Abstract
Lactobezoars are a type of bezoar composed of undigested milk and mucus. The aetiology is likely multifactorial, being classically described in association with pre-term, low-birth weight infants fed with hyperconcentrated formula. The authors present a case of lactobezoar recurrence in a pre-term infant with oesophageal atresia. To our knowledge, this is the first report of recurrence of lactobezoar.
INTRODUCTION
Lactobezoars are pathological conglomerations of undigested milk and mucus. They are usually located in the stomach of milk-fed infants, but can be found in other parts of the intestinal tract [1, 2]. Despite being a rarely reported disorder [1], it is the most common form of bezoar in neonates [3]. Since its first description in 1959, there are about 100 reported cases of gastric lactobezoar (GLB), but no reports of recurrence. In the following report, we present a case of recurrent lactobezoar and discuss our therapeutic approach, along with a brief review of GLB.
CASE REPORT
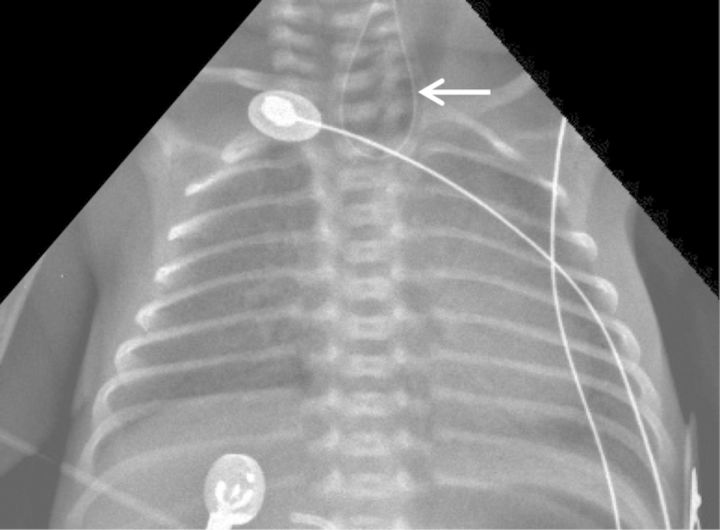
A female pre-term infant was delivered at 30 weeks of gestation by normal spontaneous vaginal delivery. Twenty-four hours before delivery the 38-year-old G2P2 mother was admitted for pre-mature labour. Routine laboratory and ultrasound exams in all trimesters were unremarkable. Ultrasound on admission showed polyhydramnios. Apgar scores were 9/9 at 1st/5th min, respectively. Birth weight was 1308 g. There was no need for resuscitation manoeuvres and she was admitted to neonatal intensive care unit on spontaneous breathing. On admission, there was evidence of excessive drooling and difficulty progressing orogastric tube. Plain chest X-ray showed curled back orogastric tube, which raised the suspicion of oesophageal atresia (Figure 1). Surgery performed on day 2 of life identified long gap oesophageal atresia with distal fistula (type III oesophageal atresia). She was submitted to ligation of the fistula and placement of gastrostomy. Initial post-operative period was unremarkable and enteric feeding was initiated on D7 post-operative with expressed and fortified breast milk and pre-term formula.
Figure 1:
Plain chest X-ray showing curled back orogastric tube at proximal oesophagus (arrow).
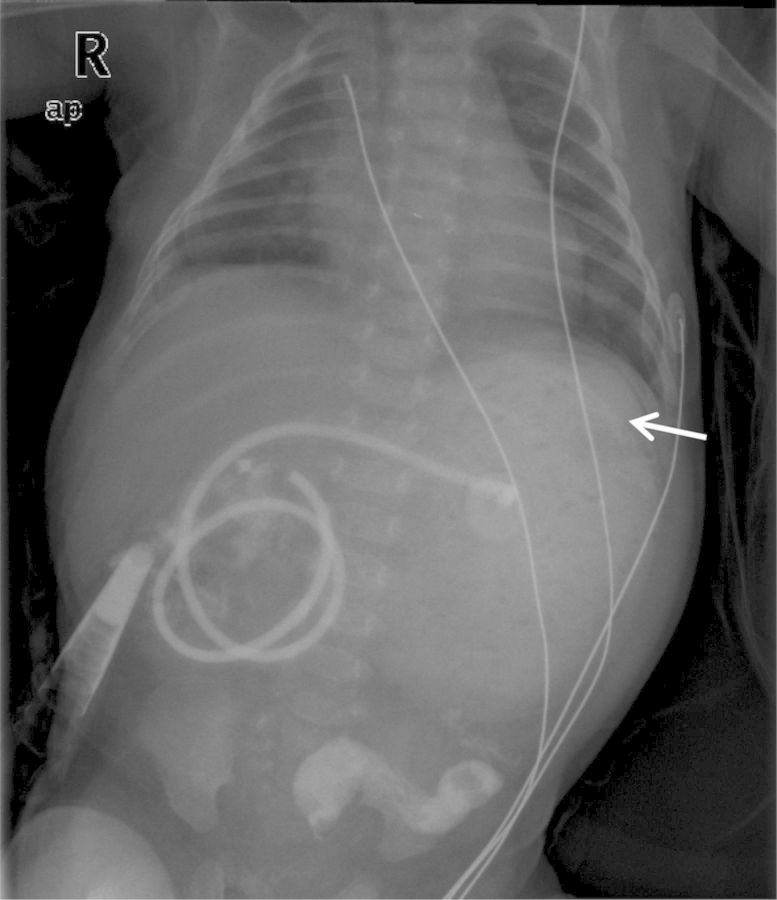
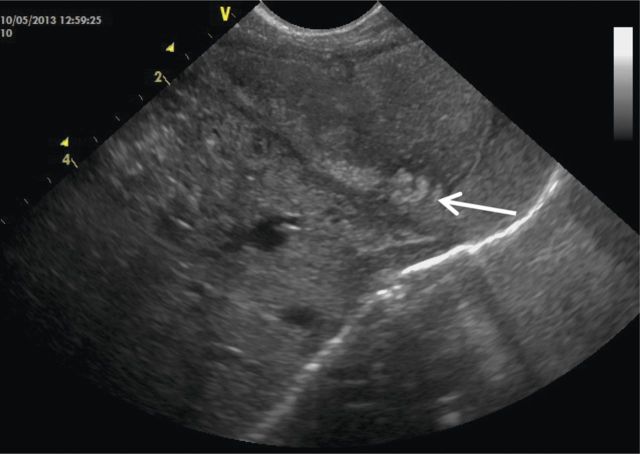
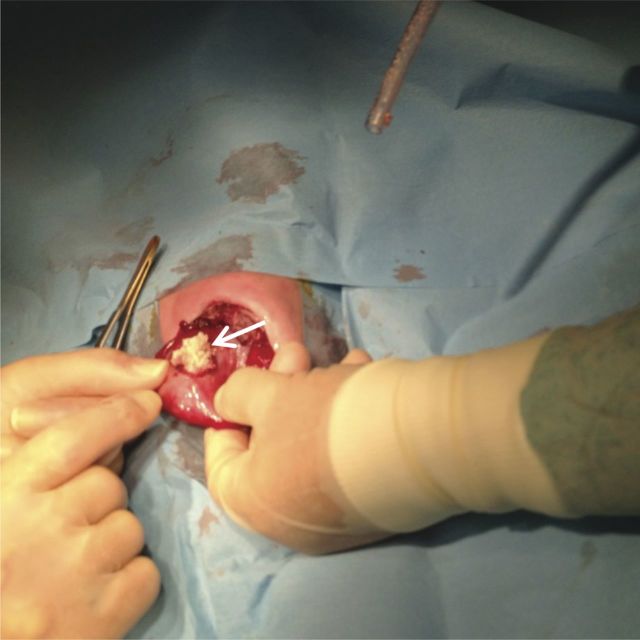
On D47 post-operative, she started persistent crying when fed. The abdomen was diffusely tender and a palpable mass was identified on left upper quadrant. Physical exam was otherwise normal. Abdominal X-ray showed left upper quadrant hypotransparency (Figure 2). Abdominal ultrasound revealed gastric free-floating echogenic content (Figure 3) and fluoroscopy was inconclusive. Conservative treatment (domperidone, ranitidine, nil by mouth and IV fluid) was administered for 48 h, but showed no improvement. On suspicion of gastrostomy complication, an exploratory laparotomy was performed, which revealed a GLB with compromised gastric mucosa that was removed through the gastrostomy (Figure 4). Her post-operative course was uneventful and enteric feeding with expressed breast milk and pre-term formula was initiated 5 days after surgery. On D7, she began extensively hydrolysed (EH) formula. On D15 post-operative, she initiated discomfort after feeding and gastric aspiration showed flocculated milk curds. Ultrasound revealed similar gastric free-floating echogenic content. Given the suspicion of newly formed lactobezoar, her own saliva was infused trough gastrostomy tube during meals, and in the following 48 h there was improvement of discomfort. Enteric nutrition with formula milk was preserved and saliva infusion was discontinued 1 month later. No other gastric complaints were identified. Oesophageal anastomosis was performed at 6 months of life. Currently, she is 14 months old and was recently diagnosed with coeliac disease, during investigation of intermittent diarrhoea and failure to thrive.
Figure 2:
Abdominal X-ray showing an opaque gastric mass with a mosque pattern, outlined by intraluminal air (arrow).
Figure 3:
Abdominal ultrasound showing echogenic gastric air trapping within an intra-gastric mass (arrow).
Figure 4:
Gastric lactobezoar revealed during exploratory laparotomy (arrow).
DISCUSSION
GLBs are known to emerge in the presence of certain conditions, but the aetiology is likely multifactorial. Prematurity and low-birth weight (LBW) are known pathogenic factors, although it has been documented in a full-term infant [4] and in toddlers [5]. Other reported causes are related to composition of formula, antacid medications and dehydration [1]. Despite the endogenous factors (prematurity and LBW) featured by our patient, exogenous pathogenic factors were also identified: she was fed with fortified breast milk and pre-term formula, which are high in caloric and protein contents and may exceed the digestive capacity of pre-mature infants. Knowing that altered digestive capacity and decreased enzyme activity are postulated causes for GLB formation [1], the absence of salivary enzymes due to oesophageal atresia, leading to altered digestion, may have influenced GLB formation. On the other hand, altered gastric emptying, a known complication of enteral tube feeding [6], may have also been a contributing factor. On the recurrence of lactobezoar, she was being fed with EH formula lower in caloric and protein content and no other risk factors were identified other than altered digestion due to oesophageal atresia and gastrostomy feeding. There is no reported association of hydrolysed formula and GLB; however, it has been described in patients who consume milk and soy-based formulas, breast milk and even cow's milk [1, 4, 5]. Nonetheless one article suggested changing to elementary formula after resolution of lactobezoar, even though recurrence had never been reported in the literature [5].
Lactobezoars may be found in asymptomatic patients but usually present with gastrointestinal symptoms, such as abdominal distension, vomiting, regurgitation, gastric residuals or palpable abdominal mass [1, 5]. More rarely it can appear as gastric outlet obstruction or perforation [3]. Diagnosis requires a high index of suspicion and experienced investigators. Abdominal ultrasound, the method of choice, reveals highly echogenic intra-bezoaric air trapping. Abdominal X-ray can be diagnostic but is inconclusive in about 30% of the cases [1]. On our case, diagnosis was made solely after exploratory laparotomy, even though in review of prior exams, there was an identifiable intra-gastric mass on abdominal X-ray (Figure 2) and ultrasound (Figure 3).
Conservative management is the gold standard, consisting of bowel rest and parenteral nutrition, with or without gastric saline lavage [1, 3]. There have been reports of successful disintegration of lactobezoars using intra-gastric N-acetylcysteine [3]. Conservative management is effective in over 85% of the cases; however, surgical measures may be necessary if the condition does not resolve within 72 h or in case of complications [1]. Even though the cause for abdominal distension was not clear in our patient, she received conservative, yet ineffective, management for 48 h before laparotomy. On the second episode, we tried a different approach, infusing saliva along with formula milk, rather than resting the bowel. There are other reports of successful GLB resolution despite continuation of enteral nutrition [1].
Given early diagnostic and treatment, the prognosis is excellent [1]. However, it remains an underdiagnosed entity and physicians should be alert in order to include GLB in the differential of non-specific gastrointestinal symptoms [3]. To our knowledge, there is only one other case report in association with oesophageal atresia [2] and no reports of recurrence. With this case, we intend to expand the comprehension about GLB pathogenesis and treatment, reinforcing that there is still much to understand about this likely underdiagnosed entity.
REFERENCES
- 1.Heinz-Erian P, Gassner I, Klein-Franke A, Jud V, Trawoeger R, Niederwanger C, et al. Gastric lactobezoar - a rare disorder? Orphanet J Rare Dis. 2012;7:3. doi: 10.1186/1750-1172-7-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bos M, Wijnen R, Blaauw I. Gastric pneumatosis and rupture caused by lactobezoar. Pediatr Int. 2013;55:757–760. doi: 10.1111/ped.12164. [DOI] [PubMed] [Google Scholar]
- 3.Bajorek S, Basaldua R, McGoogan K, Miller C, Sussman C. Neonatal gastric lactobezoar: management with N-acetylcysteine. Case Rep Pediatr. 2012;2012:412412. doi: 10.1155/2012/412412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Usmani S, Levenbrown J. Lactobezoar in a full-term breast-fed infant. Am J Gastroenterol. 1989;84:647–649. [PubMed] [Google Scholar]
- 5.DuBose T, Southgate W, Hill J. Lactobezoars: a patient series and literature review. Clin Pediatr (Phila) 2001;40:603–606. doi: 10.1177/000992280104001104. [DOI] [PubMed] [Google Scholar]
- 6.Pearce C, Duncan H. Enteral feeding. Nasogastric, nasojejunal, percutaneous endoscopic gastrostomy, or jejunostomy: its indications and limitations. Postgrad Med J. 2002;78:198–204. doi: 10.1136/pmj.78.918.198. [DOI] [PMC free article] [PubMed] [Google Scholar]