Abstract
Salmonella is an enteric pathogen that causes a range of diseases in humans. Non-typhoidal Salmonella (NTS) serovars such as Salmonella enterica serovar Typhimurium generally cause a self-limiting gastroenteritis whereas typhoidal serovars cause a systemic disease, typhoid fever. However, S. Typhimurium isolates within the multi-locus sequence type ST313 have emerged in sub-Saharan Africa as a major cause of bacteremia in humans. The S. Typhimurium ST313 lineage is phylogenetically distinct from classical S. Typhimurium lineages, such as ST19, that cause zoonotic gastroenteritis worldwide. Previous studies have shown that the ST313 lineage has undergone genome degradation when compared to the ST19 lineage, similar to that observed for typhoidal serovars. Currently, little is known about phenotypic differences between ST313 isolates and other NTS isolates. We find that representative ST313 isolates invade non-phagocytic cells less efficiently than the classical ST19 isolates that are more commonly associated with gastroenteritis. In addition, ST313 isolates induce less Caspase-1-dependent macrophage death and IL-1β release than ST19 isolates. ST313 isolates also express relatively lower levels of mRNA of the genes encoding the SPI-1 effector sopE2 and the flagellin, fliC, providing possible explanations for the decrease in invasion and inflammasome activation. The ST313 isolates have invasion and inflammatory phenotypes that are intermediate; more invasive and inflammatory than Salmonella enterica serovar Typhi and less than ST19 isolates associated with gastroenteritis. This suggests that both phenotypically and at the genomic level ST313 isolates are evolving signatures that facilitate a systemic lifestyle in humans.
Keywords: iNTS, immune evasion, genomic degradation
Isolates of Salmonella Typhimurium that cause bacteremia in humans have evolved to be less inflammatory.
Graphical Abstract Figure.
Isolates of Salmonella Typhimurium that cause bacteremia in humans have evolved to be less inflammatory.
Salmonella enterica is a Gram-negative, facultative intracellular bacterial species. More than 2300 serovars of S. enterica can cause disease in humans (Porwollik et al., 2004). The serovars are classically assigned into two categories based on the human disease they cause: typhoidal and non-typhoidal Salmonella (NTS) (de Jong et al., 2012). Typhoidal serovars (e.g. Salmonella enterica serovar Typhi and Paratyphi A) are human restricted and cause the systemic disease typhoid fever (Selander et al., 1990; Parry et al., 2002; Raffatellu et al., 2008b; Nuccio and Bäumler 2014). The NTS serovars such as Salmonella enterica serovar Typhimurium (S. Typhimurium) generally cause a self-limiting gastroenteritis in humans, which is usually zoonotic in origin (Zhang et al., 2003; de Jong et al., 2012). Occasionally, NTS serovars cause bacteremia, particularly in immunocompromised individuals (de Jong et al., 2012). Beyond this paradigm, certain animal host-adapted S. enterica serovars, such as Dublin and Choleraesuis, are still capable of infecting humans and are more often associated with bacteremia and focal extraintestinal infections than gastroenteritis in humans (Fang and Fierer 1991; Werner, Humphrey and Kamei 1979; Nuccio and Bäumler 2014).
After ingestion, Salmonella reaches the small intestine, where it crosses the epithelial barrier either through invasion of intestinal epithelial and M cells or is taken up by CD18+ immune cells (Vazquez-Torres et al., 1999; Monack et al., 2004). After crossing the epithelium, Salmonella interacts with macrophages and other immune cells (Monack et al., 2004). In humans, typhoidal serovars cause limited gastrointestinal inflammation and disseminate throughout the host (de Jong et al., 2012). Salmonella Typhi replicates and persists in macrophages at systemic sites, such as the liver and lymph nodes (Raffatellu et al., 2008b). In contrast, infections of humans with NTS predominantly results in substantial gastrointestinal inflammation and the pathogen is largely restricted to the gut and gut-associated lymphoid tissues (Harris 1972; Raffatellu et al., 2008b).
In sub-Saharan Africa, the distinctions between the disease syndromes caused by NTS and typhoidal serovars are less clear. Indeed, NTS serovars, particularly S. Typhimurium, are a common cause of systemic disease including febrile bacteremia, septicemia and meningitis (Walsh et al., 2000; Gordon et al., 2008; Kingsley et al., 2009; Morpeth, Ramadhani and Crump 2009; Sigaúque et al., 2009; Gordon 2011; Feasey et al., 2012; Okoro et al., 2012). These systemic NTS infections are often associated with malaria infection, anemia and malnutrition in children and HIV infections in adults, with a mortality rate of 20–45% being reported (Walsh et al., 2000; Gordon et al., 2008; Mackenzie et al., 2010; MacLennan et al., 2010). Phylogenetic analysis of S. Typhimurium isolates that cause systemic disease in sub-Saharan Africa revealed that they predominantly belong to a specific multi-locus sequence type, ST313, rarely found outside of sub-Saharan Africa (Kingsley et al., 2009; Okoro et al., 2012). In contrast, many common gastroenteritis-causing isolates of S. typhimurium belong to sequence type ST19 (Kingsley et al., 2009; Okoro et al., 2012).
Genomic analysis of ST313 NTS isolates reveals that this lineage possesses a distinct prophage repertoire, as well as a unique Tn21 element encoding multiple resistance genes on the virulence-associated plasmid (Kingsley et al., 2009; Okoro et al., 2012). There is also evidence of a significant level of genome degradation in the ST313 lineage, above that normally seen in ST19 isolates (Kingsley et al., 2009). It is notable that some of the degraded genes are also non-functional in the typhoidal serovars S. Typhi and S. Paratyphi A (Kingsley et al., 2009). Based on this genomic data, it has been speculated that the ST313 NTS isolates that cause systemic disease are evolving along similar lines to typhoidal serovars to become more host adapted. However, there is currently a lack of phenotypic data to support this hypothesis. Phenotypic characterizations of S. Typhimurium ST313 isolates have focused on dissecting host antibody responses and the impact of HIV and malaria coinfections (Gondwe et al., 2010; MacLennan et al., 2010; Schreiber et al., 2011; Rondini et al., 2013; Lokken et al., 2014; Mooney et al., 2014; Siggins et al., 2014). Most studies do not directly compare phenotypic differences between ST313 NTS and other NTS. Indeed, little is known about how ST313 S. Typhimurium interact with host cells or if they differ from other S. Typhimurium lineages in this regard. In this work, we compare the ability of ST313 isolates to invade epithelial cells and to activate the inflammasome with that of classical gastroenteritis-causing ST19 isolates.
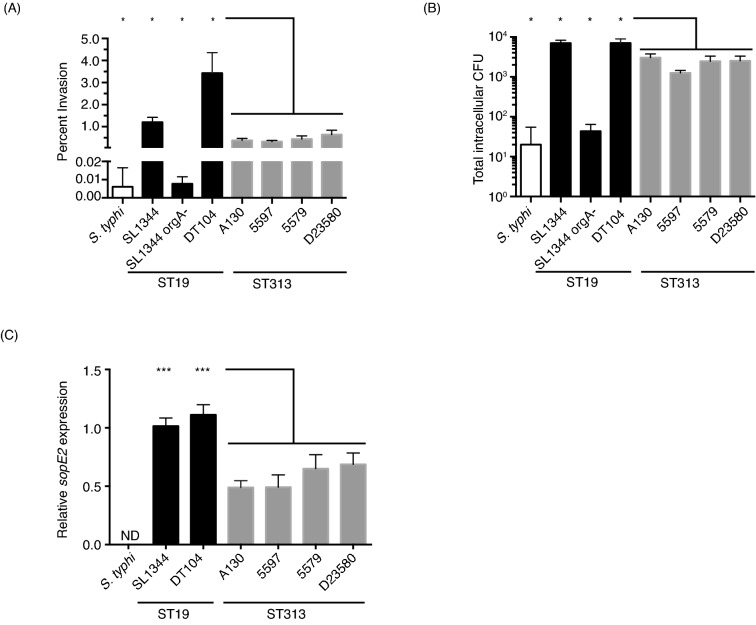
Since ST313 NTS routinely cause disseminated disease, we hypothesized that ST313 NTS are more invasive than ST19 isolates, which would allow them to breach the gut epithelium more efficiently, leading to dissemination to systemic sites. Salmonella can actively invade mammalian cells by injecting effector proteins through the Salmonella Pathogenicity Island 1 (SPI-1) type III secretion system (T3SS) into host cells (García-del Portillo and Finlay 1994; Mirold et al., 2001; Zhou and Galán 2001; Raffatellu et al., 2004). To test this hypothesis, we compared the abilities of various Salmonella isolates to invade the non-phagocytic, epithelial HeLa cell line. Our panel of Salmonella isolates included four different NTS ST313 isolates (A130, 5597, 5579 and D23580), two S. Typhimurium ST19 isolates that cause gastroenteritis (DT104 ATCC 700408 and SL1344) and a S. Typhi isolate (Ty2). The DT104 lineage is associated with a recent global epidemic of gastroenteritis (Threlfall, Ward and Rowe 1998), and SL1344 is a commonly used laboratory strain. In addition, we included an SL1344 orgA mutant (BJ66 orgA::tn5), which is unable to form a functional SPI-1 T3SS, and therefore cannot actively invade cells (Jones and Falkow 1994; Klein, Fahlen and Jones 2000; Sukhan et al., 2001). We used a gentamicin protection assay to measure invasion of HeLa cells by quantifying intracellular Salmonella at 2 h post-infection (Isberg and Falkow 1985). To induce the expression of SPI-1 genes and increase invasion rates, Salmonella isolates were subcultured into fresh media and incubated standing at 37°C before infecting cells (Lee and Falkow 1990; Bajaj et al., 1996; Ellermeier, Ellermeier and Slauch 2005). Salmonella were centrifuged onto the HeLa cells in order to synchronize infection and overcome any potential differences in motility (Hoffmann et al., 2010). The wild-type ST19 isolates, SL1344 and DT104, exhibit 1.20 ± 0.22 and 3.43 ± 0.93% invasion, respectively. In contrast the four ST313, S. Typhimurium isolates were significantly less invasive (0.32 ± 0.05 to 0.65 ± 0.20% invasion; Fig. 1a). This represents approximately a 5–10-fold decrease in invasiveness compared to the epidemic isolate DT104. In contrast, the SL1344 orgA mutant strain and S. Typhi have very low levels of invasion (<0.01% invasion; Fig. 1a and b). Similar results were obtained when the total number of intracellular Salmonella colony forming units (CFU) was measured, with significantly lower levels of intracellular ST313 NTS bacteria compared to the ST19 isolates (Fig. 1b). While different culture conditions, Salmonella strains and invasion assay procedures cause variability in the actual percent invasion published by different research groups, significantly lower levels of invasion of S. Typhi compared to S. Typhimurium have been reported in a variety of polarized (T84, Caco-2) and non-polarized (Int-407, Caco-2 and HeLa) epithelial cell lines (Galán and Curtiss 1991; Mills and Finlay 1994; Bishop et al., 2008). Taken together, our results indicate that the ability of ST313 NTS isolates to invade non-phagocytic cells is intermediate between the human-restricted S. Typhi and the zoonotic ST19 NTS isolates (Fig. 1).
Figure 1.
ST313 S. Typhimurium isolates are less invasive than ST19 S. Typhimurium isolates. (A and B) HeLa cells were seeded into 24-well plates at 200,000 cells per well. Overnight cultures of each Salmonella isolate were diluted 1:50 and subcultured standing for 3–4 h. Salmonella isolates were centrifuged onto HeLa cells at a multiplicity of infection of 10 and allowed to infect for 30 min. Cells were washed and given media containing 100 ug mL−1 gentamicin for 1.5 h. Then HeLa cells were washed, lysed and intracellular bacteria were enumerated by plating. Invasion is quantified as percent invasion over the initial input (A) and total Salmonella CFU recovered from the HeLa cells (B). Bars represent the mean and standard deviation for each isolate. Experiments were repeated three times, and data shown is a representative experiment (A and B). (C) Overnight cultures were diluted 1:50 in LB broth and subcultured standing for 3–4 h. Culture was placed in RNAprotect (Qiagen) and then RNA was extracted using hot phenol–chloroform and then ethanol precipitated. The RNeasy kit (Qiagen) was used to clean up and on-column Dnase treat the RNA. Then RNA was subjected to a second round of DNase treatment using the Turbo DNA-free kit (Invitrogen) and made into cDNA using Superscript III first-strand synthesis kit (Invitrogen). Primers were designed using Primer3Plus. All qRT-PCR was performed using Applied Biosystems 7300 real-time PCR system and FastStart SYBR Green Master Mix with Rox (Roche). Data presented is fold change over the abundance of sopE2 mRNA recovered from the SL1344 after standardization to the housekeeping gene gmk. Experiments were repeated three times and bars represent the mean with standard deviation from the data from all three experiments. (A–C) White bar represents S. Typhi strain Ty2, black bars represent ST19 NTS and gray bars represent ST313. Statistics were calculated using student's t-test with welch's correction in GraphPad Prism. P-values are given as the lowest significance level between the control isolate and the individual four ST313 isolates. *P ≤ 0.05, **P ≤ 0.01 and ***P ≤ 0.001.
The lower levels of host cell invasion exhibited by ST313 isolates compared to ST19 isolates is consistent with lower levels of the expression of at least one SPI-1 T3SS effector in ST313 isolates. To test this notion, we measured gene expression of the effectors involved in active invasion in the different isolates. Salmonella injects four effectors to actively invade mammalian cells: SipA, SopB, SopE2 and SopE (Zhou and Galán 2001; Raffatellu et al., 2004). While SipA and SopB are present in all ST19, ST313 and S. Typhi isolates, the presence of SopE and SopE2 is variable. SL1344 has a functional SopE, while DT104 does not (Mirold et al., 1999; Hopkins and Threlfall 2004). Previous studies indicated that the genomes of ST313 and ST19 isolates contain the gene encoding SopE2, but lack a functional SopE (Kingsley et al., 2009). In contrast, sopE is present in the genome of S. Typhi Ty2, but it does not contain an intact sopE2 gene (Bakshi et al., 2000; Stender et al., 2000). We measured the expression of sopE2, sipA and sopB under SPI-1-inducing conditions by qRT-PCR in the NTS isolates used in this study (Fig. 1c, data not shown, Table S2, Supporting Information; Lee and Falkow 1990; Bajaj et al., 1996; Ellermeier et al., 2005). We found no significant differences in the levels of sipA and sopB mRNA between ST19 and ST313 lineages (data not shown). However, importantly the level of sopE2 mRNA differed between ST19 and ST313 isolates (Fig. 1c). ST313 NTS isolates have significantly lower levels of sopE2 mRNA compared to the ST19 NTS isolates (1.6–2.3-fold lower than DT104; Fig. 1c). To investigate possible explanations for the reduced level of sopE2 gene expression, we analyzed the genomes of our ST313 and ST19 isolates for SNPs in known regulators of sopE2 gene expression (Table S3, Supporting Information). We did not find any obvious SNPs that could be responsible for this reduction. Interestingly the S. Typhi regulatory protein TviA downregulates the expression of SopE, which is 69% identical to SopE2 and is also a guanine nucleotide exchange factor (Friebel et al., 2001; Winter et al., 2014). Taken together, our data suggest that lower levels of expression of the invasion-inducing SPI-1 effector protein SopE2 is likely one factor responsible for the decrease in invasion by ST313 isolates.
It is perhaps counterintuitive that Salmonella isolates that cause more disseminated disease, such as S. Typhi and ST313 NTS, invade non-phagocytic cells at lower levels and have lower gene expression of key invasion effectors SopE and SopE2, respectively (Winter et al., 2014). A potential explanation for this could be that higher amounts of cellular invasion causes greater activation of the host immune response which limits dissemination of Salmonella beyond the gut and gut-associated lymphoid tissue. One of the main distinctions between typical S. Typhimurium and S. Typhi infections is that S. Typhimurium causes substantial gut inflammation while S. Typhi does not (Harris 1972; Raffatellu et al., 2008b; de Jong et al., 2012). Effectors that promote invasion, like SopE2, usually also contribute to gut inflammation in mouse and bovine animal models (Zhang et al., 2002, 2003; Raffatellu et al., 2004). Additionally, intracellular Salmonella are detected by immune surveillance systems, such as the nodosome and the inflammasome, leading to increased inflammation (Broz and Monack 2011; Keestra, Winter and Auburger 2013; Keestra and Baumler 2014).Thus, increased invasion could cause more immune activation in a variety of ways and consequently lead to host restriction of bacterial dissemination.
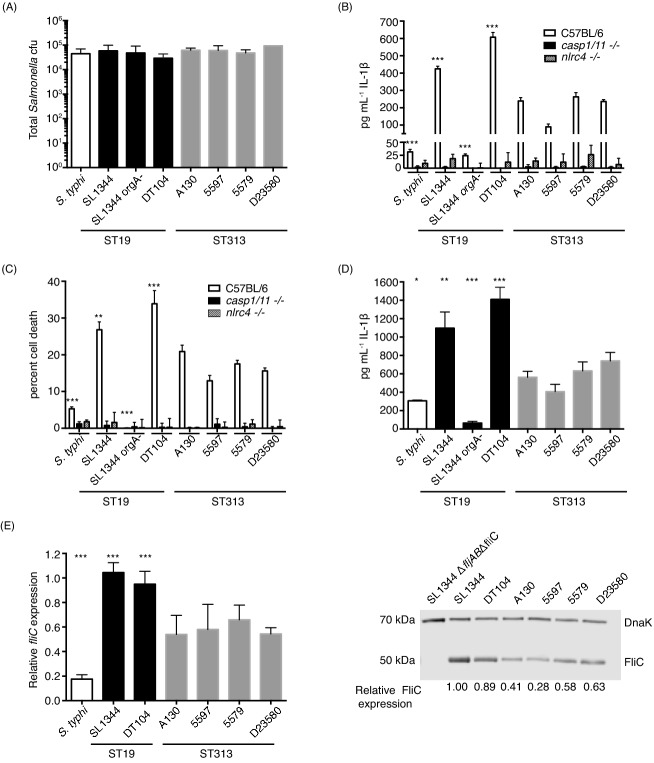
To delve more into potential differences in immune activation by the Salmonella isolates, we examined their interaction with macrophages. Macrophages are an important systemic niche for Salmonella as well as a vital part of host innate immune defenses. A key immune surveillance system for macrophages is the inflammasome, which senses cytosolic pathogen-associated molecular patterns (PAMPs) or danger signals (Broz and Monack 2011; Ng, Kortmann and Monack 2013). Host cytosolic sensors and adaptor proteins detect these signals, then recruit and activate caspase-1 or 11, which ultimately leads to the release of cytokines (IL-1β and IL-18) and sometimes pyroptotic cell death (Broz and Monack 2011; Ng et al., 2013). To characterize the interactions between the Salmonella isolates and macrophages, we infected primary bone marrow-derived macrophages (BMDM) from wild-type C57BL/6 mice. There were no significant differences between any of the isolates in total intracellular CFU at 1 h post-infection in BMDM, which is consistent with the primary macrophages being highly phagocytic (Fig. 2a). This allowed us to directly compare the levels of inflammasome activation between the various Salmonella isolates.
Figure 2.
ST313 isolates induce less NLRC4 inflammasome activation in macrophages than ST19 isolates. Bone marrow-derived C57BL/6 macrophages were differentiated for 5 days in DMEM (invitrogen) with 10% FBS (Thermo Fisher Scientific), 20% MCSF (L929 cell supernatant) and 10 mm HEPES (Invitrogen) (A–C). (A) One day before infection macrophages were seeded into 24 well at densities of 2.5 × 105 cells well−1 in DMEM with 10% FBS, 10% MSCF and 10 mM HEPES and prestimulated with 0.1 μg mL−1 LPS (invitrogen) for 16 h. Overnight cultures of each Salmonella isolate were diluted 1:50 and subcultured standing for 3–4 h. Salmonella isolates were centrifuged onto macrophages at a multiplicity of infection of 10 and allowed to infect for 1 h. Macrophages were washed, lysed and intracellular Salmonella were enumerated by plating. Total intracellular CFU of Salmonella is presented in (A). Bars represent the mean and standard deviation for each isolate. White bar represents S. typhi strain Ty2, black bars represent ST19 NTS isolates and gray bars represent ST313 NTS isolates. (B and C) C57BL/6, Casp1/11−/− or Nlrc4−/− bone marrow-derived macrophages were differentiated as above. One day before infection macrophages were seeded into 96-well plates at densities of 5 × 104 cells well−1 in DMEM with 10% FBS, 10% MSCF and 10 mM HEPES and prestimulated with 0.1 μg mL−1 LPS (invitrogen) for 16 h. Overnight cultures of each Salmonella isolate were diluted 1:50 and subcultured standing for 3–4 h. Salmonella isolates were centrifuged onto macrophages at a multiplicity of infection of 10 (B) or 20 (C). One hour post-infection, supernatants were collected and release of IL-1β (B) or lactose dehydrogenase (C) was quantified. (B) Pg ml−1 IL-1β released into the supernatant in response to infection by C57BL/6J, Casp1/11−/− or Nlrc4−/− macrophages was quantified by IL-1β ELISA (R&D Biosystems). (C) The amount of cell death in response to infection was measured using the Cytox96 non-radioactive cytoxicity kit (Promega) measuring LDH release into the supernatant compared to an uninfected total lysis control for by C57BL/6J, Casp1/11−/− or Nlrc4−/− macrophages. (B and C) Bars represent the mean and standard deviation for each isolate. White, black and hatched bars represent infection of C57BL/6, Casp1/11−/− or Nlrc4−/−, respectively. For panels (B) and (C), differences between all isolates infecting WT C57BL/6J macrophages and Casp1/11−/− or Nlrc4−/− macrophages were significant at P < 0.001, except for infection with SL1344 orgA− in C which was not significantly different. D. Human monocytic cell line U937 cells were differentiated into macrophages for 2 days with 100 nM PMA (Sigma) in RPMI supplemented with 10% FBS (Thermo Fisher Scientific). One day before infection U937 macrophages were seeded into 96 well at densities of 5.0 × 104 cells well−1 in DMEM with 10% FBS, 1 0% MSCF and 10 mM HEPES and prestimulated with 0.1 ug ml−1 LPS (invitrogen) for 16 h. Overnight cultures of each Salmonella isolate were diluted 1:50 and subcultured standing for 3–4 h. Salmonella isolates were centrifuged onto macrophages at a multiplicity of infection of 10 and allowed to infect for 1 h. One hour post-infection, supernatants were collected and release of IL-1β was quantified by ELISA (eBioscience). (A–D) Experiments were repeated at least three times, and data shown are from a representative experiment. (E) Overnight cultures were diluted 1:50 in LB broth and subcultured standing for 3–4 h. Culture was placed in RNAprotect (Qiagen) and then RNA was extracted using hot phenol–chloroform and then ethanol precipitated. The RNeasy kit (Qiagen) was used to clean-up and on-column Dnase treat the RNA. Then RNA was subjected to a second round of DNase treatment using the Turbo DNA-free kit (Invitrogen) and made into cDNA using Superscript III first-strand synthesis kit (Invitrogen). Primers were designed using Primer3Plus. All qRT-PCR was performed using Applied Biosystems 7300 real-time PCR system and FastStart SYBR Green Master Mix with Rox (Roche). Data presented is fold change over the abundance of fliC mRNA recovered from the SL1344 after standardization to the housekeeping gene gmk. Experiments were repeated three times and bars represent the mean with standard deviation from the data from all three experiments. White bar represents S. typhi strain TY2, black bars represent ST19 NTS isolates and gray bars represent ST313 NTS isolates. (A–E) Statistics were calculated using student's t-test with welch's correction in GraphPad Prism. P-values are given as the lowest significance level between the control isolate and the individual four ST313 isolates. *P ≤ 0.05, **P ≤ 0.01 and ***P ≤ 0.001. (F) Overnight cultures were diluted 1:50 in LB broth and subcultured standing for 3–4 h. Cells were lysed and proteins were separated by SDS-PAGE. Western blots were performed using anti-S. Typhimurium Flagellin FliC antibody (Invivogen) diluted 1:3750 and anti-DnaK antibody clone 8E2/2 (Enzo Life Sciences) at 1:5000. Protein levels were visualized and quantified using Licor Odyssey system. FliC levels are presented as fold change relative to SL1344 protein levels after normalization to the housekeeping protein DnaK expression. The experiment was repeated twice and data shown are from a representative experiment.
We detected inflammasome activation induced by each of the Salmonella isolates by quantifying cell death and IL-1β release after 1 h of infection of primary murine BMDM. As expected, the wild-type ST19 S. Typhimurium isolates induced high levels of IL-1β secretion in BMDM (Fig. 2b). The ST313 S. Typhimurium isolates induced intermediate levels of IL-1β secretion (Fig. 2b). In contrast, S. Typhi and the SL1344 orgA mutant induced relatively little IL-1β secretion (Fig. 2b). The levels of macrophage death following Salmonella infection showed similar trends to IL-1β release (Fig. 2c). SL1344 and DT104 induced significantly higher levels of macrophage death compared to infections with the ST313 isolates (Fig. 2c). Salmonella Typhi and SL1344 orgA mutant caused very little macrophage death (Fig. 2c). Taken together, our results indicate that the ST313 NTS isolates induce intermediate levels of proinflammatory cytokine secretion and macrophage death compared to the zoonotic ST19 NTS isolates and fully human-adapted S. Typhi.
To confirm that the cytokine release and macrophage death were due to inflammasome activation, we infected Casp1/11−/− and Nlrc4−/− macrophages and measured the amount IL-1β and cell death at 1 h post-infection (Broz and Monack 2011). It has been shown previously that when Salmonella are grown under SPI-1-inducing conditions, caspase-1 is rapidly activated and that this activation is dependent on the intracellular sensor NLRC4 (Miao et al., 2006; Broz et al., 2010). The activation of an NLRC4 inflammasome is dependent on the bacteria having an intact SPI-1 T3SS which delivers flagellin and components of the T3SS apparatus (PrgJ and PrgI) into the host cytosol ( Miao et al., 2006, 2010; Broz et al., 2010). Indeed, none of the Salmonella isolates induced significant levels of IL-1β secretion or cell death in the Casp1/11−/− or Nlrc4−/− deficient macrophages (Fig. 2b and c). These results indicate that the cytokine release and cell death induced by the isolates of Salmonella tested in this study are dependent on the NLRC4 inflammasome, and that ST313 S. Typhimurium induce NLRC4 inflammasome activation at a level that is, once again, between the levels induced by human-adapted S. Typhi and zoonotic ST19 S. Typhimurium.
To determine whether inflammasome activation induced by the different Salmonella isolates is similar in human macrophages, we infected differentiated human U937 macrophages. Inflammasome activation in U937 cells can be quantified robustly by measuring IL-1β release (Bryan et al., 2009). Infections of human macrophages with our panel of Salmonella isolates yielded a similar pattern of IL-1β release to that observed in murine macrophages. As expected, the ST19 isolates, SL1344 and DT104, elicited high levels of IL-1β secretion while S. Typhi and the SL1344 orgA mutant caused very low levels of cytokine release (Fig. 2d). Again, ST313 isolates induced intermediate levels of IL-1β release, which was significantly lower than the amount induced by ST19 isolates, but higher than the levels induced by S. Typhi (Fig. 2d). All together, our results suggest that ST313 S. Typhimurium induce an intermediate level of NLRC4 inflammasome activation in both human and mouse macrophages.
The NLRC4 inflammasome recognizes components of the SPI-1 T3SS (PrgJ and PrgI) and flagellin (Miao et al., 2006, 2010). During the first hour post-infection with S. Typhimurium SL1344, flagellin causes the majority of NLRC4 inflammasome activation (Franchi et al., 2006; Miao et al., 2006; Broz et al., 2010). The reduced levels of proinflammatory cytokine secretion and macrophage death induced by ST313 isolates compared to ST19 isolates could potentially be due to lower levels of flagellin in ST313 isolates. To test this, we measured expression of the gene that encodes the flagellin monomer, fliC, under SPI-1-inducing conditions by the Salmonella isolates (Lee and Falkow 1990; Bajaj et al., 1996; Ellermeier et al., 2005).We found that fliC expression was significantly lower in ST313 S. Typhimurium compared to ST19 S. Typhimurium (1.8–2.6-fold lower than DT104; Fig. 2e). FliC mRNA expression by S. Typhi was significantly lower than that of ST313 isolates (Fig. 2e). Thus, ST313 isolates express levels of fliC mRNA that is intermediate between ST19 isolates and S.Typhi. We next examined the genomes of ST19 and ST313 isolates for SNPs in known fliC regulators, but did not detect any SNPs that could explain the difference (Table S3, Supporting Information). Lower levels of fliC gene expression in ST313 compared to ST19 isolates suggest that the ST313 isolates produce less flagellin. Indeed, FliC protein levels during SPI-1-inducing conditions showed similar trends to the qRT-PCR data, with protein levels in ST313 isolates ranging from 30 to 70% of those seen in SL1344 (Fig. 2f). No FliC was detected in a control SL1344 mutant lacking fliC (SL1344 ΔfljABΔfliC), confirming that our staining was specific to FliC (Fig. 2f). Salmonella Typhi FliC levels are not shown because the antibody did not recognize the S. Typhi flagellin. Since flagellin causes the majority of early inflammasome activation in macrophages (Franchi et al., 2006; Miao et al., 2006; Broz et al., 2010), lower levels of FliC protein in ST313 isolates likely accounts for the decrease in NLRC4 inflammasome activation by those isolates.
In summary, ST313 isolates of S. Typhimurium are less invasive and less inflammatory compared to other S. Typhimurium isolates. However, the ST313 isolates invade non-phagocytic cells more efficiently and stimulate more inflammasome activation than S. Typhi Ty2. Lower levels of cell invasion and inflammasome activation are likely due to lower levels of mRNA expression of the genes-encoding sopE2 and flagellin (fliC), respectively. Interestingly, it is known that SopE2 and flagellin both induce the release of IL-8, an important neutrophil chemoattractant, in epithelial cells (Gewirtz et al., 2001; Huang et al., 2004). Classically, during human infections NTS induce massive influx of neutrophils into the gut lumen, while in contrast S. Typhi induces very little neutrophil transmigration (Gal-Mor, Boyle and Grassl 2014). The lower levels of sopE2 and fliC in ST313 S. Typhimurium compared to ST19 S. Typhimurium isolates could result in lower amounts of IL-8 and neutrophil influx during ST313 infection and represent another intermediate phenotype of ST313 isolates between ST19 isolates and S. Typhi. Immune evasion by S. Typhi reduces host inflammatory responses, which may allow it to effectively disseminate to systemic tissues, establish a persistent infection within a host and eventually transmit to a new host (Wangdi, Winter and Baumler 2012). We have shown here that at a cellular level, the ST313 NTS isolates are causing an infection that also displays aspects of immune evasion by downregulating fliC and sopE2 expression compared to ST19 NTS isolates.
This study adds to a growing body of work indicating that dampening flagellin expression and inflammation helps Salmonella disseminate from the gut. Previous studies have shown that the S. Typhi regulator TviA downregulates the expression of SPI-1 genes and flagellin (Winter et al.,, 2009, 2014). Introduction of a deletion or TviA repression of flagellin in S. Typhimurium causes an increase in dissemination to the spleen but does not increase the bacterial load in gut tissues in mice and chicks (Winter et al., 2010; Atif et al., 2014). In addition, T cell responses against flagellin help restrict dissemination of Salmonella expressing flagella in the mouse model (Atif et al., 2014). This indicates that SPI-1 and flagellin repression by TviA can help Salmonella bypass host restriction of dissemination to systemic sites (Winter et al., 2010; Atif et al., 2014). Although ST313 NTS isolates lack the gene that encodes TviA, it appears that these isolates elicit less of an immune response by expressing lower levels of genes that are recognized by mammalian cytosolic innate immune receptors (Miao et al., 2006; Müller et al., 2009; Broz et al., 2010; Hoffmann et al., 2010). This common theme of flagellin repression or deletion has been described in other host-adapted serovars, such as S. enterica serovar Gallinarum in chickens and S. enterica serovar Dublin in cattle (Freitas Neto et al., 2013; Yim et al., 2014). Similarly, the S. Typhimurium DT2 clade, which is restricted to feral pigeons and causes a typhoid-like disease in these animals, downregulates the expression of flagellar and SPI-1 genes at 42°C, which is the approximate body temperature of pigeons (Kingsley et al., 2013). Thus, repressing flagella gene expression to reduce inflammation seems to be a common theme in Salmonella serovars that cause invasive disease and is potentially an important step for host adaptation (Winter et al., 2009, 2010, 2014; Freitas Neto et al., 2013; Kingsley et al., 2013; Yim et al., 2014).
It is worth noting that in sub-Saharan Africa a significant proportion of the population have comorbidities such as malnutrition, malarial infection and HIV that contribute to the amount of disseminated ST313 infections (Walsh et al., 2000; Raffatellu et al., 2008a; Schreiber et al., 2011; Lokken et al., 2014; Mooney et al., 2014). For example, SIV infection blunts the TH17 response causing defects in the intestinal barrier and increasing Salmonella dissemination in rhesus macaques (Raffatellu et al., 2008a). ST313 isolates may be adapting to cause systemic disease specifically in humans with these comorbidities or these comorbidities might form a more permissive niche that ST313 NTS that allows adaptation to cause disseminated disease in immunocompetent humans.
Epidemiologically, the ST313 NTS isolates also seem to be intermediate between NTS isolates that cause gastroenteritis and S. Typhi. Kariuki et al., found that NTS isolates that cause gastroenteritis have similar pulsed field gel electrophoresis (PFGE) types as isolates that cause bacteremia in Kenya, indicating that the same PFGE type commonly cause both forms of the disease (Kariuki et al., 2006). Although ST313 routinely cause systemic disease in humans, they are not host restricted and can infect chickens (Parsons et al., 2013). Interestingly, another study in Kenya showed a lack of clonal relationship between S. Typhimurium strain types from humans (symptomatic and asymptomatic) and those isolated from animals living in close contact (Kariuki 2006). Although ST313 NTS isolates are not restricted to humans, this suggests that they can persist and possibly even transmit between humans (Kariuki 2006). Studying how ST313 NTS differ from other NTS allows us the unique opportunity to see how these isolates of this lineage are able to cause disseminated human disease and potentially adapt to new niches.
SUPPLEMENTARY DATA
Supplementary data is available at FEMSPD online.
Acknowledgments
We would like to thank Stanley Falkow and members of the Monack lab for valuable comments. We would like to thank Jens Kortmann for allowing us to use his image in our graphical abstract.
FUNDING
SEC is funded by the Stanford Graduate Fellowship and National Science Foundation Graduate Research Fellowship. GD and CO were supported by The Wellcome Trust. CO is supported by a Branco-Weiss Fellowship. This study was supported by awards AI095396 and AI08972 from NIAID to D.M.
Conflict of interest statement. None declared.
REFERENCES
- Atif SM, Winter SE, Winter MG, et al. Salmonella enterica serovar Typhi impairs CD4 T cell responses by reducing antigen availability. Infect Immun. 2014;82:2247–54. doi: 10.1128/IAI.00020-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bajaj V, Lucas RL, Hwang C, et al. Co-ordinate regulation of Salmonella typhimurium invasion genes by environmental and regulatory factors is mediated by control of hilA expression. Mol Microbiol. 1996;22:703–14. doi: 10.1046/j.1365-2958.1996.d01-1718.x. [DOI] [PubMed] [Google Scholar]
- Bakshi CS, Singh VP, Wood MW, et al. Identification of SopE2, a Salmonella secreted protein which is highly homologous to SopE and involved in bacterial invasion of epithelial cells. J Bacteriol. 2000;182:2341–4. doi: 10.1128/jb.182.8.2341-2344.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop A, House D, Perkins T, et al. Interaction of Salmonella enterica serovar Typhi with cultured epithelial cells: roles of surface structures in adhesion and invasion. Microbiology. 2008;154:1914–26. doi: 10.1099/mic.0.2008/016998-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broz P, Monack DM. Molecular mechanisms of inflammasome activation during microbial infections. Immunol Rev. 2011;243:174–90. doi: 10.1111/j.1600-065X.2011.01041.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broz P, Newton K, Lamkanfi M, et al. Redundant roles for inflammasome receptors NLRP3 and NLRC4 in host defense against Salmonella. J Exp Med. 2010;207:1745–55. doi: 10.1084/jem.20100257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryan NB, Dorfleutner A, Rojanasakul Y, et al. Activation of inflammasomes requires intracellular redistribution of the apoptotic speck-like protein containing a caspase recruitment domain. J Immunol. 2009;182:3173–82. doi: 10.4049/jimmunol.0802367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Freitas Neto OC, Setta A, Imre A, et al. A flagellated motile Salmonella Gallinarum mutant (SG Fla+) elicits a pro-inflammatory response from avian epithelial cells and macrophages and is less virulent to chickens. Vet Microbiol. 2013;165:425–33. doi: 10.1016/j.vetmic.2013.04.015. [DOI] [PubMed] [Google Scholar]
- de Jong HK, Parry CM, van der Poll T, et al. Host-pathogen interaction in invasive Salmonellosis. PLoS Pathog. 2012;8:e1002933. doi: 10.1371/journal.ppat.1002933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellermeier CD, Ellermeier JR, Slauch JM. HilD, HilC and RtsA constitute a feed forward loop that controls expression of the SPI1 type three secretion system regulator hilA in Salmonella enterica serovar Typhimurium. Mol Microbiol. 2005;57:691–705. doi: 10.1111/j.1365-2958.2005.04737.x. [DOI] [PubMed] [Google Scholar]
- Fang FC, Fierer J. Human infection with Salmonella dublin. Medicine (Baltimore) 1991;70:198–207. doi: 10.1097/00005792-199105000-00004. [DOI] [PubMed] [Google Scholar]
- Feasey NA, Dougan G, Kingsley RA, et al. Invasive non-typhoidal salmonella disease: an emerging and neglected tropical disease in Africa. Lancet. 2012;379:2489–99. doi: 10.1016/S0140-6736(11)61752-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franchi L, Amer A, Body-Malapel M, et al. Cytosolic flagellin requires Ipaf for activation of caspase-1 and interleukin 1β in salmonella-infected macrophages. Nat Immunol. 2006;7:576–82. doi: 10.1038/ni1346. [DOI] [PubMed] [Google Scholar]
- Friebel A, Ilchmann H, Aepfelbacher M, et al. SopE and SopE2 from Salmonella typhimurium activate different sets of RhoGTPases of the host cell. J Biol Chem. 2001;276:34035–40. doi: 10.1074/jbc.M100609200. [DOI] [PubMed] [Google Scholar]
- Gal-Mor O, Boyle EC, Grassl GA. Same species, different diseases: how and why typhoidal and non-typhoidal Salmonella enterica serovars differ. Front Microbiol. 2014;5:391. doi: 10.3389/fmicb.2014.00391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galán JE, Curtiss R. Distribution of the invA, -B, -C, and -D genes of Salmonella typhimurium among other Salmonella serovars: invA mutants of Salmonella typhi are deficient for entry into mammalian cells. Infect Immun. 1991;59:2901–8. doi: 10.1128/iai.59.9.2901-2908.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- García-del Portillo F, Finlay BB. Salmonella invasion of nonphagocytic cells induces formation of macropinosomes in the host cell. Infect Immun. 1994;62:4641–5. doi: 10.1128/iai.62.10.4641-4645.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gewirtz AT, Navas TA, Lyons S, et al. Cutting edge: bacterial flagellin activates basolaterally expressed TLR5 to induce epithelial proinflammatory gene expression. J Immunol. 2001;167:1882–5. doi: 10.4049/jimmunol.167.4.1882. [DOI] [PubMed] [Google Scholar]
- Gondwe EN, Molyneux ME, Goodall M, et al. Importance of antibody and complement for oxidative burst and killing of invasive nontyphoidal Salmonella by blood cells in Africans. P Natl Acad Sci USA. 2010;107:3070–5. doi: 10.1073/pnas.0910497107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon MA. Invasive nontyphoidal Salmonella disease—epidemiology, pathogenesis and diagnosis. Curr Opin Infect Dis. 2011;24:484–9. doi: 10.1097/QCO.0b013e32834a9980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon MA, Graham SM, Walsh AL, et al. Epidemics of invasive Salmonella enterica serovar enteritidis and S. enterica serovar Typhimurium infection associated with multidrug resistance among adults and children in Malawi. Clin Infect Dis. 2008;46:963–9. doi: 10.1086/529146. [DOI] [PubMed] [Google Scholar]
- Harris JC. Fecal leukocytes in diarrheal illness. Ann Intern Med. 1972;76:697–703. doi: 10.7326/0003-4819-76-5-697. [DOI] [PubMed] [Google Scholar]
- Hoffmann C, Galle M, Dilling S, et al. In macrophages, caspase-1 activation by SopE and the type III secretion system-1 of S. Typhimurium can proceed in the absence of flagellin. PLoS ONE. 2010;5:e12477. doi: 10.1371/journal.pone.0012477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins KL, Threlfall EJ. Frequency and polymorphism of sopE in isolates of Salmonella enterica belonging to the ten most prevalent serotypes in England and Wales. J Med Microbiol. 2004;53:539–43. doi: 10.1099/jmm.0.05510-0. [DOI] [PubMed] [Google Scholar]
- Huang F-C, Werne A, Li Q, et al. Cooperative interactions between flagellin and SopE2 in the epithelial interleukin-8 response to Salmonella enterica serovar typhimurium infection. Infect Immun. 2004;72:5052–62. doi: 10.1128/IAI.72.9.5052-5062.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isberg RR, Falkow S. A single genetic locus encoded by Yersinia pseudotuberculosis permits invasion of cultured animal cells by Escherichia coli K-12. Nature. 1985;317:262–4. doi: 10.1038/317262a0. [DOI] [PubMed] [Google Scholar]
- Jones BD, Falkow S. Identification and characterization of a Salmonella typhimurium oxygen-regulated gene required for bacterial internalization. Infect Immun. 1994;62:3745–52. doi: 10.1128/iai.62.9.3745-3752.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kariuki S. Invasive multidrug-resistant non-typhoidal Salmonella infections in Africa: zoonotic or anthroponotic transmission? J Med Microbiol. 2006;55:585–91. doi: 10.1099/jmm.0.46375-0. [DOI] [PubMed] [Google Scholar]
- Kariuki S, Revathi G, Kariuki N, et al. Characterisation of community acquired non-typhoidal Salmonella from bacteraemia and diarrhoeal infections in children admitted to hospital in Nairobi, Kenya. BMC Microbiol. 2006;6:101. doi: 10.1186/1471-2180-6-101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keestra AM, Baumler AJ. Detection of enteric pathogens by the nodosome. Trends Immunol. 2014;35:123–30. doi: 10.1016/j.it.2013.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keestra AM, Winter MG, Auburger JJ. Manipulation of small Rho GTPases is a pathogen-induced process detected by NOD1. Nat Med. 2013;496:233–7. doi: 10.1038/nature12025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kingsley RA, Kay S, Connor T, et al. Genome and transcriptome adaptation accompanying emergence of the definitive type 2 host-restricted Salmonella enterica serovar Typhimurium pathovar. mBio. 2013;4:e00565-13. doi: 10.1128/mBio.00565-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kingsley RA, Msefula CL, Thomson NR, et al. Epidemic multiple drug resistant Salmonella Typhimurium causing invasive disease in sub-Saharan Africa have a distinct genotype. Genome Res. 2009;19:2279–87. doi: 10.1101/gr.091017.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein JR, Fahlen TF, Jones BD. Transcriptional organization and function of invasion genes within Salmonella enterica serovar typhimurium pathogenicity island 1, including the prgH, prgI, prgJ, prgK, orgA, orgB, and orgC genes. Infect Immun. 2000;68:3368–76. doi: 10.1128/iai.68.6.3368-3376.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee CA, Falkow S. The ability of Salmonella to enter mammalian cells is affected by bacterial growth state. P Natl Acad Sci USA. 1990;87:4304–8. doi: 10.1073/pnas.87.11.4304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lokken KL, Mooney JP, Butler BP, et al. Malaria parasite infection compromises control of concurrent systemic non-typhoidal Salmonella infection via IL-10-mediated alteration of myeloid cell function. PLoS Pathog. 2014;10:e1004049. doi: 10.1371/journal.ppat.1004049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackenzie G, Ceesay SJ, Hill PC, et al. A decline in the incidence of invasive non-typhoidal Salmonella infection in the Gambia temporally associated with a decline in malaria infection. PLoS One. 2010;5:e10568. doi: 10.1371/journal.pone.0010568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLennan CA, Gilchrist JJ, Gordon MA, et al. Dysregulated humoral immunity to nontyphoidal Salmonella in HIV-infected African adults. Science. 2010;328:508–12. doi: 10.1126/science.1180346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miao EA, Alpuche-Aranda CM, Dors M, et al. Cytoplasmic flagellin activates caspase-1 and secretion of interleukin 1β via Ipaf. Nat Immunol. 2006;7:569–75. doi: 10.1038/ni1344. [DOI] [PubMed] [Google Scholar]
- Miao EA, Mao DP, Yudkovsky N, et al. Innate immune detection of the type III secretion apparatus through the NLRC4 inflammasome. P Natl Acad Sci USA. 2010;107:3076–80. doi: 10.1073/pnas.0913087107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills SD, Finlay BB. Comparison of Salmonella typhi and Salmonella typhimurium invasion, intracellular growth and localization in cultured human epithelial cells. Microb Pathogenesis. 1994;17:409–23. doi: 10.1006/mpat.1994.1086. [DOI] [PubMed] [Google Scholar]
- Mirold S, Ehrbar K, Weissmuller A, et al. Salmonella host cell invasion emerged by acquisition of a mosaic of separate genetic elements, including Salmonella pathogenicity island 1 (SPI1), SPI5, and sopE2. J Bacteriol. 2001;183:2348–58. doi: 10.1128/JB.183.7.2348-2358.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirold S, Rabsch W, Rohde M, et al. Isolation of a temperate bacteriophage encoding the type III effector protein SopE from an epidemic Salmonella typhimurium strain. P Natl Acad Sci USA. 1999;96:9845–50. doi: 10.1073/pnas.96.17.9845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monack DM, Mueller A, Falkow S. Persistent bacterial infections: the interface of the pathogen and the host immune system. Nat Rev Microbiol. 2004;2:747–65. doi: 10.1038/nrmicro955. [DOI] [PubMed] [Google Scholar]
- Mooney JP, Butler BP, Lokken KL, et al. The mucosal inflammatory response to non-typhoidal Salmonella in the intestine is blunted by IL-10 during concurrent malaria parasite infection. Mucosal Immunol. 2014;7:1302–11. doi: 10.1038/mi.2014.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morpeth SC, Ramadhani HO, Crump JA. Invasive non-Typhi Salmonella disease in Africa. Clin Infect Dis. 2009;49:606–11. doi: 10.1086/603553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller AJ, Hoffmann C, Galle M, et al. The S. Typhimurium effector SopE induces caspase-1 activation in stromal cells to initiate gut inflammation. Cell Host Microbe. 2009;6:125–36. doi: 10.1016/j.chom.2009.07.007. [DOI] [PubMed] [Google Scholar]
- Ng TM, Kortmann J, Monack DM. Policing the cytosol—bacterial-sensing inflammasome receptors and pathways. Curr Opin Immunol. 2013;25:34–9. doi: 10.1016/j.coi.2012.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nuccio SP, Bäumler AJ. Comparative analysis of Salmonella genomes identifies a metabolic network for escalating growth in the inflamed gut. mBio. 2014;5:e00929–14. doi: 10.1128/mBio.00929-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okoro CK, Kingsley RA, Connor TR, et al. Intracontinental spread of human invasive Salmonella Typhimurium pathovariants in sub-Saharan Africa. Nat Genet. 2012;44:1215–21. doi: 10.1038/ng.2423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parry CM, Hien TT, Dougan G, et al. Typhoid fever. New Engl J Med. 2002;347:1770–82. doi: 10.1056/NEJMra020201. [DOI] [PubMed] [Google Scholar]
- Parsons BN, Humphrey S, Salisbury AM, et al. Invasive non-typhoidal Salmonella Typhimurium ST313 are not host-restricted and have an invasive phenotype in experimentally infected chickens. PLoS Neglect Trop D. 2013;7:e2487. doi: 10.1371/journal.pntd.0002487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porwollik S, Boyd EF, Choy C, et al. Characterization of Salmonella enterica subspecies I genovars by use of microarrays. J Bacteriol. 2004;186:5883–98. doi: 10.1128/JB.186.17.5883-5898.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raffatellu M, Santos RL, Verhoeven DE, et al. Simian immunodeficiency virus-induced mucosal interleukin-17 deficiency promotes Salmonella dissemination from the gut. Nat Med. 2008a;14:421–8. doi: 10.1038/nm1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raffatellu M, Wilson RP, Chessa D, et al. SipA, SopA, SopB, SopD, and SopE2 contribute to Salmonella enterica serotype Typhimurium invasion of epithelial cells. Infect Immun. 2004;73:146–54. doi: 10.1128/IAI.73.1.146-154.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raffatellu M, Wilson RP, Winter SE, et al. Clinical pathogenesis of typhoid fever. J Infect Dev Ctries. 2008b;2(4):260–6. doi: 10.3855/jidc.219. [DOI] [PubMed] [Google Scholar]
- Rondini S, Lanzilao L, Necchi F, et al. Invasive African Salmonella Typhimurium induces bactericidal antibodies against O-antigens. Microb Pathogenesis. 2013;63:19–23. doi: 10.1016/j.micpath.2013.05.014. [DOI] [PubMed] [Google Scholar]
- Schreiber F, Lynn DJ, Houston A, et al. The human transcriptome during nontyphoid Salmonella and HIV coinfection reveals attenuated NF B-mediated inflammation and persistent cell cycle disruption. J Infect Dis. 2011;204:1237–45. doi: 10.1093/infdis/jir512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selander RK, Beltran P, Smith NH, et al. Genetic population structure, clonal phylogeny, and pathogenicity of Salmonella paratyphi B. Infect Immun. 1990;58:1891–901. doi: 10.1128/iai.58.6.1891-1901.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigaúque B, Roca A, Mandomando I, et al. Community-acquired bacteremia among children admitted to a rural hospital in Mozambique. Pediatr Infect Dis J. 2009;28:108–13. doi: 10.1097/INF.0b013e318187a87d. [DOI] [PubMed] [Google Scholar]
- Siggins MK, O'Shaughnessy CM, Pravin J, et al. Differential timing of antibody-mediated phagocytosis and cell-free killing of invasive African Salmonella allows immune evasion. Eur J Immunol. 2014;44:1093–8. doi: 10.1002/eji.201343529. [DOI] [PubMed] [Google Scholar]
- Stender S, Friebel A, Linder S, et al. Identification of SopE2 from Salmonella typhimurium, a conserved guanine nucleotide exchange factor for Cdc42 of the host cell. Mol Microbiol. 2000;36:1206–21. doi: 10.1046/j.1365-2958.2000.01933.x. [DOI] [PubMed] [Google Scholar]
- Sukhan A, Kubori T, Wilson J, et al. Genetic analysis of assembly of the Salmonella enterica serovar Typhimurium type III secretion-associated needle complex. J Bacteriol. 2001;183:1159–67. doi: 10.1128/JB.183.4.1159-1167.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Threlfall EJ, Ward LR, Rowe B. Multiresistant Salmonella typhimurium DT 104 and salmonella bacteraemia. Lancet. 1998;352:287–8. doi: 10.1016/s0140-6736(05)60261-9. [DOI] [PubMed] [Google Scholar]
- Vazquez-Torres A, Jones-Carson J, Fang FC, et al. Extraintestinal dissemination of Salmonella by CD18-expressing phagocytes. Nature. 1999;401:804–8. doi: 10.1038/44593. [DOI] [PubMed] [Google Scholar]
- Walsh AL, Phiri AJ, Graham SM, et al. Bacteremia in febrile Malawian children: clinical and microbiologic features. Pediatr Infect Dis J. 2000;19:312–8. doi: 10.1097/00006454-200004000-00010. [DOI] [PubMed] [Google Scholar]
- Wangdi T, Winter SE, Baumler AJ. Typhoid fever: ‘you can't hit what you can't see.’. Gut Microbes. 2012;3:88. doi: 10.4161/gmic.18602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werner SB, Humphrey GL, Kamei K. Association between raw milk and human Salmonella dublin infection. Brit Med J. 1979;2:238. doi: 10.1136/bmj.2.6184.238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winter SE, Winter MG, Godinez I, et al. A rapid change in virulence gene expression during the transition from the intestinal lumen into tissue promotes systemic dissemination of Salmonella. PLoS Pathog. 2010;6:e1001060. doi: 10.1371/journal.ppat.1001060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winter SE, Winter MG, Poon V, et al. Salmonella enterica serovar Typhi conceals the invasion-associated type three secretion system from the innate immune system by gene regulation. PLoS Pathog. 2014;10:e1004207. doi: 10.1371/journal.ppat.1004207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winter SE, Winter MG, Thiennimitr P, et al. The TviA auxiliary protein renders the Salmonella entericaserotype Typhi RcsB regulon responsive to changes in osmolarity. Mol Microbiol. 2009;74:175–93. doi: 10.1111/j.1365-2958.2009.06859.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yim L, Sasías S, Martínez A, et al. Repression of flagella is a common trait in field isolates of Salmonella enterica serovar Dublin and is associated with invasive human infections. Infect Immun. 2014;82:1465–76. doi: 10.1128/IAI.01336-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S, Kingsley RA, Santos RL, et al. Molecular pathogenesis of Salmonella enterica serotype Typhimurium-induced diarrhea. Infect Immun. 2003;71:1–12. doi: 10.1128/IAI.71.1.1-12.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S, Santos RL, Tsolis RM, et al. The Salmonella enterica serotype Typhimurium effector proteins SipA, SopA, SopB, SopD, and SopE2 act in concert to induce diarrhea in calves. Infect Immun. 2002;70:3843–55. doi: 10.1128/IAI.70.7.3843-3855.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou D, Galán J. Salmonella entry into host cells: the work in concert of type III secreted effector proteins. Microbes Infect. 2001;3:1293–8. doi: 10.1016/s1286-4579(01)01489-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.