Abstract
Gammaherpesviruses can persist in the host in the face of an aggressive immune response. T cells recognize Ags expressed in both the productive and latent phases of the virus life cycle, however little is known about their relative roles in the long-term control of the infection. In this study we used the murine gammaherpesvirus 68 model system to investigate the relative properties of CD8 T cells recognizing lytic and latent viral Ags. We report that the CD8 T cell response to lytic phase epitopes is maximal in the lungs of infected mice at ~10 days postinfection, and is of progressively lesser magnitude in the mediastinal lymph nodes and spleen. In contrast, the CD8 T cell response to the latent M2 protein is maximal at ~19 days postinfection and is most prominent in the spleen, then progressively less in the mediastinal lymph node and the lung. Latent and lytic Ag-specific CD8 T cells had markedly different cell surface phenotypes during chronic infection, with latent Ag-specific cells being predominantly CD62Lhigh or CD43 (1B11)high. Lytic Ag-specific T cells had significantly lower expression of these markers. Importantly, latent but not lytic Ag-specific T cells could kill target cells rapidly in vivo during the chronic infection. These two different sets of CD8 T cells also responded differentially to IL-7, a cytokine involved in T cell homeostasis and the maintenance of T cell memory. These data have important implications for our understanding of immunological control during chronic gammaherpesvirus infections.
Chronic gammaherpesvirus infections persist in infected individuals despite a potent antiviral immune response. One of the main strategies used to evade the immune response is the establishment of latency within host cells. Nevertheless there is effective immune surveillance during viral latency, as viral reactivation and/or outgrowth of latently infected cells occurs only when the T lymphocyte response is compromised. The mechanisms by which virus-specific T cells are able to contain the virus infection without eliminating it are poorly understood.
In the latent stage of the virus life cycle a limited number of viral gene products are expressed. In contrast, a large number of proteins are expressed during lytic cycle replication. Epitopes derived from proteins expressed in both latent and lytic phases of the virus life cycle are targets for the T cell response. Interestingly, the phenotype of lytic and latent Ag-specific T cells is markedly different in chronic EBV infection in humans. CD8 T cells specific for the lytic phase BZLF1 protein were heterogeneous with respect to CD45RO/RA and CD28 expression whereas latent epitope specific populations were uniformly CD45RO+CD28+ (1, 2). In addition, latent Ag-specific CD8 T cells were predominantly CD62Lhigh whereas lytic Ag-specific cells were mostly CD62Llow. These differences likely indicate that CD8 T cells specific for latent Ags are in a different stage of activation when compared with T cells specific for lytic phase Ags. What this represents in terms of the function of these two T cell subsets is currently unclear.
One possible reason for this difference is that lytic and latent Ag-specific CD8 T cells expand to different degrees during the primary immune response to EBV. There is a huge expansion in lytic Ag-specific CD8 T cell cells during acute infectious mononucleosis (AIM)3 but a much smaller expansion in CD8 T cells specific for the latent viral proteins (3). This may affect the subsequent phenotype of memory CD8 T cells recognizing these different epitopes. A second reason may relate to the balance between the virus and the immune response during chronic infection. Sporadic reactivation of virus from latency may continually stimulate lytic Ag-specific T cells during the chronic infection. In contrast, immunogenic latent Ags are thought to be present at a much lower abundance in AIM and their expression long term is either highly restricted or absent.
In humans it is difficult to follow the kinetics with which the latent and lytic Ag-specific T cells are induced, as patients with acute EBV infection present with AIM several weeks after initial infection. It is also impossible to perform in vivo experiments to address the differential roles these cells may be playing in chronic EBV infection. Therefore we chose to study a mouse model, murine gammaherpesvirus 68 (MHV-68). In this model the virus replicates initially in the lungs, then establishes a latent infection in B cells and other APCs (4, 5) in addition to lung epithelial cells (6). This virus is widely accepted as a model system for the study of how the immune system controls chronic gammaherpesvirus infections (7, 8).
To date no studies have directly compared the phenotype and function of lytic and latent Ag-specific CD8 T cells during MHV-68 infection. In this paper we report that these two specificities of CD8 T cells are induced with different kinetics during the acute infection and have different cell surface phenotypes during chronic MHV-68 infection. In addition, these cells have very different abilities to kill target cells in vivo and respond differentially to IL-7, a cytokine implicated in the generation and maintenance of T cell memory.
Materials and Methods
Mice and virus
MHV-68 virus (clone G2.4) was originally obtained from Dr. A. A. Nash (University of Edinburgh, U.K.). Virus was propagated and titered as previously described (9). Female BALB/c mice were purchased from The National Cancer Institute (Bethesda, MD). Mice were infected intranasally with 400 PFU of MHV-68 under anesthesia with 2,2,2-tribromoethanol. All animal experiments were approved by the Animal Care and Use Program of Dartmouth College. The bronchoalveolar lavage (BAL) was taken from animals terminally anesthetized with 2,2,2-tribromoethanol by cutting a small hole in the trachea, feeding in a catheter tube, and washing the lungs with 1 ml of HBSS three times. This washing procedure was repeated a total of three times resulting in 3 ml BAL fluid per mouse.
IFN-γ ELISPOT assay
The number of IFN-γ secreting cells was determined after stimulation with peptides in a standard ELISPOT assay. In brief, 96-well Multiscreen HA nitrocellulose plates (Millipore, Bedford, MA) were coated overnight at 4°C with 100 µl per well of rat anti-mouse IFN-γ (clone R4-6A2; BD PharMingen, San Diego, CA), at a concentration of 2 µg/ml. The plates were then washed and blocked before the addition of irradiated (3000 rad) normal BALB/c spleen cells (5 × 105 cells/well), a graded number of responder spleen cells, 10 µg/ml of the appropriate peptide and 10 U/ml recombinant human IL-2 (Tecin, National Cancer Institute). Responder cells consisted of cells from the spleen, mediastinal lymph node (MLN) and BAL taken from BALB/c mice infected for the stated period of time with MHV-68. For MLN and BAL samples the cells were pooled from three to four mice, whereas spleens were assayed from individual mice. Plates were then incubated for 24 h at 37°C and developed for 2 h with a biotinylated rat anti-mouse IFN-γ (clone XMG1.2; BD PharMingen) at a concentration of 2 µg/ml, followed by streptavidin-alkaline phosphatase (DAKO, Carpinteria, CA) at a 1/500 dilution for 1 h at room temperature. Following addition of the chromogenic substrate 5-bromo-4-chloro-3-indolyl phosphate/nitroblue tetrazolium (BCIP/NBT; Sigma-Aldrich, St. Louis, MO), visible spots were enumerated using a dissecting microscope.
MHC tetrameric reagents and analysis
The construction of folded MHC class I-peptide complexes and their tetramerization have been previously described (10). Two tetramers were used: Kd folded with peptide M291–99 (GFNKLRSTL) and Dd folded with open reading frame (ORF)65131–140 (LGPDKSGLGF). Tetramers were stored as aliquots at −80°C. B cells were removed from spleen cell samples by panning for 1 h on plates coated overnight with 100 µg/ml goat anti-mouse IgG/IgM (Jackson ImmunoResearch Laboratories, West Grove, PA). Cells were incubated with anti-CD16/CD32 Fc block (BD PharMingen) for 10 min on ice; staining with tetrameric reagents took place for 1 h at room temperature, followed by staining with anti-CD8a PerCP (clone 53-6.7) and anti-CD62L FITC (clone MEL-14) or anti-CD43 PE (clone 1B11) on ice for 20 min. Stained samples were analyzed using a FACSCalibur flow cytometer and CellQuest software (BD Immunocytometry Systems, Mountain View, CA). Control tetramers consisting of the same H chain folded with irrelevant peptides did not stain CD8 T cells from MHV-68 infected mice.
CFSE and CellTracker Orange labeling
Spleen cells were labeled with either 0.5 µM CFSE (Molecular Probes, Eugene, OR) or 10 µM CellTracker Orange (Molecular Probes). For CFSE labeling, spleen cells were incubated at a concentration of 2 × 107 cells/ml in 0.5 µM CFSE diluted in HBSS for 10 min at room temperature in the dark. Cells were washed with complete tumor medium (CTM; see Ref. 11) before use. For CellTracker Orange labeling, spleen cells were incubated at a concentration of 2 × 107 cells/ml in 10 µM CellTracker Orange diluted in CTM for 30 min at 37°C in the dark. Cells were then washed and incubated at 37°C for an additional 30 min to allow complete modification of the probe. Cells were subsequently washed with CTM before use.
In vivo cytotoxicity assay
Normal BALB/c spleen cells were used as target cells for the evaluation of in vivo cytotoxic activity. Splenocytes were lysed of erythrocytes, pulsed with 2 µg/ml peptide for 60 min at 37°C, and washed. Flu HA518–528 pulsed spleen cells were labeled with CFSE as previously described and M291–99 or ORF65131–140 pulsed spleen cells were labeled with Cell-Tracker Orange. Following fluorescent labeling, cells were resuspended at 108 cells/ml in HBSS, mixed in a 1:1 ratio (Flu HA to M2 or Flu HA to ORF65) and 2 × 107 cells were injected i.v. to BALB/c mice that had been infected with MHV-68 >40 days previously (or naive mice, used to calculate specific lysis). Mice were then sacrificed at the indicated times postinjection and their spleens were taken. Spleen cell suspensions were treated with 20 µg/ml 7-amino actinomycin D (7-AAD) (Sigma-Aldrich) for 15 min at room temperature in the dark to label dead cells and then analyzed by flow cytometry with 2 × 104 live CFSE- or CellTracker Orange-positive events being collected. Specific lysis was calculated using methods described in other reports (12). In brief, specific lysis was calculated using the formulas: ratio = (number CFSE/number CellTracker Orange); percentage of specific lysis = (1 − (ratio of naive/ratio of infected) × 100).
In vitro proliferation assay
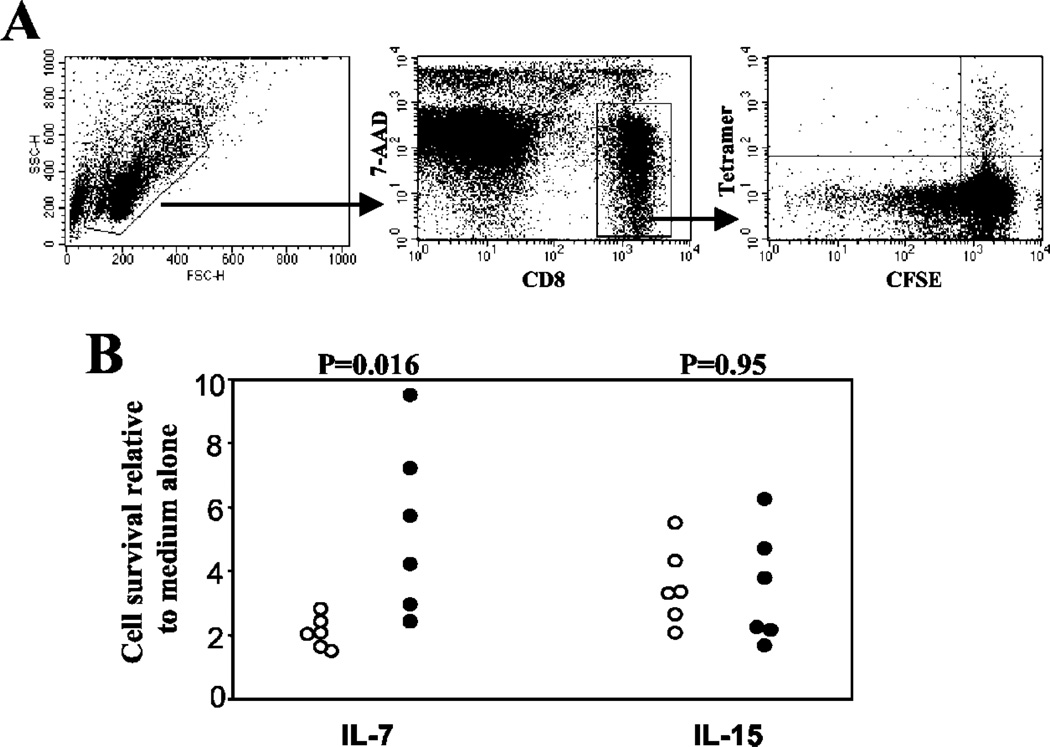
Spleen cells from mice infected with MHV-68 for >40 days were labeled with CFSE as previously described. The effects of cytokines was assessed by culturing CFSE-labeled spleen cells at 106 cells/ml for 4 days at 37°C in CTM with recombinant murine IL-7 at 25 ng/ml (Peprotech, Rocky Hill, NJ) or recombinant murine IL-15 at 25 ng/ml (Peprotech) in the absence of cognate Ag. Cells were then harvested, counted, and stained with tetramer, anti-CD8 Ab, and 7-AAD then analyzed using a FACSCalibur flow cytometer (BD Biosciences, San Jose, CA).
Statistical analysis
All data were analyzed using the Student t test and significance was determined as a p < 0.05.
Results
Different priming kinetics of lytic and latent Ag-specific T cell responses
We previously described the identification of a CD8 T cell epitope in a latency-associated protein, M2 (13, 14). This epitope was recognized only by BALB/c mice, whereas the only known epitopes in lytic cycle proteins were identified in C57BL/6 mice. Therefore it was not possible to compare directly the priming kinetics of lytic and latent Ag-specific T cells in any given strain. Recently we reported the identification of two novel epitopes within the ORF65 protein of MHV-68, which are recognized by BALB/c mice (15). One of these epitopes, ORF65131–140, was recognized by CD8 T cells and the other epitope, ORF6516–30, by CD4 T cells. ORF65 is homologous to proteins that form part of the virus capsid in other herpesviruses, therefore it can be considered a lytic cycle protein.
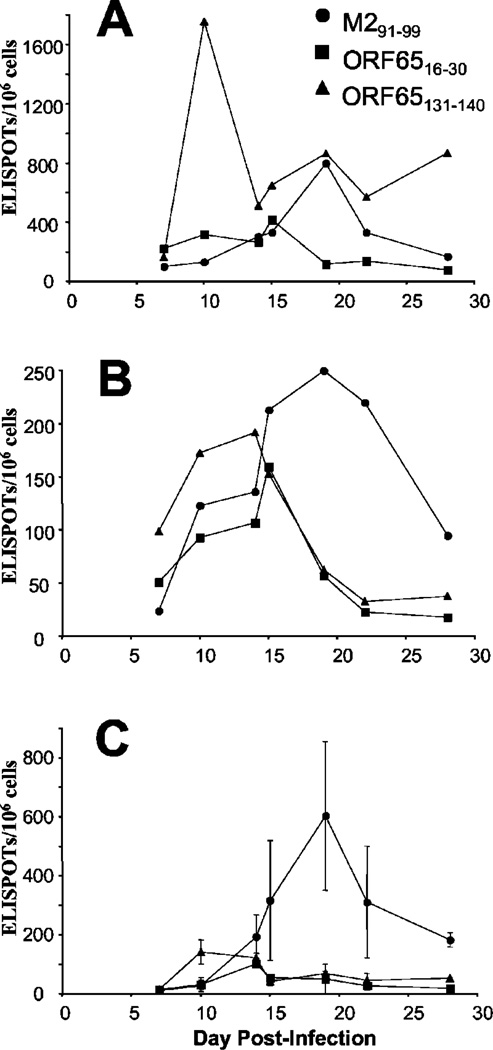
We intended to measure the kinetics with which the lytic cycle Ag-specific (ORF65) T cells were primed and compare this with the latent Ag-specific (M2) T cells. BALB/c mice were infected intranasally with MHV-68 then at the times shown spleens, MLN, and BAL were taken. The frequency of T cells specific for particular viral epitopes was then measured using an IFN-γ ELISPOT assay. In the lungs there was a substantial response to the ORF65131–140 CD8 T cell epitope at day 10 postinfection (Fig. 1A), whereas the M291–99 CD8 T cell response did not peak until 19 days postinfection. The apparent second peak in the ORF65131–140 CD8 T cell response at 19 days postinfection was not a consistent finding. Interestingly, the CD4 T cell response to the ORF6516–30 epitope was present at a much lower frequency, and appeared to develop more slowly than the CD8 T cell response to this protein. These kinetics were similar in the MLN (Fig. 1B), although at this site the ORF65131–140 CD8 T cell response was still present at 14 days postinfection and declined thereafter. Importantly, the magnitude of the M291–99-specific CD8 T cell response was greater than the response to the ORF65131–140 epitope in the MLN, however it arose with similar kinetics to those observed in the BAL. In contrast to the BAL, the magnitude of the CD4 T cell response to the ORF16–30 epitope was comparable to the two CD8 T cell epitopes in the MLN. In the spleen the magnitude of the M291–99 epitope was much larger than the responses to the other epitopes (Fig. 1C), however the kinetics were the same as those observed in the BAL and MLN. The response to the ORF65131–140 epitope was weak but still detectable between days 10 and 14 postinfection, and the response to the CD4 T cell epitope in this protein was also weak.
FIGURE 1.
Different priming kinetics for lytic and latent Ag-specific T cells. BALB/c mice were infected with 400 PFU of MHV-68, and T cell responses were measured in the BAL (A), MLN (B), and spleen (C) at the indicated times using a standard ELISPOT assay in response to the following peptides: M291–99 (●), ORF6516–30 (■) or ORF65131–140 (▲). For BAL and MLN each point represents pooled cells from three to four mice, whereas for spleen each point represents the average of three to four individual mice with error bars indicating one SD. Data is representative of two experiments.
Overall the priming kinetics were remarkably similar in all three sites examined. The CD8 T cell response to the ORF65131–140 epitope peaked first, followed by the CD4 T cell response to the ORF16–30 epitope. The CD8 T cell response to the latent M2 protein peaked later, at ~19 days postinfection. The response to the latency-associated M2 protein was mainly seen in lymphoid tissue, whereas the response to the ORF65 protein was preferentially in the lung or MLN.
Differences in phenotype between lytic and latent Ag-specific cells during chronic MHV-68 infection
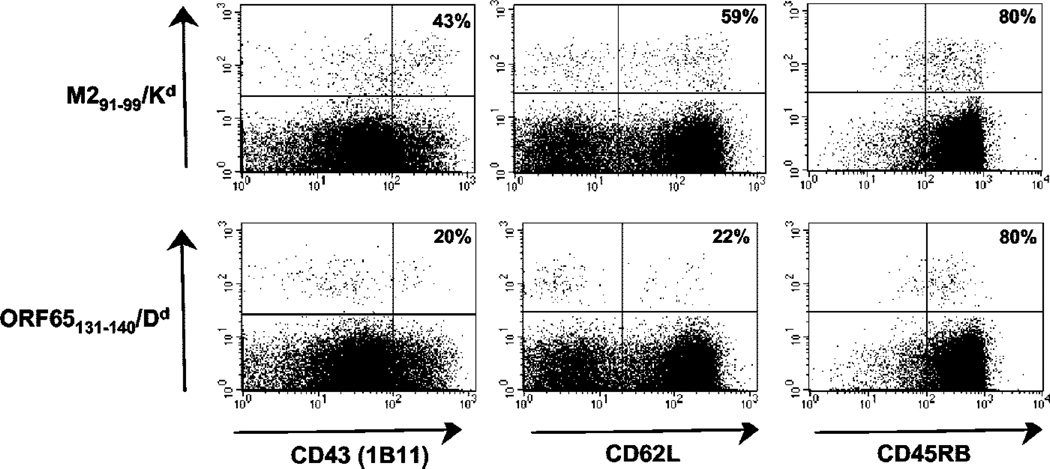
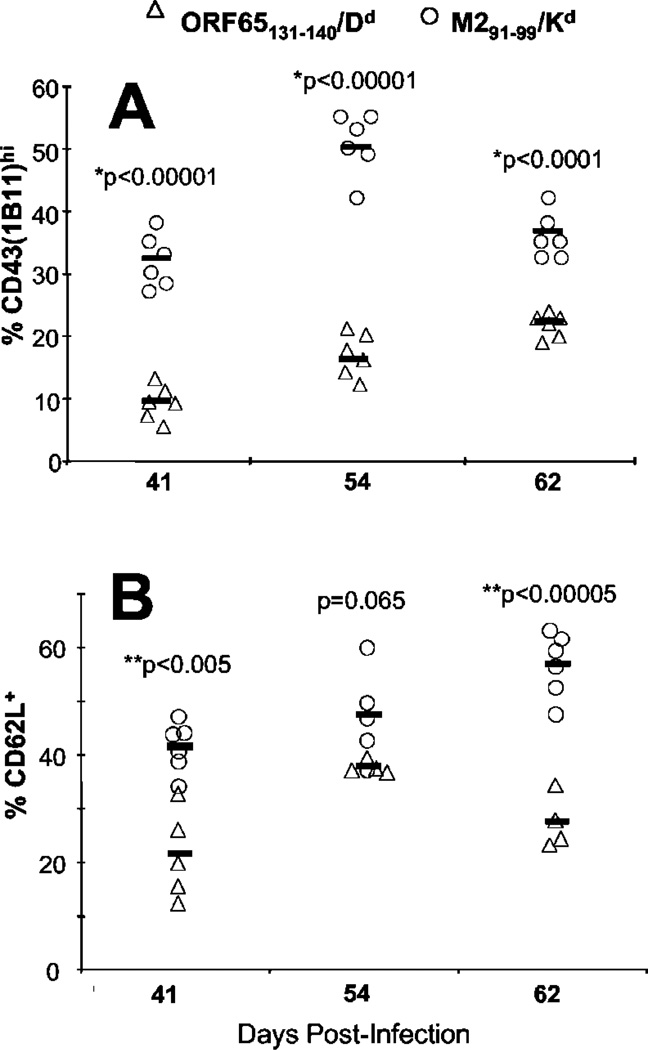
During latent infection EBV-specific T cells differ in their phenotype according to whether they recognized lytic or latent phase Ags (1, 2). To test whether this was the case during latent MHV-68 infection we infected BALB/c mice with MHV-68 then determined the phenotype of the lytic and latent Ag-specific CD8 T cells at >40 days postinfection. During the time period we studied the frequencies of M291–99/Kd- and ORF65131–140/Dd-specific T cells remained stable at ~0.9% and 0.3% of CD8 T cells, respectively. To identify lytic and latent Ag-specific T cells in the spleen we used allophycocyanin-labeled tetrameric MHC/peptide reagents loaded with the appropriate epitope, and samples were costained for CD8 and activation markers. An example of the staining is shown in Fig. 2. There was a marked difference in the phenotype in the two cell populations with respect to the markers CD43 (1B11) and CD62L (Fig. 3). The latent Ag-specific CD8 T cells had significantly higher expression of both CD43 (1B11) (Fig. 3A) and CD62L (Fig. 3B) than did the lytic Ag-specific T cells. These differences were observed consistently at all the time points studied between 40 and 60 days postinfection. In contrast no significant differences were observed with respect to other activation markers such as CD45RB, CD45RA, and CD11a (data not shown).
FIGURE 2.
Analysis of MHV-68 epitope-specific CD8+ T cells for CD43 (1B11), CD62L, and CD45RB. Splenocytes from latently infected mice (62 dpi) were stained with either the M291–99/Kd or ORF65131–140/Dd tetramer followed by Abs to CD8a and the appropriate activation marker. Results shown were gated on the CD8 population and the percentage values refer to the percentage of tetramer-positive cells that stained for the activation marker.
FIGURE 3.
CD8 T cells responding to lytic (ORF65131–140/Dd) or latent (M291–99/Kd) Ags express different levels of activation marker on their cell surface during latent MHV-68 infection. Expression of CD43 (1B11) (A) and CD62L (B) was assessed on tetramer-binding CD8+ T cells during the latent phase of the MHV-68 infection (>40 dpi). Cells stained with the M291–99/Kd tetramer (○) or the ORF65131–140/Dd tetramer (△) are shown. Each symbol represents one mouse with the thick bar indicating the mean for that group. Values for p at a given time point are shown and were determined using Student’s t test.
Differences in the CTL activity of lytic and latent Ag-specific CD8 T cells in vivo
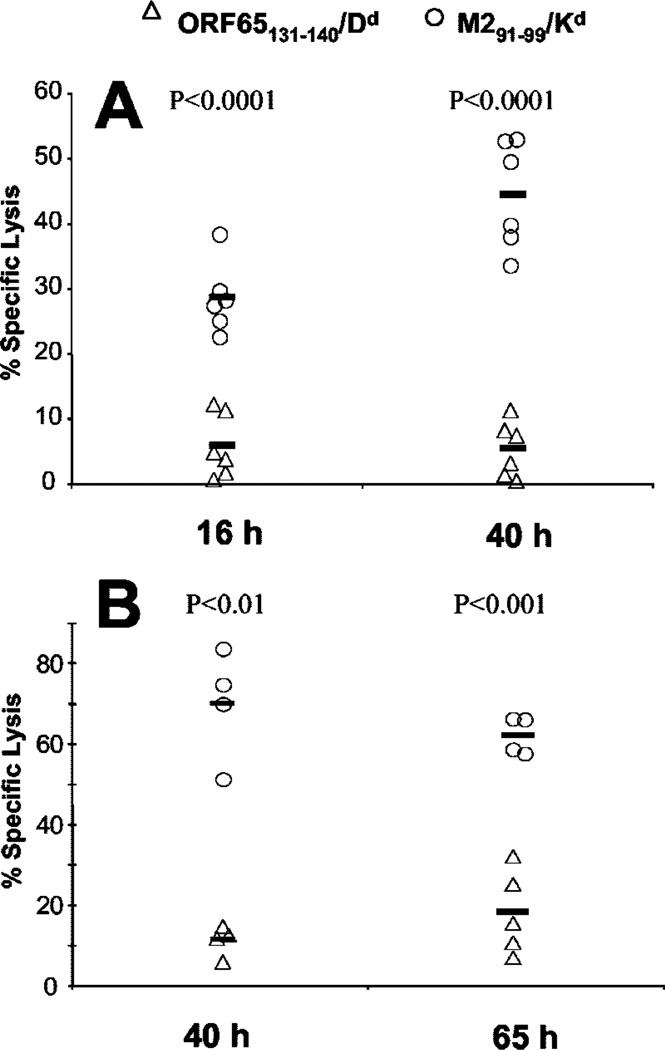
We wished to test whether the phenotypic differences between the lytic and latent Ag-specific CD8 T cells corresponded to any functional differences. The frequency of each cell population was considered too low to observe any direct ex vivo CTL activity (0.3–0.9% of CD8 T cells), and we wished to avoid any potential artifacts introduced by an in vitro restimulation before assaying CTL activity. Therefore we chose to examine CTL activity directly in vivo. To achieve this we tested the ability of latently infected mice to eliminate cells loaded with peptides representing either the lytic (ORF65131–140) or latent (M291–99) epitope. We loaded one group of normal spleen cells with the MHV-68 epitope of interest, and another with an irrelevant peptide. Each group of cells was then labeled with a different fluorescent dye (CFSE or CellTracker Orange), and the cells were mixed in a 1:1 ratio and i.v. injected into mice latently infected with MHV-68. At set times postinjection the spleens were removed and the ratio of cells detected with each epitope was determined. To distinguish between live and dead fluorescently labeled cells we included the vital dye 7-AAD. If the cells loaded with the viral epitope were killed preferentially then the ratio would change from the input 1:1 ratio. As a control the cell populations were injected into naive mice, in which the ratio should remain 1:1.
We observed substantial killing of M291–99-loaded cells at all time points studied (Fig. 4, A and B). In contrast, <20% killing of ORF65131–140-labeled cells was observed even at 65 h posttransfer. ORF65131–140/Dd-specific T cells are present at ~3-fold lower levels than M291–99/Kd-specific T cells so it was possible the failure to observe specific killing by these cells was due to low sensitivity of the assay system. To address this concern we performed control experiments in which naive mice were injected with graded numbers of a CD8 T cell line specific for the MHV-68 epitope then challenged with target cells as previously described. The level of tetramer-positive cells in the spleen was measured in recipient mice so we could achieve a frequency similar to that seen with ORF65131–140/Dd-specific cells in latently infected mice. Specific lysis (53%) was observed in these mice at 40 h after injection of target cells. This demonstrated that our in vivo CTL assay did give us the necessary degree of sensitivity to measure lysis by ORF65131–140/Dd-specific cells if they were cytolytic during the latent infection. Importantly, ORF65131–140/Dd-specific cells did have the ability to kill during the acute infection, as we observed 37.5% specific lysis using the in vivo CTL assay at 10 days postinfection.
FIGURE 4.
CD8 T cells responding to lytic (ORF65131–140/Dd) or latent (M291–99/Kd) Ags have different abilities to kill peptide-loaded targets in vivo. Latently infected BALB/c mice (>40 days postinfection with MHV-68) were given 2 × 107 fluorescently labeled splenocytes i.v. as described in Materials and Methods. In the first experiment (A) killing was analyzed 16 or 40 h later, whereas in the second experiment (B) killing was analyzed 40 or 64 h postinjection. To measure Ag-specific killing, splenocytes were harvested, incubated with 20 µg/ml 7-AAD for 15 min and then analyzed using a FACSCalibur cytometer. Specific lysis was then determined as described in Materials and Methods and the killing of M291–99 (○) and ORF65131–140 (△) labeled cells is shown in the graph. Each symbol represents one mouse with the bar indicating the average for that group. p values for a given time-point are and were determined using Student’s t test.
Response of latent and lytic Ag-specific CD8 T cells to IL-7 and IL-15
It has been shown recently by several groups that the maintenance of memory CD8 T cells depends upon IL-15 (16–18). This cytokine can induce the proliferation of memory CD8 T cells in an Ag-independent fashion, and in its absence memory CD8 T cells do not divide and self-renew in vivo. IL-7 affects the homeostatic turnover of naive T cells, but recent evidence has also shown that CD8 T cells that are unresponsive to IL-7 are very inefficient at developing memory (19). IL-15 and IL-7 have also been shown to selectively stimulate “effector memory” but not “central memory” T cells (20, 21). Given the different functional abilities of latent and lytic Ag-specific CD8 T cells in vivo, we tested whether they responded to IL-7 and IL-15 in the same way.
We removed spleens from BALB/c mice infected for >40 days with MHV-68, labeled them with CFSE, then cultured spleen cells with either IL-7 or IL-15 in the absence of cognate Ag. After 4 days in culture cells were stained as described and gated on live, CD8 T cells. M291–99/Kd-specific and ORF65131–140/Dd-specific cells were identified by tetramer staining, and the intensity of CFSE staining served as a measure of cell division (Fig. 5A). Only a small proportion of cells proliferated in response to any of these cytokines (data not shown), however there were marked differences in the survival of the two groups of CD8 T cells. The survival of both groups was enhanced by the presence of IL-15 (Fig. 5C), to approximately the same degree (3–4-fold) over medium alone. In the presence of IL-7 M291–99/Kd-specific cells survived ~2-fold over medium alone. Survival of ORF65131–140/Dd-specific cells was variable in response to IL-7, but enhanced relative to M291–99/Kd-specific cells (p = 0.016, six independent experiments shown in Fig. 5C). These data therefore demonstrate that latent and lytic Ag-specific CD8 T cells not only have different abilities to kill target cells in vivo, but they also respond differently to a memory-associated cytokine.
FIGURE 5.
Survival of lytic or latent Ag-specific CD8 T cells in response to IL-7 or IL-15 in vitro. Spleen cells from BALB/c mice infected for >40 days with MHV-68 were labeled with CFSE then placed in culture with 25 ng/ml recombinant mouse IL-15 or 25 ng/ml recombinant mouse IL-7. Four days later, the cells were harvested, counted, and stained with tetramers consisting of the M291–99/Kd epitope (○), or the ORF65131–140/Dd epitope (●) and anti-CD8a Ab. To identify dead cells, samples were incubated with 20 µg/ml 7-AAD for 15 min before analyzing on a FACSCalibur cytometer. A, Gating used for analysis of the data. B, The number of live tetramer-positive cells in the different treatment groups relative to the numbers obtained after culture with medium alone. Each point represents data from a different experiment, a total of six experiments were performed. Values for p were calculated using Student’s t test.
Discussion
To date it has been impossible to compare the phenotype and function of lytic and latent Ag-specific CD8 T cells in MHV-68 infection in any given mouse strain. Aided by our recent identification of a lytic Ag-specific CD8 T cell epitope in BALB/c mice (15) we have now performed these studies and showed that latent Ag-specific CD8 T cells have a different cell surface phenotype to lytic Ag-specific cells during chronic infection. Latent Ag-specific but not lytic Ag-specific T cells also have the ability to kill target cells rapidly in vivo. In addition lytic and latent Ag-specific T cells respond differentially to IL-7, a cytokine associated with lymphoid homeostasis and memory T cell maintenance. These data have important implications for our understanding of immune surveillance during gammaherpesvirus infections.
One important aspect of these studies is the detailed kinetics of priming of the lytic and latent Ag-specific T cells. This is difficult to measure in EBV-infected patients as they present with AIM several weeks after initial infection, by which time the T cell response has already been primed. In this study we show that the CD8 T cell response to the lytic cycle ORF65 protein is most evident in the BAL at 10 days postinfection, present to a lesser extent in the MLN and only at a low level in the spleen. In contrast, the CD8 T cell response to the latent M2 Ag shows the reverse distribution, it peaks at 19 days postinfection in the spleen, is present at a comparable level to ORF65-specific cells in the MLN, and at a lower level in the BAL. ORF65 protein is likely to be present mostly during productive replication in the lungs, and only in smaller amounts in the MLN and spleen, as virus replication is less evident in these tissues. Alternatively Ag may reach these sites by migrating dendritic cells (22, 23). M2 is expressed during latency in lymphoid tissue so this would explain the majority of the response being focused in the spleen and MLN. M2-specific cells may reach the lung either as a consequence of low-level Ag production at this site or due to the fact that activated CD8 T cells of any specificity have predisposition to migrate to the lung (24). The CD4 T cell response to the ORF65 protein was present at a low level in the BAL and spleen, but at a level only slightly lower than the CD8 T cells in the MLN. There was also a delay in the kinetics of the response, consistent with other reports that CD4 T cells respond more slowly and undertake fewer divisions than CD8 T cells (25). Interestingly the CD4 response to the ORF65 protein appeared to peak earlier in the MLN and spleen than has been reported for MHV-68 specific CD4 responses in C57BL/6 mice (26, 27). This is unlikely to be due to differences in the kinetics of expression of viral proteins from which the epitopes are derived, as ORF65 and glycoprotein 150 (which contains a CD4 epitope for C57BL/6 mice) are both late proteins (28–30). However it may be due to subtle differences between the CD4 responsiveness of BALB/c and C57BL/6 mouse strains following MHV-68 infection.
In latent EBV infection, T cells responding to epitopes present in lytic cycle proteins have a markedly different phenotype to T cells responding to latent cycle proteins (1, 2). Latent Ag-specific CD8 T cells were uniformly CD45R0+ whereas lytic Ag-specific cells were heterogeneous with respect to CD45R0 expression. Similar differences were seen with CD28 expression, the latent Ag-specific cells were predominantly CD28+ and the lytic Ag-specific cells were of a mixed phenotype. The markers CD45 and CD28 do not appear to be as useful in the mouse, however we did obtain remarkably similar data to the EBV system with respect to CD62L expression. In both EBV (1) and MHV-68 infection lytic Ag-specific CD8 T cells were ~30% CD62Lhigh compared with ~60% in the latent Ag-specific population.
CD62L is down-regulated upon activation of naive T cells and is not re-expressed for many months after Ag exposure. Thus most memory T cells are CD62Llow, however an increasing proportion re-express CD62L with time postinfection (31). CD62L expression allows T cells to migrate through high-endothelial venules into lymph nodes, and cells lacking this molecule migrate preferentially into peripheral tissues. T cells also need to express CCR7 to gain access to lymph nodes, therefore the expression of CCR7 and CD62L have become associated with central memory T cells, which mostly recirculate through lymphoid tissues, and effector-memory T cells are believed to be CD62LlowCCR7− (32). Consistent with this idea, Catalina et al. (1) reported that latent Ag-specific CD8 T cells are predominantly CCR7+ in addition to being CD62Lhigh. We were unable to study CCR7 expression in our studies due to the lack of widely available reagents to detect murine CCR7. However other investigators report that in the mouse central memory T cells coexpress CD62L and CCR7 and effector memory cells express neither marker, therefore CD62L alone can be used to discriminate between these populations (33).
It was suggested that the difference in phenotype of lytic and latent Ag-specific CD8 T cells in EBV infection was related to the extent to which they were exposed to Ag during chronic infection. Chronic low-level replication of EBV was believed to stimulate lytic Ag-specific CD8 T cells so that a proportion were driven from CD45R0+ to the CD45RAhigh and/or CD28− phenotype (2). There was believed to be less chronic exposure to latency-associated proteins therefore the latent Ag-specific T cells did not differentiate into the CD45RAhigh phenotype. In the MHV-68 system, our previously published data indicates that the latent M2 gene is not consistently expressed past 21 days postinfection, but there may be sporadic expression thereafter (14). However it is likely that virus reactivation continues intermittently throughout the latent phase of the infection (34). We present data showing T cells recognizing the latent M2 Ag can rapidly kill target cells in vivo, and this is consistent with their expression of CD43, a marker of effector T cells (35). The ability to rapidly display effector function is a hallmark of effector memory T cells, however M2-specific T cells are mostly CD62Lhigh, considered to be a marker of central memory T cells. In contrast, the lytic ORF65-specific T cells did not kill in vivo, despite the fact they are presumably stimulated by low-level Ag release. Therefore we find that, as for EBV infection, lytic and latent Ag-specific T cells cannot be classified easily as either effector or central memory cells. This classification was devised based on immune responses to Ags or infections that are cleared from the host, and it may be that T cell responses to chronic infections do not fit exactly into this model. It appears paradoxical that M2-specific CD8 T cells are more cytolytic than ORF65-specific T cells as the latter would be expected to encounter Ag more often during latent infection. However ORF65 is a late protein, expressed only after viral DNA replication has occurred (29, 30). Therefore it is likely that cells reactivating from latency may be killed by T cells specific for early or immediate early gene products before ORF65 is expressed. Although no epitopes in these early genes have yet been mapped in the BALB/c strain, several epitopes in early proteins are targeted by CD8 T cells in C57BL/6 mice (36). Therefore it is possible that ORF65-specific T cells do not encounter their Ag frequently during latency, however M2 may be sporadically expressed and restimulate M2-specific CD8 T cells, which may explain their more cytotoxic phenotype.
IL-15 and IL-7 are both important cytokines in the development and maintenance of memory CD8 T cells (16–19, 37). It has been suggested that IL-15 is more important in stimulating cell division whereas IL-7 is of more importance in promoting cell survival. However our data, and recent data from other groups, indicates that IL-15 can also act as a survival factor. Berard and colleagues (38) reported that low concentrations of IL-15 supported the survival of both naive and memory CD8 T cells, without inducing proliferation. This is probably due to the ability of IL-15 to induce Bcl-2, a molecule with anti-apoptotic function (39). Although our experiments were performed using concentrations of IL-15 in the range that normally induces cell proliferation, we observed little proliferation but an enhancement of the survival of both specificities of CD8 T cells studied. In contrast, lytic Ag-specific CD8 T cells responded more readily than latent Ag-specific cells to IL-7. Less is known about the effect of IL-7 on memory T cells, however Schluns and colleagues (19) have reported that cells deficient in IL-7Rα had a poor ability to establish memory. IL-7 was also reported to induce a low level of proliferation in both effector memory and central memory CD8 T cells (21). There is also a report that IL-7 can promote the survival of CD8 T cell clones (40). Responsiveness to IL-7 appears to correlate with expression of the IL-7Rα chain, which is high on naive T cells, low on effector cells and high on memory cells (21). We observed that lytic Ag-specific CD8 T cells responded better to IL-7 than did latent Ag-specific CD8 T cells. This may be because the M2-specific CD8 T cells were closer to effector cells, as evidenced by their ability to kill targets rapidly in vivo. In contrast, ORF65-specific CD8 T cells may be closer to classical memory cells and have higher levels of IL-7Rα and therefore higher responsiveness to IL-7. Overall, these data combined with the differential cytotoxic ability of these cells in vivo highlights the fact that lytic and latent Ag-specific T cells are persisting in different states of activation during chronic MHV-68 infection.
In conclusion, we show that in MHV-68 infection lytic and latent Ag-specific T cells are in a different state of activation with respect to cell surface markers, cytotoxicity and responsiveness to IL-7. These results confirm data obtained in EBV infection, and significantly extend them by showing a functional difference between lytic and latent Ag-specific CD8 T cells in vivo. Future studies will address whether these functional differences relate to the ability of latent or lytic Ag-specific CD8 T cells to control latent MHV-68 infection.
Footnotes
This work was supported by National Institutes of Health Grant AI51663-01 and American Cancer Society Institutional Research Grant IRG-82-003-18.
Abbreviations used in this paper: AIM, acute infectious mononucleosis; BAL, bronchoalveolar lavage; ORF, open reading frame; MLN, mediastinal lymph node; CTM, complete tumor medium; 7-AAD, 7-aminoactinomycin D.
References
- 1.Catalina MD, Sullivan JL, Brody RM, Luzuriaga K. Phenotypic and functional heterogeneity of EBV epitope-specific CD8+ T cells. J. Immunol. 2002;168:4184. doi: 10.4049/jimmunol.168.8.4184. [DOI] [PubMed] [Google Scholar]
- 2.Hislop AD, Gudgeon NH, Callan MF, Fazou C, Hasegawa H, Salmon M, Rickinson AB. EBV-specific CD8+ T cell memory: relationships between epitope specificity, cell phenotype, and immediate effector function. J. Immunol. 2001;167:2019. doi: 10.4049/jimmunol.167.4.2019. [DOI] [PubMed] [Google Scholar]
- 3.Callan MF, Steven N, Krausa P, Wilson JD, Moss PA, Gillespie GM, Bell JI, Rickinson AB, McMichael AJ. Large clonal expansions of CD8+ T cells in acute infectious mononucleosis. Nat. Med. 1996;2:906. doi: 10.1038/nm0896-906. [DOI] [PubMed] [Google Scholar]
- 4.Flano E, Husain SM, Sample JT, Woodland DL, Blackman MA. Latent murine gamma-herpesvirus infection is established in activated B cells, dendritic cells, and macrophages. J. Immunol. 2000;165:1074. doi: 10.4049/jimmunol.165.2.1074. [DOI] [PubMed] [Google Scholar]
- 5.Weck KE, Kim SS, Virgin HI, Speck SH. Macrophages are the major reservoir of latent murine gammaherpesvirus 68 in peritoneal cells. J. Virol. 1999;73:3273. doi: 10.1128/jvi.73.4.3273-3283.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stewart JP, Usherwood EJ, Ross A, Dyson H, Nash T. Lung epithelial cells are a major site of murine gammaherpesvirus persistence. J. Exp. Med. 1998;187:1941. doi: 10.1084/jem.187.12.1941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Doherty PC, Christensen JP, Belz GT, Stevenson PG, Sangster MY. Dissecting the host response to a gamma-herpesvirus. Philos. Trans. R. Soc. London B. 2001;356:581. doi: 10.1098/rstb.2000.0786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Virgin HW, Speck SH. Unraveling immunity to gamma-herpesviruses: a new model for understanding the role of immunity in chronic virus infection. Curr. Opin. Immunol. 1999;11:371. doi: 10.1016/s0952-7915(99)80063-6. [DOI] [PubMed] [Google Scholar]
- 9.Sunil-Chandra NP, Efstathiou S, Arno J, Nash AA. Virological and pathological features of mice infected with murine gamma-herpesvirus 68. J. Gen. Virol. 1992;73:2347. doi: 10.1099/0022-1317-73-9-2347. [DOI] [PubMed] [Google Scholar]
- 10.Altman JD, Moss PH, Goulder PR, Barouch DH, McHeyzer-Williams MG, Bell JI, McMichael AJ, Davis MM. Phenotypic analysis of antigen-specific T lymphocytes. Science. 1996;274:94. [PubMed] [Google Scholar]
- 11.Kappler JW, Skidmore B, White J, Marrack P. Antigen-inducible, H-2-restricted, interleukin-2-producing T cell hybridomas: lack of independent antigen and H-2 recognition. J. Exp. Med. 1981;153:1198. doi: 10.1084/jem.153.5.1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Coles RM, Mueller SN, Heath WR, Carbone FR, Brooks AG. Progression of armed CTL from draining lymph node to spleen shortly after localized infection with herpes simplex virus 1. J. Immunol. 2002;168:834. doi: 10.4049/jimmunol.168.2.834. [DOI] [PubMed] [Google Scholar]
- 13.Husain SM, Usherwood EJ, Dyson H, Coleclough C, Coppola MA, Woodland DL, Blackman MA, Stewart JP, Sample JT. Murine gammaherpesvirus M2 gene is latency-associated and its protein a target for CD8+ T lymphocytes. Proc. Natl. Acad. Sci. USA. 1999;96:7508. doi: 10.1073/pnas.96.13.7508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Usherwood EJ, Roy DJ, Ward K, Surman SL, Dutia BM, Blackman MA, Stewart JP, Woodland DL. Control of gamma-herpesvirus latency by latent antigen-specific CD8+ T cells. J. Exp. Med. 2000;192:943. doi: 10.1084/jem.192.7.943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Usherwood EJ. A new approach to epitope confirmation by sampling effector/memory T cells migrating to the lung. J. Immunol. Methods. 2002;266:135. doi: 10.1016/s0022-1759(02)00106-0. [DOI] [PubMed] [Google Scholar]
- 16.Becker TC, Wherry EJ, Boone D, Murali-Krishna K, Antia R, Ma A, Ahmed R. Interleukin 15 is required for proliferative renewal of virus-specific memory CD8 T cells. J. Exp. Med. 2002;195:1541. doi: 10.1084/jem.20020369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Judge AD, Zhang X, Fujii H, Surh CD, Sprent J. Interleukin 15 controls both proliferation and survival of a subset of memory-phenotype CD8+ T cells. J. Exp. Med. 2002;196:935. doi: 10.1084/jem.20020772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schluns KS, Williams K, Ma A, Zheng XX, Lefrancois L. Cutting edge: Requirement for IL-15 in the generation of primary and memory antigen-specific CD8 T cells. J. Immunol. 2002;168:4827. doi: 10.4049/jimmunol.168.10.4827. [DOI] [PubMed] [Google Scholar]
- 19.Schluns KS, Kieper WC, Jameson SC, Lefrancois L. Interleukin-7 mediates the homeostasis of naive and memory CD8 T cells in vivo. Nat. Immun. 2000;1:426. doi: 10.1038/80868. [DOI] [PubMed] [Google Scholar]
- 20.Geginat J, Sallusto F, Lanzavecchia A. Cytokine-driven proliferation and differentiation of human naive, central memory, and effector memory CD4+ T cells. J. Exp. Med. 2001;194:1711. doi: 10.1084/jem.194.12.1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Geginat J, Lanzavecchia A, Sallusto F. Proliferation and differentiation potential of human CD8+ memory T-cell subsets in response to antigen or homeostatic cytokines. Blood. 2003;101:4260. doi: 10.1182/blood-2002-11-3577. [DOI] [PubMed] [Google Scholar]
- 22.Usherwood EJ, Hogg TL, Woodland DL. Enumeration of antigen-presenting cells in mice infected with Sendai virus. J. Immunol. 1999;162:3350. [PubMed] [Google Scholar]
- 23.Liu L, Flano E, Usherwood EJ, Surman S, Blackman MA, Woodland DL. Lytic cycle T cell epitopes are expressed in two distinct phases during MHV-68 infection. J. Immunol. 1999;163:868. [PubMed] [Google Scholar]
- 24.Hogan RJ, Usherwood EJ, Zhong W, Roberts AA, Dutton RW, Harmsen AG, Woodland DL. Activated antigen-specific CD8+ T cells persist in the lungs following recovery from respiratory virus infections. J. Immunol. 2001;166:1813. doi: 10.4049/jimmunol.166.3.1813. [DOI] [PubMed] [Google Scholar]
- 25.Foulds KE, Zenewicz LA, Shedlock DJ, Jiang J, Troy AE, Shen H. Cutting edge: CD4 and CD8 T cells are intrinsically different in their proliferative responses. J. Immunol. 2002;168:1528. doi: 10.4049/jimmunol.168.4.1528. [DOI] [PubMed] [Google Scholar]
- 26.Flano E, Woodland DL, Blackman MA, Doherty PC. Analysis of virus-specific CD4+ T cells during long-term gammaherpesvirus infection. J. Virol. 2001 doi: 10.1128/JVI.75.16.7744-7748.2001. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Christensen JP, Doherty PC. Quantitative analysis of the acute and long-term CD4+ T-cell response to a persistent gammaherpesvirus. J. Virol. 1999;75:7744. doi: 10.1128/jvi.73.5.4279-4283.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Martinez-Guzman D, Rickabaugh T, Wu TT, Brown H, Cole S, Song MJ, Tong L, Sun R. Transcription program of murine gammaherpesvirus 68. J. Virol. 2003;77:10488. doi: 10.1128/JVI.77.19.10488-10503.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ahn JW, Powell KL, Kellam P, Alber DG. Gammaherpesvirus lytic gene expression as characterized by DNA array. J. Virol. 2002;76:6244. doi: 10.1128/JVI.76.12.6244-6256.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ebrahimi B, Dutia BM, Roberts KL, Garcia-Ramirez JJ, Dickinson P, Stewart JP, Ghazal P, Roy DJ, Nash AA. Transcriptome profile of murine gammaherpesvirus-68 lytic infection. J. Gen. Virol. 2003;84:99. doi: 10.1099/vir.0.18639-0. [DOI] [PubMed] [Google Scholar]
- 31.Tripp RA, Hou S, Doherty PC. Temporal loss of the activated L-selectin-low phenotype for virus-specific CD8+ memory T cells. J. Immunol. 1995;154:5870. [PubMed] [Google Scholar]
- 32.Sallusto F, Lenig D, Forster R, Lipp M, Lanzavecchia A. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature. 1999;401:708. doi: 10.1038/44385. [DOI] [PubMed] [Google Scholar]
- 33.Wherry EJ, Teichgraber V, Becker TC, Masopust D, Kaech SM, Antia R, von Andrian UH, Ahmed R. Lineage relationship and protective immunity of memory CD8 T cell subsets. Nat Immun. 2003;4:225. doi: 10.1038/ni889. [DOI] [PubMed] [Google Scholar]
- 34.Flano E, Kim IJ, Moore J, Woodland DL, Blackman MA. Differential gamma-herpesvirus distribution in distinct anatomical locations and cell subsets during persistent infection in mice. J. Immunol. 2003;170:3828. doi: 10.4049/jimmunol.170.7.3828. [DOI] [PubMed] [Google Scholar]
- 35.Harrington LE, Galvan M, Baum LG, Altman JD, Ahmed R. Differentiating between memory and effector CD8 T cells by altered expression of cell surface O-glycans [In Process Citation] J. Exp. Med. 2000;191:1241. doi: 10.1084/jem.191.7.1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stevenson PG, Belz GT, Altman JD, Doherty PC. Changing patterns of dominance in the CD8+ T cell response during acute and persistent murine gamma-herpesvirus infection. Eur. J. Immunol. 1999;29:1059. doi: 10.1002/(SICI)1521-4141(199904)29:04<1059::AID-IMMU1059>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 37.Prlic M, Lefrancois L, Jameson SC. Multiple choices: regulation of memory CD8 T cell generation and homeostasis by interleukin (IL)-7 and IL-15. J. Exp. Med. 2002;195:F49. doi: 10.1084/jem.20020767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Berard M, Brandt K, Paus SB, Tough DF. IL-15 promotes the survival of naive and memory phenotype CD8+ T cells. J. Immunol. 2003;170:5018. doi: 10.4049/jimmunol.170.10.5018. [DOI] [PubMed] [Google Scholar]
- 39.Wu TS, Lee JM, Lai YG, Hsu JC, Tsai CY, Lee YH, Liao NS. Reduced expression of Bcl-2 in CD8+ T cells deficient in the IL-15 receptor α-chain. J. Immunol. 2002;168:705. doi: 10.4049/jimmunol.168.2.705. [DOI] [PubMed] [Google Scholar]
- 40.Tsuda K, Toda M, Kim G, Saitoh K, Yoshimura S, Yoshida T, Taki W, Waga S, Kuribayashi K. Survival-promoting activity of IL-7 on IL-2-dependent cytotoxic T lymphocyte clones: resultant induction of G1 arrest. J. Immunol. Methods. 2000;236:37. doi: 10.1016/s0022-1759(99)00235-5. [DOI] [PubMed] [Google Scholar]