Abstract
The purpose of this study was to identify the microscopic arterial vascularization of the corpora cavernosa (CC) of the penis using computer-assisted anatomic dissection (CAAD), determine the contribution of the different penile arteries towards this vascularization, detail the nature of cavernospongiosum shunts, and locate the anastomoses between these different arteries. Tissue specimens were taken from five donors who donated their bodies to science. The specimens were fixed in 10% formalin and sliced into a series of five 5-μm sections at intervals of 200 μm. The first section was stained with hematoxylin-eosin or Masson's trichrome and the second with anti-protein S100. The cavernous artery of the penis is not the only source of arterial vascularization of the CC. In four of the five cases studied, we found two to four perforating branches arising from the dorsal arteries of the penis that join up with the cavernous artery of the penis or that are solely responsible for the vascularization of the distal third of the penis. The bulbo-urethral and urethral arteries are situated outside of the tunica albuginea of the corpus spongiosum on their lateral and dorsal sides. The anastomoses do not occur between the cavernous artery of the penis and the corpus spongiosum but between the cavernous artery of the penis and the urethral artery on the surface of the tunica albuginea. All of these arteries are accompanied by nerve branches. The CC were found to be vascularized by both cavernous and dorsal arteries of the penis. Intrapenile vascularization is organized around four arterial axes, which are anastomosed by multiple neurovascular shunts.
Keywords: cavernous artery, cavernous urethral shunt, corpora cavernosa, erection, penis
Introduction
Medical care for prostate cancers, caught early, has changed considerably. Many cancers are detected at a localized stage (Lunacek et al. 2005). Surgery is one of the therapeutic options; in this case the objective is to cure the patients by giving them an acceptable quality of life. Erectile dysfunction and urinary incontinence are the complications that alter these patients' quality of life the most (Donnellan et al. 1997).
The etiology of erectile dysfunction after a radical prostatectomy for prostate cancer is multifactorial, including both neurological and vascular elements.
The cavernous arteries traditionally represent the principal source of blood to the CC. Additional pudendal arteries may also be important (Droupy et al. 1997). The arterial vascularization of the penis was variable, highly anastomosed, with the presence of an accessory pudendal artery (70% of cases) anastomosed (also 70% of cases) with a cavernous artery originating from the internal pudendal (Benoit et al. 1999; Droupy et al. 1999b). These arteries are not terminal but highly anastomosed. The proximity of these arteries with the prostate and the bladder renders them vulnerable to injury, which may explain some erectile dysfunction after pelvic surgery.
The most frequent cause of erectile dysfunction cited is an intra-operative injury to the cavernous nerves, not the arteries.
In a series of men with normal pre-operative erectile function and post-operative erectile dysfunction, the incidence of arterial insufficiency, as assessed by Doppler ultrasound, was < 10% (Quinlan et al. 1991).
Vascular preservation techniques exist that might allow us to optimize the post-operative erectile function and to facilitate nerve preservation (Secin et al. 2007; Patel et al. 2012).
Moreover, injection of vasoactive agents into the urethra can induce an erection of the CC, thereby demonstrating the existence of vascular anastomoses between the CC and the corpus spongiosum (CS). Wagner et al. (1982) described shunts between the CC and the CS. Our team has confirmed the existence of these shunts via operating microscope dissection after intra-arterial injection of colored latex (Droupy et al. 1999a). Anastomoses have been confirmed by Costabile et al. (1998).
Other studies have reported anastomoses between the dorsal artery of the penis and the cavernous artery. Through Doppler analysis, Montorsi et al. (1997) highlighted the arterioles connecting the dorsal artery of the penis to the cavernous artery in which the blood flow went from the cavernous artery towards the dorsal artery during erection. These authors considered these arteries to be cavernous. According to Doppler studies, there are estimated to be between 30 and 90% perforating dorso-cavernous branches in healthy men. Erdogru et al. (2001), once again through Doppler studies, has shown that these dorso-cavernous anastomoses played an important role in intracavernous arterial hemodynamics in 28% of patients with erectile dysfunction. In two-thirds of 40 penile specimens, Juskiewenski et al. (1982) had found one or several accessory cavernous arteries originating from the dorsal arteries. He also remarked that the dorsal artery could terminate as the cavernous accessory artery. The origin of the vascularization of the CC has therefore several origins, since anastomoses exist.
The goal of our work was to study the microscopic arterial vascularization of the CC by computer-assisted anatomic dissection (CAAD) in order to determine the anastomoses between the different arteries of the penis in the CC, particularly the caverno-spongiosum shunts.
Materials and methods
This study was performed using tissue specimens taken from five fresh male adult cadavers between the ages of 68 and 85 who donated their bodies to science. The sample from the first subject was taken from the bladder at the root of the penis. The specimens from the four other subjects were taken from the whole pelvic region, from the bladder to the balano-preputial fold (Fig. 1). The glans was not removed in order to return the bodies to the families.
Fig. 1.
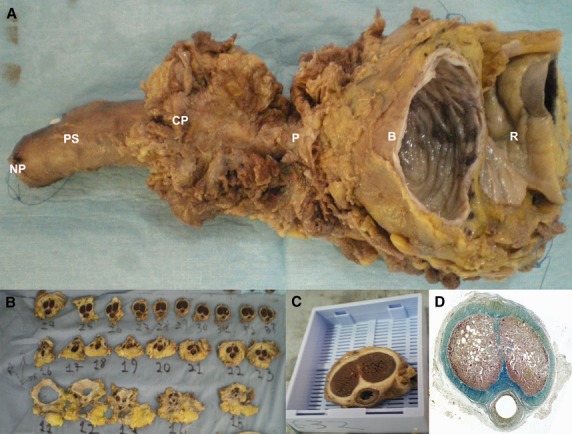
(A) Pelvic block taken from 70-year-old adult male from the bladder (B) to the neck of the penis (NP). (B) The specimen was cut into 1-cm macroscopic blocks. (C) These blocks were put in mega-cassettes (code: 38VSP59040) before being embedded in paraffin. (D) Histological section of the previous block stained by TreM and scanned at high resolution (3200 dots inch−1, dpi). R, rectum; P, prostate; CP, crura of penis; PS, penile shaft.
In each case, a Ch. 20 Foley catheter was placed in the urethra to maintain alignment of the penis. The tissue specimens were fixed in 10% formalin for 24–48 h and then washed and sliced into 5-mm-thick blocks before being enclosed in paraffin. Series of five 5-μm-thick slices at intervals of 200 μm were obtained.
In each case, the first slide was stained with hematoxylin-eosin (HE) or Masson's trichrome (TriM) and considered the reference slide. The second slide was stained with rabbit polyclonal antibody agonist S100 (code: ab15520) to identify the nerve branches. The immunohistochemical protocol has been published previously (Alsaid et al. 2009).
Three-dimensional reconstruction
Serial 2D sections, stained and immunolabeled for nerve fibers, were used to perform 3D reconstructions. Analysis of sections stained by HE or TriM allowed us to distinguish the various anatomic structures. The next sections, which were treated with anti-S100, shows that the interval between each HE or Trim and S100 sections was negligible (5 μm). Comparison with the immunolabeled sections allowed us to localize the nerve fibers. This step was controlled microscopically using a Nikon Eclipse 80i microscope loaded by the digital camera DXM1200F and available optical zooms of ×4, ×10, ×20 and ×40.
The computer system comprised a personal laptop (Windows XP), an Epson PerfectionV750 digitalization system, Silver Fast AI digitalized software, Adobe Photoshop image processing software, and WinSurf Reconstruction Software v.4.3.
The scanning resolution was 3200 dpi. Histological images of transverse section portions were regrouped in Photoshop software.
The penile anatomic structures and the nerve fibers were outlined manually in all the sections, and a 3D analysis of the location, course, and distribution of nerve fibers was performed as an animated motion picture.
Results
Bulbo-urethral arteries
The first dividing branches of the internal pudendal arteries were the bulbo-urethral arteries, which are 0.3–0.5 mm in diameter (Table 1). These ran medially through to the perineal membrane to reach the lateral sides of the tunica albuginea of the CC. The bulbo-urethral arteries received the branches that came from the dorsal artery, which ran forward along the surface of the tunica albuginea of the CC (Fig. 2). These arteries were anastomosed 10–15 times per individual with the urethral artery situated on the dorsal side of the CS.
Table 1.
Size of the different arteries of the penis and the thickness of their walls
| Arteries | Diameter | Thickness |
|---|---|---|
| Bulbo-urethral arteries (BUA) | 0.30–0.50 mm | 0.02–0.05 mm |
| Dorsal arteries of the penis (DAP) | 1.30–3.5 mm | 0.30–0.90 mm |
| Urethral arteries (UA) | 0.20–0.45 mm | 0.05–0.15 mm |
| Cavernous arteries (CA) | 0.30–1.75 mm | 0.10–0.60 mm |
Fig. 2.
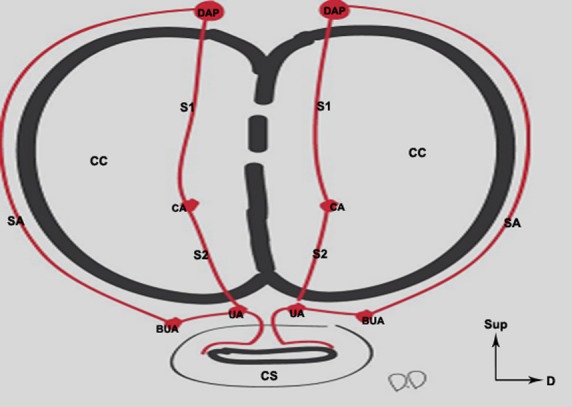
Schematic representation of the arteries of the penis and their anastomoses. The dorsal arteries of the penis (DAP) have superficial anastomoses (SA) (outside of the tunica albuginea TA) with the bulbo-urethral arteries (BUA). They give off deep branches (S1) which run through the tunica albuginea to join up with the cavernous arteries (CA) inside the corpora cavernosa (CC). The urethral arteries (UA) and the bulbo-urethral arteries are situated outside of the TA of the corpus spongiosum (CS). The anastomoses (S2) between the CAs and the UAs occurred outside of the CS, hence the term ‘cavernous urethral shunts’ used to designate these anastomoses.
The urethral arteries
The urethral arteries, originating from the internal pudendal arteries after the origin of the bulbo-urethral arteries, ran forward along the dorsal side of the tunica albuginea of the CS (Fig. 3) on both sides of the extension of the intercavernous septum at 1 and 11 o'clock. The arteries participated in the formation of a dense arterial network with the bulbo-urethral arteries on the dorsal side of the tunica albuginea of the CS (Fig. 4). They gave off branches that pierced the tunica albuginea of the CS between 1 and 11 o'clock and vascularized the CS and the urethra. They were anastomosed with the cavernous artery (Fig. 3), with diameters ranging from 0.20 to 0.45 mm. Two urethral arteries were found in four subjects and only one urethral artery was found in the fifth subject.
Fig. 3.
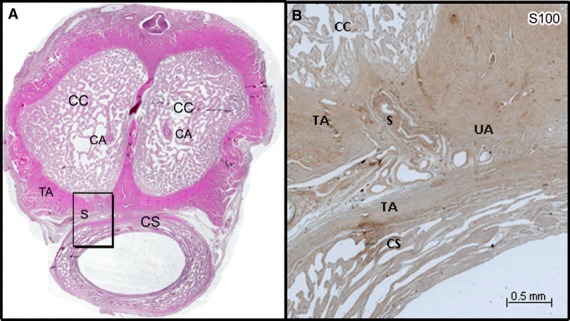
(A) Histological section of the penis of a 68-year-old man stained by HE and scanned at high resolution (3200 dpi). (B) Microscopic aspect (×10) of the shunt (S) between the CA and the UA (frame in A) immunostained with anti-protein S100. The shunt came from the CA, passed on the ventral surface of the tunica albuginea (TA) of the corpora cavernosa (CC) to join UA located on the dorsal surface of the corpus spongiosum (CS) between the two tunicae albugineae (TA) and not in the CS, hence our use of the term ‘cavernosal urethral shunt’.
Fig. 4.
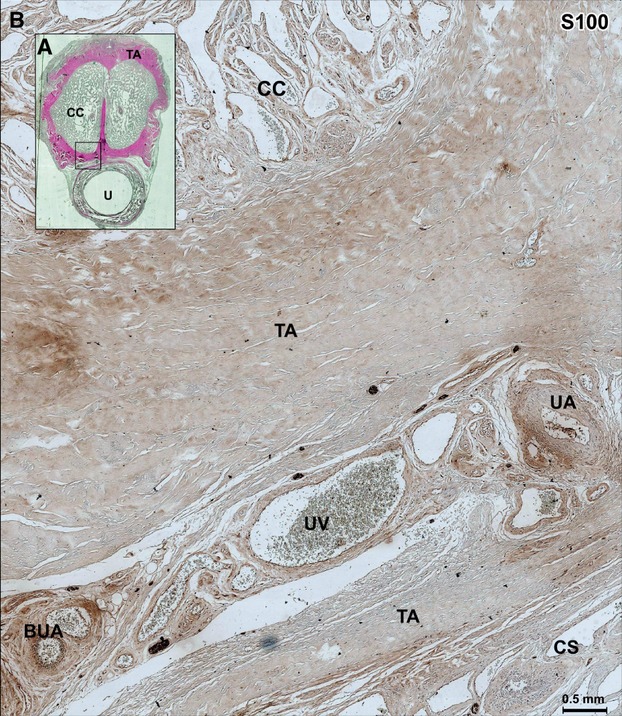
(A) Histological section through the body of the penis in 68-year-old man, stained by HE. (B) Microscopic aspect (×10) of the next section immunolabeled with S100 showing the space between the urethral artery (UA) (after its anastomosis with the shunt arising from the cavernous artery, as shown above) and the bulbo-urethral artery (BUA) (frame in A). The UA is situated on the dorsal side and outside of the corpus spongiosum (CS) between the two tunicae albugineae (TA), which receive the arterial shunt arising from the cavernous artery. The term ‘cavernous urethral shunts’ is thus a more appropriate designation for this arterial anastomosis. CC, corpora cavernosa; TA, tunica albuginea; UV, urethral vein.
The cavernous arteries
The cavernous arteries arose from the internal pudendal artery after the origin of the bulbo-urethral arteries and were situated on the dorsomedial side of the root of the tunica albuginea of the CC and laterally with respect to the deep venous branches of the penis. These arteries pierced the CC at 3 and 9 o'clock on both sides of the intercavernous septum accompanied by numerous nerve fibers, and rapidly separated into many numerous branches. They gave off retrograde branches for the crura of penis.
There were one to four cavernous arteries per CC at the distal part of the penis on both sides of the intercavernous septum. The muscular walls of these arteries were 0.1–0.6 mm thick (Table 1), thus they were contractile and were always accompanied by four to five nerve branches. The diameter of these arteries varied from 0.3 to 1.75 mm. These arteries received between two and four perforating branches, stemming from the dorsal arteries in four of our five subjects.
Arising from these cavernous arteries were four to six direct arteries piercing the ventral side of the tunica albuginea of the CC to anastomose on the dorsal side of the tunica albuginea of the CS with the urethral arteries (Figs 2 and 3), corresponding to cavernospongiosum shunts.
These anastomoses took place in the angle between the tunicae albugineae of the CC and the CS (Figs 3 and 4). In one of our subjects, these perforating arteries came from the dorsal artery before its anastomosis with the cavernous artery. We defined them as ‘cavernous urethral shunts’ for CAAD, which showed that these anastomoses occurred between the cavernous artery and the urethral artery on the surface of the tunica albuginea of the CS (Fig. 4) and not with the CS itself, as the work of Wagner et al. (1982) and our dissections would lead us to believe. The diameters of these ‘cavernous urethral shunts’ varied from 0.16 to 0.50 mm and they were accompanied by nerve branches stemming from the cavernous nerves. These shunts were found in all five of our subjects.
The dorsal arteries of the penis
The dorsal arteries encircled medially the dorsal sides of the CC between the deep veins of the penis and, laterally, the dorsal nerve branches of the penis.
During their course, they gave off circumflex branches on the lateral sides of the CC accompanied by fine venous branches of the same name. The number of these arteries varied from four to eight per penis and their diameter varied from 1.3 to 3.5 mm.
The dorsal arteries gave off branches that went around the lateral side of the tunica albuginea of the CC to anastomose with the bulbo-urethral arteries (Fig. 2).
The dorsal arteries gave off deep branches that pierced the tunica albuginea of the CC to anastomose in the CC with the cavernous artery (Figs 2 and 5). There were two to four of these perforating arteries depending upon the individual, with diameters ranging from 0.30 to 1 mm, and they were often larger than the cavernous artery at the distal extremity of the penis, and always accompanied by very fine nerve branches. These perforating arteries had a larger caliber than the ones in the ‘cavernous urethral shunt’. In two cases, they were the only arteries vascularizing the distal third of the penis.
Fig. 5.
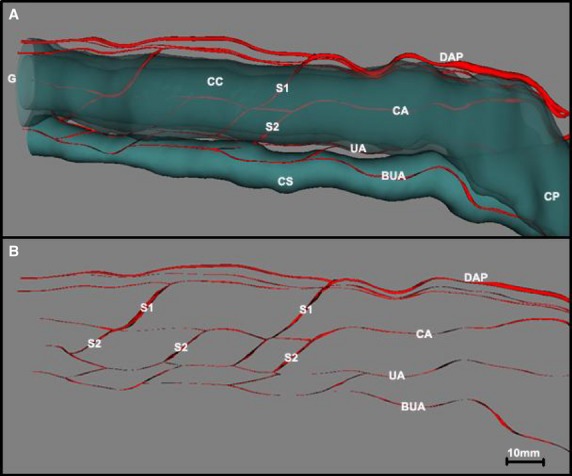
(A) Three-dimensional reconstruction of the penis of a 72-year-old human cadaver showing the trajectory of the arteries of the penis with regard to the corpora cavernosa (CC) and the corpus spongiosum (CS). (B) The same image after the CC and CS have been made transparent, showing the different anastomoses between the arteries of the penis. The dorsal arteries of the penis (DAP) played a role in the vascularization of the CC via the perforating branches (S1), which ran through the tunica albuginea (TA) to anastomose with the cavernous arteries (CA) or to ensure solely the vascularization of the one-third distal part of the CC. The bulbo-urethral arteries (BUA) and the urethral arteries (UA), highly anastomosed with each other, were situated outside of the TA of the CS. These were the UAs that received the shunt (S2) stemming from the CA, reinforcing the vascularization of the CS and the urethra. CP, crura of penis; G, glans.
In two of the five cases studied, the cavernous arteries vascularized the proximal two-thirds of the mobile part of the penis, the distal third being vascularized by a perforating branch 1 mm in diameter stemming from the dorsal artery (Fig. 5).
Discussion
Our study found anastomoses between the different arteries of the penis. These anastomoses occurred between the dorsal, the cavernous, and bulbo-urethral arteries and the urethral arteries. Many anastomoses were noted between urethral and bulbo-urethral arteries, and bulbo-urethral arteries received many branches from the dorsal arteries external to the tunica albuginea.
These anastomoses have already been described between CC and CS by Wagner et al. (1982), Murray (1798) and Soemmering (1880), but those authors did not identify the urethral arteries.
Droupy et al. (1999a) in dissection assisted by an operating microscope, described 6–10 anastomoses between the cavernous artery and the CS. Conti (1952) also described arterial anastomoses between the cavernous arteries and the urethral arteries in the distal parts of the penis. Bookstein & Lang (1987) visualized arteries connecting the cavernous arteries and the urethral arteries during erection in 13% of their patients.
We found anastomoses between the cavernous artery and the urethral artery situated on the dorsal side of the CS. These shunts, consisting of fine arterial branches, start at the cavernous artery and join up with the urethral artery situated on the dorsal side of the tunica albuginea of the CS. These communications between the cavernous artery and the urethral artery are situated outside of the tunica albuginea of the CS. We regard the term ‘cavernous urethral shunt’ as more appropriate than ‘cavernospongiosum shunts’ to designate these vessels. The bulbo-urethral arteries and the urethral arteries were situated outside of the tunica albuginea of the CS on the lateral and dorsal sides.
The arteries of the penis, except for the bulbo-urethral arteries, have muscular walls, indicative of their contractile properties (Table 1). The arterial anastomoses are accompanied by nerve branches, creating anastomotic ‘neurovascular pedicles’.
The cavernous arteries, thus, were not the sole vascularizing arteries of the CC. They served the proximal part of the CC and played a role in the vascularization of the CS and the urethra via the ‘cavernous urethral shunts’.
Our study found numerous intracavernous and extracavernous anastomoses. These anastomoses could be collateral vessels, permitting a possible revascularization of the CC and recovery of erectile function after a radical prostatectomy.
The urethral arteries, due to their position, are at potential risk for lesions during urethral or cosmetic penile surgery. Penile surgery created branches that pierced the tunica albuginea of the CS at 12 o'clock, risking injury during an internal urethrotomy (Mundy, 1993; Coursey et al. 2001).
The dorsal arteries played a role in the vascularization of the CC via the branches that perforated the tunica albuginea to join up with the cavernous artery. They presented surface anastomoses that flank the lateral sides of the tunica albuginea to join up with the bulbo-urethral artery on the lateral sides of the CS.
Conclusion
The vascularization of the CC is not carried out solely by the cavernous arteries. The cavernospongiosum shunts have anastomoses between the cavernous artery and the urethral artery. In four of the five cases studied, we found an accessory cavernous artery arising from the dorsal artery, which suggests the possible use of collateral vessels in case of injury to the accessory pudendal arteries during a radical prostatectomy.
References
- Alsaid B, Bessede T, Karam I, et al. Coexistence of adrenergic and cholinergic nerves in the inferior hypogastric plexus: anatomical and immunohistochemical study with 3D reconstruction in human male fetus. J Anat. 2009;214:645–654. doi: 10.1111/j.1469-7580.2009.01071.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benoit G, Droupy S, Quillard J, et al. Supra and infralevator neurovascular pathways to the penile CC. J Anat. 1999;195(Pt 4):605–615. doi: 10.1046/j.1469-7580.1999.19540605.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bookstein JJ, Lang EV. Penile magnification pharmacoarteriography: details of intrapenile arterial anatomy. Am J Roentgenol. 1987;148:883–888. doi: 10.2214/ajr.148.5.883. [DOI] [PubMed] [Google Scholar]
- Conti G. The erection of the human penis and its morphologico-vascular basis. Acta Anat (Basel) 1952;14:217–262. [PubMed] [Google Scholar]
- Costabile RA, Spevak M, Fishman IJ, et al. Efficacy and safety of transurethral alprostadil in patients with erectile dysfunction following radical prostatectomy. J Urol. 1998;160:1325–1328. [PubMed] [Google Scholar]
- Coursey JW, Morey AF, McAninch JW, et al. Erectile function after anterior urethroplasty. J Urol. 2001;166:2273–2276. [PubMed] [Google Scholar]
- Donnellan SM, Duncan HJ, MacGregor RJ, et al. Prospective assessment of incontinence after radical retropubic prostatectomy: objective and subjective analysis. Urology. 1997;49:225–230. doi: 10.1016/S0090-4295(96)00451-7. [DOI] [PubMed] [Google Scholar]
- Droupy S, Benoit G, Giuliano F, et al. Penile arteries in humans. Origin – distribution – variations. Surg Radiol Anat. 1997;19:161–167. doi: 10.1007/BF01627967. [DOI] [PubMed] [Google Scholar]
- Droupy S, Giuliano F, Jardin A, et al. Cavernospongious shunts: anatomical study of intrapenile vascular pathways. Eur Urol. 1999a;36:123–128. doi: 10.1159/000067983. [DOI] [PubMed] [Google Scholar]
- Droupy S, Hessel A, Benoit G, et al. Assessment of the functional role of accessory pudendal arteries in erection by transrectal color Doppler ultrasound. J Urol. 1999b;162:1987–1991. doi: 10.1016/S0022-5347(05)68084-6. [DOI] [PubMed] [Google Scholar]
- Erdogru T, Kaplancan T, Aker O, et al. Cavernosal arterial anatomic variations and its effect on penile hemodynamic status. Eur J Ultrasound. 2001;14:141–148. doi: 10.1016/s0929-8266(01)00155-0. [DOI] [PubMed] [Google Scholar]
- Juskiewenski SVP, Moscovici J, Hammoudi SBE. A study of the arterial blood supply to the penis. Anat Clin. 1982;4:101–107. [Google Scholar]
- Lunacek A, Schwentner C, Fritsch H, et al. Anatomical radical retropubic prostatectomy: ‘curtain dissection’ of the neurovascular bundle. BJU Int. 2005;95:1226–1231. doi: 10.1111/j.1464-410X.2005.05510.x. [DOI] [PubMed] [Google Scholar]
- Montorsi F, Guazzoni G, Strambi LF, et al. Recovery of spontaneous erectile function after nerve-sparing radical retropubic prostatectomy with and without early intracavernous injections of alprostadil: results of a prospective, randomized trial. J Urol. 1997;158:1408–1410. [PubMed] [Google Scholar]
- Mundy AR. Results and complications of urethroplasty and its future. Br J Urol. 1993;71:322–325. doi: 10.1111/j.1464-410x.1993.tb15951.x. [DOI] [PubMed] [Google Scholar]
- Murray AM. Arteriarum corporis humani. Edman. 1798:85–88. [Google Scholar]
- Patel VR, Schatloff O, Chauhan S, et al. The role of the prostatic vasculature as a landmark for nerve sparing during robot-assisted radical prostatectomy. Eur Urol. 2012;61:571–576. doi: 10.1016/j.eururo.2011.12.047. [DOI] [PubMed] [Google Scholar]
- Quinlan DM, Epstein JI, Carter BS, et al. Sexual function following radical prostatectomy: influence of preservation of neurovascular bundles. J Urol. 1991;145:998–1002. doi: 10.1016/s0022-5347(17)38512-9. [DOI] [PubMed] [Google Scholar]
- Secin FP, Touijer K, Mulhall J, et al. Anatomy and preservation of accessory pudendal arteries in laparoscopic radical prostatectomy. Eur Urol. 2007;51:1229–1235. doi: 10.1016/j.eururo.2006.08.030. [DOI] [PubMed] [Google Scholar]
- Soemmering STHS. De corpori humani fabrica. Tomus Quintus de Angiologia. Varrentrapp et Wenner. 1880:278–282. [Google Scholar]
- Wagner G, Willis EA, Bro-Rasmussen F, et al. New theory on the mechanism of erection involving hitherto undescribed vessels. Lancet. 1982;1:416–418. doi: 10.1016/s0140-6736(82)91618-x. [DOI] [PubMed] [Google Scholar]


