Abstract
Objectives
To estimate the frequency of pregnancy testing in emergency department (ED) visits by reproductive-aged women administered or prescribed teratogenic medications (Food and Drug Administration categories D or X), and to determine factors associated with non-receipt of a pregnancy test.
Methods
This was a retrospective cross-sectional study using 2005 through 2009 National Hospital Ambulatory Medical Care Survey data of ED visits by females ages 14 to 40 years. The number of visits was estimated where teratogenic medications were administered or prescribed and pregnancy testing was not conducted. The association of demographic and clinical factors with non-receipt of pregnancy testing was assessed using multivariable logistic regression.
Results
Of 39,859 sampled visits, representing an estimated 141.0 million ED visits by reproductive-aged females nationwide, 10.1 million (95% CI = 8.9 to 11.3 million) estimated visits were associated with administration or prescription of teratogenic medications. Of these, 22.0% (95% CI = 19.8% to 24.2%) underwent pregnancy testing. The most frequent teratogenic medications administered without pregnancy testing were: benzodiazepines (52.2%; 95% CI = 31.1% to 72.7%), antibiotics (10.7%; 95% CI = 5.0% to 16.3%), and antiepileptics (7.7%; 95% CI = 0.12% to 15.5%). The most common diagnoses associated with teratogenic drug prescription without pregnancy testing were psychiatric (16.1%; 95% CI = 13.6% to 18.6%), musculoskeletal (12.7%; 95% CI = 10.8% to 14.5%), and cardiac (9.5%; 95% CI = 7.6% to 11.3%). In multivariable analyses, visits by older (adjusted odds ratio [AOR] 0.57, 95% CI = 0.42 to 0.79), non-Hispanic white females (AOR 0.71; 95% CI = 0.54 to 0.93), visits in the Northeast region (AOR 0.60; 95% CI = 0.42 to 0.86), and visits during which teratogenic medications were administered in the ED only (AOR 0.74; 95% CI = 0.57 to 0.97) as compared to prescribed at discharge only, were less likely to have pregnancy testing.
Conclusions
A minority of ED visits by reproductive-aged women included pregnancy testing when patients were prescribed category D or X medications. Interventions are needed to ensure that pregnancy testing occurs before women are prescribed potentially teratogenic medications, as a preventable cause of infant morbidity.
INTRODUCTION
Almost 12 million prescriptions for potentially teratogenic medications are written for women of reproductive age in the United States annually,1 placing approximately 6% of U. S. pregnancies at risk for potential birth defects.2 In 1979, The U.S. Food and Drug Administration (FDA) instituted a drug classification system in which the category D or X indicates potential for teratogenicity. Although the absolute risk of birth defects with exposure is unknown, the FDA has determined that use of these drugs may confer teratogenic risk. Family planning services are provided during only 5% to 20% of visits by reproductive-aged women prescribed potentially teratogenic medications.1 Because some women may choose to terminate pregnancies exposed to teratogens,3 inadvertent exposure of a pregnant woman to a teratogen may cause hardship even when it may not result in the birth of an infant with congenital malformations.1,3
It is crucial to assess pregnancy risk when caring for reproductive-aged women and prescribing potentially teratogenic medications. This is especially important for women seeking care in the emergency department (ED), who are at high risk for unintended pregnancies, and may be unaware of their pregnancy status.4 Given that sexual histories are not always reliable5–8 and may be time-consuming in a busy ED, one of the most reliable and efficient ways to evaluate for pregnancy is with pregnancy testing. Highly accurate pregnancy testing can be performed noninvasively, quickly, and inexpensively, as a point-of-care urine test.
Failure to consider pregnancy and underuse of pregnancy testing may lead to overuse of teratogenic medications and potentially avoidable adverse effects on the fetus and/or emotional distress to the patient. The objective of this study was to determine the frequency of pregnancy testing during ED visits when women are administered or prescribed potentially teratogenic medications, and to identify patient and clinical characteristics associated with non-use of pregnancy testing.
METHODS
Study Design
This was a repeated cross-sectional analysis of the National Hospital Ambulatory Medical Care Survey (NHAMCS) from 2005 through 2009. This study was deemed exempt from full review or informed consent by our institutional review board.
Study Setting and Population
The NHAMCS is a multi-stage national probability sample survey including visits to the EDs of general and short-stay hospitals conducted by the Centers for Disease Control and Prevention, National Center for Health Statistics.9 The survey uses a four-stage probability design with samples of primary sampling units (PSUs), hospitals within PSUs, clinical and emergency service areas within hospitals, and patient visits within clinics and emergency service areas. The patient visit is the basic sampling unit. Each patient visit represents a larger number of visits. A weight is assigned to each observation and allows for the generation of nationally representative estimates. For the purposes of this study, we limited our analysis to emergency service areas or emergency departments (EDs) only.
The eligible study population included all sampled ED visits by reproductive-aged female patients who were prescribed or administered teratogenic medications, defined as category D or X by the FDA.10 Drugs are classified as category D if there is evidence of human fetal risk based on adverse reaction data from investigational or marketing experience, or studies in humans. Drugs are categorized as category X if studies in animals and humans have demonstrated fetal abnormalities, and/or there is evidence of human fetal risk based on adverse reaction data from investigational or marketing experience, and the risks involved in the use of the drugs in pregnant women clearly outweigh potential benefits. We defined reproductive-aged females as females between the ages of 14 and 40 years to avoid misclassification of women who were premenarchal or menopausal. We excluded visits during which methotrexate, misoprostol, or levonorgestrel were administered, and all visits for emergency contraception provision, sexual assault, and pregnancy complications (ectopic pregnancy, spontaneous or threatened abortion).
Outcomes
Our outcome measure was frequency of pregnancy testing among visits by female ED patients administered or prescribed teratogenic medications. NHAMCS codes up to eight medications per patient visit. Each visit was further categorized as to whether or not at least one teratogenic medication had been prescribed or administered. We also sought to identify factors associated with teratogenic drug prescription and pregnancy testing. Variables of interest included patient age, race/ethnicity, insurance status, triage level, geographic region, and whether the potentially teratogenic medications were administered in the ED only, prescribed at discharge only for use as an outpatient, or both. Age was categorized as pediatric (14 to 21 years) and adult (22 to 40 years). Triage level was categorized as Emergent, Urgent, Semi-Urgent, and Non-Urgent. These covariates were chosen because the authors believed that they may be related to pregnancy test performance, and were based on a previous study of factors associated with pregnancy testing in the ED.11
Data Analysis
The survey sample suite of programs implemented in Stata 12.1 was used for all analyses to account for the multistage cluster sampling design of the NHAMCS survey. We used descriptive statistics with appropriate survey weighting to calculate frequency of pregnancy testing in patient visits where teratogenic medications were prescribed or administered. To identify factors that might be associated with pregnancy test performance, we first considered bivariable associations with these candidate factors and testing. We performed multivariable logistic regression to estimate associations with pregnancy test performance after adjusting for all factors with p-values <0.1 on bivariable analysis. The purposeful selection of covariates strategy was used for model building.12 A goodness-of-fit test applicable to survey data, the F-adjusted mean residual test,13 was used to provide an estimate of model performance. Estimates derived from the multivariable model included adjusted odds ratios (AOR) with 95% confidence intervals (CI).
RESULTS
Overall, 39,859 sampled visits were identified, representing an estimated 141.0 million ED visits by reproductive-aged females between 2005 and 2009 (Table 1). Of these, an estimated 10.1 million visits (95% CI = 8.9 to 11.3) were associated with administration or prescription of teratogenic medications. The total numbers of Category D and Category X medications prescribed were 9.37 million (95% CI = 8.29 to 10.4), and 0.91 million (95% CI = 0.68 to 1.14), respectively.
Table 1.
Demographics of Study Population
| Demographic | Point Estimate (95% CI) | |
|---|---|---|
| Mean age | 26.7 years (26.6–26.8) | |
| Age category | 14–21 yrs | 29.2 (28.4–29.9) |
| 22–40 yrs | 70.8 (70.1–71.6) | |
| Race/ethnicity | Non-Hispanic white | 56.6 (54.3–58.8) |
| Non-Hispanic black | 28.5 (26.0–31.0) | |
| Hispanic | 13.9 (12.0–15.9) | |
| Insurance status | Private | 21.9 (20.6–23.2) |
| Non-Private | 78.1 (76.8–79.4) | |
| Triage level | Immediate | 3.3 ((2.5–4.1) |
| Emergent | 9.9 (8.9–10.9) | |
| Urgent | 44.6 (42.5–46.8) | |
| Semi-urgent | 29.9 (28.2–31.5) | |
| Non-urgent | 12.3 (11.1–13.5) | |
| Geographic region | Northeast | 17.3 (15.1–19.6) |
| Midwest | 23.0 (18.1–27.8) | |
| South | 41.9 (37.2–46.6) | |
| West | 17.8 (13.6–22.1) |
Point estimates are in % except for age
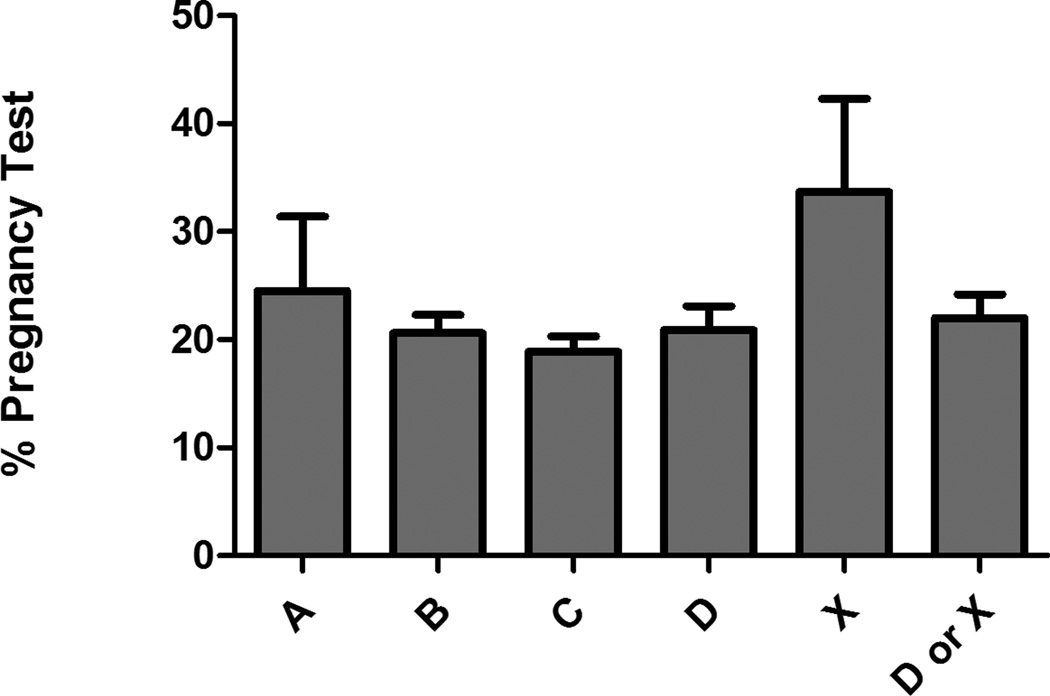
Figure 1 depicts the percentage of visits with pregnancy testing performed by FDA pregnancy category risk. Among patient visits where category D or X medications were prescribed, 22.0% (95% CI = 19.8% to 24.2%) had pregnancy testing. For Category X specifically, where the risk of teratogenic effects is highest, 33.7% (95% CI = 25.1% to 42.3%) had pregnancy testing. The most frequent teratogenic medications administered without pregnancy testing were benzodiazepines (52.2%; 95% CI = 31.8% to 72.7%), antibiotics (10.7%; 95% CI = 5.0% to 16.3%), and antiepileptics (7.7%; 95% CI = 0.12% to 15.5%). The most common diagnoses associated with teratogenic drug prescription without pregnancy testing were psychiatric (16.1%; 95% CI = 13.6% to 18.6%), musculoskeletal (12.7%; 95% CI = 10.8% to 14.5%), and cardiac (9.5%; 95% CI = 7.6% to 11.3%). Among visits associated with category D or X medication use and pregnancy testing was not performed, 51.6% (95% CI = 47.9% to 55.3%) were associated with medication administration in the ED only, 30.9% (95% CI = 27.7% to 34.0%) of visits were associated with discharge prescription of potentially teratogenic medications only, and 14.7% of visits were associated with both administration and discharge prescription of category D or X medications.
Figure 1.
Pregnancy Testing Frequency by FDA Pregnancy Risk Category
Among patient visits with administration or prescription of category D or X medications, in unadjusted analyses we observed associations of testing for pregnancy with age (p < 0.001), racial/ethnic group (p = 0.04), triage category (p = 0.03), geographic region (p = 0.02), and administration or prescription status (p = 0.09) (Table 2), but not by insurance status (p = 0.66). Table 2 provides results from our multivariable analysis. On adjusted analysis, older females were less likely to have pregnancy testing (AOR 0.57, 95% CI = 0.42 to 0.79) when compared to 14 to 21 year olds. Non-Hispanic white females were less likely to undergo pregnancy testing (AOR 0.71; 95% CI = 0.54 to 0.93) when compared to non-Hispanic black females. Furthermore, geographic differences in pregnancy testing in the setting of teratogenic medication administration were noted; pregnancy testing was less frequently performed in the Northeast region (AOR 0.60; 95% CI = 0.42 to 0.86) as compared to the Midwest. Finally, pregnancy testing was less likely to occur when potentially teratogenic medications were administered in the ED only (AOR 0.74; 95% CI = 0.57 to 0.97) as compared to when prescribed at ED discharge.
Table 2.
Bivariable and Multivariable Analysis of Factors Associated with Pregnancy Testing Among ED Visits with Category D or X Medication Administration
| Demographic | Unadjusted OR (95% CI) |
Adjusted OR (95% CI) |
|
|---|---|---|---|
| Age category | 14–21 yrs | Referent | Referent |
| 22–40 yrs | 0.59 (0.44–0.79)* | 0.9 (0.44–0.82)* | |
| Race/ethnicity | Non-Hispanic white | Referent | Referent |
| Non-Hispanic black | 1.42 (1.10–1.82)* | 1.38 (1.04–1.82)* | |
| Hispanic | 1.08 (0.77–1.51) | 1.25 (0.82–1.91) | |
| Triage level | Immediate | Referent | Referent |
| Emergent | 0.76 (0.43–1.32) | 0.86 (0.48–1.55) | |
| Urgent | 0.96 (0.57–1.60) | 1.06 (0.59–1.88) | |
| Semi-Urgent | 0.60 (0.35–1.04) | 0.61 (0.33–1.12) | |
| Non-Urgent | 0.61 (0.31–1.20) | 0.65 (0.32–1.33) | |
| Geographic region | Northeast | Referent | Referent |
| Midwest | 1.56 (1.07–2.27)* | 1.72 (1.20–2.46)* | |
| South | 1.01 (0.73–1.40) | 0.94 (0.68–1.30) | |
| West | 0.81 (0.51–1.28) | 0.80 (0.50–1.29) | |
| Medication administered and/or prescribed | Administered in ED only | Referent | Referent |
| Prescribed only | 1.28 (1.01–1.63)* | 1.35 (1.03–1.76)* | |
| Administered in ED and prescribed | 0.92 (0.62–1.38) | 0.96 (0.65–1.42) |
p<0.05
OR = odds ratio
Model goodness-of-fit: F-test = 0.664, p = 0.74
DISCUSSION
In this nationally representative cross-sectional analysis of ED visits by women of child-bearing age, we found low frequency of pregnancy testing when medications with teratogenic risk were prescribed. During the study period, over 10 million category D or X medications were prescribed to women of child-bearing age, and only 22% of these visits included pregnancy testing. Based on medication class, some of these medications may have been administered emergently, although pregnancy testing was not affected by acuity level for the visit after adjusting for other covariates.
Teratogenic drug administration is common. One in every six women in the United States receives a prescription for a potentially teratogenic medication each year,1 and approximately 6% of U.S. pregnancies are exposed to potentially teratogenic medications.2 Although the majority of potentially teratogenic medications are prescribed by primary care providers,1 we estimate over 10 million Category D or X medications were prescribed or administered to women of child-bearing age in EDs nationally.
In a study evaluating category D and X medications most frequently prescribed to reproductive-aged women in the primary care setting, benzodiazepines, anticonvulsants, antibiotics, and ‘statins’ were the most frequently prescribed.1 We found similar results in our study, with benzodiazepines and antibiotics as the most frequently prescribed medications with potential for teratogenicity. Although some population-based studies have failed to find associations of major congenital malformations with use of benzodiazepines, antibiotics, or statins, prior literature has argued that these studies are limited by sample size (as congenital malformations occur rarely even after exposure to known teratogens), recall bias, nonrandomized observational study designs, and insufficient data on pregnancy terminations.14,15 Therefore, even when a study does not demonstrate increased congenital malformations after drug exposure, teratogenic potential has still not been definitely ruled out. Furthermore, since the FDA categorizations have not yet been changed for these medications, they are still considered to have potential for teratogenicity. Finally, even when risk for congenital malformation risk may be low after drug exposure, knowledge of potential for teratogenic risk may cause undue stress and even termination of pregnancy with inadvertent exposure.3
It is concerning that the frequency of pregnancy testing was so low when prescribing teratogenic medications. Our results are similar to those of other studies that have evaluated pregnancy testing or contraception counseling when prescribing teratogenic medications in other clinical environments. A recent survey of reproductive-aged women who received prescriptions for teratogenic medications found almost 50% reported no receipt of counseling regarding birth defects.16 A survey of female veterans also found similar low rates of birth defects counseling when prescribed teratogenic medications.17
It is especially important to evaluate for pregnancy in the ED. Given the nature of the ED, it is often difficult to conduct confidential interviews with patients, making pregnancy status or contraceptive use difficult to elicit. Additionally, patient histories may be unreliable.5–8 Furthermore, women are often unaware of their pregnancy status, and ED patients tend to be at even higher risk for unplanned pregnancies.4 Pregnancy testing can be conducted as a point-of-care test in most EDs or hospital laboratories, with results available within minutes. Furthermore, although the absolute risk of a birth defect with any given teratogenic exposure may be low,18 knowledge of a patient’s pregnancy status may better inform the treating physician’s drug choice (unless required emergently).
We evaluated whether certain patient demographics or visit characteristics were associated with pregnancy testing when administered teratogenic medications. We found that child-bearing aged women of non-Hispanic black race/ethnicity were more likely to undergo pregnancy testing than women of non-Hispanic white race/ethnicity. We also found that visits by women 22 to 40 years were less likely to undergo pregnancy testing than visits by younger reproductive-aged women, despite the fact that birth rates are higher in the older age group.19 These differences suggest that providers may perceive differential pregnancy risks by race, ethnicity, and age. We also found that visits during which teratogenic medications were administered in the ED only were less likely to undergo pregnancy testing when compared to visits during which category D or X medications were prescribed. This is concerning because administering medications may assume a higher risk without the safety net of package instructions and/or pharmacist discussion of teratogenic risk. However, this may also be due to physician perception that risk of teratogenicity may be lower with ED administration of a single or few doses of a category D or X medication, as compared to repeated doses with a discharge prescription.
LIMITATIONS
Pregnancy testing may have been under-coded in the overall survey. However, the data miscoding rate among NHAMCS surveys is less than 1%,9 and beginning in 2005, data abstractors were specifically trained on pregnancy test data abstraction. Furthermore, to validate our findings, we also estimated pregnancy testing rates using the Nationwide Emergency Department Sample (NEDS), a database supported by the Agency for Healthcare Research and Quality,20 and found similar rates of pregnancy testing (unpublished data). Second, provider knowledge of sexual activity, previously documented pregnancy, recent testing, and current contraceptive use cannot be obtained from these data sources. Such knowledge might obviate the need for pregnancy testing in certain cases. However, other studies have found positive pregnancy tests even among patients who have denied sexual activity.5,21 Third, the NHAMCS database only codes eight medications per visit. Therefore, in visits during which more than eight medications were administered or prescribed, a teratogenic medication may have been missed, which would lead to an overestimate of the frequency of pregnancy testing among visits associated with teratogenic medication provision. Additionally, although some medications may have been needed on an emergent basis, pregnancy testing was not associated with triage level.
CONCLUSIONS
Millions of teratogenic medications are prescribed yearly to women of reproductive age in EDs, and only a minority undergo pregnancy testing. Future studies should focus on designing interventions to ensure that pregnancy is considered before prescription of potentially teratogenic medications.
Acknowledgments
Funding Source: This work was supported by NIH grant K23 HD070910 (MKG).
Footnotes
Conflict of Interest: None of the authors have any conflicts of interest to declare.
References
- 1.Schwarz EB, Maselli J, Norton M, Gonzales R. Prescription of teratogenic medications in United States ambulatory practices. Am J Med. 2005;118:1240–1249. doi: 10.1016/j.amjmed.2005.02.029. [DOI] [PubMed] [Google Scholar]
- 2.Andrade SE, Gurwitz JH, Davis RL, et al. Prescription drug use in pregnancy. Am J Obstet Gynecol. 2004;191:398–407. doi: 10.1016/j.ajog.2004.04.025. [DOI] [PubMed] [Google Scholar]
- 3.Koren G. The way women perceive teratogenic risk. Can J Clin Pharmacol. 2007;14:e10–e16. [PubMed] [Google Scholar]
- 4.Todd CS, Mountvarner G, Lichenstein R. Unintended pregnancy risk in an emergency department population. Contraception. 2005;71:35–39. doi: 10.1016/j.contraception.2004.08.005. [DOI] [PubMed] [Google Scholar]
- 5.Ramoska EA, Sacchetti AD, Nepp M. Reliability of patient history in determining the possibility of pregnancy. Ann Emerg Med. 1989;18:48–50. doi: 10.1016/s0196-0644(89)80310-5. [DOI] [PubMed] [Google Scholar]
- 6.Ellish NJ, Weisman CS, Celentano D, Zenilman JM. Reliability of partner reports of sexual history in a heterosexual population at a sexually transmitted diseases clinic. Sex Transm Dis. 1996;23:446–452. doi: 10.1097/00007435-199611000-00002. [DOI] [PubMed] [Google Scholar]
- 7.Van Duynhoven YT, Nagelkerke NJ, Van De Laar MJ. Reliability of self-reported sexual histories: test-retest and interpartner comparison in a sexually transmitted diseases clinic. Sex Transm Dis. 1999;26:33–42. doi: 10.1097/00007435-199901000-00006. [DOI] [PubMed] [Google Scholar]
- 8.Fisher CM, Lee MG. Comparison of adolescents' reports of sexual behavior on a survey and sexual health history calendar. J Sex Res. 2014;51(7):777–787. doi: 10.1080/00224499.2013.782482. [DOI] [PubMed] [Google Scholar]
- 9.CDC. NHAMCS scope and sample design. [Accessed Nov 1, 2014]; Available at: http://www.cdc.gov/nchs/ahcd/ahcd_scope.htm#nhamcs_scope.
- 10.Briggs GG, Freeman RK, Yaffe SJ. Drugs in Pregnancy and Lactation. 6th edition. Philadelphia, PA: Lippincott, Williams and Williams; 2001. [Google Scholar]
- 11.Goyal M, Hersh A, Luan X, et al. Frequency of pregnancy testing among adolescent emergency department visits. Acad Emerg Med. 2013;20:816–821. doi: 10.1111/acem.12186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bursac Z, Gauss CH, Williams DK, Hosmer DW. Purposeful selection of variables in logistic regression. Source Code Biol Med. 2008;3:17. doi: 10.1186/1751-0473-3-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Archer KJ, Lemeshow SL, Hosmer DW. Goodness-of-fit tests for logistic regression models when data are collected using a complex sampling design. Computational Stat Data Anal. 2007;51(9):4450–4464. [Google Scholar]
- 14.Levy A, Matok I, Gorodischer R, et al. Bias toward the null hypothesis in pregnancy drug studies that do not include data on medical terminations of pregnancy: the folic acid antagonists. J Clin Pharmacol. 2012;52:78–83. doi: 10.1177/0091270010390806. [DOI] [PubMed] [Google Scholar]
- 15.Koren G, Pastuszak A, Ito S. Drugs in pregnancy. N Engl J Med. 1998;338:1128–1137. doi: 10.1056/NEJM199804163381607. [DOI] [PubMed] [Google Scholar]
- 16.Schwarz EB, Parisi SM, Handler SM, Koren G, Shevchik G, Fischer GS. Counseling about medication-induced birth defects with clinical decision support in primary care. J Womens Health. 2013;22:817–824. doi: 10.1089/jwh.2013.4262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schwarz EB, Mattocks K, Brandt C, et al. Counseling of female veterans about risks of medication-induced birth defects. J Gen Intern Med. 2013;28(Suppl 2):S598–S603. doi: 10.1007/s11606-012-2240-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shepard TH, Brent RL, Friedman JM, et al. Update on new developments in the study of human teratogens. Teratology. 2002;65:153–161. doi: 10.1002/tera.10032. [DOI] [PubMed] [Google Scholar]
- 19.Hamilton BE, Martin JA, Ventura SJ. Births: preliminary data for 2012. Natl Vital Stat Rep. 2013;62:1–20. [PubMed] [Google Scholar]
- 20.Agency for Healthcare Research and Quality. Overview of the Nationwide Emergency Department Sample (NEDS) [Accessed Nov 1, 2014]; Available at: https://www.hcup-us.ahrq.gov/nedsoverview.jsp.
- 21.Givens TG, Jackson CL, Kulick RM. Recognition and management of pregnant adolescents in the pediatric emergency department. Pediatr Emerg Care. 1994;10:253–255. doi: 10.1097/00006565-199410000-00002. [DOI] [PubMed] [Google Scholar]