Abstract
Background
Enrolling patients in studies of pancreatic ductal adenocarcinoma (pdac) is challenging because of the high fatality of the disease. We hypothesized that a prospective clinic-based study with rapid ascertainment would result in high participation rates. Using that strategy, we established the Quebec Pancreas Cancer Study (qpcs) to investigate the genetics and causes of pdac and other periampullary tumours (pats) that are also rare and underrepresented in research studies.
Methods
Patients diagnosed with pdac or pat were introduced to the study at their initial clinical encounter, with a strategy to enrol participants within 2 weeks of diagnosis. Patient self-referrals and referrals of unaffected individuals with an increased risk of pdac were also accepted. Family histories, epidemiologic and clinical data, and biospecimens were collected. Additional relatives were enrolled in families at increased genetic risk.
Results
The first 346 completed referrals led to 306 probands being enrolled, including 190 probands affected with pdac, who represent the population focus of the qpcs. Participation rates were 88.4% for all referrals and 89.2% for pdac referrals. Family history, epidemiologic and clinical data, and biospecimens were ascertained from 91.9%, 54.6%, and 97.5% respectively of patients with pdac. Although demographics and trends in risk factors in our patients were consistent with published statistics for patients with pdac, the qpcs is enriched for families with French-Canadian ancestry (37.4%), a population with recurrent germ-line mutations in hereditary diseases.
Conclusions
Using rapid ascertainment, a pdac and pat research registry with high participation rates can be established. The qpcs is a valuable research resource and its enrichment with patients of French-Canadian ancestry provides a unique opportunity for studies of heredity in these diseases.
Keywords: Pancreatic cancer, periampullary tumours, research registry, cancer genetics, translational research
1. INTRODUCTION
Pancreatic ductal adenocarcinoma (pdac) is a lethal malignancy with a 5-year survival rate of only 6%1–3. It represents the 4th leading cause of cancer-related death in Quebec, with an estimated 1290 new cases diagnosed and 1170 deaths in 20141. These dismal statistics are attributable largely to late diagnosis: 80% of patients present with locally advanced or metastatic disease that precludes curative-intent surgical resection4. The therapeutic options currently available for such patients are largely ineffective, and even patients who present with operable disease have poor outcomes because of early distant and local recurrence5.
Despite those tragic statistics, pdac has, compared with other major cancers, been underrepresented in research studies largely because of its rapid progression and fatality—median survival being less than 4 months in the presence of metastatic disease3. As a result, high-quality epidemiologic data and biospecimens for pdac research studies have been lacking. To overcome those challenges, several prospective pdac research registries have been established in Europe and North America6. A resource of that calibre had not been established in Quebec, and the Ontario Pancreas Cancer Study (opcs) was the only reported Canadian pdac research registry7. Given the predominantly French-Canadian population in Quebec, a Quebec research registry would provide a unique resource for studies of heredity in pdac, because founder genetic effects have been reported for a number of hereditary diseases in the French-Canadian population8.
To meet this research resource need in Quebec, we established a prospective clinic-based research registry, the Quebec Pancreas Cancer Study (qpcs). We used a clinic-based approach with a rapid ascertainment strategy because we hypothesized that those methods would facilitate high participation rates. The clinical and epidemiologic variables collected by the qpcs and the opcs overlap, with the goal of fostering research synergies between those two Canadian studies.
Although the primary objective of the qpcs is to establish a Quebec research resource for genetic susceptibility studies of pdac, the data and biospecimens collected will also allow for future epidemiologic, biomarker, and cancer biology studies of pdac. In addition, the qpcs includes cases of other periampullary tumours (pats) that are also rare, underrepresented in research studies, and often difficult to treat. Characterization of such cases could prove helpful in advancing the understanding of pdac and in contributing to more global studies of biliary and gastrointestinal precancerous and cancerous conditions9,10.
Here, we describe the methods used to establish the qpcs and the results obtained after the first 374 referrals, with an emphasis on pdac.
2. METHODS
2.1. Study Participants
2.1.1. Inclusion Criteria
Men and women more than 18 years of age with a diagnosis of pdac or pat were eligible to participate. Patients with disease confirmed by histology or cytology were included, as were patients diagnosed based on clinical and axial imaging when a biopsy was either unavailable or pending. Unaffected individuals with a family history of pdac or a diagnosis of a pdac-associated genetic syndrome (hereditary breast and ovarian cancer syndrome, hereditary pancreatitis, hereditary nonpolyposis colorectal cancer, Peutz–Jeghers syndrome, or familial atypical multiple-mole melanoma)5 were also eligible to participate.
Although pdac is the primary focus of the qpcs, the registry was expanded in October 2012 to include pats, including pancreatic neuroendocrine tumours, solid pseudo-papillary epithelial neoplasms, premalignant lesions of the pancreas (for example, intraductal papillary mucinous neoplasms, mucinous cystic neoplasms), and preinvasive and malignant biliary, ampullary, gastric, and duodenal lesions. We defined “proband” as the first individual in a family to enrol in the qpcs, whether that individual was affected or unaffected. Institutional Research Board approval was obtained for the study.
2.1.2. Referrals and Enrolment
Patients with a diagnosis of pdac or pat were informed of the study by the treating hepatopancreatobiliary surgeon or oncologist at the time of their initial consultation at the McGill University Health Centre (muhc). Patients interested in participating met with the qpcs study coordinator after their initial clinic visit or were contacted within 2 weeks for an in-person or telephone interview. Referrals from cancer genetics or oncology clinics outside of the muhc, patient self-referrals, and referrals of unaffected individuals with a family history or known genetic syndrome associated with the earlier-described conditions were also accepted. Self-referrals occurred primarily through the qpcs Web page (http://www.cancerpancreas.ca). If, after 2 attempts (made twice monthly), a study candidate could not be reached in person or by telephone, and no response ensued during the following 6 months, the participant was classified as a “nonresponder.”
Probands (or their next-of-kin) are contacted annually after enrolment to obtain updated demographics, vital status, medical history, and genetic testing, as well as any changes in family history. Written informed consent from all participants was obtained at the time of enrolment.
2.1.3. Work-Up of Families
With consent from the proband, relatives (both affected and unaffected at-risk blood relatives) were invited to enrol in the qpcs when the family history was consistent with an increased risk of pdac or related conditions. If an affected family member was deceased, consent for the individual was obtained from the appropriate next-of-kin.
Genetic counselling and familial risk assessment were provided to each proband and family after enrolment. Families with a history suggestive of a genetic syndrome were referred to clinical genetics.
2.2. Data and Biospecimen Collection
2.2.1. Family History
At the time of enrolment, study participants were asked to provide a detailed family history either by interview with a genetic counsellor or by completing a family history questionnaire. In either case, a three-generation pedigree capturing both the maternal and paternal branches of the family, with details about ethnicity and family history, was obtained.
2.2.2. Epidemiology
Study participants were asked to complete a personal history questionnaire (phq), which included detailed questions about lifestyle and epidemiologic risk factors. Data collection included medical history, medication use, dietary patterns, physical activity, reproductive history, chemical exposures, and alcohol and tobacco consumption. If a participant was deceased at the time of enrolment, the next-of-kin was asked to complete the phq on the participant’s behalf.
2.2.3. Clinical Data
Participants provided the qpcs with written consent to obtain medical records for confirmation of their diagnosis (by radiologic imaging or pathology report, or both) and for collection of data relating to clinical treatments and outcomes. For participants who presented with a prior diagnosis of a genetic syndrome, clinical genetics records were obtained to confirm mutation status. For deceased family members who had a diagnosis of cancer, consent to obtain medical records was obtained from the appropriate next-of-kin. We also obtained permission to access records collected by the Province of Quebec (Régie de l’assurance maladie du Québec), which allows the qpcs to perform a death clearance analysis.
2.2.4. Biospecimens
At enrolment, probands and relatives were asked to provide blood (blood tubes: BD Biosciences, Mississauga, ON) or saliva samples (saliva collection kit: DNA Genotek, Ottawa, ON), or both, for biobanking. Blood samples were obtained either at a regular clinic appointment, by a research nurse at the time of enrolment, or preoperatively from participants who were undergoing surgical resection at the muhc. Otherwise, (for example, if a participant resided out of town), a blood kit was mailed to the participant so that blood could be drawn at a clinic closer to the participant’s home. Blood samples were then shipped and received within 24–48 hours. Alternatively, participants had the option to provide a saliva specimen, which was returned to the study coordinator at the time of enrolment or by mail. If a deceased participant had received clinical genetic testing, archived dna samples from the testing laboratory were requested for biobanking.
Blood samples were processed within 3 hours (local blood draws) or within 24–48 hours (shipped samples) from the time of collection. Plasma samples (collected only in the case of local draws) were immediately placed on ice and processed within 3 hours of collection. White blood cells were separated using an ammonium chloride red blood cell lysis buffer, and lymphocytes were isolated using a gradient lymphocyte separation medium (Ficoll-Paque: GE Healthcare, Baie d’Urfe, QC; lymphocyte separation medium: Wisent Bioproducts, St-Bruno, QC). The plasma, white blood cell pellets, and lymphocyte pellets were stored in liquid nitrogen. Extracted dna was resuspended in Tris-edta buffer and stored at 4°C.
For participants with a diagnosis of pdac or a related pat, we aimed to obtain samples of the corresponding tissues for biobanking. With patient consent, we requested archived (formalin-fixed, paraffin-embedded) tissue samples from biopsies or resection specimens (or both) from the treating hospitals. We also obtained any available archived non-tumour tissue samples that could be used as surrogate germline tissues for deceased participants enrolled by a next-of-kin.
Consent was obtained from patients undergoing resection at the muhc for collection of fresh-frozen tissue samples for tissue biobanking. Samples were collected immediately after resection. A pathologist first examined each specimen macroscopically, confirmed the diagnosis, and determined if the tumour tissue was sufficient for biobanking without compromising the clinical pathology diagnosis. When possible, paired affected (that is, tumour) and adjacent unaffected (that is, “normal”) tissues were obtained. Depending on availability and diagnosis, tumour samples collected from resection specimens were also used to establish patient-derived xenografts in immune-compromised mice.
2.2.5. Data Storage
Family history and pedigree data were stored and manipulated using a genetic data management system (Progeny Clinical, version 9: Progeny Software LLC, Delray Beach, FL, U.S.A.; http://www.progenygenetics.com/). All study participants were assigned a unique qpcs identification number to ensure anonymity and confidentiality. Biospecimen data were stored separately using the Canadian BioSample Repository software package (http://www.biosample.ca). Each biospecimen was assigned a database biobank repository number unique from the qpcs identification number. The qpcs repository is registered with the Canadian Tumour Repository Network.
3. RESULTS
Between April 1, 2012, and July 31, 2014, the qpcs received 374 patient referrals, of which 230 had pdac (Table i). Most referrals (88.5%) came from the hepatopancreatobiliary surgery and oncology clinics; the remainder came from genetics clinics or were self-referrals. Because qpcs enrolment is ongoing, 28 referrals (7.5%) were pending enrolment at July 31, 2014. Of the completed patient referrals (that is, excluding the pending enrolments), 306 probands (88.4%) had been enrolled, 20 patients (5.8%) were nonresponders, 10 patients (2.9%) had declined to participate, and 10 patients (2.9%) were palliative or deceased at the time of attempted contact, translating into participation rates of 88.4% and 89.2% for all referrals and for pdac referrals respectively.
TABLE I.
Quebec Pancreas Cancer Study referrals and enrolment between April 1, 2012, and July 31, 2014
| Variable |
Patients [n (%)]
|
|
|---|---|---|
| With pdac | Overall | |
| Referrals | 230 | 374 |
| hpb surgery and oncology clinics | 218 (94.8) | 331 (88.5) |
| Genetics clinics | 9 (3.9) | 23 (6.2) |
| Self-referral | 3 (1.3) | 20 (5.4) |
| Pending enrolment | 16 (7.0) | 28 (7.5) |
| Completed referrals | 213 (92.6) | 346 (92.5) |
| Enrolleda | 190 (89.2) | 306 (88.4) |
| At diagnosisb | 132 (69.5) | — |
| >3 Months after diagnosisb | 58 (30.5) | — |
| Family historyb | ||
| Complete | 174 (91.6) | 277 (90.5) |
| Pending | 11 (5.8) | 20 (6.5) |
| Unavailable | 5 (2.6) | 9 (2.9) |
| Personal historyb | ||
| Complete | 107 (56.6) | 172 (56.2) |
| Pending | 30 (15.8) | 48 (15.7) |
| Unavailable | 53 (27.9) | 86 (28.1) |
| Non-respondersa | 8 (3.8) | 20 (5.8) |
| Declineda | 7 (3.3) | 10 (2.9) |
| Palliative or deceaseda | 8 (3.8) | 10 (2.9) |
Percentages calculated on completed referrals.
Percentages calculated on enrolled patients.
pdac = pancreatic ductal adenocarcinoma; hpb = hepatopancreatobiliary.
Among the 306 probands enrolled, 277 provided family history data (90.5%), and 172 (56.2%) completed the phq. In addition to probands enrolled, 56 relatives of probands from 25 families were also enrolled. Considering all subjects enrolled [n = 362 (that is, 306 probands and 56 relatives of probands)], the qpcs now has 198 participants with a pdac diagnosis, 182 (91.9%) of whom have provided family history data, and 108 of whom (54.5%) have also made epidemiologic and clinical data available. Notably, most pdac-affected probands (69.5%) were enrolled within 3 months of their diagnosis.
Table ii summarizes the diagnoses of enrolled probands. Of the 306 probands successfully enrolled, 190 (62.1%) had been diagnosed with pdac; 36 (11.8%) with a related periampullary malignancy (distal or hilar cholangiocarcinoma; ampullary, gallbladder, or duodenal cancer); 3 (1.0%) with gastric cancer; 27 (8.8%) with a premalignant pancreatic lesion (intraductal papillary mucinous neoplasm, mucinous cystic neoplasm); 3 (1.0%) with pseudo-papillary epithelial neoplasms; 11 (3.6%) with pancreatic neuroendocrine tumours; and 10 (3.3%) with benign pancreatic lesions (for example, pancreatitis, microcystic serous adenoma). In one case, the final pathology diagnosis of the resected specimen was metastatic low-grade sarcoma (epithelioid hemangioendothelioma).
TABLE II.
Diagnoses of enrolled probands (n = 306)
| Diagnosis | Probands [n (%)] |
|---|---|
| Pancreatic ductal adenocarcinoma (pdac) | 190 (62.1) |
| Distal cholangiocarcinoma | 12 (3.9) |
| Hilar cholangiocarcinoma | 3 (1.0) |
| Ampullary cancer | 12 (3.9) |
| Gallbladder cancer | 6 (2.0) |
| Duodenal cancer | 3 (1.0) |
| Gastric cancer | 3 (1.0) |
| Multiple synchronous primaries | 2 (0.6) |
| Distal cholangiocarcinoma and gallbladder cancer | 1 (0.3) |
| Pancreatic neuroendocrine tumour and colorectal cancer | 1 (0.3) |
| Epithelioid hemangioendothelioma metastatic to the pancreas | 1 (0.3) |
| Mucinous cystic neoplasm of the pancreas | 3 (1.0) |
| Intraductal papillary mucinous neoplasm of the pancreas | 24 (7.8) |
| Side branch | 15 (4.9) |
| Main branch | 9 (2.9) |
| Pseudopapillary pancreatic tumour | 3 (1.0) |
| Microcystic serous adenoma of the pancreas | 5 (1.6) |
| Pancreatic neuroendocrine tumour | 11 (3.6) |
| Pancreatitis | 5 (1.6) |
| Unaffected, with increased risk of pdac | 23 (7.5) |
| Family history of pancreatic malignancy | 16 (5.2) |
| Genetic syndrome diagnosis | 6 (2.0) |
| Elevated CA19-9 | 1 (0.3) |
Of the 190 probands with a pdac diagnosis, 52 (27.4%) had tumours that were resectable, 58 (30.5%) had locally advanced disease, and 80 (42.1%) had metastatic disease at the time of enrolment. The patients with benign surgical pathologies were enrolled based on premalignant or malignant preoperative clinical diagnoses. The preoperative diagnosis for the case with the low-grade sarcoma was pdac. Multiple synchronous primary tumours were diagnosed in 2 probands. Additionally, 23 probands (7.5%) were unaffected, but were enrolled because of an increased risk of pdac: that is, because of a significant family history, a mutation carrier with a known genetic syndrome (for example, Peutz–Jeghers), or in one case, a chronically elevated serum level (>500 U/mL) of the CA19-9 tumour biomarker that was found incidentally without radiologic evidence of a lesion.
The study has ascertained 668 tissue samples from 333 of the 362 total enrolled subjects. Whole blood (plasma and buffy layer) was collected from 237 subjects (71.2%) and buffy layer alone from 20 subjects (6.0%). A blood sample could not be collected from 69 subjects (20.7%); however, unaffected tissue samples in the form of saliva, dna, or non-tumour tissue specimens (formalin-fixed, paraffin-embedded or fresh-frozen) were obtained. Affected tissue biospecimens (formalin-fixed, paraffin-embedded or fresh-frozen, or both) were collected from 189 subjects (56.8%). Notably, both affected and unaffected tissue specimens were collected from 182 subjects (54.7%).
Considering only the 198 subjects with a diagnosis of pdac (n = 198), the study obtained 401 samples from 193 subjects (97.5%), including unaffected tissue samples from 188 subjects (95.0%) and pdac-affected tissue samples from 118 subjects (59.6%). For 113 pdac-affected subjects (57.1%), the qpcs ascertained both non-tumour (that is, surrogate germline) and pdac biospecimens. Notably, biospecimens have been collected from 56 relatives in 25 families with at least 1 pdac-affected family member enrolled (n = 193), including biospecimens from 2 or more pdac-affected family members (including the proband) in 6 families.
Table iii describes the characteristics of the 198 enrolled subjects with a pdac diagnosis (that is, pdac-affected probands and pdac-affected relatives). Demographic and epidemiologic data were curated using available family history and phq data from the pdac subjects or their next-of-kin. Mean age at diagnosis was 66.1 ± 10.5 years, and the ratio of men to women was 1.4:1. Most subjects with pdac were white (79.2% maternal, 80.1% paternal), with an enrichment of cases (37.4%) having French-Canadian ancestry (at least 1 parental origin, Table iii). In addition, 56.8% of pdac-affected subjects had an ancestry (that is, French-Canadian, Ashkenazi Jewish, Greek, German, Polish, or Latvian) known to harbour recurrent germline (“founder”) mutations in the BRCA1, BRCA2, and PALB2 pdac predisposing genes (Table iii)11–19. Table iii also shows data describing education, environmental exposures, weight loss, and history of type 2 diabetes and pancreatitis for enrolled patients with pdac.
TABLE III.
Demographics and epidemiology of 198 enrolled patients with pancreatic ductal adenocarcinoma (pdac)
| Characteristic | Ptsa (n) | Value |
|---|---|---|
| Mean age (years) | 198 | 66.1±10.5 |
| Sex [n (%)] | 198 | |
| Men | 115 (58.1) | |
| Women | 83 (41.9) | |
| Education status [n (%)] | 109 | |
| High school or less | 48 (44.0) | |
| Some college or university | 13 (11.9) | |
| College or university graduate | 23 (21.1) | |
| Postgraduate degree | 25 (22.9) | |
| Ancestry with founder BRCA1 and BRCA2 mutations [n (%)] | 190 | 108 (56.8) |
| French-Canadian | 71 (37.4) | |
| Ashkenazi Jewish | 21 (11.1) | |
| Greek | 8 (4.2) | |
| German | 5 (2.6) | |
| Polish | 2 (1.1) | |
| Latvian | 1 (0.5) | |
| Weight change | 103 | |
| Loss during the year preceding enrolment [n (%)] | 89 (86.4) | |
| Mean loss during the year preceding enrolment (kg) | 11.8±23.5 | |
| Mean bmi 1 year before enrolment (kg/m2) | 29±5.61 | |
| Mean bmi at enrolment (kg/m2) | 25.6±4.5 | |
| bmi > 25 kg/m2 1 year before enrolment [n (%)] | 77 (74.8) | |
| bmi > 25 kg/m2 at enrolment [n (%)] | 53 (51.5) | |
| Patients with type 2 diabetes | 104 | |
| Diabetes diagnosed before pdac [n (%)] | 30 (28.8) | |
| Mean time of diagnosis before pdac (years) | 8.9±7.9 | |
| Mean bmi 1 year before enrolment (kg/m2) | 30.9±5.3 | |
| Mean bmi at enrolment (kg/m2) | 26.3±4.2 | |
| Patients with pancreatitis | 103 | |
| Pancreatitis diagnosed before pdac [n (%)] | 6 (5.8) | |
| Mean time of diagnosis before pdac (years) | 8±10.0 | |
| Environmental exposures | 107 | |
| Tobacco | ||
| Nonsmoker [n (%)] | 52 (48.6) | |
| Former smoker [n (%)] | 47 (43.9) | |
| Current smoker [n (%)] | 8 (7.5) | |
| Smoking history (mean years) | 24.7±14.6 | |
| Mean cigarettes daily (n) | 19.9±27.5 | |
| Exposed to second-hand smoke ≥1 hour daily for more than ≥10 years [n (%)] | 83 (77.6) | |
| Alcohol | ||
| Consumer of >1 alcoholic beverage weekly [n (%)] | 75 (70.1) | |
| Duration of consumption of >1 beverage weekly (mean years) | 32±15.4 | |
| Mean alcoholic beverages weekly (n) | 10±17.9 |
For whom data are available.
Pts = patients; bmi = body mass index.
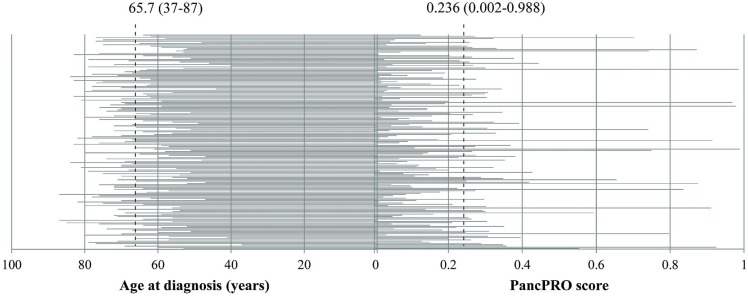
Table iv summarizes the distribution of enrolled families with a high probability of hereditary pdac. In families with an available family history and at least 1 case of pdac or otherwise at risk of pdac (n = 211), tumours in 152 patients (72.0%) were classified as sporadic because the family had no history of pdac and did not meet criteria for a genetic syndrome associated with hereditary pdac. Another 59 patients (28.0%) had an increased likelihood of hereditary pdac either because of multiple pdac diagnoses in the family [that is, familial pdac (fpdac), 18.5%], because of diagnosis of a genetic syndrome whose tumour spectrum includes pdac (3.8%), or because of young age of onset (diagnosed with pdac at 50 years of age or younger, 5.7%). Those observations are consistent with the distribution of pancpro scores for all pdac probands with an available pedigree (n = 175, Figure 1). pancpro is risk prediction tool (courtesy of BayesMendel Lab, Harvard University, Cambridge, MA, U.S.A.) designed to estimate the probability that an individual carries a deleterious mutation in a pdac susceptibility gene20.
FIGURE 1.
Ages at diagnosis (in years) and pancpro scores for all enrolled probands with a diagnosis of pancreatic ductal adenocarcinoma and an available pedigree (n = 175). Dashed lines indicate the mean age and the mean PancPRO score (ranges given in parentheses). pancpro model courtesy of BayesMendel Lab, Harvard University, Cambridge, MA, U.S.A.
TABLE IV.
Predisposition to pancreatic ductal adenocarcinoma (pdac) in 211 enrolled familiesa with available family histories
| Classification | Families [n (%)] |
|---|---|
| Sporadic pdac | 152 (72.0) |
| Non-sporadic pdac | 59 (28.0) |
| Familial pdacb | 39 (18.5) |
| 2 Affected relatives | 34 (16.1) |
| 3 Affected relatives | 2 (0.9) |
| ≥4 Affected relatives | 3 (1.4) |
| Genetic syndrome diagnosis | 8 (3.8) |
| Hereditary breast and ovarian cancer (BRCA1) | 3 (1.4) |
| Hereditary breast and ovarian cancer (BRCA2) | 2 (0.9) |
| Hereditary breast and ovarian cancer–like (PALB2) | 1 (0.5) |
| Familial atypical multiple-mole melanoma (CDKN2A) | 1 (0.5) |
| Peutz-Jeghers syndrome (STK11) | 1 (0.5) |
| Young-onset pdac (≤50 years) | 12 (5.7) |
With 1 or more cases of pdac (n = 209) or otherwise at risk of pdac (n = 2).
Defined as 2 or more pdac-affected relatives in a family, without diagnosis of a known genetic syndrome.
4. DISCUSSION
Research into pdac has been hampered by a paucity of data and biospecimens. Perhaps the most significant challenge in conducting pdac studies is recruitment of cases, because of rapid progression and death after diagnosis. The qpcs was designed as a clinic-based study with the goal of rapid case ascertainment after a pdac or pat diagnosis. Using that approach, we achieved participation rates of 88.4% for all referrals and 89.2% for referrals with a pdac diagnosis. Moreover, just 3.8% of pdac cases were palliative or deceased at the time of attempted contact. Previous population-based pdac registries have reported much lower participation rates of 35%–56%, with a significant proportion of pdac patients being deceased at the time of contact (28%–44%)7,21. In contrast to such low participation rates, the Mayo Clinic’s clinic-based Pancreatic Cancer Genetic Study has reported enrolment rates (approximately 80%) similar to those achieved by the qpcs22. A comparison of study results supports our hypothesis that a clinic-based recruitment approach with an emphasis on rapid ascertainment facilitates high participation rates in pdac studies.
Although the qpcs achieved high participation rates, the study has limitations associated with a clinic-based approach. The low proportion of palliative or deceased pdac cases at attempted contact could reflect a selection bias because our patients were referred primarily from surgery and oncology clinics, likely favouring patients with a higher performance status. The qpcs is also affected by the pattern of referrals to the muhc. In 2003, the Quebec Ministry of Health and Social Services created the Réseau universitaire intégré de santé, assigning a portion of the province’s territory to each of the four provincial medical schools. McGill’s territory includes Nunavik, the Cree Territory, Nord du Québec, Abitibi–Témiscamingue, Outaouais, western Montéregie, and western Montreal. As such, the muhc’s referrals come largely from those regions of Quebec. Despite those probable biases, the demographics of the qpcs case series are consistent with previous reports in North America1,2,23, suggesting that the qpcs is an accurate representation of the patient population with pdac. The inclusion, in addition to pdac, of related pats is a unique research resource. Further, the qpcs results demonstrate that research registries can be successfully integrated into high-volume tertiary care surgery and oncology clinics. Moreover, the enrolment of cases through surgery and oncology clinics provides unique opportunities for studies of disease heredity in the prospectively collected cases, unselected for genetic susceptibility.
A number of lifestyle and environmental risk factors are associated with pdac. Although our cohort is currently small for epidemiologic analyses, we observed trends in risk factors associated with pdac that are consistent with those previously reported5,24. We collected level of education as a surrogate for socioeconomic status, because low socioeconomic status has been associated with higher pdac mortality rates25. Of participants who returned the phq (n = 109), 44.0% reported obtaining a high school diploma or less, and 56.0% indicated having received at least some college or university education. Smoking is the most common risk factor associated with pdac. Consistent with earlier reports5, more than half the phq questionnaire responders (51.4%) reported a history of cigarette smoking. Notably, 78.5% were exposed either directly or indirectly to cigarette smoke in their lifetime (Table iii). Compared with abstention and occasional drinking, heavy alcohol consumption (≥6 drinks daily) has been associated with an increased risk of pdac26. Of our phq responders, 70.1% indicated consumption of more than 1 alcoholic beverage weekly (mean: 10.0 ± 17.9 alcoholic beverages consumed weekly).
Long-standing type 2 diabetes (>10 years) has also been shown to increase risk of pdac by a factor of 1.51, and new-onset diabetes (<3 years) can be an early symptom of the disease27,28. Furthermore, individuals with a history of chronic pancreatitis (>2 years before the pdac diagnosis) have a risk of pdac that is increased by a factor of 2.71, and new-onset pancreatitis could be a symptom of pdac-associated ductal obstruction29. In our study, 30 participants (28.9%) reported a diagnosis of type 2 diabetes before their pdac diagnosis, with a mean of 8.9 ± 7.9 years between that diagnosis and the pdac diagnosis (range: 0–25 years). A diagnosis of pancreatitis was reported by 6 participants (5.8%) at a mean of 8.0 ± 10.0 years before their pdac diagnosis (range: 0–26 years). Although our cohort is small, those observations are consistent with the results of a pooled case–control analysis showing that 6.2% of pdac patients have a history of pancreatitis29. Younger patients (<65 years of age) with a history of pancreatitis could be at increased risk for a genetic predisposition to pdac, including hereditary pancreatitis30.
Several studies have shown that an increased body mass index (bmi) is associated with pdac risk31. In the present study, 74.8% of responders with pdac were overweight (bmi > 25) and 14.5% were obese (bmi > 35) 1 year before enrolment. Interestingly, the average bmi for participants diagnosed with type 2 diabetes was higher than the average bmi for participants without diabetes, both at the time of enrolment (26.3 ± 4.20 vs. 25.6 ± 4.46) and at 1 year before enrolment (30.9 ± 5.29 vs. 29.0 ± 5.61). Because of discrepancies in the time of enrolment relative to the time of pdac diagnosis, the latter observations might be underestimated, given that weight loss is a common symptom of pdac. To account for that possibility, we also determined the mean bmi at enrolment and 1 year before enrolment (24.0 ± 4.66 and 27.2 ± 4.31 respectively) only for responders with pdac who were enrolled within 3 months of diagnosis (n = 64). Because the assessment of lifestyle and environmental exposures was retrospective and self-reported, the possibility of recall bias cannot be excluded.
Research registries for pdac have made important contributions to investigations of the genetic causes of pdac, including the discovery of novel pdac susceptibility genes6,32,33. Estimates suggest that 10% of pdac cases are attributable to Mendelian inheritance34. Although a small fraction of such hereditary cases are explained by germline mutations in known pdac susceptibility genes (for example, BRCA1, BRCA2, PALB2, CDKN2A)34, one of the most important questions in field remains the identification of the genetic causes of fpdac in which known genes are not implicated. The qpcs was designed with the goal of collecting high-quality data and bio-specimens for genetic studies of pdac. Importantly, we aimed to collect epidemiologic and clinical variables similar to those collected by the opcs7, which will allow for research collaboration by the two Canadian studies. In addition, the qpcs design has allowed us to contribute data and biospecimens to the multicentre Pancreatic Cancer Genetic Epidemiology Consortium35, as part of a larger collaborative effort to elucidate the genetics underlying fpdac.
Through our integrated genetic counselling program, the qpcs has obtained detailed pedigrees and family history data from 90.5% of enrolled probands, including 174 probands with pdac (91.6%). High-risk pdac families have been extensively characterized, including acquisition of biospecimens from affected and unaffected blood relatives. Enrolled families are followed prospectively by annual contact to monitor for new diagnoses in the family. As has been the experience of other pdac registries, the qpcs expects that its prospective approach will likely identify new (incident) cases of pdac in family members who were unaffected at the time of enrolment, whether their kindred had multiple or only 1 pdac-affected relative at the time of qpcs enrolment6. Moreover, the ascertainment of both unaffected (that is, surrogate germline) and affected tissues in 113 cases of pdac (57.1% of enrolled cases) is particularly valuable for genetic predisposition studies36. Finally, the concomitant collection of lifestyle and environmental exposure data for the participants will allow for rationalization of non-genetic contributors to pdac causation.
Notably, more than half the enrolled patients in the qpcs affected with pdac (56.8%) had an ancestry (at least 1 parental origin) known to harbour founder BRCA1 and BRCA2 mutations11–19. The founder populations represent a genetically enriched subgroup ideal for gene discovery studies8,37–39. The enrichment of the qpcs with participants of French-Canadian descent is particularly valuable because fpdac is prevalent in that population40. In addition, French-Canadian founder mutations in the BRCA1, BRCA2, and PALB2 genes have already been described11–13, providing the qpcs with a unique opportunity to study the prevalence of those founder mutations among French-Canadian participants with pdac. BRCA1- and BRCA2-associated pdac is of particular interest because those tumours can have beneficial treatment responses to dna cross-linking agents (that is, platinum salts) and parp inhibitors41–46.
The immediate research directions of the qpcs include characterizing the hereditary contribution to pdac. In particular, as a collaborative effort with the opcs, we are using next-generation sequencing to search for novel pdac susceptibility genes in fpdac kindreds. Moreover, we will take advantage of the French-Canadian patient population collected by the qpcs to look for recurrent genetic mutations in known pdac susceptibility genes, because those results could have important implications for clinical genetic screening in that population.
5. CONCLUSIONS
We have demonstrated that a rapid ascertainment protocol in a clinic-based pdac and pat research study can achieve high participation rates of 88.4% for all referrals and 89.2% for pdac referrals. After the opcs7, the qpcs is the second pdac research registry to be reported in Canada, and it provides a valuable resource available to the scientific community at large for studies of pdac and related pats. The registry will also facilitate the identification in Quebec of eligible participants for clinical screening protocols as new imaging technologies and biomarkers emerge.
6. ACKNOWLEDGMENTS
A special thank you goes to the patients and families participating in the qpcs. GZ is a clinical research scholar of the Fonds de recherche du Québec–Santé. ALS is a recipient of the Cedars Cancer Institute Fellowship award and a McGill Integrated Cancer Research Training Program award. Establishment of the qpcs has been funded by the Research Institute of the muhc, the foundations of the Royal Victoria and Montreal General hospitals, and the Department of Oncology of the muhc (to GZ). This work is dedicated to the memory of Rosalind Goodman.
7. CONFLICT OF INTEREST DISCLOSURES
We have read and understood Current Oncology’s policy on disclosing conflicts of interest, and we declare that we have none.
8. REFERENCES
- 1.Canadian Cancer Society’s Advisory Committee on Cancer Statistics . Canadian Cancer Statistics 2014. Toronto, ON: Canadian Cancer Society; 2014. [Google Scholar]
- 2.Howlader N, Noone AM, Krapcho M, et al., editors. SEER Cancer Statistics Review, 1975–2011 [Web resource] Bethesda, MD: U.S. National Cancer Institute; 2013. [Downloadable from: http://seer.cancer.gov/csr/1975_2011; cited November 26, 2014] [Google Scholar]
- 3.Hurton S, MacDonald F, Porter G, Walsh M, Molinari M. The current state of pancreatic cancer in Canada: incidence, mortality, and surgical therapy. Pancreas. 2014;43:879–85. doi: 10.1097/MPA.0000000000000147. [DOI] [PubMed] [Google Scholar]
- 4.Hidalgo M. Pancreatic cancer. N Eng J Med. 2010;362:1605–17. doi: 10.1056/NEJMra0901557. [Erratum in: N Engl J Med 2010;363:298] [DOI] [PubMed] [Google Scholar]
- 5.Wolfgang CL, Herman JM, Laheru DA, et al. Recent progress in pancreatic cancer. CA Cancer J Clin. 2013;63:318–48. doi: 10.3322/caac.21190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Klein AP. Identifying people at a high risk of developing pancreatic cancer. Nat Rev Cancer. 2013;13:66–74. doi: 10.1038/nrc3420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Borgida AE, Ashamalla S, Al-Sukhni W, et al. Management of pancreatic adenocarcinoma in Ontario, Canada: a population-based study using novel case ascertainment. Can J Surg. 2011;54:54–60. doi: 10.1503/cjs.026409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Scriver CR. Human genetics: lessons from Quebec populations. Annu Rev Genom Human Genet. 2001;2:69–101. doi: 10.1146/annurev.genom.2.1.69. [DOI] [PubMed] [Google Scholar]
- 9.Kermanshahi TR, Jayachandran P, Chang DT, Pai R. Lef-1 is frequently expressed in colorectal carcinoma and not in other gastrointestinal tract adenocarcinomas: an immunohistochemical survey of 602 gastrointestinal tract neoplasms. Appl Immunohistochem Mol Morphol. 2014;22:728–34. doi: 10.1097/PAI.0000000000000109. [DOI] [PubMed] [Google Scholar]
- 10.Keane MG, Horsfall L, Rait G, Pereira SP. A case–control study comparing the incidence of early symptoms in pancreatic and biliary tract cancer. BMJ Open. 2014;4:e005720. doi: 10.1136/bmjopen-2014-005720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Simard J, Dumont M, Moisan AM, et al. Evaluation of BRCA1 and BRCA2 mutation prevalence, risk prediction models and a multistep testing approach in French-Canadian families with high risk of breast and ovarian cancer. J Med Genet. 2007;44:107–21. doi: 10.1136/jmg.2006.044388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ghadirian P, Robidoux A, Zhang P, et al. The contribution of founder mutations to early-onset breast cancer in French-Canadian women. Clin Genet. 2009;76:421–6. doi: 10.1111/j.1399-0004.2009.01277.x. [DOI] [PubMed] [Google Scholar]
- 13.Cavallone L, Arcand SL, Maugard CM, et al. Comprehensive BRCA1 and BRCA2 mutation analyses and review of French Canadian families with at least three cases of breast cancer. Fam Cancer. 2010;9:507–17. doi: 10.1007/s10689-010-9372-3. [DOI] [PubMed] [Google Scholar]
- 14.Ferrone CR, Levine DA, Tang LH, et al. BRCA germline mutations in Jewish patients with pancreatic adenocarcinoma. J Clin Oncol. 2009;27:433–8. doi: 10.1200/JCO.2008.18.5546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Armaou S, Pertesi M, Fostira F, et al. Contribution of BRCA1 germ-line mutations to breast cancer in Greece: a hospital-based study of 987 unselected breast cancer cases. Br J Cancer. 2009;101:32–7. doi: 10.1038/sj.bjc.6605115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Janavičius R. Founder BRCA1/2 mutations in the Europe: implications for hereditary breast–ovarian cancer prevention and control. EPMA J. 2010;1:397–412. doi: 10.1007/s13167-010-0037-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Meindl A, on behalf of the German Consortium for Hereditary Breast and Ovarian Cancer Comprehensive analysis of 989 patients with breast or ovarian cancer provides BRCA1 and BRCA2 mutation profiles and frequencies for the German population. Int J Cancer. 2002;97:472–80. doi: 10.1002/ijc.1626. [DOI] [PubMed] [Google Scholar]
- 18.Brozek I, Cybulska C, Ratajska M, et al. Prevalence of the most frequent BRCA1 mutations in Polish population. J Appl Genet. 2011;52:325–30. doi: 10.1007/s13353-011-0040-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tikhomirova L, Sinicka O, Smite D, Eglitis J, Hodgson SV, Stengrevics A. High prevalence of two BRCA1 mutations, 4154delA and 5382insC, in Latvia. Fam Cancer. 2005;4:77–84. doi: 10.1007/s10689-004-2758-3. [DOI] [PubMed] [Google Scholar]
- 20.Wang W, Chen S, Brune KA, Hruban RH, Parmigiani G, Klein AP. pancpro: risk assessment for individuals with a family history of pancreatic cancer. J Clin Oncol. 2007;25:1417–22. doi: 10.1200/JCO.2006.09.2452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nkondjock A, Krewski D, Johnson KC, Ghadirian P, on behalf of the Canadian Cancer Registries Epidemiology Research Group Specific fatty acid intake and the risk of pancreatic cancer in Canada. Br J Cancer. 2005;92:971–7. doi: 10.1038/sj.bjc.6602380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McWilliams RR, Rabe KG, Olswold C, de Andrade M, Petersen GM. Risk of malignancy in first-degree relatives of patients with pancreatic carcinoma. Cancer. 2005;104:388–94. doi: 10.1002/cncr.21166. [DOI] [PubMed] [Google Scholar]
- 23.Hurton S, Macdonald F, Porter G, Walsh M, Molinari M. The current state of pancreatic cancer in Canada: incidence, mortality, and surgical therapy. Pancreas. 2014;43:879–85. doi: 10.1097/MPA.0000000000000147. [DOI] [PubMed] [Google Scholar]
- 24.Olson SH, Kurtz RC. Epidemiology of pancreatic cancer and the role of family history. J Surg Oncol. 2013;107:1–7. doi: 10.1002/jso.23149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cheung MC, Yang R, Byrne MM, Solorzano CC, Nakeeb A, Koniaris LG. Are patients of low socioeconomic status receiving suboptimal management for pancreatic adenocarcinoma? Cancer. 2010;116:723–33. doi: 10.1002/cncr.24758. [DOI] [PubMed] [Google Scholar]
- 26.Lucenteforte E, La Vecchia C, Silverman D, et al. Alcohol consumption and pancreatic cancer: a pooled analysis in the International Pancreatic Cancer Case–Control Consortium (PanC4) Ann Oncol. 2012;23:374–82. doi: 10.1093/annonc/mdr120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Huxley R, Ansary–Moghaddam A, Berrington de González A, Barzi F, Woodward M. Type-ii diabetes and pancreatic cancer: a meta-analysis of 36 studies. Br J Cancer. 2005;92:2076–83. doi: 10.1038/sj.bjc.6602619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chari ST, Leibson CL, Rabe KG, Ransom J, de Andrade M, Petersen GM. Probability of pancreatic cancer following diabetes: a population-based study. Gastroenterology. 2005;129:504–11. doi: 10.1016/j.gastro.2005.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Duell EJ, Lucenteforte E, Olson SH, et al. Pancreatitis and pancreatic cancer risk: a pooled analysis in the International Pancreatic Cancer Case–Control Consortium (PanC4) Ann Oncol. 2012;23:2964–70. doi: 10.1093/annonc/mds140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Masamune A. Genetics of pancreatitis: the 2014 update. Tohoku J Exp Med. 2014;232:69–77. doi: 10.1620/tjem.232.69. [DOI] [PubMed] [Google Scholar]
- 31.Arslan AA, Helzlsouer KJ, Kooperberg C, et al. on behalf of the Pancreatic Cancer Cohort Consortium (PanScan) Anthropometric measures, body mass index, and pancreatic cancer: a pooled analysis from the Pancreatic Cancer Cohort Consortium (PanScan) Arch Intern Med. 2010;170:791–802. doi: 10.1001/archinternmed.2010.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jones S, Hruban RH, Kamiyama M, et al. Exomic sequencing identifies PALB2 as a pancreatic cancer susceptibility gene. Science. 2009;324:217. doi: 10.1126/science.1171202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Roberts NJ, Jiao Y, Yu J, et al. ATM mutations in patients with hereditary pancreatic cancer. Cancer Discov. 2012;2:41–6. doi: 10.1158/2159-8290.CD-11-0194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Becker AE, Hernandez YG, Frucht H, Lucas AL. Pancreatic ductal adenocarcinoma: risk factors, screening, and early detection. World J Gastroenterol. 2014;20:11182–98. doi: 10.3748/wjg.v20.i32.11182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Petersen GM, de Andrade M, Goggins M, et al. Pancreatic cancer genetic epidemiology consortium. Cancer Epidemiol Biomarkers Prev. 2006;15:704–10. doi: 10.1158/1055-9965.EPI-05-0734. [DOI] [PubMed] [Google Scholar]
- 36.Knudson AG. Two genetic hits (more or less) to cancer. Nat Rev Cancer. 2001;1:157–62. doi: 10.1038/35101031. [DOI] [PubMed] [Google Scholar]
- 37.Abbott A. Manhattan versus Reykjavik. Nature. 2000;406:340–2. doi: 10.1038/35019167. [DOI] [PubMed] [Google Scholar]
- 38.Neuhausen SL. Founder populations and their uses for breast cancer genetics. Breast Cancer Res. 2000;2:77–81. doi: 10.1186/bcr36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sokolenko AP, Iyevleva AG, Preobrazhenskaya EV, et al. High prevalence and breast cancer predisposing role of the BLM c.1642 C>T (Q548X) mutation in Russia. Int J Cancer. 2012;130:2867–73. doi: 10.1002/ijc.26342. [DOI] [PubMed] [Google Scholar]
- 40.Ghadirian P, Boyle P, Simard A, Baillargeon J, Maisonneuve P, Perret C. Reported family aggregation of pancreatic cancer within a population-based case–control study in the Francophone community in Montreal, Canada. Int J Pancreatol. 1991;10:183–96. doi: 10.1007/BF02924156. [DOI] [PubMed] [Google Scholar]
- 41.Farmer H, McCabe N, Lord CJ, et al. Targeting the dna repair defect in BRCA mutant cells as a therapeutic strategy. Nature. 2005;434:917–21. doi: 10.1038/nature03445. [DOI] [PubMed] [Google Scholar]
- 42.Fong PC, Boss DS, Yap TA, et al. Inhibition of poly(adp-ribose) polymerase in tumors from BRCA mutation carriers. N Engl J Med. 2009;361:123–34. doi: 10.1056/NEJMoa0900212. [DOI] [PubMed] [Google Scholar]
- 43.Lowery MA, Kelsen DP, Stadler ZK, et al. An emerging entity: pancreatic adenocarcinoma associated with a known BRCA mutation: clinical descriptors, treatment implications, and future directions. Oncologist. 2011;16:1397–402. doi: 10.1634/theoncologist.2011-0185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Imyanitov EN, Moiseyenko VM. Drug therapy for hereditary cancers. Hered Cancer Clin Pract. 2011;9:5. doi: 10.1186/1897-4287-9-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zogopoulos G, Al-Sukhni A, Moore S, et al. Platinum-based chemotherapy in patients with pancreatic cancer and germline BRCA2 mutations [abstract] HPB (Oxford) 2011;13(suppl 1):77. [Google Scholar]
- 46.Tran B, Moore S, Zogopoulos G, et al. Platinum-based chemo-therapy in pancreatic adenocarcinoma (pc) associated with BRCA mutations: a translational case series [abstract 217] J Clin Oncol. 2012;30 doi: 10.1200/JCO.2011.39.2316. [Available online at: http://meetinglibrary.asco.org/content/87854-115; cited February 12, 2015] [DOI] [Google Scholar]