Abstract
Following peripheral nerve injury, the distal nerve is primed for regenerating axons by generating a permissive environment replete with glial cells, cytokines, and neurotrophic factors to encourage axonal growth. However, increasing evidence demonstrates regenerating axons within peripheral nerves still encounter axonal-growth inhibitors, such as chondroitin sulfate proteoglycans. Given the generally poor clinical outcomes following peripheral nerve injury and reconstruction, the use of pharmacological therapies to augment axonal regeneration and overcome inhibitory signals has gained considerable interest. Joshi et al (2014) have provided evidence for preferential or modality-specific (motor versus sensory) axonal growth and regeneration due to inhibitory signaling from Rho-associated kinase (ROCK) pathway regulation. By providing inhibition to the ROCK signaling pathway through Y-27632, they demonstrate that motor neurons regenerating their axons are impacted to a greater extent compared to sensory neurons. In light of this evidence, we briefly review the literature regarding modality-specific axonal regeneration to provide context to their findings. We also describe potential and novel barriers, such as senescent Schwann cells, which provide additional axonal-growth inhibitory factors for future consideration following peripheral nerve injury.
Keywords: axon modality, chondroitin sulfate proteoglycan, motor neuron, nerve regeneration, peripheral nerve, RhoA, ROCK, Schwann cell, sensory neuron, Y-27632
Peripheral nerve injury, axonal regeneration, and functional recovery
Functional recovery following general nerve reconstruction is often associated with poor results. Recovery following axonal regeneration is largely driven by the ability of neurons to regenerate their axons through the nerve “pathway” to reinnervate their end-organ “target”. Axonal regeneration following injury is promoted by neurotrophic factors, cytokines, and axon adhesion molecules produced by glial cells within nerve and muscle, as well as axonal regenerative programs initiated by peripheral neurons. In addition, sensory and motor axons are guided to their specific end-organ target (specificity) through both pathway and target-derived factors. These factors are spatially and temporally produced and regulated following injury (Boyd and Gordon, 2003). Recovery, then, is dependent upon the number of motor and sensory axons that successfully regenerate through the pathway and are properly matched with their respective motor endplates and sensory receptors in a timely manner.
Following injury, damaged peripheral nerve undergoes a process described by Wallerian degeneration, which prepares the distal nerve facilitating axonal regeneration. The blood-nerve barrier breaks down uniformly along the nerve within days of injury allowing large molecules to cross and enter the endoneurial space containing axons and Schwann cells (SCs) (Bruck, 1997; Olsson, 1966; Rotshenker, 2003; Seitz et al., 1985; Seitz et al., 1989). Concurrently, SCs dedifferentiate or trans-differentiate into a pro-regenerative phenotype within days. This trans-differentiation is characterized by changes in mitogen and neurotrophic factor expression and phagocytic activity (Fu and Gordon, 1997; Jessen and Mirsky, 2002, 2005; Xu et al., 2008; You et al., 1997).
In contrast to the central nervous system, where glial cells direct scarring and the persistence of myelin-based inhibitory proteins, SCs phagocytose myelin debris (Kazakova et al., 2006; Lai, 2005; Lyons et al., 2005). This dramatic change in the nerve pathway for regenerating axons is a major component responsible for facilitating axonal growth after injury in the peripheral nervous system compared to the central nervous system. However, increasing evidence demonstrates this pathway still contains, and actively expresses, inhibitory components to axonal growth, such as chondroitin sulfate proteoglycans (CSPGs) (Zuo et al., 1998a; Zuo et al., 1998b; Zuo et al., 1998c; Zuo et al., 2002). A recent study by Joshi et al. considered the role of CSPGs on modality-specific (motor vs sensory) axonal regeneration. They demonstrated differential axonal regeneration of motor axons through the nerve pathway due to CSPG signaling (Joshi et al., 2014). Their evidence suggests a role for CSPGs in axonal specificity for end-organ targets and modality-specific barriers to successful axonal regeneration through the nerve pathway. We present a general background on factors influencing modality-specific axonal regeneration to give context to their study and future directions.
Specificity of Axonal Regeneration
Regenerating axons are significantly influenced by regenerative pathway cues and their innate signaling pathways. Sensory and motor neurons express different levels of a wide range of receptors (for example, tyrosine kinases receptors) resulting in modality-specific regulation of ligand-induced signaling (Boyd and Gordon, 2003). This signaling provides a mechanism to regulate axon growth to appropriate targets. The choice axons make to grow into a motor or sensory pathway and end-organ target is generally referred to as axonal specificity.
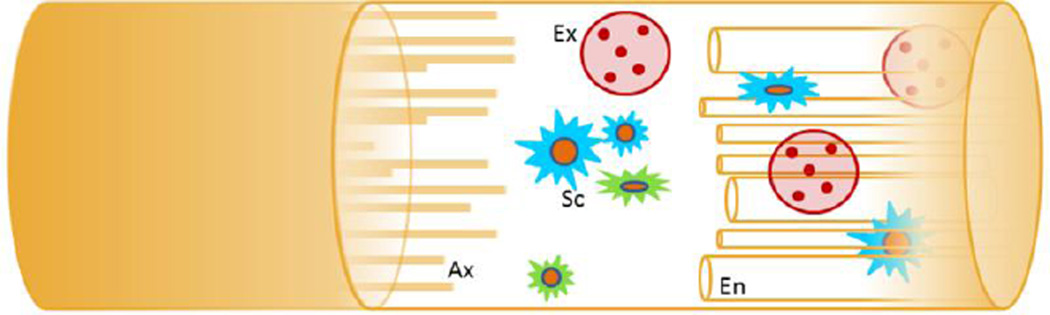
Much of what is known regarding modality-specific axonal regeneration is provided by animal models. The femoral nerve injury model is ideally suited for studying modality-specific axonal regeneration. In the femoral nerve model, motor and sensory fibers demonstrate a predictable topography distally with branches into the cutaneous saphenous nerve and the quadriceps motor branch (Brushart, 1988, 1993; Brushart et al., 1998; Brushart and Seiler, 1987; Madison et al., 1996; Martini et al., 1994). When given equal access to motor and sensory pathways, regenerating motor axons preferentially regenerate down a terminal motor pathway and reinnervate its muscle target in what has been termed preferential motor reinnervation (PMR) (Brushart, 1988, 1993). In a series of studies, Brushart, Madison, and colleagues have demonstrated that regenerating motor axons (Brushart, 1988, 1993; Madison et al., 1996), as well as afferents from the muscle spindle, preferentially regenerate down the quadriceps motor pathway even when deliberate attempts at mismatching sensory and motor paths are made (Madison et al., 1996). These studies have provided a major framework to elucidate the impact of principal components guiding axons to their specific targets (Figure 1). The components involved have included specific glial cells intrinsic to the nerve pathways (sensory and motor SCs) (Hoke et al., 2006), tropic influence from endorgans (Robinson and Madison, 2004), and basal lamina proteins and architecture inherent to sensory and motor nerves (Nichols et al., 2004). While axonal guidance is directed, to a degree, by all these factors, it is of great interest to identify a potentially predominant mechanism to establish translational efforts to improve functional recovery.
Figure 1. Pathways influencing modality-specific axonal regeneration.
Within the regenerative pathway, axons (Ax) encounter glial cells (Schwann cells (Sc)), tropic factors derived from end-organs (exosomes (Ex)), and endoneurial architecture (En). These components modulate the growth response of sensory and motor neurons and their axons as they respond to this environment.
Schwann cells
As Schwann cells (SCs) are the primary intrinsic mediators of nerve regeneration in the peripheral nervous system, they play an extensive role in regulation of axonal regeneration. In fact, the absence of SCs following nerve injury and during regeneration severely limits the quality and extent of axonal regeneration (Hall, 1986a, b). The protein expression of SCs supports axons through the deposition of basal lamina, excretion of trophic factors, and adhesion molecules that facilitate regeneration after nerve injury (Araki and Milbrandt, 1996; Bunge, 1994; Bunge et al., 1986; Friedman et al., 1992; Levi and Bunge, 1994). While SCs are usually described as myelinating and non-myelinating (Jessen and Mirsky, 2002), there is strong evidence for the existence of SC phenotypes that are distinct in motor and sensory nerves (Hoke et al., 2006; Jesuraj et al., 2012).
The concept of modality-specific SC phenotypes resulted from the original experiments considering PMR (Brushart, 1993). These sensory- and motor-derived SCs were first described based on gene expression differences between cutaneous nerve and ventral root SCs. The variations in gene and protein expression between these SC phenotypes directly support modality-matched axonal growth and regeneration (i.e. sensory neurons extend longer and more axons in sensory nerve-derived SC environments) (Hoke et al., 2006; Marquardt and Sakiyama-Elbert, 2014). In fact, the delivery of neurotrophic factors, such as glial cell-line derived neurotrophic factor (GDNF), guides and regulates the differentiation and phenotype of sensory- and motor-derived SCs (Jesuraj et al., 2014). The delineation of sensory- and motor-derived SCs has also been further refined by spatial arrangement, where SC phenotypes defined by growth factor expression vary according to a central-peripheral location (Brushart et al., 2013).
Beyond SC phenotype, the “state” of SCs can greatly affect regenerative potential. Prolonged axonal denervation of nerve leads SCs to no longer divide and possibly enter a quiescent state. This proposed state of quiescence in SCs has not been specifically characterized in detail, as there are no specific markers to identify this state, but is generally described to result in less axonal growth support and cell division (Sulaiman and Gordon, 2000, 2002). In addition to the state of quiescence, aging and stressful environments can significantly alter SCs’ state inducing senescence. Our lab recently described the accumulation of senescent SCs, defined by expression of specific senescence markers (Ben-Porath and Weinberg, 2005), following long nerve graft reconstruction (Saheb-Al-Zamani et al., 2013). Cellular senescence is a state of permanent growth arrest distinct from quiescence, which is an exit from the cell cycle and reversible. Senescent cells not only cease to proliferate, they also undergo a change in protein expression called the senescence associated secretory phenotype (SASP). The SASP of senescent cells is replete with altered expression of chemokines, cytokines, growth factors, and extracellular remodeling enzymes that radically alter the tissue microenvironment (Coppe et al., 2010; Pazolli and Stewart, 2008). Given this radical change in protein expression, SC senescence may play a pivotal role in axonal regeneration after peripheral nerve injury. Due to the known association of limited axonal regeneration with senescent SC accumulation, increased production of axonalgrowth inhibitors could drive this axonal arrest (Saheb-Al-Zamani et al., 2013). It is yet to be determined if senescence can result in differences in axonal regeneration modalities.
End-organ
While SC phenotype provides excellent intrinsic evidence to support regenerative modality regulation (Redett et al., 2005), other work suggests that tropic support derived from nerves’ direct connection with muscle (“target-derived”) is predominant (Madison et al., 2009; Robinson and Madison, 2004). Using the femoral nerve model, Madison and Robinson have shown that motor axons lose PMR and regenerate increasing numbers of motor axons into the sensory saphenous branch when the motor quadriceps branch is severed from its muscle end-organ or the regenerative distance to muscle increased (Robinson and Madison, 2004). Additionally, selectively removing Schwann cells distal to a nerve lesion while maintaining end-organ contact results in a maintenance of PMR (Madison et al., 2009). These studies provide significant evidence that tropic support from end-organs determine the specificity of axonal regeneration. However, until recently understanding the form of “tropic” support was unknown. Robinson and Madison have now described a source of diffusible trophic support provided by muscle. By crushing the distal quadriceps nerve in a femoral nerve injury model, they demonstrated the accuracy of regenerating motor neurons is dependent upon the denervated distal nerve remaining in uninterrupted continuity with muscle (Madison and Robinson, 2014). These results make a strong case for the existence of muscle-derived exosomes (nanometer sized extracellular vesicles) that are secreted and diffuse through nerve. These exosomes could provide a means to deliver contents such as RNA and proteins to motor or sensory regenerating axons (Madison et al., 2014).
Basal lamina
Peripheral nerves have general “architecture” defined by their basal lamina tubes. The motor nerve architecture, which has larger SC basal lamina tubes, was seen as a possible factor in directing PMR. It was hypothesized the larger basal lamina tubes of motor-derived nerve branches might better accommodate larger regenerating motor axons. To define the role of nerve architecture, Mackinnon’s group evaluated regeneration through isografts derived from nerve branches that support variable amounts of sensory (sural and saphenous) and motor (tibial and quadriceps) axons. While initial studies found regenerating tibial axons preferred tibial nerve grafts compared to sural nerve grafts (Brenner et al., 2006; Nichols et al., 2004), these studies were limited by inclusion of SCs. Therefore, acellular nerve grafts (ANAs) were derived from these same nerves to remove the SC influence as a factor. ANAs are conceptually appealing because their physical, chemical, and mechanical properties are similar to those of a nerve autograft (isograft) while lacking SCs. These studies revealed more robust axonal regeneration in both acellular and cellular tibial grafts in a tibial nerve injury model (Moradzadeh et al., 2008), again suggesting that nerve architecture influenced axonal regeneration modality. A final study was conducted in the femoral nerve model, which contains more distinct motor (quadriceps) and sensory (saphenous) nerve branches. In this study, when modality matched and mismatched grafts were evaluated (i.e. matched: femoral motor graft to femoral motor nerve, mismatched: femoral sensory graft to the femoral motor nerve), modality-matching had no significant effect on nerve regeneration (Kawamura et al., 2010). Overall, these sets of studies suggest nerve architecture has a minor influence on axonal regeneration when the distal nerve is connected to mixed end-organs (skin and muscle), while the graft type has little effect when the distal nerve is connected to a purely motor end organ. Therefore, increasing evidence supports end-organ-derived factors providing signals to stimulate appropriate reinnervation (Madison et al., 2009).
Influence of axonal-growth inhibitors to axon modality
While the previously described studies have focused significantly on the role of factors which encourage modality-specific axonal growth and regeneration, less has been considered on the role of axonal-growth inhibitors. As recently described by Joshi et al., sensory and motor neurons have differential sensitivity to CSPGs as evidenced by activation of Rho-associated kinases (ROCK) pathways and axon regeneration (Joshi et al., 2014). Given the profound influence of pathway-derived factors, the production of CSPGs serves as a further source of regulation in modality-specific axonal regeneration.
CSPGs play an intricate role in axon extension signaling. Axonal injury elevates levels of cyclic adenosine monophosphate (cAMP) intrinsic to the injured neuron to promote axonal extension (Cai et al., 2001; Gao et al., 2004). Small guanine nucleotide (GTP)ases of the Rho family control actin polymerization, cell growth and motility, cytokinesis, trafficking and cytoskeletal architecture. Elevated cAMP activates protein kinase A (PKA) through a transcription-dependent process, which leads to axonal elongation by inactivating ROCK (Hiraga et al., 2006; Kubo et al., 2007; Mimura et al., 2006; Yamauchi et al., 2003). Conversely, CSPGs inhibit axonal extension through activation of the ROCK pathway (Monnier et al., 2003). Sulfated proteoglycans modify axon binding surfaces reducing SC adhesion and neurite outgrowth (Hoke, 2005; Hynds and Snow, 1999; Lee et al., 2014).
Due to evidence demonstrating expression of CSPGs in peripheral nerve, pharmacologic therapies targeting the RhoA/ROCK pathway have increased in interest (Joshi et al., 2014). The recent study by Joshi et al. demonstrates motor neurons and their regenerating axons are ideal targets for this targeted therapy due to their increased responsiveness to the ROCK pathway. Motor neurons extend longer axons in cell culture and express less active and inactive RhoA protein following pharmacological therapy with Y-27632. In addition, motor neurons demonstrate strong differences in axonal regeneration through modality-specific nerve pathways. Axonal regeneration following femoral nerve crush was significantly enhanced (a nearly 100% increase in myelinated axon numbers compared to untreated) in the quadriceps motor branch while regeneration was marginally modified in a sciatic or tibial injury model (Joshi et al., 2014).
Clinical considerations impacting nerve regeneration
While many factors can contribute to poor functional recovery, including issues in the nerve “pathway” and end-organ “target” as just described, clinician’s recognize four situations. These include: proximal injuries, delayed reconstruction, use of nerve grafts (especially long nerve grafts), and lack of understanding of the topography inherent to nerve. Recently the introduction of nerve transfers has replaced a need for nerve grafts in many situations, with the results of these new procedures producing spectacular results, which is not commonly associated with nerve repairs. Transfers avoid these four situations just described by reconstructing the nerve as proximal as possible and utilizing an extensive understanding of peripheral nerve topography, which naturally separates sensory and motor nerves. Therefore, while motor specific therapies are of interest (Joshi et al., 2014), clinically, efforts are moving towards expanding indications for nerve transfer and improving the nuances of the technique.
The established nerve injury classification systems (Seddon and Sunderland) continue to guide the management of nerve-injured patients (Table 1). Peripheral nerve injuries are generally classified by compression with or without demyelination (neurapraxia, Sunderland I), axon transection with both perineurium and epineurium remaining intact (axonotmesis, Sunderland II & III), and nerve transection (neurotmesis, Sunderland IV & V) where the continuity of the epineurium is disrupted. The timely and accurate diagnosis of nerve injury is essential to provide optimal treatment for recovery. Favorable or Recoverable injuries (Neurapraxia and axonotmesis or Sunderland I, II, III) can be separated from non-favorable or non-recoverable injuries (neurotmesis or Sunderland IV & V) with at 3 months using electrodiagnostics (Mackinnon and Dellon, 1988; Sunderland, 1978). Delayed surgery for non-recoverable injuries leads to potentially irreversible atrophy of muscle and chronic denervation of SCs, both of which are correlated with poor regenerative outcomes (Fu and Gordon, 1995a, b; Gordon et al., 2011; Kobayashi et al., 1997). The recent study by Joshi et al considered nerve crush injury models (Joshi et al., 2014) (Sunderland II or axonotmesis), which limits clinical impact to recoverable injuries. However, the use of ROCK inhibitors during the observation period to potentially speed axonal motor recovery could be of great benefit if side-effects are proven minimal, as diagnostic accuracy could be improved to avoid unnecessary surgery.
Table 1.
Classification systems of peripheral nerve injury
| Recovery indication |
Recovery speed | Overall recovery |
Sunderland injury classification |
Seddon injury classification |
|---|---|---|---|---|
| Favorable | Fast (<1 month) | Complete | I | Neurapraxia |
| Favorable | Slow (~1mm/day) | Complete | II* | Axonotmesis |
| Favorable | Slow (~1mm/day) | Partial or incomplete | III | |
| Unfavorable | None | None | IV | Neurotmesis |
| Unfavorable | None | None | V | |
| Mixed | Variable | Incomplete | VI (Mackinnon) | All |
indicates animal nerve crush injury models
While nerve transfers are now used for most proximal nerve injuries, distal nerve injuries are typically reconstructed with a nerve autograft. Donor nerves that are commonly used as autografts are expendable sensory nerves, such as the sural nerve or the medial antebrachial cutaneous nerve, and the motor nerve to the gracilis muscle (Mackinnon and Dellon, 1988). Even though clinical results continue to improve, there still exist challenging nerve injuries to manage including long nerve grafts and multiple nerve injuries precluding use of nerve transfers. The recent evidence suggesting pharmacological inhibition of the ROCK pathway is effective to promote motor axonal regeneration (Joshi et al., 2014) offers potential to improve the most challenging nerve injury cases.
Highlights.
Work in Exp Neurol shows axon regenerative modality is regulated by ROCK signaling.
We review the literature regarding modality-specific axonal regeneration.
Axon modality studies tend to focus on growth encouraging factors, not inhibitors.
CSPG expression and ROCK signaling may depend on nerve spatial pathway.
Acknowledgements
The authors’ research in this manuscript was supported in part by the National Institutes of Neurological Disorders and Stroke of the National Institutes of Health under award number NS051706. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The authors would also like to thank Dr. Gwendolyn Hoben for her thoughtful review of this manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest: The authors declare no competing financial interests.
References
- Araki T, Milbrandt J. Ninjurin, a novel adhesion molecule, is induced by nerve injury and promotes axonal growth. Neuron. 1996;17:353–361. doi: 10.1016/s0896-6273(00)80166-x. [DOI] [PubMed] [Google Scholar]
- Ben-Porath I, Weinberg RA. The signals and pathways activating cellular senescence. Int J Biochem Cell Biol. 2005;37:961–976. doi: 10.1016/j.biocel.2004.10.013. [DOI] [PubMed] [Google Scholar]
- Boyd JG, Gordon T. Neurotrophic factors and their receptors in axonal regeneration and functional recovery after peripheral nerve injury. Mol Neurobiol. 2003;27:277–324. doi: 10.1385/MN:27:3:277. [DOI] [PubMed] [Google Scholar]
- Brenner MJ, Hess JR, Myckatyn TM, Hayashi A, Hunter DA, Mackinnon SE. Repair of motor nerve gaps with sensory nerve inhibits regeneration in rats. The Laryngoscope. 2006;116:1685–1692. doi: 10.1097/01.mlg.0000229469.31749.91. [DOI] [PubMed] [Google Scholar]
- Bruck W. The role of macrophages in Wallerian degeneration. Brain Pathol. 1997;7:741–752. doi: 10.1111/j.1750-3639.1997.tb01060.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brushart TM. Preferential reinnervation of motor nerves by regenerating motor axons. J Neurosci. 1988;8:1026–1031. doi: 10.1523/JNEUROSCI.08-03-01026.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brushart TM. Motor axons preferentially reinnervate motor pathways. J Neurosci. 1993;13:2730–2738. doi: 10.1523/JNEUROSCI.13-06-02730.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brushart TM, Aspalter M, Griffin JW, Redett R, Hameed H, Zhou C, Wright M, Vyas A, Hoke A. Schwann cell phenotype is regulated by axon modality and central-peripheral location, and persists in vitro. Exp Neurol. 2013;247:272–281. doi: 10.1016/j.expneurol.2013.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brushart TM, Gerber J, Kessens P, Chen YG, Royall RM. Contributions of pathway and neuron to preferential motor reinnervation. J Neurosci. 1998;18:8674–8681. doi: 10.1523/JNEUROSCI.18-21-08674.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brushart TM, Seiler WAt. Selective reinnervation of distal motor stumps by peripheral motor axons. Exp Neurol. 1987;97:289–300. doi: 10.1016/0014-4886(87)90090-2. [DOI] [PubMed] [Google Scholar]
- Bunge RP. The role of the Schwann cell in trophic support and regeneration. J Neurol. 1994;242:S19–S21. doi: 10.1007/BF00939235. [DOI] [PubMed] [Google Scholar]
- Bunge RP, Bunge MB, Eldridge CF. Linkage between axonal ensheathment and basal lamina production by Schwann cells. Annu Rev Neurosci. 1986;9:305–328. doi: 10.1146/annurev.ne.09.030186.001513. [DOI] [PubMed] [Google Scholar]
- Cai D, Qiu J, Cao Z, McAtee M, Bregman BS, Filbin MT. Neuronal cyclic AMP controls the developmental loss in ability of axons to regenerate. J Neurosci. 2001;21:4731–4739. doi: 10.1523/JNEUROSCI.21-13-04731.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coppe JP, Desprez PY, Krtolica A, Campisi J. The senescence-associated secretory phenotype: the dark side of tumor suppression. Annu Rev Pathol. 2010;5:99–118. doi: 10.1146/annurev-pathol-121808-102144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman B, Scherer SS, Rudge JS, Helgren M, Morrisey D, McClain J, Wang DY, Wiegand SJ, Furth ME, Lindsay RM, et al. Regulation of ciliary neurotrophic factor expression in myelin-related Schwann cells in vivo. Neuron. 1992;9:295–305. doi: 10.1016/0896-6273(92)90168-d. [DOI] [PubMed] [Google Scholar]
- Fu SY, Gordon T. Contributing factors to poor functional recovery after delayed nerve repair: prolonged axotomy. J Neurosci. 1995a;15:3876–3885. doi: 10.1523/JNEUROSCI.15-05-03876.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu SY, Gordon T. Contributing factors to poor functional recovery after delayed nerve repair: prolonged denervation. J Neurosci. 1995b;15:3886–3895. doi: 10.1523/JNEUROSCI.15-05-03886.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu SY, Gordon T. The cellular and molecular basis of peripheral nerve regeneration. Mol Neurobiol. 1997;14:67–116. doi: 10.1007/BF02740621. [DOI] [PubMed] [Google Scholar]
- Gao Y, Deng K, Hou J, Bryson JB, Barco A, Nikulina E, Spencer T, Mellado W, Kandel ER, Filbin MT. Activated CREB is sufficient to overcome inhibitors in myelin and promote spinal axon regeneration in vivo. Neuron. 2004;44:609–621. doi: 10.1016/j.neuron.2004.10.030. [DOI] [PubMed] [Google Scholar]
- Gordon T, Tyreman N, Raji MA. The basis for diminished functional recovery after delayed peripheral nerve repair. J Neurosci. 2011;31:5325–5334. doi: 10.1523/JNEUROSCI.6156-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall SM. The effect of inhibiting Schwann cell mitosis on the re-innervation of acellular autografts in the peripheral nervous system of the mouse. Neuropathol Appl Neurobiol. 1986a;12:401–414. doi: 10.1111/j.1365-2990.1986.tb00151.x. [DOI] [PubMed] [Google Scholar]
- Hall SM. Regeneration in cellular and acellular autografts in the peripheral nervous system. Neuropathol Appl Neurobiol. 1986b;12:27–46. doi: 10.1111/j.1365-2990.1986.tb00679.x. [DOI] [PubMed] [Google Scholar]
- Hiraga A, Kuwabara S, Doya H, Kanai K, Fujitani M, Taniguchi J, Arai K, Mori M, Hattori T, Yamashita T. Rho-kinase inhibition enhances axonal regeneration after peripheral nerve injury. J Peripher Nerv Syst. 2006;11:217–224. doi: 10.1111/j.1529-8027.2006.00091.x. [DOI] [PubMed] [Google Scholar]
- Hoke A. Proteoglycans in axonal regeneration. Exp Neurol. 2005;195:273–277. doi: 10.1016/j.expneurol.2005.06.016. [DOI] [PubMed] [Google Scholar]
- Hoke A, Redett R, Hameed H, Jari R, Zhou C, Li ZB, Griffin JW, Brushart TM. Schwann cells express motor and sensory phenotypes that regulate axon regeneration. J Neurosci. 2006;26:9646–9655. doi: 10.1523/JNEUROSCI.1620-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hynds DL, Snow DM. Neurite outgrowth inhibition by chondroitin sulfate proteoglycan: stalling/stopping exceeds turning in human neuroblastoma growth cones. Exp Neurol. 1999;160:244–255. doi: 10.1006/exnr.1999.7212. [DOI] [PubMed] [Google Scholar]
- Jessen KR, Mirsky R. Signals that determine Schwann cell identity. J Anat. 2002;200:367–376. doi: 10.1046/j.1469-7580.2002.00046.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jessen KR, Mirsky R. The origin and development of glial cells in peripheral nerves. Nature reviews. 2005;6:671–682. doi: 10.1038/nrn1746. [DOI] [PubMed] [Google Scholar]
- Jesuraj NJ, Marquardt LM, Kwasa JA, Sakiyama-Elbert SE. Glial cell line-derived neurotrophic factor promotes increased phenotypic marker expression in femoral sensory and motor-derived Schwann cell cultures. Exp Neurol. 2014;257:10–18. doi: 10.1016/j.expneurol.2014.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jesuraj NJ, Nguyen PK, Wood MD, Moore AM, Borschel GH, Mackinnon SE, Sakiyama-Elbert SE. Differential gene expression in motor and sensory Schwann cells in the rat femoral nerve. Journal of neuroscience research. 2012;90:96–104. doi: 10.1002/jnr.22752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshi AR, Bobylev I, Zhang G, Sheikh KA, Lehmann HC. Inhibition of Rho-kinase differentially affects axon regeneration of peripheral motor and sensory nerves. Exp Neurol. 2014;263C:28–38. doi: 10.1016/j.expneurol.2014.09.012. [DOI] [PubMed] [Google Scholar]
- Kawamura DH, Johnson PJ, Moore AM, Magill CK, Hunter DA, Ray WZ, Tung TH, Mackinnon SE. Matching of motor-sensory modality in the rodent femoral nerve model shows no enhanced effect on peripheral nerve regeneration. Exp Neurol. 2010;223:496–504. doi: 10.1016/j.expneurol.2010.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazakova N, Li H, Mora A, Jessen KR, Mirsky R, Richardson WD, Smith HK. A screen for mutations in zebrafish that affect myelin gene expression in Schwann cells and oligodendrocytes. Developmental biology. 2006;297:1–13. doi: 10.1016/j.ydbio.2006.03.020. [DOI] [PubMed] [Google Scholar]
- Kobayashi J, Mackinnon SE, Watanabe O, Ball DJ, Gu XM, Hunter DA, Kuzon WM., Jr The effect of duration of muscle denervation on functional recovery in the rat model. Muscle Nerve. 1997;20:858–866. doi: 10.1002/(sici)1097-4598(199707)20:7<858::aid-mus10>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- Kubo T, Hata K, Yamaguchi A, Yamashita T. Rho-ROCK inhibitors as emerging strategies to promote nerve regeneration. Current pharmaceutical design. 2007;13:2493–2499. doi: 10.2174/138161207781368657. [DOI] [PubMed] [Google Scholar]
- Lai C. Peripheral glia: Schwann cells in motion. Curr Biol. 2005;15:R332–R334. doi: 10.1016/j.cub.2005.04.024. [DOI] [PubMed] [Google Scholar]
- Lee SJ, Kalinski AL, Twiss JL. Awakening the stalled axon - surprises in CSPG gradients. Exp Neurol. 2014;254:12–17. doi: 10.1016/j.expneurol.2013.12.025. [DOI] [PubMed] [Google Scholar]
- Levi AD, Bunge RP. Studies of myelin formation after transplantation of human Schwann cells into the severe combined immunodeficient mouse. Exp Neurol. 1994;130:41–52. doi: 10.1006/exnr.1994.1183. [DOI] [PubMed] [Google Scholar]
- Lyons DA, Pogoda HM, Voas MG, Woods IG, Diamond B, Nix R, Arana N, Jacobs J, Talbot WS. erbb3 and erbb2 are essential for schwann cell migration and myelination in zebrafish. Curr Biol. 2005;15:513–524. doi: 10.1016/j.cub.2005.02.030. [DOI] [PubMed] [Google Scholar]
- Mackinnon SE, Dellon AL. Surgery of the peripheral nerve. New York: Thieme Medical Publishers; 1988. [Google Scholar]
- Madison RD, Archibald SJ, Brushart TM. Reinnervation accuracy of the rat femoral nerve by motor and sensory neurons. J Neurosci. 1996;16:5698–5703. doi: 10.1523/JNEUROSCI.16-18-05698.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madison RD, McGee C, Rawson R, Robinson GA. Extracellular vesicles from a muscle cell line (C2C12) enhance cell survival and neurite outgrowth of a motor neuron cell line (NSC-34) Journal of extracellular vesicles. 2014;3 doi: 10.3402/jev.v3.22865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madison RD, Robinson GA. Accuracy of regenerating motor neurons: influence of diffusion in denervated nerve. Neuroscience. 2014;273:128–140. doi: 10.1016/j.neuroscience.2014.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madison RD, Sofroniew MV, Robinson GA. Schwann cell influence on motor neuron regeneration accuracy. Neuroscience. 2009;163:213–221. doi: 10.1016/j.neuroscience.2009.05.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marquardt LM, Sakiyama-Elbert SE. GDNF preconditioning can overcome Schwann cell phenotypic memory. Exp Neurol. 2014 doi: 10.1016/j.expneurol.2014.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martini R, Schachner M, Brushart TM. The L2/HNK-1 carbohydrate is preferentially expressed by previously motor axon-associated Schwann cells in reinnervated peripheral nerves. J Neurosci. 1994;14:7180–7191. doi: 10.1523/JNEUROSCI.14-11-07180.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mimura F, Yamagishi S, Arimura N, Fujitani M, Kubo T, Kaibuchi K, Yamashita T. Myelin-associated glycoprotein inhibits microtubule assembly by a Rho-kinase-dependent mechanism. The Journal of biological chemistry. 2006;281:15970–15979. doi: 10.1074/jbc.M510934200. [DOI] [PubMed] [Google Scholar]
- Monnier PP, Sierra A, Schwab JM, Henke-Fahle S, Mueller BK. The Rho/ROCK pathway mediates neurite growth-inhibitory activity associated with the chondroitin sulfate proteoglycans of the CNS glial scar. Molecular and cellular neurosciences. 2003;22:319–330. doi: 10.1016/s1044-7431(02)00035-0. [DOI] [PubMed] [Google Scholar]
- Moradzadeh A, Borschel GH, Luciano JP, Whitlock EL, Hayashi A, Hunter DA, Mackinnon SE. The impact of motor and sensory nerve architecture on nerve regeneration. Exp Neurol. 2008;212:370–376. doi: 10.1016/j.expneurol.2008.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols CM, Brenner MJ, Fox IK, Tung TH, Hunter DA, Rickman SR, Mackinnon SE. Effects of motor versus sensory nerve grafts on peripheral nerve regeneration. Exp Neurol. 2004;190:347–355. doi: 10.1016/j.expneurol.2004.08.003. [DOI] [PubMed] [Google Scholar]
- Olsson Y. Studies on vascular permeability in peripheral nerves. I. Distribution of circulating fluorescent serum albumin in normal, crushed and sectioned rat sciatic nerve. Acta Neuropathol. 1966;7:1–15. doi: 10.1007/BF00686605. [DOI] [PubMed] [Google Scholar]
- Pazolli E, Stewart SA. Senescence: the good the bad and the dysfunctional. Current opinion in genetics & development. 2008;18:42–47. doi: 10.1016/j.gde.2007.12.002. [DOI] [PubMed] [Google Scholar]
- Redett R, Jari R, Crawford T, Chen YG, Rohde C, Brushart TM. Peripheral pathways regulate motoneuron collateral dynamics. J Neurosci. 2005;25:9406–9412. doi: 10.1523/JNEUROSCI.3105-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson GA, Madison RD. Motor neurons can preferentially reinnervate cutaneous pathways. Exp Neurol. 2004;190:407–413. doi: 10.1016/j.expneurol.2004.08.007. [DOI] [PubMed] [Google Scholar]
- Rotshenker S. Microglia and macrophage activation and the regulation of complement-receptor-3 (CR3/MAC-1)-mediated myelin phagocytosis in injury and disease. Journal of molecular neuroscience : MN. 2003;21:65–72. doi: 10.1385/JMN:21:1:65. [DOI] [PubMed] [Google Scholar]
- Saheb-Al-Zamani M, Yan Y, Farber SJ, Hunter DA, Newton P, Wood MD, Stewart SA, Johnson PJ, Mackinnon SE. Limited regeneration in long acellular nerve allografts is associated with increased Schwann cell senescence. Exp Neurol. 2013;247:165–177. doi: 10.1016/j.expneurol.2013.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seitz RJ, Heininger K, Schwendemann G, Toyka KV, Wechsler W. The mouse blood-brain barrier and blood-nerve barrier for IgG: a tracer study by use of the avidin-biotin system. Acta Neuropathol. 1985;68:15–21. doi: 10.1007/BF00688950. [DOI] [PubMed] [Google Scholar]
- Seitz RJ, Reiners K, Himmelmann F, Heininger K, Hartung HP, Toyka KV. The blood-nerve barrier in Wallerian degeneration: a sequential long-term study. Muscle Nerve. 1989;12:627–635. doi: 10.1002/mus.880120803. [DOI] [PubMed] [Google Scholar]
- Sulaiman OA, Gordon T. Effects of short- and long-term Schwann cell denervation on peripheral nerve regeneration, myelination, and size. Glia. 2000;32:234–246. doi: 10.1002/1098-1136(200012)32:3<234::aid-glia40>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Sulaiman OA, Gordon T. Transforming growth factor-beta and forskolin attenuate the adverse effects of long-term Schwann cell denervation on peripheral nerve regeneration in vivo. Glia. 2002;37:206–218. doi: 10.1002/glia.10022. [DOI] [PubMed] [Google Scholar]
- Sunderland S. Nerves and nerve injuries. 2nd ed. New York: Churchill Livingstone; 1978. [Google Scholar]
- Xu QG, Midha R, Martinez JA, Guo GF, Zochodne DW. Facilitated sprouting in a peripheral nerve injury. Neuroscience. 2008;152:877–887. doi: 10.1016/j.neuroscience.2008.01.060. [DOI] [PubMed] [Google Scholar]
- Yamauchi J, Chan JR, Shooter EM. Neurotrophin 3 activation of TrkC induces Schwann cell migration through the c-Jun N-terminal kinase pathway. Proc Natl Acad Sci U S A. 2003;100:14421–14426. doi: 10.1073/pnas.2336152100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- You S, Petrov T, Chung PH, Gordon T. The expression of the low affinity nerve growth factor receptor in long-term denervated Schwann cells. Glia. 1997;20:87–100. doi: 10.1002/(sici)1098-1136(199706)20:2<87::aid-glia1>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- Zuo J, Ferguson TA, Hernandez YJ, Stetler-Stevenson WG, Muir D. Neuronal matrix metalloproteinase-2 degrades and inactivates a neurite-inhibiting chondroitin sulfate proteoglycan. J Neurosci. 1998a;18:5203–5211. doi: 10.1523/JNEUROSCI.18-14-05203.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuo J, Hernandez YJ, Muir D. Chondroitin sulfate proteoglycan with neurite-inhibiting activity is up-regulated following peripheral nerve injury. Journal of neurobiology. 1998b;34:41–54. [PubMed] [Google Scholar]
- Zuo J, Neubauer D, Dyess K, Ferguson TA, Muir D. Degradation of chondroitin sulfate proteoglycan enhances the neurite-promoting potential of spinal cord tissue. Exp Neurol. 1998c;154:654–662. doi: 10.1006/exnr.1998.6951. [DOI] [PubMed] [Google Scholar]
- Zuo J, Neubauer D, Graham J, Krekoski CA, Ferguson TA, Muir D. Regeneration of axons after nerve transection repair is enhanced by degradation of chondroitin sulfate proteoglycan. Exp Neurol. 2002;176:221–228. doi: 10.1006/exnr.2002.7922. [DOI] [PubMed] [Google Scholar]