Abstract
We previously demonstrated that the enhancer of rudimentary homolog (ERH) gene is required for the expression of multiple cell cycle and DNA damage response (DDR) genes. The present study investigated the role of ERH and its target DNA damage repair genes in hepatocellular carcinoma cells. We observed positive correlation between ERH and ataxia telangiectasia and Rad3 related (ATR) expression in liver tissues. Expression of ERH, ATR as well as checkpoint kinase 1 (CHK1) were higher in HCCs than in normal liver tissues. Knocking-down ERH augmented ultraviolet light induced DNA damage in HepG2 cells. ATR protein level is reduced upon ERH depletion as a result of defect in the splicing of ATR mRNA. Consequently, the ATR effector kinase Chk1 failed to be phosphorylated upon ultraviolet light or hydroxyurea treatment in ERH knocked-down HepG2 cells. Finally, we observed Chk1 inhibitor AZD7762 enhanced the effect of doxorubicin on inhibiting growth of HCC cells in vitro and in vivo. This study suggested that ERH regulates the splicing of the DNA damage response proteins ATR in HCC cells, and targeting DNA damage response by Chk1 inhibitor augments chemotherapy to treat HCC cells.
Hepatocellular carcinoma (HCC) is the most common primary liver cancer and is the third leading cause of cancer-related death worldwide1,2. Sorafenib is the only approved systemic therapy for advanced HCC, but the median survival of HCC patients is still less than one year3. No systemic chemotherapy has shown to improve survival of advanced HCC2,4, and doxorubicin, a topoisomerase inhibitor which damages DNA, is widely used to treat advanced HCC4.
Genetic alterations are common in HCCs5,6. The DNA damage response (DDR) pathway is essential for maintenance of genomic integrity during replication and in situations of genomic stress. Dysregulation of DDR is often involved in the carcinogenesis of HCC and may contribute to HCC's resistance to chemotherapies7. Gene expression analysis has demonstrated up-regulation of DNA repair genes involved in the activation of ataxia telangiectasia and Rad3 related (ATR) kinase in HCC cells8. Thus, the DNA repair pathway could be a potential target for cancer therapy against HCC9.
Enhancer of rudimentary homolog (ERH), originally identified in Drosophila, is a highly conserved gene among metazoans10. We previously demonstrated that ERH is a novel splicing factor that regulates the mRNA splicing of the mitotic motor protein CENP-E, and knocking-down ERH in cancer cells resulted in chromosome congression defects during mitosis11. Analysis of changes in gene expression profile in colorectal cancer cells upon ERH depletion revealed the down-regulation of several additional cell cycle genes, including ATR11. ATR is a DNA damage checkpoint kinase that is activated by single stranded DNA breaks and by replication stress, and it is required for cell cycle arrest in response to DNA damage. ATR phosphorylates the checkpoint kinase 1 (CHK1), and other checkpoint proteins including RAD17 and the tumor suppressor protein BRCA112,13. CGK733, a small molecule inhibitor targeting the ATR, significantly enhances paclitaxel-induced cytotoxicity in a HCC cell line14. However, little is known about the role of ERH in DNA damage response, and its role in HCCs.
In the current study, we evaluated the roles of ERH to DNA damage response in HCC cells and we studied whether ERH and its target DNA damage response genes could be a potential therapeutic target in HCC cells.
Results
ERH and ATR expression are elevated in HCC tumors
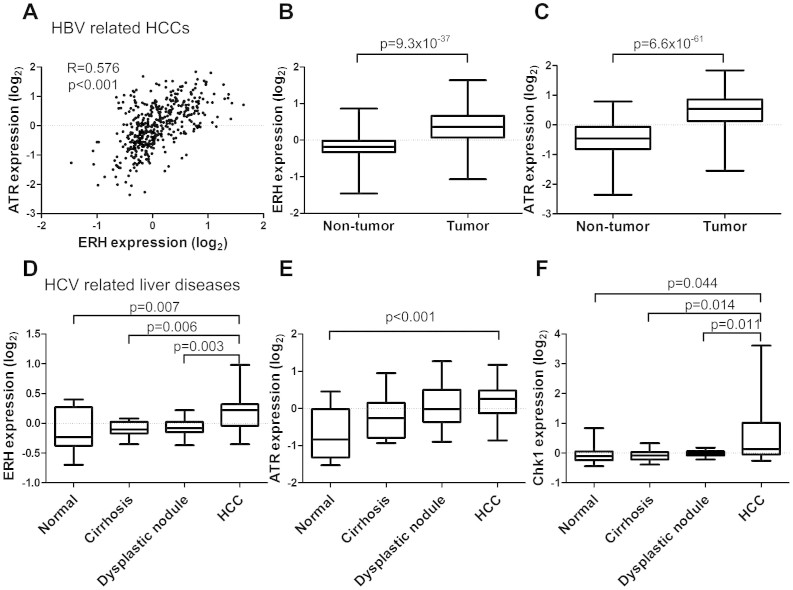
To investigate the role of ERH in regulating the DDR, we first examined the expression of ERH and ATR mRNA in HCC tumors using publicly accessible gene expression databases. The GSE14520 dataset15 contains gene expression profiles from hepatitis B (HBV) positive HCCs and matching normal liver tissues. In this dataset, expression of ERH mRNA was correlated with expression of ATR mRNA in 444 liver tissues containing HCC and non-tumor tissues (Figure 1A). ERH mRNA expression in tumor tissue was higher than in the matched normal tissue (p = 9.3 × 10−37) (Figure 1B). Similarly, ATR expression was also higher in tumor tissue compared to matched normal controls (p = 6.6 × 10−61) (Figure 1C).
Figure 1. ERH expression in HBV and HCV related HCC and non-tumor liver tissue.
(A) Relationship between mRNA expression of ERH and ATR in liver tissue (correlation coefficient = 0.576, p < 0.001 by Spearman's method). (B) ERH mRNA and (C) ATR mRNA expression in tumor part and non-tumor part in HBV related HCCs. (D) ERH mRNA, (E) ATR mRNA, and (F) CHK1 mRNA expression in HCC, normal liver, cirrhosis and dysplastic tissue of HCV carriers. The p-values were less than 0.001 by one-way ANOVA for the comparison of all 3 genes. The p-values demonstrated in the panels were the results of post-hoc analysis by Bonferroni method.
We next analyzed the GSE6764 dataset16, which contains gene expression profiles of hepatitis C (HCV) positive HCCs and normal liver tissue controls. In this dataset we also observed a correlation between ERH and ATR mRNA expression (correlation coefficient = 0.285, p = 0.013). We further observed that ERH mRNA expression was significantly higher in HCCs (n = 35) than in normal tissues (n = 10), cirrhosis (n = 13) or dysplastic lesions (n = 17), indicating that the transition from premalignant lesions to small HCC is associated with an increase of ERH expression (Figure 1D). Similarly, in these cases, ATR mRNA expression was significant higher in HCCs than in normal liver tissues (p < 0.001 by one-way ANOVA and p < 0.001 for HCC vs. normal tissue with post-hoc analysis by Bonferroni way) (Figure 1E). Chk1 is a downstream effector kinase of ATR, we thus also investigated Chk1 expression in these samples. Similar to ATR, Chk1 mRNA expression is correlated with that of ERH (correlation coefficient = 0.504, p < 0.001), and Chk1 mRNA expression was higher in HCCs compared to normal tissues, cirrhosis tissue or dysplastic lesions (Figure 1F).
ERH depletion augmented ultraviolet light (UV)-irradiation induced DNA damage in HCC cells
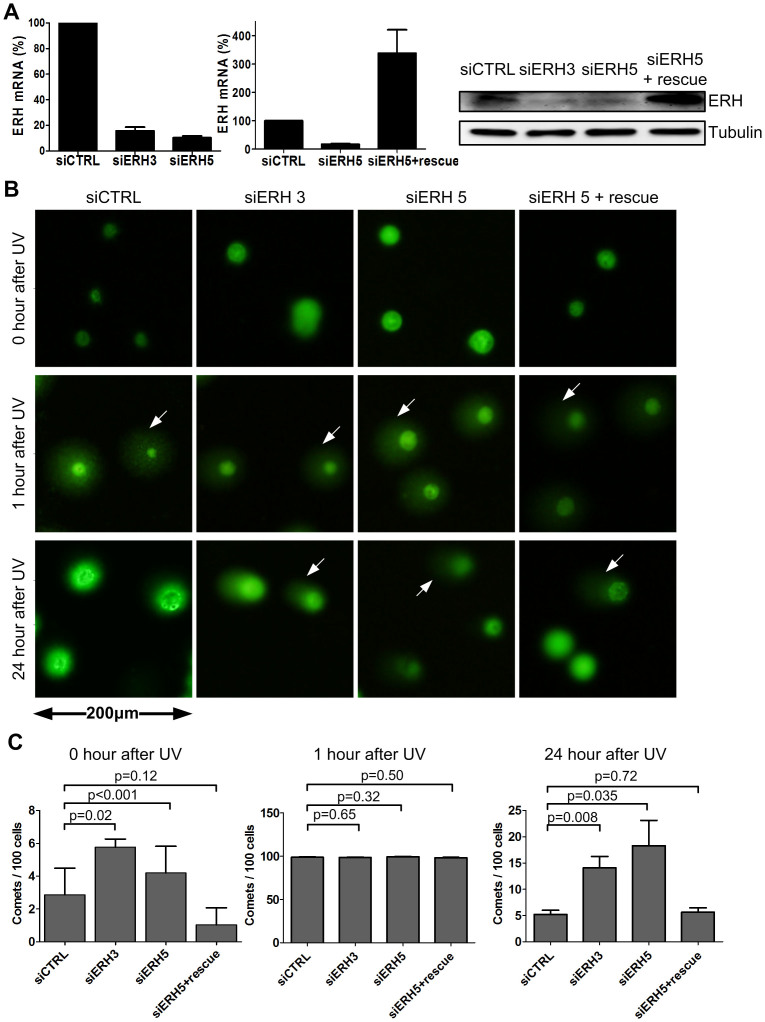
We next assessed the effect of ERH depletion on the DDR in HCC cells. We knocked down ERH in HepG2 cells using two ERH siRNAs that we had previous described11: one targeting the coding region of ERH mRNA (siERH-3) and one targeting its 3'-UTR (siERH-5) (Figure 2A, left panel). And as we showed previously11, stable expression of an ERH cDNA was able to selectively rescue against siERH-5 knockdown but not siERH-3 knockdown (Figure 2A, middle and right panels).
Figure 2. Knocking-down ERH impaired DNA-damage-repair ability.
(A) Expression of ERH mRNA and protein in HepG2 cells upon ERH siRNA transfection and in ERH open reading frame stably expressing HepG2 cells upon siRNA ERH 5 transfection (siERH 5 rescue). (B) Representative images of comet assay in HepG2 cells upon ERH knocking-down and ERH rescue, followed by one episode of UV 200 J/m2 irradiation. Cells were collected at 0, 1 and 24 hours after irradiation. (White arrows indicated damaged DNA) (C) Quantification of comet assay in figure 2B.
To investigate how ERH might influence DNA damage repair in HCC cells, we induced DNA damage using UV irradiation in cells with or without ERH depletion and measured the extent and time course of DNA damage repair using the comet assay17. We quantified DNA damage response by measuring the fraction of cells with nuclear DNA comet tails which is indicative of DNA breaks, at 0, 1 and 24 hours after UV. Knocking-down ERH in the HepG2 cells induced a modest increase of comets cells (Figure 2C, left panel). We also observed an increase of G2/M fraction of cell cycle in ERH knocked-down HCC cells (Figure S1A). Upon UV irradiation for 1 hour, almost all cells presented as having nuclear comet tails. The number of comet tails in cells transfected with control siRNA decreased to baseline level at 24 hours post UV, indicative that these cells have repaired their DNA damage. In contrast, in ERH knocking-down cells we observed a significant numbers of cells with comet tail at this later time point, indicative of persistent DNA break. This phenotype can be fully rescued by ERH cDNA, thus ruling out siRNA off-target effect (Figure 2B–C). Together these findings show that loss of ERH attenuated UV-induced DNA damage repair in HCC cells.
ERH regulates ATR mRNA splicing and expression
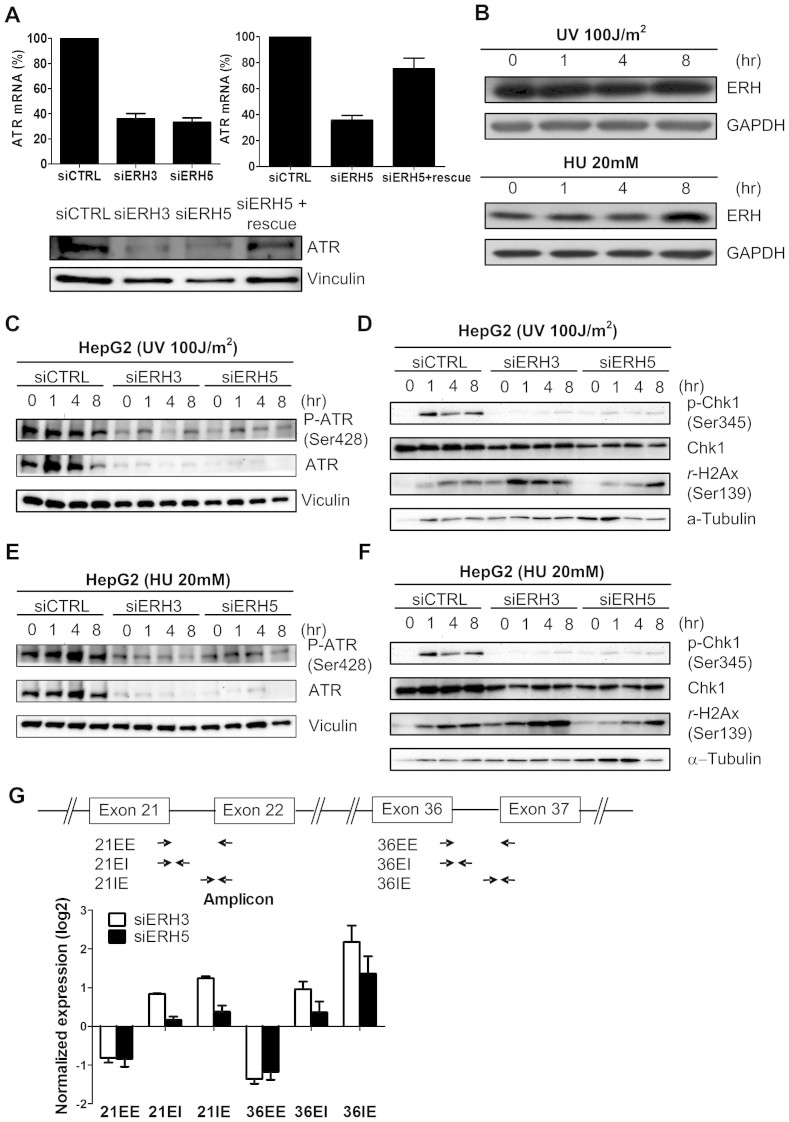
We previously demonstrated, using gene expression microarray, that ERH regulates ATR expression11. This phenomenon was also observed in both colon cancer and lung cancer cell lines (Figure S2A). We confirmed that ATR mRNA and protein expression was decreased upon transfection with ERH siRNAs in HCC cells. Once again this can be rescued by ERH cDNA, (Figure 3A), indicating that this is an on-target effect of ERH siRNAs. As ATR responds to replication stress and single-stranded DNA breaks18,19, we investigated whether knocking down ERH would affect ATR-mediated response to DNA damage in HCC cells treated with UV-radiation or with hydroxyurea (HU). First, we tested whether UV radiation or HU affect the ERH expression and found the protein level not change under treatment (Figure 3B). In cells transfected with control siRNA, UV and HU treatment led to a robust increase in phosphor-ATR in HepG2 cells, whereas ATR phosphorylation is largely abrogated in cells transfected with ERH siRNAs (Figure 3C and 3E), likely due to the loss of ATR protein in ERH depleted cells. Knocking-down ERH did not affect the expression of Chk1 (Figure S2B), but abrogated Chk1 activation as measured by its phosphorylation at the ATR-site S345 (Figure 3D and 3F). Comparable levels of γ-H2AX signals in HepG2 cells transfected with either control siRNA or ERH siRNAs indicated that this is not due to a difference in the extent of DNA damage (Figure 3D and 3F), but rather a failure of DNA damage to activate ATR signaling in these cells.
Figure 3. ERH modulated DNA damage response in HCC cells.
(A) Expression of ERH mRNA and protein in HepG2 cells upon ERH siRNA transfection and in ERH open reading frame stably expressing HepG2 cells upon siRNA ERH 5 transfection (siERH 5 rescue). (B) ERH protein expression didn't change after UV 100 J/m2 irradiation or hydroxyurea 20 mM treatment. (C)Protein expression of total and phosphorylated ATR protein upon ERH knocking-down in HepG2 cells after UV 100 J/m2 irradiation of various durations. (D) Protein expression of total and phosphorylated Chk1 protein and gamma-H2AX expression upon ERH knocking-down after UV irradiation. (E) Protein expression of total and phosphorylated ATR protein upon ERH knocking-down in HepG2 cell upon hydroxyurea 20 mM treatment of various durations. (F) Protein expression of total and phosphorylated Chk1 protein and rH2Ax expression upon ERH knocking-down after hydroxyurea treatment. (G) ATR pre-mRNA expression at the exon–intron junctions detected by qPCR with exon-spanning and splice junction primers. The schematic indicates the location of PCR primers (EE, exon–exon PCR; EI, exon–intron PCR; IE, intron–exon PCR).
In our previous study, we demonstrated that ERH is a splicing factor that regulates CENP-E mRNA expression via controlling its splicing11. We thus tested whether ERH also regulated the splicing of ATR mRNA by measuring splicing efficiency across intron 21 and intron 36. We found that, at both introns, mature mRNA level was decreased whereas unspliced mRNA level was concomitantly increased in ERH depleted cells (Figure 3G). Thus ATR mRNA splicing is subject of regulation by ERH and the loss of mature ATR mRNA could account for the loss of ATR protein upon ERH depletion. As ERH and SNRPD3 are binding partners, we also knocked down SNRPD3 and found that led to ATR mRNA and protein expression partially loss (Figure S2C and S2D).
CHK1 inhibitor AZD 7762 synergizes with doxorubicin to inhibit HCC cell proliferation
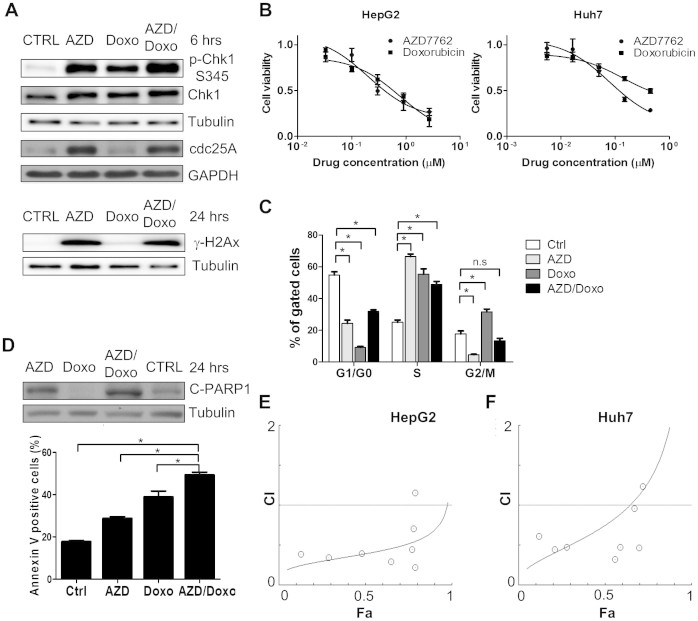
The elevated expression of ERH, ATR and CHK1 in HCCs suggests that in HCC cells combining DNA damage checkpoint inhibitors with DNA damage agents might lead to improve toxicity. We thus tested whether interfering with DNA damage response signaling could potentiate the cytotoxic effect of DNA damage agents. Knocking-down ERH by siRNAs was unable to potentiate HCC cells sensitivities to doxorubicin (Figure S3A). As ERH is undruggable, we next tested whether the Chk1 inhibitor AZD776220 could synergize with the DNA damage agent doxorubicin in HCC cells. We observed stabilization of cdc25A in HepG2 cells upon AZD7762 treatment (Figure 4A) as previous reported21, indicating successful inhibition of Chk1 signaling by AZD7762. As expected, treatment with doxorubicin or AZD7762 activated the DDR and resulted in phosphorylation of Chk1 at serine 345, which is an ATR regulated site (Figure 4A). We also observed increased γ-H2AX in HepG2 cells upon AZD7762 and combination treatment for 24 hours, suggesting DNA damage in HepG2 cells (Figure 4A). The findings were consistent with the AZD7762 treatment effect in pancreatic cancer cells22. Both AZD7762 and doxorubin inhibited proliferation of HepG2 and Huh7 cells. The IC50 to AZD7762 and Doxorubicin were 0.401 μM and 0.583 μM, respectively, for HepG2 cells, and 0.118 μM and 0.427 μM, respectively, for Huh7 cells (Figure 4B). In Huh7 cells, AZD7762 induced S phase arrest and doxorubicin induced G2/M arrest. Combination of AZD7762 and doxorubicin predominantly leads to an S phase arrest phenotype that more closely resembles AZD7762 (Figure 4C). Higher expression of cleaved PARP1 and more apoptosis cells were observed in combination treatment group compared to other groups (Figure 4D).
Figure 4. Effect of a combination of AZD7762 and doxorubicin in HCC cancer cell lines.
(A) Protein expression of γ-H2AX, cdc25 A, total and phosphorylated Chk1 S345 protein in HepG2 cells upon 6 hours of AZD7762 (AZD) 500 nM, doxorubicin (Doxo) 500 nM or combination therapy. CTRL: media only. (B) Cell viability of HepG2 cells and Huh7 cells 72 hours after various concentrations of AZD7762 or doxorubicin treatment. (C) Cell cycle distributions in the Huh7 cells 72 hours after treatment of AZD7762 500 nM, doxorubicin 500 nM, or combination therapy. (D) Apoptosis of cell, as demonstrated by protein expression of cleaved PARP1 and percentage of annexin-V positive cells, in HepG2 cells upon AZD7762, doxorubicin or combination therapy. Cells were incubated for 48 hours for annexin-V experiment. (E) Combination index (CI) in HepG2 cells upon treatment with AZD7762 and doxorubicin. The CI was calculated using cell viability data presented in the supplementary figure S2A (F) CI in Huh7 cells upon treatment with AZD7762 and doxorubicin. The CI was calculated using cell viability data presented in the supplementary figure S2B. (n.s: non-specific, *: p < 0.05).
We next tested whether AZD7762 and doxorubicin could work in synergy in HCC cells. The combination index (CI) of AZD7762 and doxorubicin were less than 1.0 mostly in HepG2 cells (Figure 4E, Figure S3B) and Huh7 cells (Figure 4F, Figure S3C), suggesting a synergistic effect in vitro.
AZD 7762 enhanced the effect of doxorubicin on HCC xenografts
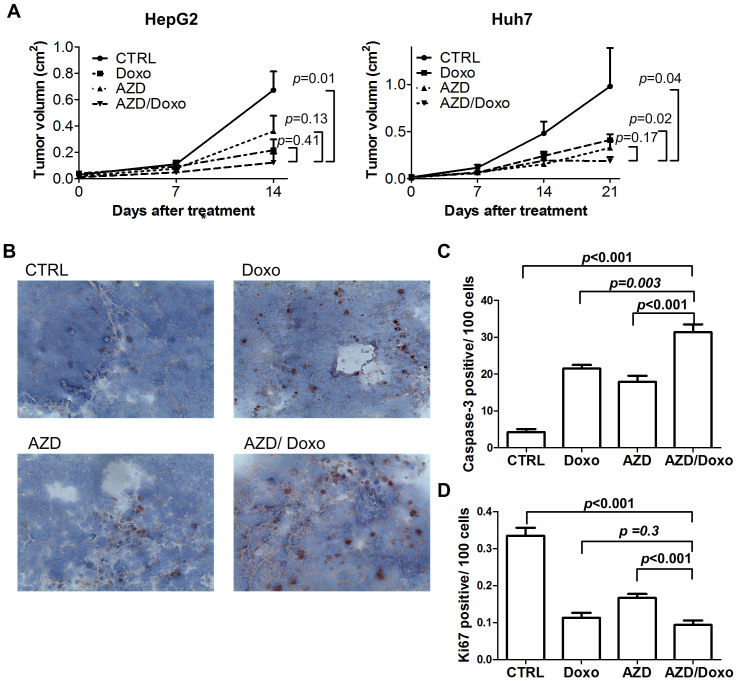
To test whether the combination of AZD7762 and doxorubicin can effectively inhibit HCC tumor growth in vivo, we treated mice bearing HCC xenografts with AZD 7762, doxorubicin, or a combination of both. Whereas either doxorubicin or AZD7762 inhibited growth of HepG2 or Huh7 xenografts, the strongest inhibition effect was observed in the combination groups (Figure 5A). We observed increased cleaved caspase-3, a marker of apoptosis, in HepG2 xenografts upon either doxorubicin or AZD7762 treatment, and the combination further increased apoptosis in the xenografts (Figure 5B and 5C). The proliferation of cancer cells in the xenografts, as indicated by the Ki-67 positive cells, decreased upon doxorubicin or AZD7762 treatment, and the combination decreased it further (Figure 5D).
Figure 5. Effect of doxorubicin and/or AZD7762 on HCC xenografts.
(A) Tumor volumes of HepG2 and Huh7 xenografts upon doxorubicin (Doxo) and/or AZD7762 (AZD) treatment. N = 5 for each group. Ctrl: vesicle. P < 0.05 for tumors on day 14 (HepG2) or day 21 (Huh7) of treatment by one-way ANOVA test, vertical bar: standard error. (B) Representative images of cleaved caspase-3 (dark brown) in HepG2 xenografts. (C) Numbers of cleaved caspase-3 cells in HepG2 xenografts. (D) Numbers of Ki-67 positive cells in HepG2 xenografts.
Discussion
Chronic HBV and HCV infection is believed to contribute to 80 % of HCC23. Several studies have reported that HBV and HCV could augment DNA damage in liver cells. The HBV X gene product HBx interferes with DNA repair by binding to damaged DNA and it sensitizes liver cells to low dose UV irradiation24. HBx also inhibits nucleotide excision repair through both p53-dependent and -independent mechanisms25,26. Hepatitis C virus interferes the DNA damage repair by inhibiting p53 activation via the protein phosphatase 2A catalytic subunit (PP2Ac)27 and by sequestering p53 in the cytoplasm through the NS2 protein28. On the other hand, high phosphorylated ATM, ATR, H2AX, and TP53 were found in occult HBV infection and HCV infection29,30. Elevated levels of DNA damage were also reported in hepatocellular carcinoma31,32,33,34. Virus related increase DNA damage and impairment of DNA damage repair both contribute to genomic instability during hepatocellular carcinogenesis, and in turn, induces the up-regulation of DNA repair genes. Here we show that ATR and CHK1 genes are up-regulated in HBV as well as HCV positive HCCs (Figure 1), and this finding is consistent with previous reports35,36. In addition, we show the up-regulation of the ERH gene in HCCs, which has not been reported in HCC previously. We also show that ERH knockdown in HCC cells leads to dysregulated DNA damage response to UV irradiation. The expression of ATR is decreased in ERH knocked-down HCC cells due to reduced ATR mRNA splicing. Our findings thus imply ERH as a novel regulator of DNA damage response genes and are important for the splicing, and thus expression, of the ATR gene.
We observed a modest but significant increase of the proportion of comet in ERH knocked-down HepG2 cells, indicating cells with DNA damage (Figure 2C). As we have previously shown that ERH depletion resulted in mitotic defects through down-regulation of CENP-E, the DNA damage induced by ERH knocking-down may be a consequence of CENP-E dysfunction. Alternatively, this could also reflect the loss of ATR expression.
We previously demonstrated that ERH regulates mRNA splicing of CENP-E mRNA through interacting with the splicesome protein SNRPD311. Here we showed that down-regulation of ERH by siRNAs also decreased the splicing efficiency of ATR mRNA that led to the loss of ATR protein. Our findings thus suggest that the in addition to CENP-E, ERH also controls the splicing of other mRNAs, and further investigation is necessary to elucidate the mechanism by which ERH operates in the cellular mRNA splicing pathway.
As no systemic chemotherapy can effectively prolong the survival of HCC patients4, we investigated whether target therapies can potentiate the effect of chemotherapies. The efficacy of anticancer reagents is dependent on the cellular DNA repair capacity. We hypothesized that up-regulation of DNA damage response genes such as ERH, ATR and CHK1 in HCC tumor cells could contribute toward resistance to DNA-damaging chemotherapy and inhibiting DNA damage response might thus overcome this resistance. We used AZD 7762 to target Chk1, the major downstream effector of the ERH-ATR axis. Chk1 inhibition has been previously shown to sensitize cancer cells toward genotoxic agents in vitro and in vivo20,35,37,38,39,40. In this study, we showed that AZD7762 induces S phase arrest and sensitizes HCC cells to doxorubicin, a well-studied chemotherapy for treating HCC4, in vitro (Figure 4). We also observed strong inhibition of growth of HCC xenografts in mice treated with a combination of doxorubicin plus AZD7762 (Figure 5), and we observed a significant more caspase-3 positive cells, suggestive of apoptosis, in tumors from mice treated with the combination. Although the clinical development of AZD7762 was terminated due to cardiac toxicity41, several other Chk1 inhibitors are under development pre-clinically or clinically42. Our findings thus support further testing of Chk1 inhibitors in combination with doxorubicin for the treat advanced HCCs.
Methods
HCC cells and reagents
HepG2 and Huh7 cells were maintained in DMEM (Gibco-BRL, Gaithersburg, MD) supplemented with 10% heat inactivated fetal bovine serum (FBS), 2 mM glutamine, 100 U/ml penicillin, and 100 μg/ml streptomycin.
To generate ERH stably expressing HepG2 cells for the rescue experiment with siERH 5, the C-terminal HA-Flag–tagged human ERH cDNA was cloned into pHAGE lentiviral vector with hygromycin resistance marker. Vector plasmids were cotransfected with packaging plasmids in 293T cells, and HepG2 cells were infected with viral supernatants containing 4 μg/mL polybrene.
AZD7762 was purchased from Selleck Chemicals (Houston, TX), and doxorubicin was from Sigma-Aldrich (St. Louis MI). Compounds were solved in DMSO at concentration of 10 mM and were stored at −20°C.
Gene expression dataset from the Gene Expression Omnibus (GEO) database analysis
The hepatocellular carcinoma patient gene expression data was available on Gene Expression Omnibus (GEO) with accession number GSE14520 and GSE6764. Expression data were normalized using the RMA method and analyzed using GeneSpring GX software (Agilent Technologies). Use and analysis of the GEO datasets were conducted in accordance with the GEO regulations.
siRNA transfection
siERH-3 and siERH-5 were obtained from Dharmacon (Lafayette, CO) and Qiagen (Venlo, Netherlands), respectively. Control siRNA was from Qiagen. Sequence for the siRNAs were as followed: siERH #3 GAACTTATGCTGACTACGA and siERH #5 GAGGATCTTGTTCAATCGGAA. Transfection of siRNA into HCC cells was performed using Lipofetamine RNAiMAX (Invitrogen, Carlsbad, CA) following manufacturer's instruction at final concentration of 10 nM.
Western Blot
Western blots were performed as described previously11. Antibodies were obtained from Abcam (ERH), Cell Signaling Technology (β-tubulin, ATR, pATR, pCHK1, r-H2Ax, PARP) and Santa Cruz (CHK1). Images of blots were captured using the FluoChem HD2 Imaging System (Alpha Innotech).
RNA extraction and real-time polymerase chain reaction (qPCR)
Total RNA from cell lines was extracted with RNeasy Mini kit (Qiagen). The RNA was reverse transcribed to DNA using High Capacity cDNA Reverse Transcription kit (Applied Biosystems, Foster City, CA). Expression of ERH and ATR mRNA was evaluated by using Power SYBR Green PCR Master Mix (Applied Biosystems). GAPDH mRNA was used as an endogenous control. Expression of RNA was analyzed using the 2−ΔΔCt method. Each RT-qPCR assay was performed in triplicates.
Primer for mRNA expression and splicing experiments were demonstrated in supplementary figure S4.
Comet Assay
The cells were stained by using the OxiSelect™ Comet Assay Kit (Cell Biolabs, Inc. San Diego, CA) according to the manufacturer's instruction. Briefly, after exposure to UV, the cells were incubated for 0, 1 and 24 hours in DMEM. Then the cells were trypsinzed by 0.25% trypsin/EDTA and resuspended to 1 × 105 cells/mL in ice-cold PBS. Combine cell samples with Comet Agarose (step 2) at 1:10 ratio, titrate to mix and immediately pipette 75 μL/well onto the OxiSelect™ Comet Slide. Slides were stored in the dark at 4°C for 15 min before adding pre-chilled lysis buffer for 45 min. The slides were immersed in freshly prepared alkaline solution (0.3 M NaOH containing 1 mM EDTA, pH >13) for 30 minutes at 4°C. Gel electrophoresis was performed at 1 V/cm for 30 minutes. The Comet slides were washed with 70% ethanol for 5 minutes, air-dried for 2.5 hours, stained with diluted Vista Green DNA Dye for 15 minutes, and then read by fluorescence microscopy using a FITC filter. At least 100 cells from each slide were analyzed. Cells were scored on a 0 to 4 scales43,44. In this study, score 1–4 cells were all calculated as comets (+) cells.
Growth inhibition assay
HepG2 and Huh7 cells were treated with various concentrations up to 2.7 μM of AZD7762, doxorubicin, or combination for 72 hours. Cell viability was determined by the MTT [3-(4,5-dimethylthiazol-2-yl)-2,5- diphenyltetrazoliumbromide] assay (Sigma-Aldrich Co). The IC50 to the drug was determined using GraphPad Prism version 5 (GraphPad Software, Inc. La Jolla, CA).
To determine the synergistic effect of AZD7762 and doxorubicin, HepG2 or Huh7 cells were treated with AZD7762 and doxorubicin at 1:1 ratio. The combination index (CI) value was determined using CompuSyn software 1.0 (ComboSyn Inc. Paramus, NJ). Synergy was defined as a CI value less than 1.045.
Cell cycle analysis
Flow cytometry was used to study cell-cycle distribution. Cells were treated with AZD7762, doxorubicin or combination treatment for 72 hours. Cell cycle analyses were performed using BD FACSCalibur™ as described previously11.
The occurrence of apoptosis was determined by the fluorescein isothiocyanate (FITC) Annexin V Apoptosis Detection Kit (Becton, Dickinson and Company) after cells were treated with AZD7762, doxorubicin or combination treatment for 48 hours.
Xenograft studies
Six-week-old male BALB/c nude mice were inoculated subcutaneously at flank with 5 × 106 HepG2 cells or 1 × 107 Huh7 cells in 100 μl PBS. When the diameter of tumors reached 5 mm, mice were randomly assigned to four treatment groups of five mice each: control (water for injection intraperitoneal (ip)), doxorubiciin (4 mg/kg twice per week ip46), AZD7762 (20 mg/kg/day on day1, 2, 4, and 5 per week, ip47), and AZD7762 + doxorubicin. The mice were treated for 2–3 weeks and the body weight and tumor volume of each mouse were monitored. Tumor volume was calculated as V = d2 × D/2, where d is the shortest and D is the longest diameter, respectively. Use of animals was conducted in accordance with the Institutional Animal Care and Use Committee (IACUC) guidelines of the College of Medicine, National Taiwan University and was approved by the IACUC of the College of Medicine, National Taiwan University.
Immunohistochemistry
Frozen sections (8 μm thick) were stained by using the NoVo Link Polymer Detection System (Leica, Biosystems Newcastle Ltd, UK), followed by AEC substrate kit (Vector Laboratories Inc. Burlingame, CA), according to the manufacturer's instruction. Hematoxylin and eosin (H&E) stain and immunohistochemistry study were performed as described previously48. Isotype antibody was used as the staining negative control. Anti-Ki-67 and anti-caspase 3 antibodies were from Santa Cruz and Cell Signaling.
Statistical analysis
We used the Spearman method to analyze correlations between variables. Comparisons of variables between two groups were performed using two tailed Student's t test. Paired t-test were used for comparing tumor and non-tumor part mRNA expression. For each cohort analyzed, ERH and ATR expression among three or more groups were performed using one-way ANOVA, followed by Bonferroni test for post hoc analysis. p-values less than 0.05 were regarded as significant.
Author Contributions
Conception and design: M.T.W., J.L. and J.C.S. Acquisition of data: M.T.W., T.H.T., S.C.W., Y.J.H. and H.L.L. Analysis and interpretation of the data: M.T.W., J.H.L., J.M.W. and J.C.S. Writing the manuscript: M.T.W., J.H.L., J.L. and J.C.S. All authors approved the final version of the manuscript.
Supplementary Material
supplementary figures
Acknowledgments
We thank Yin-Wen Shiue for comet assay technique assistance. The study was supported partly by the Liver Disease Prevention and Treatment Research Foundation, Taiwan.
References
- Ferlay J. et al. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 127, 2893–2917 (2010). [DOI] [PubMed] [Google Scholar]
- Forner A., Llovet J. M. & Bruix J. Hepatocellular carcinoma. Lancet. 379, 1245–1255 (2012). [DOI] [PubMed] [Google Scholar]
- Llovet J. M. et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 359, 378–390 (2008). [DOI] [PubMed] [Google Scholar]
- Burroughs A., Hochhauser D. & Meyer T. Systemic treatment and liver transplantation for hepatocellular carcinoma: two ends of the therapeutic spectrum. Lancet Oncol. 5, 409–418 (2004). [DOI] [PubMed] [Google Scholar]
- Kan Z. et al. Whole-genome sequencing identifies recurrent mutations in hepatocellular carcinoma. Genome Res. 23, 1422–1433 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Totoki Y. et al. High-resolution characterization of a hepatocellular carcinoma genome. Nat Genet. 43, 464–469 (2011). [DOI] [PubMed] [Google Scholar]
- Yang S. F. et al. Involvement of DNA Damage Response Pathways in Hepatocellular Carcinoma. Biomed Res Int. 2014, 153867 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yildiz G. et al. Genome-wide transcriptional reorganization associated with senescence-to-immortality switch during human hepatocellular carcinogenesis. PloS one. 8, e64016 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo G. S. et al. DNA repair and synthetic lethality. Int J Oral Sci. 3, 176–179 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wojcik E., Murphy A. M., Fares H., Dang-Vu K. & Tsubota S. I. Enhancer of rudimentaryp1, e(r)p1, a highly conserved enhancer of the rudimentary gene. Genetics. 138, 1163–1170 (1994). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weng M. T. et al. Evolutionarily conserved protein ERH controls CENP-E mRNA splicing and is required for the survival of KRAS mutant cancer cells. Proc Natl Acad Sci U S A. 109, E3659–3667 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q. et al. Chk1 is an essential kinase that is regulated by Atr and required for the G(2)/M DNA damage checkpoint. Genes Dev. 14, 1448–1459 (2000). [PMC free article] [PubMed] [Google Scholar]
- Wang X. et al. Rad17 phosphorylation is required for claspin recruitment and Chk1 activation in response to replication stress. Mol Cell. 23, 331–341 (2006). [DOI] [PubMed] [Google Scholar]
- Wang H. et al. CGK733 enhances multinucleated cell formation and cytotoxicity induced by taxol in Chk1-deficient HBV-positive hepatocellular carcinoma cells. Biochem Biophys Res Commun. 422, 103–108 (2012). [DOI] [PubMed] [Google Scholar]
- Roessler S. et al. A unique metastasis gene signature enables prediction of tumor relapse in early-stage hepatocellular carcinoma patients. Cancer Res. 70, 10202–10212 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng S., Tansey W. P., Hiebert S. W. & Zhao Z. Integrative network analysis identifies key genes and pathways in the progression of hepatitis C virus induced hepatocellular carcinoma. BMC Med Genomics. 4, 62 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olive P. L., Banath J. P. & Durand R. E. Heterogeneity in radiation-induced DNA damage and repair in tumor and normal cells measured using the "comet" assay. Radiat Res. 122, 86–94 (1990). [PubMed] [Google Scholar]
- Guo Z., Kumagai A., Wang S. X. & Dunphy W. G. Requirement for Atr in phosphorylation of Chk1 and cell cycle regulation in response to DNA replication blocks and UV-damaged DNA in Xenopus egg extracts. Gene Dev. 14, 2745–2756 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou L. & Elledge S. J. Sensing DNA damage through ATRIP recognition of RPA-ssDNA complexes. Science 300, 1542–1548 (2003). [DOI] [PubMed] [Google Scholar]
- Zabludoff S. D. et al. AZD7762, a novel checkpoint kinase inhibitor, drives checkpoint abrogation and potentiates DNA-targeted therapies. Mol Cancer Ther. 7, 2955–2966 (2008). [DOI] [PubMed] [Google Scholar]
- Hassepass I., Voit R. & Hoffmann I. Phosphorylation at serine 75 is required for UV-mediated degradation of human Cdc25A phosphatase at the S-phase checkpoint. J. Biol Chem. 278, 29824–29829 (2003). [DOI] [PubMed] [Google Scholar]
- Parsels L. A. et al. Assessment of chk1 phosphorylation as a pharmacodynamic biomarker of chk1 inhibition. Clin Cancer Res. 17, 3706–3715 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgs M. R., Chouteau P. & Lerat H. ‘Liver let die': oxidative DNA damage and hepatotropic viruses. J Gen Virol 95, 991–1004 (2014). [DOI] [PubMed] [Google Scholar]
- Capovilla A., Carmona S. & Arbuthnot P. Hepatitis B virus X-protein binds damaged DNA and sensitizes liver cells to ultraviolet irradiation. Biochem Biophys Res Commun. 232, 255–260 (1997). [DOI] [PubMed] [Google Scholar]
- Mathonnet G., Lachance S., Alaoui-Jamali M. & Drobetsky E. A. Expression of hepatitis B virus X oncoprotein inhibits transcription-coupled nucleotide excision repair in human cells. Mutat Res. 554, 305–318 (2004). [DOI] [PubMed] [Google Scholar]
- Becker S. A., Lee T. H., Butel J. S. & Slagle B. L. Hepatitis B virus X protein interferes with cellular DNA repair. J. Virol. 72, 266–272 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duong F. H. et al. Protein phosphatase 2A promotes hepatocellular carcinogenesis in the diethylnitrosamine mouse model through inhibition of p53. Carcinogenesis. 35, 114–122 (2014). [DOI] [PubMed] [Google Scholar]
- Bittar C., Shrivastava S., Bhanja Chowdhury J., Rahal P. & Ray R. B. Hepatitis C virus NS2 protein inhibits DNA damage pathway by sequestering p53 to the cytoplasm. PloS one 8, e62581 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhargava A. et al. Occult hepatitis C virus elicits mitochondrial oxidative stress in lymphocytes and triggers PI3-kinase-mediated DNA damage response. Free Radic Biol Med. 51, 1806–1814 (2011). [DOI] [PubMed] [Google Scholar]
- Bhargava A. et al. Occult hepatitis B virus infection with low viremia induces DNA damage, apoptosis and oxidative stress in peripheral blood lymphocytes. Virus Res. 153, 143–150 (2010). [DOI] [PubMed] [Google Scholar]
- Schwarz K. B. et al. Increased hepatic oxidative DNA damage in patients with hepatocellular carcinoma. Dig Dis Sci. 46, 2173–2178 (2001). [DOI] [PubMed] [Google Scholar]
- Tanaka S. et al. Increased hepatic oxidative DNA damage in patients with nonalcoholic steatohepatitis who develop hepatocellular carcinoma. J Gastroenterol. 48, 1249–1258 (2013). [DOI] [PubMed] [Google Scholar]
- Shawki S. M., Meshaal S. S., El Dash A. S., Zayed N. A. & Hanna M. O. Increased DNA damage in hepatitis C virus-related hepatocellular carcinoma. DNA Cell Biol. 33, 884–890 (2014). [DOI] [PubMed] [Google Scholar]
- Fujita N. et al. Comparison of hepatic oxidative DNA damage in patients with chronic hepatitis B and C. J. Viral Hepat. 15, 498–507 (2008). [DOI] [PubMed] [Google Scholar]
- Hong J. et al. CHK1 targets spleen tyrosine kinase (L) for proteolysis in hepatocellular carcinoma. J Clin Invest. 122, 2165–2175 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho C. C. et al. The relative contribution of CHK1 and CHK2 to Adriamycin-induced checkpoint. Exp Cell Res. 304, 1–15 (2005). [DOI] [PubMed] [Google Scholar]
- Bunch R. T. & Eastman A. Enhancement of cisplatin-induced cytotoxicity by 7-hydroxystaurosporine (UCN-01), a new G2-checkpoint inhibitor. Clin Cancer Res. 2, 791–797 (1996). [PubMed] [Google Scholar]
- Tse A. N. et al. CHIR-124, a novel potent inhibitor of Chk1, potentiates the cytotoxicity of topoisomerase I poisons in vitro and in vivo. Clin Cancer Res. 13, 591–602 (2007). [DOI] [PubMed] [Google Scholar]
- McNeely S. et al. Chk1 inhibition after replicative stress activates a double strand break response mediated by ATM and DNA-dependent protein kinase. Cell cycle. 9, 995–1004 (2010). [DOI] [PubMed] [Google Scholar]
- Landau H. J. et al. The checkpoint kinase inhibitor AZD7762 potentiates chemotherapy-induced apoptosis of p53-mutated multiple myeloma cells. Mol Cancer Ther. 11, 1781–1788 (2012). [DOI] [PubMed] [Google Scholar]
- Sausville E. et al. Phase I dose-escalation study of AZD7762, a checkpoint kinase inhibitor, in combination with gemcitabine in US patients with advanced solid tumors. Cancer Chemother Pharmacol. 73, 539–549 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrett M. D. & Collins I. Anticancer therapy with checkpoint inhibitors: what, where and when? Trends Pharmacol Sci. 32, 308–316 (2011). [DOI] [PubMed] [Google Scholar]
- Waters D. J. et al. Noninvasive prediction of prostatic DNA damage by oxidative stress challenge of peripheral blood lymphocytes. Cancer Epidermiol Biomarkers Prev. 16, 1906–1910 (2007). [DOI] [PubMed] [Google Scholar]
- Duthie S. J. & Collins A. R. The influence of cell growth, detoxifying enzymes and DNA repair on hydrogen peroxide-mediated DNA damage (measured using the comet assay) in human cells. Free Radic Biol Med. 22, 717–724 (1997). [DOI] [PubMed] [Google Scholar]
- Chou T. C. & Talalay P. Quantitative analysis of dose-effect relationships: the combined effects of multiple drugs or enzyme inhibitors. Adv Enzyme Regul. 22, 27–55 (1984). [DOI] [PubMed] [Google Scholar]
- Bandyopadhyay A. et al. Doxorubicin in combination with a small TGFbeta inhibitor: a potential novel therapy for metastatic breast cancer in mouse models. PloS one. 5, e10365 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkatesha V. A. et al. Sensitization of pancreatic cancer stem cells to gemcitabine by Chk1 inhibition. Neoplasia. 14, 519–525 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei S. C., Tsao P. N., Weng M. T., Cao Z. & Wong J. M. Flt-1 in colorectal cancer cells is required for the tumor invasive effect of placental growth factor through a p38-MMP9 pathway. J. Biomed Sci. 20, 39 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
supplementary figures