Abstract
The complex, highly integrative endocrine system regulates all aspects of somatic maintenance and reproduction and has been widely implicated as an important determinant of longevity in short-lived traditional model organisms of aging research. Genetic or experimental manipulation of hormone profiles in mice has been proven to definitively alter longevity. These hormonally induced lifespan extension mechanisms may not necessarily be relevant to humans and other long-lived organisms that naturally show successful slow aging. Long-lived species may have evolved novel anti-aging defenses germane to naturally retarding the aging process. Here we examine the available endocrine data associated with the vitamin D, insulin, grlucocorticoid and thyroid endocrine systems of naturally long-living small mammals. Generally, long-living rodents and bats maintain tightly regulated lower basal levels of these key pleiotropic hormones than shorter-lived rodents. Similarities with genetically manipulated suggest that evolutionarily wellconserved hormonal mechanisms are integrally involved in lifespan determination.
Keywords: lifespan, rodents, bats, naked mole-rat, endocrinology, insulin, vitamin D, thyroid, slow aging
1.0 Introduction
For thousands of years, the process of aging has been attributed to a progressively larger deficit of some vital regulatory substances with advancing years. Within the past two centuries, the decline in hormone levels has been touted as responsible for the age-related deterioration in physiological function and greater frailty (for historical reviews see Haber, 2004). In mammals, these pleiotropic hormones work together in a highly integrated manner to exert control over energy and ion balance, and affect metabolism, growth, repair and reproductive function. Not surprisingly, given the continued quest for human immortality, hormone replacement therapy (HRT) is often proposed as a reliable method of slowing aging (Olshanky et al., 2001; Horani et al., 2004). However HRT has, at best, yielded equivocal results, and even though it may restore hormone levels to those of young healthy adults, it does not avert aging. Indeed, HRT may even accelerate the aging process and lead to increased incidence of many age-associated diseases such as cancer and cardiovascular ailments (Roussouw et al., 2002; Manson et al., 2003).
1.1 Role of endocrines in lifespan extension
Contrary to proposals espoused by “anti-aging HRT” advocates, genetic and experimental manipulations in animal aging models reveal that those individuals with naturally low levels of key hormones (e.g., growth hormone) live longer than those with higher, albeit “normal” levels (for review see Bartke, 2007; Brown-Borg, 2007). A deficiency in pituitary hormones, and growth hormone in particular, is strongly implicated in lifespan extension. Mice homozygous for Pit1 show attenuated aging, live approximately 40% longer than wild-type, and have lower growth hormone and IGF-1 levels than wild-type (Flurkey et al., 2001). Conversely, mice over-expressing bovine growth hormone appear to age faster and show greater incidence of age-associated pathologies (Bartke, 2003).
The life-extending effects of dietary restriction (DR) also are attributed, in part, to low basal hormone levels and reduced daily fluctuations in insulin, insulin-like growth factor (IGF), thyroid hormone and sex steroids and the concomitant decline in metabolism (Longo and Finch, 2003; Masoro, 2005). A fundamental, but still unanswered, question is whether or not naturally long-living species have low or sustained high levels of hormones.
1.2 Long-living small mammals
For our size, humans are exceptionally long-living relative to most other species, surviving five times longer than predicted by size (Hulbert et al., 2007). Paradoxically, traditional animal models used in aging research are primarily chosen because they age extremely rapidly and are short-lived (Miller and Nadon, 2000), even though the principal aim of most of these studies is to discover mechanisms that will allow us to ultimately further retard human aging. These short-lived animal models may reveal lifespan extension mechanisms not necessarily relevant to organisms (like us) that naturally age slowly. Rather, long-lived species may have evolved novel anti-aging characteristics that are present throughout life, and germane to naturally retarding the aging process, thereby maintaining the low age-specific mortality that allows slow-aging organisms to achieve their impressive longevity.
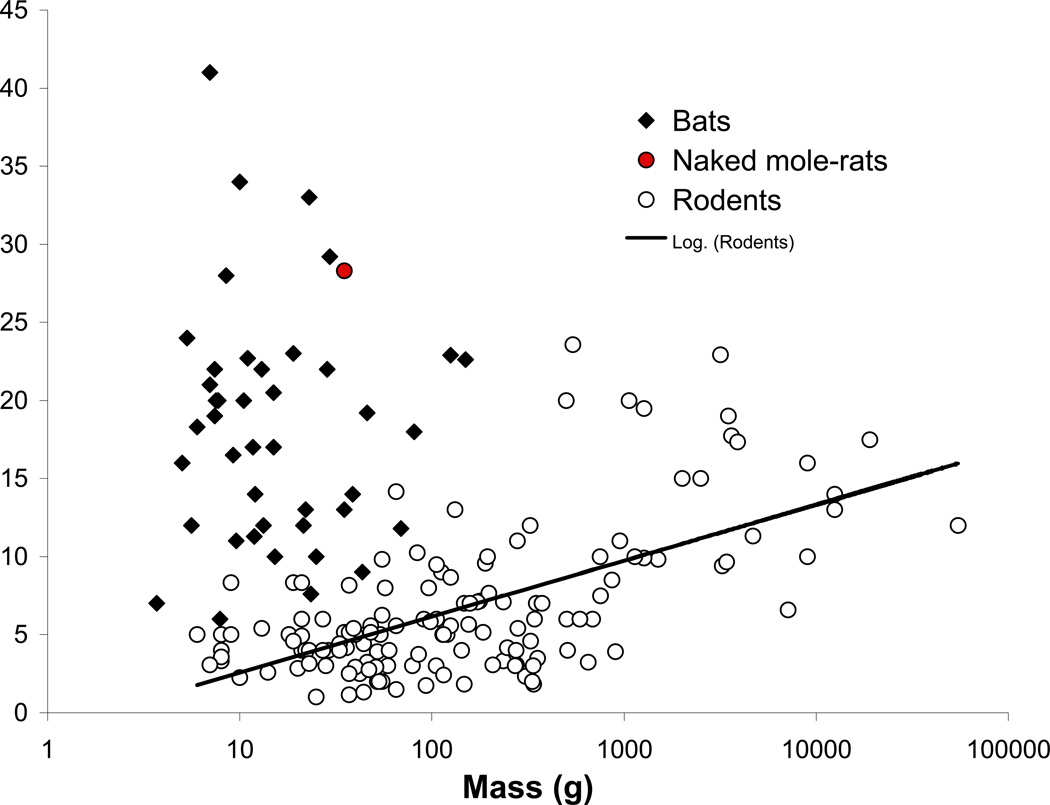
Among mammals, maximum species lifespan potential (MLSP) scales with body mass. This allometric relationship holds true for rodents as well (Fig. 1; de Magalhães, et al., 2007). The larger rodent species such as squirrels, marmots, porcupines and mole-rats frequently attain maximum life spans, exceeding twenty years (Fig. 1; Weigl, 2005), while most mouse-sized rodents in captivity live no more than four years. Surprisingly, the longest-living rodent defies this allometric relationship and is a small mouse-sized (35g) hystricognath, the naked mole-rat (Heterocephalus glaber). This species in captivity shows attenuated age-related declines in reproduction, physiological and biochemical function, maintaining good health until very near the end of their long >28.3y lifespan (O’Connor et al., 2002; Buffenstein, 2005; Cszizar et al., 2007; Buffenstein, 2008). Other reportedly long-living rodents such as certain squirrel species, porcupines and deer mice live approximately double that predicted by body mass. The naked mole-rat, like humans, lives five times longer than predicted allometrically using the equations of de Magalhães et al. (2007). Volant mammals (e.g., bats) are generally excluded from the data used to determine the mammalian allometric relationship as this distinct phylogenetic order are all extremely long-lived with several small species (e.g. Myotis lucifugus 11g) living in excess of 30 years (Fig. 1; de Magalhães et al. (2007).
Fig. 1.
Maximum lifespan as a function of body mass of small mammals. Please note that most bat species are long-lived while only a few rodent species acquire similar longevity. Most of these long-living rodents are burrow dwelling rodents, like mole-rats and squirrels.
In this paper we report on the very sparse literature addressing the comparative biology of hormone profiles pertinent to aging in long-living small mammals, and assess these limited data in the light of what is known about hormone profiles associated with extended lifespan in mutant mice models. A list of the main animals discussed in this review and their phenotypic characteristics is provided in Table 1. Most of these data were collated to address specific ecological issues and as such were collected under different conditions making interspecies longevity-associated comparative assessments difficult, nevertheless certain trends are evident and future research hopefully will confirm these appraisals. I shall focus particularly, on data from Bathyergid mole-rats and Egyptian fruit bats and little brown bats as these species are all long-lived and their endocrine profiles have been extensively studied.
Table 1.
Some phenotypic characteristics of long-living small mammals.
| MICE | AMES, SNELL |
Dietary restriction |
Deer mouse |
Golden mantle sq. |
Damara mole-rat |
Egyptian fruit bat |
Naked mole-rat |
Little brown bat |
|
|---|---|---|---|---|---|---|---|---|---|
| Body mass | 25 | 9 | 17 | 20 | 250 | 180 | 21 | 35 | 11 |
| MLSP | 3 | 4 | 4 | 8 | 10 | 16 | 23 | 28 | 34 |
| Body temp (°C) | 37 Normal |
35.5 Reduced Labile |
36 Reduced |
36 Reduced labile |
36 Reduced labile |
34 Reduced |
35 Reduced |
32 Reduced/ Labile |
33 Reduced labile |
| BMR, mass specific | Normal | Reduced | Normal | Reduced | Reduced | Reduced | Reduced | Reduced | Reduced |
| Gestation length (d) | 19 | - | - | 24 | 30 | 85 | 120 | 77 | 55 |
| Fasting glucose | Normal | Reduced | Reduced | Normal | Normal | Reduced | Reduced | Reduced | Normal |
| GTT | Normal | Abnormal | Normal | Normal | NAD | Abnormal | Abnormal | Abnormal | NAD |
| Insulin | Normal | Reduced | Reduced | Normal | Normal | Reduced | Reduced | Reduced | Normal |
| Thyroid | Normal | Reduced | Reduced | Normal Variable |
Normal Variable |
Reduced | NAD | Reduced | Normal Variable |
| Vitamin D | Normal | NAD | NAD | NAD | NAD | Reduced | Reduced | Reduced | NAD |
| Main Glucocorticoid | Corticost. | Corticost. | Corticost. | Corticost. | Cortisol | Cortisol | Cortisol | Cortisol | Cortisol |
Data are complied from multiple sources:- Mice, Ames and Snell dwarf mice and dietary restriction data are taken from Longo and Finch (2002). Comparative species information was taken from de Magalhães et al., 2005; Bauman, 1990; Bauman et al., 1987; Buffenstein et al., 1994; Buffenstein et al., 2001; Cavaleros et al., 2003; Faulkes and Bennett, 2000; Hulbert, 2000; Korine et al., 2004; Kwiecinski et al., 2001; Masoro, 1988; Ogunsua et al., 1969, Reeder et al., 2005; Stone and Wieber, 1966; Tracy et et al., 2007; Widmaier and Kunz, 1993. See text for details.
2. Hormone profiles
Rodent aging research predominantly focuses on growth hormone, insulin and insulinlike growth hormones. Given the species specificity of these peptide hormones, there are few comparative data that directly examine basal levels of these in captive or free-ranging populations and considerably fewer studies that examine these with reference to aging or MLSP. Nevertheless, there is considerable circumstantial evidence that pronounced differences in basal hormone levels in long-living species may be important determinants of lifespan (see Brown-Borg 2007).
There is also surprisingly little emphasis in aging studies on other hormones involved in homeostatic and allostatic adjustments to the internal or external milieu. Substantial evidence suggests that extended longevity associated with over-expression of the mammalian longevity gene ~Klotho (Kurosu et al., 2005) is associated not only with insulin and IGF modulation, but also with marked inhibition of the vitamin D endocrine pathway (Torres et al., 2007) and this too may be an important, albeit neglected, player in aging.
2.1 Vitamin D
Dubbed the “sunshine hormone,” vitamin D is best known for its actions regulating mineral homeostasis and bone formation, but is also widely regarded as a pleiotropic hormone affecting upon multiple processes. This precursor of the hormone 1,25 dihydroxy vitamin D (1,25(OH)2D) is “photo-synthesized” from cholesterol present in skin when exposed to ultraviolet light in a tightly regulated process, and thereafter undergoes hydroxylation primarily in the liver and kidney (Fig. 2), resulting in the formation of 1,25(OH)2D and other metabolites. Renal synthesis of 1,25(OH)2D is known to regulate calcium and phosphorus homeostasis through actions on both the gastrointestinal tract and bone, whereas 1,25(OH)2D produced in other issues is an anti-proliferative regulator of cell growth and also an immune system modulator (for review see Holick, 2004). A decline in these extra-renal functions may contribute to both age-associated and auto-immune diseases (e.g., cancer and multiple sclerosis).
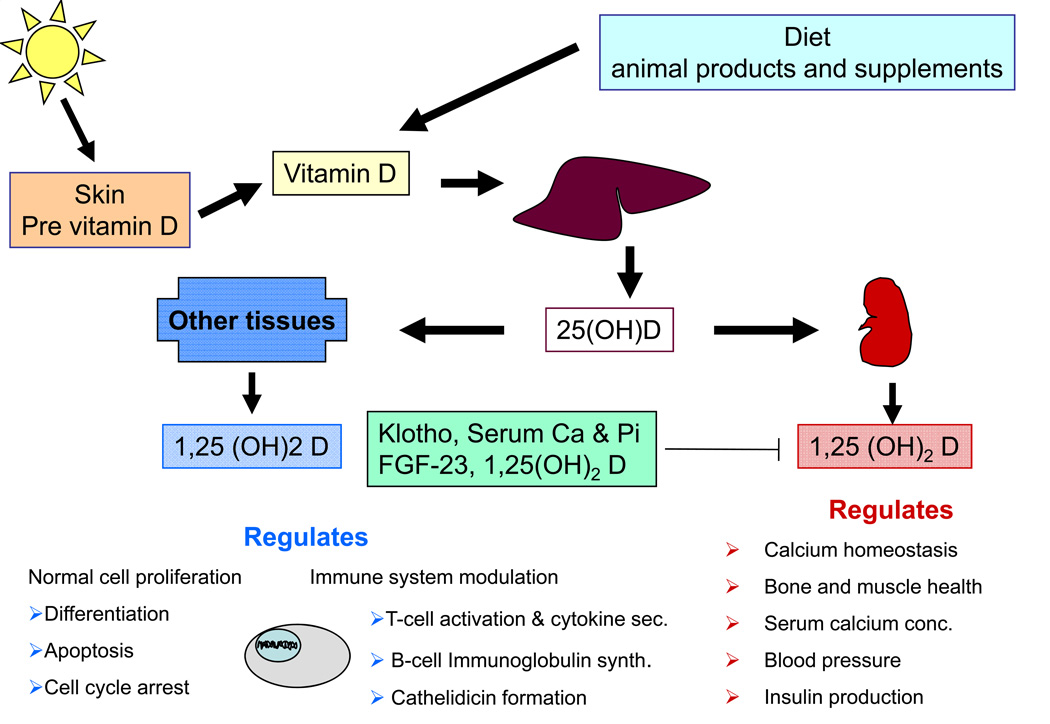
Fig. 2.
Synthesis, metabolism and functions of vitamin D. Vitamin D can be synthesized in skin during exposure to solar ultraviolet B radiation, or acquired by eating foods rich in animal fats. Vitamin D is converted by 25-hydroxylase to 25-hydroxyvitamin D [25(OH)D]. This major circulating metabolite, although biologically inactive is used as an indicator of vitamin D status. 25(OH)D is hydroxylated by1-alpha-hydroxylase to the biologically active form, 1,25-dihydroxyvitamin D [1,25(OH)2D]. Serum phosphorus, calcium, 1,25(OH)2D itself, Klotho, fibroblast growth factor 23 (FGF-23), and other factors regulate 1,25(OH)2D synthesis. Renal 1,25(OH)2D enhances intestinal calcium absorption by promoting the expression of the epithelial calcium channels and calcium-binding proteins. Furthermore, renal 1,25(OH)2D is active in bone where it is recognized by its receptor in osteoblasts, causing an increase in the expression of nuclear factor-{kappa}B ligand. This in turn promotes osteoclastic activity and assists in both the maintenance of serum calcium and phosphorus as well as in bone remodelling. The 1,25(OH)2D produced in the kidney enters the circulation and can down-regulate renin production in the kidney and stimulate insulin secretion in the beta islet cells of the pancreas. Extra-renal 1,25(OH)2D synthesis acts locally to direct normal cell proliferation by regulating a variety of genes that control proliferation, including p21 and p27, as well as genes that inhibit angiogenesis and induce differentiation and apoptosis. Modulation of the immune response is facilitated by influencing T lymphocyte synthesis and cytokine secretions, and by activating B lymphocytes thereby regulating immunoglobulin synthesis, as well as through increasing the expression of cathelicidin, a peptide capable of promoting innate immunity. Clearly actions of vitamin D may play an integral part in determining rates of organismal aging.
Given that vitamin D can only be synthesized in the presence of sunlight or acquired by eating a high animal fat diet, it is not surprising that subterranean mole-rats (Buffenstein et al., 1994) and nocturnal frugivorous (Cavaleros et al., 2003) and insectivorous bats (Kwiecinski et al., 2001) have undetectable levels of the principal circulating D-metabolite (25(OH)D) and have low levels of the hormone 1,25(OH)2D. Despite this natural state of vitamin D deficiency, both mole-rats and bats are able to tightly regulate serum calcium levels and adequately maintain mineral homeostasis using vitamin D independent mechanisms (Skinner et al., 1991; Cavaleros et al., 2003). In fact, when mole-rats were experimentally exposed to sunlight or given oral supplements, 1-alpha hydroxylase, the enzyme responsible for the formation of the active metabolite, is rapidly down-regulated thereby maintaining low levels of 1,25(OH)2D (Pitcher et al., 1994). Indeed, it may be possible that naturally low levels of circulating 1,25(OH)2D may be an important determinant of species longevity. This low basal state of 1,25(OH)2D may be associated with the longevity hormone klotho.
Overexpression of klotho in mice extends lifespan by about 30%, while mutations in klotho appear to accelerate the aging process and shorten lifespan (Kuro-o et al., 1997). Klotho modifies vitamin D metabolite status and mineral homeostasis, lowering 1,25(OH)2D levels when over expressed (Yoshida et al., 2002; Tsujikawa et al., 2003). A prominent phenotype associated with Klotho disruption is elevated serum calcium and phosphorus levels and concomitant ectopic soft tissue calcification. This is associated with discordantly high levels of 1,25(OH)2D and overexpression of 1 alpha-hydroxylase (Lanske and Razzaque, 2007). Reducing vitamin D activity in Klotho mutant mice, either by dietary restriction of foods containing vitamin D, or by genetic manipulation can rescue the premature aging-like features of these mutants. These data support the premise that high levels of vitamin D activity may accelerate the aging process.
Low levels of vitamin D in long-living species may possibly be due to klotho regulation of this endocrine system. Alternately, low levels of vitamin D may bind to an extremely sensitive high-affinity receptor inducing specifically targeted effects without activating more deleterious signaling pathways. Since insulin and the IGF-1 signaling pathway are inextricably linked with both that of klotho and 1,25(OH)2D (Kurosu et al., 2005), it is possible that many of the actions attributed to both Klotho and 1,25(OH)2D reflect downstream modulation of the insulin/IGF signaling cascade.
2.2 Insulin
The insulin signaling pathway control of aging remains to be irrefutably proven, although a wealth of data exist in support of this premise. A decline in insulin sensitivity and concomitant higher levels of blood glucose are commonly reported with aging and associated with many deleterious effects (Chahal and Drake, 2007). Conversely, reduced insulin and IGF-1 and low fasting blood glucose concentrations are frequent features associated with extended longevity in experimental dietary restriction (DR ~ Sonntag et al., 1999) and genetically modified long-lived animal models (Longo and Finch, 2003).
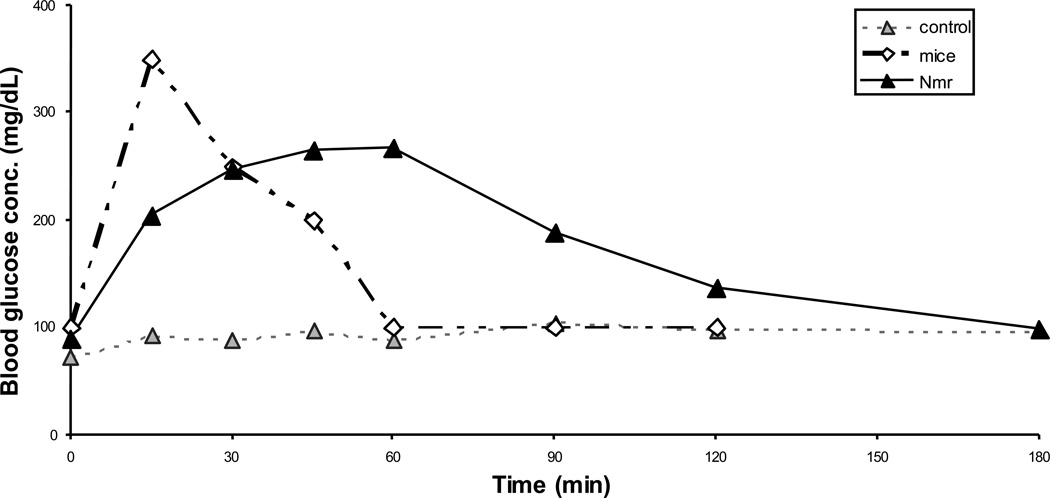
Regardless of age, naked mole-rats, like Ames and Snell dwarf mice, show an impaired glucose tolerance response (Kramer and Buffenstein, 2004), more in keeping with glucose tolerance profiles associated with either insulin resistance or insulin deficiency (Fig. 3). Despite the apparent lack of insulin and abnormal glucose handling, glycated hemoglobin levels are low and similar in both 2 and 20 year olds (Yang and Buffenstein, 2004). It is tantalizing to hypothesize that these common traits with long-lived dwarf mice reflect hormonal profiles, or specific genes characteristic of slow aging. Circulating insulin levels in naked mole-rats cannot be measured using commercially available antibodies. Yet when given a physiologically determined (body mass dependent) dose of human insulin, blood glucose rapidly drops to low levels and remains low for more than two hours. Collectively, these data suggest that the naked mole-rat may be naturally deficient in insulin yet exquisitely sensitive to this hormone if and when it is available. The abnormal glucose tolerance test, in both dwarf mutants and naked mole-rats, most likely reflects that these long-lived rodents produce only small quantities of this hormone and this is usually sufficient for their needs but are unable to rapidly regulate blood glucose when given a supraphysiological amount (as happens in the glucose tolerance test).
Fig. 3.
Glucose tolerance of naked mole-rats and mice. Glucose tolerance tests were measured in young healthy naked mole-rats and mice that had been fasted overnight. Animals were given intraperitoneal injections of either 2g glucose per kg body weight or an equivalent volume of 0.9% saline (control). Blood samples were taken immediately prior to injection and again at set time intervals after the glucose load. Blood glucose of naked mole-rats rose more slowly than mice and remained elevated for considerably longer, suggesting that naked mole-rats show some degree of either insulin insensitivity or insulin deficiency.
An alternate explanation may be that naked mole-rat insulin has a very different structure and different receptor binding properties to that of mice and rats. Although we have not yet attempted to sequence naked mole-rat insulin, there is considerable evidence to suggest that insulin structure is different in the entire hystricognath rodent suborder (Horuk et al., 1980; Beintama and Campagne 1987). These differences in insulin structure are attributed to differences in zinc binding properties within the insulin molecule. The evolutionary factors that gave rise to these deviating structural properties are unknown, but it is interesting to note that long-living new world ceboid primates, like mole-rats and guinea pigs, have insulin that does not cross-react with commonly used antibodies (Mann and Crofford, 1970) and similarly may differentially regulate down-stream signaling pathways.
King et al. (1983) reported that insulins from both the South American and African hystricognath rodents stimulate DNA synthesis to a greater level than other mammalian insulin or IGF proteins. Furthermore, they concluded that the growth promoting effects of hystricognath insulin may be mediated through a different receptor and may employ a mechanism similar to that of platelet-derived growth factor. In mice, deletion of insulin receptor substrate 2 (IRS2) in the brain (blrs −/− and bls +/−) increases longevity by 14–18% and suggests that systemic signaling of the insulin/IGF pathway in the central nervous system is involved in regulating lifespan (Taguchi et al., 2007). Indeed reduced IRS2 signaling in the brain may be an important component of lifespan extension in mutant mice with aberrant growth hormone, insulin and IGF. While is highly speculative, it is nevertheless reasonable to propose that the poor glucose tolerance observed in both long-living hystricognaths, together with their apparent hypoinsulinemia and their pronounced sensitivity to insulin injection, may reflect similar phenotypes to other animal models of lifespan extension and modified signaling pathways better suited for prolonged longevity. Surprisingly, both the transgenic klotho mice and brain IRS2 depleted mice exhibit insulin resistance yet have increased longevity. Instead of providing closure through a simple mechanistic explanation for their regulatory effects upon aging, this contradiction incites further study (Bartke 2007). Future studies assessing gene sequence, structure and mode of function of insulin, IGF and growth hormone and their receptors may hold the key to why several hystricognath species are long-lived and the mechanisms involved in this process.
Frugivorous and nectarivorous bats show many similarities with long-living rodents with respect to glucose handling. They are known to consume vast quantities of carbohydrate-rich meals that they digest rapidly and efficiently (Keegan, 1977; Tedman and Hall, 1985; Korine et al., 1996). Yet in the case of the Egyptian free-tailed bat (Roussetus aegyptiacus, the chiropteran with the most information regarding glucose metabolism) fasting blood glucose levels are low, and they also show an abnormal glucose tolerance test with blood glucose concentrations more than five-fold higher than those observed in laboratory rats and mice (Keegan, 1977; Tracy et al., 2007).
Egyptian fruit eating bats may consume more than their own body weight in fruit in a single evening. Although the pancreas of these bats has considerably more endocrine tissue than rodents and other small mammals (Michelmore et al., 1998), post-prandial levels of circulating insulin are similar to those of laboratory rats and post-prandial blood glucose levels markedly fluctuate. Both microchirpoteran and megachiropteran fruit eating bats predominantly rely on passive paracellular transport for glucose absorption (Keegan, 1977; Pappenheimer et al., 1990; Tracy et al., 2007; Caviedes-Vidal et al., 2008). This energy conserving uncontrolled process may explain the post-prandial variation in blood glucose. It is however constrained by the potentially harmful possibilities associated with passive uptake of toxins through these pores in intestinal tissue (Caviedes-Vidal et al., 2008).
Many bats, including insectivorous Angolan free-tailed bats (unpublished data); hematophagus vampire bats (Freitas et al., 2005) frugivorous Egyptian fruit bats (Keegan 1977), like experimental animal models of lifespan extension, show low fasting blood glucose levels. In the case of the vampire bats, blood glucose levels drop to precipitously low levels (30 mg/dL) after a 24h fast (Freitas et al., 2005) when compared with fasting blood glucose levels of other mammals (Eisenstein and Strack, 1971, Kettelhut et al., 1980). Indeed, these animals appear unable to adequately maintain glycemic levels during their inactive period through gluconeogenic mechanisms. Although it is not known if the marked fluctuations in blood glucose are harmful, as is the case in diabetics, given that bats only leave their roosts at night to feed, prolonged post-absorptional low blood glucose levels may contribute to extended longevity of these bats relative to most other small mammals.
Seasonal changes in insulin and glucagons levels are evident in free-ranging rodents (e.g., golden-mantle squirrels; Bauman et al., 1987, Boswell et al., 1994) and bats (Myotis lucifugus, Bauman, 1990). These are most pronounced in temperate areas where small mammals routinely enter a state of dormancy for prolonged periods. While elevated glucagons levels during hibernation most likely are involved in maintaining fasting blood glucose levels, it is less clear why insulin should peak during this period; one possibility is that it may play an important role in suppression of reproductive hormones during dormancy.
Insulin is an anabolic hormone that promotes lipogenesis and stimulates the secretion of leptin from adipose tissue. Collectively these two hormones (insulin and leptin) serve as a signal to the CNS of body fat content and nutrition status (Schwartz et al., 2001). Bats exhibit a variety of reproductive delays coinciding with winter adiposity and hibernation, and this is attributed to increased leptin (Srivastava and Krishna, 2007). Insulin and leptin signals are integrated to modulate reproduction through the release of gonadotropins thereby regulating ovarian function. Insulin deficiency diabetes has been implicated in delayed puberty type 1 diabetic girls (Elamin et al., 2006); however, both extremely high levels of these hormones (associated with insulin resistance and obesity) as well as very low levels (as commonly occur during dietary restriction) impair reproductive fertility and may impact upon longevity.
2.3 Glucocorticoids
Glucocorticoid responses to acute stressors are considered essential for species survival, facilitating the appropriate acute responses to stressors, including the regulation of behavior, energy stores and energy expenditure, as well as long term reproductive suppression under adverse conditions, thereby favoring adult survival until conditions may be more suitable to meet the high energetic demands of reproduction. As such, these stress hormones are important in Darwinian species fitness (Romero et al., 2008). Indeed, basal glucocorticoid levels are positively correlated with individual survival following extreme climate events (such as drought or extreme cold) in birds, reptiles and mammals (Brown et al., 2005; Romero, 2002; Boonstra and Singleton, 1993). However, chronically elevated glucocorticoid levels can result in numerous pathological effects including neuron death, immune suppression and reproductive malfunction. Not surprisingly age-related increases in glucocorticoids have been implicated in the aging process (Sapolsky et al., 2000; Hibberd et al., 2000), although in healthy humans, evidence for age-associated hypercortisolism, to date, is equivocal (Ferrari et al., 2008; Peeters et al., 2008).
Laboratory mice and rats appear to have lost many of the natural circannual rhythms in glucocorticoid responses and have markedly different glucocorticoid profiles to those of free-ranging species. Most free-ranging species as well as captive non-traditional small mammals that have been in captivity for several generations exhibit seasonal modulation in stress hormone responses (see Romero, 2002 for review). Seasonal fluctuations are commonly associated with breeding cycle and employment of thermolability associated with summer torpor or winter hibernation. Mammalian seasonal changes in baseline glucocorticoids are poorly documented and interspecies comparative assessments are complicated by interspecies differences in animal handling susceptibility and time spent in traps prior to measurements. Based upon the few well controlled studies, both free-ranging and captive long-living bats and rodents show a peak in glucocorticoids associated with the breeding season and show species specific gender differences (Reeder et al., 2006; Romero et al., 2008). Levels are highest at peak breeding and may function to modulate hyperphagia, energy and water balance as well as gonadal function. Extremely high levels of glucocortiocids at the end of the breeding season have been observed in small dasyurid marsupials (e.g. Antechinus stuartii) and this is generally associated with “programmed mortality” on completion of breeding in male dasyurids, most of whom die after one reproductive season. Here death is attributed to a stress induced suppressed immune response (McDonald et al., 1986). Female brown lemmings (MLSP 5y) hold the record for the highest free-ranging corticosterone levels (3000–8000ng/ml) that surpass even those of the mortality prone dasyurids. Fruit eating flying foxes (Pteropus species), although long-living (~20y), following handling stress have the highest levels of cortisol recorded for mammals (Reeder, 2006). Glucocorticoid levels this high would be fatal in most species suggesting that these species must have high circulating levels of cortisol binding protein thereby limiting the amount of free and active hormone or insensitive, low affinity receptors, as well as a down regulated negative feedback loop, in which the hypothalamo-pituitary unit is less sensitive to CRH/ACTH suppression by glucocorticoid. New world squirrel monkeys (MLSP~ 30y) similarly show high levels of plasma cortisol, lower receptor affinity and this type of glucocorticoid resistance (Chrousos et al., 1986). Interpretations of glucococorticoid levels are also complicated by the fact that there are two distinct receptors for this hormone. These receptors elicit different physiological and behavioral effects, including disparate negative feedback regulation. Further complicating data interpretation is reports that receptor responses may be influenced by previous stressful events (Romero, 2004).
Many studies addressing glucocorticoids in aging research commonly state that “rodents (meaning laboratory rats and mice) use corticosterone while humans and some other mammals (Hibberd et al., 2000; Patel and Finch, 2002) use cortisol”, with little to no discussion concerning differences in regulation or functionality among these two hormones. Comparative studies reveal that most small mammals have detectable circulating levels of both cortisol and corticosterone, although marked species variation in the relative proportions of these steroids has been reported (Pushpa, 1969; Sandor, 1969). While corticosterone predominates in short-living rats and mice, cortisol is the principal glucocorticoid in most larger mammals, as well as in bats and non-Muroid rodents (Ogunsua et al., 1969). Comparative endocrinologists have been unable to detect any evolutionary, ecological or functional significance in interspecies variation in adrenocortical secretions. Rather, this may be a phylogenetic quirk of the Muroid rodents; they secrete only 17-deoxycorticosteroids and lack adrenal 17-hydroxylase (Sandor, 1969). In long-living rodents (ground squirrels and mole-rats) and bats (e.g. Myotis and Pteropys sp), cortisol levels are at least an order of magnitude (10–50 times)greater than those of corticosterone (Boonstra et al., 2001; Ganem and Bennett, 2004; Reeder et al., 2004; 2006) whereas in shorter-living rodents this ratio is considerably lower (0.01–5). It is not known why some species have one or other glucocorticoid and others have both, albeit in different concentrations. It is possible the presence of both steroids can be explained by “leakage” of byproducts of the biosynthetic pathways, but nevertheless it is tantalizing to speculate that the relative proportion of the two steroids may lead to differential effects that may be important in species longevity.
The two glucocorticoids may show different responses to acute stressors. For example the stress of restraint in little brown bats collected prior to hibernation resulted in an increase of corticosterone, but not cortisol (Reeder et al., 2004). The opposite pattern was reported for both golden hamsters (Mesocricetus auratus; Ottenweller et al., 1985) and tree shrews (Tupaia belangeri; Collins et al., 1984). Here, acute stress resulted in a decline in the corticosterone/ cortisol ratio as a result of uncoordinated increases in these steroids, and particularly pronounced increases in cortisol rather than corticosterone. This dissociation in glucocorticoid secretion following stress induction suggests that these hormones are independently regulated (Ottenweller et al., 1985). Further evidence in support of this hypothesis is revealed in seasonal studies of golden-mantled ground squirrels hormone profiles (Boswell et al., 1994). That study reported seasonal changes in cortisol and corticosterone that were not synchronized but rather fluctuated in different directions (Boswell et al., 1994). This dichotomy may reflect differential regulation of hormone synthesis in the adrenal cortex, in particular differential expression of the enzymes responsible for shifting cholesterol towards cortisol, androgens or aldosterone, or that these hormones have different functions within the organism. Although it has been widely speculated that cortisol and corticosterone have different functions, and in particular have different effects on the immune and flight or fight response (Weber 1998), to date, in vivo studies assessing different physiological and biochemical functions of these two steroids, have been not been conclusive (M. Romero personal communication). A bolus of either cortisol, corticosterone or even a synthetic glucocorticoid derivative have similar effect and potency on the HPA axis. Qualitatively different effects of these two steroids have been observed in tissue culture (Kahri et al., 1979). Here, corticosterone reportedly inhibits cortisol production while stimulating androgen production in fetal human adrenals whereas cortisol suppresses aldosterone production but does not inhibit corticosterone secretion in fetal rat adrenals. These data are hard to interpret, since these are not typical actions and more data is needed before definitive conclusions can be drawn.
Glucocorticoid effects are mediated by two types of intracellular receptors; a high affinity mineralocorticoid (Type 1, GR) receptor and a low affinity glucocorticoid (Type 2; GC) receptor. These receptors are thought to regulate transcription of distinct, but overlapping sets of genes. Cortisol and corticosterone may have differential affinities for these receptors and hormonal effects would therefore depend upon receptor affinity, differential receptor density and hormone levels (see Sapolsky et al., 2000 for review). Furthermore, Sapolsky et al (2000) reported that basal and stress-induced glucocorticoids have very different physiological effects. At basal concentrations, the Type 1 receptor is activated, inducing permissive effects that prime other physiological systems (such as cardiovascular, immune and metabolic responses) to stress, whereas stress-induced responses act via the Type 2 receptor to stimulate or suppress specific physiological functions (e.g., norepinephrine secretion). Given the paucity of data, especially concerning different cortisol/corticosterone ratios among species and the impact thereof, clearly considerably more work remains to be done to fully understand differential roles, if any, in stress, energy and water balance, homeostasis and immune function before any definitive conclusions can be drawn about interspecies differences and the impact thereof on aging and longevity.
Age-related changes in the hypothalamo-pituitary-adrenal (HPA) axis reportedly lead to elevated levels of glucocorticoids (Chahal and Drake, 2007). These, together with elevated stress responses, have been implicated in alterations in visceral fat composition, hippocampal atrophy, altered neuronal function and other deleterious age-related changes. Indeed rising levels of glucocorticoids midlife reportedly may predict the trajectory of subsequent cognitive decline. Intraspecies variability even in genetically identical inbred rodent strains highlight the importance of environmental influences on this HPA stress pathway. Despite the far-reaching physiological significance, surprisingly little is known about how aging induces changes in this axis. Age-related changes in glucocorticoids among different species are not consistent, and even within humans conflicting age-related changes are reported (Ottenweller et al., 1990). Not surprisingly, it is not known if long-living species show differential regulation of this hormone pathway or if they display varying tissue sensitivity to these hormones. Syrian hamsters reportedly show differential changes in the cortisol/corticosterone ratio with age; basal plasma cortisol increases whereas corticosterone levels decline with age resulting in unchanged total glucocorticoids (Ottenweller et al., 1990). Adrenocorticol responses to acute and chronic stressors, however, were diminished with age in this species. Both long-lived mutant animals as well as mice and rats that are subjected to chronic caloric restriction surprisingly show elevated levels of corticosterone (Sabatini et al., 1990; Borg et al., 1995; Nelson et al., 1995; Hauck et al., 2001). These longer-living mice nevertheless exhibit attenuated declines in hippocampal neuron degeneration and concomitant effects on learning and memory generally attributed to raised levels of glucocorticoids (Sapolsky et al., 2000). Paradoxically, it appears that chronically, mildly elevated glucocorticoids provide neuron protection and may play a pivotal role in facilitating the retarded aging associated with caloric restriction. While elevated corticosterone may be necessary for caloric restricted rodents, it may not be beneficial to ad lib fed mice that are in a very different physiological state. It is possible, but as yet not experimentally proven, that the beneficial effects associated with chronically (albeit mildly) elevated glucocorticoids are mediated through differential ligand binding to the different receptor types, thereby, activating different down-stream processes.
The HPA axis is amenable to manipulation throughout lifespan such that different stressors may lead to altered density of Type 1 receptors and sensitivity of negative feedback loops. For example post natal handling stress in young rat pups may lead to permanently altered Type 1 receptor density and enhanced receptor binding as well as improved learning in aged rats (Vallee et al., 1999). Even later life manipulation for example by housing adult rats in an enriched environment continuously exposed to novel items or toys, show better maze solving abilities as well as increased hippocampal glucocorticoid receptors (Hibberd et al., 2000). The influence of early life experience on the HPA axis, no doubt contributes to the intraspecific and interspecific age-related variability and the intricacy of this important endocrine system.
Clearly the role of the different glucocorticoids in comparative biology is extremely complex and, to date, remains under studied and poorly understood. Given the multiple glucocorticoid effects (immunosuppression, neurodegeneration, insulin suppression) that impact upon the aging process, resolving why both basal and stress-induced levels of glucocorticoids differ among species with disparate longevity and how these change with age are likely to be important in understanding species differences in aging.
2.4 Thyroid hormone
The hypothalmo-pituitary-thyroid axis, primarily through its metabolic actions has been widely implicated as a mechanism modulating aging (Leitol. et al., 2002; Habra and Sarlis, 2005). Thyroid hormones in wild animals may vary considerably with season (especially in temperate regions), food availability, habitat (e.g., subterranean versus above ground dweller), and stages of life history (e.g., hibernation). It appears that, at least for prolonged periods of time, long-lived squirrels, deer mice, bats and mole-rats maintain low levels of thyroxine (Lyman et al., 1982; Hulbert et al., 1985; Kwiecinski et al., 1986; Buffenstein et al., 2001). Life extension effects associated with sustained low thyroid hormone levels have been reported in rats, whereas a reduction in lifespan occurs with sustained hyperthyroid levels (Ooka and Shinkai, 1986). Similarly, low thyroxine levels are reported in Ames and Snell dwarf mice that typically manifest many features associated with hypothyroidism, including low metabolic rate and low core body temperature (Brown-Borg et al., 1996; Flurkey et al., 2001; Hauck et al., 2001). These lifespan extension effects are reduced with thyroxine supplementation (Vergara et al., 2004).
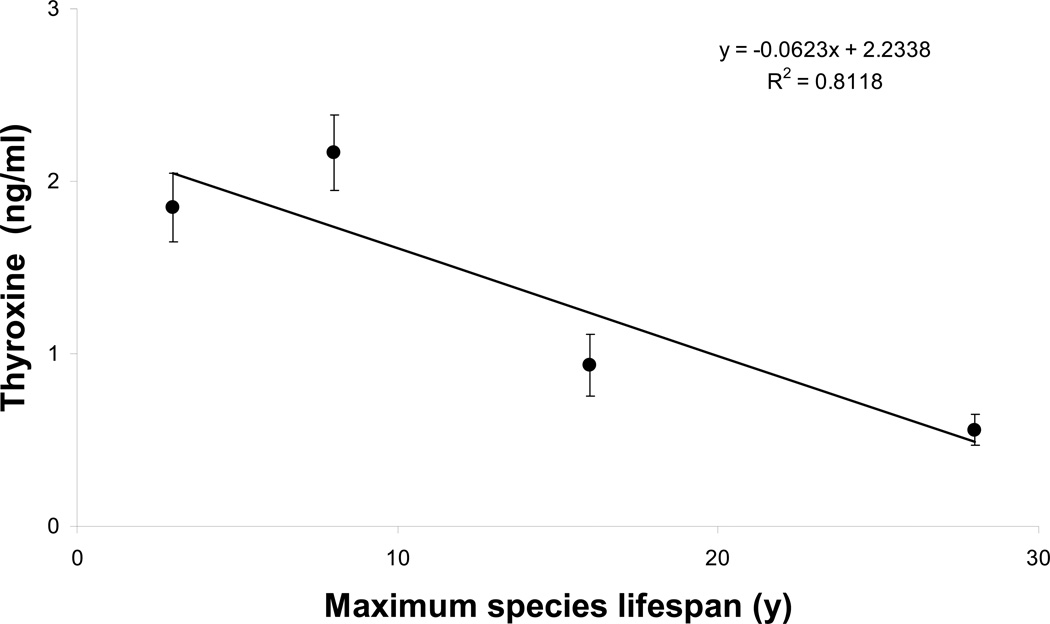
A comparative study assessing thyroxine concentration in four captive species show a strong negative correlation between circulating levels of these hormones and MLSP (Fig. 4), culminating in the naked mole-rat having the lowest levels of thyroid hormone. While this may be due to the warm housing conditions (30°C) under which naked mole-rats are usually maintained, even when housed under cooler conditions, thyroid hormones are significantly lower than those of mice (Buffenstein et al., 2001).
Fig. 4.
Free thyroxine levels of four rodents with disparate longevity (mice, 3y; guinea pigs 8y; damara mole-rats 15y and naked mole-rats 28.3y) show a negative correlation with maximum lifespan potential. Blood samples were collected at the same time of day from animals housed in our animal facility. Free thyroxine was measured using commercially available kits.
Thyroid hormone has widespread impact influencing metabolic rate and body temperature by modulating mitochondrial efficiency, membrane composition and ion permeability, and substrate cycling (Silvestri et al., 2005). Generally, the relationship between prolonged longevity and low thyroid hormone, whether natural or experimentally induced, is attributed to lower levels of ROS production and oxidative stress in response to induced changes in both obligate and facultative thermogenesis (Merry, 2002). Most long-lived rodents have low basal metabolic rates and low core body temperature, at least for a large component of their day. Deer mice, squirrels and many bat species routinely employ daily or seasonal torpor, while naked mole-rats are thermally labile when housed alone outside their natural burrow conditions (Lyman et al., 1982; Buffenstein and Yahav, 1991; Maloney et al., 1999). Similarly, dwarf mice and calorie restricted rodents have lower body temperatures and appear to be thermally labile if not housed with their normal sized relatives. This, in its own right, may be an important mechanism facilitating extended longevity.
Prolonged longevity associated with low thyroxine may also result from synergistic trophic relationships with other hormones such as leptin, insulin, and steroid hormones, including testosterone, vitamin D and the glucocorticoids. Indeed, thyroid hormone appears to impact upon the structure and function of every cell in the body as well as most hormone secretions (reviewed in Hulbert, 2000).
2.5 Reproductive hormones
There is a plethora of literature addressing age-related changes in reproductive hormones and both the menopause and andropause observed in most mammals (Packer et al., 1998). The function of the hypothalamic-pituitary-gonadal (HPG) axis retains remarkable similarity across the taxa and age-related declines in both fertility and reproductive hormone secretions commonly occur in all vertebrates (Packer et al., 1998). Although long-lived mutant rodents present with impaired fecundity (Bartke and Brown-Borg, 2004), experimental models of extended longevity (dietary restriction) delay the age of reproductive cessation (Nelson et al., 1985; McShane and Wise, 1996). Dietary restriction and its concomitant down-regulation of the HPG hormones however also slow the rate of reproductive maturation and fertility (Nelson et al., 1995).
Both long-living bats and rodents in both temperate and tropical regions show temporal down regulation of the HPG axis, associated with seasonal breeding, hibernation and employment of torpor under harsh climatic conditions (Bernard and Cumming, 1997; Reeder et al., 2004; 2006). Synchronization and control of seasonal breeding is under the complex control of both photoperiod and/or nutritional status and multiple regulatory hormones are involved in this process, including insulin, glucocorticoids, adrenal sex steroids, thyroxine, leptin and melatonin all of which may influence gonadotrophin releasing hormone (GnRH secretion) and the responsiveness to the inhibitory effects of sex steroid hormones (for review see Karsch et al., 1995; Lehman et al., 1997; Yoshimura, 2006).
Many bat species uncouple the reproductive demands of mating and pregnancy by employing spermatogenesis in the summer, store sperm in their reproductive tracts and may delay ovulation, fertilization, implantation, and/or in utero fetal development until) the next spring (see Kawamoto (2003) for review). This unusual delayed ovulation and or implantation phenomenon is not restricted to Chiroptera and has been observed in some long-living rodents (Clarke, 1981). These unusual features may result in concomitant sustained low reproductive hormone levels. This, together with long gestation periods, may be an important trait of prolonged longevity observed in these species.
Long-living naked mole-rats normally live in reproductively suppressed social groups such that only the dominant female within a colony breeds. The breeding female shows no sign of reproductive senescence and may produce more than 1000 offspring during her long life (Buffenstein, 2008). Although the same size as mice, these long-living species take ~ four times as long to reach reproductive maturity (Faulkes and Bennett, 2001), and most individuals, with the exception of those breeding, maintain exceedingly low HPG hormones. Despite these “caste” differences in reproductive hormone profiles, both breeders and non-breeders have similar lifespans. One possible explanation for this is that gestation in naked mole-rats is considerably longer (77 days) than of mice that (19–20 days) and this may reflect sustained lower hormonal levels throughout pregnancy and slower rates of fetal growth. Similar prolonged gestation periods are reported in bats with a mode of 120 days for 66 species (Hayssen et al., 1993) and this may in part reflect lower resting body temperatures and slower rates of fetal development in addition to sustained lower levels of hormones associated with reproduction.
Age-related changes in reproductive hormones from both the HPG and HPA axes are observed in most mammals, regardless of gender and are associated with a plethora of “menopause/ andropause-associated” non-reproductive pathophysiology (such as memory loss and sarcopenia). These effects are most pronounced in females and primarily attributed to loss of sex steroids, and estrogen in particular. Long-living rodents and bats continue to reproduce for many more years than do shorter-living mammals. In the case of the naked mole-rat, a reproductive senescence is not evident; indeed histological examination of the ovaries from a wild caught individual that had been in captivity for 28 years showed numerous follicles in differing stages of development including large Graaffian follicles suggestive that this female was about to undergo ovulation when she died. Prolonged reproductive lifespan has also been observed in caloric restricted rodents who maintain estrous cycles at an age when most of their age-matched controls have stopped cycling (Nelson et al., 1985; McShane and Wise, 1996; Wu et al., 2005). McShane and Wise (1996) proposed that this extension of reproductive lifespan results from the preservation of the neuroendocrine axis since gonadotrophin secretions were sustained and even enhanced with caloric restriction while those in age-matched controls showed a decline in luteinizing hormone (LH) concentration and pulse amplitude. Age-related hypothalamic and pituitary changes may also be directly involved in reproductive cessation and non-reproductive menopausal effects, such as the cognitive decline associated with menopause (Bellino 2006). Both LH and follicle stimulating hormone (FSH) as well as hypothalamic gonadotrophin releasing hormone (GnRH) may affect cognition and beta amyloid induced neurofibrillary tangle development (Bowen et al., 2004) and LH suppression in an aged transgenic mouse model for Alzheimer’s disease markedly reduced beta amyloid deposition and the decline in cognitive function (Casadesus et al., 2006).
Clearly, there is still much to be learnt about age-related changes in reproductive hormones and the impact thereof and while there is tremendous variation in reproductive strategies in both rodents and bats, these long-living species may elucidate mechanisms critical to the understanding of attenuated reproductive aging.
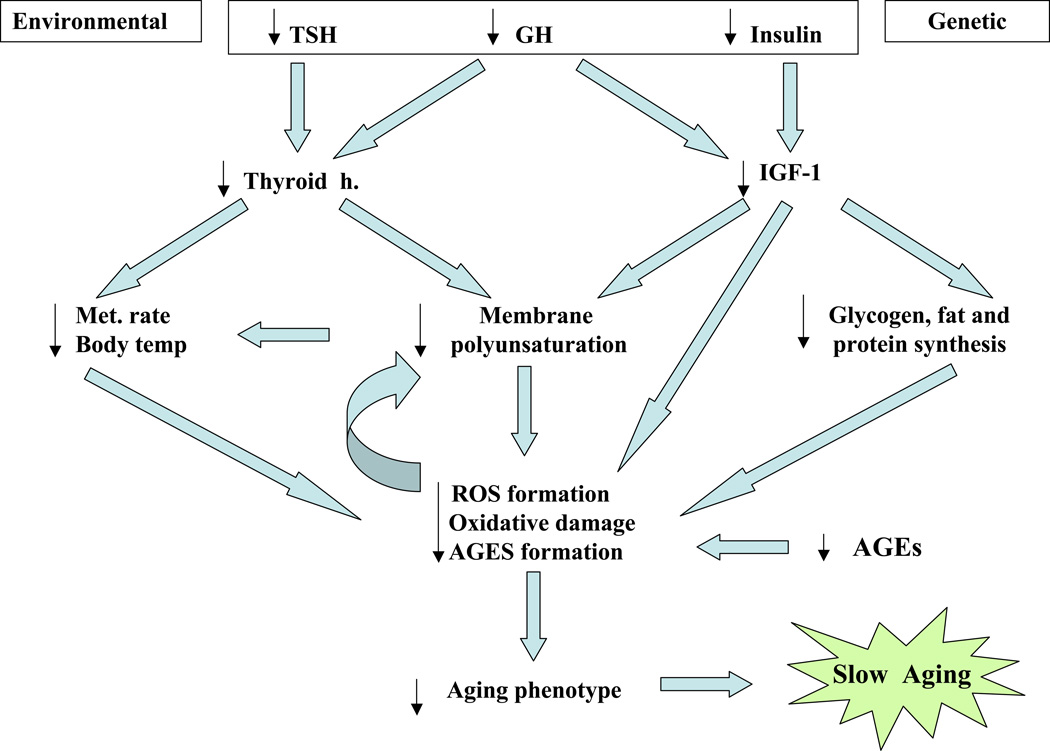
3. Conclusion
Reduced hormone secretion and enhanced hormone sensitivity appear to be common traits in long-living rodents and may be pivotal determinants of slow aging. Low hormone levels may reflect high affinity-specific receptors and greater control over specific targeted functions. All the endocrine systems discussed above work in a highly integrative manner to induce their pleiotropic effects. One of the ways in which the insulin, thyroid and vitamin D endocrine systems work together may be through regulatory effects on membrane composition (Fig. 5). Both thyroxine and insulin concentrations influence desaturase enzyme activities, which in turn promote the polyunsaturation of acyl chains. Highly polyunsaturated membranes facilitate greater membrane permeability, and higher metabolic rates and, as a consequence, higher amounts of oxidative damage (Hulbert et al., 2007). Changes in membrane composition not only will influence the susceptibility to ROS attack, but also may have another very important function: modulating the function of resident membrane proteins and the sensitivity of membrane-bound receptors. These factors impact cellular processes, signal transduction pathways and the immune response (Stulnig, 2003; Ovide-Bordeaux and Grynberg, 2004). Low levels of these hormones may therefore lower cellular metabolic requirements as well as the peroxidation index, facilitating the resistance to oxidative stress commonly observed in long-lived rodents (Harper et al., 2007). Similarly, since all the hormones discussed above are known to affect mitochondrial biogenesis and cell proliferation (Berdanier, 2006), they are likely to affect both mitochondrial functional efficiency and genomic maintenance. Species with low hormone levels may in turn exhibit attenuated damage accrual, contributing to their prolonged longevity.
Fig. 5.
Low hormone levels may promote slow aging through modulation of body temperature, glucose metabolism and membrane composition, membrane permeability and signal transduction pathways.
Clearly, given the paucity of data in long-living species, considerable work is still necessary to pinpoint the role of endocrines as determinants of longevity. Our limited research suggests that further investigations will likely yield fruitful findings that may not only confirm the importance of maintaining low hormone levels in healthy and slow-aging mammals but also point to the molecular mechanisms to be emulated in anti-aging therapy.
Acknowledgements
Our work is supported by grants from the NIH/NIA, Ellison Foundation and the Paul Glenn Foundation. We apologize to those whose work could not be cited through inadvertent omission or because of space limitations.
Alphabetic list of abbreviations
- HRT
hormone replacement therapy
- NMR
naked mole-rat
- MLSP
maximum lifespan
- LQ
longevity quotient
- BMR
basal metabolic rate
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bartke A. Is growth hormone deficiency a beneficial adaptation to aging? Evidence from experimental animals. Trends Endocrinol. Metab. 2003;14:340–344. doi: 10.1016/s1043-2760(03)00115-2. [DOI] [PubMed] [Google Scholar]
- Bartke A. New findings in gene knockout, mutant and transgenic mice. Exp. Gerontol. 2007;43:11–14. doi: 10.1016/j.exger.2007.10.009. [DOI] [PubMed] [Google Scholar]
- Bartke A, Brown-Borg H. Life extension in the dwarf mouse. Curr. Top. Dev. Biol. 2004;63:189–225. doi: 10.1016/S0070-2153(04)63006-7. [DOI] [PubMed] [Google Scholar]
- Bauman WA, Meryn S, Florant GL. Pancreatic hormones in the non hibernating and hibernating golden mantled ground squirrel. Comp. Biochem. Physiol. A. 1987;86:241–244. doi: 10.1016/0300-9629(87)90324-0. [DOI] [PubMed] [Google Scholar]
- Bauman WA. Seasonal changes in pancreatic insulin and glucagon in the little brown bat (Myotis lucifugus) Pancreas. 1990;5:342–346. doi: 10.1097/00006676-199005000-00015. [DOI] [PubMed] [Google Scholar]
- Beintema JJ, Campagne RN. Molecular evolution of rodent insulins. Mol. Biol. Evol. 1987;4:10–18. doi: 10.1093/oxfordjournals.molbev.a040424. [DOI] [PubMed] [Google Scholar]
- Bellino FL. Advances in endocrinology of aging research, 2005–2006. Exp. Gerontol. 2006;41:1228–1233. doi: 10.1016/j.exger.2006.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berdanier CD. Mitochondrial gene expression: influence of nutrients and hormones. Exp. Biol. Med. 2006;231:593–1601. doi: 10.1177/153537020623101003. [DOI] [PubMed] [Google Scholar]
- Bernard RT, Cumming GS. African bats: evolution of reproductive patterns and delays. Q. Rev. Biol. 1997;72:253–274. doi: 10.1086/419859. [DOI] [PubMed] [Google Scholar]
- Boonstra R, Hubbs AH, Lacey EA, McColl CJ. Seasonal changes in glucocorticoid and testosterone concentrations in free-living arctic ground squirrels from the boreal forest of the Yukon. Can. J. Zool. 2001;79:49–58. [Google Scholar]
- Boonstra R, Singleton GR. Population declines in the snowshoe hare and the role of stress. Gen. Comp. Endocrinol. 1993;91:126–143. doi: 10.1006/gcen.1993.1113. [DOI] [PubMed] [Google Scholar]
- Borg KE, Brown-Borg HM, Bartke A. Assessment of the primary adrenal cortical and pancreatic hormone basal levels in relation to plasma glucose and age in the unstressed Ames dwarf mouse. Proc. Soc. Exp. Biol. Med. 1995;210:126–133. doi: 10.3181/00379727-210-43931. [DOI] [PubMed] [Google Scholar]
- Boswell T, Woods SC, Kenagy GJ. Seasonal changes in body mass, insulin, and glucocorticoids of free-living golden-mantled ground squirrels. Gen. Comp. Endocrinol. 1994;96:339–346. doi: 10.1006/gcen.1994.1189. [DOI] [PubMed] [Google Scholar]
- Bowen RL, Verdile G, Liu T, Parlow AF, Perry G, Smith MA, Martins RN, Atwood CS. Luteinizing hormone, a reproductive regulator that modulates the processing of amyloid-beta precursor protein and amyloid-beta deposition. J. Biol. Chem. 2004;279:20539–20545. doi: 10.1074/jbc.M311993200. [DOI] [PubMed] [Google Scholar]
- Brown CR, Brown MB, Raouf SA, Smith LC, Wingfield JC. Effects of endogenous steroid hormone levels on annual survival in cliff swallows. Ecol. 2005;86:1034–1046. [Google Scholar]
- Brown-Borg HM, Borg KE, Meliska CJ, Bartke A. Dwarf mice and the ageing process. Nature. 1996;384:33. doi: 10.1038/384033a0. [DOI] [PubMed] [Google Scholar]
- Brown-Borg HM. Hormonal regulation of longevity in mammals. Ageing Res. Rev. 2007;6:28–45. doi: 10.1016/j.arr.2007.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buffenstein R. The naked mole-rat: a new long-living model for human aging research. J. Gerontol. A Biol. Sci. Med. Sci. 2005;60:1369–1377. doi: 10.1093/gerona/60.11.1369. [DOI] [PubMed] [Google Scholar]
- Buffenstein R. Negligible senescence in the longest living rodent, the naked mole-rat; insights from a successfully aging species. J. Comp. Physiol. 2008 doi: 10.1007/s00360-007-0237-5. (in press). [DOI] [PubMed] [Google Scholar]
- Buffenstein R, Jarvis JUM, Opperman LA, Cavaleros M, Ross FP, Pettifor JM. Subterranean mole-rats naturally have an impoverished calciol status, yet synthesize calciol metabolites and calbindins. Eur. J. Endo. 1994;130:402–409. doi: 10.1530/eje.0.1300402. [DOI] [PubMed] [Google Scholar]
- Buffenstein R, Yahav S. Is the naked mole-rat, Heterocephalus glaber, a poikilothermic or poorly thermoregulating endothermic mammal? J. therm. Biol. 1991;16:227–232. [Google Scholar]
- Buffenstein R, Woodley R, Thomadakis C, Daly TJM, Gray DA. Cold-induced changes in thyroid function in a poikilothermic mammal, the naked mole-rat. Am. J. Physiol. 2001;280:R149–R155. doi: 10.1152/ajpregu.2001.280.1.R149. [DOI] [PubMed] [Google Scholar]
- Casadesus G, Webber KM, Atwood CS, Pappolla MA, Perry G, Bowen RL, Smith MA. Luteinizing hormone modulates cognition and amyloid-beta deposition in Alzheimer APP transgenic mice. Biochim. Biophys. Acta. 2006;1762:447–452. doi: 10.1016/j.bbadis.2006.01.008. [DOI] [PubMed] [Google Scholar]
- Caviedes-Vidal E, Karasov WH, Chediack JG, Fasulo V, Cruz-Neto AP, Otani L. Paracellular absorption: a bat breaks the mammal paradigm. PLoS ONE. 2008;9:e1425. doi: 10.1371/journal.pone.0001425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavaleros M, Buffenstein R, Ross FP, Pettifor JM. Vitamin D metabolism in a frugiverous nocturnal mammal, the Egyptian fruit bat (Rousettus aegyptiacus) Gen. Comp. Endoc. 2003;133:109–117. doi: 10.1016/s0016-6480(03)00150-3. [DOI] [PubMed] [Google Scholar]
- Chahal HS, Drake WM. The endocrine system and ageing. J. Pathol. 2007;211:173–180. doi: 10.1002/path.2110. [DOI] [PubMed] [Google Scholar]
- Chrousos GP, Loriaux DL, Tomita M, Brandon DD, Renquist D, Albertson B, Lipsett MB. The new world primates as animal models of glucocorticoid resistance. Adv. Exp. Med. Biol. 1986;196:129–144. doi: 10.1007/978-1-4684-5101-6_9. [DOI] [PubMed] [Google Scholar]
- Clark JR. Physiological problems of seasonal breeding in eutherian mammals. Oxford Rev. Reprod. Biol. 1981;3:244–312. [Google Scholar]
- Collins PM, Tsang WN, Metzger JM. Influence of stress on adrenocortical function in the male tree shrew (Tupaia belangeri) Gen. Comp. Endocrinol. 1984;55:450–457. doi: 10.1016/0016-6480(84)90017-0. [DOI] [PubMed] [Google Scholar]
- Csiszar A, Labinskyy N, Orosz Z, Buffenstein R, Ungvari Z. Vascular aging in the longest-living rodent, the naked mole-rat. Am. J. Physiol. Heart Circ. Physiol. 2007;293:H919–H927. doi: 10.1152/ajpheart.01287.2006. [DOI] [PubMed] [Google Scholar]
- de Magalhães JP, Costa J, Toussaint O. "HAGR: the Human Ageing Genomic Resources". Nucleic Acids Research. 2005;33:D537–D543. doi: 10.1093/nar/gki017. (Database Issue) [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Magalhães JP, Costa J, Church GM. An analysis of the relationship between metabolism, developmental schedules, and longevity using phylogenetic independent contrasts. J. Gerontol. A Biol. Sci. Med. Sci. 2007;62:149–160. doi: 10.1093/gerona/62.2.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenstein AB, Strack I. Effect of high protein feeding on gluconeogenesis in rat liver. Diabetes. 1971;20:577–585. doi: 10.2337/diab.20.9.577. [DOI] [PubMed] [Google Scholar]
- Elamin A, Hussein O, Tuvemo T. Growth, puberty, and final height in children with Type 1 diabetes. J. Diab. Compl. 2006;20:252–256. doi: 10.1016/j.jdiacomp.2005.07.001. [DOI] [PubMed] [Google Scholar]
- Faulkes CG, Bennett NC. Family values, group dynamics and social control of reproduction in African mole-rats. T.R.E.E. 2001;16:184–190. doi: 10.1016/s0169-5347(01)02116-4. [DOI] [PubMed] [Google Scholar]
- Ferrari E, Cravello L, Falvo F, Barili L, Solerte SB, Fioravanti M, Magri F. Neuroendocrine features in extreme longevity. Exp. Gerontol. 2008;43:88–94. doi: 10.1016/j.exger.2007.06.010. [DOI] [PubMed] [Google Scholar]
- Flurkey K, Papaconstantinou J, Miller RA, Harrison DE. Lifespan extension and delayed immune and collagen aging in mutant mice with defects in growth hormone production. Proc. Natl. Acad. Sci. U. S. A. 2001;98:6736–6741. doi: 10.1073/pnas.111158898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freitas MB, Passos CB, Vasconcelos RB, Pinheiro EC. Effects of short-term fasting on energy reserves of vampire bats (Desmodus rotundus) Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2005;140:59–62. doi: 10.1016/j.cbpc.2004.09.023. [DOI] [PubMed] [Google Scholar]
- Ganem G, Bennett NC. Tolerance to unfamiliar conspecifics varies with social organization in female African mole-rats. Physiol. Behav. 2004;82:555–562. doi: 10.1016/j.physbeh.2004.05.002. [DOI] [PubMed] [Google Scholar]
- Haber C. Life extension and history: the continual search for the fountain of youth. J. Gerontol. A Biol. Sci. Med. Sci. 2004;59:B515–B522. doi: 10.1093/gerona/59.6.b515. [DOI] [PubMed] [Google Scholar]
- Habra M, Sarlis NJ. Thyroid and aging. Rev. Endocr. Metab. Disord. 2005;6:145–154. doi: 10.1007/s11154-005-1494-9. [DOI] [PubMed] [Google Scholar]
- Harper JM, Salmon AB, Leiser SF, Galecki AT, Miller RA. Skin-derived fibroblasts from long-lived species are resistant to some, but not all, lethal stresses and to the mitochondrial inhibitor rotenone. Aging Cell. 2007;6:1–13. doi: 10.1111/j.1474-9726.2006.00255.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauck SJ, Hunter WS, Danilovich N, Kopchick JJ, Bartke A. Reduced levels of thyroid hormones, insulin, and glucose, and lower body core temperature in the growth hormone receptor/binding protein knockout mouse. Exp. Biol. Med. 2001;226:552–558. doi: 10.1177/153537020122600607. [DOI] [PubMed] [Google Scholar]
- Hayssen V. Empirical and theoretical constraints on the evolution of lactation. J. Dairy Sci. 1993;76:3213–3233. doi: 10.3168/jds.S0022-0302(93)77659-6. [DOI] [PubMed] [Google Scholar]
- Hibberd C, Yau JL, Seckl JR. Glucocorticoids and the ageing hippocampus. J. Anat. 2000;197:553–562. doi: 10.1046/j.1469-7580.2000.19740553.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holick MF. Sunlight and vitamin D for bone health and prevention of autoimmune diseases, cancers, and cardiovascular disease. Am. J. Clin. Nutr. 2004;80:1678S–1688S. doi: 10.1093/ajcn/80.6.1678S. [DOI] [PubMed] [Google Scholar]
- Horani MH, Morley JE. Hormonal fountains of youth. Clin. Geriatr. Med. 2004;20:275–292. doi: 10.1016/j.cger.2004.02.002. [DOI] [PubMed] [Google Scholar]
- Horuk R, Blundell TL, Lazarus NR, Neville RW, Stone D, Wollmer A. A monomeric insulin from the porcupine (Hystrix cristata), an Old World hystricomorph. Nature. 1980;286:822–824. doi: 10.1038/286822a0. [DOI] [PubMed] [Google Scholar]
- Hulbert AJ. Thyroid hormones and their effects: a new perspective. Biol. Rev. Camb. Philos. Soc. 2000;75:519–631. doi: 10.1017/s146479310000556x. [DOI] [PubMed] [Google Scholar]
- Hulbert AJ, Pamplona R, Buffenstein R, Buttemer W. Life and Death: metabolic rate, membrane composition and life span of animals. Physiol. Rev. 2007;87:1175–1213. doi: 10.1152/physrev.00047.2006. [DOI] [PubMed] [Google Scholar]
- Hulbert AJ, Hinds DS, MacMillen RE. Minimal metabolism, summit metabolism and plasma thyroxine in rodents from different environments. Comp. Biochem. Physiol. A. 1985;81:687–693. doi: 10.1016/0300-9629(85)91048-5. [DOI] [PubMed] [Google Scholar]
- Kahri AI, Voutilainen R, Salmenperä M. Different biological action of corticosteroids, corticosterone and cortisol, as a base of zonal function of adrenal cortex. Acta Endocrinol (Copenh) 1979;91:329–337. doi: 10.1530/acta.0.0910329. [DOI] [PubMed] [Google Scholar]
- Karsch FJ, Dahl GE, Hachigian TM, Thrun LA. Involvement of thyroid hormones in seasonal reproduction. J. Reprod. Fertil. Suppl. 1995;49:409–422. [PubMed] [Google Scholar]
- Kawamoto K. Endocrine control of the reproductive activity in hibernating bats. Zoo. Sci. 2003;20:1057–1069. doi: 10.2108/zsj.20.1057. [DOI] [PubMed] [Google Scholar]
- Keegan DJ. Aspects of the assimilation of sugars by Rousettus aegyptiacus . Comp. Biochem. Physiol. 1977;58A:349–352. [Google Scholar]
- Kenagy GJ, Place NJ. Seasonal changes in plasma glucocorticosteroids of free-living female yellow-pine chipmunks: effects of reproduction and capture and handling. Gen. Comp. Endocrinol. 2000;117:189–199. doi: 10.1006/gcen.1999.7397. [DOI] [PubMed] [Google Scholar]
- Kettelhut IC, Foss MC, Migliorini RH. Glucose homeostasis in a carnivorous animal (cat) and in rats fed a high protein diet. Am. J. Physiol. 1980;239:R437–R444. doi: 10.1152/ajpregu.1980.239.5.R437. [DOI] [PubMed] [Google Scholar]
- King GL, Kahn CR, Heldin CH. Sharing of biological effect and receptors between guinea pig insulin and platelet-derived growth factor. Proc. Natl. Acad. Sci. U. S. A. 1983;80:1308–1312. doi: 10.1073/pnas.80.5.1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korrine C, Speakman J, Arad Z. Reproductive energetics of captive and free ranging Egyptian fruit bats (Rousettus aegyptiacus) Ecol. 2004;85:220–230. [Google Scholar]
- Kramer B, Buffenstein R. The pancreas of the naked mole-rat (Heterocephalus glaber): an ultrastructural and immunocytochemical study of the endocrine component of thermoneutral and cold acclimated animals. Gen. Comp. Endocr. 2004;139:206–214. doi: 10.1016/j.ygcen.2004.09.006. [DOI] [PubMed] [Google Scholar]
- Kuro-o M, Matsumura Y, Aizawa H, Kawaguchi H, Suga T, Utsugi T, Ohyama Y, Kurabayashi M, Kaname T, Kume E, Iwasaki H, Iida A, Shiraki-Iida T, Nishikawa S, Nagai R, Nabeshima YI. Mutation of the mouse klotho gene leads to a syndrome resembling ageing. Nature. 1997;390:45–51. doi: 10.1038/36285. [DOI] [PubMed] [Google Scholar]
- Kurosu H, Yamamoto M, Clark JD, Pastor JV, Nandi A, Gurnani P, McGuinness OP, Chikuda H, Yamaguchi M, Kawaguchi H, Shimomura I, Takayama Y, Herz J, Kahn CR, Rosenblatt KP, Kuroo M. Suppression of aging in mice by the hormone Klotho. Science. 2005;309:1829–1833. doi: 10.1126/science.1112766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwiecinski GG, Zhiren L, Chen TC, Holick MF. Observations on serum 25-hydroxyvitamin D and calcium concentrations from wild-caught and captive neotropical bats, Artibeus jamaicensis. Gen. Comp. Endocrinol. 2001;122:225–231. doi: 10.1006/gcen.2001.7635. [DOI] [PubMed] [Google Scholar]
- Kwiecinski GG, Damassa DA, Gustafson AW. Control of sex steroid-binding protein (SBP) in the male little brown bat: relationship of plasma thyroxine levels to the induction of plasma SBP in immature males. J. Endocrinol. 1986;110:271–278. doi: 10.1677/joe.0.1100271. [DOI] [PubMed] [Google Scholar]
- Lanske B, Razzaque MS. Vitamin D and aging: old concepts and new insights. J. Nutr. Biochem. 2007;18:771–777. doi: 10.1016/j.jnutbio.2007.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehman MN, Goodman RL, Karsch FJ, Jackson GL, Berriman SJ, Jansen HT. The GnRH system of seasonal breeders: anatomy and plasticity. Brain Res Bull. 1997;44:445–57. doi: 10.1016/s0361-9230(97)00225-6. [DOI] [PubMed] [Google Scholar]
- Leitol H, Behrends J, Brabant G. The thyroid axis in ageing. Novartis Found. Symp. 2002;242:193–201. [PubMed] [Google Scholar]
- Longo VD, Finch CE. Evolutionary medicine: from dwarf model systems to healthy centenarians? Science. 2003;299:1342–1346. doi: 10.1126/science.1077991. [DOI] [PubMed] [Google Scholar]
- Lyman CP, Willis JS, Malan A, Wang LCH. Hibernation and Torpor in Mammals and Birds. NY: Academic Press; 1982. [Google Scholar]
- McShane TM, Wise PM. Life-long moderate caloric restriction prolongs reproductive life span in rats without interrupting estrous cyclicity: effects on the gonadotropin-releasing hormone/luteinizing hormone axis. Biol. Reprod. 1996;54:70–75. doi: 10.1095/biolreprod54.1.70. [DOI] [PubMed] [Google Scholar]
- McDonald IR, Lee AK, Than KA, Martin RW. Failure of glucocorticoid feedback in males of a population of small marsupials (Antechinus swainsonii) during the period of mating. J. Endocrinol. 1986;108:63–68. doi: 10.1677/joe.0.1080063. [DOI] [PubMed] [Google Scholar]
- Maloney SK, Bronner GN, Buffenstein R. Thermoregulation in the Angolan free-tailed bat (Mops condylurus): a small mammal that uses hot roosts. Physiol. Biochem. Zool. 1999;72:385–396. doi: 10.1086/316677. [DOI] [PubMed] [Google Scholar]
- Mann GV, Crofford OB. Insulin levels in primates by immunoassay. Science. 1970;169:1312–1313. doi: 10.1126/science.169.3952.1312. [DOI] [PubMed] [Google Scholar]
- Manson JE, Hsia J, Johnson KC, Rossouw JE, Assaf AR, Lasser NL, Trevisan M, Black HR, Heckbert SR, Detrano R, Strickland OL, Wong ND, Crouse JR, Stein E, Cushman M. Women's Health Initiative Investigators. Estrogen plus progestin and the risk of coronary heart disease. N. Engl. J. Med. 2003;349:523–534. doi: 10.1056/NEJMoa030808. [DOI] [PubMed] [Google Scholar]
- Mariotti S. Thyroid function and aging: do serum 3,5,3'-triiodothyronine and thyroid-stimulating hormone concentrations give the Janus response? J. Clin. Endocrinol. Metab. 2005;90:6735–6737. doi: 10.1210/jc.2005-2214. [DOI] [PubMed] [Google Scholar]
- Masoro EJ. Food restriction in rodents: An evaluation of its role in the study of aging. J. Gerontol. Biol. Sci. 1988;43:B59–B64. doi: 10.1093/geronj/43.3.b59. [DOI] [PubMed] [Google Scholar]
- Masoro EJ. Overview of caloric restriction and ageing. Mech. Ageing Dev. 2005;126:913–922. doi: 10.1016/j.mad.2005.03.012. [DOI] [PubMed] [Google Scholar]
- McShane TM, Wise PM. Life-long moderate caloric restriction prolongs reproductive life span in rats without interrupting estrous cyclicity: effects on the gonadotropin-releasing hormone/luteinizing hormone axis. Biol. Reprod. 1996;54:40–75. doi: 10.1095/biolreprod54.1.70. [DOI] [PubMed] [Google Scholar]
- Merry BJ. Molecular mechanisms linking calorie restriction and longevity. Int. J. Biochem. Cell. Biol. 2002;34:1340–1354. doi: 10.1016/s1357-2725(02)00038-9. [DOI] [PubMed] [Google Scholar]
- Michelmore AJ, Keegan DJ, Kramer B. Immunocytochemical identification of endocrine cells in the pancreas of the fruit bat, Rousettus aegyptiacus . Gen. Comp. Endocrinol. 1998;110:319–325. doi: 10.1006/gcen.1998.7077. [DOI] [PubMed] [Google Scholar]
- Miller RA, Nadon NL. Principles of animal use for gerontological research. J. Gerontol. A Biol. Sci. Med. Sci. 2000;55:B117–B123. doi: 10.1093/gerona/55.3.B117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson JF, Gosden RG, Felicio LS. Effect of dietary restriction on estrous cyclicity and follicular reserves in aging C57BL/6J mice. Biol. Reprod. 1985;32:515–522. doi: 10.1095/biolreprod32.3.515. [DOI] [PubMed] [Google Scholar]
- Nelson JF, Karelus K, Bergman MD, Felicio LS. Neuroendocrine involvement in aging: evidence from studies of reproductive aging and caloric restriction. Neurobiol. Aging. 1995;16:837–843. doi: 10.1016/0197-4580(95)00072-m. [DOI] [PubMed] [Google Scholar]
- O’Connor TP, Lee A, Jarvis JUM, Buffenstein R. Prolonged longevity in naked mole-rats: Age-related changes in metabolism, body composition and gastrointestinal function. Comp. Biochem. Physiol. 2002;133:835–842. doi: 10.1016/s1095-6433(02)00198-8. [DOI] [PubMed] [Google Scholar]
- Ogunsua AO, De Nicola AF, Traikov H, Birmingham MK, Levine S. Adrenal steroid biosynthesis by different species of mouselike rodents. Gen. Comp. Endo. 1969;16:192–199. doi: 10.1016/0016-6480(71)90031-1. [DOI] [PubMed] [Google Scholar]
- Olshansky JS, Carnes BA, Butler RN. If humans were built to last. Sci. Am. 2001;284:50–55. doi: 10.1038/scientificamerican0301-50. [DOI] [PubMed] [Google Scholar]
- Ooka H, Shinkai T. Effects of chronic hyperthyroidism on the lifespan of the rat. Mech. Ageing Dev. 1986;33:275–282. doi: 10.1016/0047-6374(86)90052-7. [DOI] [PubMed] [Google Scholar]
- Ottenweller JE, Tapp WN, Burke JM, Natelson BH. Plasma cortisol and corticosterone concentrations in the golden hamster (Mesocricetus auratus) Life Sci. 1985;37:1551–1558. doi: 10.1016/0024-3205(85)90188-2. [DOI] [PubMed] [Google Scholar]
- Ottenweller JE, Tapp WN, Pitman DL, Natelson BH. Interactions among the effects of aging, chronic disease, and stress on adrenocortical function in Syrian hamsters. Endocrinology. 1990;126:102–109. doi: 10.1210/endo-126-1-102. [DOI] [PubMed] [Google Scholar]
- Ovide-Bordeaux S, Grynberg A. Docosahexaenoic acid affects insulin deficiency- and insulin resistance-induced alterations in cardiac mitochondria. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2004;286:R519–R527. doi: 10.1152/ajpregu.00303.2003. [DOI] [PubMed] [Google Scholar]
- Packer C, Tatar M, Collins A. Reproductive cessation in female mammals. Nature. 1998;392:807–811. doi: 10.1038/33910. [DOI] [PubMed] [Google Scholar]
- Pappenheimer JR. Paracellular intestinal absorption of glucose, creatinine, and mannitol in normal animals: relation to body size. Am. J. Physiol. 1990;259:G290–G299. doi: 10.1152/ajpgi.1990.259.2.G290. [DOI] [PubMed] [Google Scholar]
- Patel NV, Finch CV. The glucocorticoid paradox of caloric restriction in slowing brain aging. Neurobiol Aging. 2002;23:707–717. doi: 10.1016/s0197-4580(02)00017-9. [DOI] [PubMed] [Google Scholar]
- Peeters GM, van Schoor NM, van Rossum EF, Visser M, Lips P. The relationship between cortisol, muscle mass and muscle strength in older persons and the rle of genetic variations in the glucocorticois receptor. Clin. Endocrinol. Epub. 2008 doi: 10.1111/j.1365-2265.2008.03212.x. [DOI] [PubMed] [Google Scholar]
- Pitcher T, Sergeev IN, Buffenstein R. Vitamin D metabolism in the damara mole-rat is altered by sunlight yet mineral metabolism is unaffected. J. Endocr. 1994;143:367–374. doi: 10.1677/joe.0.1430367. [DOI] [PubMed] [Google Scholar]
- Pushpa S. Occurrence and function of corticosteroids in some selected mammalian species. Gen. Comp. Endo. 1969;2:317–324. (S) [Google Scholar]
- Reeder DM, Kosteczko NS, Kunz TH, Widmaier EP. Changes in baseline and stress-induced glucocorticold levels during the active period in free-ranging male and female little brown bat, Myotis lucifugus (Chiroptera : Vespertilionidae) Gen. Comp. Endo. 2004;136:260–269. doi: 10.1016/j.ygcen.2003.12.020. [DOI] [PubMed] [Google Scholar]
- Reeder DM, Raff H, Kunz TH, Widmaier EP. Characterization of pituitary-adrenocortical activity in the Malayan flying fox (Pteropus vampyrus) J. Comp. Physiol. B. 2006;176:513–519. doi: 10.1007/s00360-006-0073-z. [DOI] [PubMed] [Google Scholar]
- Romero LM. Seasonal changes in plasma glucocorticoid concentrations in free-living vertebrates. Gen. Comp. Endo. 2002;128:1–24. doi: 10.1016/s0016-6480(02)00064-3. [DOI] [PubMed] [Google Scholar]
- Romero LM. Physiological stress in ecology: lessons from biomedical research. T.R.E.E. 2004;19:249–255. doi: 10.1016/j.tree.2004.03.008. [DOI] [PubMed] [Google Scholar]
- Romero LM, Meister CJ, Cyr NE, Kenagy GJ, Wingfield JC. Seasonal glucocorticoid responses to capture in wild free-living mammals. Am. J. Physiol. Reg. Integr. Comp. Physiol. 2008;294:R614–R622. doi: 10.1152/ajpregu.00752.2007. [DOI] [PubMed] [Google Scholar]
- Rossouw JE. Hormones for coronary disease-full circle. Lancet. 2002;360:1996–1997. doi: 10.1016/S0140-6736(02)12030-7. [DOI] [PubMed] [Google Scholar]
- Sabatino F, Masoro EJ, McMahan CA, Kuhn RW. Assessment of the role of the glucocorticoid systeem in aging processes and in the action of food restriction. J. Gerontol. Biol. Sci. 1991;46:B171–B179. doi: 10.1093/geronj/46.5.b171. [DOI] [PubMed] [Google Scholar]
- Sandor T. A comparative survey of steroids and steroidogenic pathways throughout the vertebrates. Gen. Comp. Endo. 1969;2:284–298. (S) [Google Scholar]
- Sapolsky RM, Romero LM, Munck AU. How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory, and preparative actions. Endocr. Rev. 2000;21:55–89. doi: 10.1210/edrv.21.1.0389. [DOI] [PubMed] [Google Scholar]
- Schwartz MW. Brain pathways controlling food intake and body weight. Exp. Biol. Med. 2001;226:978–981. doi: 10.1177/153537020122601103. [DOI] [PubMed] [Google Scholar]
- Silvestri E, Moreno M, Lombardi A, Ragni M, de Lange P, Alexson SE, Lanni A, Goglia F. Thyroid-hormone effects on putative biochemical pathways involved in UCP3 activation in rat skeletal muscle mitochondria. F.E.B.S. Lett. 2005;579:1639–1645. doi: 10.1016/j.febslet.2005.02.022. [DOI] [PubMed] [Google Scholar]
- Skinner DC, Moodley GP, Buffenstein R. Is vitamin D3 essential for mineral metabolism in the damara mole rat (Cryptomys damarensis)? Gen. Comp. Endocr. 1991;81:500–505. doi: 10.1016/0016-6480(91)90178-9. [DOI] [PubMed] [Google Scholar]
- Sonntag WE, Lynch CD, Cefalu WT, Ingram RL, Bennett SA, Thornton PL, Khan AS. Pleiotropic effects of growth hormone and insulin-like growth factor (IGF)-1 on biological aging: inferences from moderate caloric-restricted animals. J. Gerontol. A Biol. Sci. Med. Sci. 1999;54:B521–B538. doi: 10.1093/gerona/54.12.b521. [DOI] [PubMed] [Google Scholar]
- Srivastava RK, Krishna A. Adiposity associated rise in leptin impairs ovarian activity during winter dormancy in vespertilionid bat, Scotophilus heathi reproduction. 2007;133:165–176. doi: 10.1530/rep.1.01019. [DOI] [PubMed] [Google Scholar]
- Stone RC, Wiebers JE. Body Weight and Temperature Regulation of Myotis lucifugus at a Low Temperature of 10° C. J. Mamm. 1966;47:520–521. [PubMed] [Google Scholar]
- Stulnig TM. Immunomodulation by polyunsaturated fatty acids: mechanisms and effects. Int. Arch. Allergy Immunol. 2003;132:310–321. doi: 10.1159/000074898. [DOI] [PubMed] [Google Scholar]
- Taguchi A, Wartschow LM, White MF. Brain IRS2 signaling coordinates life span and nutrient homeostasis. Science. 2007;317:369–372. doi: 10.1126/science.1142179. [DOI] [PubMed] [Google Scholar]
- Tedman RA, Hall LS. The morphology of the gastrointestinal tract and food transit time in fruit bats Pteropus alecto and P. poliocephalus (Megachiroptera) Aust. J. Zool. 1985;33:625–640. [Google Scholar]
- Torres PU, Prié D, Molina-Blétry V, Beck L, Silve C, Friedlander G. Klotho: an antiaging protein involved in mineral and vitamin D metabolism. Kidney Int. 2007;71:730–737. doi: 10.1038/sj.ki.5002163. [DOI] [PubMed] [Google Scholar]
- Tracy CR, McWhorter TJ, Korine C, Wojciechowski MS, Pinshow B, Karasov WH. Absorption of sugars in the Egyptian fruit bat (Rousettus aegyptiacus): a paradox explained. J. Exp. Biol. 2007;210:1726–1734. doi: 10.1242/jeb.02766. [DOI] [PubMed] [Google Scholar]
- Tsujikawa H, Kurotaki Y, Fujimori T, Fukuda K, Nabeshima Y. Klotho, a gene related to a syndrome resembling human premature aging, functions in a negative regulatory circuit of vitamin D endocrine system. Mol. Endocrinol. 2003;17:2393–2403. doi: 10.1210/me.2003-0048. [DOI] [PubMed] [Google Scholar]
- Vallée M, MacCari S, Dellu F, Simon H, Le Moal M, Mayo W. Long-term effects of prenatal stress and postnatal handling on age-related glucocorticoid secretion and cognitive performance: a longitudinal study in the rat. Eur. J. Neurosci. 1999;11:2906–2916. doi: 10.1046/j.1460-9568.1999.00705.x. [DOI] [PubMed] [Google Scholar]
- Vergara M, Smith-Wheelock M, Harper JM, Sigler R, Miller RA. Hormone-treated Snell dwarf mice regain fertility but remain long-lived and disease resistant. J. Gerontol. A Biol. Sci. Med. Sci. 2004;59:1244–1250. doi: 10.1093/gerona/59.12.1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber C. Cortisol’s purpose. Med. Hypotheses. 51:289–291. doi: 10.1016/s0306-9877(98)90049-4. [DOI] [PubMed] [Google Scholar]
- Weigl R. Longevity of Mammals in Captivity; from the Living Collections of the World. Kleine Senckenberg-Reihe, Stuttgart. 2005 [Google Scholar]
- Widmaier EP, Kunz TH. Basal, diurnal, and stress-induced levels of glucose and glucocorticoids in captive bats. J. Exp. Zool. 1993;265:533–540. doi: 10.1002/jez.1402650509. [DOI] [PubMed] [Google Scholar]
- Wu JM, Zelinski MB, Ingram DK, Ottinger MA. Ovarian aging and menopause: current theories, hypotheses, and research models. Exp. Biol. Med. 2005;230:818–828. doi: 10.1177/153537020523001106. [DOI] [PubMed] [Google Scholar]
- Yang T, Buffenstein R. Effect of aging on glycated hemoglobin and blood glucose concentration in naked mole-rats. FASEB. 2004;18:A1301–A1302. Suppl. [Google Scholar]
- Yoshida T, Fujimori T, Nabeshima Y. Mediation of unusually high concentrations of 1,25-dihydroxyvitamin D in homozygous klotho mutant mice by increased expression of renal 1alpha-hydroxylase gene. Endocr. 2002;143:683–689. doi: 10.1210/endo.143.2.8657. [DOI] [PubMed] [Google Scholar]
- Yoshimura T. Molecular mechanism of the photoperiodic response of gonads in birds and mammals. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2006;144:345–350. doi: 10.1016/j.cbpa.2005.09.009. [DOI] [PubMed] [Google Scholar]