Abstract
Across Canada, introduction of the Pap test for cervical cancer screening, followed by mammography for breast cancer screening and, more recently, the fecal occult blood test for colorectal cancer screening, has contributed to a reduction in cancer mortality. However, another contribution of screening has been disparities in cancer mortality between certain populations. Here, we explore the disparities associated with breast and cervical cancer screening and preliminary data concerning disparities in colorectal cancer screening.
Although some disparities in screening utilization have been successfully reduced over time (for example, mammography and Pap test screening in rural and remote populations), screening utilization data for other populations (for example, low-income groups) clearly indicate that disparities have existed and continue to exist across Canada. Organized screening programs in Canada have been able to successfully engage 80% of women for regular cervical cancer screening and 70% of women for regular mammography screening, but of the women who remain to be reached or engaged in regular screening, those with the least resources, those who are the most isolated, and those who are least culturally integrated into Canadian society as a whole are over-represented. Population differences are also observed for utilization of colorectal cancer screening services.
The research literature on interventions to promote screening utilization provides some evidence about what can be done to increase participation in organized screening by vulnerable populations. Adaption and adoption of evidence-based screening promotion interventions can increase the utilization of available screening services by populations that have experienced the greatest burden of disease with the least access to screening services.
Keywords: Cancer screening, cancer screening disparities
1. INTRODUCTION
Finding cancers in the earliest stages of development allows for a greater probability of cure, a longer period of disease-free survival, and fewer cancer deaths1,2. For a few cancers, screening tests for average-risk populations, when available and implemented in population-based programs for asymptomatic individuals, can in and of themselves lead to reduced cancer mortality3–5. Although organized screening programs are able to deliver services more efficiently and more equitably, it recognized that in most countries, the meaning of “organized screening” varies widely and therefore includes a mix of organized and opportunistic screening6.
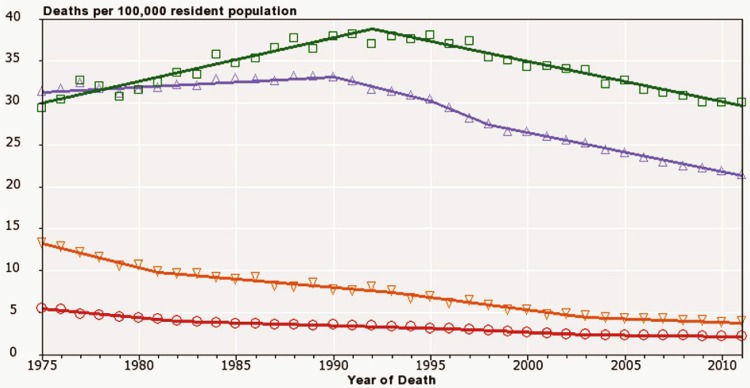
The first of the average-risk screening tests was the Papanicolaou (Pap) test for cervical cancer; it was followed by mammography for breast cancer and, more recently, by fecal occult blood testing or fecal immunochemical testing for colorectal cancer. These screening technologies have been, and continue to be, refined over time, but their introduction across Canada and around the world has contributed to a reduction in cancer mortality7. However, as with many new technologies or tests introduced into the general population, adoption and implementation varies from one group to another, contributing to disparities in cancer mortality for various populations8. For example, in the United States, the gap between African American and overall American breast cancer rates first appeared soon after the introduction of mammography screening in the 1980s, and the gap between African American and overall American cervical cancer mortality rates only recently began to narrow some 30 years after introduction of the Pap test for cervical cancer screening (Figure 1).
FIGURE 1.
Historical trends in U.S. female mortality from cervical and breast cancer, 1975–2011, all ages. Open squares = breast cancer, black and Hispanic; open triangles = breast cancer, all races; open reverse triangles = cervical cancer, black and Hispanic; open circles = all races. Source: Death data provided by the U.S. National Vital Statistics System public-use data file. Death rates calculated by the U.S. National Cancer Institute (nci) using seer*Stat. Rates are age-adjusted to the 2000 U.S. standard population (19 groups: <1. 1–4, 5–9, ... 80–84, ≥85 years). Population counts for denominators are based on census populations as modified by the nci. The populations included with the data release have been adjusted for the population shifts resulting from Hurricanes Katrina and Rita for 62 counties and parishes in Alabama, Mississippi, Louisiana, and Texas. 1969–2012 U.S. Population Data File is used with mortality data.
In Canada, despite near-universal access to health care in all provinces and territories, significant disparities of income, rural or urban residence, and immigration status have been observed across the cancer control continuum for measures ranging from risk factor and screening utilization behaviours through diagnostic and treatment service access and utilization9. Here, we explore those disparities with respect to breast and cervical cancer screening in recent times and with respect to preliminary data about colorectal cancer screening, which has more recently been introduced in Canada as part of population-based organized screening programs.
2. METHODS
Canadian utilization rates for screening were calculated using data from the Canadian Community Health Survey, a cross-sectional survey of the non-institutionalized Canadian population 12 years of age or older in all provinces and territories, excepting members of the regular Canadian Forces and residents of First Nations reserves, Canadian Forces bases, and some remote areas. The estimates presented are adjusted using sampling weights to represent the overall population. For cervical and breast cancer screening, data from 2003, 2005, 2008, and 2012 were available for all provinces and territories; for colorectal cancer screening, full data were available for 2008 and 2012.
Cervical cancer screening estimates included women 21–69 years of age who had not had a hysterectomy and who had received at least 1 Pap test within the preceding 3 years. Breast cancer screening estimates included women 50–69 years of age screened with mammography in the preceding 2 years for asymptomatic reasons (not to investigate previously detected lumps, breast problems, or as follow-up to breast cancer treatment or other specified reasons). Colorectal screening estimates included men and women who completed a fecal test in the preceding 2 years for asymptomatic reasons (that is, not to investigate symptoms).
To investigate screening disparities, we examined three stratification variables: household income quintile, geography, and immigrant status. Income quintiles were available only for 2005 and excluded the territories. Geography was divided into four categories for the analyses: urban (most densely populated), rural, rural remote, and rural very remote (least densely populated). The three rural categories were based on the census concept of metropolitan-influenced zones, measured by the proportion of the workforce that commutes to the urban centre for work (http://www12.statcan.ca/census-recensement/2011/ref/dict/geo010-eng.cfm). Immigrant status was examined using three categories: Canadian-born and length of time in Canada since immigration being either less than 10 years or 10 years or more.
Statistical analyses were conducted using the SAS software application (version 9.3: SAS Institute, Cary, NC, U.S.A.) and the Joinpoint regression program (version 4.1.1: Statistical Methodology and Applications Branch, U.S. National Cancer Institute, Bethesda, MD, U.S.A.) for Windows. Proportions were compared using 2-sided hypothesis testing for difference in proportions, assuming normality. Joinpoint was used to analyze linear trends within and between stratification groups.
To explore the extent to which intervention research evidence about cancer screening promotion could inform efforts to address disparities, the applicable research literature (focusing on breast, cervical, and colorectal cancer screening utilization) in relation to low income, rural and remote residence, and immigrant populations was reviewed. The research literature relating to prostate cancer screening interventions was not considered here, because there are no current recommendations in Canada or the United States to implement population-based prostate cancer screening. Structured searches of Ovid medline (1946 to October 2014), cinahl, psycinfo, the Cochrane Database of Systematic Reviews, and the NHS Economic Evaluation Database at the Cochrane Library were conducted in October 2014. A combination of key words and free-text terms related to cancer screening and disparities were used. Articles were limited to evaluations of intervention studies published in the English language from 1990 to the date of the search. All titles and abstracts were initially screened for inclusion by one reviewer; an in-depth screening of full-text articles followed. Extraction of data from included articles was guided by a template developed for the review. No quality assessment of the included articles was performed.
3. RESULTS
3.1. Income Disparities
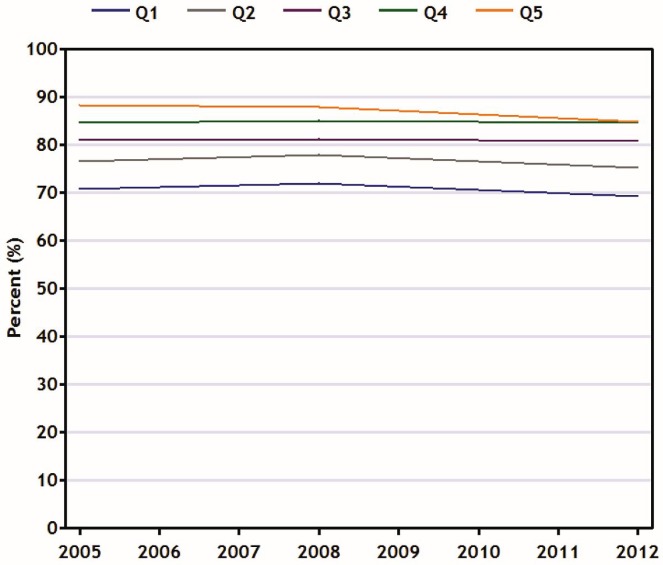
Figure 2 shows that, in recent years and across all income quintiles, the percentage of age-eligible women reporting a Pap test in the preceding 3 years has changed little. The percentage in the highest income quintile (Q5) declined slightly but nonsignificantly over time; the percentages in the other income quintiles remained relatively stable. As a result, the prevalence rate of Pap test utilization has remained between 16% and 18% lower among women in the lowest income quintile than among women in the highest income quintile (test for difference in trend between Q5 and Q1: p = 0.02).
FIGURE 2.
Percentage of women in Canada (21–69 years of age) reporting having had at least 1 Pap test in the preceding 3 years, by household income quintile, 2005–2012. Data source: Canadian Community Health Survey.
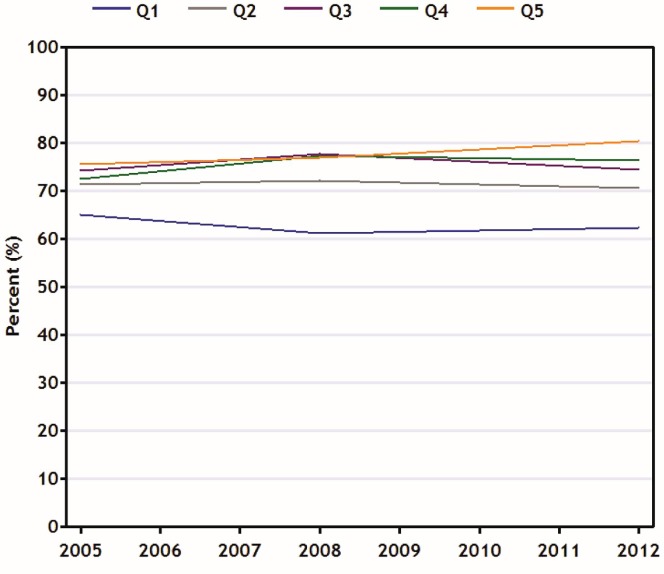
Figure 3 illustrates differences in mammography screening among age-eligible women by income quintile, although the pattern is somewhat different. Mammography screening rates among the Q5 women rose over time to 80% in 2012 from 75% in 2005, while rates for women from the lowest income quintiles dropped slightly to 62% from 65% (statistically nonsignificant). The evidence therefore suggests that the disparity in the prevalence of mammography utilization for Q1 women compared with Q5 women has grown to 18% from 11% (p = 0.06).
FIGURE 3.
Percentage of eligiblea women in Canada (50–69 years of age) reporting having undergone screening mammography in the preceding 2 years, by household income quintile, 2005–2012.
a Those not having a previous lump, not being followed after breast cancer treatment, and not undergoing mammography because of a breast problem or other specified reason. Data source: Canadian Community Health Survey.
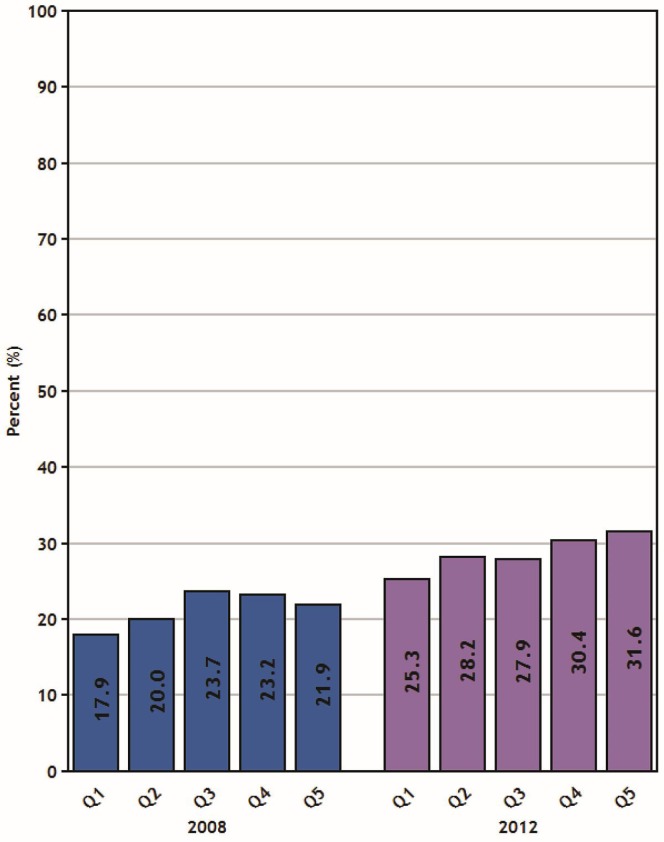
For colorectal cancer screening, a review of Canadian Community Health Survey data from the years 2008 and 2012 showed significant increases in biannual fecal test utilization rates for all income quintiles (all p < 0.01). Significantly different rates of fecal test screening were observed when comparing all Q1 participants (men and women combined: 18% in 2008 and 25% in 2012) with participants from the two highest income quintiles in both 2008 (Q4: 23%; Q5: 22%; p = 0.01) and 2012 (Q4: 30%; Q5: 32%; p < 0.001). The gap between Q1 and Q5 widened to 6% from 4% during that time, but was not statistically significantly different (Figure 4).
FIGURE 4.
Percentage of the population in Canada (50–74 years of age) reporting a screening fecal testa in the preceding 2 years, by household income quintile, 2008 and 2012 reporting years.
a Excludes tests performed to investigate symptoms. Data source: Canadian Community Health Survey.
3.2. Geographic Disparities
Turning to variations in screening rates by geographic residence, the prevalence of breast and cervical cancer screening was not significantly different for the various populations—a trend that persisted over time. However, for colorectal cancer screening, statistically significant differences were observed in 2008 between urban and rural remote populations (22% and 18% respectively, p < 0.001). Differences were not observed in 2008 between urban and rural (19%) or rural very remote (20%), or in 2012 between rural (22%) and rural remote (31%), rural and rural very remote (31%), and rural and urban (29%): p < 0.001 in each case. Table i shows absolute percentages for the geographic and immigrant population disparities.
TABLE I.
Percentage of individuals screened for cervical, breast, or colorectal cancer, by geography and immigrant status (Data source: Statistics Canada, Canadian Community Health Survey)
| Cancer screening site | Year | Geography (%) | Immigrant status (%) | |||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||
| Urban | Rural | Rural remote | Rural very remote | In Canada for | Canadian-born | |||
|
| ||||||||
| <10 Years | ≥10 Years | |||||||
| Cervical (ages: 21–69 years) | 2003 | 80.7 | 80.9 | 80.8 | 80.4 | 61.0 | 76.0 | 83.3 |
| 2005 | 79.1 | 82.4 | 78.0 | 79.2 | 59.6 | 75.7 | 81.2 | |
| 2008 | 79.5 | 77.5 | 78.0 | 79.8 | 58.4 | 74.0 | 82.3 | |
| 2012 | 78.8 | 79.1 | 78.9 | 78.9 | 65.0 | 71.7 | 81.8 | |
| Breast (ages: 50–69 years) | 2003 | 72.3 | 71.7 | 71.0 | 71.0 | 51.9 | 70.9 | 72.8 |
| 2005 | 71.7 | 74.3 | 70.0 | 70.8 | 58.5 | 72.0 | 72.0 | |
| 2008 | 72.8 | 67.8 | 71.2 | 71.1 | 40.6a | 70.0 | 74.2 | |
| 2012 | 73.1 | 76.9 | 69.7 | 69.9 | 71.5 | 71.1 | 73.4 | |
| Colorectal (ages: 50–74 years) | 2008 | 21.6 | 18.9 | 17.5 | 20.1 | —b | 24.5 | 20.2 |
| 2012 | 28.9 | 21.8 | 30.8 | 30.6 | 23.1a | 32.6 | 28.0 | |
Interpret with caution because of large variability in the estimate.
Suppressed because of statistical unreliability caused by small numbers.
3.3. Immigrant Disparities
From 2003 to 2012, immigrant women residing in Canada for less than 10 years had a consistently lower Pap test prevalence than either immigrant women residing in Canada for 10 years or more or Canadian-born women; however, because of small numbers, those differences were not statistically significant. The differences declined from 2003 to 2012 (to 7% from 15% and to 17% from 22% respectively), but those trends were not statistically significant.
In earlier years, similar patterns were observed for screening mammography, with a lower screening prevalence among new immigrant women; however, the differences were not always statistically significant. By 2012, no differences between the three groups were observed: new immigrant women were reporting a prevalence of mammography utilization (72%) similar to that in the other two groups (71% for immigrant women ≥10 years and 73% for Canadian-born women).
In 2008, new immigrant respondents to the Canadian Community Health Survey were insufficient in number to reliably estimate utilization of colorectal cancer screening. However, in 2012, the sample of newer immigrants had grown sufficiently to estimate a fecal test utilization rate of 23%, which was not significantly different from that for immigrant respondents who had been in Canada for 10 or more years (33%) or for Canadian-born respondents (28%).
4. EVIDENCE FROM RESEARCH INTO SCREENING PROMOTION INTERVENTIONS
As noted in the Methods section, publications specifically focused on disparities in cancer screening utilization by low-income, rural and remote, and immigrant populations were reviewed10–41. Table ii summarizes, by cancer screening and vulnerable population type, the number of screening promotion intervention studies found. Most of the literature reports studies conducted in the United States, and yet only limited organized population-based screening is conducted through the U.S. Centers for Disease Control and Prevention (cdc). However, that agency’s 2015 budget42 included an increase to support a new cancer demonstration project to facilitate the transition from existing screening programs to a more population-based model. The cdc will develop and implement innovative strategies to increase population-level screening rates for recommended breast, cervical, and colorectal cancer screenings. Thus, the screening promotion interventions being evaluated in the United States could have relevance in Canada for engaging underserved, vulnerable, and “hard-to-reach” populations in organized screening programs.
TABLE II.
Intervention studies promoting cancer screening for underserved populations
| Cancer screening site | Underserved population type | ||
|---|---|---|---|
|
| |||
| Income disparity | Geographic disparity | Immigrant disparity | |
| Cervical | 0 | 1 | 3 |
| Breast | 13 | 3 | 3 |
| Colorectal | 2 | 4 | 3 |
| TOTAL | 15 | 8 | 9 |
Looking at the number of studies found in the review, it is clear that relatively little research to evaluate approaches that specifically enhance screening uptake in such populations has been completed. As such, it is difficult to summarize effective strategies for particular vulnerable populations. Consistent with the literature on implementation, interventions that use a number of different strategies seem to be most effective, and almost all the studies reviewed for the present work used multiple intervention approaches. Some recommendations proposed for enhancing screening in low-income populations included the development of additional educational materials aimed at individuals with a low literacy level13; the use of patient activation, including empowering patients to ask their health care providers about screening41; and the promise of patient navigators18,36 or lay health advisors20 as approaches to reduce disparities.
For rural or remote populations, strategies to overcome challenges such as distance to screening clinics, not enough family practitioners or specialists (for example, endoscopists)38, and the cost of disseminating information have included telehealth approaches (for example, teleradiology)40, itinerant screening clinics39, and the tailoring of national campaigns to rural audiences37. For immigrant populations, approaches such as use of lay health workers and culturally focused media30; cultural awareness training for health care providers, combined with outreach workers who can help to overcome language and cultural barriers26; patient navigators34; and multilingual health educators who can explain material and assist with barriers29,35 have shown promise.
The Guide to Community Preventive Services, a program also supported by the cdc, conducts systematic reviews of intervention efficacy studies for many disease prevention and health promotion interventions, including those for breast, cervical, and colorectal cancer screening. However, their systematic reviews of cancer screening have not focused on interventions targeted to reducing disparities for particular vulnerable populations, in large part because studies for any particular population are insufficient in number to permit a robust review.
For overall screening approaches, the cdc Guide has found evidence for and recommended a number of screening promotion intervention approaches43:
Sending client reminders (such as letters, postcards, or e-mail or telephone messages) to patients to increase screening rates for all three cancers
Developing small-media videos and print materials such as letters, brochures, and newsletters that can educate and motivate people to ask for screening for all three cancers (These materials can be distributed in community settings or health care systems and do not have to be tailored to the recipients.)
Holding individual education sessions that can help people to overcome barriers to screening for all three cancers (Health care professionals, lay health advisors, or volunteers can conduct sessions by telephone or in person in a variety of settings. One-on-one education is often supported by small media such as brochures or client reminders.)
Providing group education, which can increase mammography screening for breast cancer (In group education, a health professional or trained layperson leads a lecture, presentation, or other interactive session in a church, home, seniors centre, or other setting.)
Removal of other barriers to screening, which is supported by strong evidence in breast and colorectal cancer screening (Examples include keeping flexible clinic hours, working in nonclinical settings such as mobile mammography vans, and offering on-site translation, transportation, patient navigators, and other administrative services.)
Recruiting health care providers to encourage patients to be screened for cancer (Assessing how many patients receive screening services and providing feedback on performance can boost screening rates for all three cancers.)
An online resource for finding interventions tailored to particular vulnerable populations is the Research-tested Intervention Programs Web site (http://rtips.cancer.gov/rtips/index.do), where, in addition to program descriptions and evaluation research data, the screening promotion materials from many of the programs can be obtained directly or ordered on compact disc. The Research-tested Intervention Programs have been linked directly to the Guide to Community Preventive Service recommendations and vice versa, and efforts are being made in Canada to encourage cancer prevention and control intervention researchers to submit their intervention program materials and evaluation findings to the U.S. site for review so that they can be added to the repository.
5. CONCLUSIONS
Although some screening utilization disparities (for example, for mammography and Pap test screening in rural and remote populations) have been successfully reduced over time, screening utilization data for other populations (low-income populations, for instance) clearly indicate that disparities have existed and continue to exist across Canada. Population-based screening programs in Canada have been able to successfully engage 80% of women for regular cervical cancer screening and 70% for regular mammography screening. However, the women that remain to be reached or engaged in regular screening are overrep-resented among those that have the least resources, are the most isolated, and are the least culturally integrated into Canadian society as a whole. Correspondingly, they experience cancer that is more advanced in stage at presentation and poorer outcomes. Differences in the utilization of colorectal cancer screening services, which more recently became available in Canada, were also observed and thus might be expected to reflect larger disparities than the disparities seen for screening services that have had more time for infrastructure and human resource development and implementation.
In the United States, based on its 2010 National Health Interview Survey, the breast cancer screening rate was 72%, below the Healthy People 2020 target of 81%. The cervical cancer screening rate was 83%, below the target of 93%. And the colorectal cancer screening rate was 59%, below the target of 71%. Screening rates are lower in Asian Americans (64% for breast cancer, 75% for cervical cancer, and 47% for colorectal cancer) than in other groups, and rates in the Asian subgroups (Chinese, Filipino, and other Asian) varied. Hispanic Americans were less likely to be screened than non-Hispanic Americans (70% for breast cancer, 79% for cervical cancer, and 47% for colorectal cancer), and rates among the Hispanic subgroups (Puerto Rican, Mexican, Mexican American, Central or South American, and other Hispanic) also varied44.
In Canada, the ethnic groups that have historically experienced the most limited access to, and utilization of, cancer screening services have been the First Nations, Inuit, and Métis peoples. No longitudinal databases are available for an examination of historical trends in cancer screening utilization among Canada’s first peoples, and thus their cancer screening experiences could not be included here. The Canadian Partnership Against Cancer is working with those communities, investing in the development of collaborative strategies to improve their experiences and outcomes across the cancer control continuum.
Although the research into screening promotion interventions provides some evidence about what can be done to increase participation in population-based screening by vulnerable populations, a one-size-fits-all approach appears unlikely to be effective. Rather, each province and territory in Canada can carefully examine the demographic and geographic characteristics of its “hard-to-reach” populations eligible for breast, cervical, and colorectal cancer screening, review the intervention approaches that have been developed and tested for similar populations, and adapt and adopt evidence-based screening promotion interventions to integrate into their organized cancer screening programs. When delivered in an organized program, these population-based cancer screening services remain the most effective way to reduce the burden of cancer. However, additional targeted approaches are needed to increase the utilization of available screening services among populations that historically have not equitably benefited from those resources. The Canadian Partnership Against Cancer’s support for and regular convening of Canadian breast, cervical, colorectal—and, most recently, lung—cancer screening networks, engaging all 10 provinces and 3 territories, provides numerous opportunities to exchange targeted intervention approaches to cancer screening promotion and improve the integration of lessons learned from science with the lessons from practice and policy across Canada.
6. ACKNOWLEDGMENTS
The authors express their sincere thanks to the members of breast, cervical, and colorectal cancer screening networks who took the time to review the manuscript and provide valuable edits and comments.
7. CONFLICT OF INTEREST DISCLOSURES
We have read and understood Current Oncology’s policy on disclosing conflicts of interest, and we declare that we have none.
8. REFERENCES
- 1.Hung MC, Liu MT, Cheng YM, Wang JD. Estimation of savings of life-years and cost from early detection of cervical cancer: a follow-up study using nationwide databases for the period 2002–2009. BMC Cancer. 2014;14:505. doi: 10.1186/1471-2407-14-505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tria Tirona M. Breast cancer screening update. Am Fam Physician. 2013;87:274–8. [PubMed] [Google Scholar]
- 3.U.S. Preventive Services Task Force (uspstf). Home > Recommendations for Primary Care Practice > Published Recommendations > Recommendation Summary > Cervical Cancer: Screening [Web page] Rockville, MD: USPSTF; 2003. [Available at: http://www.uspreventiveservicestaskforce.org/Page/Topic/recommendation-summary/cervical-cancer-screening; cited March 16, 2015] [Google Scholar]
- 4.U.S. Preventive Services Task Force (uspstf). Home > Recommendations for Primary Care Practice > Published Recommendations > Recommendation Summary > Colorectal Cancer: Screening [Web page] Rockville, MD: USPSTF; 2003. [Available at: http://www.uspreventiveservicestaskforce.org/uspstf/uspscolo.htm; cited March 16, 2015] [Google Scholar]
- 5.U.S. Preventive Services Task Force (uspstf). Home > Recommendations for Primary Care Practice > Published Recommendations > Recommendation Summary > Breast Cancer: Screening [Web page] Rockville, MD: USPSTF; 2003. [Available at: http://www.uspreventiveservicestaskforce.org/Page/Topic/recommendation-summary/breast-cancer-screening; cited March 16, 2015] [Google Scholar]
- 6.Williams JH, Carter SM, Rychetnik L. “Organised” cervical screening 45 years on: how consistent are organised screening practices? Eur J Cancer. 2014;50:3029–38. doi: 10.1016/j.ejca.2014.09.005. [DOI] [PubMed] [Google Scholar]
- 7.Elwood JM, Sutcliffe SB. Cancer Control. Oxford, UK: Oxford University Press; 2010. p. xiv.p. 469. [Google Scholar]
- 8.Kerner JF. Integrating research, practice, and policy: what we see depends on where we stand. J Public Health Manag Pract. 2008;14:193–8. doi: 10.1097/01.PHH.0000311899.11197.db. [DOI] [PubMed] [Google Scholar]
- 9.Canadian Partnership Against Cancer (cpac) Examining Disparities in Cancer Control: A System Performance Special Focus Report. Toronto, ON: CPAC; 2014. [Google Scholar]
- 10.Ahmed NU, Haber G, Semenya KA, Hargreaves MK. Randomized controlled trial of mammography intervention in insured very low-income women. Cancer Epidemiol Biomarkers Prev. 2010;19:1790–8. doi: 10.1158/1055-9965.EPI-10-0141. [DOI] [PubMed] [Google Scholar]
- 11.Ansell D, Lacey L, Whitman S, Chen E, Phillips C. A nurse-delivered intervention to reduce barriers to breast and cervical cancer screening in Chicago inner city clinics. Public Health Rep. 1994;109:104–11. [PMC free article] [PubMed] [Google Scholar]
- 12.Crane LA, Leakey TA, Ehrsam G, Rimer BK, Warnecke RB. Effectiveness and cost-effectiveness of multiple outcalls to promote mammography among low-income women. Cancer Epidemiol Biomarkers Prev. 2000;9:923–31. [PubMed] [Google Scholar]
- 13.Coleman E, Lord J, Heard J, et al. The Delta project: increasing breast cancer screening among rural minority and older women by targeting rural healthcare providers. Oncol Nurs Forum. 2003;30:669–77. doi: 10.1188/03.ONF.669-677. [DOI] [PubMed] [Google Scholar]
- 14.Dietrich AJ, Tobin JN, Cassells A, et al. Telephone care management to improve cancer screening among low-income women: a randomized, controlled trial. Ann Intern Med. 2006;144:563–71. doi: 10.7326/0003-4819-144-8-200604180-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kreuter MW, Holmes K, Alcaraz K, et al. Comparing narrative and informational videos to increase mammography in low-income African American women. Patient Educ Couns. 2010;81(suppl):S6–14. doi: 10.1016/j.pec.2010.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Deavenport A, Modeste N, Marshak HH, Neish C. Closing the gap in mammogram screening: an experimental intervention among low-income Hispanic women in community health clinics. Health Educ Behav. 2011;38:452–61. doi: 10.1177/1090198110375037. [DOI] [PubMed] [Google Scholar]
- 17.Hendren S, Winters P, Humiston S, et al. Randomized, controlled trial of a multimodal intervention to improve cancer screening rates in a safety-net primary care practice. J Gen Intern Med. 2014;29:41–9. doi: 10.1007/s11606-013-2506-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Phillips CE, Rothstein JD, Beaver K, Sherman BJ, Freund KM, Battaglia TA. Patient navigation to increase mammography screening among inner city women. J Gen Intern Med. 2011;26:123–9. doi: 10.1007/s11606-010-1527-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Paskett ED, Tatum CM, D’Agostino R, Jr, et al. Community-based interventions to improve breast and cervical cancer screening: results of the Forsyth County Cancer Screening (focas) Project. Cancer Epidemiol Biomarkers Prev. 1999;8:453–9. [PubMed] [Google Scholar]
- 20.Paskett E, Tatum C, Rushing J, et al. Randomized trial of an intervention to improve mammography utilization among a triracial rural population of women. J Natl Cancer Inst. 2006;98:1226–37. doi: 10.1093/jnci/djj333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Slater JS, Henly GA, Ha CN, et al. Effect of direct mail as a population-based strategy to increase mammography use among low-income underinsured women ages 40 to 64 years. Cancer Epidemiol Biomarkers Prev. 2005;14:2346–52. doi: 10.1158/1055-9965.EPI-05-0034. [DOI] [PubMed] [Google Scholar]
- 22.Slater JS, Nim Ha C, Malone ME, et al. A randomized community trial to increase mammography utilization among low-income women living in public housing. Prev Med. 1998;27:862–70. doi: 10.1006/pmed.1998.0370. [DOI] [PubMed] [Google Scholar]
- 23.Andersen MR, Yasui Y, Meischke H, Kuniyuki A, Etzioni R, Urban N. The effectiveness of mammography promotion by volunteers in rural communities. Am J Prev Med. 2000;18:199–207. doi: 10.1016/S0749-3797(99)00161-0. [DOI] [PubMed] [Google Scholar]
- 24.Sparks BT, Ragheb NE, Given BA, Swanson GM. Breast cancer screening in rural populations: a pilot study. J Rural Health. 1996;12:120–9. doi: 10.1111/j.1748-0361.1996.tb00783.x. [DOI] [PubMed] [Google Scholar]
- 25.West DS, Greene P, Pulley L, et al. Stepped-care, community clinic interventions to promote mammography use among low-income rural African American women. Health Educ Behav. 2004;31(suppl):29S–44S. doi: 10.1177/1090198104266033. [DOI] [PubMed] [Google Scholar]
- 26.Lu M, Moritz S, Lorenzetti D, Sykes L, Straus S, Quan H. A systematic review of interventions to increase breast and cervical cancer screening uptake among Asian women. BMC Public Health. 2012;12:413. doi: 10.1186/1471-2458-12-413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pruthi S, Shmidt E, Sherman MM, Neal L, Wahner–Roedler D. Promoting a breast cancer screening clinic for underserved women: a community collaboration. Ethn Dis. 2010;20:463–6. [PubMed] [Google Scholar]
- 28.Wang J, Liang W, Schwartz MD, Lee MM, Kreling B, Mandelblatt JS. Development and evaluation of a culturally tailored educational video: changing breast cancer-related behaviors in Chinese women. Health Educ Behav. 2008;35:806–20. doi: 10.1177/1090198106296768. [DOI] [PubMed] [Google Scholar]
- 29.Jackson J, Do H, Chitnarong K, et al. Development of cervical cancer control interventions for Chinese immigrants. J Immigr Health. 2002;4:147–57. doi: 10.1023/A:1015650901458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mock J, McPhee SJ, Nguyen T, et al. Effective lay health worker outreach and media-based education for promoting cervical cancer screening among Vietnamese American women. Am J Public Health. 2007;97:1693–700. doi: 10.2105/AJPH.2006.086470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Taylor VM, Carey Jackson JC, Yasui Y, et al. Evaluation of an outreach intervention to promote cervical cancer screening among Cambodian American women. Cancer Detect Prev. 2002;26:320–7. doi: 10.1016/S0361-090X(02)00055-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zehbe I, Moeller H, Severini A, et al. Feasibility of self-sampling and human papillomavirus testing for cervical cancer screening in First Nation women from Northwest Ontario, Canada: a pilot study. BMJ Open. 2011;1:e000030. doi: 10.1136/bmjopen-2010-000030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nguyen TT, Love MB, Liang C, et al. A pilot study of lay health worker outreach and colorectal cancer screening among Chinese Americans. J Cancer Educ. 2010;25:405–12. doi: 10.1007/s13187-010-0064-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Percac–Lima S, Grant RW, Green AR, et al. A culturally tailored navigator program for colorectal cancer screening in a community health center: a randomized, controlled trial. J Gen Intern Med. 2009;24:211–17. doi: 10.1007/s11606-008-0864-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tu SP, Taylor V, Yasui Y, et al. Promoting culturally appropriate colorectal cancer screening through a health educator: a randomized controlled trial. Cancer. 2006;107:959–66. doi: 10.1002/cncr.22091. [DOI] [PubMed] [Google Scholar]
- 36.Lasser KE, Murillo J, Medlin E, et al. A multilevel intervention to promote colorectal cancer screening among community health center patients: results of a pilot study. BMC Fam Pract. 2009;10:37. doi: 10.1186/1471-2296-10-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Campo S, Askelson NM, Routsong T, Graaf LJ, Losch M, Smith H. The green acres effect: the need for a new colorectal cancer screening campaign tailored to rural audiences. Health Educ Behav. 2008;35:749–62. doi: 10.1177/1090198108320358. [DOI] [PubMed] [Google Scholar]
- 38.Cotterill M, Gasparelli R, Kirby E. Colorectal cancer detection in a rural community. Development of a colonoscopy screening program. Can Fam Physician. 2005;51:1224–8. [PMC free article] [PubMed] [Google Scholar]
- 39.Redwood D, Provost E, Perdue D, Haverkamp D, Espey D. The last frontier: innovative efforts to reduce colorectal cancer disparities among the remote Alaska Native population. Gastrointest Endosc. 2012;75:474–80. doi: 10.1016/j.gie.2011.12.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lefere P, Silva C, Gryspeerdt S, et al. Teleradiology based ct colonography to screen a population group of a remote island; at average risk for colorectal cancer. Eur J Radiol. 2013;82:e262–7. doi: 10.1016/j.ejrad.2013.02.010. [DOI] [PubMed] [Google Scholar]
- 41.Katz ML, Fisher JL, Fleming K, Paskett ED. Patient activation increases colorectal cancer screening rates: a randomized trial among low-income minority patients. Cancer Epidemiol Biomarkers Prev. 2012;21:45–52. doi: 10.1158/1055-9965.EPI-11-0815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.United States, Department of Health and Human Services, Centers for Disease Control and Prevention (cdc) Justification of Estimates for Appropriation Committees. Atlanta, GA: CDC; n.d. [Available online at: http://www.cdc.gov/fmo/topic/Budget%20Information/appropriations_budget_form_pdf/FY2015_CJ_CDC_FINAL.pdf; cited March 13, 2015] [Google Scholar]
- 43.The Guide to Community Preventive Services. What Works: Cancer Prevention and Control: Cancer Screening. Evidence-Based Interventions for Your Community. Atlanta, GA: The Guide to Community Preventive Services; 2012. [Google Scholar]
- 44.Centers for Disease Control and Prevention Cancer screening—United States, 2010. MMWR Morb Mortal Wkly Rep. 2012;61:41–5. [PubMed] [Google Scholar]