, Kiss1 was localized to germ cells, with no activity in the Leydig cells of Kiss1 transgenic mice. However, when sections of testes were visualized with a well characterized and highly specific kisspeptin antibody, there was strong immunoreactivity in Leydig cells with no staining in germ cells (11).
These data raise questions about where within the testes kisspeptin is present, when during testicular development it appears, how it is regulated, and what it does. In this study, we investigate the developmental appearance of kisspeptin in testes and provide data suggesting central, neuroendocrine regulation of its expression and secretion.
Materials and Methods
Reagents
HEPES, Hanks' balanced salt solution, Percoll, hCG, LHRH agonist, and collagenase were obtained from Sigma-Aldrich. Medium 199 was from Gibco BRL. BSA was obtained from ICN Biomedicals, Inc. Bovine LH (USDA-bLH-B-6) was provided by the United States Department of Agriculture Animal Hormone Program (Beltsville, MD).
Animals
C57BL/6 mice were housed in Johns Hopkins University Medical Research Building at 22°C, 14-hour light, 10-hour dark environment. They were fed standard chow diet and had free access to water. All experiments were carried out in accordance with the guidelines established by the National Institutes of Health Guide for the Care and Use of Laboratory Animals. Procedures were approved by the Johns Hopkins Animal Care and Use Committee. Adult (2–4 mo old) or postnatal (7, 14, 21, or 28 d old) male mice were used in this study.
In vivo studies
Adult male mice were divided into 4 different groups (n = 3–4/group). First samples were collected from mice via a mandibular blood draw right before treatments. Single doses of hCG and saline were injected ip in each group. LHRH agonist was injected to the dorsal neck area. Testosterone (DELATESTRYL) was injected ip on 3 consecutive days. Two hours after the last injections, animals were killed, a second blood sample was obtained, and testes were snap frozen in liquid nitrogen. Experiments were repeated 2 times. In developmental studies, male animals were killed on postnatal day (PND)7, PND14, PND21, or PND28 (n = 3–4). The frozen testes were stored at −80°C until analysis. Hypothalamic nuclei regions, AVPV and ARC, are obtained as described by Quennell et al (12). Briefly, hypothalami were dissected and then divided in half in the coronal plane immediately anterior to the pituitary stalk. AVPV neurons are embedded within the anterior part, and the posterior part contained the ARC neurons. Brain cortex and cerebellum were collected in blocks with the same amount of tissue present in the hypothalamic block. Subcutaneous fat was obtained from the abdominal area. Frozen tissues were stored at −80°C until analysis.
Primary Leydig cell isolation and in vitro LH stimulation
Primary Leydig cells were isolated from the testes of 10 adult mice following established procedures (13). In brief, the testes were dissected and washed in cold PBS buffer. Testes then were decapsulated and digested in the dissociation buffer (M-199 medium with 2.2-g/L HEPES, 1.0-g/L BSA, 2.2-g/L sodium bicarbonate, containing collagenase, 0.5 mg/mL; Sigma-Aldrich) at 34°C with shaking for 30 minutes. To separate the interstitial cells from the seminiferous tubules, BSA was added to produce 1% final BSA concentration. The solution was then settled for 1 minute, and supernatants were transferred to a new tube. The interstitial cells were pelleted by centrifugation at 1000 rpm for 5 minutes. Leydig cells were purified by Percoll gradient separation (55% Percoll, 27 000g for 1 h). After centrifugation, Leydig cells with a density of 1.07 and heavier were harvested. The cells were further purified by BSA gradient centrifugation (0%–10% BSA at 450g for 10 min). The final purity of the Leydig cells, determined by staining the cells for 3β-hydroxysteroid dehydrogenase (3β-HSD) activity, was consistently about 90%. Freshly isolated Leydig cells were suspended in M-199 culture media (0.1% BSA) and plated in 24-well culture plates (2 × 105 cells/well; Becton Dickson) and cultured at 34°C. Cells were stimulated with ovine LH (20 ng/mL) for different period of times (up to 18 h). By the end of culture, the medium was collected and frozen for testosterone assay, and cells were used for total RNA isolation. The experiments were repeated 3 times with the cells isolated from different animals.
Total RNA extraction, reverse transcription, and quantitative PCR
Testes and primary Leydig cell RNA were extracted by TRIzol (Invitrogen) according to the manufacturer's protocol. Total RNA (1 μg) was reverse transcribed (iScript cDNA Synthesis kit; Bio-Rad) to cDNA. mRNA levels of StAR and 18S were measured by Quantitative Real-Time PCR according to the manufacturer's protocol (IQ SYBR Green; Bio-Rad). Relative mRNA levels of Kiss1 and Gapdh were measured by TaqMan assay according to the manufacturer's protocol (IQTM Supermix; Bio-Rad). Primers for StAR were sense 5′-CCCAAAGAAGGCATAGCAAG-3′ and antisense 5′-GCTGAATCCCCCAAACTTCT-3′, for Kiss1 sense 5′-AGCTGCTGCTTCTCCTCTGT3′ and antisense 5′-GGACTGCTGGCCTGTGGAT-3′, for Gapdh sense 5′-GGGCATCTTGGGCTACACT-3′, and antisense 5′-GGCATCGAAGGTGGAAGAGT-3′, and for 18S sense 5′-GCATGGCCGTTCTTAGTTGG-3′, and antisense 5′-TGGCAGAGTCTCGTTCGTTA-3′. TaqMan probe sequence for Kiss1 was 5′-CCGCTGGCAAAAGTGAAGCCTGG-3′ labeled with FAM and for Gapdh was 5′-AGGACCAGGTTGTCTCCTGCGA-3′ labeled with Cal Gold 540, both with Black Hole QuencherTM 1 (BHQ1).
Immunohistochemistry
The testis was dissected from adult or postnatal mice and fixed in Bouin's solution overnight. Tissue was then cryopreserved in 30% sucrose solution and frozen. Tissue was immersed in Tissue-Tek CRYO-OCT Compound (Fisher Scientific) and sectioned to 6-μm thickness using cryostat (HM550; MICROM GmbH) at −20°C. Sections were rinsed 3 times with PBS (Corning cellgro) and subsequently incubated in a humid chamber with blocking solution containing 5% normal goat serum (Vector Laboratories, Inc) and 0.3% Triton X-100 in PBS for 1 hour at room temperature. Sections were incubated overnight at 4°C with primary kisspeptin antibody (AB9754; Millipore) dissolved 1:500 to 1:1000 in blocking solution. After washing 3 times with PBS, sections were incubated for 1 hour at room temperature with goat antirabbit IgG conjugated to Alexa Fluor 488 (A11034; Invitrogen) dissolved 1:2000 to 1:5000 in blocking solution. Sections were washed 3 times with PBS and mounted with vectashield mounting medium with DAPI (Vector Laboratories, Inc) and photographed with an AxioCamMR camera and exported to AxioVision software.
Western blottings
Proteins (30–40 mg) from testes or primary Leydig cell lysates were separated by a 4%–20% gradient SDS-PAGE. The separated proteins were transferred to a PVDF membrane in a semidry blotting chamber according to the manufacturer's protocol (Bio-Rad). Blots were blocked with 5% milk in Tris-buffered saline solution (pH 7.6) containing 0.05% Tween 20 and probed with rabbit primary antibodies against kisspeptin (ab19028; Abcam), or steroidogenic acute regulatory (StAR) (8449P; Cell Signaling), and mouse primary antibody against β-actin (sc-47778 horseradish peroxidase; Santa Cruz Biotechnology, Inc). Signals were detected by enhanced chemiluminescence plus Western Blotting Detection System (GE Healthcare).
3β-HSD staining
To examine 3β-HSD enzyme activity, cells were washed in PBS and dried at room temperature for 20 minutes. Staining solution (0.4 mm 5β-androstan-3β-ol-17-one steroid substrate, 1-mg/mL nicotinamide adenine dinucleotide, and 0.2-mg/mL tetranitro blue tetrazolium) was added to slides for 40 minutes and then removed by 2 successive washes in PBS. The slides were placed in 10% formalin for 30 minutes (14).
Hormone assays
Serum LH and FSH were measured using a Milliplex MAP immunoassay (Mouse Pituitary panel; Millipore) on a Luminex 200IS platform (Luminex Corp). Testosterone in the medium was assayed by RIA. The sensitivity and intra- and interassay coefficients of variation of the RIA were 13 pg/tube, 8.9% and 13.6%, respectively. Serum kisspeptin was measured using Kisspeptin-13 (human) RIA kit (catalog number S-2233.0001; Peninsula Laboratories International, Inc), which has cross-reactivity between rodent and human (15).
Statistical analysis
All data were analyzed with unpaired 2-tailed Student's t test or one-way ANOVA with Tukey post hoc multiple comparison using Prism Software (GraphPad Software, Inc). Data are expressed as mean ± SEM, and P < .05 was defined as statistically significant.
Results
Kisspeptin is expressed and translated in testicular Leydig cells
We performed an initial tissue panel expression analysis of Kiss1 mRNA (Figure 1A) to determine relative levels of expression. Quantitative real-time RT-PCR measurement of Kiss1 after normalizing to the housekeeping gene Gapdh showed that Kiss1 expression is 17 times greater in testes compared with the male ARC (P < .05). To determine whether expression of the Kiss1 gene was translated into protein in the testes, we performed immunohistochemistry of mouse testicular tissue using a highly specific kisspeptin antibody. Figure 1B shows that kisspeptin protein localized to the interstitial compartment of the testis. At higher magnification (Figure 1C), it is apparent that staining was in the Leydig cells and not in cells within the seminiferous tubules.
Figure 1. Expression of Kiss1 mRNA in mouse tissues.
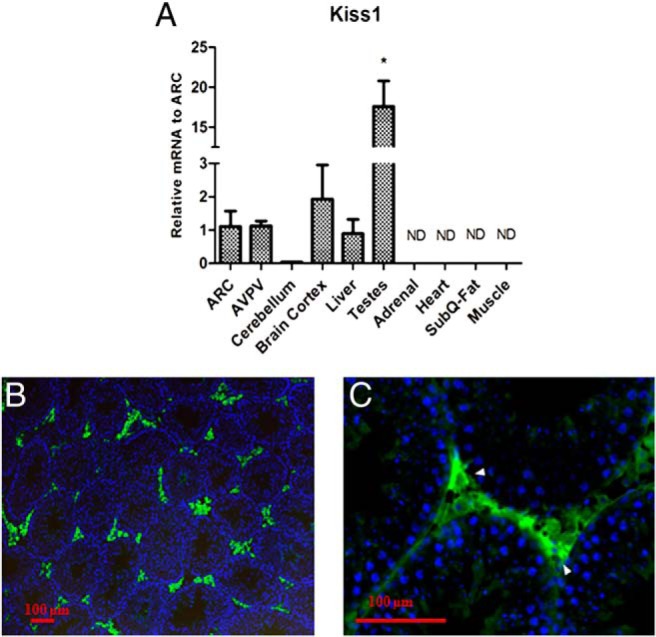
A, Tissue panel of Kiss1 mRNA expression. The bar graph represents the ratio of Kiss1/glyceraldehyde-3-phosphate dehydrogenase (Gapdh) mRNA normalized to the average of the ARC mRNA (n = 3). B and C, Immunostaining of a histologic section from an adult testis with kisspeptin antibody showing kisspeptin is present in interstitial cells (B, 10×; C, 40×). ND, not distinguished.
Developmental expression and translation of kisspeptin
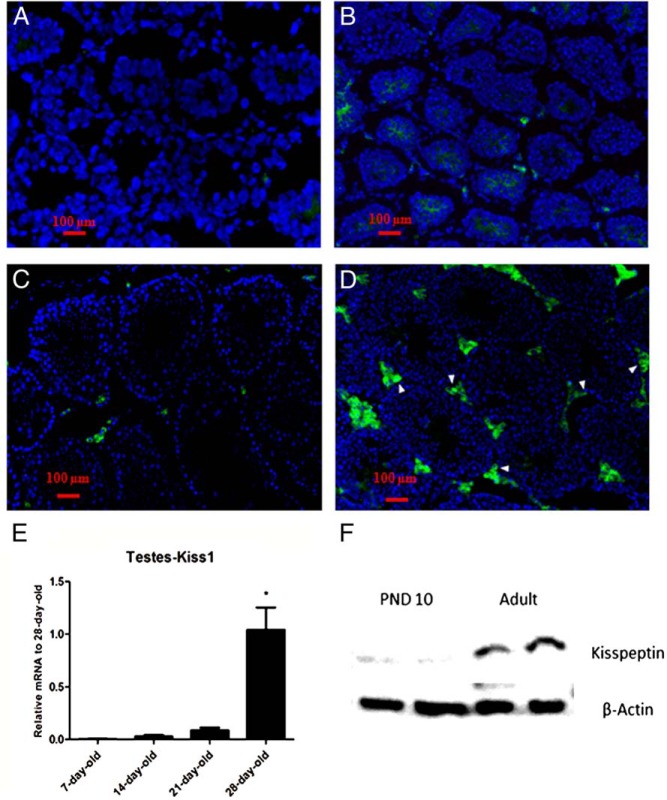
We next examined the developmental expression pattern of Kiss1 on PND7 (Figure 2A), PND14 (Figure 2B), PND21 (Figure 2C), and PND28 (Figure 2D) using cryosectioned testes immunostained for kisspeptin. Quantification of mRNA levels is seen in Figure 2E. Kisspeptin mRNA was not detected in early postnatal testes (PND7), increased somewhat through PND21, and then increased substantially by PND28, correlating with the onset of puberty. Of note, spermatogenesis in mice starts not earlier than 3 weeks postnatal (16). To determine whether there also were increases in kisspeptin protein, Western blot analysis of kisspeptin in testicular lysates from neonatal (PND10) and adult testes were compared. As seen in Figure 2F, increased kisspeptin was seen in the adult as compared with the neonatal testes, consistent with the mRNA analyses.
Figure 2. Developmental expression of kisspeptin in interstitial cells of mouse testes.
Histologic sections from testes of 7-day-old (A), 14-day-old (B), 21-day-old (C), and 28-day-old (D) mice were immunostained with kisspeptin antibody. Arrowheads in the photo denote KP-immunoreactive site in the tissue. E, The bar graph represents the relative mRNA expression of Kiss1 at different ages relative to 28-day-old mice (n = 4 in each group). F, Representative Western blot analysis of kisspeptin in testes lysate of 10-day-old and adult mice.
Regulation of Leydig cell Kiss1 expression by LH, in vitro analyses
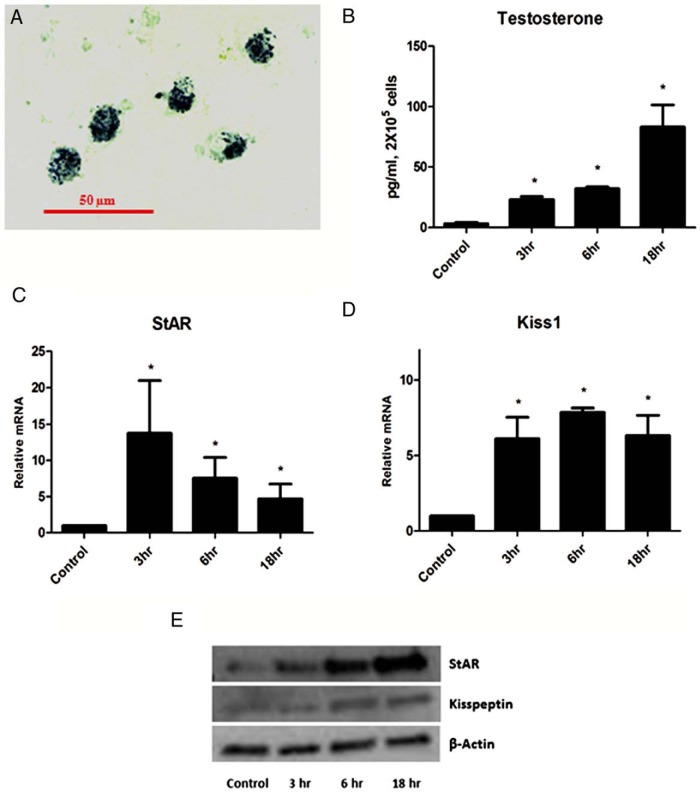
The observation of increased Kiss1 expression from the postnatal to the prepubertal period, combined with previous studies indicating increased testosterone production in response to increase circulating LH during this period, led us to hypothesize that increased Kiss1 expression might be in response to LH. To test this, we isolated Leydig cells from adult mice and incubated the cells with maximally stimulating LH (20 ng/mL). The cells were over 80% pure, as determined by their staining for 3β-HSD (Figure 3A). Cells incubated with LH for 3–18 hours produced increasing amounts of testosterone (P < .01) (Figure 3C), and StAR mRNA expression was increased (P < .05) (Figure 3B), serving as positive controls for LH action. Incubation of the cells with LH was found to increase Kiss1 mRNA levels in the cells (P < .05) (Figure 3D), although the pattern of elevation differed from that of testosterone. That is, in the case of Kiss1, a significant increase was seen at 3 hours and, then, sustained through 18 hours. Western blot analysis of kisspeptin and StAR were also performed (Figure 3E) and showed that protein levels were elevated after 3 hours (StAR) or 6 hours (kisspeptin) of incubation with LH.
Figure 3. In vitro LH stimulation of Leydig cells.
A, 3β-HSD staining of Leydig cells isolated from the mice testes. B, Testosterone production by Leydig cells at different times after LH (20 ng/mL) stimulation. StAR (C) and Kiss1 (D) mRNA expression normalized to 18S after LH stimulation.*, significant difference compared with control. E, Western blot analysis of kisspeptin and StAR after stimulation of primary Leydig cells with LH (20 ng/mL).
Regulation of Leydig cell Kiss1 expression by LH, in vivo analyses
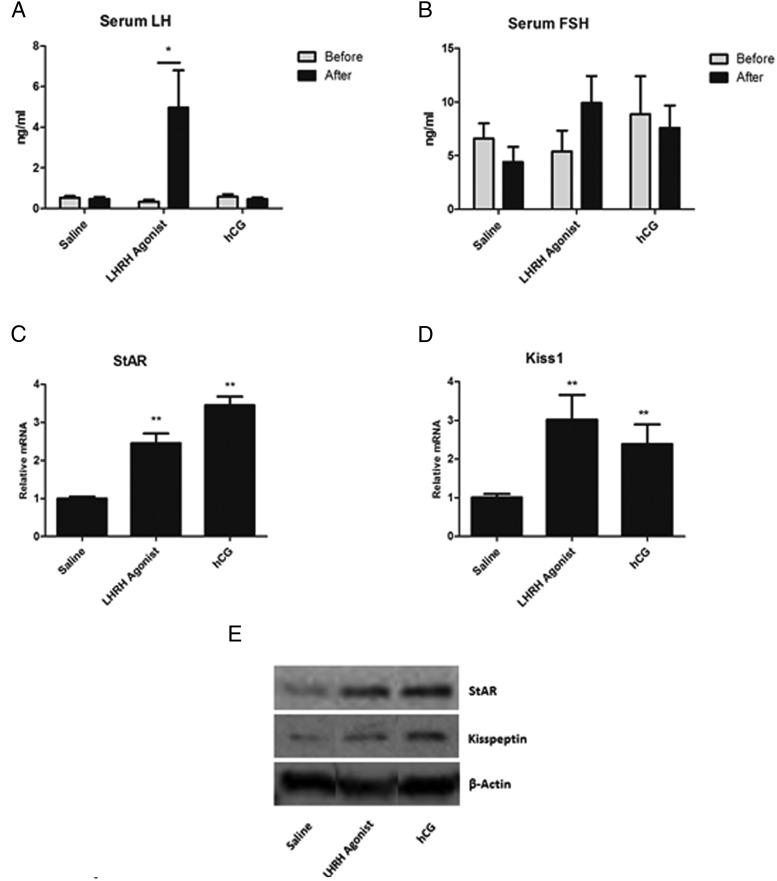
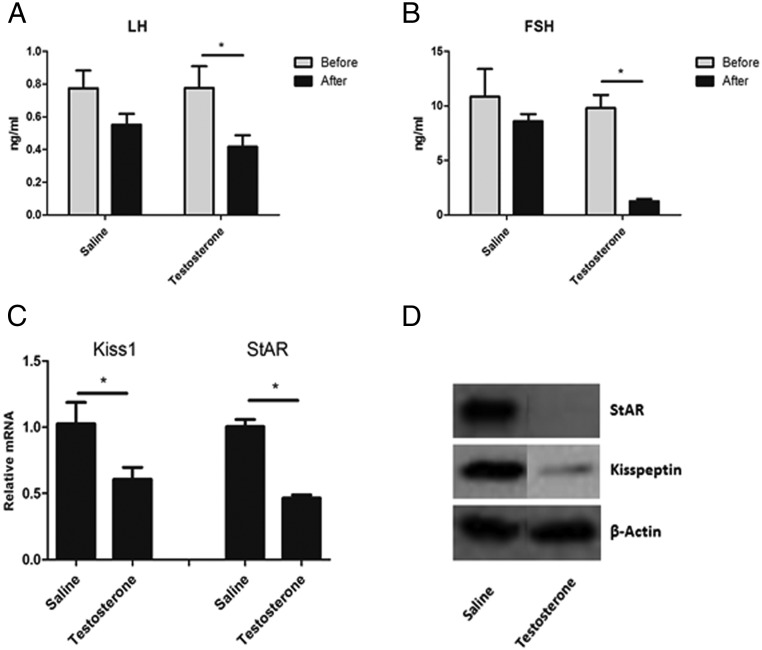
As shown above, in vitro studies suggested a role for LH in the regulation of Leydig cell Kiss1 expression. To investigate whether LH plays this role in vivo, we first asked whether enhancement of endogenous LH production in vivo would increase kisspeptin expression in the testes. To this end, mice were sc injected with LHRH agonist (in a single dose of 0.2 ng/g body weight) (17) or administered hCG (10 IU ip). As expected, there was a robust increase in serum LH (P < .05) (Figure 4A) after LHRH stimulation compared with saline injection, as well as in levels of intratesticular StAR mRNA (Figure 4C). Because the cAMP/protein kinase A signaling pathway activated by LH/hCG is the most well-defined mechanism to regulate StAR expression (18), StAR was thus used as a control for the LH/hCG effects on Leydig cell gene expression. There was no change in serum FSH (Figure 4B). Consistent with the in vitro results, increased LH resulting from LHRH administration was found to be associated with significant increase in testicular Kiss1 mRNA expression (P < .05) (Figure 4D). This also was the case when hCG was administered to the mice (P < .05) (Figure 4D). Western blot analysis of the testicular lysate (Figure 4E) showed that StAR and kisspeptin increased after 2 hours of LH, consistent with in vitro results. In a second group of mice, the effect of LH suppression on Kiss1 expression was assessed. Given that LH increase in vivo resulted in increases in Kiss 1 mRNA, we hypothesized that its suppression would result in the reverse. To suppress LH, mice were injected with testosterone on 3 consecutive days (0.5 mg/10 g/d). As expected, this resulted in reduced serum LH (Figure 5A) and FSH (Figure 5B) levels, as well as in reduced StAR (P < .05) (Figure 5C). As hypothesized, the decrease in LH was associated with decreased Kiss1 (P < .05) (Figure 5C). Protein levels of StAR and kisspeptin were also suppressed (Figure 5D) by 3 days of testosterone injections.
Figure 4. Endocrine regulation of testicular kisspeptin.
A single dose of LHRH agonist (0.2 ng/g), hCG (10 IU), or saline was injected in animals. Tissues were harvested 2 hours after injection. A and B, Serum concentrations of LH (A) and FSH (B) before and after treatments. C and D, mRNA expression of StAR (C) and Kiss1(D) normalized to Gapdh in testes after treatment. *, P < .05; **, statistically significant difference compared with the saline treated group (n = 3–4 for each group). E, Representative Western blot analysis showing the effects of 2 hours of treatment with LHRH agonist and hCG on kisspeptin and StAR protein levels in the testes.
Figure 5. Testosterone was injected for 3 days in a dose of 0.5 mg/10 g/d.
Tissues were harvested 2 hours after the last injection. A and B, Serum concentrations of LH (A) and FSH (B) before and after treatments. C, mRNA expression of Kiss1 and StAR normalized to Gapdh in testes after treatment. *, P < .05. D, Representative Western blot analysis showing the effects of 3 days of testosterone on kisspeptin and StAR protein expression in testes.
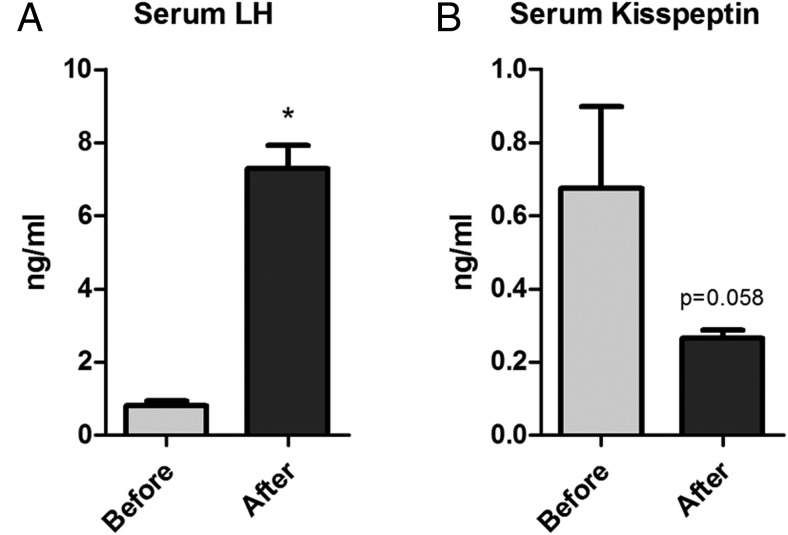
In light of the observation that the testicular expression of Kiss1 is very high in the testes relative to the other tissues that were analyzed (Figure 1A), we asked whether testicular kisspeptin might be secreted from Leydig cells into the peripheral circulation. To test this, serum was obtained from mice before and 7 days after castration, and serum LH and kisspeptin were measured. Serum LH was significantly increased after gonadectomy, as expected (0.8040 ± 0.1236 vs 7.288 ± 0.6259, P < .05) (Figure 6A). Despite the increased LH, however, the serum kisspeptin levels decreased by about 2.5-fold after castration, presumably as a consequence of the absence of the testes (0.6758 ± 0.2232 vs 0.2648 ± 0.02268, P = .058) (Figure 6B). These results suggest that testicular kisspeptin is, in fact, secreted into the serum.
Figure 6. Serum concentration of LH (A) and kisspeptin (B); before and 7 days after gonadectomy.
*, significant difference between groups (n = 4).
Discussion
It is well established that kisspeptin, through its binding to specific G protein-coupled receptors in the hypothalamus, is integrally involved in initiating the secretion of GnRH, and in this way is critically important in the initiation of puberty and ultimately in male fertility. Several recent studies have presented evidence regarding the presence of kisspeptin in peripheral tissues, including the testes. In contrast to its well-established central function, however, its functions in tissues outside the central reproductive axis remain uncertain. In the testes, the cell type(s) that express kisspeptin, its regulation, and its possible physiologic role remain either controversial or unknown. Hence, we sought to characterize the expression pattern of testicular kisspeptin and define its regulated role in the reproductive axis.
The studies that we conducted demonstrated that, in the mouse, the expression of Kiss1 mRNA in the testes was by far the highest of all tissues examined. For example, we found that the expression of Kiss1 mRNA was 17 times greater in testes than in the ARC in the brain. Immunohistochemical analysis from testicular tissue sections showed that kisspeptin is highly present in Leydig cells and localized to these cells. This result confirms the initial finding by Anjum et al (10) of kisspeptin expression in Leydig cells. We further provide evidence that testicular kisspeptin increases along a developmental paradigm paralleling pubertal development in the mouse. Robust expression of Kiss1 mRNA and protein in interstitial cells appears at PND28, the time for initiation of puberty in our C57BL/6 mice colony (19). In contrast to our observation of Kiss1 localization in Leydig cells, Mei et al (11) reported that after 4 weeks of age, Kiss1 mRNA was seen in germ cells within the seminiferous tubules and not in Leydig cells, although the results of immunohistochemical staining of adult mouse testes using a specific kisspeptin antibody revealed the presence of kisspeptin in Leydig cells. These authors suggested that the observation of kisspeptin in adult Leydig cells may be an artifact. Our results are not in agreement with this conclusion. Rather, our morphological studies, of both developmental expression of kisspeptin and its location in the adult testis, show clearly that kisspeptin is localized to Leydig cells. This conclusion is supported by our studies of isolated primary Leydig cells from the adult testis. These cells were similarly found to express kisspeptin mRNA. Additionally, in vivo studies showed that Leydig cells, which responded to increased LH by increased steroid production, also responded with increased Kiss1 expression. In studies in which LH was suppressed, there was reduced Kiss1 expression. Taken together, these studies show that Kiss1 is produced by Leydig cells and that the neuroendocrine hormone LH plays a significant role in Kiss1 mRNA regulation in Leydig cells both in vivo and in vitro. Although it is not known whether Leydig cell kisspeptin expression is initiated via a hormonal regulatory signal alone, or also by an autoregulatory process, the responsiveness of Leydig cell kisspeptin to LH and its increased expression corresponding with central activation of the reproductive axis at puberty suggests that the signal for Leydig cell kisspeptin expression is neuroendocrine-mediated LH secretion.
To define a physiological or pharmacological role for peripheral kisspeptin, several studies tested the use of exogenous kisspeptin on testicular maturation and testosterone production. Chronic administration of kp-10 to prepubertal male rats decreased serum LH levels, testosterone, and sperm maturation and resulted in degeneration of seminiferous tubules (20). In 2 other studies, chronic administration of kisspeptin to mice decreased seminal fructose concentrations (21) and increased prostatic epithelial cellular atrophy (22), demonstrating that long-term kp-10 administration negatively regulates gonadal maturation. Thompson et al (23) tested single subcutaneous kisspeptin-54 as well as continuous SC administration of kp-54. Surprisingly, both single dose and chronic administration of kisspeptin resulted in dose-dependent testicular degeneration. However, pretreatment of animals with cetrorelix blocked kisspeptin-induced testicular degeneration, suggesting that the potentiating effect of exogenous kisspeptin is centrally mediated (23). All of these studies were performed to test the potential pharmacological effects or side effects of kisspeptin as a potent stimulator of the hypothalamic-pituitary-gonadal axis, and none of them considered the testes as the source of peripheral kisspeptin. Indeed, our data obtained by measuring serum kisspeptin after castration suggested that testicular kisspeptin is a source of circulating kisspeptin.
The Kiss1 receptor is not only expressed in different regions of brain in rodents and primates (24–27) but also in peripheral tissues such as the liver, intestine, pancreas, ovary, and placenta (27–30). Pinto et al demonstrated that KISS1R is also present in human spermatozoa along with kisspeptin (8), and β-galctosidase activity was detected in spermatids of the kiss1R-lacz reporter mouse testis (11). Based on our data, we propose that during the initiation of puberty, the increase in LH secretion from the pituitary induces kisspeptin expression and secretion from Leydig cells, which then enhances spermatogenesis. This idea is supported by Pinto et al, who reported that kisspeptin induced sperm motility and hyperactivation and that the effects were blunted with a KISS1R antagonist, peptide 234 (8).
Although the functional role of testicular kisspeptin secretion remains elusive, we suggest that it may convey a feedback signal to control the central neuroendocrine axis and perhaps integrate metabolic and reproductive function. With regard to the latter, emerging data support the concept that central kisspeptin could be a link between metabolism and reproduction (31). Recent data also implicates peripheral kisspeptin is playing a significant role in metabolic function. Using genetically modified mouse models, Song et al (32) showed that kisspeptin is expressed in the liver and functions as a hormone to suppress glucose-stimulated insulin secretion from pancreatic β-cells. Importantly, this study revealed that supraphysiologic doses of kisspeptin stimulate glucose-stimulated insulin secretion, independent of Kiss1R signaling (32).
In conclusion, kisspeptin is present in Leydig cells, developmentally responsive, and regulated by neuroendocrine mechanisms controlling LH. We propose that Leydig cell kisspeptin may play a pivotal role in peripheral and neuroendocrine modulation of male reproduction.
Acknowledgments
This work was supported by National Institutes of Health grants R01HD068777 and U01HD066432 to S.R., and T32 DK07751 to S.S.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- ARC
- arcuate nucleus of the hypothalamus
- AVPV
- anteroventral periventricular nucleus of the hypothalamus
- 3β-HSD
- 3β-hydroxysteroid dehydrogenase
- hCG
- human chorionic gonadotropin
- Kiss1
- kisspeptin1
- KISS1R
- kisspeptin1 receptor
- KP-10
- kisspeptin-10
- LHRH
- luteinizing hormone-releasing hormone
- M-199
- medium 199
- PND
- postnatal day
- StAR
- steroidogenic acute regulatory.
References
- 1. Seminara SB, Messager S, Chatzidaki EE, et al. The GPR54 gene as a regulator of puberty. N Engl J Med. 2003;349(17):1614–1627. [DOI] [PubMed] [Google Scholar]
- 2. Pinilla L, Aguilar E, Dieguez C, Millar RP, Tena-Sempere M. Kisspeptins and reproduction: physiological roles and regulatory mechanisms. Physiol Rev. 2012;92(3):1235–1231. [DOI] [PubMed] [Google Scholar]
- 3. d'Anglemont de Tassigny X, Colledge WH. The role of kisspeptin signaling in reproduction. Physiology (Bethesda). 2010;25(4):207–217. [DOI] [PubMed] [Google Scholar]
- 4. Hauge-Evans AC, Richardson CC, Milne HM, Christie MR, Persaud SJ, Jones PM. A role for kisspeptin in islet function. Diabetologia. 2006;49(9):2131–2135. [DOI] [PubMed] [Google Scholar]
- 5. Lee JH, Miele ME, Hicks DJ, et al. KiSS-1, a novel human malignant melanoma metastasis-suppressor gene. J Natl Cancer Inst. 1996;88(23):1731–1737. [DOI] [PubMed] [Google Scholar]
- 6. Brown RE, Imran SA, Ur E, Wilkinson M. KiSS-1 mRNA in adipose tissue is regulated by sex hormones and food intake. Mol Cell Endocrinol. 2008;281(1–2):64–72. [DOI] [PubMed] [Google Scholar]
- 7. Nakamura Y, Aoki S, Xing Y, Sasano H, Rainey WE. Metastin stimulates aldosterone synthesis in human adrenal cells. Reprod Sci. 2007;14(8):836–845. [DOI] [PubMed] [Google Scholar]
- 8. Pinto FM, Cejudo-Román A, Ravina CG, et al. Characterization of the kisspeptin system in human spermatozoa. Int J Androl. 2012;35(1):63–73. [DOI] [PubMed] [Google Scholar]
- 9. Tariq AR, Shahab M, Clarke IJ, et al. Kiss1 and Kiss1 receptor expression in the rhesus monkey testis: a possible local regulator of testicular function. Cent Eur J Biol. 2013;8(10):968–974. [Google Scholar]
- 10. Anjum S, Krishna A, Sridaran R, Tsutsui K. Localization of gonadotropin-releasing hormone (GnRH), gonadotropin-inhibitory hormone (GnIH), kisspeptin and GnRH receptor and their possible roles in testicular activities from birth to senescence in mice. J Exp Zool A Ecol Genet Physiol. 2012;317(10):630–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Mei H, Doran J, Kyle V, Yeo SH, Colledge WH. Does kisspeptin signaling have a role in the testes? Front Endocrinol (Lausanne). 2013;4:19830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Quennell JH, Howell CS, Roa J, Augustine RA, Grattan DR, Anderson GM. Leptin deficiency and diet-induced obesity reduce hypothalamic kisspeptin expression in mice. Endocrinology. 2011;152(4):1541–1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Salva A, Klinefelter GR, Hardy MP. Purification of rat leydig cells: increased yields after unit-gravity sedimentation of collagenase-dispersed interstitial cells. J Androl. 2001;22(4):665–671. [PubMed] [Google Scholar]
- 14. Stanley E, Lin CY, Jin S, et al. Identification, proliferation, and differentiation of adult leydig stem cells. Endocrinology. 2012;153(10):5002–5010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kinsey-Jones JS, Beale KE, Cuenco J, et al. Quantification of rat kisspeptin using a novel radioimmunoassay. PLoS One. 2014;9(5):e9761120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Nebel BR, Amarose AP, Hacket EM. Calendar of gametogenic development in the prepuberal male mouse. Science. 1961;134(3482):832–833. [DOI] [PubMed] [Google Scholar]
- 17. Wu S, Chen Y, Fajobi T, et al. Conditional knockout of the androgen receptor in gonadotropes reveals crucial roles for androgen in gonadotropin synthesis and surge in female mice. Mol Endocrinol. 2014;28(10):1670–1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Stocco D. The role of StAR in leydig cell steroidogenesis. In: Payne AH, Hardy M, eds. Contemporary Endocrinology: The Leydig Cell in Health and Disease. 2007;149–155. [Google Scholar]
- 19. Wu S, Divall S, Hoffman GE, Le WW, Wagner KU, Wolfe A. Jak2 is necessary for neuroendocrine control of female reproduction. J Neurosci. 2011;31(1):184–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ramzan F, Qureshi IZ. Intraperitoneal kisspeptin-10 administration induces dose-dependent degenerative changes in maturing rat testes. Life Sci. 2011;88(5–6):246–256. [DOI] [PubMed] [Google Scholar]
- 21. Ramzan F, Khan MA, Ramzan MH. The effect of chronic kisspeptin administration on seminal fructose levels in male mice. Endocrine. 2014;45(1):144–147. [DOI] [PubMed] [Google Scholar]
- 22. Ramzan F, Qureshi IZ, Ramzan M, Ramzan MH, Ramzan F. Kisspeptin-10 induces dose dependent degeneration in prepubertal rat prostate gland. Prostate. 2013;73(7):690–699. [DOI] [PubMed] [Google Scholar]
- 23. Thompson EL, Amber V, Stamp GW, et al. Kisspeptin-54 at high doses acutely induces testicular degeneration in adult male rats via central mechanisms. Br J Pharmacol. 2009;156(4):609–625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Shahab M, Mastronardi C, Seminara SB, Crowley WF, Ojeda SR, Plant TM. Increased hypothalamic GPR54 signaling: a potential mechanism for initiation of puberty in primates. Proc Natl Acad Sci USA. 2005;102(6):2129–2134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wahab F, Ullah F, Chan YM, Seminara SB, Shahab M. Decrease in hypothalamic Kiss1 and Kiss1r expression: a potential mechanism for fasting-induced suppression of the HPG axis in the adult male rhesus monkey (Macaca mulatta). Horm Metab Res. 2011;43(2):81–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kotani M, Detheux M, Vandenbogaerde A, et al. The metastasis suppressor gene KiSS-1 encodes kisspeptins, the natural ligands of the orphan G protein-coupled receptor GPR54. J Biol Chem. 2001;276(37):34631–34636. [DOI] [PubMed] [Google Scholar]
- 27. Muir AI, Chamberlain L, Elshourbagy NA, et al. AXOR12, a novel human G protein-coupled receptor, activated by the peptide KiSS-1. J Biol Chem. 2001;276(31):28969–28975. [DOI] [PubMed] [Google Scholar]
- 28. Lee DK, Nguyen T, O'Neill GP, et al. Discovery of a receptor related to the galanin receptors. FEBS Lett. 1999;446(1):103–107. [DOI] [PubMed] [Google Scholar]
- 29. Ramaswamy S, Guerriero KA, Gibbs RB, Plant TM. Structural interactions between kisspeptin and GnRH neurons in the mediobasal hypothalamus of the male rhesus monkey (Macaca mulatta) as revealed by double immunofluorescence and confocal microscopy. Endocrinology. 2008;149(9):4387–4395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Shibata M, Friedman RL, Ramaswamy S, Plant TM. Evidence that down regulation of hypothalamic KiSS-1 expression is involved in the negative feedback action of testosterone to regulate luteinising hormone secretion in the adult male rhesus monkey (Macaca mulatta). J Neuroendocrinol. 2007;19(6):432–438. [DOI] [PubMed] [Google Scholar]
- 31. Wahab F, Atika B, Shahab M. Kisspeptin as a link between metabolism and reproduction: evidences from rodent and primate studies. Metabolism. 2013;62(7):898–910. [DOI] [PubMed] [Google Scholar]
- 32. Song WJ, Mondal P, Wolfe A, et al. Glucagon regulates hepatic kisspeptin to impair insulin secretion. Cell Metab. 2014;19(4):667–681. [DOI] [PMC free article] [PubMed] [Google Scholar]