Abstract
LH signaling is required for oocyte maturation in fish and other vertebrates. However, the downstream factors mediating LH signaling are largely unexplored in fish. In this study, we investigated whether IGFs could mediate LH action on oocyte maturation in zebrafish. Our results show that all igfs, including igf1, igf2a, igf2b, and igf3, are dynamically expressed during folliculogenesis, with the expression of igf3 reaching its maximal level in full grown stage follicles. The expression of igfs is regulated by LH through a cAMP pathway in intact follicles as well as in primary cultured follicular cells, with igf3 expression being the most sensitive to human chorionic gonadotropin (hCG) treatment. Moreover, recombinant zebrafish IGF-2a, IGF-2b, and IGF-3 proteins significantly enhanced oocyte maturation via IGF-1 receptors (IGF-1rs), with IGF-3 exhibiting the most potent stimulatory action on oocyte maturation. Furthermore, we have demonstrated that IGF-3 or hCG treatment could stimulate IGF-1rs phosphorylation, and hCG-induced oocyte maturation could be attenuated by IGF-1r inhibitors as well as by an anti-IGF-3 antiserum in vitro and in vivo, indicating that the IGF system especially IGF-3 plays a crucial role in mediating LH action on oocyte maturation. In addition, igf3 expression is significantly attenuated in LH β-subunit (lhb) mutant zebrafish and treatment with recombinant IGF-3 could partially rescue the oocyte maturation defects of the lhb mutants in vitro and in vivo. Collectively, our results clearly demonstrated that IGFs, particularly the gonad-specific IGF-3, act as important mediators of LH action on oocyte maturation in zebrafish.
Female fertility requires precise regulation of oocyte meiosis. Fish oocytes, as in other vertebrates, undergo meiosis but are subsequently arrested at the first meiotic prophase stage until gonadotropin (LH) released from the pituitary arrives to resume the meiotic cell cycle. In response to this LH surge, the oocytes proceed from prophase to second metaphase. This prophase-to-metaphase transition is characterized by germinal vesicle breakdown (GVBD), which is indicative of oocyte maturation. Much is still unknown about the signaling events in the ovary that regulate this process (1).
It has been demonstrated that LH acts via interaction with its cognate LH receptor, a member of the large superfamily of G protein-coupled receptors (2, 3). However, LH receptor is mainly expressed in the granulosa cells but not in the oocytes. Therefore, LH action on oocyte is indirect and mediators are needed to transduce the signaling events from the granulosa cells into the oocyte (1, 4, 5). In fact, LH was shown to be capable of inducing oocyte maturation in intact follicles but not in defolliculated (denuded) oocytes lacking granulosa cells. In fish and amphibians, it is widely accepted that maturation-inducing hormones (MIHs) synthesized in the follicular layer (containing granulosa cells and theca cells) initiate the LH-induced maturation events. Progesterone and androgens are considered effective MIHs in amphibians. In most fish, 17α,20β-dihydroxy-4-pregnen-3-one (DHP) is considered the MIH (1). MIH can bind to its receptor to activate various signal transduction pathways. This signaling event ultimately induces the activation of maturation-promoting factor (MPF), which catalyzes the entry of the oocyte into M phase of meiosis I and meiosis II (6).
In recent years, increasing evidence suggested that local paracrine factors are also involved in the regulation of meiotic reinitiation in oocytes (7). For example, the epidermal growth factor (EGF)-like family has recently been shown to be essential mediators of LH action on oocyte maturation in mammals (8, 9). In fish, increasing evidence supported the involvement of the activin family in mediating LH-induced oocyte maturation (10–12). However, as a major group of growth factors, the potential roles of the IGF family in mediating LH action in oocyte maturation have been largely unexplored. Furthermore, a newly discovered gonad-specific IGF, namely IGF-3, in teleosts was reported by our group (13). Interestingly, the expression of igf3 could be up-regulated by human chorionic gonadotropin (hCG) treatment and IGF-3 could induce oocyte maturation in vitro (14). Although these results suggest the involvement of IGF-3 in inducing oocyte maturation, the in vivo actions of IGF-3 on oocyte maturation have not been investigated. Moreover, whether this novel IGF subtype could mediate LH action on oocyte maturation remains unknown. In addition, the roles of other IGF subtypes in oocyte maturation have not been looked at. In this study, we have systematically investigated the expression, regulation, and action of the zebrafish IGFs in the ovary and their potential roles in mediating LH action on oocyte maturation. Here, we provide the first in vitro and in vivo evidence that IGFs, particularly IGF-3, are important mediators of the LH action on oocyte maturation in zebrafish.
Materials and Methods
Animals
For gene knockout studies, AB strain zebrafish (Danio rerio) were used. For other experiments, zebrafish were purchased locally. The lhb mutant line was obtained using our modified transcription activator-like effector nucleases system (Supplemental Figure 1) (15). All fish were maintained under 14-hour light, 10-hour dark cycles, in circulating freshwater aquaria at 26°C–28°C. Fish were fed twice daily with newly hatched brine shrimp (Brine Shrimp Direct). Fish experiments were conducted in accordance to the regulations of the Animal Experimentation Ethics Committee of The Chinese University of Hong Kong.
Chemicals
Analytical Reagent grade chemicals and hCG were obtained from Sigma-Aldrich, culture media from Gibco, and enzymes from Promega. The IGF-1r inhibitor (NVP-ADW742) was obtained for Selleck Chemicals, and another IGF-1r inhibitor (NVP-AEW541) was the gift from Novartis Co.
RNA isolation and RT-PCR
Total RNA samples were isolated from ovarian follicles of zebrafish using TRIzol Reagent (Invitrogen). The amount and purity of the RNA were determined on a NanoDrop 2000C Spectrophotometer (Thermo). For real-time PCR, the internal standards for target genes, namely elongation factor-1α and glyceraldehyde-3-phosphate dehydrogenase (gapdh), were prepared by amplification of cDNA fragments with the gene specific primers (Table 1). Real-time PCR was carried out as described in Ref. 14. For semiquantitative RT-PCR analysis, PCR was carried out on a Thermal Cycler 9600 (Eppendorf).
Table 1.
Primers Used in This Study
| Gene | Sequence(5′ to 3′ direction) | Strand | Application |
|---|---|---|---|
| igf1 | AGGTCACACAACCGTGGCATC | S | Real-time PCR |
| TAGTTTCTGCCCCCTGTGTTTCC | AS | ||
| igf2a | GGTCTTCCCAGTGTCACAGGCTC | S | Real-time PCR |
| TGCTCCTCATCTTGGATTTTCTC | AS | ||
| igf2b | CATCATTCTGTTTGCCATACCTG | S | Real-time PCR |
| ACACAAACTGTAGAGCGTCCACC | AS | ||
| igf3 | GCCAAACGCCTTCAGATAATGC | S | Real-time PCR |
| GCTGCTCCAGGTTTGCCTATGT | AS | ||
| ef1a | GGCTGACTGTGCTGTGCTGATTG | S | Real-time PCR |
| CTTGTCGGTGGGACGGCTAGG | AS | ||
| 18s | CCTGAGAAACGGCTACCACATCC | S | RT-PCR |
| AGCAACTTTAGTATACGCTATTGGAG | AS | ||
| gapdh | CGACCTCACCTGCCGCCTTACA | S | Real-time PCR |
| GTCATTGAGGGAGATGCCAGCG | AS |
Abbreviation: ef1a, elongation factor-1α.
Expression and purification of zebrafish recombinant IGF-2a, IGF-2b, and IGF-3
The expression and purification of recombinant zebrafish IGF-2a, IGF-2b, and IGF-3 was carried out as described previously (14). Briefly, zebrafish igf2a, igf2b, and igf3 cDNAs corresponding to the predicted mature peptides were synthesized (Genscript) and cloned into the pProEX-HTb vector. One liter of recombinant Escherichia coli harboring this plasmid was grown in Lysogeny Broth medium at 37°C until the absorbance value reached 0.5–0.8 at 600 nm. Cells were collected 3 hours after induction by isopropylthio-β-galactoside, and the collected cells were resuspended in 1× PBS. After sonication, the total lysate was divided into the soluble fraction and insoluble fraction by centrifugation. SDS-PAGE was carried out to check the expression of the IGF. The insoluble fraction was kept and dissolved in 1× PBS with 8M urea. Recombinant IGF was affinity purified with nickel-nitrilotriacetic agarose (QIAGEN). The purified protein was dissolved in 1× PBS with 8M urea (pH 4.5) and concentrated in a concentrator (Millipore). The concentrated protein was dialyzed in a tubing of a molecular weight cut-off of 3500 Da (Pierce) as follows: 1× PBS with 4M urea at room temperature for 24 hours; 1× PBS with 2M urea at 4°C for 24 hours; 1× PBS with 1M urea and 0.4M L-arginine at 4°C for 24 hours; and 1× PBS with 0.4M L-arginine at 4°C for 24 hours. The refolded IGF was collected and concentrated. The concentration was determined by the bicinchoninic acid Protein Assay kit (Pierce).
Western blot analysis
Lysates of ovarian follicles were firstly separated by 10% SDS-PAGE gels. The separated proteins were transferred onto polyvinylidene fluoride membranes and immunoblotted with the primary antibodies against total-IGF-1 receptor (IGF-1r), phospho-IGF-1r (Tyr1161), and β-actin (Santa Cruz Biotechnology, Inc) and cyclin B (Cell Signaling). The protein bands were visualized by a Western blotting kit (Millipore) after incubation with the secondary antibody conjugated with horseradish peroxidase.
Isolation of ovarian follicles
The staging system that we have adopted is based on the original definition of Selman et al (16) as modified by Wang and Ge (17) and Pang and Thomas (18). The ovaries were dissected out from 15–20 female zebrafish after anesthetization and decapitation and placed in a 100-mm culture dish containing 60% Leibovitz L-15 medium. Follicles of different stages were manually isolated and grouped into the next stages: primary growth (PG) (stage I; below 0.1 mm in diameter), previtellogenic (PV) (stage II or cortical alveolus stage; ∼0.30 mm in diameter), early vitellogenic (∼0.40 mm in diameter), midvitellogenic (∼0.50 mm in diameter), and full grown but immature (FG) (∼0.65 mm in diameter). The isolation process normally lasted for 4–6 hours at room temperature before incubation and drug treatment at 28°C for different periods of time.
Ovarian follicle incubations
Zebrafish were killed and ovaries excised as described above. Follicles of different stages were separated gently and incubated (30–40 follicles/well) in 24-well culture plates at 28°C. After treatment, follicles that underwent GVBD were identified by their ooplasmic clearing (due to proteolytic cleavage of vitellogenin). Each group had 4 replicate wells, and each experiment was repeated 3 times. For IGF-1r inhibition studies, the separated follicles were pretreated with the inhibitors (10μM) before the addition of other agents.
Primary culture of ovarian follicular cells
Primary culture of zebrafish ovarian follicular cells was performed according to an established protocol (10). Briefly, follicles of around vitellogenic stage from 15 to 25 females were carefully selected and cultured in 25 cm2 flask for 6 days in M199 medium plus 10% fetal bovine serum under the condition of 28°C and 5% CO2. The medium was changed on the third day. After 6 days, the cells were subcultured in 24-well plates at a density of 100 000 cells per well for 24 hours before hormone and drug treatment.
Removal of follicular cells from ovarian follicles
The follicular cells (theca cell and granulosa cell layers) were removed from the ovarian follicles by enzymatic digestion (19, 20). Intact follicles were incubated in 60% L-15 medium containing collagenase (100 μg/mL) for 1 hour at room temperature with mild agitation and repeated (20–30 times) gentle pipetting of the follicles through a narrow pipette (1 mm in diameter) during the incubation. The resulting denuded oocytes were washed 3 times with fresh 60% L-15 medium before transferred to wells for the in vitro oocyte maturation bioassay. Removal of the follicular cells was confirmed by staining the denuded oocytes with propidium iodide (PI) and observation under a fluorescent microscope.
Intraperitoneal injection into adult zebrafish
The procedure of Kinkel et al (21) was followed with minor modifications. Briefly, after fasting and anesthetization, zebrafish were quickly placed on an agar gel plate. Using a microinjection system (WPI), 2-μL hCG (5 IU/μL) with or without 4-μL (10mM) NVP-ADW742, or 2-μL recombinant IGF (0.5 μg/μL), or 2-μL 60% Leibovitz L-15 medium (control) was carefully injected into the midline between the pelvic fins. At least 5 fish were used in each group. After injection, the fish were immediately transferred back to the water tank for recovery. For quantification of oocyte maturation in vivo, the ovaries were carefully dissected out from the fish. All FG stage and mature follicles were manually isolated according to their size and morphology. The extent of GVBD in each fish was calculated as the number of mature follicles/(number of mature follicles + number of FG stage follicles).
Statistical analysis
All data were expressed as mean values ± SEM. P < .05 was considered statistically significant using one-way ANOVA, followed by Fisher's least significant difference test using the GraphPad Instat software (GraphPad Software). Statistical comparisons of the expression levels between wild-type and mutant fish was conducted using an unpaired 2-tailed Student's t test. All experiments were performed 2 or 3 times to confirm the results.
Results
Differential gene expression of the 4 igfs during zebrafish folliculogenesis
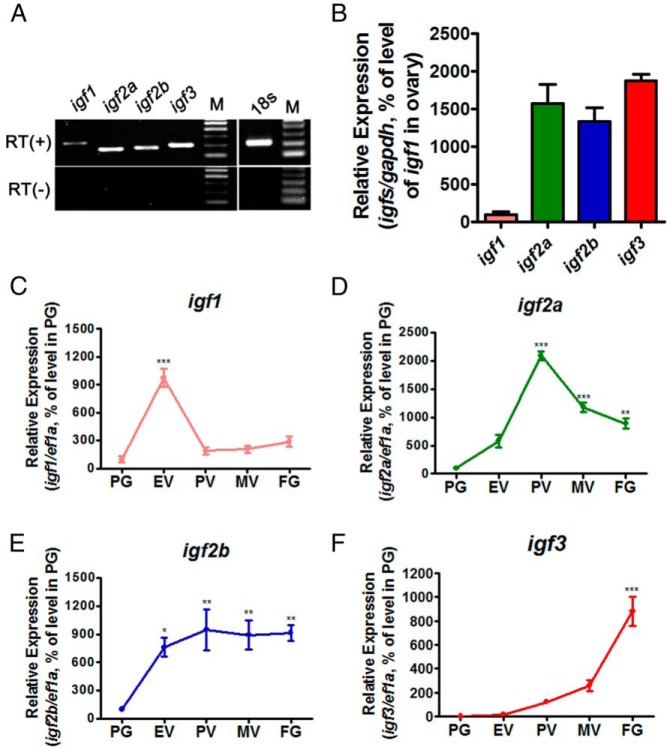
RT-PCR was performed to examine the gene expression profiles of the 4 igfs in the mature zebrafish ovary. Transcripts of all 4 igfs could be detected with igf1 eliciting extremely low level (Figure 1A). The results were confirmed by quantitative real-time PCR. The mRNA level of igf1 was barely detectable as compared with the other igfs (Figure 1B).
Figure 1.
Expression of igf1, igf2a, igf2b, and igf3 during folliculogenesis. A, RT-PCR detection of igf1, igf2a, igf2b, and igf3 expression in the ovary of adult fish. RT(+), RNA with reverse transcription; RT(−), RNA without reverse transcription; M, marker. B, Real-time PCR detection of igf1, igf2a, igf2b, and igf3 expression in the ovary of adult fish. C–F, Temporal expression of igf1 (C), igf2a (D), igf2b (E), and igf3 (F) in the follicles of different stages isolated from the ovaries of adult fish; each value representing the mean value ± SEM of quadruplicate assays of 3 independent experiments (*, P < .05; **, P < .01; ***, P < .001 vs control).
The expression profiles of all the 4 igfs in the follicles of different stages of development during folliculogenesis were further assessed using real-time PCR. The level of igf1 increased from PG to early vitellogenic stage and then decreased rapidly thereafter (Figure 1C). The expression of igf2a started to increase from PG stage, reaching its highest level in PV stage and then decreased afterwards (Figure 1D). In contrast, igf2b increased from PG to PV stage and plateaued thereafter (Figure 1E). The expression of igf3 was low in the early stages of follicle development but dramatically increased in FG stage (Figure 1F). These data indicate that igfs are dynamically expressed during folliculogenesis in zebrafish.
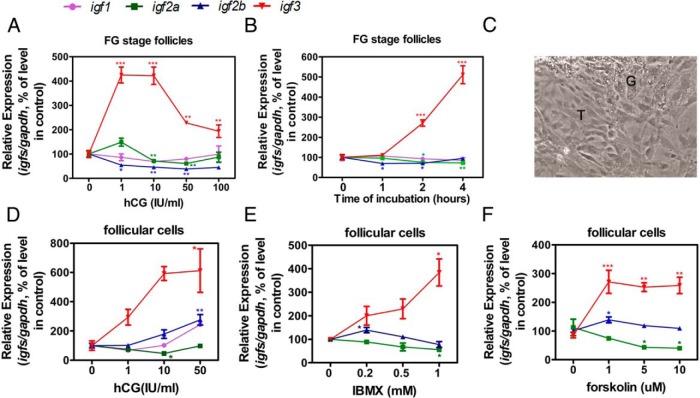
Differential regulation of the 4 igfs in FG stage follicles and in cultured follicular cells by hCG
To examine whether gonadotropin regulates the expression of igfs in zebrafish follicles, FG stage follicles were treated with hCG. Treatment of such follicles with hCG for 2 hours caused no change in the expression of igf1 and some small changes in the expression of igf2a and igf2b (Figure 2A). The expression of igf2a and igf2b was in fact slightly decreased by hCG. In big contrast, hCG drastically stimulated the expression of igf3, and hCG at higher concentrations elicited a strong desensitizing action on the expression of igf3. Time-course study yielded largely similar results (Figure 2B). The most responsive ligand towards stimulation by hCG was igf3, and all the other igfs exhibited either no or small changes. Notably, the expression of igf2a and igf2b was slightly decreased, but igf3 was greatly increased by hCG in both dose and time-dependent manners in FG stage follicles.
Figure 2.
Regulation of igf expression by hCG. A, Dose dependence of hCG action (2-h treatment) on the expression of igf1, igf2a, igf2b, and igf3 in FG stage follicles. B, Time dependence of hCG action (at 10 IU/mL of hCG) on the expression of igf1, igf2a, igf2b, and igf3 in FG stage follicles. C, Morphology of primary cultured zebrafish ovarian follicular cells after 6 days of incubation. The 2 types of epithelial cells present in the culture with distinct morphologies. G, granulosa cells; T, theca cells. D, Dose response of hCG action (2-h treatment) on the expression of igf1, igf2a, igf2b, and igf3 in primary cultured follicular cells. E, Dose response of IBMX action (2-h treatment) on the expression of igf1, igf2a, igf2b, and igf3 in primary cultured follicular cells. F, Dose response of forskolin action (2-h treatment) on the expression of igf2a, igf2b, and igf3 in primary cultured follicular cells. The expression levels of igfs were normalized to gapdh and expressed as a percentage of the control. Each value represents the mean value ± SEM of quintuplicate assays from 3 independent experiments (*, P < .05; **, P < .01; ***, P < .001 vs control).
To further investigate the regulation of IGF expression by gonadotropin, a primary culture of zebrafish ovarian follicular cells was established (Figure 2C). The 2 types of epithelial cells (granulosa cells and theca cells) could be observed in the culture with distinct morphology. The cultured ovarian follicular cells were treated with different concentrations of hCG for 2 hours (Figure 2D). Again, igf3 stood out as the most responsive towards stimulation by hCG.
Because cAMP is considered the principal second messenger involved in gonadotropin signaling, we have also studied the effects of elevated intracellular cAMP levels on the expression of igfs. Two cAMP agonists, 3-isobutyl-1-methylxanthine (IBMX) and forskolin, were used. Similar to the action of hCG, IBMX and forskolin treatment caused relatively small changes in the expression of igf2a and igf2b but up-regulated igf3 expression in the cultured follicular cells (Figure 2, E and F).
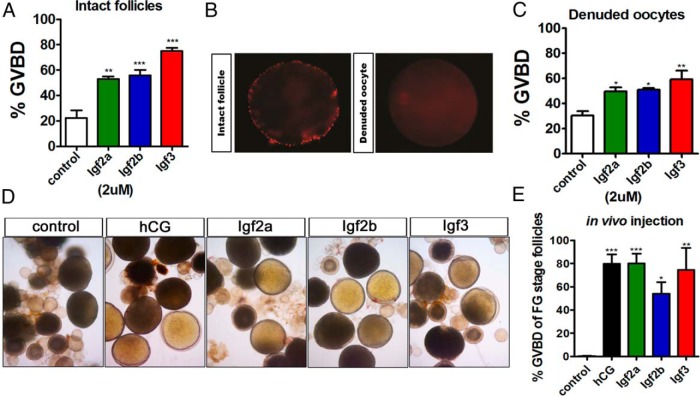
Induction of oocyte maturation by recombinant zebrafish IGF-2a, IGF-2b, and IGF-3 in vitro and in vivo
In order to analyze the biological functions of those IGFs expressed in the mature zebrafish ovaries, recombinant proteins were prepared from a bacterial expression system (Supplemental Figure 2). After treatment of FG stage follicles with these purified recombinant proteins, IGF-2a, IGF-2b, or IGF-3 proteins were found to significantly stimulate zebrafish oocyte maturation, with IGF-3 exhibiting the most potent action (Figure 3A). To investigate whether the IGFs exerted their action directly on oocytes, the ovarian follicular layers were removed by collagenase treatment. Successful removal of the ovarian follicular cell layers was confirmed by PI staining and observation under a fluorescent microscope (Figure 3B). Treatment of the denuded oocytes by recombinant IGF-2a, IGF-2b, or IGF-3 could also significantly stimulate oocyte maturation (Figure 3C). These results suggest that IGFs exert their action on oocyte maturation directly on the oocyte itself. To further test the in vivo action of IGFs on oocyte maturation, we have injected the recombinant IGF-2a, IGF-2b, or IGF-3 into adult zebrafish. In the control group, GVBD did not happen (Figure 3D). However, GVBD was evident in the ovaries of zebrafish injected with hCG or with the recombinant IGFs (Figure 3D). Quantitation of oocyte maturation in vivo assessed in the injected zebrafish indicated significant induction of oocyte maturation by hCG or by the recombinant IGFs (Figure 3E). Taken together, these data indicate that IGFs could induce oocyte maturation in vitro and in vivo.
Figure 3.
Recombinant zebrafish IGFs stimulate oocyte maturation in vitro and in vivo. A, Incubation of intact FG follicles with 2μM recombinant zebrafish IGF-2a, IGF-2b, or IGF-3 protein for 17 hours significantly induced GVBD. B, PI staining of intact follicles (left panel) and denuded oocytes (right panel). C, Incubation of denuded oocytes with 2μM recombinant zebrafish IGF-2a, IGF-2b, or IGF-3 protein for 4 hours significantly induced GVBD. D, Gross morphology of ovaries dissected from adult zebrafish after injection of 10-IU hCG or 1ug recombinant zebrafish IGF-2a, IGF-2b, or IGF-3 protein into zebrafish after 4 hours. Each picture is the representative result of triplicate assays from 3 independent experiments. E, Quantitative assessment of oocyte maturation induction by injection of 10-IU hCG or 1ug recombinant zebrafish IGF-2a, IGF-2b, or IGF-3 protein after 4 hours. Each value represents the mean value ± SEM of quintuplicate assays from 3 independent experiments (*, P < .05; **, P < .01; ***, P < .001 vs control).
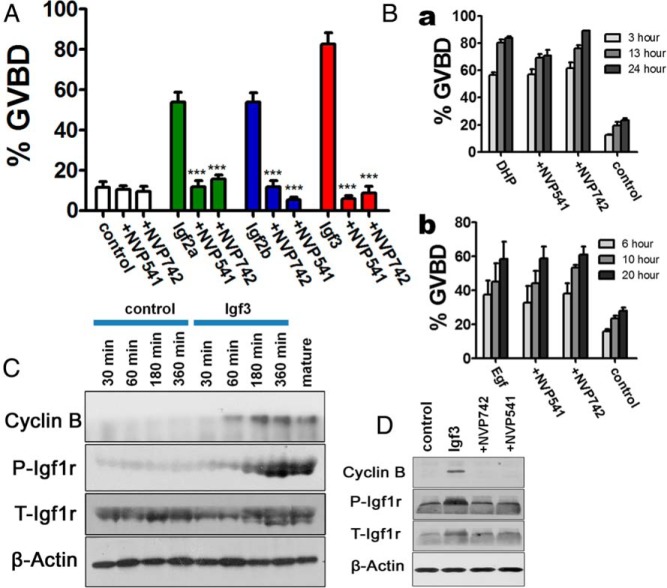
Induction of oocyte maturation by IGFs through activating IGF-1r in zebrafish
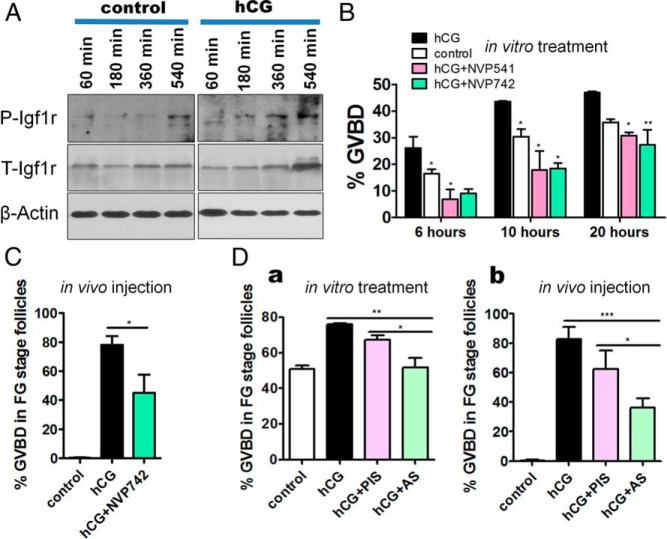
Next, we investigated whether the IGFs induced oocyte maturation via activating IGF receptor. Two IGF-1r inhibitors (NVP-AEW541 and NVP-ADW742) were employed to block IGF-1r action. After treatment with either inhibitor, the action of recombinant IGF-2a, IGF-2b, or IGF-3 on zebrafish oocyte maturation was completely abolished (Figure 4A). The action of IGF-3 on oocyte maturation could be blocked by both inhibitors in a dose-dependent manner (Supplemental Figure 3). This effective blockade by the inhibitors could not be explained by the toxicity of the inhibitors on the follicles, because the action of DHP or EGF on oocyte maturation was not affected by either inhibitor (Figure 4B). Western blotting was used to confirm the action of the IGF on the IGF-1r. It was found that phosphorylation of IGF-1r could also be stimulated by IGF-3 in a time-dependent manner, and the expression of cyclin B, a component of MPF, was also concomitantly activated (Figure 4C), indicating that IGF could induce the activity of MPF and GVBD by stimulating IGF-1r signaling. An elevated level of phosphorylated IGF-1rs could also be observed in the naturally mature oocytes as compared with that in FG stage follicles (Figure 4C), suggesting that activation of IGF-1r is a natural phenomenon required for oocyte maturation. Cyclin B expression induced by IGF-3 was further detected in the presence or absence of IGF-1r inhibitors by Western blotting. The results show that the expression of cyclin B and activation of IGF-1r could be totally blocked by IGF-1r inhibitors (Figure 4D), indicating that the induction of cyclin B by IGF-3 is dependent on the activation of IGF-1r. All the above data indicate that IGFs induce oocyte maturation through activating IGF-1r in zebrafish.
Figure 4.
IGFs stimulate oocyte maturation through activating IGF-1rs in zebrafish. A, Inhibition of IGF-induced (at 2μM) GVBD by IGF-1r inhibitors (10μM NVP-AEW541 or NVP-ADW742). B, DHP-induced (at 10 ng/mL) (a) and EGF-induced (at 100 ng/mL) (b) oocyte maturation was not significantly blocked by IGF-1r inhibitors (10μM). C, Western blotting results demonstrated the stimulation of IGF-3 (at 2μM) on IGF-1r phosphorylation and expression of cyclin B. Numbers on top stand for the treatment time. Mature, mature oocytes. D, Western blotting results demonstrated the stimulation of cyclin B by IGF-3 depends on the activation of IGF-1r. Each value represents the mean value ± SEM of triplicate assays from 3 independent experiments (***, P < .001 vs control).
Attenuation of hCG-induced oocyte maturation by IGF-1r inhibitors and anti-IGF-3 antiserum in vitro and in vivo
We further investigated whether the induction of oocyte maturation by LH requires IGF-1r activation. Treatment of FG stage follicles with hCG could enhance IGF-1r phosphorylation in a time-dependent manner (Figure 5A), suggesting that hCG could activate IGF-1r. The time course of hCG-induced IGF-1r activation actually lags behind IGF-3-induced IGF-1r phosphorylation. Moreover, hCG-induced oocyte maturation of follicles in vitro could be attenuated by treatment with IGF-1r inhibitors (NVP-AEW541 or NVP-ADW742) (Figure 5B). Furthermore, administration of hCG into adult zebrafish could induce oocyte maturation and coninjection of hCG with an IGF-1r inhibitor (NVP-ADW742) could significantly block the stimulatory action of hCG in vivo (Figure 5C). Meanwhile, we have also employed an anti-IGF-3 antiserum to neutralize the action of IGF-3. The production of this anti-IGF-3 antiserum has been reported by us previously (14). Our results clearly indicated that hCG induced-oocyte maturation could be significantly blocked by this anti-IGF-3 antiserum but not by the control preimmune serum in vitro (Figure 5Da) and in vivo (Figure 5Db). Taken together, these data strongly suggest the sequence of events in LH induced oocyte maturation, from stimulation of igf3 gene expression to IGF-1r activation.
Figure 5.
IGF-1r phosphorylation could be activated by hCG and hCG-induced oocyte maturation could be blocked by IGF-1r inhibitors in vitro and in vivo. A, Phosphorylation of IGF-1rs activated by treatment with hCG (at 50 IU/mL) in FG stage follicles. B, Inhibition of hCG-induced (50 IU/mL) GVBD by in vitro treatment of FG stage follicles in vitro with IGF-1r inhibitors (10μM NVP-AEW541 or NVP-ADW742). C, Significant inhibition of hCG-induced oocyte maturation after in vivo administration of hCG (10 IU/fish) with or without IGF-1r inhibitor (10mM NVP-ADW742, 4 μL/fish). D, Inhibition of hCG-induced (50 IU/mL) GVBD by treatment with an anti-IGF-3 antiserum (AS) (1:25 dilution) but not by the preimmune serum (PIS) (1:25 dilution) of FG stage follicles in vitro for 18 hours (a). Inhibition of hCG-induced oocyte maturation in vivo after injection of hCG (10 IU/fish) by AS or PIS (1.5 μL/fish) (b). Each value represents the mean value ± SEM of quintuplicate assays from 3 independent experiments (*, P < .05; **, P < .01 vs control).
Regulation of the 4 igfs in lhb knockout fish and rescue of defects in oocyte maturation by IGF-3 in lhb knockout fish
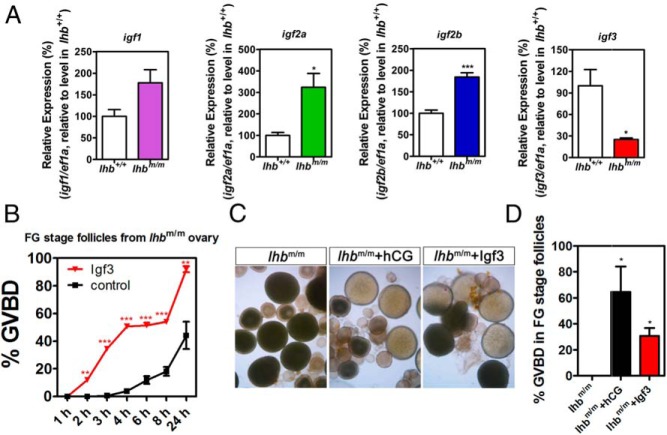
Finally, the significance of IGF signaling during oocyte maturation was investigated in vivo using lhb-deficient zebrafish. We have obtained the lhb homozygous mutant zebrafish using TALENs (Supplemental Figure 1) (15). The ovaries of the lhb mutant fish contain many FG stage follicles but no mature oocytes, indicating that endogenous LH signaling is essential for final oocyte maturation. We have examined the expression of all the 4 igfs in FG stage follicles of both the wild-type and homozygous mutant fish (Figure 6A). Compared with the wild-type controls, igf1 level was slightly increased, and both igf2a and igf2b were significantly increased in the homozygous lhb mutant fish (Figure 6A). In sharp contrast, igf3 expression level was dramatically decreased in the mutant fish. These data provide strong in vivo evidence to support that IGFs, particularly IGF-3, constitute important downstream molecules of LH signaling during oocyte maturation. To substantiate this further, we have isolated FG stage follicles from the lhb mutant fish. Treatment of these follicles in vitro with recombinant zebrafish IGF-3 could induce oocyte maturation (Figure 6B). We also tested whether in vivo administration of IGF-3 could rescue the oocyte maturation defects observed in the lhb mutant fish. Injection of hCG into lhb mutant fish was used as a positive control. After administration of hCG (10 IU/fish) or recombinant IGF-3 (1 μg/fish) to the homozygous lhb mutant female fish, mature oocyte could be observed in the ovary 4 hours after injection (Figure 6, C and D). These results indicate that IGF-3 acts as an important downstream mediator of LH action on oocyte maturation in zebrafish.
Figure 6.
Expression of igfs in FG stage follicles of lhb mutant fish and partial rescue of defects in oocyte maturation by IGF-3. A, Relative expression of igf1, igf2a, igf2b, and igf3 in FG stage follicles of the wild-type (lhb+/+) and lhb mutant (lhbm/m) zebrafish. B, Recombinant zebrafish IGF-3 protein (2μM) induced maturation of FG follicles isolated from the ovary of lhb mutant fish. Each value represents the mean value ± SEM of triplicate assays from 3 independent experiments (*, P < .05; **, P < .01; ***, P < .001 vs control). C, Gross morphology of ovaries dissected from lhb mutant (lhbm/m) zebrafish after administration of hCG (10 IU/fish) or IGF-3 (1ug/fish). Each picture is the representative result of triplicate assays from 3 independent experiments. D, Quantitative assessment of oocyte maturation induction in lhbm/m zebrafish by injection of 10-IU hCG or 1ug recombinant zebrafish IGF-3 protein after 4 hours. Each value represents the mean value ± SEM of triplicate assays from 3 independent experiments. (*, P < .05 vs control).
Discussion
It is known for a long time that oocyte maturation is controlled by LH signaling in fish, but the downstream factors mediating LH action remain to be fully elucidated. In this study, we have demonstrated that IGF ligands, particularly the gonad-specific IGF-3, are important mediators of LH action on oocyte maturation in zebrafish. The pivotal role of IGF-3 in this process can be appreciated by the next findings: 1) IGF-3 is specifically and abundantly expressed in the ovaries; 2) its expression is dramatically increased during follicle maturation; 3) its expression is very sensitive to LH stimulation in vitro and in vivo; 4) its expression could be regulated by LH in the follicular cells; 5) recombinant IGF-3 protein could enhance oocyte maturation; 6) pharmacological blockade of IGF receptor could attenuate LH-induced oocyte maturation; 7) neutralization of IGF-3 by anti-IGF-3 antiserum could attenuate LH-induced oocyte maturation; and 8) administration of IGF-3 could partially rescue the oocyte maturation defects of lhb-deficient fish. Based on these findings, we propose that IGF-3 acts as an important mediator of LH signaling during oocyte maturation in zebrafish (Figure 7).
Figure 7.
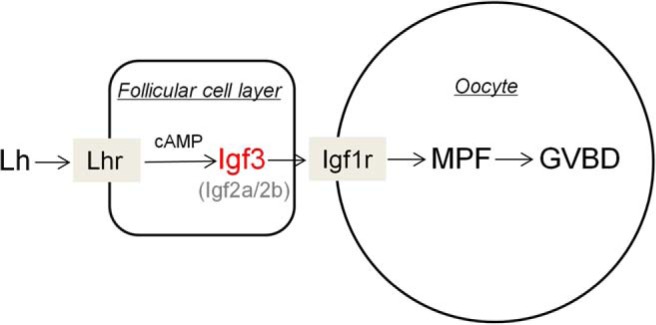
IGFs mediate LH action on oocyte maturation: a proposed model. IGF-2a, IGF-2b, and IGF-3 are all expressed in the follicular cells and subject to regulation by LH signaling, with IGF-3 being the most important IGF subtype in mediating LH action on oocyte maturation.
Four IGF ligands, including IGF-1, IGF-2a, IGF-2b, and IGF-3, have been identified in zebrafish. All of them could be detected in the ovary but at very different levels. The level of igf1 mRNA is extremely low in the mature ovary, indicating that IGF-1 probably plays a minor role in the adult ovary. Its relative insensitivity to stimulation by hCG and cAMP agonists also attests to its insignificant physiological role in the adult zebrafish ovary. However, a transient increase of igf1 expression from PG to PV stage follicles was noticed during folliculogenesis (Figure 1C), indicating the possible involvement of IGF-1 in the transition of these stages. Larger increases in igf2a and igf2b expression were observed during early folliculogenesis (Figure 1, D and E), indicating the involvement of these 2 IGF subtypes in this process. The most remarkable feature in the expression profiles of these growth factors during folliculogenesis is the sharp increase in igf3 expression at the later stage follicles, suggesting that igf3 is involved in the regulation of oocyte maturation. In support of this supposition, our subsequent findings confirmed that IGF-3 could indeed stimulate oocyte maturation in vitro and in vivo. Among all the IGFs ligands expressed in the ovary, IGF-3 is the most responsive to hCG stimulation (Figure 2), consistent with the recent results of Irwin et al (22). Using primary cultured zebrafish follicular cells, we have demonstrated again that IGF-3 is the most responsive to regulation by hCG and cAMP agonists. The differential regulation of igfs by LH was further substantiated by assessment of the expression profiles of the IGF ligands in zebrafish mutant lines in which the lhb gene has been knocked out (15). Among all the IGF ligands expressed in the ovary, only igf3 expression level was dramatically decreased in the intact FG stage follicles obtained from lhb mutant fish. In conclusion, our observations on the expression and regulation of igfs in the ovary indicate that LH could regulate the expression of igfs in the ovary, particularly igf3, through a cAMP mechanism to induce oocyte maturation.
The actions of recombinant zebrafish IGF-2a, IGF-2b, and IGF-3 on oocyte maturation were assessed. The recombinant IGFs were found to significantly stimulate oocyte maturation, with IGF-3 exhibiting the most potent stimulatory action. We have further investigated whether the IGFs activate oocyte maturation through the follicular cells indirectly or on the oocyte directly. After removing the theca and granulosa cells surrounding the oocyte, the stimulatory actions of the recombinant zebrafish IGFs on oocyte maturation of the denuded oocytes could still be observed. This is consistent with a previous report in Cyprinus carpio where oocyte maturation could be stimulated by IGF-1 in denuded oocytes (23), suggesting the direct action of fish IGFs on the oocyte. Because igf1rs have been found to be expressed in both the follicular cells and oocyte (24), we further investigated whether IGFs activate oocyte maturation through the IGF-1rs in zebrafish. Two specific IGF-1r inhibitors previously demonstrated to be able to successfully block the phosphorylation of IGF-1rs in zebrafish (25) were employed. Our results clearly demonstrated that the action of the IGFs including IGF-3 on oocyte maturation could be effectively blocked by these 2 IGF-1r inhibitors. Furthermore, phosphorylation of IGF-1rs could be stimulated by IGF-3, as observed in the LH-stimulated follicles as well as in the naturally mature oocytes, suggesting that IGF-3, produced in response to LH stimulation, induces oocyte maturation through IGF-1rs in zebrafish.
We have further investigated how important the IGF system serves as mediator of LH action in zebrafish oocyte maturation. Although some reports support the notion that IGFs act as potent regulators of meiotic maturation in fish and amphibia (26–29), our present study provides the first solid evidence demonstrating the importance of IGFs in mediating the action of LH in zebrafish follicles. Using the same IGF-1r inhibitors that have been employed to effectively block IGF action on oocyte maturation, the action of hCG on oocyte maturation could also be significantly blocked by both inhibitors. Such effect was further demonstrated by coadministration of IGF-1r inhibitor and hCG into adult zebrafish in vivo. Moreover, hCG-induced oocyte maturation could be significantly inhibited by an anti-IGF-3 antiserum but not by the control preimmune serum in vitro and in vivo. Furthermore, we have found that the defects of oocyte maturation in lhb mutant fish could be rescued by IGF-3 in vitro and in vivo. These results establish beyond doubts that IGF-3 serves as a crucial mediator of LH action on oocyte maturation in zebrafish. At this point in time, it is still hard to conclude on the specific role of IGF-2a and IGF-2b in LH-induced oocyte maturation. But in view of their expression profiles in response to hCG stimulation, their relative importance in LH-induced oocyte maturation is probably not as high as IGF-3.
It is widely accepted in fish that LH induces oocyte maturation by stimulating the production of MIH (DHP in most fishes) in follicular cells, and MIH binds to its membrane-bound MIH receptors to stimulate MPF activity (1). However, recent studies also demonstrated the involvement of several growth factors in this process. Findings of the present study provide direct evidence that IGFs mediate this LH-induced oocyte maturation. Other growth factors, including members of the activin system (12), TGF-β1 (30, 31), TNF (32, 33), etc, have also been reported to be mediators of this process. It has been shown that both the action of LH and DHP on oocyte maturation could be inhibited by blockade of activin signaling by follistatin, and the membrane-bound MIH receptors could be up-regulated by activin (10, 12, 17, 35–40), suggesting that the activin system and DHP signaling converge to induce fish oocyte maturation. However, results of our study show that only the action of LH but not that of DHP could be inhibited by blockade of IGF signaling by IGF-1r inhibitors. Furthermore, a recent study in zebrafish reported that the stimulation of hCG on the production of DHP was not affected by cotreatment with IGF-1r inhibitor (NVP-AEW541) (22). We also have data showing that steroidogenesis is not required for IGF-3-induced oocyte maturation (Supplemental Figure 4). These results indicate that the action of the IGF ligands on oocyte maturation is probably independent of activin and DHP signaling. This is the first demonstration that the IGF system could act in parallel with steroids to induce oocyte maturation in any vertebrate species.
Until now, IGF-3 has been identified in several fish species, including zebrafish (13), tilapia (13, 41, 42), medaka (13), and rainbow trout (43). The expression pattern of IGF-3 in the ovarian follicles is similar in both zebrafish and tilapia, and its regulation by LH via a cAMP pathway has also been reported in both fish species (13, 14, 22, 24, 42). Here, the definitive role of IGF-3 in LH-induced oocyte maturation was further demonstrated in this study. These results indicate the role of IGF-3 in serving as a downstream mediator of LH action in the induction of final oocyte maturation might well be a common phenomenon in fishes. Further studies are highly warranted. Despite the absence of IGF-3 in mammalian species, the significant role of the IGF system in regulating oocyte maturation has been demonstrated in mammals (44). IGF-1 mutant female mice are infertile, due to an arrest of follicular development before the maturation stage and failure to ovulate even after administration of gonadotropins (45). The induction of oocyte maturation by either IGF-1 and IGF-2 has been reported in many species of mammals (46–49). Many studies have also demonstrated the regulation of IGFs by LH in mammalian ovary. For example, igf2 as the major form of igf in human ovary could be regulated by LH in granulosa cells (50, 51). In the mouse ovary, igf1 as the major form of igf could be up-regulated by LH (52). Considering the interplay of IGFs and gonadotropin reported in mammals (34, 53), and the importance of IGF-3 in fish oocyte maturation as revealed in this study, it would be interesting to revisit the role of the IGF system in LH-induced oocyte maturation in mammals.
In summary, we provide both in vitro and in vivo evidence demonstrating that IGF-3 acts as a crucial mediator of LH-induced oocyte maturation in zebrafish. These results not only supply valuable information on the role of IGF on oocyte maturation but also provide insights into a better understanding on the signaling pathway of meiotic resumption in female reproduction in other species.
Additional material
Supplementary data supplied by authors.
Acknowledgments
We thank Ms Josie Lai and Ms Kathy W.Y. Sham for technical assistance. We also thank Novartis Co for supplying us the IGF-1r inhibitor (NVP-AEW541).
This work was supported by the General Research Fund Grant CUHK 462511 of the Hong Kong Research Grants Council, a grant for enhancing the Research Capability of Young Teachers in Northwest Normal University (NWNU-LKQN-12–8), and a Shenzhen Municipal R&D Fund of Science and Technology Grant SZRIC 0413007.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- DHP
- 17α,20β-dihydroxy-4-pregnen-3-one
- EGF
- epidermal growth factor
- FG
- full grown but immature
- gapdh
- glyceraldehyde-3-phosphate dehydrogenase
- GVBD
- germinal vesicle breakdown
- hCG
- human chorionic gonadotropin
- IBMX
- 3-isobutyl-1-methylxanthine
- IGF-1r
- IGF-1 receptor
- MIH
- maturation-inducing hormone
- MPF
- maturation-promoting factor
- PG
- primary growth
- PI
- propidium iodide
- PV
- previtellogenic.
References
- 1. Nagahama Y, Yamashita M. Regulation of oocyte maturation in fish. Dev Growth Differ. 2008;50(suppl 1):S195–S219. [DOI] [PubMed] [Google Scholar]
- 2. Ascoli M, Fanelli F, Segaloff DL. The lutropin/choriogonadotropin receptor, a 2002 perspective. Endocr Rev. 2002;23:141–174. [DOI] [PubMed] [Google Scholar]
- 3. Richards JS. New signaling pathways for hormones and cyclic adenosine 3′,5′-monophosphate action in endocrine cells. Mol Endocrinol. 2001;15:209–218. [DOI] [PubMed] [Google Scholar]
- 4. Mehlmann LM. Stops and starts in mammalian oocytes: recent advances in understanding the regulation of meiotic arrest and oocyte maturation. Reproduction. 2005;130:791–799. [DOI] [PubMed] [Google Scholar]
- 5. Nagahama Y, Yoshikuni M, Yamashita M, Tokumoto T, Katsu Y. Regulation of oocyte growth and maturation in fish. Curr Top Dev Biol. 1995;30:103–145. [DOI] [PubMed] [Google Scholar]
- 6. Kishimoto T. Cell-cycle control during meiotic maturation. Curr Opin Cell Biol. 2003;15:654–663. [DOI] [PubMed] [Google Scholar]
- 7. Yu SMY, Ge W. The GH-IGF axis and its potential role in the ovary of zebrafish, Danio rerio. Biol Reprod. 2007;77:1. [Google Scholar]
- 8. Park JY, Su YQ, Ariga M, Law E, Jin SL, Conti M. EGF-like growth factors as mediators of LH action in the ovulatory follicle. Science. 2004;303:682–684. [DOI] [PubMed] [Google Scholar]
- 9. Jamnongjit M, Gill A, Hammes SR. Epidermal growth factor receptor signaling is required for normal ovarian steroidogenesis and oocyte maturation. Proc Natl Acad Sci USA. 2005;102:16257–16262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Pang Y, Ge W. Gonadotropin regulation of activin βA and activin type IIA receptor expression in the ovarian follicle cells of the zebrafish, Danio rerio. Mol Cell Endocrinol. 2002;188:195–205. [DOI] [PubMed] [Google Scholar]
- 11. Pang Y, Ge W. Gonadotropin and activin enhance maturational competence of oocytes in the zebrafish (Danio rerio). Biol Reprod. 2002;66:259–265. [DOI] [PubMed] [Google Scholar]
- 12. Pang Y, Ge W. Activin stimulation of zebrafish oocyte maturation in vitro and its potential role in mediating gonadotropin-induced oocyte maturation. Biol Reprod. 1999;61:987–992. [DOI] [PubMed] [Google Scholar]
- 13. Wang DS, Jiao B, Hu C, Huang X, Liu Z, Cheng CH. Discovery of a gonad-specific IGF subtype in teleost. Biochem Biophys Res Commun. 2008;367:336–341. [DOI] [PubMed] [Google Scholar]
- 14. Li J, Liu Z, Wang D, Cheng CH. Insulin-like growth factor 3 is involved in oocyte maturation in zebrafish. Biol Reprod. 2011;84:476–486. [DOI] [PubMed] [Google Scholar]
- 15. Chu L, Li J, Liu Y, Hu W, Cheng CH. Targeted gene disruption in zebrafish reveals noncanonical functions of LH signaling in reproduction. Mol Endocrinol. 2014;28:1785–1795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Selman K, Wallace RA, Sarka A, Qi X. Stages of oocyte development in the zebrafish, Brachydanio rerio. J Morphol. 1993;218:22. [DOI] [PubMed] [Google Scholar]
- 17. Wang Y, Ge W. Developmental profiles of activin βA, βB, and follistatin expression in the zebrafish ovary: evidence for their differential roles during sexual maturation and ovulatory cycle. Biol Reprod. 2004;71:2056–2064. [DOI] [PubMed] [Google Scholar]
- 18. Pang Y, Thomas P. Role of G protein-coupled estrogen receptor 1, GPER, in inhibition of oocyte maturation by endogenous estrogens in zebrafish. Dev Biol. 2010;342:194–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Das D, Khan PP, Maitra S. Participation of PI3-kinase/Akt signalling in insulin stimulation of p34cdc2 activation in zebrafish oocyte: phosphodiesterase 3 as a potential downstream target. Mol Cell Endocrinol. 2013;374:46–55. [DOI] [PubMed] [Google Scholar]
- 20. Peyton C, Thomas P. Involvement of epidermal growth factor receptor signaling in estrogen inhibition of oocyte maturation mediated through the G protein-coupled estrogen receptor (Gper) in zebrafish (Danio rerio). Biol Reprod. 2011;85:42–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kinkel MD, Eames SC, Philipson LH, Prince VE. Intraperitoneal injection into adult zebrafish. J Vis Exp. 2010;42:2126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Irwin DA, Van Der Kraak G. Regulation and actions of insulin-like growth factors in the ovary of zebrafish (Danio rerio). Gen Comp Endocrinol. 2012;177:187–194. [DOI] [PubMed] [Google Scholar]
- 23. Paul S, Pramanick K, Kundu S, Bandyopadhyay A, Mukherjee D. Involvement of PI3 kinase and MAP kinase in IGF-I- and insulin-induced oocyte maturation in Cyprinus carpio. Mol Cell Endocrinol. 2009;309:93–100. [DOI] [PubMed] [Google Scholar]
- 24. Nelson SN, Van Der Kraak G. Characterization and regulation of the insulin-like growth factor (IGF) system in the zebrafish (Danio rerio) ovary. Gen Comp Endocrinol. 2010;168:111–120. [DOI] [PubMed] [Google Scholar]
- 25. Chablais F, Jazwinska A. IGF signaling between blastema and wound epidermis is required for fin regeneration. Development. 2010;137:871–879. [DOI] [PubMed] [Google Scholar]
- 26. Hainaut P, Kowalski A, Giorgetti S, Baron V, Van Obberghen E. Insulin and insulin-like-growth-factor-I (IGF-I) receptors in Xenopus laevis oocytes. Comparison with insulin receptors from liver and muscle. Biochem J. 1991;273(pt 3):673–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kagawa H, Kobayashi M, Hasegawa Y, Aida K. Insulin and insulin-like growth factors I and II induce final maturation of oocytes of red seabream, Pagrus major, in vitro. Gen Comp Endocrinol. 1994;95:293–300. [DOI] [PubMed] [Google Scholar]
- 28. Maestro MA, Méndez E, Párrizas M, Gutiérrez J. Characterization of insulin and insulin-like growth factor-I ovarian receptors during the reproductive cycle of carp (Cyprinus carpio). Biol Reprod. 1997;56:1126–1132. [DOI] [PubMed] [Google Scholar]
- 29. Mukhejee D, Mukherjee D, Sen U, Paul S, Bhattacharyya SP. In vitro effects of insulin-like growth factors and insulin on oocyte maturation and maturation-inducing steroid production in ovarian follicles of common carp, Cyprinus carpio. Comp Biochem Phys A. 2006;144:63–77. [DOI] [PubMed] [Google Scholar]
- 30. Kohli G, Clelland E, Peng C. Potential targets of transforming growth factor-β1 during inhibition of oocyte maturation in zebrafish. Reprod Biol Endocrinol. 2005;3:53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kohli G, Hu S, Clelland E, Di Muccio T, Rothenstein J, Peng C. Cloning of transforming growth factor-β 1 (TGF-β 1) and its type II receptor from zebrafish ovary and role of TGF-β 1 in oocyte maturation. Endocrinology. 2003;144:1931–1941. [DOI] [PubMed] [Google Scholar]
- 32. Crespo D, Bonnet E, Roher N, et al. Cellular and molecular evidence for a role of tumor necrosis factor α in the ovulatory mechanism of trout. Reprod Biol Endocrinol. 2010;8:34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Crespo D, Mañanós EL, Roher N, MacKenzie SA, Planas JV. Tumor necrosis factor α may act as an intraovarian mediator of luteinizing hormone-induced oocyte maturation in trout. Biol Reprod. 2012;86:1–12. [DOI] [PubMed] [Google Scholar]
- 34. Lackey BR, Gray SL, Henricks DM. The insulin-like growth factor (IGF) system and gonadotropin regulation: actions and interactions. Cytokine Growth Factor Rev. 1999;10:201–217. [DOI] [PubMed] [Google Scholar]
- 35. Wang Y, Ge W. Involvement of cyclic adenosine 3′,5′-monophosphate in the differential regulation of activin βA and βB expression by gonadotropin in the zebrafish ovarian follicle cells. Endocrinology. 2003;144:491–499. [DOI] [PubMed] [Google Scholar]
- 36. Wang Y, Ge W. Spatial expression patterns of activin and its signaling system in the zebrafish ovarian follicle: evidence for paracrine action of activin on the oocytes. Biol Reprod. 2003;69:1998–2006. [DOI] [PubMed] [Google Scholar]
- 37. Wu T, Patel H, Mukai S, et al. Activin, inhibin, and follistatin in zebrafish ovary: expression and role in oocyte maturation. Biol Reprod. 2000;62:1585–1592. [DOI] [PubMed] [Google Scholar]
- 38. DiMuccio T, Mukai ST, Clelland E, et al. Cloning of a second form of activin-βA cDNA and regulation of activin-βA subunits and activin type II receptor mRNA expression by gonadotropin in the zebrafish ovary. Gen Comp Endocrinol. 2005;143:287–299. [DOI] [PubMed] [Google Scholar]
- 39. Tan Q, Balofsky A, Weisz K, Peng C. Role of activin, transforming growth factor-β and bone morphogenetic protein 15 in regulating zebrafish oocyte maturation. Comp Biochem Physiol A Mol Integr Physiol. 2009;153:18–23. [DOI] [PubMed] [Google Scholar]
- 40. Tan Q, Zagrodny A, Bernaudo S, Peng C. Regulation of membrane progestin receptors in the zebrafish ovary by gonadotropin, activin, TGF-β and BMP-15. Mol Cell Endocrinol. 2009;312:72–79. [DOI] [PubMed] [Google Scholar]
- 41. Berishvili G, Baroiller JF, Eppler E, Reinecke M. Insulin-like growth factor-3 (IGF-3) in male and female gonads of the tilapia: development and regulation of gene expression by growth hormone (GH) and 17α-ethinylestradiol (EE2). Gen Comp Endocrinol. 2010;167:128–134. [DOI] [PubMed] [Google Scholar]
- 42. Li M, Wu F, Gu Y, et al. Insulin-like growth factor 3 regulates expression of genes encoding steroidogenic enzymes and key transcription factors in the Nile tilapia gonad. Biol Reprod. 2012;86:163:161–110. [DOI] [PubMed] [Google Scholar]
- 43. Sambroni E, Rolland AD, Lareyre JJ, Le Gac F. FSH and LH have common and distinct effects on gene expression in rainbow trout testis. J Mol Endocrinol. 2013;50:1–18. [DOI] [PubMed] [Google Scholar]
- 44. Silva JR, Figueiredo JR, van den Hurk R. Involvement of growth hormone (GH) and insulin-like growth factor (IGF) system in ovarian folliculogenesis. Theriogenology. 2009;71:1193–1208. [DOI] [PubMed] [Google Scholar]
- 45. Baker J, Hardy MP, Zhou J, et al. Effects of an Igf1 gene null mutation on mouse reproduction. Mol Endocrinol. 1996;10:903–918. [DOI] [PubMed] [Google Scholar]
- 46. Kiapekou E, Loutradis D, Drakakis P, Zapanti E, Mastorakos G, Antsaklis A. Effects of GH and IGF-I on the in vitro maturation of mouse oocytes. Hormones (Athens). 2005;4:155–160. [DOI] [PubMed] [Google Scholar]
- 47. Gómez E, Tarín JJ, Pellicer A. Oocyte maturation in humans: the role of gonadotropins and growth factors. Fertil steril. 1993;60:40–46. [PubMed] [Google Scholar]
- 48. Grupen CG, Nagashima H, Nottle MB. Role of epidermal growth factor and insulin-like growth factor-I on porcine oocyte maturation and embryonic development in vitro. Reprod Fertil Dev. 1997;9:571–575. [DOI] [PubMed] [Google Scholar]
- 49. Guler A, Poulin N, Mermillod P, Terqui M, Cognié Y. Effect of growth factors, EGF and IGF-I, and estradiol on in vitro maturation of sheep oocytes. Theriogenology. 2000;54:209–218. [DOI] [PubMed] [Google Scholar]
- 50. Ramasharma K, Li CH. Human pituitary and placental hormones control human insulin-like growth factor II secretion in human granulosa cells. Proc Natl Acad Sci USA. 1987;84:2643–2647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Voutilainen R, Miller WL. Coordinate tropic hormone regulation of mRNAs for insulin-like growth factor II and the cholesterol side-chain-cleavage enzyme, P450scc, in human steroidogenic tissues. Proc Natl Acad Sci USA. 1987;84:1590–1594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Hsu CJ, Hammond JM. Gonadotropins and estradiol stimulate immunoreactive insulin-like growth factor-I production by porcine granulosa cells in vitro. Endocrinology. 1987;120:198–207. [DOI] [PubMed] [Google Scholar]
- 53. Kwintkiewicz J, Giudice LC. The interplay of insulin-like growth factors, gonadotropins, and endocrine disruptors in ovarian follicular development and function. Semin Reprod Med. 2009;27:43–51. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.