Abstract
Background
Silicone breast implants have long been used for breast augmentation and reconstruction. During this time, these medical devices have gone through a number of modifications to improve their safety, quality, and clinical outcome performance.
Objectives
The authors conducted a 10-year study to determine the safety and effectiveness of Natrelle 410 silicone breast implants.
Methods
This prospective, multicenter study enrolled 941 subjects who were undergoing either augmentation, augmentation revision, reconstruction, or reconstruction revision. Data on complications, reoperations, explantations, and subject satisfaction were collected at annual clinic visits, and one-third of subjects underwent biennial magnetic resonance imaging (MRI) to screen for implant rupture. The authors used the Kaplan-Meier estimator to calculate risk rates for local complications, reoperations, and explantations.
Results
Capsular contracture rates increased approximately 1% per year from the previously reported 6-year rates. The rates were significantly lower than those from the Natrelle round gel core study. The overall rate of confirmed ruptured implants in subjects who underwent MRI was 5.7%. Eleven late seromas were reported. The most common reason for explantation was a subject requesting a size or style change. Satisfaction rates remained high through 10 years, with most subjects saying they were somewhat or definitely satisfied with their implants.
Conclusions
This 10-year prospective trial demonstrated the long-term safety and effectiveness of Natrelle 410 anatomical form-stable implants. The complication rates were low and the satisfaction rates were high.
Level of Evidence: 1
 Therapeutic
Therapeutic
Silicone gel–filled breast implants have been commercially available for 50 years.1 During this time, these medical devices have gone through a number of modifications to improve their safety, quality, and clinical outcome performance. The most objective clinical assessment of these outcome parameters in the United States was obtained from investigational device exemption studies that were approved by the US Food and Drug Administration (FDA). These exemptions allowed use of medical devices in American women before FDA approval when there was a specific need. The Natrelle 410 anatomical form-stable silicone-filled breast implant (Allergan, Inc., Irvine, California), which was introduced in Europe in 1993 as the Biodimensional Style 410 implant, was the first anatomically shaped silicone implant to be designed and brought to the market.
The initial 3-year and subsequent 6-year clinical data on these implants from an investigational device exemption study initiated in February 2001 have been published previously.2,3 The Natrelle 410 silicone gel breast implants were approved by the FDA on February 20, 2013, and the 10-year study supporting their approval has been completed. The present report provides updated clinical data through the completion of the study and represents the first publication of a completed investigational device exemption study that provides prospective, long-term data on highly cohesive, form-stable breast implants.
METHODS
In this 10-year prospective, multicenter study, we examined the safety and effectiveness of Natrelle 410 breast implants for augmentation, reconstruction, and revision. Written informed consent was obtained from all subjects, and multiple institutional review boards provided approval. (the list of the approving institutional review boards is available online at http://asj.oxfordjournals.org/supplemental.) The study was registered at ClinicalTrials.gov (identifier: NCT00690339). Before the study was initiated, the study sponsor (Allergan, Inc.) conducted a start-up meeting at each investigational site and also provided written instructions to investigators and study coordinators. In addition, site visits were conducted throughout the study to ensure adherence to study guidelines.
The study design, inclusion/exclusion criteria, subject demographics, surgical details, and safety and effectiveness results through 6 years have been published previously;2–4 thus, the present report will focus on the 10-year results. Data were captured prospectively at the initial clinic visit and at visits 4 weeks and 6 months after implantation, as well as annually thereafter for 10 years. Magnetic resonance imaging (MRI) was conducted 1, 3, 5, 7, and 10 years after implantation in a subset of subjects to assess silent rupture, with the worst-case rupture assessment by either the local facility radiologist or central reviewer radiologist used to calculate rupture rates. Kaplan-Meier risk rates were calculated for local complications, reoperations, and implant removal/replacement. These risk rates represent the cumulative risk of a subject experiencing an adverse event at any time through 10 years. The primary measure of effectiveness at 10 years was subject satisfaction. At each study visit, subjects were queried verbally about satisfaction by the investigator or study coordinator, and their responses were recorded on a case report form. Subject satisfaction was evaluated using a nonvalidated 5-point scale ranging from 1 (definitely dissatisfied) to 5 (definitely satisfied).
RESULTS
Subjects
Between February 2001 and February 2002, we enrolled 941 women (492 undergoing primary augmentation, 156 undergoing augmentation revision, 225 undergoing primary reconstruction, and 68 undergoing reconstruction revision) at 48 US sites. Of those, 316 subjects were included in the MRI cohort. More than 90% of subjects were white, and the median body mass indices (weight in kilograms divided by height in meters squared) were 20.6, 21.0, 22.6, and 22.4 in the augmentation, augmentation revision, reconstruction, and reconstruction revision cohorts, respectively.
For subjects undergoing breast augmentation, the indication for implant placement was dissatisfaction with breast size/shape in 79.1%, asymmetry in 10.6%, ptosis in 6.7%, and aplasia in 3.7%. All of the reconstruction procedures occurred after mastectomy except for 1 that was performed after breast trauma. Concurrent procedures were performed in 7.2% of augmentation, 75.2% of augmentation revision, 53.7% of reconstruction, and 75.2% of reconstruction revision surgeries, with capsulectomies being the procedure most often performed. Accompanying mastopexies were performed in 6.6% of augmentation procedures and 17.7% of augmentation revision procedures.
The inframammary fold was the most common incision site for women undergoing augmentation (86.8%) or augmentation revision (75.5%), whereas the mastectomy scar was the most common incision site for women undergoing reconstruction (74.9%) or reconstruction revision (54.0%). Partial submuscular placement was the most frequent location in all cohorts (80.5%, 65.8%, 59.9%, and 60.2% for the augmentation, augmentation revision, reconstruction, and reconstruction revision cohorts, respectively). In the augmentation and augmentation revision cohorts, subglandular placement was the second most frequent placement (15.7% and 28.4%, respectively), whereas in the reconstruction and reconstruction revision cohorts, it was complete submuscular placement (27.7% and 31.9%, respectively).
Pocket irrigation with administration of medications (most commonly antibiotics) was performed for the majority of surgeries (61.1%, 66.5%, 79.4%, and 84.1% for the augmentation, augmentation revision, reconstruction, and reconstruction revision cohorts, respectively). Most subjects also received parenteral antibiotics (88.6% for women undergoing augmentation, 93.6% for women undergoing augmentation revision, 93.3% for women undergoing reconstruction, and 97.1% for women undergoing reconstruction revision. Drains were placed in 4.3%, 45.2%, 65.5%, and 61.9% of the augmentation, augmentation revision, reconstruction, and reconstruction revision surgeries, respectively.
Follow-up rates at 10 years were 65.8% in the augmentation cohort, 55.3% in the augmentation revision cohort, 81.1% in the reconstruction cohort, and 78.4% in the reconstruction revision cohort. The by-implant compliance rate for the 10-year MRI was 70.1% for augmentation, 64.2% for augmentation revision, 72.3% for reconstruction, and 80.0% for reconstruction revision subjects.
Safety
Capsular contracture rates (Baker scale grades III and IV) by subject increased approximately 1% per year from the previously reported 6-year rates.3 The final 10-year rates were 9.2% for augmentation, 11.9% for augmentation revision, 14.5% for reconstruction, and 26.8% for reconstruction revision (Table 1, Figure 1). The overall rupture rate (suspected and confirmed) in the MRI cohort was 16.4% (9.7% of implants). Of the 38 implants included as suspected ruptures, 26 were confirmed to have ruptured at explantation and 12 remained in vivo, so the rupture was unconfirmed. Of those, 8 had MRI evidence of a probable rupture and 4 had indeterminate MRI evidence. In the MRI cohort across all indications, 9.4% of subjects had ruptures and 5.7% of implants ruptured (Table 1, Figure 2). All of the ruptures were intracapsular, with no reports of extracapsular rupture or migrated gel.
Table 1.
Kaplan-Meier Key Risk Rates by Subject Group Through 10 Yearsa
| Complication | Augmentation Cohort, % (95% CI) (n = 492) | Augmentation Revision Cohort, % (95% CI) (n = 156) | Reconstruction Cohort, % (95% CI) (n = 225) | Reconstruction Revision Cohort, % (95% CI) (n = 68) |
|---|---|---|---|---|
| Key risk rates | ||||
| Reoperation | 29.7 (25.6-34.3) | 47.3 (39.2-56.0) | 54.6 (47.9-61.6) | 48.5 (37.0-61.5) |
| Implant removal with replacement | 16.8 (13.6-20.8) | 27.8 (21.0-36.2) | 34.3 (28.0-41.6) | 39.3 (28.2-52.9) |
| Implant removal without replacement | 3.3 (1.9-5.7) | 5.9 (2.8-12.2) | 6.7 (3.8-11.7) | 4.9 (1.2-18.7) |
| Implant rupture (MRI cohort) | 17.7 (11.7-26.4) | 14.7 (5.4-36.4) | 12.4 (6.0-24.4) | 19.6 (7.8-44.4) |
| Confirmed implant rupture (MRI cohort) | 10.2 (6.0-17.0) | 5.2 (1.3-19.4) | 9.5 (4.4-19.9) | 10.1 (2.6-34.7) |
| Capsular contracture (Baker grade III/IV) | 9.2 (6.7-12.6) | 11.9 (7.2-19.3) | 14.5 (10.1-20.6) | 26.8 (16.8-41.1) |
| Additional risk rates occurring in ≥2.0% of subjects | ||||
| Implant malposition | 4.7 (3.1-7.3) | 9.1 (5.2-15.6) | 5.7 (3.1-10.5) | 8.0 (3.0-20.5) |
| Breast pain | 4.5 (2.8-7.1) | 5.2 (2.3-11.5) | 8.2 (4.9-13.7) | 7.8 (2.9-20.4) |
| Swelling | 4.0 (2.5-6.3) | 2.7 (1.0-7.1) | 5.3 (2.8-9.7) | 3.2 (0.8-12.4) |
| Infection | 1.7 (0.8-3.3) | 2.1 (0.7-6.3) | 6.1 (3.5-10.7) | 8.5 (3.6-19.5) |
| Seroma/fluid accumulation | 1.6 (0.8-3.3) | 3.2 (1.2-8.4) | 2.8 (1.1-6.6) | 6.2 (2.4-15.8) |
| Hypertrophic/abnormal scarring | 1.4 (0.6-3.0) | 3.7 (1.5-8.8) | 4.8 (2.6-8.7) | 3.2 (0.8-12.3) |
| Hematoma | 1.3 (0.6-2.9) | 2.0 (0.6-6.0) | 1.0 (0.3-4.0) | 0 |
| Delayed wound healing | 1.1 (0.4-2.5) | 1.3 (0.3-5.1) | 0.5 (0.1-3.3) | 2.9 (0.7-11.3) |
| Asymmetry | 1.2 (0.5-2.9) | 6.9 (3.6-13.1) | 12.4 (8.4-18.1) | 17.4 (9.6-30.5) |
| Wrinkling/rippling | 0.9 (0.3-2.4) | 3.7 (1.5-8.6) | 6.2 (3.3-11.4) | 12.8 (6.1-25.6) |
| Redness | 0.6 (0.2-2.0) | 0 | 0.9 (0.2-3.7) | 4.9 (1.6-14.7) |
| Implant palpability/visibility | 0.3 (0.0-1.8) | 1.4 (0.3-5.4) | 1.2 (0.3-4.7) | 4.2 (1.0-16.5) |
| Upper pole fullness | 0 | 0.7 (0.1-4.5) | 4.2 (2.2-7.8) | 1.5 (0.2-10.1) |
CI, confidence interval; MRI, magnetic resonance imaging.
aData are presented as rate (95% CI).
Figure 1.
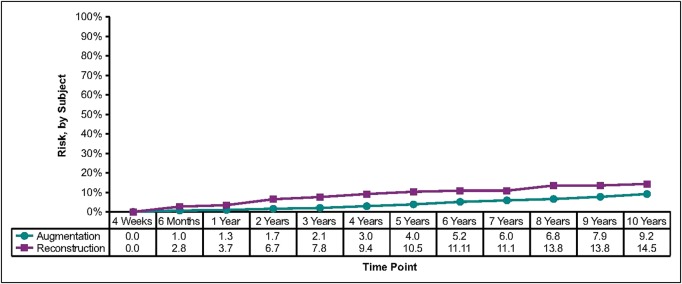
These Kaplan-Meier curves show the risk of capsular contracture through 10 years.
Figure 2.
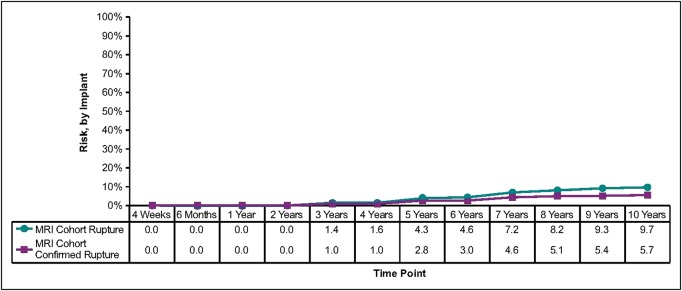
These Kaplan-Meier curves show the risk of implant rupture through 10 years. MRI, magnetic resonance imaging.
The rates of all complications remained low through 10 years, aside from capsular contracture and rupture in <5% of women undergoing augmentation. For women who underwent augmentation revision, the only other major complications (>5%) were implant malposition (9.1%) and asymmetry (6.9%). The seroma rate ranged from 1.6% in augmentation subjects to 6.2% in reconstruction revision subjects. Eleven seromas occurred >1 year after implantation, which means that 0.6% of the 1760 devices implanted developed a late seroma (ranging from 0.4% in augmentation subjects to 0.9% in reconstruction revision subjects). Onset dates for these seromas ranged from 1.4 to 7.6 years after implantation, with most occurring at approximately 6 years.
A single case of breast implant–associated anaplastic large cell lymphoma (BIA-ALCL) was reported. The subject had a strong family history of breast cancer and fibrocystic disease and had been diagnosed with intraductal hyperplasia before a simple mastectomy. She had undergone breast reconstruction and was in the 10th year of her follow-up when treatment of a late seroma led to the discovery of ALCL. The cells were detected in the seroma fluid and the subject underwent bilateral implant removal and complete capsulectomies. No tumor cells were detected in the capsules, the subsequent positron emission tomography scan was normal, and no adjuvant treatment was given. The subject has shown no evidence of recurrence in 3 years of follow-up.
Reoperation rates increased approximately 3% per year from the 6-year rates, and the most common reasons for reoperation were subjects requesting a style or size change (augmentation), capsular contracture and implant malposition (augmentation revision), scarring (reconstruction), and capsular contracture (reconstruction revision). Explantations were most often performed in response to subjects requesting a style or size change in all cohorts except the reconstruction revision cohort, in which capsular contracture resulted in more explantations (Table 2). No gel fractures were reported at explantation. Eighty-seven percent of subjects had their implants replaced after removal, and of those who had another 410 implant as the replacement, 60% went to a larger implant, whereas 15% went to a smaller implant.
Table 2.
Primary Reasons for Reoperation and Explantation
| Reason | % of Augmentation Cohort | % of Augmentation Revision Cohort | % of Reconstruction Cohort | % of Reconstruction Revision Cohort |
|---|---|---|---|---|
| Reasons for reoperation occurring in >8% of reoperations | ||||
| No. of reoperations | 167 | 83 | 163 | 40 |
| Subject request for style/size change | 13.2 | 8.4 | 7.4 | 10.0 |
| Capsular contracture | 11.4 | 14.5 | 12.3 | 22.5 |
| Suspected rupture | 11.4 | 12.0 | 9.8 | 7.5 |
| Implant malposition | 10.2 | 14.5 | 12.3 | 10.0 |
| Scarring | 9.0 | 8.4 | 19.0 | 2.5 |
| Need for biopsy | 8.4 | 13.3 | 7.4 | 5.0 |
| Ptosis | 7.8 | 8.4 | 3.7 | 0 |
| Reasons for implant removal (with or without replacement) occurring in >8% of explantations | ||||
| No. of explantations | 153 | 78 | 115 | 40 |
| Subject request for style/size change | 34.0 | 24.4 | 20.9 | 20.0 |
| Suspected rupture | 13.7 | 16.7 | 14.8 | 7.5 |
| Ptosis | 11.1 | 5.1 | 1.7 | 0 |
| Capsular contracture | 9.8 | 23.1 | 15.7 | 25.0 |
| Asymmetry | 4.6 | 6.4 | 11.3 | 2.5 |
| Implant malposition | 4.6 | 9.0 | 11.3 | 7.5 |
| Wrinkling/rippling | 0.7 | 0 | 5.2 | 15.0 |
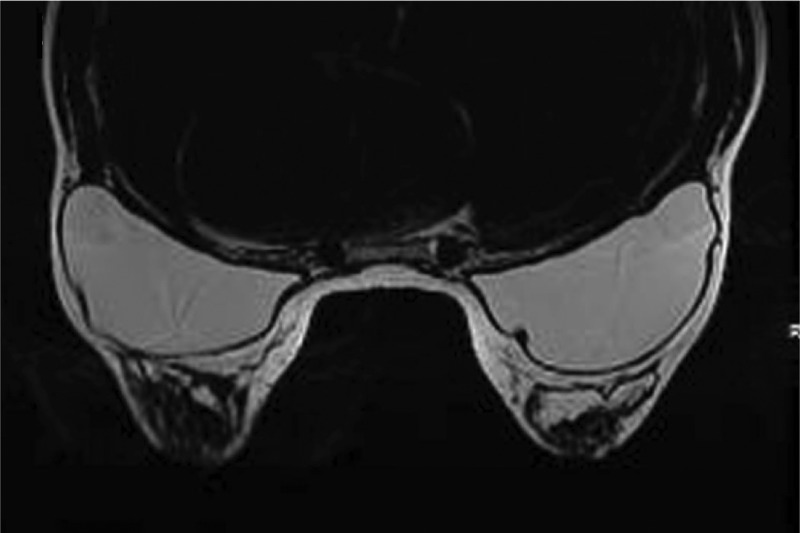
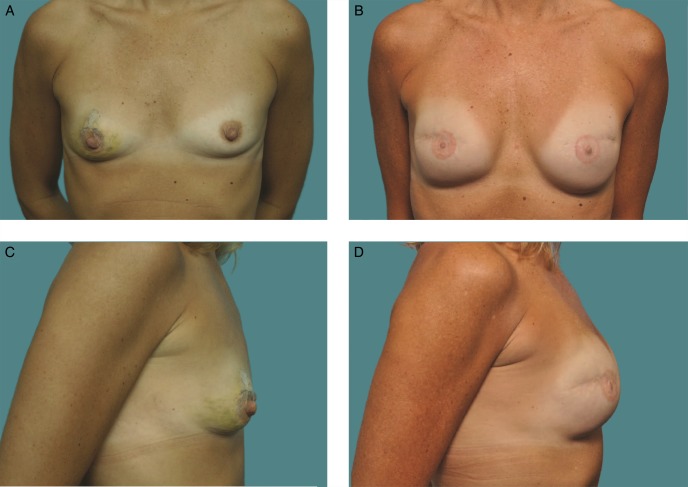
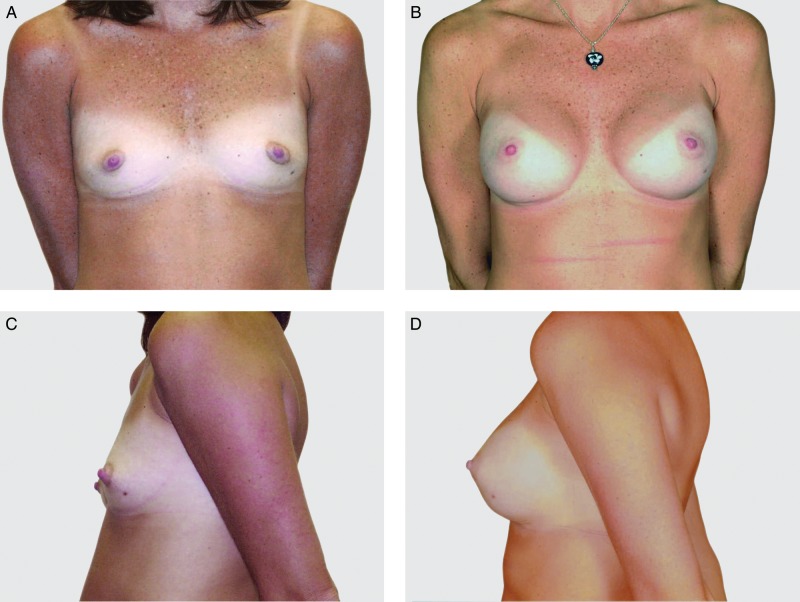
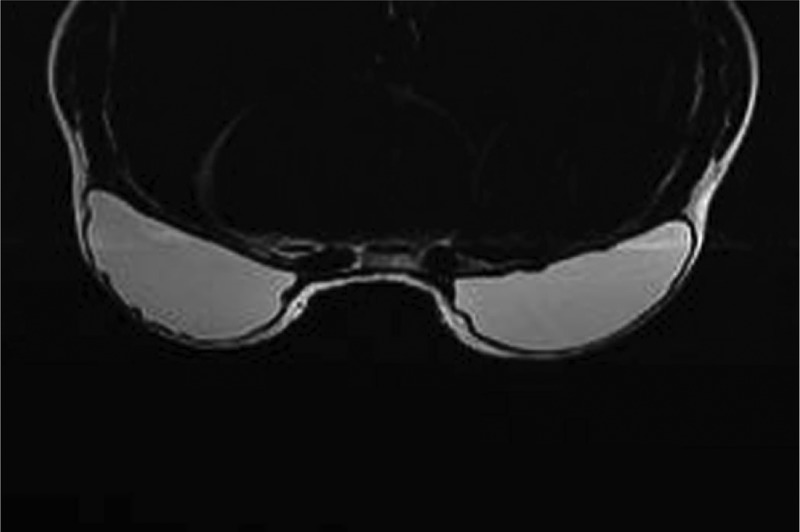
Investigators performed device assessments and reported that at 10 years, the shape of the breast reflected the shape of the implant for 97.3%, 95.8%, 91.8%, and 100% of the augmentation, augmentation revision, reconstruction, and reconstruction revision cohorts, respectively. Similarly, they reported that the implants had maintained their original position for 96.9%, 93.0%, 94.8%, and 94.9% of subjects, respectively. This was visually demonstrated with photographic examples of subjects at baseline and at 10 years, along with an accompanying MRI image from the 10-year visit (Figures 3–6).
Figure 4.
Magnetic resonance imaging scan taken 10 years after breast augmentation with 255-cc Natrelle 410 moderate height, full projection implants in the 33-year-old woman shown in Figure 3.
Figure 5.
This 36-year-old woman is shown before (A, C) and 10 years after (B, D) breast reconstruction with a bilateral neopectoral pocket conversion and placement of 255-cc Natrelle 410 full height, full projection implants.
Figure 3.
This 33-year-old woman is shown before (A, C) and 10 years (B, D) after breast augmentation with 255-cc Natrelle 410 moderate height, full projection implants.
Figure 6.
Magnetic resonance imaging scan taken 10 years after breast reconstruction with a bilateral neopectoral pocket conversion and placement of 255-cc Natrelle 410 full height, full projection implants in the 36-year-old woman shown in Figure 5.
Satisfaction
Satisfaction rates remained high through 10 years, with 96.2%, 87.5%, 93.3%, and 90.0% of subjects saying they were somewhat or definitely satisfied with their implants for the augmentation, augmentation revision, reconstruction, and reconstruction revision cohorts, respectively (Table 3). Investigators provided similarly high satisfaction scores.
Table 3.
Subject Satisfaction at 10 Years
| Satisfaction Level | % of Augmentation Cohort | % of Augmentation Revision Cohort | % of Reconstruction Cohort | % of Reconstruction Revision Cohort |
|---|---|---|---|---|
| Definitely dissatisfied (1) | 1.4 | 2.8 | 1.5 | 0 |
| Somewhat dissatisfied (2) | 2.1 | 8.3 | 2.2 | 7.5 |
| Neither satisfied nor dissatisfied (3) | 0.3 | 1.4 | 3.0 | 2.5 |
| Somewhat satisfied (4) | 7.2 | 16.7 | 17.9 | 22.5 |
| Definitely satisfied (5) | 89.0 | 70.8 | 75.4 | 67.5 |
DISCUSSION
The Natrelle 410 implant was the first fifth-generation breast implant developed,2 and it differs from prior implants in a number of ways. Rather than a single-shaped, mandrel-designed implant differentiated only by the size (volume) of the round mandrel, the Natrelle 410 is anatomically shaped, with 12 dimensional options that allow for a combination of 3 different heights and 4 different projections. This diversity of implant types (termed “cells”) allows for an individualized approach to suit patient needs. Four of the 12 cells were available and used for the current investigational device exemption study: moderate height and projection, full height and projection, moderate height and full projection, and full height and moderate projection. The 410 implant is differentiated from the fourth-generation implants by its highly cohesive, form-stable silicone gel filler (TruForm 3 gel; Allergan, Inc.).5,6 A greater degree of crosslinking of the silicone polymers during the manufacturing process determines the firmness, or cohesivity, of the silicone gel. When oriented in a vertical position, the 410 implants are said to be form-stable, maintaining their dimensions and form (ie, gel distribution within the shell), and are characterized by the lack of collapse of the upper pole of the breast implant.5,6 The textured surface of the 410 implant, termed Biocell (Allergan, Inc.), was specifically created to help maintain implant stability and position within the body. Biocell is a macrotexture surface with irregular depressions that have an average diameter of 300 μm,7 and it demonstrates an adhesive effect with surrounding native tissue after surgical implantation.8 Together, these properties mark a distinct departure from previous generations of breast implants by providing adequate shape stability to prevent significant implant collapse and a sufficiently textured surface to help hold implant position.
One of the most commonly reported complications in breast surgery is capsular contracture. The risk of this complication increases over time9–11 and is reflected in the slightly increased capsular contracture rates reported herein from 6 years to 10 years.3 However, the 10-year rates (9.2%, 11.9%, 14.5%, and 26.8% for the augmentation, augmentation revision, reconstruction, and reconstruction revision cohorts, respectively) are lower than those observed in the Natrelle round gel (fourth generation) core study, which included predominately smooth implants (56.2%).12 The capsular contracture rate for the 410 implant at 10 years was 51% lower (9.2% vs 18.9%) than that reported at 10 years in the Natrelle round gel core study for primary breast augmentation.12 Further, a 59% lower incidence of capsular contracture was noted for breast augmentation revision with the 410 implant compared with the Natrelle round gel implant (11.9% vs 28.7%, respectively) at 10 years.12 Similarly, in 6-year clinical trial data of the form-stable Contour Profile Gel (CPG) implant (Mentor Worldwide LLC, Santa Barbara, California), lower contracture rates were reported for the CPG implant than for Mentor's predominately smooth-surface round gel breast implants.13 Although these studies were not direct comparisons between form-stable and non–form-stable implants and no causation can be assigned, it is of interest that form-stable implants appeared to have lower rates of capsular contracture.
Careful preoperative planning and improvements in surgical technique also have been reported to decrease rates of capsular contracture. Most 410 implants used for augmentation and augmentation revision in this study were placed via an incision in the inframammary fold, which has been independently shown to have lower capsular contracture rates than other incision placement locations.14–16 Submuscular placement and antibiotic pocket irrigation is advocated, as well as the “no-touch” technique of implantation. Although it cannot be documented that this process was utilized in every subject in this study, these principles and techniques were taught for the use of this implant and were conveyed to the independent investigators. These factors have also been independently documented to reduce capsular contracture with all implants.17,18
Incorporation of these techniques facilitates the characteristics of the implant, which reduces capsular contracture. The direct contact with the Biocell textured surface, supported by the firmness of the highly cohesive gel, and its engagement with a surgically created vascular host pocket frequently allows for tissue adherence and immobility with softness.8,19 These findings, first established with Biocell tissue expanders, are more likely to occur with the 410 implant than with less cohesive, round, textured implants in a less precise surgical pocket. Furthermore, the reduced rate of capsular contracture with the Natrelle 410 implant and the clinically appreciated softness has been described as providing a one breast feel as compared with a round implant moving in its larger pocket separate from the movement of the overlying breast and soft tissue.20 Although the firmer gel of the 410 implant could mask the feel of some mild capsular contracture formation, it is clear that when the 410 implant was used properly, it had the lowest 10-year rate of capsular contracture of any implant studied thus far in an FDA-approved registration trial. These data also further supported the recent finding that Biocell-covered 410 implants have a statistically lower rate of capsular contracture than do smooth fourth-generation round gel implants used for primary augmentation.21 These results are in agreement with those from previous publications, which showed improved rates of capsular contracture in textured implants compared with smooth implants.22–28
Although these implants feel soft in the body, the 10-year data show a very low rate of implant rippling or wrinkling (0.9% for augmentation, 3.7% for augmentation revision, 6.2% for reconstruction, and 12.8% for reconstruction revision). These data corroborated previously published data, which demonstrated that the Natrelle 410 implants have low rates of wrinkling compared with other-shaped implants. Indeed, in a comparative study, Jewell and Jewell29 reported a 5-fold higher rate of rippling in the CPG implant group compared with the 410 implant group. In a separate study of the 410 implant, wrinkling was reported in 5% of subjects after a mean of 8 years.30
Another important finding in this 10-year study is the low malposition rate of the shaped 410 implant, reported herein at 4.7% for augmentation, 9.1% for augmentation revision, 5.7% for reconstruction, and 8.0% for reconstruction revision. It should be noted that rotation is a subset of malposition, and thus the rate of postoperative rotation is even lower. This low malposition rate likely reflects the utility of Biocell in maintaining the correct anatomical alignment within the recipient and minimizing postoperative implant rotation.8,31 However, this tissue adherence to the Biocell surface does not always occur, and therefore rotation of the shaped device is possible. To this point, in a recent study using high-resolution ultrasound rather than MRI, Schafer et al32 reported higher-than-expected rotation rates for shaped breast implants (21% and 25% for the 410 and CPG implants, respectively). The study authors noted this was a dynamic process, with the majority of rotations expected to self-reverse.33
The 10-year seroma rate was also quite low in our study (1.6% for augmentation, 3.2% for augmentation revision, 2.8% for reconstruction, and 6.2% for reconstruction revision), with 11 seromas occurring >1 year after surgery (0.6%). The confirmed rupture rate in the MRI cohort for all indications was 9.4% for subjects and 5.7% for implants at 10 years, and there was no report of extracapsular silicone gel through the 10 years of the study.
In recent years, increased awareness of BIA-ALCL, a rare ALCL subset, has been noted. In 1 study, the incidence of BIA-ALCL was estimated to be 0.1-0.3 per 100,000 women with implants per year.34 Although the etiology of this condition is not yet known, it is distinct from systemic ALCL and typically characterized by early detection, an indolent course, and high rates of complete remission after treatment via implant removal and capsulectomy.35 Cases of BIA-ALCL have been reported across implant types (smooth, textured, saline, silicone), across manufacturers, and in subjects with and without prior implant history; however, more cases have been reported with textured-surface devices. In the present study, 1 subject in her 10th year of follow-up presented with BIA-ALCL that was detected in seroma fluid. She was conservatively treated with bilateral capsulectomies and implant removal. She remains disease-free without any evidence of recurrence.
Despite the many advantages reported herein of the firmer, greater crosslinked silicone gel implant compared with fourth-generation round implants, it has been questioned whether these implants impart a firmer feel within the body. Perhaps one can accept this tradeoff for a lower capsular contracture rate, with a documented safe implant, and a better shape to the breast, particularly in subjects who have minimal breast tissue and a tighter tissue envelope or in reconstruction subjects in whom the entire breast is essentially an implant. Although our clinical data did not directly capture subject-perceived firmness, 96.2% of augmentation subjects reported they were somewhat or definitely satisfied with their implants at 10 years. These percentages were 87.5% for women who underwent augmentation revision, 93.3% for women who underwent reconstruction, and 90.0% for women who underwent reconstruction revision. In addition, in an earlier publication that focused on augmentation, Gladfelter and Murphy4 showed that women who had undergone augmentation reported improved satisfaction with the feel of their breasts after implantation. There were similar levels of improvement in subject satisfaction with regard to breast feel among subjects who underwent reconstruction.
It is important to note that there is a potential for bias in studies that rely on self-reported data. The investigators in the present study were experienced surgeons who had specific experience with shaped breast implants. Although there may be inconsistency in reporting among investigators within the study, individual investigators are likely to be fairly consistent in their reporting. Despite the limitations inherent to studies such as this, the results of controlled, monitored studies are likely less biased than are results obtained from retrospective reviews. It is also worth noting that the present study was source data monitored and conducted under the purview of the FDA.
CONCLUSION
In the present 10-year prospective trial, Natrelle 410 anatomical form-stable implants demonstrated long-term safety and effectiveness, with low complication rates and high satisfaction rates. These implants provide further options to match physician and patient needs.
Funding
Allergan, Inc. designed and funded the study. Editorial assistance was provided by Evidence Scientific Solutions and was funded by Allergan, Inc. Statistical analysis of the data was performed by Ramkumar Krish, MS, of Allergan, Inc.
Disclosures
Dr Maxwell is an Allergan, Inc. consultant, royalty recipient, and stockholder. Drs Bengtson and Van Natta are consultants for Allergan, Inc. and received research support for conducting this study. Ms Murphy is an Allergan, Inc. employee and stockholder.
Acknowledgments
The principal investigators who performed the implantations in the study were James Andersen, Duarte, California; Eric P. Bachelor, Pleasanton, California; Frank E. Barone, Toledo, Ohio; Bradley Bengtson, Grand Rapids, Michigan; Lewis H. Berger, Tampa, Florida; Scott R. Brundage, Grand Rapids, Michigan; Louis P. Bucky, Philadelphia, Pennsylvania; Athleo L. Cambre, Los Angeles, California; William M. Carpenter, Dallas, Texas; Mike Columbus, Cincinnati, Ohio; Strawford H. Dees III, Biloxi, Mississippi; Marc Alan Drimmer, Princeton, New Jersey; Gloria Duda, McLean, Virginia; Walter L. Erhardt, Jr, Albany, Georgia; Michael Fasching, Plymouth, Minnesota; Stephen W. Gordon, Las Vegas, Nevada; Dennis C. Hammond, Grand Rapids, Michigan; Robert A. Hein, Oklahoma City, Oklahoma; T. Roderick Hester, Jr, Atlanta, Georgia; Stanley M. Jackson, Puyallup, Washington; Robert Jacobs, Port Jefferson Station, New York; Mark L. Jewell, Eugene, Oregon; Perry Johnson, Omaha, Nebraska; Shujaat Ali Khan, Fort Worth, Texas; William D. Leighton, Scottsdale, Arizona; Tim Love, Oklahoma City, Oklahoma; G. Patrick Maxwell, Nashville, Tennessee; Patricia A. McGuire, Creve Coeur, Missouri; Steven Morris, Midland, Michigan; Jay S. Orringer, Beverly Hills, California; Mary A. Powers, Long Beach, California; Oscar Ramirez, Timonium, Maryland; Douglas Reavie, San Diego, California; James Romanelli, Huntington, New York; Marc Salzman, Louisville, Kentucky; Stephen J. Scully, North Andover, Massachusetts; Kenneth C. Shestak, Pittsburgh, Pennsylvania; Wendell Smoot, La Jolla, California; Scott Spear, Washington, DC; Scott A. Spiro, West Orange, New Jersey; Brett Stompro, Danville, California; Louis L. Strock, Fort Worth, Texas; John Tebbetts, Dallas, Texas; Steven Teitelbaum, Santa Monica, California; Randall J. Telman, Grand Rapids, Michigan; Bruce Van Natta, Indianapolis, Indiana; and Hillard Warm, Port Jefferson Station, New York.
REFERENCES
- 1.Jewell ML. Silicone gel breast implants at 50: the state of the science. Aesthet Surg J. 2012;32(8):1031–1034. doi: 10.1177/1090820X12461649. [DOI] [PubMed] [Google Scholar]
- 2.Bengtson BP, Van Natta BW, Murphy DK, Slicton A, Maxwell GP for the Style 410 U.S. Core Clinical Study Group. Style 410 highly cohesive silicone breast implant core study results at 3 years. Plast Reconstr Surg. 2007;120(7 Suppl 1):40S–48S. doi: 10.1097/01.prs.0000286666.29101.11. [DOI] [PubMed] [Google Scholar]
- 3.Maxwell GP, Van Natta BW, Murphy DK, Slicton A, Bengtson BP. Natrelle Style 410 form-stable silicone breast implants: core study results at 6 years. Aesthet Surg J. 2012;32(6):709–717. doi: 10.1177/1090820X12452423. [DOI] [PubMed] [Google Scholar]
- 4.Gladfelter J, Murphy D. Breast augmentation motivations and satisfaction: a prospective study of more than 3,000 silicone implantations. Plast Surg Nurs. 2008;28(4):170–174. doi: 10.1097/PSN.0b013e31818ea7e0. quiz 175-176. [DOI] [PubMed] [Google Scholar]
- 5.Maxwell GP, Hartley W. Breast augmentation. In: Mathes SJ, Hentz VR, editors. Plastic Surgery. 2nd ed. Amsterdam: Elsevier; 2006. pp. 1–33. [Google Scholar]
- 6.Maxwell GP, Baker MR, Gabriel A. Augmentation mammaplasty: general considerations. In: Spear SL, editor. Surgery of the Breast: Principles and Art. 3rd ed. Baltimore, MD: Lippincott Williams & Wilkins; 2010. p. 1227. [Google Scholar]
- 7.Barone FE, Perry L, Keller T, Maxwell GP. The biomechanical and histopathologic effects of surface texturing with silicone and polyurethane in tissue implantation and expansion. Plast Reconstr Surg. 1992;90(1):77–86. doi: 10.1097/00006534-199207000-00012. [DOI] [PubMed] [Google Scholar]
- 8.Danino AM, Basmacioglu P, Saito S, et al. Comparison of the capsular response to the Biocell RTV and Mentor 1600 Siltex breast implant surface texturing: a scanning electron microscopic study. Plast Reconstr Surg. 2001;108(7):2047–2052. doi: 10.1097/00006534-200112000-00032. [DOI] [PubMed] [Google Scholar]
- 9.Handel N, Cordray T, Gutierrez J, Jensen JA. A long-term study of outcomes, complications, and patient satisfaction with breast implants. Plast Reconstr Surg. 2006;117(3):757–767. doi: 10.1097/01.prs.0000201457.00772.1d. discussion 768-772. [DOI] [PubMed] [Google Scholar]
- 10.Cunningham B. The Mentor Core Study on silicone MemoryGel breast implants. Plast Reconstruct Surg. 2007;120(7 Suppl 1):19S–29S. doi: 10.1097/01.prs.0000286574.88752.04. [DOI] [PubMed] [Google Scholar]
- 11.Cunningham B, McCue J. Safety and effectiveness of Mentor's MemoryGel implants at 6 years. Aesthetic Plast Surg. 2009;33(3):440–444. doi: 10.1007/s00266-009-9364-6. [DOI] [PubMed] [Google Scholar]
- 12.Spear SL, Murphy DK on behalf of the Allergan Silicone Breast Implant U.S. Core Clinical Study Group. Natrelle round silicone breast implants: core study results at 10 years. Plast Reconstr Surg. 2014;133(6):1354–1361. doi: 10.1097/PRS.0000000000000021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hammond DC, Migliori MM, Caplin DA, Garcia ME, Phillips CA. Mentor Contour Profile Gel implants: clinical outcomes at 6 years. Plast Reconstr Surg. 2012;129(6):1381–1391. doi: 10.1097/PRS.0b013e31824ecbf0. [DOI] [PubMed] [Google Scholar]
- 14.Jacobson JM, Gatti ME, Schaffner AD, Hill LM, Spear SL. Effect of incision choice on outcomes in primary breast augmentation. Aesthet Surg J. 2012;32(4):456–462. doi: 10.1177/1090820X12444267. [DOI] [PubMed] [Google Scholar]
- 15.Henriksen TF, Fryzek JP, Hölmich LR, et al. Surgical intervention and capsular contracture after breast augmentation: a prospective study of risk factors. Ann Plast Surg. 2005;54(4):343–351. doi: 10.1097/01.sap.0000151459.07978.fa. [DOI] [PubMed] [Google Scholar]
- 16.Wiener TC. Relationship of incision choice to capsular contracture. Aesthetic Plast Surg. 2008;32(2):303–306. doi: 10.1007/s00266-007-9061-2. [DOI] [PubMed] [Google Scholar]
- 17.Blount AL, Martin MD, Lineberry KD, Kettaneh N, Alfonso DR. Capsular contracture rate in a low-risk population after primary augmentation mammaplasty. Aesthet Surg J. 2013;33(4):516–521. doi: 10.1177/1090820X13484465. [DOI] [PubMed] [Google Scholar]
- 18.Adams WP, Jr, Mallucci P. Breast augmentation. Plast Reconstr Surg. 2012;130(4):597e–611e. doi: 10.1097/PRS.0b013e318262f607. [DOI] [PubMed] [Google Scholar]
- 19.Maxwell GP, Falcone PA. Eighty-four consecutive breast reconstructions using a textured silicone tissue expander. Plast Reconstr Surg. 1992;89(6):1022–1034. discussion 1035-1036. [PubMed] [Google Scholar]
- 20.Brown MH, Shenker R, Silver SA. Cohesive silicone gel breast implants in aesthetic and reconstructive breast surgery. Plast Reconstr Surg. 2005;116(3):768–779. doi: 10.1097/01.prs.0000176259.66948.e7. discussion 780-781. [DOI] [PubMed] [Google Scholar]
- 21.Namnoum JD, Largent J, Kaplan HM, Oefelein MG, Brown MH. Primary breast augmentation clinical trial outcomes stratified by surgical incision, anatomical placement and implant device type. J Plast Reconstr Aesthet Surg. 2013;66(9):1165–1172. doi: 10.1016/j.bjps.2013.04.046. [DOI] [PubMed] [Google Scholar]
- 22.Asplund O, Gylbert L, Jurell G, Ward C. Textured or smooth implants for submuscular breast augmentation: a controlled study. Plast Reconstr Surg. 1996;97(6):1200–1206. doi: 10.1097/00006534-199605000-00015. [DOI] [PubMed] [Google Scholar]
- 23.Barnsley GP, Sigurdson LJ, Barnsley SE. Textured surface breast implants in the prevention of capsular contracture among breast augmentation patients: a meta-analysis of randomized controlled trials. Plast Reconstr Surg. 2006;117(7):2182–2190. doi: 10.1097/01.prs.0000218184.47372.d5. [DOI] [PubMed] [Google Scholar]
- 24.Wong CH, Samuel M, Tan BK, Song C. Capsular contracture in subglandular breast augmentation with textured versus smooth breast implants: a systematic review. Plast Reconstr Surg. 2006;118(5):1224–1236. doi: 10.1097/01.prs.0000237013.50283.d2. [DOI] [PubMed] [Google Scholar]
- 25.Hakelius L, Ohlsén L. Tendency to capsular contracture around smooth and textured gel-filled silicone mammary implants: a five-year follow-up. Plast Reconstr Surg. 1997;100(6):1566–1569. doi: 10.1097/00006534-199711000-00030. [DOI] [PubMed] [Google Scholar]
- 26.Spear SL, Elmaraghy M, Hess C. Textured-surface saline-filled silicone breast implants for augmentation mammaplasty. Plast Reconstr Surg. 2000;105(4):1542–1552. discussion 1553-1554. [PubMed] [Google Scholar]
- 27.Collis N, Coleman D, Foo IT, Sharpe DT. Ten-year review of a prospective randomized controlled trial of textured versus smooth subglandular silicone gel breast implants. Plast Reconstr Surg. 2000;106(4):786–791. doi: 10.1097/00006534-200009040-00005. [DOI] [PubMed] [Google Scholar]
- 28.Burkhardt BR, Demas CP. The effect of Siltex texturing and povidone-iodine irrigation on capsular contracture around saline inflatable breast implants. Plast Reconstr Surg. 1994;93(1):123–128. discussion 129-130. [PubMed] [Google Scholar]
- 29.Jewell ML, Jewell JL. A comparison of outcomes involving highly cohesive, form-stable breast implants from two manufacturers in patients undergoing primary breast augmentation. Aesthet Surg J. 2010;30(1):51–65. doi: 10.1177/1090820X09360700. [DOI] [PubMed] [Google Scholar]
- 30.Hedén P, Bronz G, Elberg JJ, et al. Long-term safety and effectiveness of Style 410 highly cohesive silicone breast implants. Aesthetic Plast Surg. 2009;33(3):430–436. doi: 10.1007/s00266-009-9360-x. discussion 437-438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bengtson BP. The highly cohesive, Style 410 form-stable gel implant for primary breast augmentation. In: Spear SL, editor. Surgery of the Breast: Principles and Art. 3rd ed. Baltimore, MD: Lippincott Williams & Wilkins; 2010. p. 1346. [Google Scholar]
- 32.Schafer M, Adams WP, Jr, Chase S. Detection of breast implant rotation using in-office high-resolution ultrasound [abstract 1846503] J Ultrasound Med. 2014;33(Suppl):S31. [Google Scholar]
- 33.Harrison P. Ultrasound fast alternative to MRI for breast implant status. 2014 http://www.medscape.com/viewarticle/822918. Accessed May 19, 2014. [Google Scholar]
- 34.de Jong D, Vasmel WL, de Boer JP, et al. Anaplastic large-cell lymphoma in women with breast implants. JAMA. 2008;300(17):2030–2035. doi: 10.1001/jama.2008.585. [DOI] [PubMed] [Google Scholar]
- 35.Miranda RN, Aladily TN, Prince HM, et al. Breast implant-associated anaplastic large-cell lymphoma: long-term follow-up of 60 patients. J Clin Oncol. 2014;32(2):114–120. doi: 10.1200/JCO.2013.52.7911. [DOI] [PMC free article] [PubMed] [Google Scholar]