Abstract
The endocervix has both anatomical and biological functions that participate in the delicate balance between tolerance necessary for conception and protection from pathogens. Our goal was to develop a robust 3-dimensional (3D) endocervix model that was a reliable representation of the in vivo tissues and to identify the physiological responses to changing levels of steroid hormones during a 28-day time period. Human endocervical cells were grown on polystyrene scaffolds, and the morphologic and hormonal responses of cultured cells were assessed in response to fluctuating levels of estradiol (E2) or progesterone (P4). Morphologically, the 3D cultures were composed of a mixed population of cells, including epithelial and stromal cells. Treatment with E2 and P4 (d 28) increased cell growth and proliferation as compared with no treatment control. Cells expressed estrogen receptor and P4 receptor and produced both neutral and acidic mucins, including Mucin 16. In addition, a 45-plex Luminex assay identified numerous factors secreted and regulated by hormones. Specifically, IL-1β and leukemia inhibitory factor significantly decreased in the presence of E2 and P4 as compared with the no hormone control at day 26. Cotreatment with RU486 (mifepristone) attenuated the inhibition of IL-1β and leukemia inhibitory factor secretion. In summary, a robust, novel 3D endocervical culture was developed, and physiologic responses to the menstrual cycle mimic of E2 and P4 levels for a period of 28 days were identified.
During the reproductive lifespan of a woman, the ovary synthesizes and secretes sex steroid hormones and peptides in a programmed manner. The 2 defining hormones of the menstrual cycle, estrogen and progesterone (P4) rise and fall, on average, every 28 days until follicle maturation ceases to occur at menopause. At each cycle, estradiol (E2) gradually increases during the follicular (proliferative) phase with the highest serum levels at the preovulatory stage (d 14) (1). The luteal (secretory) phase of the menstrual cycle is marked by decreased levels of E2 and a gradual increase in P4 typically peaking at day 21 of the cycle followed by a steep decline, if there is no pregnancy, to mark the end of a 28-day cycle (1, 2). The effects of estrogen and P4 on reproductive tissues are dramatic, because they can regulate cell, tissue, and organ function.
The endocervix is the tissue that lines the cervical canal, connecting the uterine cavity to the vagina (Figure 1). The endocervix is lined with a layer of columnar epithelium, which produces and secretes mucus, forming a protective barrier to the outer environment. Depending on the hormonal influence, cervical mucus is permissive or refractory to sperm transport, because the composition, viscosity, and pH of the mucus varies during the menstrual cycle (3–5). Around the time of ovulation, cervical mucus is thin, alkaline, and permissive to sperm, whereas after ovulation, the mucus becomes thick, more acidic, and acts as a barrier to sperm. Moreover, high P4 levels have been linked to increased susceptibility to HIV infection due to the composition of mucins in the endocervix, decrease in secretion of chemokines and cytokines and endogenously produced antimicrobials (6–9).
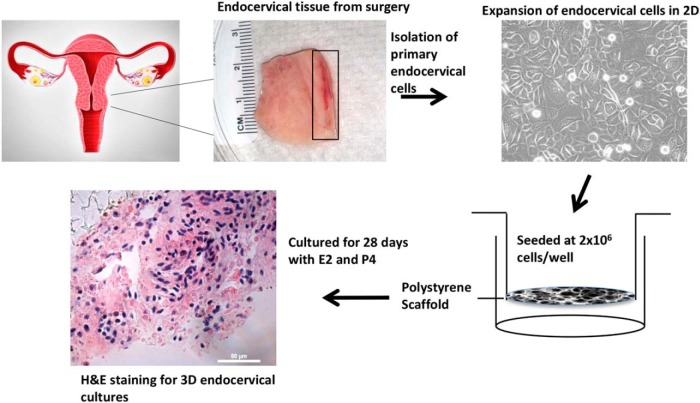
Figure 1. Establishment of 3D cultures of the human endocervix.
The endocervix tissue was obtained postsurgery, and tissue was enzymatically digested. Cells were cultured on 2D culture plates and expanded. Cells were trypsinized and seeded at 2 × 106 cells/well onto the polystyrene scaffold membrane. Cells were cultured for 28 days in the presence of steroid hormones. The 3D units were fixed and processed for H&E staining.
In contrast to other reproductive tissues, the endocervix has not been studied in extensive detail. In vitro models of the endocervix in the form of tissue explant cultures, or 2-dimensional (2D) cell cultures, have been used to study the influence of pathogens, immunity, and hormones (10). Moreover, these cultures are usually employed for a relatively short-time period (hours–days) due to degradation of tissue or cellular integrity after long-term cultures. Hormone treatments usually last for 24 or 48 hours, and single doses are tested, which provide only a snapshot of the effects of the hormones.
In this study, we demonstrate the establishment of a novel and robust 3-dimensional (3D) culture system of the human endocervix cultured in the presence of fluctuating levels of estrogen and P4 in order to mimic a 28-day menstrual cycle. We demonstrate that these 3D units, in response to hormones, proliferate, express estrogen receptor (ER) and P4 receptor (PR), produce mucus, and secrete cytokines and growth factors. Novel secretory factors specifically driven by P4 have been identified. This new model will allow us to study the protective and permissive functions of the endocervix as it relates to infection and fertility in a more physiological way.
Materials and Methods
Tissue collection
Endocervical tissue samples were collected from women undergoing routine hysterectomies at Northwestern University Prentice Women's Hospital, according to an Institutional Review Board-approved protocol. Written consent was obtained from all women included in the study.
Isolation and expansion of primary human endocervical cells
The mucosal epithelium and the underlying stroma of the endocervix were separated from the muscular tissue, minced under sterile conditions into 1- to 2-mm fragments, and subjected to enzymatic digestion with 1.51-mg/mL collagenase I (Invitrogen) and 5-mg/mL deoxyribonuclease I (DNase I) (Sigma) in 20-mL Hanks' balanced salt solution (Invitrogen). After digestion for 1–2 hours at 37°C shaker (100 rpm), cells were washed, resuspended in keratinocyte-serum-free medium (KGM-Gold, Keratinocyte Growth Medium Bullet kit; Lonza), plated on plastic culture dishes, and cultured at 37°C in a humidified atmosphere containing 5% CO2. Medium was changed every 2–3 days.
3D cell cultures and hormonal treatment
A commercially available, highly porous polystyrene scaffold, Alvetex (Reinnervate Ltd) was used for 3D cultures. The membranes are 200 μm thick with pore sizes of 36–40 μm, and the 12-well transwell format was used. Scaffolds were incubated with sterile filtered ethanol (70%) for 5 minutes as a pretreatment, washed twice with medium and left in medium. Primary endocervical cells were trypsinized from 2D cell cultures, and each scaffold transwell was seeded with 2 × 106 cells in a total volume of 100-μL medium (Figure 1). The cells were allowed to attach for 1 hour, and medium was gently added to the lower chamber for complete coverage with 3.5-mL medium. Medium was changed every 48 hours.
To represent a 28-day human menstrual cycle in vitro, a stepwise hormone regimen was added to the cells. For the first 7 days, cells were incubated with 0.1nM E2, followed by 1nM E2 for the next 7 days (Figure 2). Then, cells were treated for 1nM E2 and 10nM P4 for an additional 7 days, followed by 0.1nM E2 and 50nM P4 for 5 days. Then, media without hormones were added for the final 2 days for a total culture period of 28 days. Medium was collected every 48 hours and stored at −20°C. For PR antagonist studies, cells were treated with 100nM RU486 (Sigma), which was added on day 21. To visualize the cells on scaffold, cells were infected with the adenovirus containing pAD-eGFP-RLC (Cell Imaging Core, Northwestern University) (11) by adding 2 μL of amplified virus to 3.5 mL of medium per transwell for 24 hours.
Figure 2. Cell viability after a step hormone treatment for 28 days.
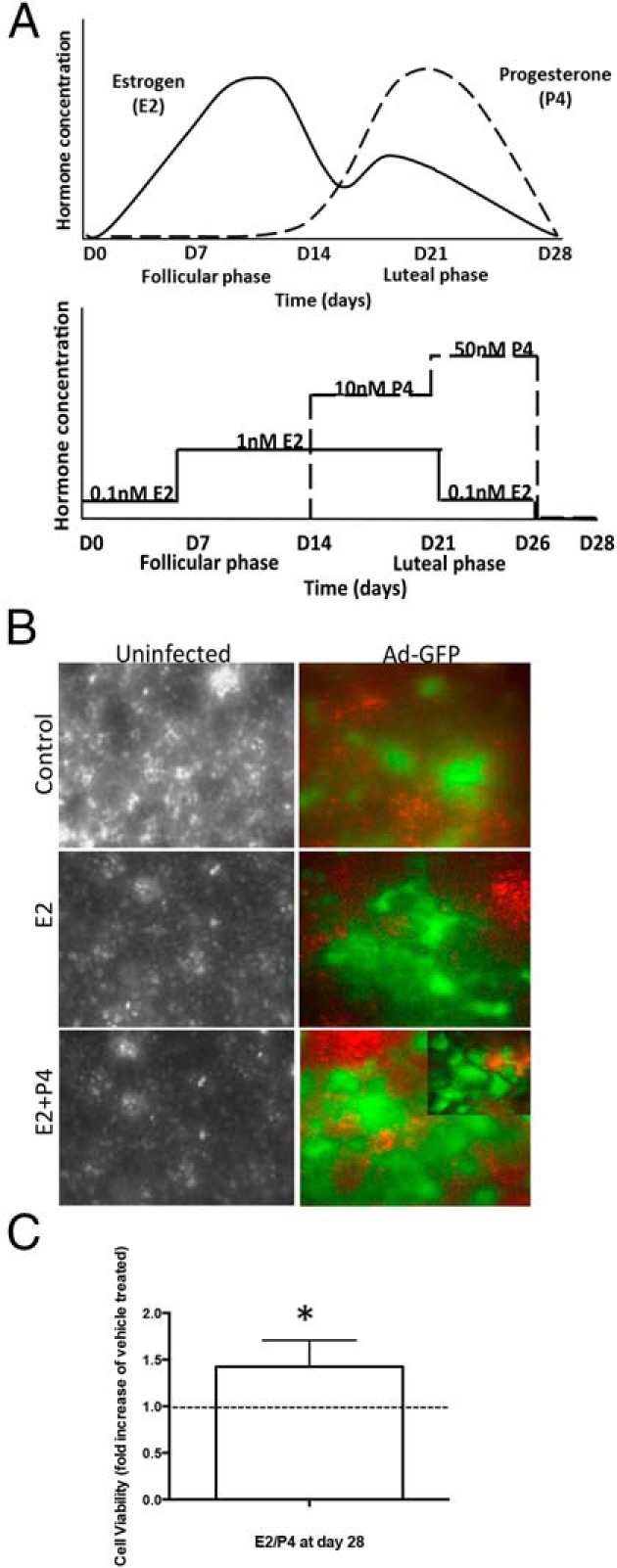
A, Changes in levels of E2 and progesterone during the menstrual cycle are depicted (1). The 28-day step hormone treatment with E2 and P4 is shown; 0.1nM E2 for 7 days, 1nM E2 for 7 days, 1nM E2 + 10nM P4 for 7 days, 0.1nM E2 + 50nM P4 for 5 days, and medium only (no hormones) for 2 days. B, Endocervical cells were infected with 2 μL of pAD-eGFP-RLC (Ad-GFP) at day 0 of hormonal treatment, and medium was changed the next day. The viable cells are shown in green and background is shown in red. The cultured cells were observed by brightfield microscopy. C, Cell viability was measured by Alamar blue staining in cultures at day 28. Data represent the fold increase from vehicle-treated control (n = 4 patient samples) (*, P < .05).
Cell viability
The Alamar Blue Cell Viability Assay (Invitrogen) that measures metabolic activity was used to measure cell viability. Briefly, 350 μL of Alamar Blue reagent were added directly to the cells at the end of 28-day hormonal treatment. Cells were incubated at 37°C, 5% CO2 for 4 hours in the dark, and the fluorescence was measured at 570/585 nm.
Immunohistochemistry
Scaffolds were washed twice in phosphate-buffered saline, covered with histogel (Fisher Scientific), and fixed in 4% paraformaldehyde for 5 hours. Processing and hematoxylin and eosin (H&E) staining was performed at the Northwestern University Center for Reproductive Sciences Histology Core. Fixed cells were processed using an automated tissue processor (Leica) and embedded in paraffin. Serial sections were cut 10 μm thick, mounted on glass slides, and stained with H&E using a Leica Autostainer XL (Leica Microsystems). Antigen retrieval was performed by treatment for 10 minutes in buffer containing 0.05% Trypsin and 1% CaCl2 at pH 7.6. After blocking, sections were incubated with primary antibodies to PR (1:200; Dako), ER (1:100; Abgent), vimentin (1:200; Abcam), pan cytokeratin (1:500; Abcam), Ki67 (1:100; Abcam), and MUC16 (1:100; Abcam) overnight at 4°C. Slides were rinsed, and horseradish peroxidase (HRP)-conjugated secondary antibodies were applied for 30 minutes. HRP activity was detected using 3,3-diaminobenzidine (DAB) in 1:50 dilution (Dako) as substrate for 30–60 seconds and then counterstained with hematoxylin. Sections incubated with dilution buffer without primary antibody were used as the negative control. For immunofluorescent staining, antigen retrieval was performed by treatment for 10 minutes in buffer containing 0.05% Trypsin and 1% CaCl at pH 7.6. Sections were washed 2× in Tris-Buffered Saline and Tween 20 (TBST), blocked with protein block for 30 minutes (Dako), and incubated with primary antibodies described above. Slides were rinsed in Tris-Buffered Saline and Tween 20 and incubated with fluorescent secondary antibodies (1:250; Invitrogen) for 1 hour. Slides were visualized and images captured with Leica DM5000B Microscope.
Staining for neutral and acidic mucins
Sections stained with periodic acid Schiff (PAS) (Abcam) were previously deparaffinized and oxidized in 1% periodic acid for 10 minutes, followed by several rinses in distilled water. Staining was carried out in Schiff's reagent at 4°C for 30 minutes followed by rinsing in distilled water. Sections were then counterstained with hematoxylin for 2 minutes, washed, dehydrated, and mounted. In addition, deparaffinized and hydrated sections were treated with acetic acid solution for 3 minutes, stained with Alcian blue (Abcam) for 30 minutes, followed by a wash in running tap water for 10 minutes. After counterstaining with Safranin O solution for 5 minutes, sections were washed, dehydrated, and mounted.
Cytokine/chemokine quantitation
ProcartaPlex Human Cytokine/Chemokine/Growth Factor Panel 1 (45-plex) (Affymetrix eBioscience) was used to measure levels of secreted cytokines and chemokines. For quantification, duplicate standards produced a curve for each analyte, from which concentrations of samples were extrapolated. Medium alone was used to establish background levels of cytokines and chemokines. The levels of leukemia inhibitory factor (LIF), IL-1β (Abcam), and MUC16 (R&D Systems) were measured using single ELISAs and performed according to manufacturer's protocol.
Statistical analysis
Statistical analysis was performed using Prism software (v5; GraphPad). P ≤ 0.05 was considered significant. The Alamar Blue cell viability assay data were analyzed using 1 sample t test (n = 4). Luminex data (pg/mL) were first transformed to fold changes of hormone treated compared with vehicle-treated control. Only secreted factors that showed consistent detectable ranges of above 25 pg/mL in at least 3 out of 5 patient samples were statistically analyzed. Fold changes at day 14 were compared with day 26 or day 28, and fold changes at day 26 were compared with day 28 using an unpaired t test with Welch's correction.
Results
Establishment of an ex vivo 3D endocervix
Human endocervix tissues obtained postsurgery were enzymatically digested and expanded in 2D cultures to obtain a sufficient number of cells (Figure 1). Two million cells were seeded on the highly porous polystyrene scaffolds, Alvetex membranes (Figure 1) (12–14), and treated with E2 and P4 in a stepwise fashion to mimic a 28-day menstrual cycle in vitro (Figure 2A). At 14-day (E2 only) and 28-day (E2+P4) time points, viable endocervical cells were present, as visualized with GFP florescence (Figure 2B). The cell viability assay demonstrated viable cells at 28 days. This assay also showed a subtle but significant increase in cell viability with E2+P4 treatment at day 28 compared with vehicle-treated control (P < .05) (Figure 2C). These data demonstrate that endocervical cells cultured on the 3D scaffolds survive and proliferate in response to a 28-day culture with hormones.
Histological analysis of 3D cultures
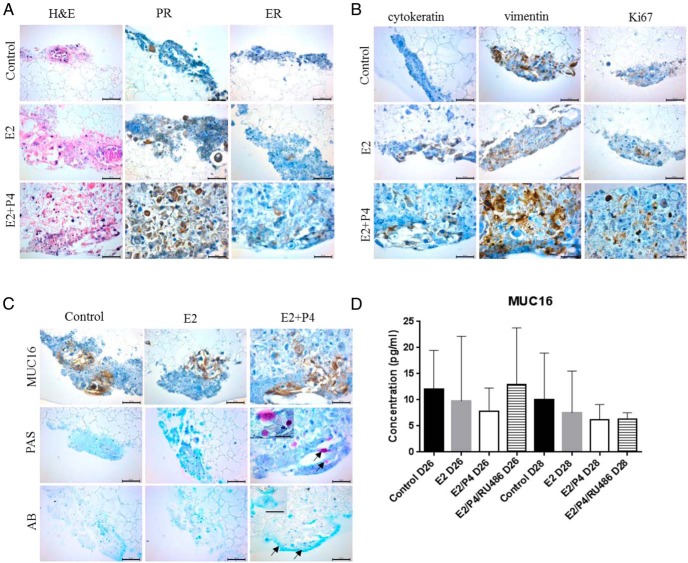
H&E staining of the 3D cultures revealed the presence of a mixed population of cells (Figure 3). Pan cytokeratin and vimentin staining showed both epithelial and stromal cells, respectively, present in the endocervical cultures at day 14 (E2) and day 28 (E2+P4). In addition, cells expressed both PR and ER, which by visual assessment, increased in the presence of E2 and P4 as compared with control. Furthermore, the Ki67 staining was evident in the cultures with increased staining in the presence of E2, or E2 and P4 as compared with control (Figure 3), indicative of active proliferation of cells, even after 28 days of culture. An increase in both cell size and number were observed in E2+P4 treatment group (Figure 3, Supplemental Figure 1).
Figure 3. Expression of endocervical cell markers.
Cells in the polystyrene scaffold were fixed, processed and (A) stained with H&E at day 14 (E2 only) and day 28 (control and E2+P4). Immunohistochemical staining for estrogen receptor (ER) and progesterone receptor (PR) (A) and pan cytokeratin, vimentin, and Ki67 (B) was done. C, Mucin 16 (MUC16) levels were detected by IHC staining, neutral mucins by Periodic acid-Schiff (PAS) staining and acidic mucins by Alcian blue (AB) at day 14 (E2) and day 28 (control and E2+P4) in 3D endocervical cells. The PAS stain revealed the presence of goblet cells (arrow). AB staining detected acidic mucins (arrow). Figures are representative of at least 3 independent experiments. Scale bars represent 25 μm (inset) and 50 μm. D, Cells were treated with 100nM RU486. Levels of MUC16 released into the culture medium were measured by ELISA. Data are mean ± SD from 3 patient samples.
Expression and secretion of endocervical mucus
One of the major functional properties of the endocervix is the production and secretion of mucus. Immunohistochemical staining showed production of the mucin, Mucin 16 (MUC16) (Figure 3C). Estrogen and P4 did not appear to affect levels of MUC16 compared with control cultures. In addition, levels of MUC16 secreted into the culture medium did not significantly change at day 26 and day 28 of the hormone treatment regimen compared with untreated cultures (Figure 3D). Moreover, the PR antagonist, RU486, in the presence of E2 and P4 did not significantly alter levels of secreted MUC16 (Figure 3D), indicating that hormones were not modulating the production and secretion of MUC16 in the 3D endocervix cultures. In contrast, the neutral mucins, as measured by PAS staining, increased with E2 and P4 (d 28) treatment compared with control or E2 (d 14) treatments (Figure 3C). This stain also detected the presence of goblet cells at the end of the 28-day hormonal treatment (Figure 3C). The acidic mucins were detected using the Alcian blue stain. In contrast to the PAS stain, no difference in the levels of acidic mucins with the hormonal treatments was observed (Figure 3C).
Cytokine/chemokine profile of 3D cultures
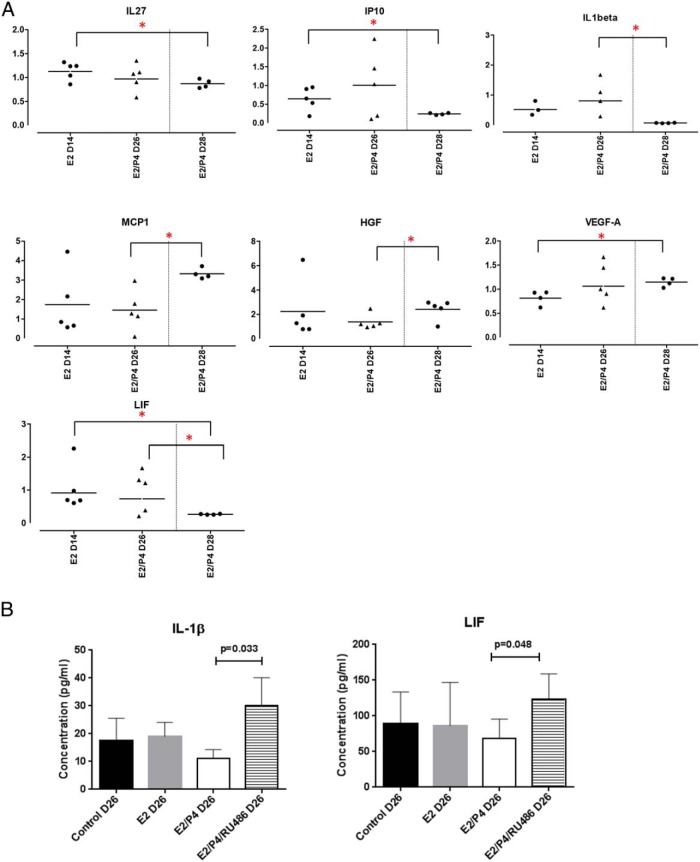
The cytokine/chemokines secreted by the 3D endocervical cultures were measured using a 45-Plex Luminex assay. Levels of factors secreted into the culture medium at day 14, 26, and 28 were measured during the step hormone treatment. Out of 45 factors, 36 were detectable by this assay (Supplemental Figure 2). There was a wide range in concentrations of factors secreted by the 3D cultures; 17 factors measured less than 25 pg/mL consistently within the replicates (Supplemental Figure 2). Due to the variation among patient samples, data were expressed as fold changes of hormone treated with its respective control (vehicle treated) (Figure 4A). Fold changes were then compared between the 3 time points, day 14, 26, and 28. Seven factors, IL-27, IP10, IL-1β, MCP1, HGF, VEGFA, and LIF, showed fold changes that were statistically different between day 14 and day 28 (IL-27, IP10, VEGFA, and LIF) or between day 26 and day 28 (IL-1β, MCP1, HGF, and LIF). Hormonal regulation of the 2 factors, IL-1β and LIF were further investigated using single ELISA assays, different patient samples and treatment with the PR antagonist, RU486 (mifepristone). In these studies, E2 and P4 treatment showed a trend to decrease the secretion of these factors at day 26 (Figure 4B), which did not reach statistical significance. Treatment of cultures with RU486 starting on day 21 increased IL-1β and LIF levels compared with E2/P4, which was statistically significant (Figure 4B), indicating that regulation of IL-1β and LIF occurs through the PR.
Figure 4. Hormonal regulation of IL-1β and LIF.
Endocervical cells in 3D were treated with the step hormone treatment. A, A 45-plex Luminex assay was performed, using medium collected at D14, D26, and D28. Data are presented as fold changes from vehicle controls (n ≥ 4 patient samples). *, P < .05. B, IL-1β and LIF levels were measured by ELISA. RU486 was added to the cells at D21. Data are mean ± SD from 3 patient samples. Statistical comparisons between treatments control vs E2, E2 vs E2/P4, and E2/P4 vs E2/P4/RU486 were performed. P values are indicated.
Discussion
To our knowledge this is the first study that examines the effects of a long-term, stepwise hormonal treatment on 3D endocervical cultures. Over the course of 28 days, cells retained tissue-specific functional properties, such as mucus production and cytokine secretion. This system is a useful tool to study the endocervix under conditions that mimic the in vivo tissues during a menstrual cycle. Treating cells for 28 days with fluctuating levels of E2 and P4 will result in cellular and molecular changes that drastically differ from treating cells for 24 hours with a single dose of E2 and P4. This study showed that endocervical cells grew in size and number in response to E2 and P4 for 28 days. Whether these hormones increase migration and/or scaffold penetration remains unclear. Nevertheless, given the relatively little information regarding the influence of estrogen and P4 on the endocervix and the interest in the role that hormones play on pathogen susceptibility as well as sperm transport within the female reproductive tract, this model system would be a significant improvement to the conventional in vitro systems used thus far.
Mucus is composed of water, electrolytes, and large structural proteins called mucins. Three gel-forming (MUC5B, MUC5AC, and MUC6) and 2 transmembrane (MUC16 and MUC1) mucins are highly expressed in cervical mucus (15). There is limited information regarding hormonal regulation of mucins in endocervical mucus. Our studies do not support the regulation of MUC16 protein levels with hormones in primary endocervical cells. A more in depth investigation is needed, however, to clarify the role of steroid hormones on MUC16 and other mucins in the endocervix, because posttranslational modifications of the protein, including glycosylation, as well as mucin assembly, may be modified by female hormones. In cervical mucus, the major alterations observed during the menstrual cycle were in the mucin O-glycosylation with more than 50 different neutral, sialylated, and sulfated oligosaccharides (15). The relative abundance of neutral oligosaccharides increased before and after ovulation, whereas acidic oligosaccharides were increased only during ovulation in cervical mucus (15). We observed neutral mucins were highly expressed in the presence of estrogen and P4 (luteal phase), supporting these studies.
Studies show that the immune system is suppressed during the luteal phase of the menstrual cycle, increasing susceptibility to infections (16–18). Recently, Goode et al demonstrated that depot medroxy P4 acetate (DMPA) treatment of rhesus macaques resulted in increased receptors and markers of cells susceptible to HIV and altered the inflammatory factors in the endocervix (6). In our study, numerous cytokines and chemokines were modulated. Specifically, levels of IL-1β and LIF decreased with E2 and P4 compared with untreated cells and the effects of RU486 implicated PR to primarily be responsible for the decrease. Studies have shown the modulation of IL-1β and LIF with hormones in the endometrium (19–22), with limited investigation in the endocervix. IL-1β concentrations were highest preceding the midcycle E2 peak in cervical mucus (8, 23). The levels of IL-1β were increased in vaginal washings obtained in the follicular phase as compare with luteal phase of menstrual cycle (24). In rhesus macaques, vaginal swabs revealed an increase of IL-1β with DMPA compared with E2 treatment (6). The different responses of this study compared with ours are most likely due to differences in experimental design and species. The rhesus macaques were injected with either E2 or DMPA alone, lacking the sequential treatments of E2 priming before DMPA. Also, the cytokines and chemokines were measured in vaginal swabs, which include endocervix, ectocervix, and vaginal secreted factors. With regards to LIF, there is little known of its regulation by steroid hormones in the endocervix, because much of the investigation has been done in the endometrium. LIF has been studied in the context of embryo implantation/development (25) and possess both antiinflammatory and proinflammatory properties (26, 27). Studies carried out in rhesus macaques and humans demonstrated LIF produced in the endometrium is regulated by P4 (28–30). Thus, it is apparent that more research is needed with regards to the endocervix and how hormones impact its permissive and barrier functions.
The 3D endocervix model we developed provides a tool to study the impact of drugs, toxicants, vaccines, and pathogens on the endocervix. Furthermore, the effect of hormones on drug action would be important to decipher. The advancement of medical technology as it pertains to the female reproductive tract greatly depends on tools and models that accurately represent the tissues in women, given the ethical issues associated with research of reproductive tissues. The ex vivo endocervix model presented in this study is an advancement to current tools available.
Acknowledgments
We thank Dr Elizabeth C. Sefton for outstanding administrative support; Stacy Druschitz and Saurabh S. Malpani for their efforts consenting patients, collecting tissue, and ensuring that our study remains within regulatory compliance; and Megan Romero and Keisha Barreto from the Ovarian Histology Core at Northwestern University for their technical expertise.
This work was supported by National Institutes of Health Grants NIEHS/OWRH/UH2ES022920 and NCATS/NIEHS/NICHD/OWHR/UH3TR001207.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- 2D
- 2 dimensional
- 3D
- 3 dimensional
- DMPA
- depot medroxy P4 acetate
- E2
- estradiol
- ER
- estrogen receptor
- GFP
- Green Fluorescence Protein
- H&E
- hematoxylin and eosin
- HGF
- hepatocyte growth factor
- IP10
- interferon gamma induced protein 10
- Ki67
- the proliferation marker
- LIF
- leukemia inhibitory factor
- MCP-1
- Monocyte chemotactic protein-1
- MUC16
- Mucin 16
- P4
- progesterone
- PAS
- periodic acid Schiff
- PR
- P4 receptor
- RU486
- mifepristone
- VEGFA
- Vascular endothelial growth factor A.
References
- 1. Klein NA, Houmard BS, Hansen KR, et al. Age-related analysis of inhibin A, inhibin B, and activin a relative to the intercycle monotropic follicle-stimulating hormone rise in normal ovulatory women. J Clin Endocrinol Metab. 2004;89:2977–2981. [DOI] [PubMed] [Google Scholar]
- 2. Hoff JD, Quigley ME, Yen SS. Hormonal dynamics at midcycle: a reevaluation. J Clin Endocrinol Metab. 1983;57:792–796. [DOI] [PubMed] [Google Scholar]
- 3. Gipson IK. Mucins of the human endocervix. Front Biosci. 2001;6:D1245–D1255. [DOI] [PubMed] [Google Scholar]
- 4. Gipson IK, Spurr-Michaud SJ, Senchyna M, Ritter R, 3rd, Schaumberg D. Comparison of mucin levels at the ocular surface of postmenopausal women with and without a history of dry eye. Cornea. 2011;30:1346–1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Argüeso P, Spurr-Michaud S, Tisdale A, Gipson IK. Variation in the amount of T antigen and N-acetyllactosamine oligosaccharides in human cervical mucus secretions with the menstrual cycle. J Clin Endocrinol Metab. 2002;87:5641–5648. [DOI] [PubMed] [Google Scholar]
- 6. Goode D, Aravantinou M, Jarl S, et al. Sex hormones selectively impact the endocervical mucosal microenvironment: implications for HIV transmission. PLoS One. 2014;9:e97767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Heffron R, Donnell D, Rees H, et al. , Partners in Prevention HSVHIVTST. Use of hormonal contraceptives and risk of HIV-1 transmission: a prospective cohort study. Lancet Infect Dis. 2012;12:19–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kutteh WH, Moldoveanu Z, Mestecky J. Mucosal immunity in the female reproductive tract: correlation of immunoglobulins, cytokines, and reproductive hormones in human cervical mucus around the time of ovulation. AIDS Res Hum Retroviruses. 1998;14(suppl 1):S51–S55. [PubMed] [Google Scholar]
- 9. Keller MJ, Guzman E, Hazrati E, et al. PRO 2000 elicits a decline in genital tract immune mediators without compromising intrinsic antimicrobial activity. Aids. 2007;21:467–476. [DOI] [PubMed] [Google Scholar]
- 10. Herbst-Kralovetz MM, Quayle AJ, Ficarra M, et al. Quantification and comparison of toll-like receptor expression and responsiveness in primary and immortalized human female lower genital tract epithelia. Am J Reprod Immunol. 2008;59:212–224. [DOI] [PubMed] [Google Scholar]
- 11. Khuon S, Liang L, Dettman RW, Sporn PH, Wysolmerski RB, Chew TL. Myosin light chain kinase mediates transcellular intravasation of breast cancer cells through the underlying endothelial cells: a three-dimensional FRET study. J Cell Sci. 2010;123:431–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Knight E, Murray B, Carnachan R, Przyborski S. Alvetex®: polystyrene scaffold technology for routine three dimensional cell culture. Methods Mol Biol. 2011;695:323–340. [DOI] [PubMed] [Google Scholar]
- 13. Bokhari M, Carnachan RJ, Cameron NR, Przyborski SA. Culture of HepG2 liver cells on three dimensional polystyrene scaffolds enhances cell structure and function during toxicological challenge. J Anat. 2007;211:567–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Stevanato L, Sinden JD. The effects of microRNAs on human neural stem cell differentiation in two- and three-dimensional cultures. Stem Cell Res Ther. 2014;5:49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Andersch-Björkman Y, Thomsson KA, Holmén Larsson JM, Ekerhovd E, Hansson GC. Large scale identification of proteins, mucins, and their O-glycosylation in the endocervical mucus during the menstrual cycle. Mol Cell Proteomics. 2007;6:708–716. [DOI] [PubMed] [Google Scholar]
- 16. Wira CR, Fahey JV. A new strategy to understand how HIV infects women: identification of a window of vulnerability during the menstrual cycle. Aids. 2008;22:1909–1917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hughes GC, Thomas S, Li C, Kaja MK, Clark EA. Cutting edge: progesterone regulates IFN-α production by plasmacytoid dendritic cells. J Immunol. 2008;180:2029–2033. [DOI] [PubMed] [Google Scholar]
- 18. Miyaura H, Iwata M. Direct and indirect inhibition of Th1 development by progesterone and glucocorticoids. J Immunol. 2002;168:1087–1094. [DOI] [PubMed] [Google Scholar]
- 19. von Wolff M, Thaler CJ, Strowitzki T, Broome J, Stolz W, Tabibzadeh S. Regulated expression of cytokines in human endometrium throughout the menstrual cycle: dysregulation in habitual abortion. Mol Hum Reprod. 2000;6:627–634. [DOI] [PubMed] [Google Scholar]
- 20. Schaefer TM, Wright JA, Pioli PA, Wira CR. IL-1β-mediated proinflammatory responses are inhibited by estradiol via down-regulation of IL-1 receptor type I in uterine epithelial cells. J Immunol. 2005;175:6509–6516. [DOI] [PubMed] [Google Scholar]
- 21. Shuya LL, Menkhorst EM, Yap J, Li P, Lane N, Dimitriadis E. Leukemia inhibitory factor enhances endometrial stromal cell decidualization in humans and mice. PLoS One. 2011;6:e25288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Chen DB, Hilsenrath R, Yang ZM, et al. Leukaemia inhibitory factor in human endometrium during the menstrual cycle: cellular origin and action on production of glandular epithelial cell prostaglandin in vitro. Hum Reprod. 1995;10:911–918. [DOI] [PubMed] [Google Scholar]
- 23. Kanai T, Fukuda-Miki M, Shimoya K, et al. Increased interleukin-1 and interleukin-1 receptor antagonist levels in cervical mucus in the ovulatory phase in comparison with the follicular phase. Gynecol Obstet Invest. 1997;43:166–170. [DOI] [PubMed] [Google Scholar]
- 24. Al-Harthi L, Wright DJ, Anderson D, et al. The impact of the ovulatory cycle on cytokine production: evaluation of systemic, cervicovaginal, and salivary compartments. J Interferon Cytokine Res. 2000;20:719–724. [DOI] [PubMed] [Google Scholar]
- 25. Piccinni MP. T-cell cytokines in pregnancy. Am J Reprod Immunol. 2002;47:289–294. [DOI] [PubMed] [Google Scholar]
- 26. Ulich TR, Fann MJ, Patterson PH, et al. Intratracheal injection of LPS and cytokines. V. LPS induces expression of LIF and LIF inhibits acute inflammation. Am J Physiol. 1994;267:L442–L446. [DOI] [PubMed] [Google Scholar]
- 27. Waring PM, Carroll GJ, Kandiah DA, Buirski G, Metcalf D. Increased levels of leukemia inhibitory factor in synovial fluid from patients with rheumatoid arthritis and other inflammatory arthritides. Arthritis Rheum. 1993;36:911–915. [DOI] [PubMed] [Google Scholar]
- 28. Ace CI, Okulicz WC. Differential gene regulation by estrogen and progesterone in the primate endometrium. Mol Cell Endocrinol. 1995;115:95–103. [DOI] [PubMed] [Google Scholar]
- 29. Cullinan EB, Abbondanzo SJ, Anderson PS, Pollard JW, Lessey BA, Stewart CL. Leukemia inhibitory factor (LIF) and LIF receptor expression in human endometrium suggests a potential autocrine/paracrine function in regulating embryo implantation. Proc Natl Acad Sci USA. 1996;93:3115–3120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Okulicz WC, Ace CI, Longcope C, Tast J. Analysis of differential gene regulation in adequate versus inadequate secretory-phase endometrial complementary deoxyribonucleic acid populations from the rhesus monkey. Endocrinology. 1996;137:4844–4850. [DOI] [PubMed] [Google Scholar]