Abstract
Background
Failure to include participants of diverse race and ethnicity (i.e. those other than European Caucasian, non-Hispanic) in clinical trials impedes the safe development of new therapies given the potential for racial/ethnicity-related variations in treatment response. Increasing diversity is problematic for low prevalence diseases, where most community-based approaches do not reach those with the disease.
Purpose
Increase racial/ethnic diversity of participants in a Parkinson's disease therapeutic trial.
Methods
We incorporated a randomized Ancillary Trial into the multisite National Institute of Neurologic Disorders and Stroke Exploratory Trials in Parkinson's Disease Long-Term Study 1. Movement disorders clinics already participating in long-term trial 1 were eligible and were the unit of randomization and analysis. At least 14% of adult residents over age 55 and living within 30 miles of the eligible site were from a diverse population, or there was a near-by zip code with a highly diverse population. Eligible sites also agreed to be randomized. The intervention was designed to increase community physicians’ trust in long-term trial 1 investigators and address recruitment barriers in diverse populations. Primary outcomes included percentage of participants from diverse racial/ethnic groups enrolled in long-term trial 1, and qualitative findings from key informant interviews of the Ancillary Trial investigators and coordinators at the end of the trial.
Results
The Ancillary Trial stopped early for lack of efficacy, conditional power less than 1%. The 17 intervention sites had 12.6% diverse participants compared to 15.6% in 15 control clinics; odds ratio 0.82 (95% confidence interval = 0.32−2.16). In key informant interviews, high enrollers of diverse participants reported more use of existing physician relationships, untargeted community outreach, and extensive efforts to overcome participants’ barriers. Low enrollers reported more use of patients in their practices and placed more responsibility for low enrollment on prospective participants.
Limitations
The Ancillary Trial included only those with Parkinson's disease. Whether our findings generalize to trials in other low prevalence diseases is unknown.
Conclusions
Increasing diversity in Parkinson's disease clinical trials requires new paradigms for trial investigator and coordinator interactions with community physicians and prospective trial participants.
Introduction
Lack of racial and ethnic diversity in clinical trials impedes the safe development of new therapies given the potential for racial/ethnicity related variations in treatment response [1,2]. Diversity has been successfully achieved in many clinical trials for common diseases such as hypertension and diabetes through faith-based organizations, beauty parlors, barbershops, and other community venues [3,4]. For diseases of low prevalence (<5%), such as Parkinson's Disease (PD), amyotrophic lateral sclerosis (ALS), Huntington's, and early-onset Alzheimer's disease, community recruitment strategies have a low likelihood of reaching those with the disease. Clinical trials for diseases of low prevalence usually are based in specialty clinics where more patients with the condition of interest may be seen; yet those from diverse racial and ethnic groups often have limited access to specialty care [5] and may be deterred by institutional barriers to referral [3].
As part of the National Institute of Neurologic Disorders and Stroke (NINDS) Exploratory Trials in Parkinson's Disease Long-Term Study 1 (NET-PD LS-1), we conducted an Ancillary Trial of a recruitment intervention to increase diversity in the patients enrolled in LS-1. We hypothesized that increasing community physicians’ referrals to LS-1 would be critical to increasing recruitment of racially and ethnically diverse participants. We report the Ancillary Trial results.
Methods
Purpose
We defined diverse participants as participants of self-defined race/ethnicity other than European Caucasian, non-Hispanic, using the categories defined in the National Institutes of Health (NIH) Target Enrollment Table. Previously, NET-PD conducted two randomized Phase II futility trials designed to discard treatments that were futile to carry forward into Phase III. In these two previous Phase II futility trials [6,7], 9% of all participants were from diverse populations, consistent with other trials in PD [8]. The goal of the LS-1 Ancillary Trial was to increase participant diversity in the next NET-PD trial, LS-1. LS-1 is a 5-year placebo-controlled Phase III randomized trial of creatine as a treatment to slow PD progression. Creatine was chosen from among four treatments studied using the Phase II futility trials. The Ancillary Trial was conducted at a subset of the movement disorders or PD clinics where LS-1 was conducted. We tested the hypothesis that LS-1 intervention sites would enroll a greater proportion of diverse participants than LS-1 control sites.
Site eligibility criteria
For LS-1 clinics to be eligible for the Ancillary Trial, at least 14% of adult residents over age 55 living within 30 miles of the site were from a diverse population, or there was a near-by zip code with a highly diverse population. Eligible clinics also agreed to be randomized.
Intervention
The prevalence of PD in the general population in industrialized countries is 1% [9]. Thus, the intervention was not focused on the lay community but on local neurologists, internists, and primary care physicians practicing in areas within 30 miles of the trial site. The physician focus was broad given the shortage of community neurologists near many sites. The intervention was based on literature suggesting a physician's advice or referral is a strong incentive for patient participation in clinical research [4,10,11], findings from our focus groups with PD patients, literature regarding the attitudes of minorities toward participation in medical research [12], and literature regarding community physicians’ attitudes toward referral of their patients to medical research [13–18].
NINDS funded 15% of a recruitment coordinator for sites randomized to intervention in the first year (6 h/week) and 10% (4 h/week) in the second year of the Ancillary Trial. The recruitment coordinator was to have a strong connection to the community, as defined by the site. Funds were provided for a continuing medical education (CME) event for local physicians in the target area, including funds for an outside speaker. CME included information on current treatment of PD, LS-1, race/ethnicity-based therapeutics, and approaches designed to reduce community physician concerns about medical research. Community physicians received a sample letter to mail to their PD patients regarding LS-1. A business agreement meeting Health Insurance Portability and Accountability Act (HIPAA) requirements was made available to physicians who wanted help in reviewing their charts to identify PD patients. Initial patient contacts were through the local physician's office. Local physicians received access to an informational website with the CME presentation, information on frequently asked questions from patients, and information regarding the specifics of LS-1.
Intervention clinic specialists and study coordinators received training in a separate session at the general pretrial training for LS-1 investigators. After the start of LS-1, a 3-month Ancillary Trial start up period ensued during which recruitment coordinators were hired. Recruitment coordinators participated in separate conference calls for training and ongoing problem-solving conference calls. Principal investigators were encouraged to join the calls. Training included information on the protocol, the Ancillary Trial processes, and approaches to overcoming barriers to clinical trial participation [3,19,20]. To assist in identifying community physicians, recruitment coordinators received a list of zip codes for the diverse populations within 30 miles of their site.
The NINDS Clinical Research Collaboration [21] agreed to facilitate contact between recruitment coordinators and local physicians where the collaboration had existing partnerships, but no partnerships could be identified. NINDS developed public information brochures about LS-1 in English, and during the Ancillary Trial, it provided Spanish versions. Community physicians were asked to place the brochures in patient areas in their offices.
Control
Control clinics implemented any recruitment procedures they wished and received the NINDS brochures. NINDS committed funds to extend the intervention to the control clinics for future NET-PD studies if the intervention proved to be successful.
Human subjects
All patient-specific materials (advertisements, patient information brochures, and sample letters to patients) were submitted for Institutional Review Board (IRB) approval as an amendment to LS-1 prior to the start of the Ancillary Trial in each site. No additional patient data were collected for the Ancillary Trial. The Data and Safety Monitoring Board (DSMB) for LS-1 reviewed the Ancillary Trial at each meeting.
Outcomes
Primary trial outcomes were the percentage of diverse participants enrolled in LS-1 and qualitative data from key informant interviews of investigators and coordinators at the end of the trial. All LS-1 sites routinely collected data on race/ethnicity at the time of randomization and data on recruitment activities. Key informant interviews focused on methods used to recruit diverse participants and perceived facilitators and barriers to increasing recruitment diversity in both intervention and control clinics.
Secondary outcomes included number of participants enrolled in the intervention and control sites regardless of race/ethnicity, number and type of recruitment activities, and percentage of minority participants enrolled at the end of LS-1 recruitment. Recruitment activity logs were collected through a secure web-based interface every 2 months.
Randomization and blinding
Unit of randomization and analysis for the Ancillary Trial was the LS-1 clinical site. Prior to randomization, eligible sites were paired on three characteristics: geographic location, diverse participants enrolled in previous NET-PD Phase II futility trials, and density of diverse populations within 30 miles of the site. Within each pair, allocation to intervention was determined via simple randomization using a random number generator. Because of the nature of the intervention, sites could not be blinded to the intervention, but were asked not to discuss the intervention with those outside the intervention sites.
Sample size
All LS-1 sites committed to enroll two participants of any race/ethnicity per month over 24 months. Given the required trial sample size (1741) and the number of participating sites (46) sites, each site was required to recruit 38 patients. Assuming 32 clusters in the Ancillary Trial (17 intervention and 15 control, average cluster size 38) and assuming a moderate to high within-site correlation (ρ of 0.1, 0.05, or 0.01), two-sample test of proportions, two-sided α of 0.05, we had 60% to 90% power to detect a difference, if the true effect of the intervention had been an absolute 9.4% difference in the proportions between groups. Based on experience in the NET-PD Phase II futility trials, the expected proportion of diverse participants in the control sites used in the sample size calculations was 9%.
Data analyses
Data for analysis excluded data collected during the 3-month start-up period. Proportions of diverse participants recruited in intervention and control sites were compared using a logistic regression model and generalized estimating equations’ methodology to adjust for clustering, more powerful than the test of proportions used in sample size calculation. No interim analysis was planned for efficacy. Average number of total participants enrolled in intervention and control sites was compared using a two-sample t-test. Recruitment activities, tabulated by intervention and control sites, provided a descriptive assessment of control site contamination and intervention site protocol adherence.
Two persons external to the Ancillary Trial with expertise in qualitative methods independently coded key informant interviews. Coders categorized data into major and supporting subthemes and identified associated informant quotations, some included in this report, reflecting the theme. Differences in coding were discussed and adjudicated by the coders.
Results
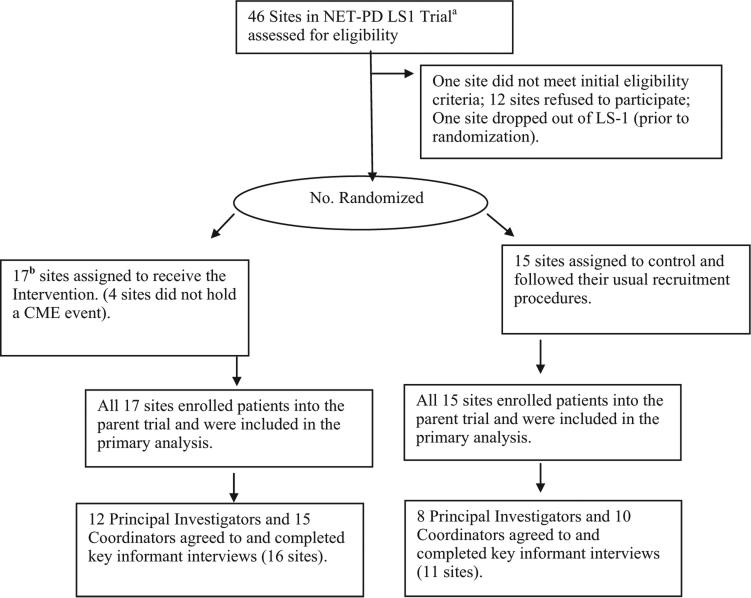
The Ancillary Trial began on 13 June 2007. Figure 1 gives the patient flow diagram for the Ancillary Trial as recommended by Consolidated Standards of Reporting Trials (CONSORT). In total, 17 intervention and 15 control sites were randomized and followed until the end of LS-1 recruitment. Due to drug supply issues, LS-1 enrollment lagged. In October 2008, the Ancillary Trial was expected to require funding for a third year to achieve the required sample size. The DSMB requested a conditional power analysis to assess the futility of continuing the LS-1 Ancillary Trial [22]. As a criterion for futility, the DSMB pre-specified conditional power less than 35%. During the Ancillary Trial, the proportion of diverse participants randomized in the control sites exceeded the proportion randomized in the intervention sites (odds ratio = 0.82, 95% confidence interval (CI) = 0.32, 2.16, p > 0.7). The computed conditional power was less than 1%. The DSMB recommended stopping the Ancillary Trial and the NINDS approved the recommendation. At the time the Ancillary Trial was stopped, 349 participants were enrolled by the 17 intervention sites and 257 participants were enrolled by the 15 control sites. Key informant interviews of trial investigators and coordinators began in December 2008 and ended in March 2009. Final recruitment to the NET-PD LS-1 trial concluded in June 2010.
Figure 1. NET-PD LS-1 Ancillary Trial flow diagram.
NET-PD LS-1, Neurologic Disorders and Stroke Exploratory Trials in Parkinson's Disease Long-Term Study 1.
aSome sites were consortiums but were randomized as a single site.
bInitially, 31 sites agreed to be randomized resulting in 16 intervention and 15 control sites; before the trial began, another site agreed to randomization and was randomized to intervention resulting in 17 intervention and 15 control sites.
Based on available screening logs, 58% of 286 potential participants from diverse groups were screened and enrolled and 73% of 2152 European Caucasians. Table 1 gives reasons for exclusion among those screened. In the diverse group, 37% of those screened did not meet trial eligibility criteria as compared to 25% in the European Caucasian, non-Hispanic group. Of the European Caucasian, non-Hispanics, 60% were either not interested or gave no reason as compared to 50% of the total diverse group. Table 2 provides the distribution of those enrolled by race/ethnicity for intervention and control sites. Using data from the beginning of LS-1 recruitment in March 2008 to the end of LS-1 recruitment in June 2010, no delayed effect of the intervention was observed.
Table 1.
Primary reason for exclusion among those screened for the NINDS NET-PD LS-1 triala
| European Caucasian, non-Hispanic | Diverse populations | |
|---|---|---|
| Total no. of patients screenedb | 2152 | 286 |
| No. of patients excluded (% Excluded) | 576 (27) | 121 (42) |
| Primary reason for exclusionb | No. (% of those excluded) | No. (% of those excluded) |
| a. Unknown | 233 (40.5) | 41 (33.9) |
| b. Eligibility criteria not met | 143 (24.8) | 45 (37.2) |
| i. Inclusion criteria | 77 (13.4) | 30 (24.8) |
| ii. Medical condition | 18 (3.1) | 1 (0.8) |
| iii. Taking exclusionary medications | 22 (3.8) | 1 (0.8) |
| iv. PD too advanced | 9 (1.6) | 5 (4.1) |
| v. Diagnosis uncertain | 12 (2.1) | 5 (4.1) |
| vi. Inability to consent | 1 (0.2) | 1 (0.8) |
| vii. Enrolled in another study | 4 (0.7) | 0 (0.0) |
| viii. Non-English speaking | 0 (0.0) | 2 (1.7) |
| c. Not interested | 110 (19.1) | 19 (15.7) |
| d. Travel requirements | 27 (4.7) | 4 (3.3) |
| e. Protocol too time intensive | 23 (4.0) | 1 (0.8) |
| f. Concern about receiving placebo | 14 (2.4) | 3 (2.5) |
| g. Medication side effect concerns | 6 (1.0) | 0 (0.0) |
| h. Doctor advised decline | 5 (0.9) | 3 (2.5) |
| i. Lost to follow-up before randomization | 4 (0.7) | 1 (0.8) |
| j. Withdrew consent before randomization | 4 (0.7) | 0 (0.0) |
| k. Family advised decline | 3 (0.5) | 2 (1.7) |
| l. Other | 4 (0.7) | 2 (1.7) |
| Total | 576 (100.0) | 121 (100.0) |
PD, Parkinson's disease.
Each prospective participant is in the table only once. Column percentages sum to 100%.
Due to a change in Institutional Review Board (IRB) policy, there was a temporary stop on site reporting of screening information to the Clinical Coordination Center (CCC) that included date of birth, and specific race and/or ethnicity until data could be de-identified. During this time, most sites continued to collect the information and then sent de-identified data to the CCC. Also, all data that resided at the CCC prior to the policy change were de-identified. A few sites stopped collecting data during the de-identification period, so we do not have a complete ascertainment of all those screened, but the missing information should not affect the comparisons between racial/ethnic groups.
Table 2.
Race/ethnicity of participants enrolled in NINDS NET-PD LS-1 ancillary trial sites and all LS-1 sitesa
| Enrolled participants by self-identified race/ethnicity | During Ancillary Trial recruitmentb |
During all NINDS NET-PD LS-1 recruitmentsc |
||||
|---|---|---|---|---|---|---|
| Ancillary Trial sites only |
Ancillary Trial sites only |
All sites |
||||
| Intervention sites (17) No. (%)d | Control sites (15) No. (%) | Intervention sites (17) No. (%) | Control sites (15) # (%) | Total (32) No. (%) | Total (46) No. (%) | |
| European Caucasian, non-Hispanic | 305 (87.4) | 216 (84.1) | 584 (88.6) | 513 (87.0) | 1097 (87.8) | 1571 (90.2) |
| African American | 21 (6.0) | 12 (0.8) | 32 (4.9) | 6 (1.0) | 38 (3.0) | 40 (2.3) |
| Hispanic/Latino | 5 (1.4) | 28 (11.0) | 2 (1.8) | 56 (9.5) | 68 (5.4) | 75 (4.3) |
| Asian | 16 (4.6) | 7 (2.7) | 25 (3.8) | 10 (1.7) | 35 (2.8) | 42 (2.4) |
| Other | 2 (0.6) | 4 (1.6) | 6 (0.9) | 5 (0.9) | 11 (0.9) | 13 (0.8) |
| Total diverse populations | 44 (12.6) | 41 (16.0) | 75 (11.4) | 77 (13.1) | 152 (12.2) | 170 (9.8) |
| Odds ratio (C.L.)e | 0.76 (0.50,1.16) | 0.86 (0.63,1.16) | ||||
Four participants changed sites after randomization but were retained in the site where they were randomized for this analysis.
12 June – 22 October 2008.
13 March 2007 – 28 May 2010. Participants in the Ancillary Trial are subset of the total participants in LS-1.
Numerator = number of participants enrolled in the race/ethnic group, denominator = total number of participants enrolled in the specified sites (intervention, control, or all sites) during the specified time period.
Ratio of odds of enrolling diverse participants in intervention sites to odds of enrolling diverse participants in control sites (95% confidence limits), taking clustering within site into account.
Table 3 presents enrollment of diverse participants in intervention and control sites by baseline site characteristics. The late addition of one intervention site induced a slight regional imbalance, but in general, differences in site baseline characteristics could not explain the lack of an intervention effect. Most Ancillary Trial sites participated in two previous NET-PD Phase II futility trials [6,7]. In the previous Phase II trials, participating sites from the intervention arm enrolled 15.3% diverse participants out of 111 Phase II trial participants, and participating sites from the control arm enrolled 12.9% out of 120.
Table 3.
Enrollment in NINDS NET-PD LS-1 Ancillary Triala intervention and control sites by baseline site characteristics
| Baseline site characteristics | Ancillary Trial intervention sites |
Ancillary Trial control sites |
||||
|---|---|---|---|---|---|---|
| No. of sites | Total no. enrolled | Diverse participants (%) | No. of sites | Total No. enrolled | Diverse participants (%) | |
| Geographic region | ||||||
| Northeast | 3 | 60 | 25.0 | 4 | 67 | 3.0 |
| Southeast | 7 | 149 | 5.4 | 4 | 52 | 26.9 |
| Midwest | 3 | 60 | 6.7 | 4 | 78 | 7.7 |
| Southwest and West | 4 | 80 | 21.3 | 3 | 60 | 31.7 |
| Enrollment of minorities in previous NET-PD Phase II futility trials [6,7] | ||||||
| 0 % | 9 | 229 | 9.2 | 7 | 90 | 5.6 |
| 1% - <10% | 0 | 0 | 0 | 3 | 64 | 10.9 |
| 10% - <20% | 1 | 12 | 8.3 | 0 | 0 | 0 |
| 20% - <90% | 2 | 35 | 54.3 | 2 | 45 | 46.7 |
| Site not in NET-PD futility study | 5 | 73 | 4.1 | 3 | 58 | 13.8 |
| Percent diverse populationsb living within 30 miles of sitec | ||||||
| <20% | 2 | 39 | 5.1 | 3 | 40 | 12.5 |
| 20%-< 25% | 3 | 48 | 4.2 | 2 | 34 | 2.9 |
| 25%-< 30% | 1 | 35 | 5.7 | 1 | 18 | 11.1 |
| 30%-< 0% | 3 | 67 | 9.0 | 2 | 37 | 2.7 |
| 40% or more | 8 | 160 | 20.0 | 6 | 114 | 27.2 |
12 June 2007 to 22 October 2008.
Self-identified as being from a population other than Caucasian, non-Hispanic.
Results exclude Calgary site due to insufficient information on this site.
During the Ancillary Trial, the 12 eligible sites that refused to be randomized enrolled 186 participants, 3.2% from diverse populations. Sites agreeing to participate in the Ancillary Trial randomized 58% of the total participants, regardless of race/ethnicity, an average of 20.5 (SD = 8.2) per site, compared with a site average of 17.1 (SD = 6.0) in those sites not participating in the Ancillary Trial. While these data suggest a possible positive impact on overall recruitment, the difference was not statistically sig- nificant (p > 0.19). The Ancillary Trial was not powered to detect a difference in overall enrollment.
Site recruitment activity logs showed more contacts with local physicians’ offices and more CME presentations in the intervention sites (Table 4), suggesting that sites attempted to implement the intervention. Despite extensive coordinator efforts, attendance at the CME events was low, generally 0 to 3 community-based physicians per event with the exception of one site. Several intervention sites did not include outside speakers given the small projected attendance or principal investigators’ concerns that using an outside speaker would not enhance their own local relationships. Most sites did not find Parkinson's support groups helpful as members were unlikely to come from diverse populations or were at a stage of disease too advanced to be eligible for LS-1.
Table 4.
Average number of recruitment activities based on activities loga
| Activities | Intervention (16 sitesb) |
Control (15 sites) |
||
|---|---|---|---|---|
| No. of sitesc | mean (SD) | No. of sites | mean (SD) | |
| Chart or database review | 15 | 8.69 (4.33) | 13 | 8.07 (4.86) |
| Lay media/lay presentation | 12 | 3.31 (2.70) | 13 | 3.60 (2.41) |
| Local physicians' office contact | 14 | 3.56 (2.19) | 5 | 0.93 (1.98) |
| Medical group presentation | 13 | 2.63 (2.13) | 4 | 0.87 (1.92) |
| Ad/flyer | 9 | 1.56 (1.86) | 10 | 1.73 (2.40) |
| Institutional media | 12 | 1.31 (1.01) | 8 | 1.20 (1.86) |
| Other | 10 | 1.81 (1.87) | 8 | 1.40 (1.84) |
SD, standard deviation
NET-PD LS-1 Ancillary Trial intervention and control sites, data collected from 12 June 2007 to 28 October 2008.
One intervention site provided no information.
Number of sites participating in the specified activity.
Given the lack of a detectable intervention effect, sites were pooled to provide qualitative key informant data on high (n = 8) and low enrollers (n = 19; Table 5). Use of targeted recruitment in churches and other venues did not differentiate between high and low enrolling sites and those who used these methods achieved limited success. Low enrollers reported more use of brochures and newsletters. Low enrollers indicated that diverse patients refused because they did not want to be treated as guinea pigs, because of the length of LS-1, because LS-1 medication could be purchased over the counter, or because of economic barriers such as the cost of transportation. LS-1 staff at some low enrollers appeared to be unaware that NINDS provided LS-1 participants with support for transportation as needed. Some low enrollers considered recruiting diverse participants too costly in terms of the enroller's time and effort required to overcome barriers.
Table 5.
Qualitative key informant interviews of high versus low enrollers in the NET-PD LS-1 Ancillary Triala
| Recruitment methods | High enrollersb N = 8 n (% using method) | Low enrollersc N = 19 n (% using method) |
|---|---|---|
| Existing physician - physician (or HMOd) relationship | 7 (88) | 11 (58) |
| Doctor-doctor letters/phone call | 6 (75) | 11 (58) |
| Physician's dinner | 6 (75) | 10 (53) |
| Community outreach (untargeted, i.e., Health Fairs) | 6 (75) | 3(16) |
| Outreach-support groups, patient symposium | 5 (63) | 14(74) |
| Approached existing patients | 4 (50) | 17 (89) |
| Advertising - flyers, brochures, posters, newsletter | 4 (50) | 16 (84) |
| Advertising-TV/radio - either ads or interviews | 3 (38) | 9 (47) |
| Other physician meeting | 3 (38) | 6 (32) |
| Visiting physician's office | 3 (38) | 4 (21) |
| Community outreach (targeted, i.e., churches) | 2 (25) | 7 (37) |
| Contacted patients through site database | 1 (13) | 4 (21) |
| Visited community clinic/sees patients at community clinic | 0 (0) | 5(26) |
Five sites would not be interviewed.
High is defined as enrolling greater than 14% or more participants from diverse populations (i.e., greater than previous mean percentage of diverse participants enrolled in NET-PD pilot studies by sites in the Ancillary Trial); range for high enrolling sites was 14.3% to 57.9%.
Low is defined as enrolling ≤ 14%; 7 sites recruited no one from diverse populations; range was 0% to 11.1%.
Health Maintenance Organization.
High enrollers reported more untargeted community outreach such as health fairs and media advertising, more letters and telephone calls to community physicians, and more use of existing physician or Health Maintenance Organization (HMO) relationships. Mailing brochures to local physicians was seen as ineffective by high enrollers who saw relationship building as a long process. Reflecting a theme identified in the key informant qualitative analysis, one high enroller observed, “One thing that we have learned is that you can't go someplace once and leave off brochures. You have to ...tactfully sort of be on their radar constantly, ...when I see community neurologists at journal club or, ...in conferences, I'm always reminding them to refer patients when they see somebody that might be eligible and thanking them for their referrals. We always send a letter when we get a referral.” High enrollers found ways to overcome participant-related barriers. Consistent with another theme identified in the qualitative analysis, one high enroller stated, “I think that the basic issue in my neighborhood is with minorities it takes time, time, time, patience, patience, and care and care. Those are the magic things.”
Discussion
In our Ancillary Trial, we encouraged intervention site investigators to overcome barriers to referral by hiring recruitment coordinators with a relationship to the community and building relationships with community physicians. There are many reports on community physicians’ reluctance to refer diverse patients to clinical research because of lack of trust in medical researchers including fear of loss of patients to the specialists conducting the studies [15–18,23]. A systematic review of barriers to cancer clinical trial recruitment for diverse patients identified provider-related barriers such as trust in sponsor/investigator, lack of physician awareness of the trial, provider's attitudes/beliefs, provider's communication and method of presentation of the trial, and provider-related stress [20]. We believe that engagement of community physicians failed for several reasons. First, there is no shortcut to building relationships with community physicians, as others have found in building relationships with members of diverse communities [3]. Second, while a Cochrane review suggests CME may have a small effect on referrals [24], no effect was observed in the Ancillary Trial. The CME events for community physicians were short-term, ineffective interventions that, at best, could only begin to introduce the specialty care sites to the community physicians, required too much effort from the community physicians and were poorly attended. Although high enrollers slightly more often hosted physician dinners, only a few found dinners helpful. Third, in many low enrolling sites, the principal investigator relied on the recruitment coordinator and did little direct personal follow-up with community physicians.
Questions and comments by participants in the key informant interviews indicated that the telephone training program for recruitment coordinators did not provide enough information on trial procedures and also did not provide the communication skills needed by the coordinators. Other approaches to training appear to be required. In clinical trials, there is usually a primary study coordinator paid by funds from the trial sponsor. These study coordinators often make the first patient contact. We focused on the recruitment coordinators and provided limited training to the primary study coordinators. The addition of a recruitment coordinator at a small percent effort made hiring difficult at a single site and was costly when replicated across the many intervention sites. Enhancing the skills of the existing study coordinator may be a more practical and effective solution than adding a recruitment coordinator. The additional time required for recruitment for both the specialists and study coordinators also needs to be considered in budgeting for future trials.
Both specialists and coordinators in low enrolling sites often placed responsibility on the prospective diverse participants rather than finding ways to address their issues. In a different study, oncologists in specialty care clinics were surveyed regarding barriers to enrollment of older patients with breast carcinoma in clinical trials. Oncologists gave responses similar to low enrollers in the Ancillary Trial. Oncologists placed responsibility on the patients, for example, “older patients could not understand directions, could not get to the sites,” and so on, rather than addressing prospective participants’ issues [25]. In the Ancillary Trial, the high proportion of African Americans recruited in the intervention sites and of Hispanic/Latinos recruited in the control sites appeared to be related to site investigator attitudes rather than a differential response to the intervention. Two of the three highest enrolling physicians and coordinators were European Caucasian, non-Hispanic, consistent with literature suggesting it is researchers’ attitude rather than race/ethnicity that helps reduce recruitment barriers [3].
The qualitative findings in this trial are strengthened by using a mixed methods approach, combining qualitative research with the quantitative data from the randomized trial. Our mixed methods approach is unique in testing recruitment interventions.
NET-PD's low enrollment of diverse populations has been replicated in numerous PD trials, despite evidence suggesting PD incidence and prevalence are similar across racial/ethnic groups [8]. New paradigms may be required to educate local physicians regarding the diagnosis of early PD and to overcome local physicians’ reluctance to refer patients to specialty clinics or to research studies. Studies suggest that less than 20% of the general population learn about available trials from their own physicians [26]. To increase research participation, a general training program on clinical research was conducted for National Medical Association (NMA) physicians, who are predominantly African American, but was not shown to be effective [27]. Consistent with our trial, a recent Cochrane Review [28] indicated that general referrals to secondary care were not improved by handing out guidelines (brochures in our study) with general referral forms. The Cochrane Review reported improvement when healthcare specialists taught about referrals, provided second opinions before referral, or provided enhanced services before referral.
Limitations
The Ancillary Trial was conducted only among patients with PD. Whether our findings relate to trials in other diseases of low prevalence is unknown. However, the issues identified are consistent with other reports on barriers to recruitment of racial/ethnic minorities to clinical trials. It is unknown whether the differences in proportion excluded for failing to meet trial eligibility criteria (Table 1) reflect true differences in prevalence of exclusion criteria or differences in physician or coordinator perceptions of eligibility since little detail was provided.
Conclusion
Increasing participant diversity in PD clinical trials requires new paradigms for investigator and study coordinator interactions with community physicians and prospective trial participants. New approaches must include site-specific solutions to address the diverse cultures of local physicians, and their prospective participants should be tested using rigorous comparative methods such as clinical trials and tested in multiple less common diseases. Findings from the Ancillary Trial may be applicable in other trials targeting low prevalence diseases.
Acknowledgments
For details of the Net-PD Steering Committee, Scientific Oversight Board, Data and Safety Monitoring Board and Recruitment (intervention sites) or Study Coordinators (control sites), and Principal Investigators please refer the online supplementary material Appendix.
Funding
Drs Tilley, Mainous, Smith, Ford, Diaz, and the NET-PD LS-1 Ancillary Trial Investigators were supported, in part, by grants U01NS043127, U01NS043128, and U10NS44415-44555 from the National Institute of Neurologic Disorders and Stroke. Drs Tilley, Mainous, Smith, Ford, and Diaz were supported, in part, by grant P30AG21677 from the National Institute on Aging. Drs Tilley, Mainous, Smith, Ford, Diaz, Pickelsimer, and Soderstrom were supported, in part, by a grant from the Duke Endowment.
References
- 1.Taylor JS, Ellis GR. Racial differences in responses to drug treatment: Implications for pharmacotherapy of heart failure. Am J Cardiovasc Drugs. 2002;2:389–99. doi: 10.2165/00129784-200202060-00004. [DOI] [PubMed] [Google Scholar]
- 2.Bjornsson TD, Wagner JA, Donahue SR, et al. A review and assessment of potential sources of ethnic differences in drug responsiveness. J Clin Pharmacol. 2003;43:943–67. doi: 10.1177/0091270003256065. [DOI] [PubMed] [Google Scholar]
- 3.Curry L, Jackson J. Recruitment and retention of diverse ethnic and racial groups in health research: An evolving science. In: Curry L, Jackson J, editors. The Science of Inclusion: Recruiting and Retaining Racial and Ethnic Elders in Health Research. The Gerontological Society of America; Washington, DC: 2003. pp. 1–7. [Google Scholar]
- 4.Levkoff S, Sanchez H. Lessons learned about minority recruitment and retention from the Centers on Minority Aging and Health Promotion. Gerontologist. 2003;43(1):18–26. doi: 10.1093/geront/43.1.18. [DOI] [PubMed] [Google Scholar]
- 5.Bluestein J, Weiss LJ. Visits to specialists under medicare: Socioeconomic advantage and access to care. J Health Care Poor U. 1998;9:153–69. doi: 10.1353/hpu.2010.0451. [DOI] [PubMed] [Google Scholar]
- 6.NINDS NET-PD Investigators A randomized, double-blind, futility clinical trial of creatine and minocycline in early Parkinson disease. Neurology. 2006;66:664–71. doi: 10.1212/01.wnl.0000201252.57661.e1. [DOI] [PubMed] [Google Scholar]
- 7.NINDS NET-PD Investigators A randomized clinical trial of coenzyme Q10 and GPI-1485 in early Parkinson’s disease. Neurology. 2007;68:20–8. doi: 10.1212/01.wnl.0000250355.28474.8e. [DOI] [PubMed] [Google Scholar]
- 8.Schneider MG, Swearingen CJ, Schulman L, et al. Minority enrollment in Parkinson’s disease clinical trials. Parkinsonism Relat D. 2009;15:258–62. doi: 10.1016/j.parkreldis.2008.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.de Lau LM, Breteler MB. Epidemiology of Parkinson’s disease. Lancet Neurol. 2006;5:525–35. doi: 10.1016/S1474-4422(06)70471-9. [DOI] [PubMed] [Google Scholar]
- 10.Jenkins V, Fallowfield L. Reasons for accepting or declining to participate in randomized clinical trials for cancer therapy. Br J Cancer. 2000;82:1783–88. doi: 10.1054/bjoc.2000.1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Daugherty C, Ratain MJ, Grochowski E, et al. Perceptions of cancer patients and their physicians involved in phase I trials. J Clin Oncol. 1995;13:1062–72. doi: 10.1200/JCO.1995.13.5.1062. [DOI] [PubMed] [Google Scholar]
- 12.Albrecht TL, Eggly SS, Gleason ME, et al. Influence of clinical communication on patients’ decision making on participation in clinical trials. J Clin Oncol. 2008;26:2666–73. doi: 10.1200/JCO.2007.14.8114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mainous AG, III, Smith DW, Geesey ME, et al. Development of a measure to assess patient trust in medical researchers. Ann Fam Med. 2004;4:247–52. doi: 10.1370/afm.541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mainous AG, III, Smith DW, Geesey ME, et al. Factors influencing physician referrals of patients to clinical trials. J Natl Med Assoc. 2008;100:1298–303. doi: 10.1016/s0027-9684(15)31508-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McCaskill-Stevens W, Pinto H, Marcus AC, et al. Recruiting minority cancer patients into cancer clinical trials: A pilot project involving the Eastern Cooperative Oncology Group and the National Medical Association. J Clin Oncol. 1999;17:1029–39. doi: 10.1200/JCO.1999.17.3.1029. [DOI] [PubMed] [Google Scholar]
- 16.Royal CD, Baffoe-Bonnie A, Kittles R, et al. Recruitment experience in the first phase of the African American Hereditary Prostate Cancer (AAHPC) Study. Ann Epidemiol. 2000;10(suppl 8):S68–S77. doi: 10.1016/s1047-2797(00)00194-0. Abstract. PMCID: 11189095. [DOI] [PubMed] [Google Scholar]
- 17.Siminoff LA, Step MM. A communication model of shared decision making: Accounting for cancer treatment decisions. Health Psychol. 2005;24(suppl 4):S99–S105. doi: 10.1037/0278-6133.24.4.S99. Abstract. PMCID: 16045427. [DOI] [PubMed] [Google Scholar]
- 18.Hudson SV, Momperousse D, Leventhal H. Physician perspectives on cancer clinical trials and barriers to minority recruitment. Cancer Ctl. 2005;12(suppl 2):93–6. doi: 10.1177/1073274805012004S14. [DOI] [PubMed] [Google Scholar]
- 19.Shavers VL, Lynch CF, Burmeister LF. Racial differences in factors that influence the willingness to participate in medical research studies. Ann Epidemiol. 2002;12:248–56. doi: 10.1016/s1047-2797(01)00265-4. [DOI] [PubMed] [Google Scholar]
- 20.Ford JG, Howerton MW, Lai GY, et al. Barriers to recruiting underrepresented populations to cancer clinical trials: A systematic review. Cancer. 2008;112:228–42. doi: 10.1002/cncr.23157. [DOI] [PubMed] [Google Scholar]
- 21.NINDS Clinical Research Collaboration [3 Feb 2012]; Available at: http://www.ninds.nih.gov/research/clinical_research/crc.htm.
- 22.Lan KKG, Wittes J. The B-value: A tool for monitoring data. Biometrics. 1988;44:579–85. [PubMed] [Google Scholar]
- 23.Lynch GF, Gorelick PB, Raman R, Leurgans S. A pilot survey of African-American physician perceptions about clinical trials. J Natl Med Assoc. 2001;93(suppl 12):8S–13S. [PMC free article] [PubMed] [Google Scholar]
- 24.Forsetlund L, Bjørndal A, Rashidian A, et al. Continuing education meetings and workshops: Effects on professional practice and health care outcomes. Cochrane Database of Syst Rev. 2009;2 doi: 10.1002/14651858.CD003030.pub2. Art. No. CD003030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kornblith AB, Kemeny M, Peterson BL, et al. Survey of oncologists’ perceptions of barriers to accrual of older patients with breast carcinoma to clinical trials. Cancer. 2002;95:989–96. doi: 10.1002/cncr.10792. [DOI] [PubMed] [Google Scholar]
- 26.Bain LJ. Crossroads in clinical trials. NeuroRx. 2005;2:525–8. doi: 10.1602/neurorx.2.3.525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Powell JH, Fleming Y, Walker-McGill CL, et al. The Project IMPACT experience to date: Increasing minority participation and awareness of clinical trials. J Natl Med Assoc. 2008;100:178–87. doi: 10.1016/s0027-9684(15)31206-2. [DOI] [PubMed] [Google Scholar]
- 28.Akbari A, Mayhew A, Al-Alawi MA, et al. Interventions to improve outpatient referrals from primary care to secondary care. Cochrane DB Syst Rev. 2008;4 doi: 10.1002/14651858.CD005471.pub2. Art. No. CD005471. [DOI] [PMC free article] [PubMed] [Google Scholar]