Abstract
Background
The aim of this study was to evaluate the efficiency of the combination of Paris and Vienna classifications in a follow-up study of gastric epithelial neoplasia (GEN) patients.
Material/Methods
This study was conducted between January 2003 and September 2010, during which 170 biopsy-proven GEN patients were followed up by gastroenterologists and pathologists according to our follow-up regimen (modified Vienna classification).
Results
In total, 161 patients with low-grade neoplasia (LGN) and 9 patients with high-grade neoplasia (HGN) were randomly enrolled in our study. Eighteen patients with depressed appearance were observed, of which 9 patients had HGN and 9 patients had low-grade dysplasia (LGD). Three patients with type 0-IIa were observed with low-grade adenoma (LGA), and type 0–I was observed in 2 patients with LGN. Endoscopic or surgical treatments were performed to avoid potential malignancy or bleeding. Two patients with ulcer lesions, 2 patients with non-depressed type 0 appearance, and 3 patients without visible lesions were shown to have higher-grade lesions during follow-up. The misdiagnosis rate of forceps biopsy – 62.07% – was determined by comparing pre- and post-resection diagnoses of 29 patients.
Conclusions
The combination of the Paris and Vienna classifications for GEN may optimize the follow-up routines for patients with suspicious precancerous lesions and may significantly improve the detection of early gastric cancer (EGC) while helping gastroenterologists select the best therapy option.
MeSH Keywords: Endoscopy, Gastrointestinal; Follow-Up Studies; Pathology; Stomach Neoplasms
Background
Gastric cancer is a malignancy associated with considerable morbidity and mortality worldwide. The prognosis of advanced gastric cancer is significantly worse compared with early gastric cancer (EGC) [1]; therefore, how to detect and treat EGC is an important issue around the world. Since the inflammation-atrophy-metaplasia-dysplasia-carcinoma sequence was raised as a human model of gastric carcinogenesis [2], dysplasia, which is the last step of the development process for precancerous lesions, has been the major topic of discussion between endoscopists and pathologists.
Dysplasia was precisely defined by an international IBD-dysplasia morphology study group as lesions showing “unequivocal, non-invasive (confined within the basement membrane), neoplastic transformation of the epithelium excluding all reactive changes” and was then applied extensively to the whole gastrointestinal tract [3]. Dysplasia mainly depends upon the recognition of morphological features such as cytological and architectural changes in routinely processed hematoxylin and eosin (H&E)-stained sections [4]. Because large discrepancies existed between Western and Japanese pathologists, a series of meetings were held in Padova and Vienna for developing common terminologies for gastrointestinal epithelial neoplasia (GEN) [5–8]. Recently, Korean pathologists have made an effort to achieve an improved diagnostic consensus for GEN [9]. The revised Vienna classification categorizes biopsy-proven dysplasias into 5 groups and gives recommendations for each group [6]. The cut-off for choosing between endoscopic resection or follow-up for low-grade neoplasia (LGN) was not clearly established, while the choice of management for higher or lower grades was generally accepted.
In contrast to the pathological perspective of the Vienna classification of GEN, the Paris classification is an endoscopic/macroscopic standard for GEN [10]. In the outstanding clinical research performed by Japanese experts, the medical term “type 0” was defined for the endoscopic gross appearance of any tumor resembling early carcinoma, including adenoma/dysplasia and advanced carcinoma [11]. Not all type 0 lesions are malignant; for example, a type 0–IIa lesion can be a juvenile or proliferative polyp, and a type 0–III lesion can be a benign ulcer [10].
The above indicates that diagnosis of a precancerous lesion cannot be accomplished by pathology or endoscopy alone. We presumed that combining the 2 procedures may be a more optimal method during our follow-up study.
Material and Methods
Patients
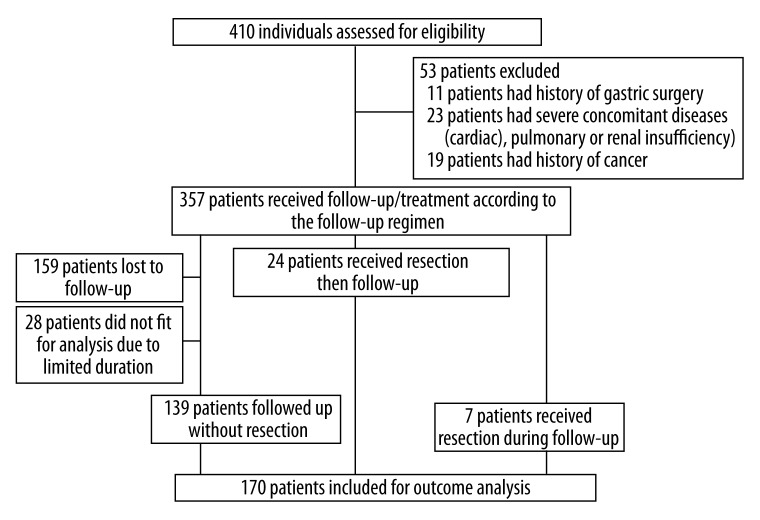
Our prospective study was conducted in the Second Affiliated Hospital of Zhejiang University School of Medicine. Patients who had undergone gastroscopy and biopsy-proven GEN were included between January 2003 and September 2010. Exclusion criteria included patients with a past history of cancer, those who have been using NSAIDs medications, those who have previously undergone surgical or endoscopic treatments, and those with severe concomitant illnesses (e.g., cardiac, respiratory, hepatic, or renal insufficiency). A total of 410 consecutive patients were enrolled in our prospective study. A total of 170 patients with a follow-up of more than 12 months (at least 3 examinations, mean 28 months, range 12–80 months) were finally documented. There were 101 male patients (mean age 56 years, range 29–81 years) and 69 female patients (mean age 56 years, range 33–82 years). Written informed consent was obtained from all of the above patients (see detailed flowchart in Figure 1).
Figure 1.
Flow of study participants.
Endoscopic assessment
The clinical study was carried out with conventional single-channel endoscopes (Olympus GIF-XQ 240 and GIF-XQ 260; Olympus Optical Co., Ltd, Tokyo, Japan).
The macroscopic description of GEN was classified into the following 3 types: type 0 (including type 0–I, IIa, IIb, and type 0–IIc, IIa+IIc), no visible lesions (including patients without specific lesions but with background lesions such as erosion, atrophy, and superficial gastritis), and the ulcer type (including type 0–III; before we knew the pathologic results, we used “ulcer” instead of “type 0–III”). Type 0 lesions were divided into depressed and non-depressed categories.
The stomach was divided into 5 sections: cardia, fundus, body, antrum, and pylorus. The cross-sectional circumference was divided into 4 equal sections: greater curvature, lesser curvature, anterior wall, and posterior wall [12].
The detailed information (e.g., size and place) should be added after the macroscopic description.
Biopsies were taken from visible lesions ranging 2–5, and biopsies were randomly taken from 2 sites with less curvature in the gastric antrum if there were no visible lesions. Biopsies were taken from the same place during follow-up.
Histological assessment and H. pylori testing
To minimize intra-procedural variation, all forceps biopsies were evaluated by the same experienced pathologist. All biopsy samples were fixed in 10% formalin and embedded in paraffin. Sections were cut into 4-μm thickness for H&E staining. Endoscopic mucosal resection (EMR), endoscopic submucosal dissection (ESD) or polypectomy specimens were washed in normal saline, fixed in 10% formalin, sectioned serially at 2-mm intervals, and entirely embedded in paraffin. Surgical specimens were fixed in 10% formalin, after recording lesion location, number, size and macroscopic type, and each lesion was removed, embedded in paraffin, and sectioned along the longitudinal axis for H&E staining. Lymph nodes around the stomach were processed in the same way, and the number of metastases was recorded.
Helicobacter pylori infection was defined as positive if a breath test, an H. pylori culture, or a histological evaluation according to the updated Sydney system [13] was positive. Breath tests for H. pylori were performed 1 month later after withdrawal of PPIs, bismuth, and any antibiotics in case of a false-negative result. The biopsy specimens for H. pylori tests were taken from the lesser curvature of the antrum.
Medical treatments
Most patients mainly had problems such as abdominal distension, acid regurgitation, and dyspepsia. Proton pump inhibitors (PPIs) and mucosal-protective drugs were prescribed to patients to relieve their symptoms. If the H. pylori test was positive, patients received H. pylori eradication therapy for 2 weeks until a negative result was obtained.
Endoscopic and surgical treatments
Endoscopic resection included ESD or EMR, with patients under intravenous sedation. With the application of marking dots around the lesion and necessary hemostatic treatment such as endoscopic clipping or thermocoagulation, every lesion was completely removed with negative margins and no complications.
EMR was performed with a snare and the Endocut mode (50 W) of an electrosurgical generator (PSD-60; Olympus), after a saline solution containing 0.005 mg/mL epinephrine was injected into the submucosa beneath the lesion.
ESD was performed with an insulated tip knife (Olympus). After a 10% glycerin solution that contained 0.005 mg/mL epinephrine was injected into the submucosa, a circumferential incision of the mucosa was then made outside the marking dots. The whole tissue, including some submucosa connective tissue, was dissected en bloc from the muscle layer [14].
Patients with HGN, or LGA but suspicious for potential malignancy received surgical gastrectomy due to incomplete endoscopic resection. The endoscopist marked the lesion locations with forceps or methylene blue staining.
Follow-up regimen
According to the Vienna classification (Revised, 2002) [7], biopsy-proven LGA or dysplasia belongs to category 3 (low-grade GEN), and high-grade adenoma or dysplasia belongs to category 4 (high-grade GEN).
Previous reports strongly suggested that high-grade adenoma/dysplasia (HGD, category 4 in the Vienna classification) is highly predictive of invasive carcinoma (category 5), which either coexists or appears thereafter. Therefore, complete endoscopic or surgical resection is strongly recommended for patients with HGD, regardless of the macroscopic type [2,6,7].
We focused on the patients who have biopsy-proved dysplasia during the enrollment process. Patients with Paris classification (which may indicate dysplasia in pathology) were paid special attention. We followed up this group of patients every half year. Patients were enrolled into our group as soon as dysplasia was found. Patients without dysplasia in 2-year follow-up were not traced thereafter.
Gastric adenoma is a benign tumor of the glandular epithelium with varying degrees of cellular atypia and has papillary and/or tubular structures [15]. Gastric adenomas tend to develop into invasive adenocarcinoma [13–16]. Tumor size and grading of dysplasia have been indicated as prognostic factors of gastric adenomas [17], and the definite malignant potential of adenomas is indicative for endoscopic resection even if the adenomas are low-grade.
While the recommendations for follow-up or endoscopic resection for category 3 (LGA/LGD) listed by the Vienna classification (Revised, 2002) seem very reasonable for managing LGN, endoscopists and gastroenterologists are still confused about how to proceed. No consensus or guideline for the detailed management of biopsy-proven LGN has yet been reached. Based on the present follow-up, we subdivided patients of this group into several categories with different recommendations. For patients who had no visible lesions, follow-up alone was recommended. For type 0 lesions, endoscopic resection was recommended for depressed lesions, and close follow-up was suitable for non-depressed lesions.
Ulcer lesions should be carefully examined. Biopsy-proven HGN surrounding an ulcer lesion demonstrate potential malignancy and should be removed immediately. While LGD is not very indicative, it could indicate gastric cancer or inflammatory changes. Therefore, close follow-up should be initiated, and re-biopsy should be performed until healing takes place or higher-level neoplasia is observed. This regimen is summarized in Table 1.
Table 1.
Modified Vienna classification (our follow-up and management regimen).
| Category | Diagnosis | Clinical management |
|---|---|---|
| 3 | Low grade neoplasia | Endoscopic resection or follow-up |
| 3.1 | Low grade adenoma | Endoscopic resection |
| 3.2 | Low grade dysplasia | |
| No visible lesion group | Follow up (resection if potential for bleeding) every year | |
| Non-depressed type 0 group | Closely follow-up | |
| Depressed type 0 group | Endoscopic resection | |
| Ulcer lesions | Rebiopsies, closely follow-up | |
| 4 | High grade neoplasia | Endoscopic resection/surgery |
| 4.1 | High grade adenoma/dysplasia | |
| 4.2 | Non-invasive carcinoma (carcinoma in situ) | |
| 4.3 | Suspicious for invasive carcinoma | |
| 4.4 | Intramucosal carcinoma | |
| 5 | Submucosal invasion by carcinoma | Surgical resection |
Results
Based on the biopsy-proven findings, 170 patients were identified with GEN – 161 patients with LGN and 9 patients with HGN (Table 2).
Table 2.
Demographic data and clinicopathological characteristics from 170 consecutive patients with biopsy-diagnosed gastric epithelial neoplasia (No. of H.pylori positive in the brackets).
| Low grade neoplasia (n=161) | High grade neoplasia (n=9) | Total (n=170) | |
|---|---|---|---|
| Demographics | |||
| Sex (men/women) | 95/66 | 6/3 | 101/69 |
| Age, mean ±SD (years) | 56.2±10.8 | 61.2±9.6 | 56.4±10.8 |
| Endoscopic feature, no.(%) | |||
| No visible lesion group | 132 (36) | 0 | 132 (36) |
| Type 0 | 18 (6) | 9 (2) | 27 (8) |
| Non-depressed type 0 | 9 (3) | 0 | 12 (3) |
| Depressed type 0 | 9 (3) | 9 (2) | 15 (5) |
| Size of lesion, mean ±SD (mm) | 1.30±0.50 | 1.51±0.59 | 1.37±0.53 |
| Background lesion | |||
| Atrophic gastritis | 16 (5) | 7 (1) | 23 (6) |
| Non-atrophic gastritis | 2 (1) | 2 (1) | 4 (2) |
| Location of lesion | |||
| Fundus | 0 | 1 (0) | 1 (0) |
| Body | 4 (1) | 2 (0) | 6 (1) |
| Antrum | 14 (5) | 6 (2) | 20 (7) |
| Surface color | |||
| Normal mucosa | 4 (0) | 0 | 4 (0) |
| Erythema | 14 (6) | 9 (2) | 23 (8) |
| Surface nodularity | |||
| Smooth | 9 (3) | 9 (2) | 18 (5) |
| Nodular | 9 (3) | 0 | 9 (3) |
| Ulcer | 11 (7) | 0 | 11 (7) |
| Single | 7 (5) | / | 7 (5) |
| Multiple | 4 (2) | / | 4 (2) |
| Background lesion | |||
| Atrophic gastritis | 1 (0) | / | 1 (0) |
| Non-atrophic gastritis | 10 (7) | / | 10 (7) |
| Location of lesion | |||
| Fundus | 1 (1) | / | 1 (1) |
| Body | 1 (1) | / | 1 (1) |
| Antrum | 9 (5) | / | 9 (5) |
| Pathological feature, no. (%) | |||
| Low grade dysplasia | 157 (48) | / | 157 (48) |
| Low grade adenoma | 4 (1) | / | 4 (1) |
According to our follow-up and treatment regimen, 24 patients had risk factors for malignancy changes or the potential for bleeding. Twenty-three of these patients received endoscopic resections or surgery. One patient had biopsy-proven LGA and type 0–IIa+IIc appearance, but ESD failed to remove the lesion; therefore, the patient chose the follow-up regimen (Table 3).
Table 3.
24 patients received interventional treatments after initial evaluations.
| Patients need resection at enrollment | Managements | Outcomes |
|---|---|---|
| 9 HGN | 7 surgery,1 EMR and 1 ESD | 1 advanced gastric carcinoma,6 early cancer, 1 HGD, 1 LGD |
| 3 LGA with non-depressed type 0 appearance | 2 EMR, 1 ESD | 2 LGA, 1 HGA |
| 1 LGA with depressed type 0 appearance | ESD | Failed, then chose follow-up |
| 9 LGD with depressed type 0 appearance | 1 surgery, 2 EMR and 6 ESD | 1 hyperplastic polyp, 2 LGD, 3 HGD, 1 HGA, 2 early cancer |
| 2 LGD with type 0-I lesion potential for bleeding | 1 EMR, 1 polypectomy | 1 hyperplastic polyp, 1 juvenile polyp with LGD |
HGN – high grade neoplasia; EMR – endoscopic mucosa resection; ESD – endoscopic submucosal dissection; HGD – high grade dysplasia; LGD – low grade dysplasia; LGA – low grade adenoma; HGA – high grade adenoma.
In total, 146 patients with LGD were documented. Two patients with ulcer lesions had biopsy-proven LGD, but re-biopsy within 1 month suggested HGD, while intramucosal carcinoma and high-grade dysplasia were proven with gastrectomy. Another 9 patients with ulcer lesions healed in the absence of neoplasia.
Two patients with type 0–IIa lesions and biopsy-proven LGD became LGA in 1 year and underwent endoscopic resection. Another 2 patients with type 0–IIa lesions disappeared endoscopically and pathologically during the follow-up. Three patients with LGD but no visible lesion progressed endoscopically or pathologically. One patient had a type IIc lesion in 1 year and received ESD, while the other 2 had biopsies of LGA and received EMR or ESD after 2- and 7-year follow-up, respectively.
The rest of the 128 non-type 0 patients did not progress endoscopically or pathologically. Eighty patients regressed spontaneously from the first follow-up examination; 31 of these patients regressed after a period of fluctuation between normal status and dysplasia and 18 of these patients persisted with LGD.
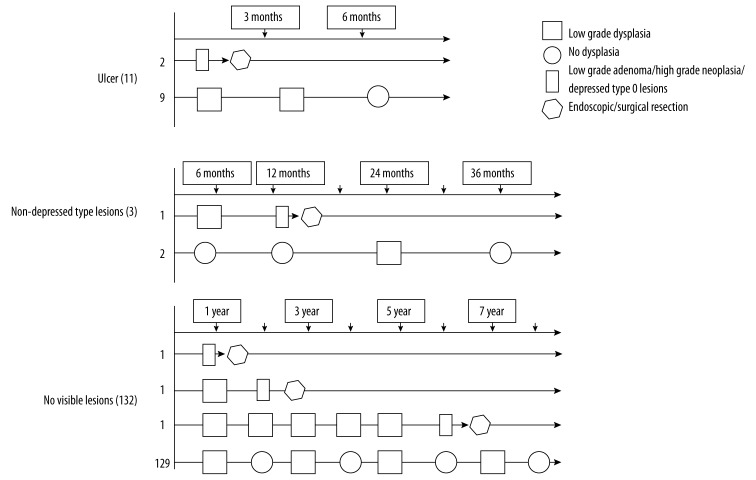
The detailed follow-up processes are described in Figure 2.
Figure 2.
Follow-up chart for low-grade neoplasia.
Figure 3 shows the endoscopic and histological follow-up record of a typical case with a major discrepancy between biopsy and final diagnosis.
Figure 3.
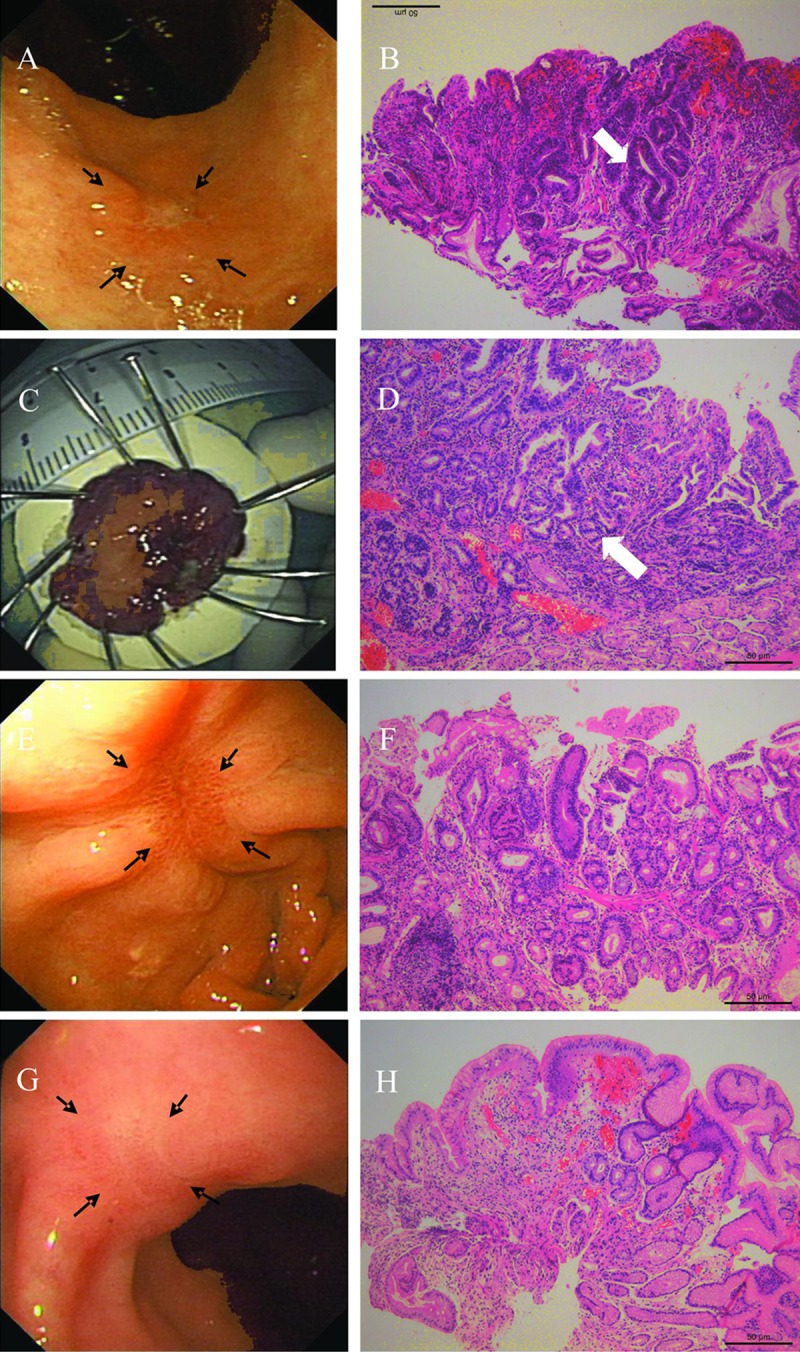
Follow-up process of one patient. (A) A 15-mm in size, superficial, and shallow depressed (type 0–IIa+IIc type in the Paris classification) lesion at the antrum (black arrows). (B) Histological morphology of low-grade dysplasia (white arrow) with the initial biopsy specimen, elongated gastric glands, and hyperplasia of fibrous tissue at the lamina propria (H&E, original magnification ×100). (C) The lesion was removed by ESD. (D) Histological features of cancerous foci in the ESD specimen. Crowding glandular cells, with obvious structural and cellular atypia (white arrow) (H&E, original magnification ×100). (E) The red ESD scar 1 month later (black arrows). (F) Histological findings suggested regenerative activity of glandular cells. (G) The white ESD scar 9 months later (black arrows). (H) Histological findings suggested elongated glandular cells with inflammatory cell infiltration and an absence of dysplasia.
Efficiency of our follow-up and treatment regimen
There were totally 4 patients who had type 0 lesions without dysplasia which were not traced after 2-year follow-up, and 4 patients who had no dysplasia initially and were found to have dysplasia in follow-up were thus finally enrolled into our study.
In total, there were 29 patients of gastric mucosal neoplasia who received endoscopic or surgical resections in our follow-up study, including 24 patients at enrollment (Table 3). The indications for interventional therapy were: HGN, LGA, depressed type 0 lesions with LGD, and other lesions with potential for bleeding.
By comparing the biopsy and post-resection diagnoses, the misdiagnosis rate was 62.07% (18/29), and the under-diagnosis rate was 51.72% (15/29), with 38.9% (7/18) in the LGN group and 72.7% (8/11) in the HGN group, respectively (Table 4).
Table 4.
Biopsy and post-resection diagnosis of 29 patients who received interventional treatment.
| No. | Genda | Age | Macroscopic | Biopsy-diag | Vienna | Management | Final-diag | Vienna |
|---|---|---|---|---|---|---|---|---|
| 1 | F | 59 | I | LGD | Category 3.2 | EMR | Hyperplastic polyp | / |
| 2 | F | 44 | I | LGD | Category 3.2 | Polypectomy | Juvenile,LGD | / |
| 3 | F | 55 | IIa | LGA | Category 3.1 | EMR | LGA | Category 3.1 |
| 4 | F | 55 | IIa | LGA | Category 3.1 | EMR | LGA | Category 3.1 |
| 5 | M | 68 | IIa | LGA | Category 3.1 | ESD | LGA | Category 3.1 |
| 6 | M | 45 | IIa | LGA | Category 3.1 | EMR | LGA | Category 3.1 |
| 7 | F | 60 | IIa | LGA | Category 3.1 | EMR | LGA | Category 3.1 |
| 8 | M | 78 | IIa | LGA | Category 3.1 | ESD | LGA | Category 3.1 |
| 9 | M | 56 | IIa | LGA | Category 3.1 | EMR | HGA | Category 4.1 |
| 10 | M | 49 | IIa+IIc | LGD | Category 3.2 | EMR | Hyperplastic polyp | / |
| 11 | M | 59 | IIa+IIc | LGD | Category 3.2 | ESD | LGD | Category 3.2 |
| 12 | F | 56 | IIa+IIc | LGD | Category 3.2 | ESD | LGD | Category 3.2 |
| 13 | M | 34 | IIa+IIc | LGD | Category 3.2 | EMR | HGA | Category 4.1 |
| 14 | M | 62 | IIa+IIc | LGD | Category 3.2 | ESD | HGD | Category 4.1 |
| 15 | F | 56 | IIc | LGD | Category 3.2 | ESD | HGD | Category 4.1 |
| 16 | M | 51 | IIa+IIc | LGD | Category 3.2 | SURGERY | HGD | Category 4.1 |
| 17 | F | 64 | IIa+IIc | LGD | Category 3.2 | ESD | Intramucosal ca | Category 4.4 |
| 18 | M | 71 | IIa+IIc | LGD | Category 3.2 | ESD | Intramucosal ca | Category 4.4 |
| 19 | M | 55 | IIa+IIc | HGD | Category 4.1 | EMR | LGD | Category 3.2 |
| 20 | M | 74 | IIc | HGD | Category 4.1 | SURGERY | HGD | Category 4.1 |
| 21 | F | 72 | IIa+IIc | HGD | Category 4.1 | SURGERY | Intramucosal ca | Category 4.4 |
| 22 | F | 45 | IIc | HGD | Category 4.1 | SURGERY | Intramucosal ca | Category 4.4 |
| 23 | M | 54 | IIa+IIc | HGD | Category 4.1 | SURGERY | Intramucosal ca | Category 4.4 |
| 24 | F | 70 | IIc | HGD | Category 4.1 | SURGERY | Intramucosal ca | Category 4.4 |
| 25 | M | 56 | IIc | HGD | Category 4.1 | ESD | Intramucosal ca | Category 4.4 |
| 26 | M | 69 | IIa+IIc | HGD | Category 4.1 | SURGERY | Submucosal ca | Category 5 |
| 27 | M | 56 | IIa+IIc | HGD | Category 4.1 | SURGERY | Advan.Ca | Type 2* |
| 28 | M | 78 | Ulcer | HGD | Category 4.1 | SURGERY | HGD | Category 4.1 |
| 29 | M | 68 | Ulcer | HGD | Category 4.1 | SURGERY | Intramucosal ca | Category 4.4 |
Type 2 is ulcerative type of macroscopic classifications of advanced gastric cancer according to Japanese Gastric Cancer Association, means ulcerated tumors with raised margins surrounded by a thickened gastric wall with clear margins.
F – female; M – male; EMR – endoscopic mucosa resection; ESD – endoscopic submucosal dissection; HGD – high grade dysplasia; LGD – low grade dysplasia; LGA – low grade adenoma; HGA – high grade adenoma; ca: carcinoma.
Discussion
Dysplasia was initially proposed by an international IBD-dysplasia morphology study group as lesions showing “unequivocal, non-invasive (confined within the basement membrane), neoplastic transformation of the epithelium excluding all reactive changes”. However, the presence of dysplasia was confusing. Diagnosis of dysplasia mainly depends on the recognition of morphological features such as cytological and architectural changes in routinely processed hematoxylin and eosin-stained sections, and our pathologists could not clearly differentiate these changes. Consequently, most endoscopists or gastroenterologists will be confused about dysplasia, and a definite diagnosis of a neoplastic lesion cannot be determined; thus, resection cannot be confidently recommended. Based on the consensus reached at the international pathological conferences held in Padova and Vienna, biopsy-proven HGN became a definite indication for resection, while LGD/LGN was still confusing. Previous studies showed that most cases of LGN disappeared due to over-diagnosing regenerative (i.e., inflammatory) lesions as neoplastic lesions [18]. In fact, we determined that most “dysplasia” specimens in pathological reports were randomly biopsied, and no visible lesions were observed in the endoscopic examination. There were 132/170 (77.6%) patients with LGN but no visible lesions; of the 132 cases, 111 (84.1%) regressed and 18 (13.6%) persisted, which agreed with previous studies. Depending on the recognition of outcomes in this group of neoplasia, we can presume that patients with neoplasia and no visible lesions may display reactive or regenerative inflammatory changes and can be reversed with or without medications.
The Paris classification of superficial neoplastic lesions assisted us greatly in recognizing “real neoplastic dysplasia”. Since the macroscopic classification of early gastric carcinomas was established by Japanese experts, the prefix “type 0” (e.g., 0–I, 0–IIa) has been widely used to distinguish early and superficial carcinomas from advanced carcinomas and is becoming the general practice in detecting endoscopic gross appearances of any tumor resembling early carcinoma, including adenoma/dysplasia and advanced carcinoma [11]. To extend the useful methodology of detecting precursor lesions endoscopically, Japanese experts convinced other participants to reach a consensus regarding the Paris endoscopic classification of superficial neoplastic lesions in 2002. “Superficial” neoplasia includes neoplastic lesions with no invasion in the lamina propria and carcinoma with invasion of the lamina propria and a depth of penetration limited to the mucosa (stomach and esophagus) or the submucosa (large bowel). Almost every superficial neoplasia has a “superficial” morphology, but not every lesion of superficial morphology suggests neoplasias (e.g., hyperplastic polyps in the GI tract have little or no potential for transformation into neoplastic lesions but always have a protruding morphology). It is reasonable that polypoid precursors play a minor role in the development of advanced gastric cancer, while depressed non-polypoid lesions (type 0–IIc) are the usual precursors [10]. Cho et al. [19] analyzed 236 LGD lesions treated with endoscopic resection and showed that depressed morphology, surface erythema, or a size of 1 cm or greater were risk factors for HGD/carcinoma; thus, endoscopic resection is recommended even with biopsy-proven LGD. Based on the above, we showed that the Paris classification is an efficient and accurate way to screen neoplastic and non-neoplastic lesions, and depressed appearance can be a specific indication for endoscopic or surgical resection. In our study, 4 patients who had type 0 lesions without dysplasia were not traced after 2-year follow-up, and 4 patients who had no dysplasia initially and found to have dysplasia thus were finally enrolled into our study. Eighteen patients with type 0 lesions and LGN were divided into 2 groups: 9 patients with non-depressed lesions and 9 with depressed lesions; clinical management revealed that 6/9 of the latter group were progressing to cancer (Table 3).
Gastric adenomas are benign epithelial neoplastic tumors with a glandular organization that are characterized by localized proliferation of adenomatous epithelium with tubular and/or papillary structures [15]. Kamiya et al. reviewed 74 patients in the 1980s and observed gastric adenomas that underwent malignant changes with gradual transformation from moderate to severe dysplasia [16]. Kasuga et al. recently retrospectively reviewed 231 gastric adenomas by comparing forceps biopsy and post-resection diagnoses and recommended en bloc resection even when forceps biopsy indicated LGA [14]. In our study, we subdivided LGN into LGA and LGD groups to treat them. Seven patients with biopsy-proven LGA were resected quickly (except for 1 who failed in ESD), including 1 patient with high-grade adenoma and 6 with LGAs.
Gastric ulcers have been frequently noted for their probability of malignancy. There are 2 hypotheses regarding malignant ulcers: first, long-time chronic ulcers lead to malignant changes, and second, the carcinoma itself presented with ulcer appearance [20]. There is no doubt that biopsy-proven HGN should be treated immediately, while in the case of biopsy-proven LGN, we can re-biopsy the lesion if suspicious for carcinoma or perform close follow-up within 1 month. In our study, 2 patients with LGN progressed to HGN within 1 month, which means that misdiagnosis took place the first time but the proper diagnosis was later discovered through close follow-up. There were 4 patients who developed multiple ulcers with LGN after the initial examinations, which may not indicate gastric cancer; however, close follow-up was required to exclude gastric lymphoma.
From our follow-up chart of 146 patients, except for 129 non-visible-lesion patients fluctuating between “LGD” and “normal”, 3 patients progressed to LGA or HGN ranging from 1 to 7 years, and 1 patient with non-depressed lesions progressed to LGA but finally had the lesions resected. Although the regression ratio was very high in the LGN group, a few did progress to HGN even after 6 years, which would be a possible sign of gastric cancer. We recommend using a follow-up strategy (we recommend at least once a year) for all cases of this group. For patients who have visible lesions (e.g., non-depressed type 0 lesions) with LGN, we recommend that the first follow-up should be initiated in 3–6 months to reduce the possibility of misdiagnosing gastric adenomas. Special attention should be paid to ulcer lesions with LGN, as we mentioned above. The follow-up interval should be strictly controlled for 1 month until definite endoscopic mucosal healing occurs.
In total, 29 patients received en bloc resection. All patients had 1 of the following indications: biopsy-proven HGN, biopsy-proven LGA, biopsy-proven LGD with depressed type 0 appearance, or potential for bleeding. By comparing the forceps biopsy and post-resection diagnoses, we determined that the misdiagnosis rate was 62.07% (18/29), and the under-diagnosis rate was 51.72% (15/29), with 38.9% (7/18) in the LGN group and 72.7% (8/11) in the HGN group. Endoscopic forceps biopsy (EFB) may cause errors in histological diagnosis and thus cause significant discrepancy between EFB and en bloc resection or surgical diagnosis [21]. Forceps biopsy errors are the leading cause of the high misdiagnosis rate, excluding variance among endoscopists and pathologists. In follow-up using the Paris classification and endoscopic techniques (EMR or ESD), with increasing attention to the probability of biopsy misdiagnosis, the problems seem to be solved to a certain extend; however, overtreatment can occur.
The limitations of the present study include the following points. First, we enrolled 410 patients in the initial examination; however, due to poor patient compliance, only 170 patients could complete our follow-up. Second, medications for H. pylori eradication were prescribed in our follow-up group, and the efficiency of medications for reversing low-grade gastric neoplasia have not been well evaluated; thus, we could not exclude the possibility that medications influenced the regression of gastric neoplasia. We plan to continue our study by obtaining results from patients on a larger scale.
Conclusions
Diagnosis of GEN by endoscopy or pathology alone was shown be insufficiently accurate. Additionally, the combination of the Vienna and Paris classifications could be helpful for follow-up and timely treatment of suspicious precancerous lesions and for reducing the misdiagnosis rate of EGC, especially in countries where, unlike Japan, the early detection and treatment rates for gastric cancer are low and advanced equipment (such as narrow-band imaging, NBI, or magnifying endoscopy) or detection experience are lacking.
Footnotes
Statement
All the authors declare that they have no conflict of interest.
Source of support: This research was supported by grants from the National Health Key Special Fund (No. 200802112), the Health Department Fund (No. 2007A093), the Traditional Chinese Medicine Bureau Fund (No. 2007ZA019), the Natural Science Fund of Zhejiang Province (No. Y2080001 and Y12H160121), the Zhejiang Province Education Department Fund (No. Y201121724 and Y201328489), the Zhejiang Province Health Department Fund (No. 201343550), the Key Project of Zhejiang Province (No. 2009C03012-5 and 2013C03044-5), and the National Natural Science Foundation of China (general project No. 81372302)
Reference
- 1.Wang ZQ, Cai Q, Jiang ZY, et al. Prognostic role of MicroRNA-21 in gastric cancer: A meta-analysis. Med Sci Monit. 2015;21:1668–74. doi: 10.12659/MSM.892096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Correa P. A human model of gastric carcinogenesis. Cancer Res. 1988;48:3554–60. [PubMed] [Google Scholar]
- 3.Riddell RH, Goldman H, Ransohoff DF, et al. Dysplasia in inflammatory bowel disease: standardized classification with provisional clinical applications. Hum Pathol. 1983;14:931–68. doi: 10.1016/s0046-8177(83)80175-0. [DOI] [PubMed] [Google Scholar]
- 4.Geboes K, Sagaert X, Geboes KP. Dysplasia Intraepithelial Neoplasia and Neoplasia without Dysplasia in the Digestive Tract. European Gastroenterology & Hepatology Review. 2011;7(1):37–42. [Google Scholar]
- 5.Rugge M, Correa P, Dixon MF, et al. Gastric dysplasia: the Padova international classification. Am J Surg Pathol. 2000;24:167–76. doi: 10.1097/00000478-200002000-00001. [DOI] [PubMed] [Google Scholar]
- 6.Schlemper RJ, Riddell RH, Kato Y, et al. The Vienna classification of gastrointestinal epithelial neoplasia. Gut. 2000;47:251–55. doi: 10.1136/gut.47.2.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dixon MF. Gastrointestinal epithelial neoplasia: Vienna revisited. Gut. 2002;51:130–31. doi: 10.1136/gut.51.1.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schlemper RJ, Kato Y, Stolte M. Review of histological classifications of gastrointestinal epithelial neoplasia: differences in diagnosis of early carcinomas between Japanese and Western pathologists. J Gastroenterol. 2001;36:445–56. doi: 10.1007/s005350170067. [DOI] [PubMed] [Google Scholar]
- 9.Kim JM, Cho MY, Sohn JH, et al. Diagnosis of gastric epithelial neoplasia: Dilemma for Korean pathologists. World J of Gastroenterol. 2011;17:2602–10. doi: 10.3748/wjg.v17.i21.2602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Inoue H, Kashida H, Kudo S, et al. The Paris endoscopic classification of superficial neoplastic lesions: esophagus, stomach, and colon: November 30 to December 1, 2002. Gastrointest Endosc. 2003;58:S3–4. doi: 10.1016/s0016-5107(03)02159-x. [DOI] [PubMed] [Google Scholar]
- 11.Schlemper RJ, Hirata I, Dixon MF. The macroscopic classification of early neoplasia of the digestive tract. Endoscopy. 2002;34:163–68. doi: 10.1055/s-2002-19855. [DOI] [PubMed] [Google Scholar]
- 12.Dixon MF, Genta RM, Yardley JH, Correa P. Classification and grading of gastritis: the updated Sydney system. Am J Surg Pathol. 1996;20:1161–81. doi: 10.1097/00000478-199610000-00001. [DOI] [PubMed] [Google Scholar]
- 13.Association JGC. Japanese classification of gastric carcinoma. Gastric Cancer. (3rd English edition) 2011;14:101–12. doi: 10.1007/s10120-011-0041-5. [DOI] [PubMed] [Google Scholar]
- 14.Kasuga A, Yamamoto Y, Fujisaki J, et al. Clinical characterization of gastric lesions initially diagnosed as low-grade adenomas on forceps biopsy. Dig Endosc. 2012;24:331–38. doi: 10.1111/j.1443-1661.2012.01238.x. [DOI] [PubMed] [Google Scholar]
- 15.Nakamura K, Sakaguchi H, Enjoji M. Depressed adenoma of the stomach. Cancer. 1988;62:2197–202. doi: 10.1002/1097-0142(19881115)62:10<2197::aid-cncr2820621021>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 16.Kamiya T, Morishita T, Asakura H, et al. Long-term follow-up study on gastric adenoma and its relation to gastric protruded carcinoma. Cancer. 1982;50:2496–503. doi: 10.1002/1097-0142(19821201)50:11<2496::aid-cncr2820501140>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 17.Rocco A, Caruso R, Toracchio S, et al. Gastric adenomas: relationship between clinicopathological findings, Helicobacter pylori infection, APC mutations and COX-2 expression. Ann Oncol. 2006;17:vii, 103–8. doi: 10.1093/annonc/mdl961. [DOI] [PubMed] [Google Scholar]
- 18.Rugge M, Cassaro M, Di Mario F, et al. The long term outcome of gastric non-invasive neoplasia. Gut. 2003;52:1111–16. doi: 10.1136/gut.52.8.1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cho SJ, Choi I, Kim C, et al. Risk of high-grade dysplasia or carcinoma in gastric biopsy-proven low-grade dysplasia: an analysis using the Vienna classification. Endoscopy. 2011;43:465–71. doi: 10.1055/s-0030-1256236. [DOI] [PubMed] [Google Scholar]
- 20.Morson BC, Sobin LH, Grundmann E, et al. Precancerous conditions and epithelial dysplasia in the stomach. J Clin Pathol. 1980;33:711–21. doi: 10.1136/jcp.33.8.711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee CK, Chung I-K, Lee S-H, et al. Is endoscopic forceps biopsy enough for a definitive diagnosis of gastric epithelial neoplasia? J Gastroenterol Hepatol. 2010;25:1507–13. doi: 10.1111/j.1440-1746.2010.006367.x. [DOI] [PubMed] [Google Scholar]