Abstract
We report a case of Strongyloides stercoralis hyperinfection in an immunosuppressed individual occurring in a non-endemic area. Geographic risk is not sufficient to rule out Strongyloidiasis in susceptible individuals presenting with severe pulmonary disease.
INTRODUCTION
The diagnosis of severe pulmonary disease in immunosuppressed patients is challenging due to the broad differential diagnosis. Epidemiological factors are often considered when prioritizing infectious etiologies. Strongyloides stercoralis is an intestinal nematode endemic in many tropical and subtropical areas. Most commonly it produces an acute gastrointestinal illness, but it may also remain dormant for many years. Immunosuppression, however, can lead to the development of a fulminant and often fatal hyperinfection syndrome predominantly affecting the lungs. We report an unexpected case of respiratory failure from Strongyloides hyperinfection occurring in a patient without apparent geographic risk.
CASE REPORT
A 50-year-old man was admitted to hospital with dyspnea, malaise and a rising serum creatinine 4 months following cadaveric renal transplantation. At home a week prior, he had developed nausea, cephalgia and neck stiffness. Blood cultures performed in the Emergency Department were negative and the symptoms eventually subsided after ∼3 days. He subsequently developed a pruritic, maculopapular rash attributed to ciprofloxacin use for a persistent post-operative fluid collection, which was thought to be infected with Pseudomonas aeruginosa.
The patient had developed a reno-vasculitic syndrome 16 years prior to admission initially treated with cyclophosphamide and corticosteroids. He was then lost to follow-up for 9 years when he presented with end-stage renal disease and had been on hemodialysis for 5 years prior to receiving a cadaveric renal transplant. The latter was complicated by delayed graft function and P. aeruginosa sepsis treated with piperacillin-tazobactam and ciprofloxacin. His admission medications were amlodipine, atorvastatin, mycophenyllate mofetil, prednisone, tacrolimus and aerosolized pentamidine. He reported an allergy to sulfa drugs. He had lived his entire life in Northern Ontario and had never traveled south of Toronto, Canada, where he received his renal transplant. He denied any travel to the Southern United States, the Caribbean or overseas. He was married and was HIV negative at the time of transplantation.
On admission he was afebrile, but markedly dyspneic with an oxygen saturation of 85% on room air. There was no jugular venous distension or peripheral edema and the lungs were initially clear. His incision appeared to be healing and his neurological examination was unremarkable. He has a persistent mild maculopapular rash on the thighs and trunk. Laboratory investigations revealed a white blood cell of 14.0 × 109 with 11.7 × 109 neutrophils, 5.9 × 109 bands, a hemoglobin of 7.8 × 1012 and a serum creatinine of 234 µmol/l. Peripheral blood eosinophilia was (1.4 × 109/l, WBC: 10.3 × 109/l) present on admission, and resolved, over 5 days, by the time of ICU transfer (0.1 × 109/l, WBC: 14.0 × 109/l).
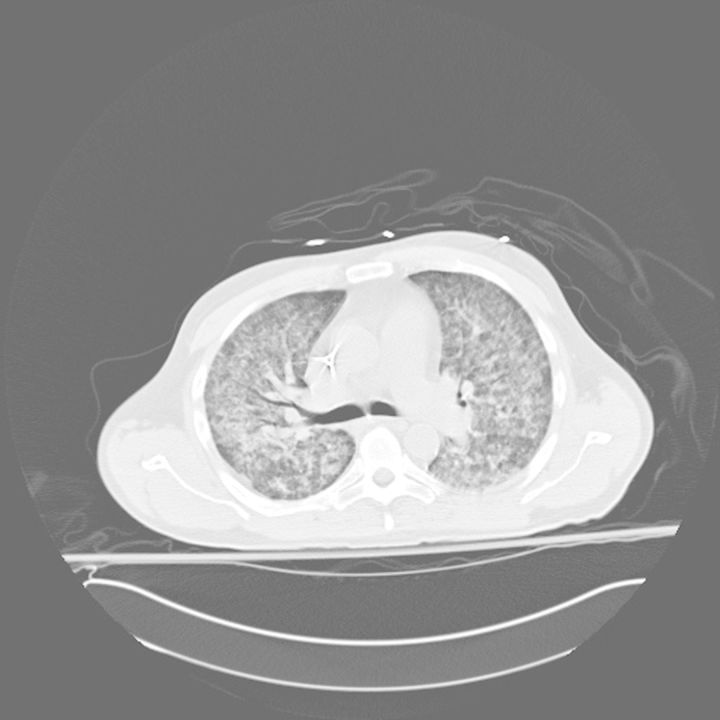
Over the 5 days following admission, the patient's dyspnea continued to worsen. Radiographs and computed tomography of the chest (Fig. 1) demonstrated rapidly evolving bilateral interstitial and airspace disease despite the initiation of ceftriaxone and azithromycin for community acquired pneumonia, in line with current North American guidelines. He was eventually required mechanical ventilation for respiratory failure. Bronchoalveolar lavage (BAL) performed during a fiberoptic flexible bronchoscopy then revealed the presence of S. stercoralis eggs, rhabditiform and filariform larvae and adult females on a wet mount preparation, as well as rhabditiform and filariform larvae on cytology stains. BAL studies for bacteria, Nocardia sp, fungus, cytomegalovirus and acid fast bacterial all remained negative. Blood cultures were positive only for a S. epidermidis and later for E. faecium, which was also present in the urine. Stool studies were unfortunately not sent. A diagnosis was of Strongyloides hyperinfection syndrome and treatment with ivermectin 15 mg daily and albendazole 400 mg bid. Despite this, over the course of the ensuing week the patient developed severe septic shock requiring vasopressin and norepinephrine infusions and worsening acute respiratory distress syndrome (ARDS) requiring prone ventilation. Blood cultures nevertheless remained negative. Pneumothorax, pneumomediastinum and pneumoperitoneum developed as a result of very high airway pressures from severe ARDS. The patient eventually suffered a fatal cardiac arrest 2 weeks after admission. No post-mortem was performed at the request of the family.
Figure 1:
Unenhanced computed tomogram image of thorax demonstrating diffuse ground glass opacification with subpleural sparing and septal thickening consistent with an atypical pneumonia.
DISCUSSION
Our case of Strongyloides hyperinfection syndrome is noteworthy because our patient did not present with any of the classic epidemiologic risk factors apart from immunosuppression. He had lived his entire life in Northwestern Ontario, Canada, a non-endemic area, and he had not endorsed a history of susceptible travel, nor was he from an ethnic group that would have brought him in frequent contact with individuals who might be infected. Possible routes of transmission include acquisition at the time of transplant from an infected organ, or by contact with an asymptomatic carrier outside any endemic area.
Strongyloides is an intestinal nematode endemic in tropical and subtropical areas, as well as some temperate areas, such as the southeastern USA, but not in any part of Canada. Acquisition is normally through the skin from contaminated soil. It most commonly causes a localized gastrointestinal infection, but may also remain clinically dormant for decades, maintained by low-grade autoinfection. Immunosuppression, particularly with impaired Th-2 responses, can amplify this cycle producing the hyperinfection syndrome [1, 2]. Hyperinfection classically presents with severe gastrointestinal symptoms (up to 91% of cases), pulmonary disease (36% of cases) and skin rash (25%) [3]. Eosinophilia is a classic manifestation but may be present in only 30% of hyperinfection cases [4]. Interestingly, our patient initially presented with eosinophilia, which resolved as his condition worsened. The absence of eosinophilia in hyperinfection is considered a poor prognostic sign [1, 2]. The mortality is generally reported at ∼87%, but may be as low as 25% with early diagnosis [4].
While person-to-person transmission has been speculated, cases clearly demonstrating this have been rare. The possibility of such transmission has generally been inferred from clustering of case within families in endemic areas [5], and as such, a confounding common environmental source cannot be excluded. Czachor and Jonas [5] described transmission of Strongyloides from an immunosuppressed, hyperinfected individual, who had grown up in an endemic area to a normal host. Our case might represent the reverse scenario, implicating transmission from a minimally symptomatic individual, to a highly susceptible host.
Timely diagnosis is challenging and requires a high index of suspicion. The diagnosis is usually made by stool examination, but a single stool specimen may be negative in 70% of cases. Multiple samples increase the yield (up to seven may be required), as do the Bearmann and Harada-Mori concentration techniques. The agar culture method appears to have the highest sensitivity at 96%. However, these techniques are time consuming and are not readily available in all laboratories [6]. Newer serological assays have been designed to detect antibodies to filariform larval antigens by the enzyme immunoassay (EIA). They appear to have very good performance characteristics with a sensitivity and specificity of 91.2 and 93.3%, respectively [7]. Thus, EIA may be useful for the diagnosis of active disease, screening at risk populations and tracking response to treatment [7, 8]. Stools were not tested in our patient. Data are lacking on the sensitivity of BAL compared with post-mortem examination. The patient was treated with both albendazole and ivermectin as currently recommended [2], but his clinical instability prevented repeat bronchoscopy to assess his response to treatment.
Current guidelines recommend pre-transplant screening only of recipients from endemic areas [9]. While relatively rare, the potentially high mortality of hyperinfection may warrant more aggressive screening of both donors and recipients [7].
Immunosuppression is the principal risk factor for Strongyloides hyperinfection, and is sufficient to consider the diagnosis in any immunocompromised patient. Clinicians practicing in temperate climates frequently do not consider helminthic infections in their differential diagnosis given the low prevalence of such organisms in these areas. However, with more population movement worldwide, the possibility of infection from outside endemic areas is likely to increase either by person-to-person transmission or by transplantation of infected organs. Given that the mode of acquisition may not be obvious, and that perseverance may be required to make the diagnosis, a high index of suspicion should be maintained for strongyloidiasis. In particular, the finding of eosinophilia should have prompted an earlier search for a possible helminthic infection. Clinicians should, therefore, not rely solely on geographic risks to rule out strongyloidiasis in an immunocompromised patient with pulmonary disease.
ACKNOWLEDGMENTS
The authors wish to thank Dr Marc Shelly (University of Rochester Medical Center) and Dr David Bowton (Wake Forest School of Medicine) for their helpful comments on the manuscript.
REFERENCES
- 1.Concha R, Harrington W, Rogers AI. Intestinal strongyloidiasis: recognition, management, and determinants of outcome. J Clin Gastroenterol. 2005;39:203–211. doi: 10.1097/01.mcg.0000152779.68900.33. [DOI] [PubMed] [Google Scholar]
- 2.Keiser PB, Nutman TB. Strongyloides stercoralis in the immunocompromised population. Clin Microbiol Rev. 2004;17:208–217. doi: 10.1128/CMR.17.1.208-217.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fardet L, Généreau T, Poirot J-L, Guidet B, Kettaneh A, Cabane J. Severe strongyloidiasis in corticosteroid-treated patients: case series and literature review. J Infect. 2007;54:18–27. doi: 10.1016/j.jinf.2006.01.016. [DOI] [PubMed] [Google Scholar]
- 4.Adedayo O, Grell G, Bellot P. Hyperinfective strongyloidiasis in the medical ward: review of 27 cases in 5 years. South Med J. 2002;95:711–716. [PubMed] [Google Scholar]
- 5.Czachor JS, Jonas AP. Transmission of Strongyloides steracolis person to person. J Travel Med. 2000;7:211–212. doi: 10.2310/7060.2000.00063. [DOI] [PubMed] [Google Scholar]
- 6.Siddiqui AA, Berk SL. Diagnosis of Strongyloides stercoralis infection. Clin Infect Dis. 2001;33:1040–1047. doi: 10.1086/322707. [DOI] [PubMed] [Google Scholar]
- 7.Bon B, Houze S, Talabani H, Magne D, Belkadi G, Develoux M, et al. Evaluation of a rapid enzyme-linked immunosorbent assay for diagnosis of strongyloidiasis. J Clin Microbiol. 2010;48:1716–1719. doi: 10.1128/JCM.02364-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Loutfy MR, Wilson M, Keystone JS, Kain KC. Serology and eosinophil count in the diagnosis and management of strongyloidiasis in a non-endemic area. Am J Trop Med Hyg. 2002;66:749–752. doi: 10.4269/ajtmh.2002.66.749. [DOI] [PubMed] [Google Scholar]
- 9.Avery RK. Recipient screening prior to solid-organ transplantation. Clin Infect Dis. 2002;35:1513–1519. doi: 10.1086/344777. [DOI] [PubMed] [Google Scholar]